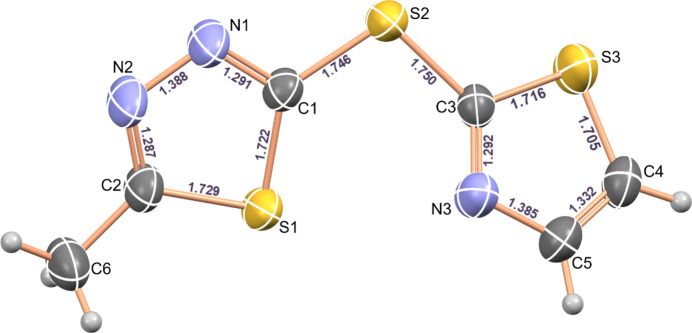
The title compound contains two biologically active heterocyclic rings, 1,3,4-thiadiazole and 1,3-thiazole, connected via a sulfur atom. The packing is consolidated by non-classical intermolecular C—H⋯N hydrogen bonds and π–π stacking interactions.
Keywords: 1,3,4-thiadiazole; 1,3-thiazole; C—H⋯N interaction; crystal structure; Hirshfeld surface analysis
Abstract
The title compound, C6H5N3S3, consists of two biologically relevant heterocyclic units, suggesting potential biological activity and possible use as a ligand in metal complexation. The compound crystallizes in the monoclinic space group P21/c and features non-classical intermolecular C—H⋯N hydrogen bonds, along with π–π stacking interactions that contribute to the crystal cohesion. Hirshfeld surface analysis highlights significant intermolecular interactions including, among others, N⋯H/H⋯N, S⋯H/H⋯S, and S⋯C/C⋯S contacts.
1. Chemical context
Derivatives combining 1,3,4-thiadiazole and 1,3-thiazole moieties offer significant potential in medicinal chemistry due to their enhanced biological activity, pharmacokinetic profiles and structural versatility. This class of compounds is being actively explored in various therapeutic areas, including their use as antimicrobial (Booq et al., 2021 ▸; Hussain et al., 2022 ▸), anticancer (Shaikh et al., 2024 ▸; Altıntop et al., 2017 ▸; Dawood et al., 2013 ▸), anti-inflammatory (Arshad et al., 2022 ▸) and neuroprotective agents. With ongoing research into their SAR, bioavailability, and environmental impact, these derivatives are promising candidates for the next generation of drug development.
The structural fusion of 1,3,4-thiadiazole and 1,3-thiazole is expected to have synergistic biological effects due to their different modes of action. Thiadiazoles are often involved in enzyme inhibition and interaction with metal ions, while thiazoles enhance interactions with biological targets such as nucleic acids or proteins.
Herein, we report the synthesis and crystal structure of a new heterocyclic compound with combination of 1,3,4-thiadiazole and 1,3-thiazole fragments. This 2-thiazole-substituted derivative can act as a chelating ligand.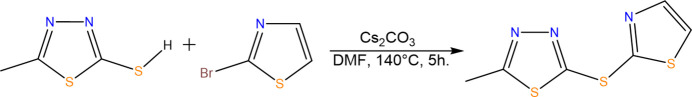
2. Structural commentary
The title compound (Fig. 1 ▸) crystallizes in the monoclinic system, space group P21/c. The molecular structure of the compound is shown in Fig. 1 ▸. The geometric parameters of the thiadiazole and thiazole rings are close to standard values and the values reported for related structures. (Renier et al., 2023 ▸; Luqman et al., 2016 ▸; Burnett et al., 2015 ▸; Dani et al., 2014 ▸; Weidner et al., 2008 ▸; Jumal et al., 2006 ▸; Kennedy et al., 2004 ▸; Hipler et al., 2003 ▸). The N—N and endocyclic C—S bonds are shorter than classical single bonds (1.4 and 1.81 Å), indicating partial double-bond character. At the same time, the C=N bonds are somewhat longer (∼0.02 Å) than the corresponding double bond, as a result of conjugation within the ring systems. These facts confirm the aromaticity of both rings. The exocyclic C—S bond is shortened since it includes carbon atoms with sp2 hybridization. Deviation of the bond angles from 120° in the 1,3,4-thiadiazole and 1,3-thiazole rings is a common feature in five-membered rings (Bharty et al., 2012 ▸). The C—S—C bond angles in the 1,3,4-thiadiazole and 1,3-thiazole rings of the title compound are 86.62 (8) and 89.25 (9)°, respectively, and the C1—S2—C3 bond angle outside the ring is 103.82 (8)°. The thiadiazole and thiazole rings do not lie in the same plane, subtending a dihedral angle of 32.61 (10)°. No intramolecular hydrogen bonds are observed.
Figure 1.
A view of the molecular structure of 5-methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole, showing the atom labeling and bond lengths. Displacement ellipsoids are drawn at the 50% probability level.
3. Supramolecular features and energy framework calculations
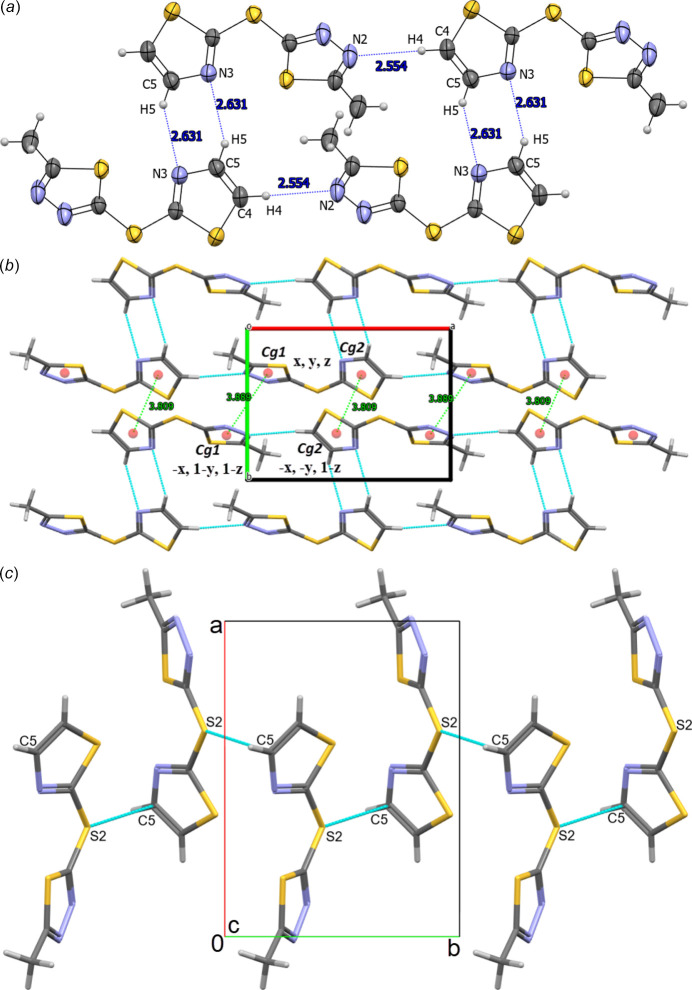
The crystal packing is consolidated by C5—H5⋯N3ii hydrogen bonds [symmetry code: (ii) x + 1, y, z + 1], forming a six-membered  (6) ring motif (Grabowski, 2020 ▸; Etter et al., 1990 ▸). Along the a-axis direction, cohesion of the crystal packing is achieved by C4—H4⋯N2i hydrogen bonds [symmetry code: (i) x + 1, y, z] between the methine group of the 1,3-thiazole ring and the nitrogen atom of the 1,3,4-thiadiazole ring of a nearby molecule. The geometrical parameters of intermolecular hydrogen bonds are shown in Table 1 ▸ and Fig. 2 ▸a.
(6) ring motif (Grabowski, 2020 ▸; Etter et al., 1990 ▸). Along the a-axis direction, cohesion of the crystal packing is achieved by C4—H4⋯N2i hydrogen bonds [symmetry code: (i) x + 1, y, z] between the methine group of the 1,3-thiazole ring and the nitrogen atom of the 1,3,4-thiadiazole ring of a nearby molecule. The geometrical parameters of intermolecular hydrogen bonds are shown in Table 1 ▸ and Fig. 2 ▸a.
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C4—H4⋯N2i | 0.93 | 2.55 | 3.472 (2) | 169 |
| C5—H5⋯N3ii | 0.93 | 2.63 | 3.392 (3) | 139 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Figure 2.
(a) Overview of intermolecular C4—H4⋯N2 and C5—H5⋯N3 hydrogen bonds (shown in blue), (b) a view of the π–π stacking interactions (hydrogen bonds are shown in blue and π–π stacking interactions are shown in green). and (c) Highlight of S2⋯C5 chalcogen interactions (dashed lines) along the b-axis direction, with relevant atoms labeled.
In the supramolecular structure of the compound, weak π–π-stacking interactions are found (Fig. 2 ▸b) between thiadiazole rings (symmetry operation −x, 1 − y, 1 − z) with an intracentroid distance of 3.889 (9) Å and between thiazole rings (symmetry operation −x, −y, 1 − z) with a centroid-to-centroid distance of 3.809 (9) Å. Similarly, the structure also exhibits intermolecular chalcogen bond between C5 of the thiazole ring and the bridging S2 atom [C5⋯S2(1 − x, −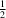 + y,
+ y,  − z) = 3.491 (2) Å] (Fig. 2 ▸c).
− z) = 3.491 (2) Å] (Fig. 2 ▸c).
The interaction energies of the hydrogen-bond system were calculated within the molecules using the B3LYP method (B3LYP/6-31G (d, p) in CrystalExplorer 21.5 (Spackman et al., 2021 ▸). The total energy (Etot) is the sum of Coulombic (Eele), polar (Epol), dispersion (Edis) and repulsive (Erep) contributions. The four energy components were scaled in the total energy: Etot = 1.057Eele + 0.74Epol + 0.871Edis + 0.618Erep. The interaction energies were investigated for a 3.8 Å cluster around the reference molecule. The results give a total interaction energy of −141 kJ mol−1 involving electrostatic (−74.3 kJ mol−1), polarization (−12.2 kJ mol−1), dispersion (−146.9 kJ mol−1) and repulsion (125 kJ mol−1) components.
4. Hirshfeld surface analysis
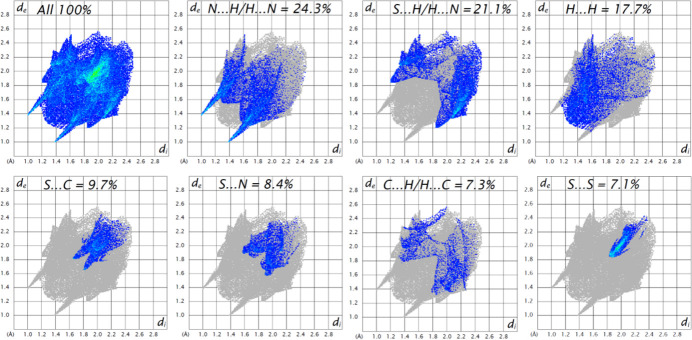
To further investigate the intermolecular interactions present in the title compound, a Hirshfeld surface analysis was performed, and the two-dimensional (2D) fingerprint plots were generated with CrystalExplorer17 (Spackman et al., 2021 ▸). Fig. 3 ▸ shows the three-dimensional (3D) Hirshfeld surface of the complex plotted over dnorm (normalized contact distance). The hydrogen-bond interactions given in Table 1 ▸ play a key role in the molecular packing of the complex.
Figure 3.
View of the three-dimensional Hirshfeld surface of the molecule plotted over dnorm.
The overall 2D fingerprint plot and those divided into interatomic interactions are shown in Fig. 4 ▸. The Hirshfeld surface analysis shows that 24.3% of the intermolecular interactions are from N⋯H/H⋯N contacts, 21.1% from S⋯H/H⋯S contacts, 17.7% from H⋯H contacts and 9.7% are from S⋯C/C⋯S contacts, while other contributions are from C⋯H/H⋯C, S⋯C/C⋯S and S⋯N/N⋯S contacts (Fig. 4 ▸).
Figure 4.
The full two-dimensional fingerprint plot for the title compound, showing all interactions, and those delineated into separate interactions with the percentage contributions of various interatomic contacts occurring in the crystal.
5. Database survey
A survey of the Cambridge Structural Database performed using ConQuest software (CSD, Version 5.46, last updated November 2024; Groom et al., 2016 ▸) revealed that 122 crystal structures have been reported for the 2-methyl-1,3,4-thiadiazole-5-thiol fragment; among them, 73 structures are related to organometallic compounds. There are mostly organic thiol-substituted compounds reported, because of the good reactivity of the thiol group. In addition, there are three organic structures based on the 2-methyl-1,3,4-thiadiazole-5-thiol fragment (CILHAI, Dani et al., 2013 ▸; GEXWOY, Zhao et al., 2010 ▸; XICMOO, Cabral et al., 2018 ▸), which can bind in a bidentate manner with metal atoms to form six-membered rings. Similar to C6H5N3S3, chalcogen-bonding interactions were observed in both structures. In CILHAI, S—N chalcogen interactions occur where both nitrogen atoms of the thiadiazole ring interact with the bridging sulfur atom and the sulfur atom of an adjacent thiadiazole ring. In XICMOO, a chalcogen interaction is present between a sulfur atom and a carbon atom of a neighboring benzene ring.
6. Synthesis and crystallization
A solution of 5-methyl-1,3,4-thiadiazole-2-thiol (0.01 mol) and 2-bromothiazole (0.01 mol) in DMF (10 ml) in presence of cesium carbonate was stirred for 5 h at 413 K. DMF was distilled off with a rotary evaporator. The resulting brown concentrate was dissolved in DCM/MeOH and separated by flash column chromatography. The synthesized amorphous product 2-methyl-5-(1,3-thiazol-2-ylsulfanyl)-1,3,4-thiadiazole was light yellow in color (m.p. 327 K). Further recrystallization gave crystals suitable for X-ray diffraction (yield: 60%).
7. Refinement
Crystal data, data collection and structure refinement details are summarized in Table 2 ▸. H atoms were positioned geometrically (C—H = 0.93–0.96 Å) and refined as riding with Uiso(H) = 1.2Ueq(C) or 1.5Ueq(C-methyl).
Table 2. Experimental details.
| Crystal data | |
| Chemical formula | C6H5N3S3 |
| M r | 215.31 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 296 |
| a, b, c (Å) | 10.6463 (2), 7.7151 (2), 11.1774 (3) |
| β (°) | 103.272 (1) |
| V (Å3) | 893.56 (4) |
| Z | 4 |
| Radiation type | Mo Kα |
| μ (mm−1) | 0.77 |
| Crystal size (mm) | 0.13 × 0.1 × 0.06 |
| Data collection | |
| Diffractometer | Bruker D8 VENTURE Kappa Duo PHOTON II CPAD |
| Absorption correction | Multi-scan (SADABS; Krause et al., 2015 ▸) |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 18027, 2296, 1988 |
| R int | 0.052 |
| (sin θ/λ)max (Å−1) | 0.677 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.034, 0.088, 1.05 |
| No. of reflections | 2296 |
| No. of parameters | 110 |
| H-atom treatment | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.50, −0.48 |
Supplementary Material
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989025004980/dx2067sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989025004980/dx2067Isup3.hkl
CCDC reference: 2455808
Additional supporting information: crystallographic information; 3D view; checkCIF report
Acknowledgments
BT would like to acknowledge a CSIR–TWAS fellowship and also the FAIRE programme provided by the Cambridge Crystallographic Data Centre (CCDC) for the use of the Cambridge Structural Database (CSD) and associated software.
supplementary crystallographic information
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Crystal data
| C6H5N3S3 | F(000) = 440 |
| Mr = 215.31 | Dx = 1.600 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 10.6463 (2) Å | Cell parameters from 8027 reflections |
| b = 7.7151 (2) Å | θ = 3.5–28.7° |
| c = 11.1774 (3) Å | µ = 0.77 mm−1 |
| β = 103.272 (1)° | T = 296 K |
| V = 893.56 (4) Å3 | Block, colourless |
| Z = 4 | 0.13 × 0.1 × 0.06 mm |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Data collection
| Bruker D8 VENTURE Kappa Duo PHOTON II CPAD diffractometer | 1988 reflections with I > 2σ(I) |
| φ and ω scans | Rint = 0.052 |
| Absorption correction: multi-scan (SADABS; Krause et al., 2015) | θmax = 28.7°, θmin = 3.6° |
| h = −13→14 | |
| 18027 measured reflections | k = −10→10 |
| 2296 independent reflections | l = −15→15 |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.034 | H-atom parameters constrained |
| wR(F2) = 0.088 | w = 1/[σ2(Fo2) + (0.0307P)2 + 0.4384P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max = 0.001 |
| 2296 reflections | Δρmax = 0.50 e Å−3 |
| 110 parameters | Δρmin = −0.48 e Å−3 |
| 0 restraints |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.18439 (4) | 0.24766 (7) | 0.46938 (4) | 0.04704 (14) | |
| S2 | 0.34737 (4) | 0.41561 (7) | 0.71120 (4) | 0.04642 (14) | |
| S3 | 0.61254 (5) | 0.45960 (7) | 0.67293 (6) | 0.05644 (16) | |
| N1 | 0.10297 (15) | 0.3506 (3) | 0.65446 (15) | 0.0558 (4) | |
| N2 | −0.00530 (15) | 0.2958 (3) | 0.56825 (16) | 0.0591 (5) | |
| N3 | 0.46784 (15) | 0.2117 (2) | 0.57237 (16) | 0.0497 (4) | |
| C1 | 0.20708 (15) | 0.3340 (2) | 0.61488 (15) | 0.0384 (3) | |
| C2 | 0.02170 (17) | 0.2417 (3) | 0.46812 (17) | 0.0449 (4) | |
| C3 | 0.47049 (15) | 0.3452 (2) | 0.64267 (14) | 0.0358 (3) | |
| C4 | 0.67326 (17) | 0.3165 (3) | 0.58452 (17) | 0.0460 (4) | |
| H4 | 0.756109 | 0.320593 | 0.570753 | 0.055* | |
| C5 | 0.58447 (17) | 0.1979 (3) | 0.53809 (18) | 0.0479 (4) | |
| H5 | 0.599905 | 0.110958 | 0.485673 | 0.058* | |
| C6 | −0.07813 (19) | 0.1783 (4) | 0.3609 (2) | 0.0606 (6) | |
| H6A | −0.089445 | 0.261709 | 0.295572 | 0.091* | |
| H6B | −0.158370 | 0.162416 | 0.384862 | 0.091* | |
| H6C | −0.050886 | 0.069815 | 0.333151 | 0.091* |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0320 (2) | 0.0677 (3) | 0.0452 (2) | −0.00771 (18) | 0.01673 (17) | −0.0140 (2) |
| S2 | 0.0357 (2) | 0.0601 (3) | 0.0451 (2) | −0.00303 (18) | 0.01261 (17) | −0.0143 (2) |
| S3 | 0.0371 (2) | 0.0589 (3) | 0.0750 (4) | −0.0130 (2) | 0.0165 (2) | −0.0186 (3) |
| N1 | 0.0346 (7) | 0.0911 (13) | 0.0452 (8) | −0.0027 (8) | 0.0164 (6) | −0.0116 (9) |
| N2 | 0.0312 (7) | 0.0991 (14) | 0.0502 (9) | −0.0052 (8) | 0.0160 (7) | −0.0104 (9) |
| N3 | 0.0372 (7) | 0.0557 (9) | 0.0591 (9) | −0.0067 (7) | 0.0175 (7) | −0.0144 (8) |
| C1 | 0.0336 (7) | 0.0455 (9) | 0.0382 (8) | 0.0005 (6) | 0.0128 (6) | 0.0004 (7) |
| C2 | 0.0313 (8) | 0.0600 (11) | 0.0456 (9) | −0.0036 (7) | 0.0131 (7) | −0.0005 (8) |
| C3 | 0.0292 (7) | 0.0421 (8) | 0.0354 (7) | 0.0000 (6) | 0.0058 (6) | 0.0023 (6) |
| C4 | 0.0319 (8) | 0.0571 (11) | 0.0507 (10) | 0.0024 (7) | 0.0131 (7) | 0.0058 (8) |
| C5 | 0.0387 (9) | 0.0568 (11) | 0.0512 (10) | 0.0035 (8) | 0.0163 (8) | −0.0054 (8) |
| C6 | 0.0377 (9) | 0.0926 (17) | 0.0509 (11) | −0.0108 (10) | 0.0087 (8) | −0.0101 (11) |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Geometric parameters (Å, º)
| S1—C1 | 1.7222 (17) | N3—C3 | 1.292 (2) |
| S1—C2 | 1.7295 (17) | N3—C5 | 1.385 (2) |
| S2—C1 | 1.7460 (17) | C2—C6 | 1.490 (3) |
| S2—C3 | 1.7496 (16) | C4—H4 | 0.9300 |
| S3—C3 | 1.7162 (16) | C4—C5 | 1.332 (3) |
| S3—C4 | 1.705 (2) | C5—H5 | 0.9300 |
| N1—N2 | 1.388 (2) | C6—H6A | 0.9600 |
| N1—C1 | 1.291 (2) | C6—H6B | 0.9600 |
| N2—C2 | 1.287 (2) | C6—H6C | 0.9600 |
| C1—S1—C2 | 86.62 (8) | N3—C3—S3 | 115.16 (12) |
| C1—S2—C3 | 103.82 (8) | S3—C4—H4 | 125.0 |
| C4—S3—C3 | 89.25 (9) | C5—C4—S3 | 109.93 (13) |
| C1—N1—N2 | 111.92 (15) | C5—C4—H4 | 125.0 |
| C2—N2—N1 | 112.76 (15) | N3—C5—H5 | 121.9 |
| C3—N3—C5 | 109.47 (15) | C4—C5—N3 | 116.16 (17) |
| S1—C1—S2 | 129.53 (9) | C4—C5—H5 | 121.9 |
| N1—C1—S1 | 114.64 (13) | C2—C6—H6A | 109.5 |
| N1—C1—S2 | 115.66 (14) | C2—C6—H6B | 109.5 |
| N2—C2—S1 | 114.04 (14) | C2—C6—H6C | 109.5 |
| N2—C2—C6 | 123.05 (17) | H6A—C6—H6B | 109.5 |
| C6—C2—S1 | 122.91 (14) | H6A—C6—H6C | 109.5 |
| S3—C3—S2 | 117.96 (10) | H6B—C6—H6C | 109.5 |
| N3—C3—S2 | 126.86 (13) | ||
| S3—C4—C5—N3 | −1.7 (2) | C2—S1—C1—S2 | −173.80 (14) |
| N1—N2—C2—S1 | 1.3 (3) | C2—S1—C1—N1 | 1.12 (17) |
| N1—N2—C2—C6 | −179.1 (2) | C3—S2—C1—S1 | −13.37 (15) |
| N2—N1—C1—S1 | −0.7 (2) | C3—S2—C1—N1 | 171.75 (15) |
| N2—N1—C1—S2 | 174.98 (15) | C3—S3—C4—C5 | 1.02 (15) |
| C1—S1—C2—N2 | −1.35 (17) | C3—N3—C5—C4 | 1.6 (3) |
| C1—S1—C2—C6 | 179.1 (2) | C4—S3—C3—S2 | 178.37 (11) |
| C1—S2—C3—S3 | 156.31 (10) | C4—S3—C3—N3 | −0.16 (15) |
| C1—S2—C3—N3 | −25.35 (18) | C5—N3—C3—S2 | −179.10 (14) |
| C1—N1—N2—C2 | −0.4 (3) | C5—N3—C3—S3 | −0.7 (2) |
5-Methyl-2-[(1,3-thiazol-2-yl)sulfanyl]-1,3,4-thiadiazole. Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C4—H4···N2i | 0.93 | 2.55 | 3.472 (2) | 169 |
| C5—H5···N3ii | 0.93 | 2.63 | 3.392 (3) | 139 |
Symmetry codes: (i) x+1, y, z; (ii) −x+1, −y, −z+1.
References
- Altıntop, M. D., Ciftci, H. I., Radwan, M. O., Sever, B., Kaplancıklı, Z. A., Ali, T. F. & Özdemir, A. (2017). Molecules23, 59. https://doi.org/10.3390/molecules23010059
- Arshad, M. F., Alam, A., Alshammari, A. A., Alhazza, M. B., Alzimam, I. M., Alam, M. A., Mustafa, G., Ansari, M. S., Alotaibi, A. M., Alotaibi, A. A., Kumar, S., Asdaq, S. M. B., Imran, M., Deb, P. K., Venugopala, K. N. & Jomah, S. (2022). Molecules27, 3994–3994. [DOI] [PMC free article] [PubMed]
- Bharty, M. K., Bharti, A., Dani, R. K., Kushawaha, S. K., Dulare, R. & Singh, N. K. (2012). Polyhedron41, 52–60.
- Booq, R. Y., Tawfik, E. A., Alfassam, H. A., Alfahad, A. J. & Alyamani, E. J. (2021). Antibiotics10, 1480. [DOI] [PMC free article] [PubMed]
- Bruker (2016). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Burnett, M. E., Johnston, H. M. & Green, K. N. (2015). Acta Cryst. C71, 1074–1079. [DOI] [PubMed]
- Cabral, L. I., Brás, E. M., Henriques, M. S., Marques, C., Frija, L. M., Barreira, L., Paixão, J. A., Fausto, R. & Cristiano, M. L. S. (2018). Chem. A Eur. J.24, 3251–3262. [DOI] [PubMed]
- Dani, R. K., Bharty, M. K., Kushawaha, S. K., Paswan, S., Prakash, O., Singh, R. K. & Singh, N. K. (2013). J. Mol. Struct.1054–1055, 251–261.
- Dani, R. K., Bharty, M. K., Paswan, S., Singh, S. & Singh, N. K. (2014). Inorg. Chim. Acta421, 519–530.
- Dawood, K. M., Eldebss, T. M., El-Zahabi, H. S., Yousef, M. H. & Metz, P. (2013). Eur. J. Med. Chem.70, 740–749. [DOI] [PubMed]
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
- Etter, M. C., MacDonald, J. C. & Bernstein, J. (1990). Acta Cryst. B46, 256–262. [DOI] [PubMed]
- Grabowski, S. J. (2020). Crystals10, 130–130.
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Hipler, F., Winter, M. & Fischer, R. A. (2003). J. Mol. Struct.658, 179–191.
- Hussain, Z., Pengfei, S., Yimin, L., Shasha, L., Zehao, L., Yifan, Y., Linhui, L., Linying, Z. & Yong, W. (2022). Pathogens and Disease80(1), 1–11. [DOI] [PubMed]
- Jumal, J. & Yamin, B. M. (2006). Acta Cryst. E62, o2893–o2894.
- Kennedy, A. R., Khalaf, A. I., Suckling, C. J. & Waigh, R. D. (2004). Acta Cryst. E60, o1510–o1512.
- Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. (2015). J. Appl. Cryst.48, 3–10. [DOI] [PMC free article] [PubMed]
- Luqman, A., Blair, V. L., Brammananth, R., Crellin, P. K., Coppel, R. L. & Andrews, P. C. (2016). Eur. J. Inorg. Chem. pp. 2738–2749.
- Renier, O., Bousrez, G., Smetana, V., Mudring, A. V. & Rogers, R. D. (2023). CrystEngComm25, 530–540.
- Shaikh, S. A., Wakchaure, S. N., Labhade, S. R., Kale, R. R., Alavala, R. R., Chobe, S. S., Jain, K. S., Labhade, H. S. & Bhanushali, D. D. (2024). BMC Chem.18, 119–119. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2015a). Acta Cryst. A71, 3–8.
- Sheldrick, G. M. (2015b). Acta Cryst. C71, 3–8.
- Spackman, P. R., Turner, M. J., McKinnon, J. J., Wolff, S. K., Grimwood, D. J., Jayatilaka, D. & Spackman, M. A. (2021). J. Appl. Cryst.54, 1006–1011. [DOI] [PMC free article] [PubMed]
- Weidner, T., Ballav, N., Zharnikov, M., Priebe, A., Long, N. J., Maurer, J., Winter, R., Rothenberger, A., Fenske, D., Rother, D., Bruhn, C., Fink, H. & Siemeling, U. (2008). Chem. Eur. J.14, 4346–4360. [DOI] [PubMed]
- Zhao, Y., Ouyang, G. P., Xu, W. M., Jin, L. H. & Yuan, K. (2010). Chin. J. Org. Chem.30, 1093–1097.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I. DOI: 10.1107/S2056989025004980/dx2067sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989025004980/dx2067Isup3.hkl
CCDC reference: 2455808
Additional supporting information: crystallographic information; 3D view; checkCIF report