Abstract
Mammalian polo-like kinase (Plk) acts at various stages in early and late mitosis. Plk is phosphorylated and activated in mitosis, and the proper subcellular localization of Plk is essential for mitotic regulation. We have observed that overexpression of the C-terminal domain of Plk is more effective than wild-type or kinase-defective Plk in causing mitotic delay or arrest. The specific activity of Plk with C-terminal deletions or substitution of aspartate for threonine-210 is increased severalfold relative to wild type. We show in this communication that the C-terminal domain can bind to full-length or the catalytic domain of Plk and inhibit its kinase activity, and that this binding is disrupted when threonine-210 is substituted with an aspartic acid residue. The C-terminal domain binds unphosphorylated Plk from G2 arrested cells, but not phosphorylated Plk from mitotic cells. Green fluorescent protein–C-terminal Plk is localized at the centrosome and the midbody of transfected cells as shown previously for full-length enzyme. These and other data indicate that although the C terminus serves to regulate Plk kinase activity, the localization of the C terminus at the centrosome and other sites in transfected cells may block the correct localization of endogenous Plk.
Keywords: intramolecular binding‖inhibition‖mitosis‖mammalian cells
Cell-cycle progression in eukaryotic cells depends on the periodic control of various cyclin-dependent kinases (cdks) (for reviews see refs. 1 and 2). Other conserved serine/threonine protein kinases, including the polo-like kinases (Plks), also play important roles in the regulation of cell-cycle events (3). Plks have high homology in their N-terminal catalytic domain as well as highly conserved sequences, often referred to as a polo-box(es), in the C-terminal noncatalytic domain (4). In Drosophila and yeast, Plk is involved in bipolar spindle assembly, spindle pole organization (5, 6), and cytokinesis (7). Microinjection of Plk antibody into mammalian cells indicates that Plk functions in centrosome maturation (8). Plk activity is required for degradation of cyclin B (9, 10), onset of mitosis, and exit from mitosis (3, 11, 12). The polo-box motif(s) have not been observed in proteins other than Plks and its functions have not yet been fully characterized. Expression in budding yeast of mammalian Plk with mutations in the polo-box shows that it is required for localization to spindle poles and the bud neck. Moreover, Cdc5 and Plk with polo-box mutations fail to support mitotic functions in budding yeast (13, 14).
The protein level of Plk fluctuates in a cell-cycle-dependent manner, increasing in S and G2/M, and rapidly decreasing at the end of mitosis (15, 16). In addition, the specific protein kinase activity of mitotic Plk is severalfold greater than that of interphase Plk, and it appears that phosphorylation of Plk is an important posttranslational regulatory event (15–17). Xenopus polo-like kinase kinase1 (xPlkk1) phosphorylates and activates Plx1 in vitro (18). The activating phosphorylation event may occur on T210 of Plk, which is located in the T-loop or activation loop of the enzyme; mutation of this residue in Plk (T201 in Plx1) to aspartic acid to mimic the effect of phosphorylation elevates kinase activity, and Plx T201V is not activated in vivo (19–21).
Recently, mammalian Plk was reported to be phosphorylated by STE20-related protein kinases in vitro (22). In addition to positive regulation by upstream kinases, there is evidence that the C terminus of Plk also regulated its activity. Previously, Lee and Erikson (17) and Mundt et al. (20) found that Plk with a deleted C terminus possesses severalfold increased kinase activity when it is expressed in Sf9 cells and when it is translated in vitro.
The study reported here indicates directly the functions of the C-terminal domain in mammalian cell systems. We present evidence that the C-terminal region of Plk regulates its kinase activity through intramolecular binding. In addition, the Plk C-terminal domain expressed in vivo is correctly localized to the centrosome and blocks progression through mitosis. These data demonstrate that the C terminus of Plk has a critical role both in the regulation of its protein kinase activity and in localization of the enzyme, which influence progression through mitosis.
Materials and Methods
Cell Culture and DNA Transfections.
HeLa and FT210 cells were maintained in DMEM (GIBCO) supplemented with 10% (vol/vol) FBS (HyClone) at 37°C and 33°C, respectively. For G2 and mitotic arrest, FT210 cells (23) were incubated at 39°C in the absence of and in presence of 200 ng/ml nocodazole for 18 h, respectively. Sf9 cells were grown in monolayer at 28°C in IPL-41 insect medium (Sigma) supplemented with 10% (vol/vol) FBS. High Five (Hi5) cells were grown in monolayer at 28°C in EX-CELL 401 medium (JRH Biosciences, Lenexa, KS).
The standard calcium chloride technique of transfection into HeLa cells was carried out according to the method of Chen and Okayama (24) except for the use of Hepes-buffered saline (HBS). Cells were transfected with 20–30 μg of plasmid DNA per 100-mm plate. For fluorescence-activated cell sorter (FACS) analysis and fluorescence microscopy, 2–3 μg of pEGFP-F vector (CLONTECH, 6074-1) was cotransfected as a marker.
Preparation of Epitope-Tagged Plk Constructs.
The full-length DNAs of murine wild-type Plk and of the corresponding K82M mutant were amplified from Ycplac111-GAL1-HA (13) clones by PCR using 5′-GAATTCCTATGAATGCAGTGGCC-3′ and 5′-GAATTCTAGGAGGCCTTGAGGC-3′ as forward and reverse primers, which contain EcoRI sites. T210D mutant of Plk was created by PCR with a pair of oligonucleotides with the following sequences: 5′-AACGAAAGAAGGACTTGTGTGG-3′ and 5′-CCACACAAGTCCTTCTTTCGTT-3′. The C-terminal construct (amino acids 306–603) was generated by PCR using 5′-GAATTCACTTCTGGCTACATCCCC-3′ as forward primer and the same reverse primer as used for the full-length Plk construct. All PCR products were cloned into pGEX vector (Promega) and sequenced. Proteins were expressed in Escherichia coli by IPTG induction, and were confirmed by Western blot with anti-Plk antibody. The Plk ΔC1–401 construct was generated by the double digestion of pGEX-Plk WT with EcoRI and BamHI. To generate baculovirus for expression in insect cells, the constructs were recloned from pGEX to pAcGHLT vector (PharMingen), and transfected into Sf9 cells with BaculoGold (PharMingen). For expression in mammalian cells, the various DNA constructs were recloned from pGEX into pCMV-Tag2 vector (Stratagene), which encodes eight amino acids (DYKDDDDK) of the FLAG epitope at the N terminus.
Flow Cytometry Analyses and Fluorescence Microscopy.
Transfected cells were detached from the plates by trypsinization, fixed in ice-cold 80% ethanol for 16–24 h at 4°C, and stained in PBS supplemented with 15 μg/ml of propidium iodide and 100 μg/ml of RNase A for 30 min at room temperature. Bivariate measurements of green [green fluorescent protein (GFP)] and red (PI-DNA content) fluorescence were made with FACScan (Becton Dickinson). A gate was set to select GFP-positive cells with a green fluorescent signal at least 40 times stronger than that in negative cells. Data were collected [>7,000 events with the FL1 signal (GFP)] and analyzed with CELLQUEST software (Becton Dickinson) and MODFIT LT 2.0 (Verity Software House, Topsham, ME) (25, 26).
For fluorescence microscopy, HeLa cells were seeded onto cover slips and 24 h later were transfected as previously described. Forty hours posttransfection, the cells were fixed in 4% paraformaldehyde-PBS solution followed by treatment with ice-cold methanol. After washing with PBS-0.1% Triton X-100 (PBST), the cells were incubated for 2 h at room temperature in PBST supplemented with propidium iodide (10 μg/ml). Immunostaining of γ-tubulin was performed using a mouse anti-γ-tubulin antibody (Sigma, T6557) and a Cy3-conjugated goat anti-mouse antibody (Jackson ImmunoResearch). The mounted coverslips were analyzed by fluorescence microscopy, and the visualized cells and images were captured on the LSM510 imaging system (Zeiss).
Preparation of Cell Lysates.
Cells were lysed in Nonidet P-40 extraction buffer (0.5% Nonidet P-40/50 mM Hepes, pH7.4/150 mM NaCl/1 mM DTT/5 mM EGTA/1 mM EDTA/20 mM NaF/20 mM β-glycerophosphate/0.5 mM Na3VO4/20 mM p-nitrophenyl phosphate) supplemented with protease inhibitors. The lysate was centrifuged at 15,000 × g for 20 min, and the supernatant was recovered.
Immunoprecipitation and Kinase Reactions.
About 1 mg in 1 ml of clarified cell lysates was immunoprecipitated for kinase reactions. The following antibodies and dilution conditions were used: monoclonal anti-FLAG (Sigma) at 2 μg/ml; monoclonal anti-Plk (Zymed) at 2.5 μg/ml. Protein A- or G-Sepharose (Zymed) was added and incubated for an additional 2–3 h to adsorb the antibody. Kinase reactions were carried out as described (20). Briefly, the kinase activity of Plk was measured in a solution (50 mM Tris⋅Cl, pH7.5/10 mM MgCl2/5 mM DTT/2 mM EGTA/0.5 mM Na3VO4/20 mM p-nitrophenyl phosphate) supplemented with 4 μg of casein (Sigma) and 25 μM ATP (10 μCi of [γ-32P]ATP; 1 Ci = 37 GBq) at 30°C for 30 min.
In Vitro Binding Assay.
In the binding assay, ≈1–2 μg of glutatione S-transferase (GST)-fused C terminus Plk proteins was incubated with 2 mg/ml of mutant FLAG-tagged Plk-transfected HeLa cell lysate in Nonidet P-40 extraction buffer (except 1% Nonidet P-40) at 4°C for various times. For quantification of the proteins expressed, 10% of total lysates (about 100–200 μg) were analyzed on SDS/PAGE and by Western blot. The GST-fused protein was adsorbed with glutathione-agarose (Sigma) and the associated proteins were detected by western analysis.
Results
Expression of the C-Terminal Domain of Plk Induces Mitotic Arrest.
In yeast, the conserved C terminus of Plk is required for its localization and mitotic functions (13, 14, 16). To investigate the physiological effect of the C-terminal domain on biological activity in mammalian cells, HeLa cells were transfected with various FLAG epitope-tagged constructs and pEGFP-F, an expression construct encoding a farnesylated enhanced GFP as a transfection marker. Cellular DNA was stained with propidium iodide, and cell-cycle profiles of transfected cells were determined by flow cytometry.
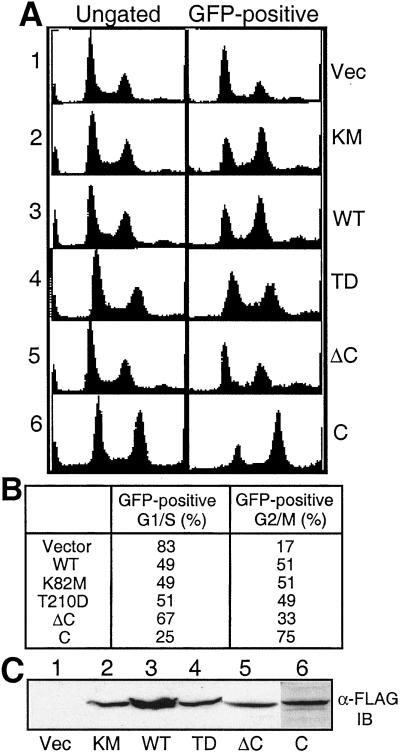
A majority (83%) of cells transfected with the empty vector were in the G1/S phase of the cell cycle. Analysis of control cells revealed 17% had a DNA content corresponding to the G2/M population (Fig. 1 A1, and B). Expression of either WT or K82M Plk resulted in an increase (51%) in the G2-M population (Fig. 1 A, 2 and 3, and B), consistent with a previous report by Mundt et al. (17). The constitutively active T210D construct had an effect similar to that of WT and K82M (Fig. 1 A4 and B), whereas the C-terminal truncation mutant (ΔC1–401) produced less of an effect on the accumulation of G2/M cells than full-length constructs (Fig. 1A5). Strikingly, expression of the C-terminal domain (C306–603) resulted in the accumulation of 75% of the cells with G2/M DNA content (Fig. 1 A6 and B).
Figure 1.
Transient expression of the C-terminal domain in HeLa cells induces mitotic arrest. (A) HeLa cells were cotransfected with pEGFP-F and various pCMV-FLAG-Plk constructs and fixed as described in Materials and Methods. DNA profiles of GFP-positive cells were determined by fluorescence-activated cell sorter (FACS) analysis. Left (ungated) and Right (GFP-positive) represent the DNA profiles from total cell populations and GFP-positive transfected cells after gating, respectively. 1, DNA profiles from transfectants with pCMV-FLAG vector; 2, with pCMV-FLAG-Plk K82M; 3, with pCMV-FLAG-Plk WT; 4, with pCMV-FLAG-Plk T210D; 5, with pCMV-FLAG-ΔC1–401; 6, with pCMV-FLAG-C306–603. (B) The percent of G2/M phase cells determined from the GFP-positive population by ModFit analysis. Data represent three independent experiments. (C) Western blot analysis of expressed proteins from transfected cell lysates with anti-FLAG antibody.
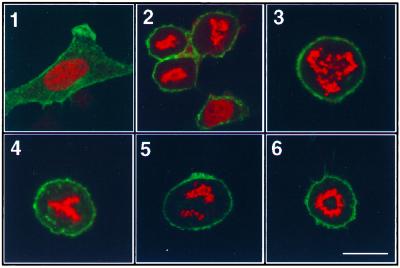
Figure 2.
Expression of the C-terminal domain induces accumulation of cells with mitotic phenotypes. (A) A membrane-bound EGFP (green) expression construct was used as the transfection marker, and the DNA was labeled with propidium iodide (red) after transfection as previously described. Most of the cells transfected with control vector are in interphase (1). Cells transfected with the C306–603 construct accumulate in M-phase (2). In some transfected mitotic cells, chromosomes appear to be randomly distributed (3), misaligned (4), or unevenly segregated (5). The circular chromosome is similar to the phenotype first observed in Drosophila polo mutants (6). (Scale bar, 15 μm.)
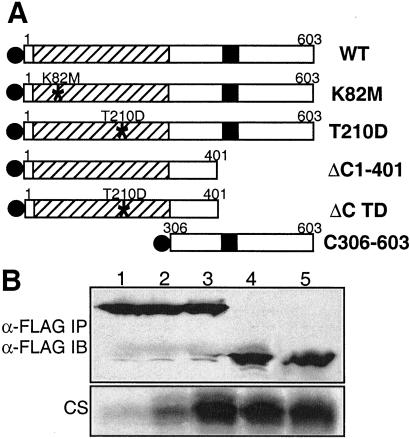
Figure 3.
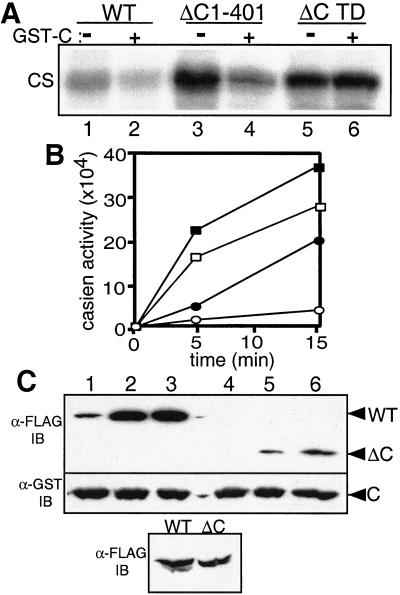
Schematic representation of Plk1 proteins and kinase activity of immunocomplexes. (A) Plk wild-type and mutant proteins used in this study. The catalytic domain and polo-box are represented by deviant lined box and filled box, respectively. FLAG epitopes on the N-termini are shown as filled circles. From top to bottom: full-length wild-type Plk (WT); kinase-defective mutant Plk (K82M); Plk mutated Thr-210 to Asp (T210D); Plk deleted of the C-terminal amino acids 402–603 (ΔC1–401); N-terminal domain containing the mutation, T210D (ΔC TD); Plk deleted of the N-terminal amino acids 1–305 (C306–603). (B) Kinase activities of Plk proteins immunoprecipitated from transfected HeLa cell lysates. The amounts of immunoprecipitated FLAG-Plk proteins were detected with anti-FLAG antibody (Upper). The kinase activity of FLAG-Plk was measured by incubation with casein for 30 min as described in Materials and Methods (Lower, CS). 1, FLAG-Plk K82M; 2, FLAG-Plk WT; 3, FLAG-Plk T210D; 4, FLAG-N terminus Plk (ΔC1–401); 5, FLAG-N terminus Plk containing T210D mutation (ΔC TD).
To further characterize the cell-cycle and cytological effects of the inhibitory C-terminal domain, HeLa cells transfected with either the vector or the Plk C-terminal domain constructs and the pEGFP-F transfection marker were stained with propidium iodide and visualized by fluorescence microscopy. Cells transfected with the control vector appeared to cycle normally and most of the cells were in G1/S phase (Fig. 2A1). In contrast, nearly half of the cells transfected with C-terminal domain constructs were in mitosis (Fig. 2A2), and many transfected cells displayed aberrant chromosome alignment and segregation (Fig. 2A, 3–6). Immunostaining of centrosomes with γ-tubulin antibody revealed the monopolar or multipolar spindle segregation (data not shown) from these abnormal cells. These findings indicate that normal mitotic progression is disrupted by expression of the C-terminal domain of Plk.
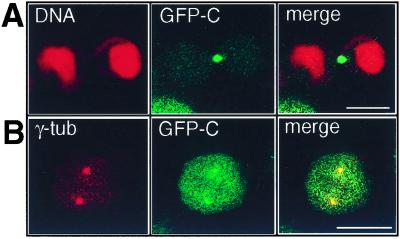
Figure 6.
Localization of GFP-Plk C terminus in HeLa cells. HeLa cells were transfected with GFP-C, fixed, and analyzed as described in Materials and Methods. (A) The central midbody is intensely labeled with GFP in a telophase cell (GFP-C, green). The chromosomal DNA is stained with propidium iodide (DNA, red). (B) GFP-C is localized predominantly at centrosomes in a prometaphase cell (GFP-C). γ-tubulin on centrosomes is stained with anti-γ-tubulin antibody. (Scale bars, 15 μm.)
The C-Terminal Domain of Plk Binds to Plk and Inhibits Its Kinase Activity.
Previous results suggested that Plk catalytic activity is regulated by its C terminus. A C-terminal deletion mutant of Plk immunoprecipitated from Sf9 cells or translated in vitro in a cell-free system, has severalfold higher catalytic activity than wild-type Plk (17, 20).
In this study, we have confirmed the increased activity of T210D and C-terminal deletion mutants of Plk in mammalian cells. The structure of the various Plk constructs analyzed is illustrated schematically in Fig. 3A. These recombinant proteins were expressed in HeLa cells by transfection, immunoprecipitated with anti-FLAG antibody, and their kinase activity assayed with casein as a substrate. Substitution of Thr-210 with Asp increased the kinase activity several- fold (Fig. 3B, lane 3), and a C-terminal deletion mutant (ΔC1–401) also displayed increased kinase activity in HeLa cells (lane 4). Plk ΔC1–401 containing substitution of Thr-210 with Asp (ΔC TD) had a level of activity similar to that of the T210D and ΔC1–401 mutants (lane 5), indicating that the effects of the modification of Thr-210 and deletion of the C-terminal region are not additive.
Our first direct evidence that C-terminal domain participates in intramolecular interactions came from yeast two-hybrid screens; with the C-terminal amino acids 323–499 as a bait, a prey encoding amino acids 501–603 was frequently detected. In a complementary screen using the amino acids 501–603 as bait, preys encoding the catalytic domain and the polo-box (amino acids 238–510) were identified (C.-Y.L., M. L. Madson, and R.L.E., unpublished data). To determine whether its C-terminal domain can bind to Plk and affect its kinase activity, we created a construct of a GST fused to C-terminal sequences (C306–603, Fig. 3A), and used this purified protein in binding and kinase assays.
FLAG-Plk WT and FLAG-ΔC1–401 were immunoprecipitated from transfected HeLa cells and assayed for casein kinase activity in the presence of the GST-C terminus (C306–603). The catalytic activity of both wild-type and ΔC1–401 decreased on incubation with GST-C306–603 (Fig. 4A, lanes 2 and 4). In contrast, the kinase activity of a C-terminal deletion containing the Thr-210 to Asp substitution (FLAG-ΔC TD) was not affected by the GST-C terminus (lanes 5 and 6).
Figure 4.
The C-terminal domain of Plk inhibits its kinase activity. (A) Inhibitory effect of the C-terminal domain. In in vitro kinase assays, immunoprecipitated FLAG-Plk WT and FLAG-N-terminal domains from transfected HeLa cells were incubated with or without 1–2 μg of C-terminal domain protein C306–603 (GST-C), which was purified from High Five (Hi5) cells. The Plk activity was measured with casein as a substrate (CS). 1, 3, and 5, incubated without GST-C; 2, 4, and 6, incubated with GST-C. (B) Time course of the wild-type and N-terminal Plk kinase activity. Purified wild-type and N-terminal Plk proteins from Hi5 cells were preincubated with or without an equal molar level of purified C-terminal protein for 20 min at 30°C, and then assayed for protein kinase activity. The reactions were stopped by adding SDS/PAGE sample buffer. The samples were boiled and resolved by PAGE. The casein bands were excised from the gel and the radioactivity was quantitated by liquid scintillation spectrometry. Filled squares, ΔC1–401 protein; open squares, ΔC1–401 with C306–603; filled circles, Plk WT; open circles, Plk WT with C306–603. (C) Binding of the C terminus to wild-type and N-terminal Plk. GST-fused C-terminal construct, expressed in Hi5 cells, was incubated with HeLa cell lysates, which had been transfected with FLAG-tagged Plk constructs. After incubation for various times, GST-fused proteins were precipitated with glutathione-agarose beads. 1 and 4, incubation for 5 min; 2 and 5, incubation for 30 min; 3 and 6, incubation for 2 h. The wild-type (1–3) and N-terminal (4–6) Plk proteins associated with the GST-C-terminal domain were detected by Western blot analysis (α-FLAG IB). After stripping the FLAG signal, the membrane was incubated with GST antibody to quantify the GST-C protein (α-GST IB). The levels of wild-type and N-terminal Plk (WT and ΔC) in 10% of total lysates were analyzed by Western analysis (α-FLAG IB).
Additional analysis with the FLAG-tagged Plk constructs expressed in HeLa cells indicated that the activity of the purified ΔC1–401 mutant was greater than that of wild-type Plk (Fig. 4B, filled square and circle). The kinase activities of both wild-type Plk and the ΔC1–401 mutant decreased on incubation with C306–603 protein. Incubation with C306–603 inhibited about 78% and 36% of wild-type and ΔC1–401 respectively (Fig. 4B, open square and circle). It should be noted that our unpublished results show that the C terminus is not a substrate of Plk and thus is not a competitive inhibitor for casein.
To determine whether the difference in inhibition is due to a difference in binding to the C-terminal domain, experiments to measure interaction were carried out using GST-pull down assays. GST-C306–603 was incubated with extracts of cells expressing FLAG-Plk WT or FLAG-ΔC1–401 for various times (Fig. 4C). Much less ΔC1–401 than WT was bound to the GST construct. This may explain the lower level of inhibition of ΔC1–401 by the C terminus (Fig. 4C, lanes 4–6). To exclude the possibility that the C-terminal domain is involved in the dimerization of Plk, different epitope-tagged Plk constructs (HA and FLAG) were cotransfected into HeLa cells. After immunoprecipitation with anti-HA antibody, no bound FLAG-Plk was detected (data not shown).
Plk Thr-210 Modification and C306–603 Binding.
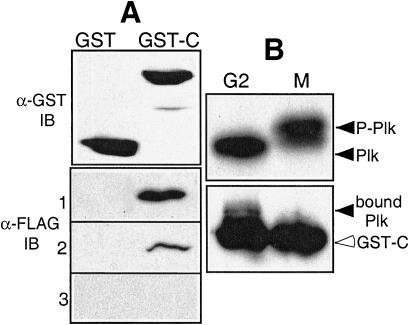
To establish the relationship between modification of Plk and binding of the C-terminal domain, we first tested whether substitution of Thr-210 with an acidic residue, which appears to mimic phosphorylation, would attenuate binding of C306–603. GST or GST-C306–603 was incubated with extracts of cells expressing FLAG-tagged WT, ΔC1–401, or ΔC TD. As shown in Fig. 5A, GST did not bind any FLAG-tagged Plk constructs. Wild-type Plk and ΔC1–401 were readily detected in association with GST C306–603. In contrast, ΔC TD did not associate with GST-C306–603. These data indicate that the T210D mutation and perhaps also phosphorylation can dissociate C-terminal sequences from the catalytic domain.
Figure 5.
The C-terminal domain of Plk binds to Plk, and does not bind to phosphorylated mitotic Plk. (A) GST-fused C-terminal protein C306–603 (GST-C) purified from High Five cells was incubated with lysates of HeLa cells that had been transfected with various FLAG-tagged Plk constructs. 1, FLAG-Plk WT protein bound to GST-C; 2, FLAG-ΔC1–401 protein bound to GST-C; 3, FLAG-ΔC1–401 protein (T210D) bound to GST-C. The Plk proteins associated with the GST-C-terminal domain were detected by Western blot analysis with anti-FLAG antibody (α-FLAG IB). The amount of GST or GST-C protein used was detected with anti-GST antibody (α-GST IB). (B) Binding of endogenous Plk to C-terminal domain (GST-C). GST-fused C306–603 protein was incubated with lysates of FT210 cells that were arrested in G2 or mitosis as previously described, and was collected on glutathione-agarose. Endogenous Plks displayed phosphorylated M-phase and dephosphorylated G2 forms (Upper) and endogenous Plk (filled arrowhead) associated with the C306–603 protein (GST-C, opened arrowhead) were detected by Western blot with anti-Plk antibody (Lower). The cell lysates were analyzed on 8% SDS/PAGE gel (acryl:bis = 120:1) (15).
We examined the effect of Plk phosphorylation on C-terminal domain binding, using mitotic- and G2-arrested FT210 cell lysates in the GST-pull down assay. Plk is phosphorylated in nocodazole-treated cells and migrates more slowly on SDS/PAGE (Fig. 5B Upper, M). When FT210 cells are cultured at the restrictive temperature, they lack Cdc2 protein kinase activity and are arrested in G2, and Plk is unphosphorylated in these cells (Upper, G2). When the purified C-terminal domain was incubated with mitotic- or G2-arrested cell lysates, unphosphorylated Plk from the G2-arrested cells associated with the C terminus, whereas activated Plk from the mitotic cell lysate was undetected (Fig. 5B Lower). These findings indicate that the phosphorylated form of Plk or aspartic acid at position 210 attenuates the interaction with the C-terminal region, and that phosphorylation of Thr-210 releases the activation loop from C-terminal inhibition and contributes to the activation of Plk.
Localization of GFP-C306–603 in Transfected Cells.
To further evaluate the significance of the phenotype observed in the transfected cells shown in Figs. 1 and 2, and the data on regulation of protein kinase activity in vitro, the localization of the C terminus was analyzed. The images presented in Fig. 6 show that GFP-C-306–603 is localized at the midbody and the centrosome (Fig. 6 A and B). These locations were previously observed for full-length Plk in mammalian cells (16, 27) and provide additional support for the observation that mutations in the polo-box of Plk disrupt normal localization in Saccharomyces cerevisiae (13, 14). It should be noted that because transfected cells are heterogeneous with regard to protein expression, these images were obtained from cells that are apparently expressing low levels of GFP-C306–603, based on the intensity of the GFP signal. Other cells in the population display a more intense signal and the GFP-C terminus appears to be more diffusely distributed. The cells expressing higher levels of C306–603 appear to be arrested in mitosis as shown in Fig. 2.
Discussion
Reversible phosphorylation is a key regulatory mechanism for protein kinases, including members of the Plk family. Phosphorylation of Thr-210 in the catalytic domain of Plk serves to activate Plk (Y.-J.J. and R.L.E., unpublished data). In addition to phosphorylation, the C terminus of Plk (amino acids 306–603) also participates in regulation of its kinase activity. In this communication, we show that cells transfected with the C-terminal domain arrested in mitosis and displayed aberrant chromosome alignment and segregation, reminiscent of the mutant phenotype reported for Drosophila polo (Figs. 1 and 2; refs. 5 and 6). This finding caused us to focus on the potential of the C terminus to regulate the protein kinase activity of Plk.
C-terminal deletion mutants of Plk expressed in Sf9 cells (20), in a cell-free system (17), or in mammalian cells (Fig. 3, lane 4) have increased catalytic activity, indicating that the C-terminal domain may inhibit Plk activity. Thus, it is possible that the C terminus disrupts the cell cycle by inhibiting endogenous Plk activity. The potential of the C terminus to regulate Plk may result from binding negative regulators of Plk, or through intramolecular interactions of the C terminus with the catalytic domain. Two-hybrid screens and in vitro binding experiments (Figs. 4C and 5A) suggest that the C-terminal domain participates in intramolecular interaction and is involved in the inhibitory regulation of Plk activity (Fig. 4 B and C). The effect of modification of Thr-210 and deletion of the C terminus is not additive (Fig. 3B, lane 5), indicating that phosphorylation and C-terminal deletions have, in part, a similar influence on catalytic activity. This possibility is supported by the observation that the kinase activity of the catalytic domain with the T210D mutation is not inhibited by C-terminal domain (Fig. 4A, lane 6), nor does it bind the C terminus (Fig. 5A). This result is consistent with the observation that phosphorylated Plk from mitotic cells did not bind the C terminus (Fig. 5B), suggesting that a result of the phosphorylation of Thr-210 is the release from inhibition by the C-terminal domain. Moreover, attempts to detect interaction between full-length phosphorylated Plk and the C terminus in transfected cells that are arrested in M-phase have failed. Thus, although the C terminus contributes to regulating the catalytic activity of unphosphorylated Plk, it does not interact with the phosphorylated form of the M-phase enzyme.
Disruption of the cell cycle by the C terminus may result from competition for localization sites of Plk, however. We have examined the subcellular localization of Plk C terminus in HeLa cells and detected GFP-Plk C-terminal protein in association with the midbody of the cleavage furrow between dividing cells (Fig. 6A) and with centrosomes (Fig. 6B). Although we captured these images from cells that expressed low levels of the GFP construct, other cells that expressed higher levels of the GFP-C terminus showed abnormal mitotic phenotypes (Fig. 2A). It is unclear whether the localization of the C terminus is due to its interaction with structural proteins in the centrosome or the midbody, or to colocalizing with endogenous Plk at these sites, because we have shown that full-length Plk binds the C terminus. However, at these stages in the cell cycle, Plk is highly phosphorylated and this activated form of Plk cannot bind the C terminus tightly (Fig. 5). Moreover, expression of the C-terminal domain with mutations in the polo box that disrupt normal localization at the centrosome does not result in the accumulation of an excessive number of cells with a G2/M DNA content (data not shown).
This observation suggests the phenotype of cells expressing the C terminus is the result of binding and occupying sites normally required for endogenous Plk localization and function. It appears there is a multiplicity of phenotypic abnormalities during metaphase or anaphase in cells expressing high levels of the C terminus (Fig. 2, 2–6), which may be the result of defective spindle formation. It is likely that the C terminus disrupts endogenous Plk localization with a variety of targets that include but are not limited to the centrosome and the central spindle or to the midbody. Previously, others have reported that wild-type and kinase-defective Plk both produced an increase in mitotic index and multinucleated cells (17). These authors also suggest that this may be caused by titration of proteins interacting with Plk. The large number of cells arrested in mitosis suggests that checkpoint mechanisms may have been activated because of centrosome and spindle abnormalities (28, 29), which might be caused by expression of the C terminus of Plk.
An unsolved puzzle raised by these data are the strong interaction found between full-length Plk and the C-terminal domain (Fig. 4C). This result suggests to us that the C terminus may contribute to dimerization of Plk; however, we have failed to detect robust interactions between full-length Plks with HA or FLAG tags coexpressed in cells. It will be important to determine the residues in the C terminus required for the in vitro interaction of the C terminus with full-length enzyme and for the cell-cycle perturbations observed. Identification of such sequences may provide the basis to design specific inhibitors of polo-like kinase. In summary, we have provided evidence for an autoinhibition and a subcellular localization of the C terminus of Plk, and demonstrated that the C-terminal domain is sufficient for disrupting cell-cycle progression and mitotic processes.
Acknowledgments
We thank E. Erikson for helpful discussion and critical reading of this manuscript. This work was supported by National Institutes of Health Grant GM59172. R.L.E. is the John F. Drum American Cancer Society Research Professor.
Abbreviations
- Plk
polo-like kinase
- GST
glutathione S-transferase
- GFP
green fluorescent protein
- EGFP
enhanced GFP
- WT
wild type
References
- 1.Morgan D O. Nature (London) 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 2.Nigg EA. BioEssays. 1995;17:471–480. doi: 10.1002/bies.950170603. [DOI] [PubMed] [Google Scholar]
- 3.Glover D M, Hagan I M, Tavares A M. Genes Dev. 1998;12:3777–3787. doi: 10.1101/gad.12.24.3777. [DOI] [PubMed] [Google Scholar]
- 4.Clay F J, McEwen S J, Bertoncello I, Wilks A F, Dunn A R. Proc Natl Acad Sci USA. 1993;90:4882–4886. doi: 10.1073/pnas.90.11.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunkel C E, Glover D M. J Cell Sci. 1988;89:25–38. doi: 10.1242/jcs.89.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Llamazares S, Moreira A, Tavares A, Girdham C, Spruce B A, Gonzalez C, Karess R E, Glover D M, Sunkel C E. Genes Dev. 1991;5:2153–2165. doi: 10.1101/gad.5.12a.2153. [DOI] [PubMed] [Google Scholar]
- 7.Ohkura H, Hagan I M, Glover D M. Genes Dev. 1995;9:1059–1073. doi: 10.1101/gad.9.9.1059. [DOI] [PubMed] [Google Scholar]
- 8.Lane R J, Nigg E A. J Cell Biol. 1996;135:1701–1713. doi: 10.1083/jcb.135.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Descombes P, Nigg E A. EMBO J. 1998;17:1328–1335. doi: 10.1093/emboj/17.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirayama M, Zachariae R, Ciosk R, Nasmyth K. EMBO J. 1998;17:1336–1349. doi: 10.1093/emboj/17.5.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumagai A, Dunphy W G. Science. 1996;273:1377–1380. doi: 10.1126/science.273.5280.1377. [DOI] [PubMed] [Google Scholar]
- 12.Nigg E A. Curr Opin Cell Biol. 1998;10:776–783. doi: 10.1016/s0955-0674(98)80121-x. [DOI] [PubMed] [Google Scholar]
- 13.Lee K S, Grenfell T Z, Yarm F R, Erikson R L. Proc Natl Acad Sci USA. 1998;95:9301–9306. doi: 10.1073/pnas.95.16.9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee K S, Song S, Erikson R L. Proc Natl Acad Sci USA. 1999;96:14360–14365. doi: 10.1073/pnas.96.25.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamanaka R, Smith M R, O'Connor P M, Maloid S, Mihalic K, Spivak J L, Longo D L, Ferris D K. J Biol Chem. 1995;270:21086–21091. doi: 10.1074/jbc.270.36.21086. [DOI] [PubMed] [Google Scholar]
- 16.Lee K S, Yuan Y L, Kuriyama R, Erikson R L. Mol Cell Biol. 1995;15:7143–7151. doi: 10.1128/mcb.15.12.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundt K E, Golsteyn R M, Lane H A, Nigg E A. Biochem Biophys Res Commun. 1997;239:377–385. doi: 10.1006/bbrc.1997.7378. [DOI] [PubMed] [Google Scholar]
- 18.Qian Y-W, Erikson E, Maller J L. Science. 1998;282:1701–1704. doi: 10.1126/science.282.5394.1701. [DOI] [PubMed] [Google Scholar]
- 19.Qian Y-W, Erikson E, Maller J L. Mol Cell Biol. 1999;19:8625–8632. doi: 10.1128/mcb.19.12.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee K S, Erikson R L. Mol Cell Biol. 1997;17:3408–3417. doi: 10.1128/mcb.17.6.3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian Y-W, Erikson E, Maller J L. Mol Cell Biol. 1998;18:4262–4271. doi: 10.1128/mcb.18.7.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellinger-Ziegelbauer H, Karasuyama H, Yamada E, Tsujikawa K, Todokoro K, Nishida E. Genes Cells. 2000;5:491–498. doi: 10.1046/j.1365-2443.2000.00337.x. [DOI] [PubMed] [Google Scholar]
- 23.Th'ng J P, Wright P S, Hamaguchi J, Lee M G, Norbury C J, Nurse P, Bradbury E M. Cell. 1990;63:313–324. doi: 10.1016/0092-8674(90)90164-a. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Okayama H. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang W, Hunter T. BioTechniques. 1998;24:348–354. [PubMed] [Google Scholar]
- 26.Pestov D G, Polonskaia M, Lau L F. BioTechniques. 1999;26:102–106. doi: 10.2144/99261st04. [DOI] [PubMed] [Google Scholar]
- 27.Arnaud L, Pines J, Nigg E A. Chromosoma. 1998;107:424–429. doi: 10.1007/s004120050326. [DOI] [PubMed] [Google Scholar]
- 28.Gardner R D, Burke D J. Trends Cell Biol. 2000;10:154–158. doi: 10.1016/s0962-8924(00)01727-x. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson M M, Tavares A A, Ohkura H, Deak P, Glover D M. J Cell Biol. 2001;153:663–676. doi: 10.1083/jcb.153.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]