Abstract
Acute tubulointerstitial nephritis (ATIN) is a leading cause of acute kidney injury (AKI) and acute kidney disease. It is characterized by interstitial inflammation and tubular injury, often triggered by medications, infections, or autoimmune disorders. Prompt diagnosis and treatment are crucial to prevent irreversible kidney damage; however, nonspecific clinical and laboratory findings pose diagnostic challenges. Although kidney biopsy remains the gold standard, its invasive nature and potential complications necessitate the exploration of alternative noninvasive strategies.
Emerging biomarkers offer promising noninvasive tools for diagnosing ATIN and differentiating it from other causes of AKI and acute kidney disease. Biomarker applications, as an alternative, are viewed through the lens of distinct immune reaction subtypes, including variations in type IV hypersensitivity mechanisms. Biomarkers such as urinary CXC chemokine ligand (CXCL)9 and cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-9 reflect T-cell polarization and specific inflammatory pathways, shedding light on T helper (Th)1- and Th2-mediated immune responses. Among these, the urinary CXCL9-to-creatinine ratio demonstrates high sensitivity and specificity, with well-defined thresholds guiding clinical decisions. Urinary retinol-binding protein and serum C-reactive protein (CRP) have also been explored, particularly in immune checkpoint inhibitor (ICPI)-associated AKI. However, their nonspecificity and overlap with other AKI etiologies limit their utility in isolating ATIN-specific pathways.
This review highlights the need for integrating biomarker-based approaches with a broader understanding of immune heterogeneity and histologic correlation to improve diagnostic precision. Future studies should focus on validating biomarker panels that capture diverse inflammatory endotypes, enabling early diagnosis and personalized management. By acknowledging the complexity of immune reactions underlying ATIN, this approach aims to enhance clinical decision-making while minimizing the need for invasive diagnostics, ultimately improving patient outcomes.
Keywords: acute kidney injury, acute tubulointerstitial nephritis, immune endotypes, type iv hypersensitivity, urinary biomarkers
The tubular structures and connective tissue in the renal parenchyma, excluding the glomeruli, are referred to as the tubulointerstitial area. Although this region does not directly participate in plasma filtration, the tubulointerstitium plays a critical primary or secondary role in fundamental renal functions such as glomerular filtration, electrolyte and acid-base balance, drug and toxin excretion, and hormonal activity.1
Kidney damage characterized by inflammation in the tubulointerstitial area, independent of glomerular diseases, is defined as tubulointerstitial nephritis. ATIN often manifests as AKI with a sudden and significant decline in renal function, although slower loss of kidney function akin to acute kidney disease may also ensue. When a kidney biopsy is performed, it typically reveals edema, tubular damage, tubulitis, and inflammation enriched with mononuclear cells such as T lymphocytes as well as eosinophils, plasma cells, mast cells, and macrophages in the tubulointerstitial area. Interstitial fibrosis and tubular atrophy are less prominent in the acute phase but may develop over time with ongoing inflammation.
ATIN can also develop in the course of various glomerular diseases, but these cases are not diagnosed as separate ATIN entities. It is important to recognize that kidney biopsy is typically only performed in patients with a significant loss of kidney function, potentially overlooking milder or subclinical cases. As a result, current definitions reflect only a subset of symptomatic patients and may not fully capture the true prevalence and diversity of ATIN.
Epidemiologic data on ATIN are limited to detected or incidental cases. Although early diagnosis can prevent permanent kidney damage and end-stage kidney disease, missed cases pose significant risks. Enhanced diagnostic methods are needed to improve early detection, support timely intervention, and provide a clearer epidemiologic understanding.
Based on kidney biopsy records and registries, the incidence of ATIN ranges between 1% and 3% for all patients biopsied, and 15% and 27% for patients who undergo biopsy due to AKI.1 In addition, 2% of chronic kidney disease (CKD) cases and 3% to 4% of incident end-stage kidney disease cases are associated with ATIN. Although these rates seem low, considering that CKD has an overall prevalence of 10% to 15%, ATIN potentially affects millions of people.2 Notably, a recent global analysis highlighted that anticancer therapies, particularly immunotherapies, have significantly contributed to the rising incidence of ATIN and AKI in recent years.3
Pathogenesis
The unique microanatomy and perfusion properties of the tubulointerstitium facilitate the accumulation of circulating molecules, predisposing to hypersensitivity reactions that contribute to ATIN pathogenesis.2 Although delayed-type cellular hypersensitivity (type 4) plays a prominent role, other hypersensitivity types or mixed reactions are also possible (Table 1).4
Table 1.
Updated nomenclature of hypersensitivity reactions as proposed by the EAACI position papera
| Type | Mechanism | Clinical examples | Key players |
|---|---|---|---|
| Type I: immediate (IgE-mediated) | IgE antibodies bind to allergens, leading to mast cell and basophil degranulation and mediator release. | Allergic rhinitis, asthma, atopic dermatitis, anaphylaxis, food, venom, and drug allergies. | Th2 cells, basophils, mast cells, IgE antibodies. |
| Type II: antibody-mediated cytotoxic | IgG or IgM antibodies bind to cell surface antigens, activating complement or engaging immune cells to induce cell lysis. | Hemolytic anemia, Goodpasture syndrome, myasthenia gravis, autoimmune neutropenia, and transfusion reactions. | IgG/IgM antibodies, complement system, NK cells, macrophages. |
| Type III: immune complex–mediated | Formation of antigen-antibody complexes deposit in tissues, triggering complement activation and inflammation. | Systemic lupus erythematosus, rheumatoid arthritis, post-streptococcal glomerulonephritis, hypersensitivity pneumonitis, serum sickness. | IgG/IgM antibodies, immune complexes, neutrophils, complement system. |
| Type IVa: T1 immune response | T1 immune response mediated by Th1 and Tc1 cells, involves macrophage activation and granuloma formation. | Allergic contact dermatitis, chronic hypersensitivity pneumonitis, granulomatous diseases, celiac disease. | Th1, ILC1, Tc1 cells, macrophages, IFN-γ, TNF-α, granuloma formation. |
| Type IVb: T2 immune response | T2 immune response mediated by Th2 cells, involves eosinophilic inflammation and chronic tissue damage. | Chronic asthma, atopic dermatitis, allergic rhinitis, eosinophilic esophagitis, DRESS. | Th2 cells, ILC2, Tc2, eosinophils, mast cells, IL-4, IL-5, IL-13. |
| Type IVc: T3 immune response | T3 immune response mediated by Th17 cells, involves neutrophilic inflammation and extracellular trap formation. | Psoriasis, neutrophilic asthma, AGEP. | Th17, Tc17 cells, ILC3, neutrophils, IL-17, IL-22, extracellular traps. |
| Type V: epithelial barrier defects | Primary defects in epithelial barriers allow allergens and pathogens to penetrate, causing chronic inflammation. | Atopic dermatitis, asthma, chronic rhinosinusitis, food protein-induced enterocolitis syndrome, eosinophilic esophagitis, and celiac disease. | Epithelial cells, innate immune cells, Th2 cells, ILC2, alarmins (IL-25, IL-33, TSLP). |
| Type VI: metabolic-induced immune dysregulation | Metabolic disturbances influence immune responses, leading to chronic low-grade inflammation and barrier leakiness. | Obesity-related asthma, metabolic syndrome–associated inflammation, diabetes-related immune dysregulation. | Adipose tissue-derived cytokines, macrophages, innate immune cells, dysbiosis. |
| Type VII: direct response to chemicals | Direct activation of immune cells by chemicals without traditional allergen-antibody interactions, often through GPCR pathways. | NSAID-exacerbated respiratory and cutaneous diseases, aspirin-exacerbated respiratory disease, drug-induced reactions. | Mast cells, basophils, GPCR pathways (e.g., MRGPRX2), chemical stimuli. |
AGEP, acute generalized exanthematous pustulosis; DRESS, severe drug reaction with eosinophilia and systemic symptoms; EAACI, European Academy of Allergy and Clinical Immunology; GPCR, G protein-coupled receptor; IL, interleukin; ILC, innate lymphoid cell; MRGPCRX2, Mas-related GPCR X2; NK, natural killer cell; NSAID, nonsteroidal anti-inflammatory drug; Tc1, cytotoxic T cell 1; Th, T helper; TSLP, thymic stromal lymphopoietin.
This table was created based on the source article titled "Nomenclature of allergic diseases and hypersensitivity reactions: Adapted to modern needs: An EAACI position paper", providing a detailed overview of the modern classification of hypersensitivity reactions, including mechanisms, clinical examples, and key immune players as proposed by the EAACI.
Histologic analysis typically shows T-cell–predominant mononuclear cell infiltration without significant immune deposits, underscoring the importance of cellular immunity in ATIN. However, ATIN may involve multiple hypersensitivity mechanisms, contributing to its clinical and immunologic heterogeneity.4
Experimental models and clinical cases indicate that type 2 hypersensitivity responses against in situ antigens, such as tubular basement membrane (TBM) antigens, megalin, glycoprotein 3M-1, and uromodulin, can trigger ATIN.5, 6, 7, 8 In addition, the deposition of circulating immune complexes and local immune complex formation may induce type 3 hypersensitivity reactions, promoting inflammation and structural damage.9
Delayed-Type Cellular Hypersensitivity and Phenotypic Evolution of the Immune Response
In type 4 hypersensitivity, antigen exposure activates immune cells (e.g., epithelial cells, innate lymphoid cells, dendritic cells, macrophages, and T cells), leading to inflammation. Depending on antigenic stimulation, this response may evolve into chronic phenotypes, potentially resulting in fibrosis and permanent damage.4
Type 4 hypersensitivity is subclassified into type 4a, 4b, and 4c reactions, driven by specific T cells, mediators, and cellular interactions (Table 1 and Figure 1).4 The pharmacologic interaction with immune receptors concept describes how certain drugs can directly activate T cells through reversible binding to T-cell receptors or human leukocyte antigen (HLA) molecules, contributing to ATIN pathogenesis.4
Figure 1.
T-cell activation and differentiation. The process of T-cell activation begins with antigen recognition by the T-cell receptor (TCR) in the presence of costimulatory signals provided by antigen-presenting cells (APCs). On activation, naive T cells proliferate and differentiate into distinct effector subsets, including CD4+ helper T cells (e.g., Th1, Th2, Th17, Treg), depending on the cytokine milieu and signaling pathways. These differentiated T cells mediate immune responses by producing cytokines, assisting other immune cells, or directly targeting infected or malignant cells. CXCL, CXC chemokine ligand; IFN, interferon; IL, interleukin; MHCII, major histocompatibility complex II; TGF, transforming growth factor; Th, T helper; TNF, tumor necrosis factor; Treg, regulatory T cell.
The Role of Th9 and Th22 Cells4
Th9 cells contribute to immune tolerance and type 2 inflammation by producing IL-9, which induces eosinophilic inflammation and activates mast cells.4 Th22 cells secrete IL-22, promoting epithelial and stromal cell proliferation, thus aiding in tissue repair and protection. These hypersensitivity subtypes are interconnected, leading to diverse clinical presentations. Immunologic assessments in ATIN cases often reveal unique cellular and cytokine profiles, with elevated eosinophil and IgE levels in some patients, indicating varied underlying mechanisms.
Etiology
As previously reviewed, various antigenic stimuli reach the tubulointerstitium, and either directly activate immune cells or trigger immune responses through antigen-presenting cells leading to the development of ATIN. The etiology of ATIN can be categorized as drug-induced (approximately two-thirds of the cases), secondary to infections, associated with systemic diseases, malignancies, idiopathic causes, and transplantation-related causes.
Drug-Induced ATIN
Since the 1950s, the widespread use of drugs has increased rapidly, significantly altering the etiology of ATIN, and this trend is expected to continue.10 In a single-center study conducted between 1993 and 2011, antibiotics were responsible for 49% of biopsy-confirmed ATIN cases, proton pump inhibitors (PPIs) for 14%, and nonsteroidal antiinflammatory drugs (NSAIDs) for 11%.11 Among the most frequently detected drugs were omeprazole (12%), amoxicillin (8%), and ciprofloxacin (8%). In another study, medications accounted for 71% of ATIN cases, with 35% related to antibiotics, 35% to PPIs, 20% to NSAIDs, and 10% to other drugs.12
Trends in drug use, geographic differences, and changes in medical practices can alter the distribution of drug-induced ATIN over time. For example, the widespread use of newly developed drugs or a better understanding of the side-effect profiles of existing medications may bring different drug classes into prominence in the future. ICPIs (8 agents target the PD-1/PD-L1 signaling pathway or the CTLA-4 pathway) are a typical example of this shift.13 Although these drugs enhance the immune system's attack on cancer by preventing cancer cells from evading T cells, they also cause nonspecific immune disinhibition, resulting in frequent and sometimes severe adverse events (59%–85%). ATIN associated with ICPIs typically develops approximately 14 weeks (range: 6–37 weeks) after the initiation of treatment. In 69% of these cases, patients are also using other potentially nephrotoxic drugs. ATIN is identified as the cause of AKI in 83% of the 151 biopsied cases among 429 patients with ICPI-AKI.14,15 Following therapy, most patients (64%) show clinical improvement with either complete or partial renal recovery, although mild kidney impairment persists at 12 months.
Vaccines, including COVID-19 and human papillomavirus vaccines, have been associated with rare cases of biopsy-proven ATIN.16, 17, 18 Recent pharmacovigilance data from VigiBase (1967–2022) show a significant increase in reports of vaccine-associated renal adverse events, including AKI, glomerulonephritis, and ATIN, particularly after 2020.19 COVID-19 mRNA vaccines exhibited significant disproportionality for ATIN (reporting odds ratio: 2.43), as did human papillomavirus vaccines (reporting odds ratio: 1.75). Although a direct causal relationship remains unproven, the temporal association, disproportionate reporting, and immunologic findings suggest more than a coincidental link. These findings highlight the need for vigilance, further research, and careful clinical monitoring to distinguish true vaccine-related reactions from coincidental occurrences.
ATIN Associated With Specific Drugs
The characteristics of drug-induced ATIN cases vary depending on the medication involved. Some examples include the following:
Rifampin-Induced ATIN
Usually associated with prior rifampin exposure or long-term use. Cases may present with fever, gastrointestinal symptoms, and myalgia, with some experiencing hemolysis, thrombocytopenia, and hepatitis. Although anti-rifampin antibodies may be positive, renal biopsies show negative immunofluorescence.20
Beta-Lactam Antibiotics
These cases often present with the classical symptom triad of fever, skin rash, and arthralgia, along with peripheral eosinophilia. Individuals with previous reactions to the same drug group are at higher risk for ATIN.
HLA Genotype Associations
Significant relationships have been identified between certain drugs and specific HLA genotypes21:
Class I HLA Genotypes
Associated with allopurinol, phenobarbital, carbamazepine, lamotrigine, amoxicillin-clavulanate, ticlopidine, dapsone, oxcarbazepine, nevirapine, cotrimoxazole, and abacavir.
Class II HLA Genotypes
Linked with hypersensitivity reactions to drugs such as carbamazepine, allopurinol, phenytoin, and lapatinib. Genotyping may become an important tool for preventing severe or fatal adverse drug reactions in the future.
NSAID-Induced ATIN
Unlike other causes, NSAID-induced cases may present with nephrotic-range proteinuria.22 Half of the reported cases are associated with fenoprofen, and ATIN typically develops after long-term use (average: 6 months).
PPIs and ATIN
The use of PPIs has been associated with both ATIN and the progression of CKD, although the actual lesion is currently unknown.23 Despite their widespread use, the potential role of PPIs in contributing to chronic kidney damage may go unnoticed because of a lack of clinical awareness.
Infection-Related ATIN
Microorganisms can lead to ATIN by directly infecting renal parenchyma, causing acute pyelonephritis, or more commonly, by triggering an immune response in the tubulointerstitium. A wide variety of bacteria, viruses, parasites, mycoplasma, and chlamydia are associated with ATIN. Infections often present with systemic symptoms, making it difficult to distinguish between the effects of the infection itself and the immune response. In addition, the antibiotics used to treat these infections can also contribute to ATIN.24
AKI is commonly observed in infections such as COVID-19, HIV, leptospirosis, and hantavirus. Hantavirus, a zoonotic disease transmitted by rodents, can cause severe back pain, thrombocytopenia, and nephrotic-range proteinuria. In biopsy specimens from hantavirus-related ATIN cases, interstitial inflammation, congestion, and hemorrhage may be detected.2 Higher viral loads may contribute to infection-related ATIN, as suggested by studies on HIV and COVID-19.25,26 In HIV-infected patients, chronic tubulointerstitial nephritis was among the histologic findings, with higher viral loads, diabetes mellitus, and older age being risk factors for kidney disease, indicating a potential link between viral replication and renal outcomes. In COVID-19, a higher SARS-CoV-2 viral load in urine sediment correlated with increased AKI incidence and higher mortality, supporting the idea that viral load may influence kidney injury severity. The spectrum of HIV-related kidney injury is particularly complex, involving direct viral effects, immune-mediated damage, drug toxicity, and contributions from opportunistic infections and comorbidities.27
Certain bacterial infections are characterized by specific immunologic finding. In Table 2, we show the infections and their specific immunologic findings.24
Table 2.
Immunologic findings in specific bacterial infections
| Infection | Specific immunologic findings |
|---|---|
| Staphylococcus infections | Low C3 levels with normal C4 levels and low-titer positive antinuclear antibodies. |
| Streptococcus infections | Kidney biopsy shows strong and widespread positive signals of anti-streptococcal pyrogenic exotoxin B antibodies in tubular epithelial cells and the tubulointerstitium. |
| Other infectious agents | Positivity for IgM-type antibodies. |
In almost all cases, improvement in kidney function is achieved once the underlying infection is adequately treated. However, it is crucial to closely monitor both the infection itself, and the effects of the treatments administered, because they may have significant implications for kidney health.
ATIN Associated With Systemic Diseases
Certain systemic diseases can lead to ATIN by triggering inflammation and immune activity in the kidneys.2,28,29 Examples are reviewed in Table 3. In these systemic diseases, unlike other etiologies, immunofluorescence studies may reveal positive immune deposit staining in the tubulointerstitial area, the TBM, and tubular epithelium. Diagnosis is relatively easier to achieve because of the typically prominent systemic manifestations. However, these conditions carry a higher risk of ATIN progressing to chronic injury, recurrence, and fibrosis, potentially resulting in permanent kidney damage.
Table 3.
Systemic diseases associated with ATIN
| Systemic disease | Key features and findings |
|---|---|
| Sarcoidosis | Mononuclear interstitial infiltrates and noncaseating granulomas (not universally present) in the kidney. |
| Systemic lupus erythematosus (SLE) | Isolated ATIN without glomerular involvement is rare; biopsies show granular immune complex deposits along the TBM. |
| Sjögren’s syndrome | Lymphocytic interstitial infiltrate with chronic tubular damage; AKI is less common. |
| Scleroderma | Rarely results in ATIN due to systemic involvement. |
| TINU syndrome | More frequent in adolescents and young adults; causes severe renal and ocular inflammation. Prognosis is favorable with therapy.86,87 |
| IgG4-related interstitial nephritis | Seen in men aged > 50 years; involves IgG4-positive plasma cell infiltration, inflammatory masses, and ureteral stenosis. It responds well to corticosteroids. |
AKI, acute kidney injury; ATIN, acute tubulointerstitial nephritis; TBM, tubular basement membrane; TINU, tubulointerstitial nephritis and uveitis.
Anti-TBM Antibody-Associated ATIN
Anti-TBM antibody-associated ATIN is caused by an immune response against the tubulointerstitial nephritis antigen located in the TBM.30,31 Linear staining on immunofluorescence is a hallmark feature. Although uncommon, this condition has been reported in drug-related cases and in patients with membranous nephropathy.
Anti–Low-Density Lipoprotein Receptor-Related Protein 2 (Megalin) Antibody Nephropathy (Anti–Brush Border Antibody Disease)
Anti–low-density lipoprotein receptor-related protein 2 antibody nephropathy, also known as anti–brush border antibody disease, is an increasingly recognized autoimmune tubulointerstitial kidney disease, primarily affecting elderly individuals.32 The disease is driven by circulating autoantibodies against the brush border of proximal tubular epithelial cells, with anti–low-density lipoprotein receptor-related protein 2 antibody (megalin) identified as the principal autoantigen.25 Diagnosis is established through indirect immunofluorescence staining that reveals IgG deposition along the TBM and proximal tubular brush borders.
Histologically, kidney biopsies demonstrate widespread tubular damage, loss of the brush border, a variable degree of mixed interstitial inflammation, and regenerative changes. Granular IgG, C3, and C5b9 staining is observed along the TBM and Bowman’s capsule, with IgG4 being the predominant immunoglobulin in deposits. Electron microscopy reveals amorphous dense deposits within the TBM and segmental subepithelial deposits in some glomerular basement membranes; PLA2R and THSD7A staining are negative. Most reported cases involve elderly patients, with findings including IgG4 positive granular immune staining along the TBM and tubulointerstitium, accompanied by IgG4 positive plasma cell infiltration.33, 34, 35 These observations suggest a potential overlap with the IgG4-related disease spectrum. In addition, some patients exhibit positive antinuclear antibodies without meeting diagnostic criteria for lupus.25
A recent study by Murphy et al.36 (2024) involving 67 patients with anti–brush border antibody disease expanded the clinicopathologic spectrum of the disease, particularly highlighting its frequent association with other kidney diseases. Notably, 56.7% of patients in this cohort had a concurrent kidney disease, including membranous nephropathy, IgA nephropathy, lupus nephritis, and crescentic glomerulonephritis. This study emphasized the poor prognosis of anti–brush border antibody disease, with 52.8% of patients requiring dialysis or dying during follow-up, especially among untreated individuals. In addition, it suggests that combination immunosuppressive therapy, particularly corticosteroids with additional agents, may improve outcomes.36
ATIN in Agricultural Communities and Mesoamerican Nephropathy
Mesoamerican nephropathy is a progressive form of CKD predominantly affecting young male agricultural workers in Central America, especially along the Pacific coast.37 Histopathologic findings early in disease are classical ATIN, but over time include tubular atrophy, interstitial fibrosis, and glomerular ischemia.38 The etiology of Mesoamerican nephropathy is multifactorial, with proposed contributing factors such as recurrent heat stress and dehydration from strenuous labor in high temperatures, leading to repeated AKI episodes that may progress to CKD. Additional factors such as exposure to agrochemicals (pesticides and glyphosate) and heavy metals have been suggested, though evidence remains inconclusive. Genetic predisposition may also play a role; however, further research is needed to clarify its significance.38 Clinically, Mesoamerican nephropathy often progresses asymptomatically to advanced CKD, with patients exhibiting reduced glomerular filtration rate and minimal proteinuria. Preventive measures focusing on reducing heat stress, ensuring adequate hydration, and minimizing exposure to potential nephrotoxins are essential.38
ATIN and Illicit Substances
Unregulated herbal products and bodybuilding supplements, often marketed as health drugs, can contribute to ATIN. Clinicians should also be aware of the potential role of illicit drugs and stimulants in causing ATIN, especially in younger individuals.39
Histologic Features of Atin
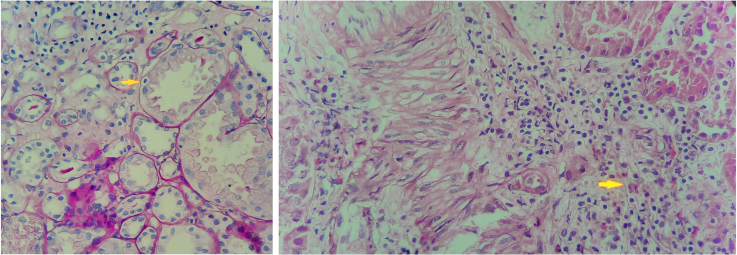
Kidney biopsy is the gold standard for diagnosing ATIN. Pathologically, ATIN is characterized by interstitial edema, tubulitis, and cellular infiltration predominantly composed of T lymphocytes.40 The composition of inflammatory infiltrates varies between cases and may include macrophages, plasma cells, and eosinophils (Figure 2). However, neutrophils are typically sparse, a feature that generally differentiates ATIN from infection. Cellular infiltrates may involve the entire interstitium or remain patchy, but glomeruli and blood vessels generally appear normal unless there is another concomitant disease.
Figure 2.
Kidney biopsies. (Left). Lymphocytes infiltrating the proximal tubule (tubulitis) (yellow arrow), accompanied by atrophic tubules and lymphocyte-dominant interstitial inflammation (periodic acid–Schiff stain, ×100). (Right) Acute tubulointerstitial nephritis of the acute hypersensitivity type. Mixed inflammation with eosinophilic leukocytes (yellow arrow) in the interstitium, accompanied by edema and a neutrophilic cast at the 9 o’clock position (hematoxylin and eosin stain, ×100).
In drug-induced cases, nonnecrotizing granulomas may develop, requiring differential diagnosis to rule out sarcoidosis, tuberculosis, and other infections.41 Unlike ATIN, granulomas in sarcoidosis are usually well-defined, numerous, and confluent. In infectious cases, specific inclusions of viruses, bacteria, or fungi may be present; and necrotizing granulomas can occur in tuberculosis, with pathogens identified by staining or culture methods.
Under light microscopy, the interstitial space shows diffuse or patchy edema, along with lymphocytic dominant cellular infiltration, accompanied by tubulitis.40 Tubulitis involves the invasion of TBMs and epithelial cells by T cells, often associated with acute tubular injury.40 Although electron microscopy does not provide specific findings, NSAID-induced ATIN may exhibit diffuse effacement of podocyte foot processes and a minimal change disease lesion.42
Immunofluorescence studies typically do not reveal specific staining. However, in some cases, granular or linear IgG and complement deposits may be detected along the TBM. Homogeneous linear IgG deposition may suggest anti-TBM disease, though it has also been observed in rare drug-induced ATIN cases, warranting differentiation from necrotizing glomerulonephritis or vasculitis—especially in biopsies without glomeruli.40
Clinical Manifestations
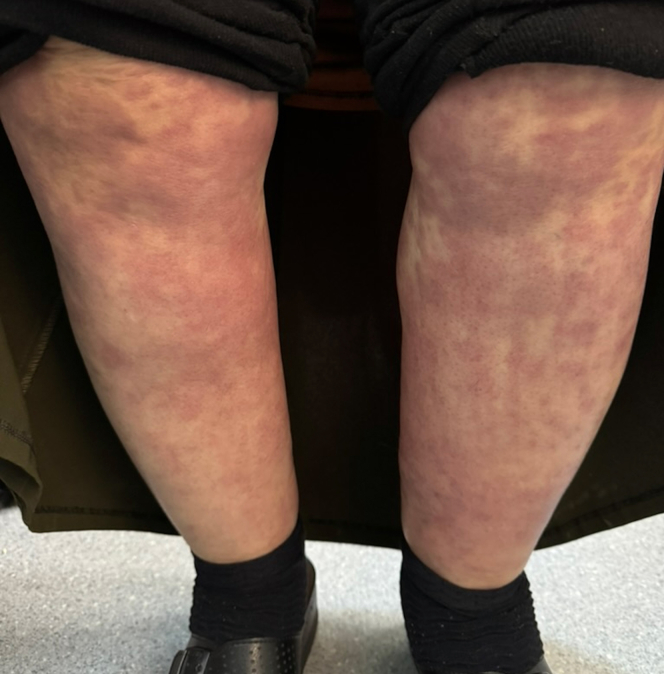
The clinical presentation of ATIN involves both symptoms related to the underlying condition and those related to ATIN. Identifying the timing and progression of symptoms offers key insights into the clinical picture. For example, fever, rash (Figure 3), eosinophilia, AKI, and elevated transaminases developing on the 10th day of beta-lactam antibiotic treatment may suggest drug reaction with eosinophilia and systemic symptoms syndrome.43 However, the presentation of ATIN is often ambiguous and nonspecific, particularly in hospitalized patients with comorbidities. This diagnostic challenge makes it difficult to distinguish ATIN from acute tubular injury/necrosis or other potential lesions, likely contributing to its underdiagnosis.44
Figure 3.
Blanching macular rash in a patient, observed after administration of an oral medication.
Identifying reliable diagnostic markers without a kidney biopsy remains challenging. Therefore, analyzing clinical and laboratory findings in patients with biopsy-confirmed ATIN is essential for better understanding of the condition. In Table 4, we describe the clinical and laboratory findings observed in patients diagnosed with ATIN.44,45 However, these findings are neither specific or sensitive for ATIN.
Table 4.
Clinical, laboratory, and urine diagnostic findings in ATIN
| Category | Finding | Details/prevalence |
|---|---|---|
| Clinical Features | Triad | Fever, rash, and eosinophilia (observed in up to 10%) |
| Rash | 22%–27% | |
| Fever | 15%–36% | |
| Oliguria | 50% | |
| Leukocytosis, eosinophilia | 23%–36% | |
| Pyuria, hematuria | Present in 50%–80% of cases | |
| Proteinuria | Present in ∼90% of cases; nephrotic range is rare | |
| Urine Microscopy | WBC casts | 3%–14% |
| RBC casts | Up to 29% | |
| RTE and granular casts | Up to 86% | |
| Bland urine sediment | ∼20% | |
| Urine Eosinophils | Presence | Sensitivity: 31%; Specificity: 68% |
| Note | Also observed in ATN, GN, and other renal diseases | |
| Urine Chemistries | FENa | Can be >1% or <1% |
| FEUrea | Can be >35% or <35% |
ATIN, acute tubulointerstitial nephritis; ATN, acute tubular necrosis; FENa, fractional excretion of sodium; FEUrea, fractional excretion of urea; GN, glomerulonephritis; RBC, red blood cell; RTE, renal tubular epithelial cell; WBC, white blood cell.
Adapted Nussbaum et al.,44 Clin Kidney J 2019).
Interestingly, in about 20% of biopsy-confirmed cases, urine sediment may be bland and unremarkable, mimicking prerenal azotemia.24 In addition, the triad of fever, rash, and eosinophilia is observed in <10% of cases.24 Eosinophiluria, which is neither a sensitive or specific test, can only be detected under specific conditions, and erythrocyte casts may not always provide definitive differentiation from glomerular diseases.46,47 Proteinuria is commonly low level (tubular), but may reach nephrotic levels with a concomitant glomerulopathy.
Other nonspecific findings include back or flank pain, which occurs in 25% to 40% of patients due to interstitial edema and stretching of the renal capsule.26 Similarly, arthralgia is reported in 25% to 40% of cases as an extrarenal symptom.26
Biomarkers
The primary utility of biomarkers lies in their ability to differentiate ATIN from other etiologies, such as acute tubular injury and glomerulonephritis.48 However, overlapping biomarker profiles in conditions with tubulointerstitial inflammation complicates interpretation.
Different hypersensitivity types (e.g., types 1, 2, 3, 4, or mixed) may produce distinct biomarker patterns. Whereas type 4a hypersensitivity may be detected through T-cell–related markers such as urinary CXCL9 and CXCL10, other types may require biomarkers for humoral or eosinophilic activity. Therefore, biomarker studies should align with the specific immune mechanisms to enhance diagnostic accuracy and clinical relevance.
Ideally, biomarkers should not only support diagnosis but also reflect histologic patterns and disease stages. Given the evolving nature of histologic lesions and inflammation, sampling timing is crucial. Biomarkers should complement clinical judgment within a broader decision-making framework. Future research should focus on biomarker panels that accurately match distinct histologic patterns, akin to kidney biopsy assessments.
ATIN is characterized by immune-mediated inflammation, and distinct immune endotypes have been described based on the polarization of CD4+ T cells.49 The Th1 (type 4a) endotype is driven by the activation of naive CD4+ T cells by antigen-presenting cells, primarily dendritic cells, in the presence of IL-12 and IL-18.49 Differentiated Th1 cells produce high levels of interferon-γ, which activates macrophages and induces the production of proinflammatory cytokines such as TNF-⍺ and chemokines, including CXCL9 (monokine induced by gamma interferon, MIG), CXCL10, and CXCL11. These chemokines, secreted by endothelial cells, fibroblasts, and epithelial cells, play a pivotal role in recruiting immune cells, including T cells and natural killer cells, to the site of inflammation.
Elevated urinary levels of CXCL9, CXCL10, CXCL11, and TNF-⍺ are strongly associated with ATIN and reflect an interferon-γ signature.50 Among these, urinary CXCL9 has emerged as a particularly promising biomarker. Recent studies using internal and external validation cohorts have established cutoff values for the urinary CXCL9-to-creatinine ratio to guide clinical decision-making. Values >58.9 ng/g strongly suggest ATIN, values <14.2 ng/g effectively rule it out, and intermediate levels between 14.2 and 58.9 ng/g identify cases where kidney biopsy may be warranted.51 These findings highlight the diagnostic potential of CXCL9 and its utility in differentiating ATIN from other causes of AKI.
The Th2 (type 4b) endotype is driven by the activation of naive CD4+ T cells by antigen-presenting cells in the presence of IL-4 and thymic stromal protein.49,52 This pathway results in the production of cytokines such as IL-4, IL-5, IL-9, IL-13, and IL-31, which mediate diverse immune responses. IL-4 enhances B-cell activation, promotes IgE class switching, and upregulates major histocompatibility complex class II expression on antigen-presenting cells.49 IL-5 recruits and activates eosinophils, which release toxic granules and reactive oxygen species, contributing to tissue injury.53 IL-9 activates mast cells, leading to the release of histamine and proinflammatory mediators such as TNF-⍺ and IL-6.54 IL-13, in combination with IL-4, polarizes macrophages to an M2 phenotype, promoting tissue remodeling and fibrosis.55 IL-31 drives pruritus and inflammation through activation of sensory neurons and immune cells.56
Elevated urinary levels of IL-9 and TNF-⍺ have been identified as potential predictors of ATIN.57 Eosinophiluria, while observed in some ATIN patients, is no longer considered a useful marker. Combining urinary CXCL9 with IL-9 and TNF-⍺ has been shown to improve diagnostic accuracy, likely reflecting the distinct Th1 and Th2 endotypes of hypersensitivity rather than a simple coexistence of elevated biomarkers.51
The Th17 (type 4c) endotype is mediated by CD4+ T cells differentiated in the presence of IL-6, IL-23, and TGF-β.49 Th17 cells secrete cytokines such as IL-17A, IL-17F, IL-21, and IL-22, which promote neutrophil recruitment, macrophage activation, and epithelial proliferation.58 These cytokines induce the production of chemokines (CXCL1, CXCL2, and CXCL8) and cytokines (IL-6, granulocyte-macrophage colony-stimulating factor) by renal parenchymal cells, amplifying the inflammatory response.58 Histologic analyses of 25 patients with omeprazole-induced ATIN have revealed IL-17A/F expression in 44% to 48% of cases and interstitial infiltrates comprising lymphoctes and macrophages (68%) or mixed lymphocytes, macrophages, and neutrophils.59 These findings underscore the role of Th17-driven inflammation in ATIN pathogenesis and highlight its potential as a target for biomarker development.
A recent study explored the potential clinical utility of elevated serum CRP and urinary retinol-binding protein-to-creatinine ratio in distinguishing ICPI-related AKI from other forms of AKI. The study included 52 patients with ICPI-associated AKI, of whom only 18 underwent kidney biopsy.60 Significantly higher levels of both CRP and urinary retinol-binding protein-to-creatinine ratio in ICPI-related AKI compared with non–ICPI-AKI were noted, suggesting their potential as diagnostic biomarkers.60 However, CRP is a nonspecific marker of systemic inflammation that is elevated in a wide range of conditions, both infectious and noninfectious, limiting its specificity for ICPI-related ATIN. Similarly, retinol-binding protein, which is produced in the liver, is freely filtered by the glomeruli and fully reabsorbed in the proximal tubules via the megalin-cubulin complex.61 Therefore, it primarily serves as a marker of proximal tubular injury rather than a specific indicator of ATIN.62 Furthermore, the observed higher creatinine levels in ICPI-AKI compared with controls likely reflect more severe proximal tubular injury rather than specific histopathologic features of ATIN.60 The lack of biopsy confirmation in the majority of cases raises concerns about the reliability of these biomarkers in differentiating ATIN from other AKI etiologies.60 Currently, the diagnostic value of CRP and urinary retinol-binding protein-to-creatinine ratio remains uncertain and requires further validation in larger, biopsy-confirmed cohorts.
The polarization of T cell subsets and their roles in ATIN pathogenesis remain areas for further research. Investigating urinary immune-related biomarkers through the perspective of type 4 hypersensitivity endotypes and various hypersensitivity reactions offers a promising approach to enhancing their clinical utility.4 Although significant progress has been made in identifying potential biomarkers, routine clinical implementation requires further validation. Robust studies correlating biomarker profiles with histologic findings and clinical outcomes are essential to establish their diagnostic and prognostic value. By integrating biomarkers into a comprehensive diagnostic framework, clinicians can improve the accuracy and timeliness of ATIN diagnosis.
Imaging
Ultrasound may reveal increased kidney size due to interstitial edema; however, this finding is not specific to ATIN. Gallium-67, a radiotracer that binds to inflammatory proteins such as lactoferrin, accumulates at sites of inflammation and can highlight interstitial cellular infiltration in the kidneys.63 Several studies have demonstrated the clinical utility of Gallium-67 scanning in differentiating ATIN from other causes of AKI.64, 65, 66, 67 Low gallium uptake makes ATIN unlikely, whereas high uptake is consistent with the diagnosis. However, false positive and negative results make this test suboptimal.
18F-fluorodeoxyglucose (FDG) uptake, which is significantly increased in inflamed tissues, has been observed in the renal cortex of patients with ATIN. Case reports of ICPI-related ATIN have shown markedly elevated FDG uptake in the renal cortex compared with baseline.68, 69, 70 The distribution of lesions in ATIN is more diffuse compared with the pyramidal pattern typical of pyelonephritis, reflecting the scattered nature of ATIN pathology.71 In another study, 9 patients with ICPI-AKI, 24 with non–ICPI-AKI, and 20 with ICPI-treated without AKI were studied with FDG scan. Of the 9 patients with ICPI-AKI, 3 had biopsy-proven ATIN, and 6 had clinically-adjudicated ATIN.72 The patients with non–ICPI-AKI had prerenal AKI (n = 10), ischemic or septic acute tubular necrosis (n = 10), or other AKI etiologies (n = 4). Patients with ICPI-AKI had a significantly increased F18-FDG uptake compared with both control groups with an area under the curve of 0.97 (95% CI: 0.93–1.00). The study was limited by lack of biopsy in 6 of 9 patients and the need for a baseline FDG scan for comparison.
Diffusion-weighted and blood oxygen level–dependent magnetic resonance imaging, which are classical functional imaging sequences, have shown potential as adjunctive modalities in a limited number of cases, warranting further investigation.73
Although further studies are needed to establish definitive standards for these imaging modalities, and their uptake patterns are not necessarily specific to the underlying mechanism of kidney inflammation, their integration with clinical parameters shows promise in improving the differentiation of ATIN from other causes of AKI.
Treatment
The primary therapeutic goal in ATIN is to prevent permanent damage by promptly identifying and eliminating the causative factor and initiating fast-acting immunosuppressive therapy. Although removal of the offending agent often leads to kidney function recovery (Figure 4), spontaneous remission often does not occur in complex cases with polypharmacy or significant comorbidities. Given that interstitial inflammation can progress to fibrosis within 7 days, as demonstrated in animal models, rapid suppression of the inflammatory process is critical.74,75
Figure 4.
An updated approach to the diagnosis and management of suspected acute tubulointerstitial nephritis (ATIN). CXCL, CXC chemokine ligand; IFTA, interstitial fibrosis tubular atrophy; IL, interleukin; PET-CT, positron emission tomography-computed tomography; TNF, tumor necrosis factor.
Corticosteroids remain the cornerstone of ATIN treatment, typically initiated with oral prednisolone or prednisone at 60 mg/d or 0.5 to 1 mg/kg/d. In drug-induced ATIN, treatment duration generally ranges from 7 to 10 days to a maximum of 6 to 8 weeks, with a tailored tapering strategy. A multicenter retrospective study by Fernandez-Juárez et al.75 found no benefit to extending corticosteroid therapy beyond 8 weeks. An exception exists for ICPI-associated ATIN, where treatment may extend to 3 to 6 months.76 Shorter regimens have also shown benefit in ICPI-associated ATIN, highlighting the need for individualized treatment durations.77
In severe AKI cases, i.v. pulse steroids can be administered, though no significant advantage over oral high-dose regimens has been demonstrated.76,78 Treatment planning should account for patient factors such as age, frailty, comorbidities, and potential side effects of glucocorticoids, especially in older patients with increased risks of infections, osteoporosis, diabetes, thromboembolic events, and hypertension.
A recent study of 166 biopsy-confirmed ATIN cases found that medications were the primary cause (67%), with corticosteroids used in 81% of patients but showing no significant impact on kidney recovery.79 Overall, 76% achieved recovery within 6 months, with better outcomes in drug-related ATIN (81%) compared with other causes (66%, P = 0.04). Severe interstitial fibrosis and tubular atrophy and dialysis dependence were strong predictors of poor prognosis, highlighting the limited role of immunosuppression in advanced cases.
Although agents such as azathioprine, mycophenolate mofetil, and infliximab have been used in select cases, their delayed onset—often taking weeks—limits their utility in ATIN, where treatment is typically needed for no more than 2 to 8 weeks.80 Notably, anti-TNF agents such as infliximab have a relatively rapid onset in conditions such as rheumatoid arthritis; however, their efficacy and safety in ATIN are not well-established, and there have been reports of these agents inducing kidney injury.81, 82, 83 Infliximab has been useful in corticosteroid resistant ICPI-associated ATIN. However, their routine use as alternatives to corticosteroids in ATIN is not recommended; in corticosteroid-dependent or -resistant cases, mycophenolate mofetil or targeted T-cell therapies such as antithymocyte globulin may significantly alter the disease course.84,85 In cases where severe interstitial fibrosis and tubular atrophy (>50% to 75%) are present, the potential for renal recovery is significantly diminished, rendering the use of corticosteroids or other immunosuppressive therapies generally unbeneficial.79 In such scenarios, therapeutic efforts should focus on supportive care and management of underlying conditions rather than aggressive immunosuppression.86
Prognosis and Follow-Up
Early diagnosis, removal of causative factors, and prompt treatment often but not always lead to a favorable prognosis. Despite elimination of the causative agent and corticosteroid therapy, patients may end up with CKD and rarely end-stage kidney disease, requiring kidney replacement therapy. In addition, re-exposure to the same or similar triggers can cause relapse, highlighting the need for patient education about these risks and notation of allergy in the medical record. Long-term follow-up is crucial in systemic disease–associated cases to prevent potential complications.
Conclusion
ATIN is a significant cause of AKI with diverse triggers, including drugs, infections, and systemic diseases. Although type 4 hypersensitivity is a key mechanism, mixed hypersensitivity reactions contribute to its complexity. Early diagnosis and prompt treatment, primarily through causative agent removal and corticosteroid use, are critical to preserving kidney function. Given the nonspecific clinical presentation, advanced biomarkers and tailored therapies are needed. A multidisciplinary approach and further research into immune pathways and targeted treatments will enhance outcomes in this evolving clinical landscape.
Disclosure
TS has received speaker honorariums from AstraZeneca, Amgen, Sanofi, Nobel Ilac, Baxter, Boehringer Ingelheim, Abdi Ibrahim-Otsuka, Alexion, and Astellas, none of which are associated with this work. MAP declared no conflicting interests.
Acknowledgments
We extend our heartfelt gratitude to Dr. Şeyhmus Kaya, Nephropathologist, for his generosity in providing the illustrative images in Figure 2.
Contributor Information
Tuncay Sahutoglu, Email: tu_cay83@yahoo.com.
Mark A. Perazella, Email: Mark.perazella@yale.edu.
References
- 1.Praga M., González E. Acute interstitial nephritis. Kidney Int. 2010;77:956–961. doi: 10.1038/ki.2010.89. [DOI] [PubMed] [Google Scholar]
- 2.MacGinley R., Rossert J.A. In: Comprehensive Clinical Nephrology. 7th ed. Johnson R.J., Floege J., Tonelli M., editors. Elsevier; 2023. Acute interstitial nephritis; pp. 754–762. [Google Scholar]
- 3.Yoon S.Y., Lee S., Lee K., et al. Global burden of anticancer drug-induced acute kidney injury and tubulointerstitial nephritis from 1967 to 2023. Sci Rep. 2024;14 doi: 10.1038/s41598-024-67020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jutel M., Agache I., Zemelka-Wiacek M., et al. Nomenclature of allergic diseases and hypersensitivity reactions: adapted to modern needs: an EAACI position paper. Allergy. 2023;78:2851–2874. doi: 10.1111/all.15889. [DOI] [PubMed] [Google Scholar]
- 5.Clayman M.D., Martinez-Hernandez A., Michaud L., et al. Isolation and characterization of the nephritogenic antigen producing anti-tubular basement membrane disease. J Exp Med. 1985;161:290–305. doi: 10.1084/jem.161.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ebrahimi N., Najafian B., Chen Wongworawat Y., Norouzi S., Abdipour A. Anti-tubular basement membrane antibody nephritis manifesting in a patient with chronic lymphocytic leukemia: a very rare case report. J Investig Med High Impact Case Rep. 2024;12 doi: 10.1177/23247096241281612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gamayo A., Hecox D., Dicker L., et al. Anti-LRP2 nephropathy with concurrent kidney infiltration by lymphoma. Clin Kidney J. 2020;13:468–472. doi: 10.1093/ckj/sfz166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scolari F., Izzi C., Ghiggeri G.M. Uromodulin: from monogenic to multifactorial diseases. Nephrol Dial Transplant. 2015;30:1250–1256. doi: 10.1093/ndt/gfu300. [DOI] [PubMed] [Google Scholar]
- 9.Tokumoto M., Fukuda K., Shinozaki M., et al. Acute interstitial nephritis with immune complex deposition and MHC class II antigen presentation along the tubular basement membrane. Nephrol Dial Transplant. 1999;14:2210–2215. doi: 10.1093/ndt/14.9.2210. [DOI] [PubMed] [Google Scholar]
- 10.Praga M., Sevillano A., Auñón P., González E. Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant. 2015;30:1472–1479. doi: 10.1093/ndt/gfu326. [DOI] [PubMed] [Google Scholar]
- 11.Muriithi A.K., Leung N., Valeri A.M., et al. Biopsy-proven acute interstitial nephritis, 1993–2011: A case series. Am J Kidney Dis. 2014;64:558–566. doi: 10.1053/j.ajkd.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 12.Valluri A., Hetherington L., Mcquarrie E., et al. Acute tubulointerstitial nephritis in Scotland. Q J M. 2015;108:527–532. doi: 10.1093/qjmed/hcu236. [DOI] [PubMed] [Google Scholar]
- 13.Herrmann S.M., Perazella M.A. Immune checkpoint inhibitors and immune-related adverse renal events. Kidney Int Rep. 2020;5:1139–1148. doi: 10.1016/j.ekir.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hultin S., Nahar K., Menzies A.M., et al. Histological diagnosis of immune checkpoint inhibitor induced acute renal injury in patients with metastatic melanoma: a retrospective case series report. BMC Nephrol. 2020;21:391. doi: 10.1186/s12882-020-02044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta S., Short S.A.P., Sise M.E., et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaubschlager T., Rajora N., Diep S., et al. De novo or recurrent glomerulonephritis and acute tubulointerstitial nephritis after COVID-19 vaccination: a report of six cases from a single center. Clin Nephrol. 2022;97:289–297. doi: 10.5414/CN110794. [DOI] [PubMed] [Google Scholar]
- 17.Czerlau C., Bocchi F., Saganas C., Vogt B. Acute interstitial nephritis after messenger RNA-based vaccination. Clin Kidney J. 2022;15:174–176. doi: 10.1093/ckj/sfab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakaoka S., Tsubata S., Adachi Y. Acute tubulointerstitial nephritis due to human papillomavirus vaccination. JMA J. 2024;7:130–132. doi: 10.31662/jmaj.2023-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang H.S., Lee H., Yoon S.Y., et al. Global burden of vaccine-associated kidney injury using an international pharmacovigilance database. Sci Rep. 2025;15:5177. doi: 10.1038/s41598-025-88713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthukumar T., Jayakumar M., Fernando E.M., Muthusethupathi M.A. Acute renal failure due to rifampicin: a study of 25 patients. Am J Kidney Dis. 2002;40:690–696. doi: 10.1053/ajkd.2002.35675. [DOI] [PubMed] [Google Scholar]
- 21.Kloypan C., Koomdee N., Satapornpong P., Tempark T., Biswas M., Sukasem C. A comprehensive review of HLA and severe cutaneous adverse drug reactions: implication for clinical pharmacogenomics and precision medicine. Pharmaceuticals (Basel) 2021;14:1077. doi: 10.3390/ph14111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mérida E., Praga M. NSAIDs and nephrotic syndrome. Clin J Am Soc Nephrol. 2019;14:1280–1282. doi: 10.2215/CJN.08090719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray S., Delaney M., Muller A.F. Proton pump inhibitors and acute interstitial nephritis. BMJ. 2010;341 doi: 10.1136/bmj.c4412. [DOI] [PubMed] [Google Scholar]
- 24.Muhammad A., Zhang Y., Huang L., et al. The diagnosis of acute interstitial nephritis caused by infection versus antibiotic-induced interstitial nephritis: a narrative review. Clin Kidney J. 2024;17 doi: 10.1093/ckj/sfae054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giri K., Sahay M., Ismail K., Kavadi A., Rama E., Gowrishankar S. The high burden of asymptomatic kidney diseases in individuals with HIV: a prospective study from a Tertiary Care Center in India. Indian J Nephrol. 2024;34:623–629. doi: 10.25259/ijn_10_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caceres P.S., Savickas G., Murray S.L., et al. High SARS-CoV-2 viral load in urine sediment correlates with acute kidney injury and poor COVID-19 outcome. J Am Soc Nephrol. 2021;32:2517–2528. doi: 10.1681/ASN.2021010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivera F.B., Ansay M.F.M., Golbin J.M., et al. HIV-associated nephropathy in 2022. Glomerular Dis. 2023;3:1–11. doi: 10.1159/000526868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caravaca-Fontán F., Fernández-Juárez G., Praga M. Acute kidney injury in interstitial nephritis. Curr Opin Crit Care. 2019;25:558–564. doi: 10.1097/MCC.0000000000000654. [DOI] [PubMed] [Google Scholar]
- 29.Stylianou K., Maragkaki E., Tzanakakis M., Stratakis S., Gakiopoulou H., Daphnis E. Acute interstitial nephritis and membranous nephropathy in the context of IgG4-related disease. Case Rep Nephrol Dial. 2014;5:44–48. doi: 10.1159/000369924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lusco M.A., Fogo A.B., Najafian B., Alpers C.E. AJKD atlas of renal pathology: anti–tubular basement membrane antibody disease. Am J Kidney Dis. 2017;70:e3–e4. doi: 10.1053/j.ajkd.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Rifai A.O., Denig K.M., Caza T., et al. Antitubular basement membrane antibody disease associated with nivolumab infusion and concomitant acute pyelonephritis leading to acute kidney injury : a case report and literature review. Case Rep Nephrol. 2023;2023:1–5. doi: 10.1155/2023/6681756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvanajscak Z., Murphy J.D., Larsen C.P., Padala S.A. Anti–brush border antibody disease (anti-LRP2 nephropathy) associated with lupus nephritis. Kidney Int Rep. 2020;5:1590–1594. doi: 10.1016/j.ekir.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larsen C.P., Trivin-Avillach C., Coles P., et al. LDL receptor-related Protein 2 (megalin) as a target antigen in human kidney anti-brush border antibody disease. J Am Soc Nephrol. 2018;29:644–653. doi: 10.1681/ASN.2017060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gallan A.J., Garg A., Collins A.B., et al. Anti-LRP2 nephropathy. Kidney Int Rep. 2020;5:2365–2370. doi: 10.1016/j.ekir.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dinesh K.P., Raniele D., Michels K., et al. Anti-LRP2 nephropathy with abundant IgG4-positive plasma cells: a case report. Am J Kidney Dis. 2019;74:132–137. doi: 10.1053/j.ajkd.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 36.Murphy J.D., Caza T.N., Cassol C.A., et al. Clinicopathologic features of antibrush border antibody disease. Kidney Int Rep. 2024;9:370–382. doi: 10.1016/j.ekir.2023.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lou-Meda R., Alvarez-Elías A.C., Bonilla-Félix M. Mesoamerican endemic nephropathy (MeN): A disease reported in adults that may start since childhood? Semin Nephrol. 2022;42 doi: 10.1016/j.semnephrol.2023.151337. [DOI] [PubMed] [Google Scholar]
- 38.Johnson R.J., Wesseling C., Newman L.S. Chronic kidney disease of unknown cause in agricultural communities. N Engl J Med. 2019;380:1843–1852. doi: 10.1056/NEJMra1813869. [DOI] [PubMed] [Google Scholar]
- 39.Acharya R., Zeng X., Upadhyay K. Synthetic cannabinoid-associated acute interstitial nephritis: an emerging cause of pediatric acute kidney injury? Clin Nephrol Case Stud. 2023;11:55–60. doi: 10.5414/CNCS111063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fogo A.B., Lusco M.A., Najafian B., Alpers C.E. AJKD atlas of renal pathology: acute interstitial nephritis. Am J Kidney Dis. 2016;67:e35–e36. doi: 10.1053/j.ajkd.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Janssen U., Naderi S., Amann K. Idiopathic granulomatous interstitial nephritis and isolated renal sarcoidosis: two diagnoses of exclusion. Sage Open Med. 2021;9 doi: 10.1177/20503121211038470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ravnskov U. Glomerular, tubular and interstitial nephritis associated with non-steroidal antiinflammatory drugs. Evidence of a common mechanism. Br J Clin Pharmacol. 1999;47:203–210. doi: 10.1046/j.1365-2125.1999.00869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calle A.M., Aguirre N., Ardila J.C., Cardona V.R. DRESS syndrome: a literature review and treatment algorithm. World Allergy Organ J. 2023;16 doi: 10.1016/j.waojou.2022.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nussbaum E.Z., Perazella M.A. Diagnosing acute interstitial nephritis: considerations for clinicians. Clin Kidney J. 2019;12:808–813. doi: 10.1093/ckj/sfz080. [DOI] [Google Scholar]
- 45.Raghavan R., Eknoyan G. Acute interstitial nephritis - a reappraisal and update. Clin Nephrol. 2014;82:149–162. doi: 10.5414/cn108386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perazella M.A., Bomback A.S. Urinary eosinophils in AIN: farewell to an old biomarker? Clin J Am Soc Nephrol. 2013;8:1841–1843. doi: 10.2215/CJN.08620813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muriithi A.K., Nasr S.H., Leung N. Utility of urine eosinophils in the diagnosis of acute interstitial nephritis. Clin J Am Soc Nephrol. 2013;8:1857–1862. doi: 10.2215/CJN.01330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perazella M.A. Clinical approach to diagnosing acute and chronic tubulointerstitial disease. Adv Chronic Kidney Dis. 2017;24:57–63. doi: 10.1053/j.ackd.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Gutcher I., Becher B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J Clin Invest. 2007;117:1119–1127. doi: 10.1172/JCI31720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martinez Valenzuela L., Draibe J., Bestard O., et al. Urinary cytokines reflect renal inflammation in acute tubulointerstitial nephritis: a multiplex bead-based assay assessment. J Clin Med. 2021;10:2986. doi: 10.3390/jcm10132986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moledina D.G., Obeid W., Smith R.N., et al. Identification and validation of urinary CXCL9 as a biomarker for diagnosis of acute interstitial nephritis. J Clin Invest. 2023;133 doi: 10.1172/JCI168950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ebina-Shibuya R., Leonard W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat Rev Immunol. 2023;23:24–37. doi: 10.1038/s41577-022-00735-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lombardi C., Comberiati P., Ridolo E., et al. Anti-IL-5 pathway agents in eosinophilic-associated disorders across the lifespan. Drugs. 2024;84:661–684. doi: 10.1007/s40265-024-02037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noelle R.J., Nowak E.C. Cellular sources and immune functions of interleukin-9. Nat Rev Immunol. 2010;10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M.-Z., Wang X., Wang Y., et al. IL-4/IL-13–mediated polarization of renal macrophages/dendritic cells to an M2a phenotype is essential for recovery from acute kidney injury. Kidney Int. 2017;91:375–386. doi: 10.1016/j.kint.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feld M., Garcia R., Buddenkotte J., et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. 2016;138:500.e24. doi: 10.1016/j.jaci.2016.02.020. 508.e24. [DOI] [PubMed] [Google Scholar]
- 57.Moledina D.G., Wilson F.P., Pober J.S., et al. Urine TNF-α and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ouyang W., Kolls J.K., Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–467. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berney-Meyer L., Hung N., Slatter T., Schollum J.B., Kitching A.R., Walker R.J. Omeprazole-induced acute interstitial nephritis: a possible Th1-Th17-mediated injury? Nephrology (Carlton) 2014;19:359–365. doi: 10.1111/nep.12226. [DOI] [PubMed] [Google Scholar]
- 60.Isik B., Alexander M.P., Manohar S., et al. Biomarkers, clinical features, and rechallenge for immune checkpoint inhibitor renal immune-related adverse events. Kidney Int Rep. 2021;6:1022–1031. doi: 10.1016/j.ekir.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marinó M., Andrews D., Brown D., Mccluskey R.T. Transcytosis of Retinol-Binding Protein across Renal Proximal Tubule Cells after megalin (gp 330)-Mediated Endocytosis. J Am Soc Nephrol. 2001;12:637–648. doi: 10.1681/ASN.V124637. [DOI] [PubMed] [Google Scholar]
- 62.Phanish M.K., Chapman A.N., Yates S., et al. Evaluation of urinary biomarkers of proximal tubular injury, inflammation, and fibrosis in patients with albuminuric and nonalbuminuric diabetic kidney disease. Kidney Int Rep. 2021;6:1355–1367. doi: 10.1016/j.ekir.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hoffer P.B., Huberty J., Khayam-Bashi H. The association of Ga-67 and lactoferrin. J Nucl Med. 1977;18:713–717. [PubMed] [Google Scholar]
- 64.Baker M.L., Perazella M.A. In: Tubulointerstitial Nephritis. Atta M.G., Perazella M.A., editors. Springer International Publishing; 2022. Imaging modalities for acute tubulointerstitial nephritis; pp. 257–266. [DOI] [Google Scholar]
- 65.Graham F., Lord M., Froment D., Cardinal H., Bollée G. The use of gallium-67 scintigraphy in the diagnosis of acute interstitial nephritis. Clin Kidney J. 2016;9:76–81. doi: 10.1093/ckj/sfv129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joaquim A.I., Mendes G.E.F., Ribeiro P.F.F., Baptista M.A.F., Burdmann E.A. Ga-67 scintigraphy in the differential diagnosis between acute interstitial nephritis and acute tubular necrosis: an experimental study. Nephrol Dial Transplant. 2010;25:3277–3282. doi: 10.1093/ndt/gfq152. [DOI] [PubMed] [Google Scholar]
- 67.Matsumura M., Okada A., Yokoyama H., et al. Usefulness of gallium-67 scintigraphy for evaluating the histopathological activity in interstitial nephritis. Clin Exp Nephrol. 2023;27:251–261. doi: 10.1007/s10157-022-02302-0. [DOI] [PubMed] [Google Scholar]
- 68.Qualls D., Seethapathy H., Bates H., et al. Positron emission tomography as an adjuvant diagnostic test in the evaluation of checkpoint inhibitor-associated acute interstitial nephritis. J Immunother Cancer. 2019;7:356. doi: 10.1186/s40425-019-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miao J., Alexander M.P., Zoghby Z.M. Acute interstitial nephritis on positron-emission tomography-computed tomography imaging. Kidney Med. 2022;4 doi: 10.1016/j.xkme.2022.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Manohar S., Albright R.C. Interstitial nephritis in immune checkpoint inhibitor therapy. Kidney Int. 2019;96:252. doi: 10.1016/j.kint.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 71.Wan C.H., Tseng J.R., Lee M.H., Yang L.Y., Yen T.C. Clinical utility of FDG PET/CT in acute complicated pyelonephritis—results from an observational study. Eur J Nucl Med Mol Imaging. 2018;45:462–470. doi: 10.1007/s00259-017-3835-9. [DOI] [PubMed] [Google Scholar]
- 72.Gupta S., Green-Lingren O., Bhimaniya S., et al. F18-FDG PET imaging as a diagnostic tool for immune checkpoint inhibitor-associated acute kidney injury. J Clin Invest. 2024;134 doi: 10.1172/JCI182275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Su T., Yang X., Wang R., Yang L., Wang X. Characteristics of diffusion-weighted and blood oxygen level-dependent magnetic resonance imaging in tubulointerstitial nephritis: an initial experience. BMC Nephrol. 2021;22:237. doi: 10.1186/s12882-021-02435-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.González E., Gutiérrez E., Galeano C., et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 2008;73:940–946. doi: 10.1038/sj.ki.5002776. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez-Juarez G., Perez J.V., Caravaca-Fontán F., et al. Duration of treatment with corticosteroids and recovery of kidney function in acute interstitial nephritis. Clin J Am Soc Nephrol. 2018;13:1851–1858. doi: 10.2215/CJN.01390118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Donati A., Krishnan N. Should corticosteroids be used to treat biopsy-proven drug-induced acute interstitial nephritis? Kidney360. 2022;3:1306–1309. doi: 10.34067/KID.0006642021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta S., Garcia-Carro C., Prosek J.M., et al. Shorter versus longer corticosteroid duration and recurrent immune checkpoint inhibitor-associated AKI. J Immunother Cancer. 2022;10 doi: 10.1136/jitc-2022-005646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ramachandran R., Kumar K., Nada R., Jha V., Gupta K., Kohli H. Drug-induced acute interstitial nephritis: a clinicopathological study and comparative trial of steroid regimens. Indian J Nephrol. 2015;25:281–286. doi: 10.4103/0971-4065.147766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Miao J., Thongprayoon C., Krisanapan P., Buglioni A., Craici I.M., Cheungpasitporn W. Clinicopathological characteristics and kidney outcomes in biopsy-confirmed acute interstitial nephritis. Kidney Int Rep. 2024;9:3542–3552. doi: 10.1016/j.ekir.2024.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caravaca-Fontán F., Shabaka A., Sánchez-Álamo B., et al. Recurrent acute interstitial nephritis: what lies beneath. Clin Kidney J. 2021;14:197–204. doi: 10.1093/ckj/sfaa018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alghamdi N., Alshehri F., Alhazza S., et al. Acute tubulointerstitial nephritis associated with infliximab therapy in a patient with Crohn’s disease: a case report. CEN Case Rep. 2025;14:266–270. doi: 10.1007/s13730-024-00943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mahjoubi Y.S., Kaabi W., Lakhoua G., et al. Tubulointerstitial nephritis induced by adalimumab. Turk J Nephrol. 2024;33:301–302. doi: 10.5152/turkjnephrol.2024.23620. [DOI] [Google Scholar]
- 83.Plant R., Rafi Ahmed A., Mchale T., Giblin L. A case of adalimumab-induced granulomatous interstitial nephritis. Cureus. 2021;13 doi: 10.7759/cureus.15986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Preddie D.C., Markowitz G.S., Radhakrishnan J., et al. Mycophenolate mofetil for the treatment of interstitial nephritis. Clin J Am Soc Nephrol. 2006;1:718–722. doi: 10.2215/CJN.01711105. [DOI] [PubMed] [Google Scholar]
- 85.Shao T., Weinstein J., Jothy S., Goldstein M. Severe acute interstitial nephritis: response to therapy with antithymocyte globulin. Kidney Int Rep. 2017;2:138–141. doi: 10.1016/j.ekir.2016.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hommos M.S., Rule A.D. Should we always defer treatment of kidney disease when there is extensive interstitial fibrosis on biopsy? Am J Nephrol. 2016;44:286–288. doi: 10.1159/000449513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Clive D.M., Vanguri V.K. The syndrome of tubulointerstitial nephritis with uveitis (TINU) Am J Kidney Dis. 2018;72:118–128. doi: 10.1053/j.ajkd.2017.11.013. [DOI] [PubMed] [Google Scholar]