Abstract
Loss of Pax 6 function leads to an eyeless phenotype in both mammals and insects, and ectopic expression of both the Drosophila and the mouse gene leads to the induction of ectopic eyes in Drosophila, which suggested to us that Pax 6 might be a universal master control gene for eye morphogenesis. Here, we report the reciprocal experiment in which the RNAs of the Drosophila Pax 6 homologs, eyeless and twin of eyeless, are transferred into a vertebrate embryo; i.e., early Xenopus embryos at the 2- and 16-cell stages. In both cases, ectopic eye structures are formed. To understand the genetic program specifying eye morphogenesis, we have analyzed the regulatory mechanisms of Pax 6 expression that initiates eye development. Previously, we have demonstrated that Notch signaling regulates the expression of eyeless and twin of eyeless in Drosophila. Here, we show that in Xenopus, activation of Notch signaling also induces eye-related gene expression, including Pax 6, in isolated animal caps. In Xenopus embryos, the activation of Notch signaling causes eye duplications and proximal eye defects, which are also induced by overexpression of eyeless and twin of eyeless. These findings indicate that the gene regulatory cascade is similar in vertebrates and invertebrates.
In the animal kingdom, a large variety of different eye types are found. In vertebrates, the development of the camera-type eye with a single lens depends on coordinated inductive interactions between the presumptive neural retina and the surface ectoderm that will give rise to the lens (1). In contrast to the single lens eye of vertebrates, the development of the compound eye of Drosophila is initiated in a small group of epithelial cells in the embryo that form the eye part of eye-antennal imaginal disk after proliferation during larval stages (2). Despite these differences, recent studies have demonstrated that the genetic programs specifying eye development are conserved during evolution (3–5). The striking examples are the Pax 6 genes, which are key regulators for eye morphogenesis in both animal phyla (6).
The Pax 6 genes code for transcription factors containing two highly conserved DNA-binding motifs, a paired domain and a paired-type homeodomain (7, 8). Mutations in Pax 6 result in eye malformations known as Small eye in mice (9), Aniridia in humans (10, 11), and eyeless in Drosophila (12). By targeted expression of eyeless, ectopic eyes can be induced on the antennae, the legs, and the wings of the fly (13). Misexpression of Pax 6 in Xenopus also results in the formation of ectopic eye structures (14, 15). To test whether Pax 6 is a universal master control gene for eye morphogenesis, we have expressed the mouse Pax 6 gene in Drosophila leading to the induction of well developed compound eyes (13). Here, we report a reciprocal experiment in which we express the Drosophila Pax 6 homologs, eyeless and twin of eyeless (16), in Xenopus embryos and obtain vertebrate eye structures. To understand the genetic hierarchy controlling eye development, we have begun to compare the Pax 6 downstream target genes in the various animal phyla. A direct target gene for eyeless, sine oculis (so; ref, 17), constitutes a positive feedback loop for eyeless with eyes absent and dachshund in Drosophila eye development (18–22). A member of the sine oculis family, Six 3, is a downstream target gene of Pax 6 in vertebrate eye development (15) and makes a positive feedback loop for Pax 6 with Rx (23), another homeobox gene (24). However, the Drosophila gene optix, which is more closely related to so than Six 3, is capable of inducing ectopic eyes by an eyeless-independent mechanism (25). In planarians, Pax 6 participates in eye development (26), and the sine oculis homolog is essential for eye regeneration (27), suggesting that the basic elements of the genetic pathway are conserved in these prototypic eyes. These observations have suggested that the regulatory network has been adapted to the control of development of different eye types during evolution (28). To understand the genetic program specifying eye development, it is important to study the regulatory mechanisms for Pax 6 expression, which initiates eye development. Previously, we have demonstrated that Notch signaling regulates the expression of eyeless and twin of eyeless (29); the latter is known to be an upstream regulator for eyeless by directly regulating the eye-specific enhancer of the eyeless gene in the embryo (16, 30). The Notch signaling pathway defines an evolutionarily conserved cell–cell interaction mechanism that throughout development controls the ability of precursor cells to respond to developmental signals (31). Here, we show that in Xenopus the activation of Notch signaling activates Pax 6 and other eye-related genes in isolated animal caps, and induces eye duplications as well as proximal eye defects in embryos that are also induced by misexpression of the Drosophila homologs of Pax 6, eyeless and twin of eyeless. Furthermore, the misexpression of eyeless and twin of eyeless results in the formation of ectopic eye structures in Xenopus embryos. These results suggest that the regulation of Pax 6 expression and the function of Pax 6 in eye development are conserved in vertebrates and invertebrates.
Materials and Methods
Construction of Expression Vectors.
Drosophila eyeless-pNRRX and twin of eyeless-pNRRX were constructed as follows: the ORF of eyeless was amplified with primers (5′-CATGTTTACATTGCAACCAACTCC-3′ and 5′-GCTAGACCCACGGTGAGTAGAAACA-3′) by using the eyeless-pNHT4 plasmid as a template by PCR (12). The ORF of twin of eyeless was PCR-amplified with primers (5′-CATGATGCTAACAACTGAACACATAA-3′ and 5′-ATCACTGAAGACGTGGCCA-3′) by using the twin of eyeless-pBluescript SK(−) plasmid as a template (16). These fragments were cloned into the EcoRV sites of pNRRX; the pNRRX vector is a modified pBluescript II SK(−) (Stratagene). The fragment of Xglobin 5′-untranslated region and 3′-untranslated region of pSP64T vector (32) was subcloned into the PstI/HindIII sites of pBluescript II SK(−), and the NotI–EcoRI–EcoRV–XhoI linker was inserted into the BglII site of the Xglobin-untranslated region fragment. Xenopus Notch ΔE-pCS2(+) was described before (33). Green fluorescent protein (GFP) (CLONTECH) was subcloned into the BglII site of pSP64T (32).
Microinjections and Histology.
Capped synthetic RNAs of eyeless-pNRRX, twin of eyeless-pNRRX, Notch ΔE-pCS2(+), and GFP-psp64T were in vitro transcribed by using T7 or SP6 RNA polymerase (Stratagene). For animal cap assays, in vitro synthesized RNAs were injected into both blastomeres of 2-cell stage Xenopus embryos. Animal caps were isolated at the late-blastula stage (stage 9) and cultured in Steinberg's solution. For the histological analyses, the synthesized RNAs were injected into one of the blastomeres of 2-cell-stage or 16-cell-stage embryos. Respectively, the embryos were grown to stage 41–42 or stage 48 and examined histologically by using hematoxylin and eosin staining.
Reverse Transcriptase (RT)-PCR Analysis.
Total RNA was extracted with TRIZOL Reagent (GIBCO/BRL) from cultured animal caps. First-strand cDNA was synthesized from 1 μg RNA with an Oligo-(dT) primer by using Superscript II reverse transcriptase (GIBCO/BRL). A negative control excluding enzyme was included for each sample. One-tenth of the cDNA obtained was used for each PCR reaction. Primers and PCR conditions used in this study were as follows: β-crystallin, forward 5′-TGAGTACCCACGTTGGGATAC-3′ and reverse 5′-AGCCTGGATACTGATATCCAACC-3′ (29 cycles) (14); EF-1α, forward 5′-TTGCCACACTGCTCACATTGCTTGC-3′ and reverse 5′-ATCCTGCTGCCTTCTTTTCCACTGC-3′ (21 cycles) (34); N-CAM, forward 5′-CACAGTTCCACCAAATGC-3′ and reverse 5′-GGAATCAAGCGGTACAGA-3′ (27 cycles) (34); ornitin dehydrocarboxylase, forward 5′-GTCAATGATGGAGTGTATGGATC-3′ and reverse 5′-TCCATTCCGCTCTCCTGAGCAC-3′ (23 cycles) (35); Pax 6, forward 5′-AGTGTCCTCATTCACATCGG-3′ and reverse 5′-GATTCTTGGCTGGATCATGG-3′ (25 cycles) (two different length products are amplified by these primers) (36–38); Rx, forward 5′-ATACAGCAGAGAAGAGCTGGC-3′ and reverse 5′-AAGGTTGGAGAGCATGACC-3′ (25 cycles) (these primers fail to distinguish Xrx1 from Xrx2) (24); and Six 3, forward 5′-GCTTCAGCACCAGTCTATCG-3′ and reverse 5′-AAGGAATCATGCAATGGCC-3′ (25 cycles) (15).
Results
The Two Pax 6 Homologs of Drosophila, eyeless and twin of eyeless, Induce Eye Structures in Xenopus Embryos and Eye-Related Gene Expression in Animal Caps.
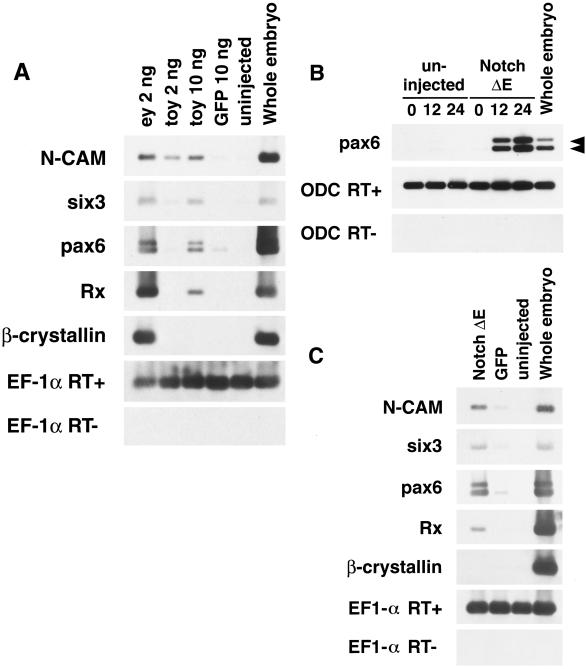
In Drosophila, expression of Drosophila Pax 6 homologs, eyeless and twin of eyeless, and mouse Pax 6 in imaginal discs result in the formation of ectopic eyes (13, 16), and the misexpression of Pax 6 in Xenopus embryos also results in the formation of ectopic eye structures (14, 15). As a reciprocal experiment, we examined the effects of misexpression of Drosophila Pax 6 homologs in Xenopus embryos. First, we injected eyeless and twin of eyeless RNA into one of the two blastomeres at the 2-cell stage and allowed the embryos to develop until stage 41 to 42. In addition to duplications of lenses and neural retina cell layers (Fig. 1F), expansion of the retinal pigment epithelium (RPE) from the endogenous eye region was observed in embryos injected with eyeless RNA (Fig. 1B and Fig. 2). Misexpression of twin of eyeless has almost no effect on expansion of the RPE under these conditions (Table 1). Histological cross sections of the embryos revealed that misexpression of eyeless induces the ectopic formation of lenses, RPE, and neural retina in the body trunk (Fig. 2B) and developmental defects including lack of lens, ectopic RPE formation, and hyperproliferation of the neural retina in the region of the original eye (Fig. 2C). Next, we injected eyeless and twin of eyeless RNA into one animal blastomere at the 16-cell stage and allowed the embryos to develop until stage 48. As shown in Fig. 3 A–C, embryos injected either with eyeless or twin of eyeless RNA at the 16-cell stage show ectopic formation of RPE. The frequency of ectopic RPE formation of eyeless-injected embryos was much higher than that of twin of eyeless-injected embryos (Table 2), and eyeless was found to induce ectopic lenses, whereas twin of eyeless failed to do so (Fig. 3A). The coinjection analyses of a lineage tracer RNA encoding nuclear β-galactosidase together with that encoding eyeless and twin of eyeless revealed that the ectopic formation of RPE and lens are cell-autonomous effects caused by eyeless and twin of eyeless (Fig. 3 E–I). These results indicate that the two Drosophila homologs of Pax 6, eyeless and twin of eyeless, induce eye structures in Xenopus embryos in a cell-autonomous manner. To determine whether eyeless and twin of eyeless induce the expression of genes implicated in early eye development, we injected RNA for eyeless and twin of eyeless into both blastomeres of 2-cell stage embryos and analyzed the expression of eye-related genes, N-CAM (neural cell adhesion molecule), Six 3, endogenous Pax 6, Rx, and β-crystallin in the animal cap ectoderm (Fig. 4A). The misexpression of eyeless induced the expression of N-CAM, Six 3, endogenous Pax 6, Rx, and β-crystallin in the animal caps. Twin of eyeless requires five times more RNA than eyeless to induce the expression of N-CAM, Six3, Pax6, and Rx, and the expression of β-crystallin was not induced in the animal cap by the injection of twin of eyeless RNA even at high doses, which is in accordance with the phenotypical analyses (Tables 1 and 2; Fig. 3). No expression of N-CAM, Six 3, Pax 6, Rx, and β-crystallin was observed in animal caps isolated from uninjected embryos, and a very weak or no expression of these genes was found in the animal caps isolated from control embryos injected with GFP RNA. These results indicate that the two Drosophila homologs of Pax 6, eyeless and twin of eyeless, are sufficient to induce eye-related gene expression including endogenous Pax 6 in the isolated animal caps of Xenopus embryos.
Figure 1.
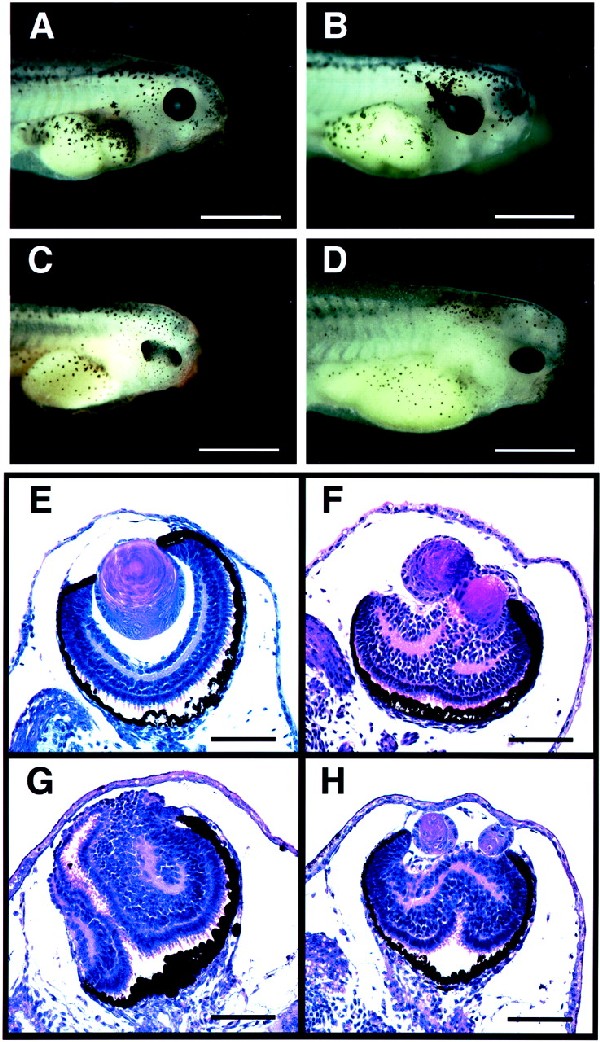
Embryos injected with eyeless, twin of eyeless, and an activated form of Notch receptor RNA at the 2-cell stage. Embryos were injected with eyeless (ey), twin of eyeless (toy), and an activated form of Xenopus Notch receptor (Notch ΔE) RNA into one blastomere at the 2-cell stage and fixed at stage 41 to 42. Bars indicate 1 mm in A–D and 0.1 mm in E–H. Cross sections are stained with hematoxylin and eosin. (A) Control embryo uninjected. (B) Embryo injected with 0.5 ng of ey RNA, showing expansion of RPE. (C) Embryo injected with 2 ng of toy RNA, showing a duplication of the retina. (D) Embryo injected with 5 ng of Notch ΔE RNA, showing the duplication of the retina (H) and the hyperplasia of neural tube. (E) Cross section of an eye of a control embryo. (F) Cross section of an embryo injected with 0.25 ng of ey RNA, showing the duplication of lenses with the increased number of cells in the neural retina. (G) Cross section of an embryo injected with 5 ng of toy RNA, showing the duplication of the retina with an increase in the number of neural retina cells. (H) Cross section of an embryo injected with 5 ng of Notch ΔE RNA, showing the duplication of the eye with a duplicated retina and two lenses. The phenotype is similar to that of an embryo injected with 0.25 ng of ey RNA in F.
Figure 2.
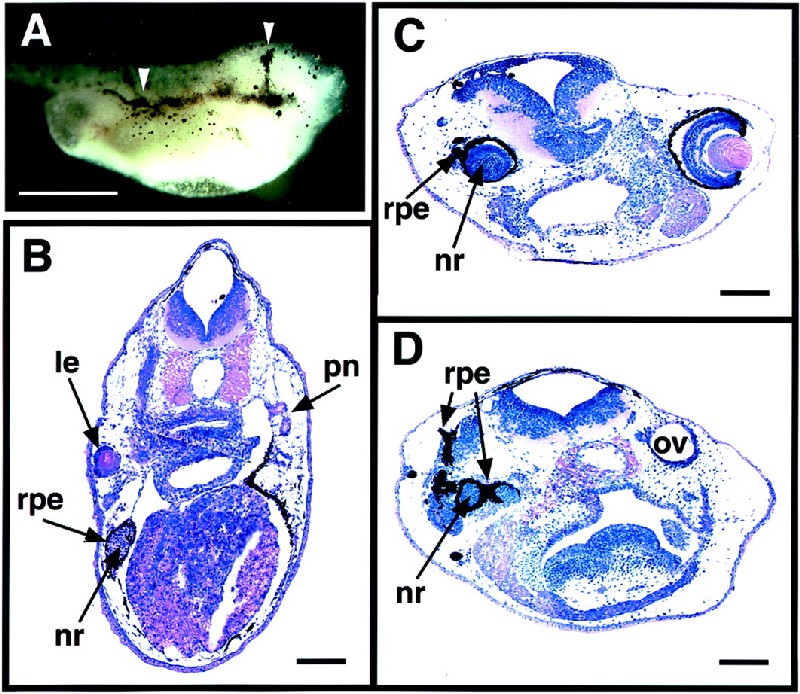
Ectopic eye structures formed in embryos injected with eyeless RNA at the 2-cell stage. Xenopus embryos were injected with various amounts of eyeless RNA into one of the two blastomeres at the 2-cell stage and fixed at stage 41 to 42. Bars indicate 1 mm in A and 0.1 mm in B–D. (A) Embryo injected with 2 ng of ey RNA. Arrows indicate the extension of the RPE toward the midline and along the body trunk. (B) Cross section of an embryo injected with 0.25 ng of ey RNA, showing the ectopic formation of a lens, RPE, and neural retina in the trunk region at the pronephros level. (C) cross section of an embryo injected with 0.5 ng of ey RNA, showing a developmental defect in the region of the original eye with the lack of a lens, the ectopic formation of RPE, and the hyperproliferation in the neural retina. (D) More posterior cross section of the same embryo as in C, showing the ectopic formation of RPE and neural retina in the region of the original otic vesicle. le, lens; nr, neural retina; ov, otic vesicle; pn, pronephros.
Table 1.
Summary of the eye phenotypes of embryos that are injected with RNA for eyeless, twin of eyeless, and an activated form of Notch receptor at the 2-cell stage
| Normal | Duplication | Eye expansion | Other | Dead | Total | |
|---|---|---|---|---|---|---|
| ey | ||||||
| 100 pg | 91% (29) | 0% (0) | 0% (0) | 9% (3) | 0% (0) | 32 |
| 250 pg | 44% (14) | 44% (14) | 6% (2) | 6% (2) | 0% (0) | 32 |
| 500 pg | 23% (22) | 23% (22) | 39% (37) | 12% (11) | 2% (2) | 94 |
| 1 ng | 0% (0) | 2% (1) | 91% (58) | 6% (4) | 2% (1) | 64 |
| toy | ||||||
| 100 pg | 100% (32) | 0% (0) | 0% (0) | 0% (0) | 0% (0) | 32 |
| 500 pg | 87% (82) | 2% (2) | 0% (0) | 4% (4) | 6% (6) | 94 |
| 1 ng | 31% (10) | 50% (16) | 0% (0) | 13% (4) | 6% (2) | 32 |
| 2 ng | 6% (2) | 81% (26) | 3% (1) | 0% (0) | 9% (3) | 32 |
| Notch E | ||||||
| 1 ng | 30% (11) | 22% (8) | 0% (0) | 43% (16) | 5% (2) | 37 |
| 5 ng | 38% (26) | 26% (18) | 0% (0) | 29% (20) | 6% (4) | 68 |
Embryos were injected with eyeless (ey), twin of eyeless (toy), and an activated form of Xenopus Notch receptor (Notch) RNA into one blastomere at the 2-cell stage and allowed to develop to stage 41 to 42. Typical examples of the duplication of eyes and eye expansion are presented in Figs. 1F and 2A, respectively. The other phenotypes are mainly caused by head defects.
Figure 3.
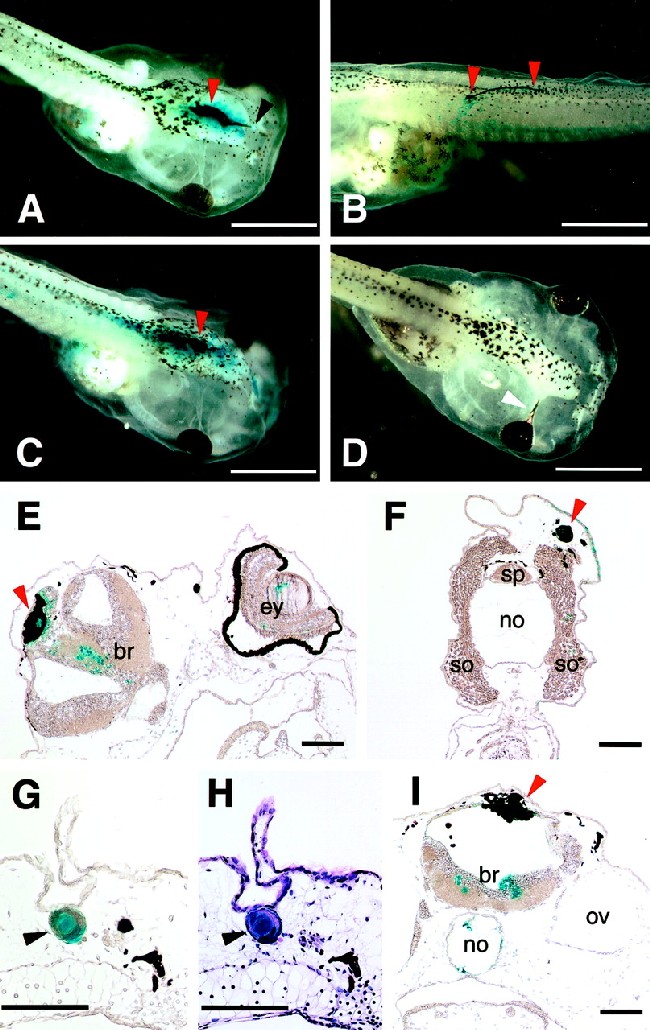
Embryos injected with eyeless, twin of eyeless, and an activated form of Notch receptor RNA at the 16-cell stage. Embryos were injected with eyeless, twin of eyeless, and an activated form of Xenopus Notch receptor (Notch ΔE) RNA into one of the animal pole blastomeres at the 16-cell stage and fixed at stage 48. In total, 0.25 ng of nuclear localized β-galactosidase RNA was coinjected and detected by X-gal staining. Bars indicate 1 mm in A–D and 0.1 mm in E–I. (A) Embryo injected with 0.25 ng of ey RNA. Arrows indicate the ectopic formation of a lens in black and RPE in red. (B) Embryo injected with 0.25 ng of ey RNA. Arrows indicate the ectopic formation of RPE. (C) Embryo injected with 0.5 ng of toy RNA. Arrow indicates the ectopic formation of RPE. (D) Embryo injected with 0.5 ng of Notch ΔE RNA. Arrow indicates the proximal eye defect. (E) Cross section of the same embryo as in A. The ectopic RPE (red arrow) colocalizes with nuclear β-galactosidase. (F) Cross section of same embryo as in B. The ectopic RPE (red arrow) colocalizes with nuclear β-galactosidase. (G) Cross section of the ectopic lens of embryo (A). The ectopic lens (black arrow) colocalizes with nuclear β-galactosidase. (H) Cross section shown in G stained with hematoxylin and eosin. (I) Cross section of same embryo as in C. The ectopic RPE (red arrow) colocalizes with nuclear β-galactosidase. br, brain; ey, eye; no, notochord; ov, otic vesicle; so, somite; sp, spinal cord.
Table 2.
Summary of the phenotypes of embryos that are injected with RNA for eyeless, twin of eyeless, and an activated form of Notch receptor at the 16-cell stage
| Normal | Ectopic RPE | Eye defect | Other | Dead | Total | |
|---|---|---|---|---|---|---|
| ey | ||||||
| 100 pg | 86% (49) | 2% (1) | 2% (1) | 0% (0) | 11% (6) | 57 |
| 125 pg | 68% (40) | 15% (9) | 14% (8) | 2% (1) | 2% (1) | 59 |
| 250 pg | 25% (14) | 25% (14) | 38% (21) | 5% (3) | 13% (7) | 56 |
| toy | ||||||
| 100 pg | 91% (48) | 0% (0) | 0% (0) | 2% (1) | 8% (4) | 53 |
| 500 pg | 80% (90) | 3% (3) | 9% (10) | 1% (1) | 9% (10) | 113 |
| notch_E | ||||||
| 500 pg | 83% (45) | 0% (0) | 11% (6) | 4% (2) | 2% (1) | 54 |
| 2 ng | 86% (96) | 0% (0) | 8% (9) | 3% (3) | 5% (5) | 111 |
Embryos were injected with eyeless (ey), twin of eyeless (toy), and an activated form of Xenopus Notch receptor (Notch) RNA into one animal pole blastomere at the 16-cell stage and allowed to develop to stage 48. Typical examples of the ectopic RPE and the proximal eye defect are presented in Fig. 3 A and D, respectively. The other phenotypes are mainly caused by head defects.
Figure 4.
Induction of eye-related gene expression including Pax 6 in Xenopus animal caps by overexpression of Drosophila Pax 6 genes, eyeless and twin of eyeless, and by activation of Notch signaling. (A) Induction of eye-related gene expression in the animal caps by the injection of eyeless and twin of eyeless RNA. Embryos were injected with 1 ng of ey and 1 and 5 ng of toy RNA into each of the two blastomeres at the 2-cell stage. Animal cap ectoderm was isolated at late blastula (stage 9) and processed for RT-PCR at the time when control embryos have reached stage 28. The misexpression of ey induces the expression of neural cell adhesion molecule (N-CAM), Six 3, endogenous Pax 6, Rx, and β-crystallin in the animal caps. Toy requires five times more RNA than ey to induce the expression of N-CAM, Six3, Pax6, and Rx, which is in agreement with our morphological analyses (Tables 1 and 2). The expression of β-crystallin was not induced in the animal cap by the injection of toy RNA. GFP 10 ng, 5 ng of green fluorescent protein RNA (total 10 ng) was injected into each of the two blastomeres at the 2-cell stage as a negative control. Uninjected, uninjected animal cap control. Whole embryo, whole embryo positive control. An elongation factor 1α (EF-1α) cDNA product was amplified as an internal loading control for animal caps. RT−, reverse transcriptase minus negative control. (B) Induction of Pax 6 expression in the animal caps by the activation of Notch signaling. RNA encoding an activated form of Xenopus Notch receptor (Notch ΔE) was injected into both blastomeres of 2-cell stage embryos. Animal cap ectoderm was isolated at late blastula (stage 9) and processed for RT-PCR after 0, 12, and 24 h. The induction of Pax 6 expression was observed after 12 h in the animal caps injected with Notch ΔE RNA (2 ng). Uninjected, uninjected animal cap control. Whole embryo, whole embryo positive control at the same stage of 24-h culture of animal caps. An ornitin dehydrocarboxylase cDNA product was amplified as an internal loading control for animal caps. RNA for noggin was injected as a positive control for the induction of Pax 6 in animal caps. RT−, reverse transcriptase minus negative control. Arrow heads indicate two isomers of Xenopus Pax 6. (C) Induction of eye-related gene expression in the animal caps by the activation of Notch signaling. RNA (5 ng) for Xenopus Notch ΔE RNA was injected into both blastomeres of 2-cell stage embryos. Animal cap ectoderm was isolated at late blastula (stage 9) and processed for RT-PCR at the time in which control embryos show stage 28. The activation of Notch signaling induces the expression of N-CAM, Six 3, Pax 6, and Rx, but not the expression of β-crystallin in the animal caps. The pattern of the induction is similar to that of animal caps injected with 10 ng of toy RNA (A), in agreement with our phenotypical analyses (Table 1). GFP, 5 ng of green fluorescent protein RNA was injected into both blastomeres of 2-cell embryos as the negative control. Uninjected, uninjected animal cap control. Whole embryo, whole embryo positive control. EF-1α cDNA product was amplified as an internal loading control for animal caps. RT−, reverse transcriptase minus negative control.
Induction of Eye Duplications and Proximal Eye Defects by Activation of Notch Signaling.
To determine the role of Notch signaling in vertebrate eye development, we injected a constitutively activated form of Xenopus Notch receptor (Notch ΔE) RNA into one of the two blastomeres at the 2-cell stage and allowed the embryos to develop until stage 41 to 42. As shown in Fig. 1 D and H, the activation of Notch signaling induced duplications of lenses with a massive increase in the number of neural retina cells, in addition to the hyperplasia of the neural tube that has been reported before (33). These duplications are similar to ones observed in embryos injected with eyeless and twin of eyeless RNA described above (Fig. 1 F and G). Sometimes, the formation of the lens is inhibited under these conditions (Fig. 1G). These results are summarized in Table 1. Chow et al. (15) have found that embryos injected into one of the animal blastomeres at the 16-cell stage with Xenopus Pax 6 also displayed eye-related phenotypes, proximal eye defects, ectopic formation of retinal pigment epithelium, and ectopic formation of eye and lens structures at high frequencies at stage 48. Following the same conditions, we injected Notch ΔE, eyeless, and twin of eyeless RNA into one animal blastomere at the 16-cell stage and allowed the embryos to develop until stage 48 (Fig. 3 and Table 2). As shown in Fig. 3D, the activation of Notch signaling induced the proximal eye defects, which appeared as an extension of the endogenous RPE toward the midline. The phenotype was similar to that observed with overexpression of Pax 6 and Rx in Xenopus embryos (15, 24). These results suggest that Notch signaling participates in the eye development via induction of Pax 6 and Rx expression.
Induction of Eye-Related Gene Expression Including Pax 6 in the Isolated Animal Caps of Xenopus by the Activation of Notch Signaling.
In Drosophila, the expression of eyeless is restricted to the region anterior to the morphogenetic furrow in the eye part of wild-type eye-antennal discs (12). The ectopic expression of eyeless is induced in eye-antennal discs by the activation of Notch signaling resulting from the expression of a constitutively activated form of Drosophila Notch receptor (Nact) in eye-antennal discs (29). To examine the potential role of Notch signaling on the induction of the Pax 6 gene in vertebrates, we injected RNA for Xenopus Notch ΔE into both blastomeres of 2-cell Xenopus embryos and analyzed the expression of Pax 6 in the animal cap ectoderm isolated at late blastula (stage 9). In this well established animal cap assay, the animal caps are not exposed to the inductive signals from the mesodermal tissues, and they develop as epithelia that fail to express eye-related genes including Pax 6. As shown in Fig. 4B, RT-PCR analysis revealed that the activation of Notch signaling induces the expression of two isomers of Pax 6 in the isolated animal caps from 12 h after the isolation of animal caps. Almost no expression of Pax 6 was observed in the animal caps isolated from uninjected control embryos. Next, we investigated whether the activation of Notch signaling induces other eye-related genes like N-CAM, Six 3, Rx, and β-crystallin under these conditions. In vertebrates, the homeobox genes Rx and Six 3 are expressed in the neural plate and in the developing eyes (24, 39). Injection of Rx RNA into Xenopus embryos induces ectopic RPE (24). Six 3 is a member of the sine oculis family and is sufficient to induce ectopic lens formation in medaka and zebrafish (40, 41). As shown in Fig. 4C, the activation of Notch signaling induced the expression of N-CAM, Six 3, and Rx but not the expression of β-crystallin in the isolated animal caps. No expression of N-CAM, Six 3, and Rx was observed in the animal caps isolated from uninjected embryos. A very weak or no expression of these genes was observed in the animal caps isolated from GFP encoding RNA-injected control embryos. These results indicate that the activation of Notch signaling is capable of inducing eye-related gene expression including Pax 6 in the isolated animal caps of Xenopus.
Discussion
Evolutionary Conservation of the Function of Pax 6 in Eye Development.
In this paper, we demonstrate that the Drosophila homologs of Pax 6, eyeless and twin of eyeless, are sufficient to induce eye structures in Xenopus embryos, indicating that Drosophila Pax 6 homologs are functional equivalents of vertebrate Pax 6 in vertebrate eye development. Because eyeless and twin of eyeless induce the expression of endogenous Pax 6 in the isolated animal caps, they presumably activate the positive feedback loop for Pax 6, which has been shown to be implicated in eye development. In Drosophila, the ectopic expression of mouse Pax 6 in imaginal discs activates a similar positive feedback loop for eyeless that comprises eyeless, sine oculis, eyes absent, and dachshund (16, 20). Therefore, the function of Pax 6 genes seems to be conserved in eye development of vertebrates and invertebrates. We found that both Pax 6 homologs, eyeless and twin of eyeless, induce ectopic eye structures in Xenopus embryo. However, judging from the frequency of the ectopic induction of eye-related structures, eyeless has a stronger activity for the induction than twin of eyeless. Eyeless is capable of inducing ectopic lenses, whereas twin of eyeless failed to do so, even at higher concentrations. Corresponding to these phenotypical analyses, in the animal cap assays eyeless also shows stronger activity for induction of eye-related gene expression than twin of eyeless. Particularly, β-crystallin expression was only induced by misexpression of eyeless and not by twin of eyeless. Overexpression of vertebrate Pax 6 induces ectopic eye structures with lens and ectopic expression of β-crystallin in Xenopus embryos (15). These observations indicate that eyeless is more closely related to vertebrate Pax 6 with respect to eye induction than twin of eyeless. Because two Pax 6 genes are only found in holometabolous insects and not in hemimetabolous or apterygote insects (28), the functional divergence of these genes seems to have occurred during insect evolution. The twin of eyeless protein is more similar to the vertebrate Pax 6 proteins than to the eyeless protein, particularly in its overall length and at the C-terminal region (16). However, the eyeless protein is more closely related in the paired domain to vertebrate Pax 6 proteins than the twin of eyeless protein (16). Recently, we found that in Drosophila the paired domain of eyeless is essential for its ectopic eye induction, and the expression of a truncated eyeless protein lacking the homeodomain is sufficient to induce ectopic eyes (42). Therefore, the paired domain of Pax 6 seems to be critical for its conserved function in eye induction.
Regulatory Mechanisms for Expression of Pax 6 in Eye Development.
We found that activation of Notch signaling is sufficient to induce Pax 6 expression in the isolated animal caps of Xenopus. In the embryos, the activation of Notch signaling induces eye duplications and proximal eye defects that are also induced by misexpression of Pax 6 as well as eyeless and twin of eyeless. These results suggest that Notch signaling participates in vertebrate eye development in a Pax 6-dependent manner. Overexpression of Drosophila Pax 6 homologs induces ectopic eye structures in the head and trunk region, whereas activation of Notch signaling induces eye defects in a restricted region of the head around the original eyes, suggesting a context-dependent manner for Pax 6 induction by Notch signaling. In Drosophila, Notch signaling regulates the expression of several key regulators for eye, antenna, leg, and wing morphogenesis in a context-dependent manner (29). For example, the activation of Notch signaling induces eyeless and twin of eyeless in eye-antennal discs and induces hyperplasia of the original eyes, as well as ectopic eyes on the rostral membrane, which is derived from the antennal discs. In the presence of Antennapedia protein, a homeotic gene product specifying the identity of the second thoracic segment (43, 44), the activation of Notch signaling induces expression of vestigial, a key regulator for wing development (45), and ectopic wing structures on the head (29). These observations suggest the possible conservation of a regulatory mechanism for Pax 6 gene expression in eye development of vertebrates and invertebrates. Consistent with our findings, Xu et al. (46) have reported that cis-regulatory elements of Pax 6 genes are functionally conserved in vertebrates and invertebrates.
Acknowledgments
We thank D. Ish-Horowicz and C. Kintner for Notch ΔE plasmid; R. Harland and W. Smith for Xnoggin plasmid; and R. Lang for discussion. This work was supported by the Kantons of Basel, the Swiss National Science Foundation, Core Research for Evolution Science and Technology of the Japan Science and Technology Corporation, The Mitsubishi Foundation, The Sumitomo Foundation, and a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Abbreviations
- GFP
green fluorescent protein
- RT
reverse transcriptase
- RPE
retinal pigment epithelium
References
- 1.Spemann H. Embryonic Development and Induction. New York: Yale Univ. Press; 1938. [Google Scholar]
- 2.Younossi-Hartenstein A, Tepass U, Hartenstein V. Roux's Arch Dev Biol. 1993;203:60–73. doi: 10.1007/BF00539891. [DOI] [PubMed] [Google Scholar]
- 3.Halder G, Callaerts P, Gehring W J. Curr Opin Genet Dev. 1995;5:602–609. doi: 10.1016/0959-437x(95)80029-8. [DOI] [PubMed] [Google Scholar]
- 4.Gehring W J. Genes Cells. 1996;1:11–15. doi: 10.1046/j.1365-2443.1996.11011.x. [DOI] [PubMed] [Google Scholar]
- 5.Mathers P H, Jamrich M. Cell Mol Life Sci. 2000;57:186–194. doi: 10.1007/PL00000683. [DOI] [PubMed] [Google Scholar]
- 6.Callaerts P, Halder G, Gehring W J. Annu Rev Neurosci. 1997;20:483–532. doi: 10.1146/annurev.neuro.20.1.483. [DOI] [PubMed] [Google Scholar]
- 7.Ton C C, Hirvonen H, Miwa H, Weil M M, Monaghan P, Jordan T, van Heyningen V, Hastie N D, Meijers-Heijboer H, Drechsler M, et al. Cell. 1991;67:1059–1074. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- 8.Walther C, Gruss P. Development (Cambridge, UK) 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 9.Hill R E, Favor J, Hogan B L, Ton C C, Saunders G F, Hanson I M, Prosser J, Jordan T, Hastie N D, van Heyningen V. Nature (London) 1991;354:522–525. doi: 10.1038/354522a0. [DOI] [PubMed] [Google Scholar]
- 10.Glaser T, Walton D S, Maas R L. Nat Genet. 1992;2:232–239. doi: 10.1038/ng1192-232. [DOI] [PubMed] [Google Scholar]
- 11.Jordan T, Hanson I, Zaletayev D, Hodgson S, Prosser J, Seawright A, Hastie N, van Heyningen V. Nat Genet. 1992;1:328–332. doi: 10.1038/ng0892-328. [DOI] [PubMed] [Google Scholar]
- 12.Quiring R, Walldorf U, Kloter U, Gehring W J. Science. 1994;265:785–789. doi: 10.1126/science.7914031. [DOI] [PubMed] [Google Scholar]
- 13.Halder G, Callaerts P, Gehring W J. Science. 1995;267:1788–1792. doi: 10.1126/science.7892602. [DOI] [PubMed] [Google Scholar]
- 14.Altmann C R, Chow R L, Lang R A, Hemmati-Brivanlou A. Dev Biol. 1997;185:119–123. doi: 10.1006/dbio.1997.8573. [DOI] [PubMed] [Google Scholar]
- 15.Chow R L, Altmann C R, Lang R A, Hemmati-Brivanlou A. Development (Cambridge, UK) 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- 16.Czerny T, Halder G, Kloter U, Souabni A, Gehring W J, Busslinger M. Mol Cell. 1999;3:297–307. doi: 10.1016/s1097-2765(00)80457-8. [DOI] [PubMed] [Google Scholar]
- 17.Niimi T, Seimiya M, Kloter U, Flister S, Gehring W J. Development (Cambridge, UK) 1999;126:2253–2260. doi: 10.1242/dev.126.10.2253. [DOI] [PubMed] [Google Scholar]
- 18.Bonini N M, Bui Q T, Gray-Board G L, Warrick J M. Development (Cambridge, UK) 1997;124:4819–4826. doi: 10.1242/dev.124.23.4819. [DOI] [PubMed] [Google Scholar]
- 19.Chen R, Amoui M, Zhang Z, Mardon G. Cell. 1997;91:893–903. doi: 10.1016/s0092-8674(00)80481-x. [DOI] [PubMed] [Google Scholar]
- 20.Halder G, Callaerts P, Flister S, Walldorf U, Kloter U, Gehring W J. Development (Cambridge, UK) 1998;125:2181–2191. doi: 10.1242/dev.125.12.2181. [DOI] [PubMed] [Google Scholar]
- 21.Pignoni F, Hu B, Zavitz K H, Xiao J, Garrity P A, Zipursky S L. Cell. 1997;91:881–891. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- 22.Shen W, Mardon G. Development (Cambridge, UK) 1997;124:45–52. doi: 10.1242/dev.124.1.45. [DOI] [PubMed] [Google Scholar]
- 23.Bernier G, Panitz F, Zhou X, Hollemann T, Gruss P, Pieler T. Mech Dev. 2000;93:59–69. doi: 10.1016/s0925-4773(00)00271-9. [DOI] [PubMed] [Google Scholar]
- 24.Mathers P H, Grinberg A, Mahon K A, Jamrich M. Nature (London) 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- 25.Seimiya M, Gehring W J. Development (Cambridge, UK) 2000;127:1879–1886. doi: 10.1242/dev.127.9.1879. [DOI] [PubMed] [Google Scholar]
- 26.Callaerts P, Munoz-Marmol A M, Glardon S, Castillo E, Sun H, Li W H, Gehring W J, Salo E. Proc Natl Acad Sci USA. 1999;96:558–563. doi: 10.1073/pnas.96.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pineda D, Gonzalez J, Callaerts P, Ikeo K, Gehring W J, Salo E. Proc Natl Acad Sci USA. 2000;97:4525–4529. doi: 10.1073/pnas.97.9.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gehring W J, Ikeo K. Trends Genet. 1999;15:371–377. doi: 10.1016/s0168-9525(99)01776-x. [DOI] [PubMed] [Google Scholar]
- 29.Kurata S, Go M J, Artavanis-Tsakonas S, Gehring W J. Proc Natl Acad Sci USA. 2000;97:2117–2122. doi: 10.1073/pnas.040556497. . (First Published February 11, 2000; 10.1073/pnas.040556497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hauck B, Gehring W J, Walldorf U. Proc Natl Acad Sci USA. 1999;96:564–569. doi: 10.1073/pnas.96.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artavanis-Tsakonas S, Rand M D, Lake R J. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- 32.Krieg P A, Melton D A. Nucleic Acids Res. 1984;12:7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coffman C R, Skoglund P, Harris W A, Kintner C R. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- 34.Hemmati-Brivanlou A, Melton D A. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 35.Bouwmeester T, Kim S, Sasai Y, Lu B, De Robertis E M. Nature (London) 1996;382:595–601. doi: 10.1038/382595a0. [DOI] [PubMed] [Google Scholar]
- 36.Hirsch N, Harris W A. J Neurobiol. 1997;32:45–61. [PubMed] [Google Scholar]
- 37.Li H, Tierney C, Wen L, Wu J Y, Rao Y. Development (Cambridge, UK) 1997;124:603–615. doi: 10.1242/dev.124.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hollemann T, Bellefroid E, Pieler T. Development (Cambridge, UK) 1998;125:2425–2432. doi: 10.1242/dev.125.13.2425. [DOI] [PubMed] [Google Scholar]
- 39.Loosli F, Koster R W, Carl M, Krone A, Wittbrodt J. Mech Dev. 1998;74:159–164. doi: 10.1016/s0925-4773(98)00055-0. [DOI] [PubMed] [Google Scholar]
- 40.Oliver G, Loosli F, Koster R, Wittbrodt J, Gruss P. Mech Dev. 1996;60:223–239. doi: 10.1016/s0925-4773(96)00632-6. [DOI] [PubMed] [Google Scholar]
- 41.Loosli F, Winkler S, Wittbrodt J. Genes Dev. 1999;13:649–654. doi: 10.1101/gad.13.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punzo C, Kurata S, Gehring W J. Genes Dev. 2001;15:1716–1723. doi: 10.1101/gad.196401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Struhl G. Proc Natl Acad Sci USA. 1982;79:7380–7384. doi: 10.1073/pnas.79.23.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schneuwly S, Klemenz R, Gehring W J. Nature (London) 1987;325:816–818. doi: 10.1038/325816a0. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Sebring A, Esch J J, Kraus M E, Vorwerk K, Magee J, Carroll S B. Nature (London) 1996;382:133–138. doi: 10.1038/382133a0. [DOI] [PubMed] [Google Scholar]
- 46.Xu P X, Zhang X, Heaney S, Yoon A, Michelson A M, Maas R L. Development (Cambridge, UK) 1999;126:383–395. doi: 10.1242/dev.126.2.383. [DOI] [PubMed] [Google Scholar]