Abstract
Background
Heavy metals especially cadmium (Cd), has become a matter of concern for environmentalists due to extensive industrialization and poor management of industrial waste. As a toxic pollutant, Cd has ability to deteriorate soil quality and hence disturbs the plant growth and yield. Co-composted biochar (COMBI) has been reported as an excellent organic amendment for improving soil quality, crop productivity and amelioration of heavy metals polluted soil. Therefore, an experiment was performed to assess the potential of co-composted biochar to enhance sunflower growth under Cd stressed soil. Different concentrations 0, 30 and 60 mg kg-1 Cd and normal, modified and co-composted biochar at the rate of 1% (w/w) were applied to soil.
Results
The application of normal and modified biochar considerably improved the sunflower growth, yield, physiology and biochemistry and decreased the Cd uptake in plant tissues. Among applied amendments, co-composted biochar showed better results, by increasing the crop agronomic parameters ranging from 115 to 132%, as compared to control treatment under Cd stress. The chlorophyll content, water use efficiency (WUE), photosynthetic rate (A), transpiration rate (E), stomatal conductance (gs), sub-stomatal conductance (Ci), relative water content (RWC), and electrolyte leakage (EL) were improved by 122, 117, 126, 133, 128, 131, 123, and 121%, respectively, when co-composted biochar was used compared to the control. Moreover, stress related metabolites and antioxidant enzyme essays showed increase in proline content, soluble sugars, lipid peroxidation, catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD) and glutathione reductase (GR) by using co-composted biochar by 123, 121, 118, 128, 124, 133 and 126%, respectively, in Cd (60 mg kg-1) contaminated soil. In addition to this, a prominent reduction in accumulation of Cd in the root (66%), shoot (77%) and grain (94%) was observed due to its immobilization in soil (121%) under the influence of co-composted biochar application.
Conclusion
The results of this study revealed that application of biochar could improve crop growth and immobilize Cd in soil and co-composted biochar could be adopted as a better strategy to remediate the heavy metal stressed soils. It can be considered as an effective practical approach to transform agricultural waste materials into organic soil amendments to be applied for sustainable agricultural practices in polluted soil.
Keywords: Cadmium, Sunflower, Co-composted biochar, Immobilization, Remediation, Sustainable agriculture
Introduction
The potentially toxic elements (PTEs) contamination particularly heavy metals in the soil have been increased during the last few decades mainly due to the industrial revolution and population explosion, which has consequences for environmental sustainability [1, 2]. Among most hazardous pollutants, heavy metals (HMs) are provoking havoc once introduced into the environment because of their long-term persistence in the environment [3, 4]. The absorption of essential nutrients by crop highly affected by heavy metals accumulation in soil, cause reduction in yield or even failure of crop [5]. Cadmium (Cd) is more dangerous than others heavy metals because it negatively affects the soil characteristics as well as threaten all living beings like plants, animals and especially human life [6]. Anthropogenic sources of Cd present in environment owe to combustion emissions, metal industry, traffic, sewage sludge, mining and incidents [7]. Degraded organic matter and Cd built up in soil disturbs its balance of essential nutrients which affects crop germination, stunts plant growth, and ultimately cause reduction in crop production [8]. Cd in soil and plants reduces the overall growth of plant and productivity mainly due to the interruption of cellular procedures occurring in plants like photosynthesis, respiration, transpiration, stomatal conductance, protein synthesis, antioxidant activity, nutrients uptake etc [9, 10].
In Faisalabad district, Pakistan, chief culprit of high Cadmium (Cd) concentration in soil is industrial discharge causing concentration of Cd by greater than 200% in the soil flooded with this wastewater [11, 12]. From soil, Cd is impacting humans and animal life by bio-magnification [13] causing lethal effects even when present at very low concentrations as it’s a known mutagenic and carcinogenic element [14, 15]. The excessive accumulation of Cd in edible parts of plant is a major challenge to prevent humans from the toxic effects of Cd [16]. Various physical, chemical and biological methods to minimize lethal impacts of HMs in plants have been adopted so far [17]; however, organic amendment’s use have gained great attention owing to their capability to decrease bioavailability of HMs. Among all organic amendments being applied, use of biochar is on the rise due to unique properties it possesses [18, 19].
Biochar is a product of pyrolysis in which organic material produced from animals or plants are heated (350–750 °C) under specific supply conditions of oxygen [20, 21]. It is used to improve the certain properties of soil i.e., soil structure, water and nutrient holding capacity, porosity, water infiltration, pH, organic matter as well as inorganic and organic pollutants removal [22, 23]. Heavy metals (HMs) contamination decreases soil fertility while biochar application increases the organic matter (OM) concentration in soil and ultimately increased fertility of soil [24]. Biochar addition to soil immobilizes the HMs and minimizes their availability and translocation to plants [25], thereby improving soil qualities [26]. Use of biochar amendments in soil improved (Cd) immobilization and decrease its bioaccumulation, ultimately enhance rice yield [27]. Application of biochar on the agricultural land also resulted in an increase of microbial activity to accelerate plant growth and yield by managing stresses like diseases, nutrients leaching and deficiency, mitigation of degraded soil [28, 29]. Under conditions like reduced functional groups, the biochar has low capacity to adsorb contaminants. Some earlier studies have reported that modified sorbent performs more efficiently to adsorb HMs than pristine biochar [18, 30].
Currently, modification procedures for effectively removal of contaminants which is most commonly enhanced by its surface modification with polyethylenimine (PEI). The high adsorption capacity for heavy metals (HMs) owing to presence of amine group [31, 32], are studied to improve sorption ability of biochar [33, 34]. Soil properties as well as physiological and bio-chemical features of plant adversely affected by salt stress. However, salt accumulation in soil and plant uptake reduced by residual impacts of microbial modified biochar along with nitrogen (N) fertilizer by improving soil properties as well as plant’s physiological and bio-chemical attributes [35]. Activation of biochar by use of potassium hydroxide and sodium hydroxide may produce further C-, H-, O- functional groups like hydroxyl group and increases the sorption capacity by complexation mechanism with enlarged biochar surface area [10, 36]. Application of microbial modified biochar improve the physico-chemical soil properties by immobilization as well as transformation of soil pollutants [37]. Therefore, surface modification of biochar is an important practice to enhance HMs adsorption in soil [38]. The functional group properties of biochar surface can be further augmented by combining with enriched compost product [39, 40]. Compost, usually produced under aerobic conditions through microbial oxidation of crop residues, organic wastes, and animal manure is also used to reclaim polluted soil [41, 42]. The synergistic effect of co-composted biochar (COMBI), which is rarely reported [43, 44], has more organic matter, C content and cation exchange capacity as well, compared to the compost or biochar applied individually [45–47]. It was reported that COMBI could decrease loss of nutrients from agricultural ecosystem and optimize recycling of organic resources for mitigation of climate change, leading to enhance agricultural-productivity [48]. COMBI support soil health, reduce lead uptake in Brassica napus and ultimately improving growth, Physiology as well as biochemical characteristics of plant [49].
Previous studies focused on application of biochar for mitigation of cadmium (Cd) contaminated soil, however, information on how co-composted and modified biochar enhances crop production and minimizes Cd uptake is still lacking. We hypothesized that the co-composted biochar (COMBI) would retrieve the badly effects of Cd and enhance the growth of crops by altering its antioxidants and metabolic profiles. The current study objectives were to (i) evaluate the impacts of normal, modified and co-composted biochar on growth and physiological alterations of sunflower under Cd stress and (ii) assess the capacity of applied amendments to retrieve negative effects of Cd and improve antioxidant, water relations and stress related metabolites of sunflower grown as a test crop under varying levels of Cd.
Materials and methods
Preparation of normal, modified, and co-composted biochar
The feedstock material rice husk collected from Institute of Soil and Environmental Sciences (ISES), University of Agriculture Faisalabad (UAF), was ground after drying, and subjected to muffle furnace (Nabertherm B 180, Lilienthal, Germany) at 450 °C pyrolysis temperature [50]. Prepared normal biochar further modified by following study [51, 52]. Prior to modification, normal biochar first treated in 3 mol L−1 solution of KOH at the rate of 1:10 (w/v) by adding 20 g biochar with 200 ml solution of KOH in flask and stirred at 160 rpm. at room temperature for a period of 1 h. Subsequently, deionized water was used to rinse biochar until pH of elution reached approximately 7.0. Then biochar dried at 353 K for a period of 12 h and then kept in desiccator before further use. Then KOH treated biochar added with 100 ml of 10% (w/v) polyethyleneimine (PEI) / methanol solution and stirred at 160 rpm. and 303 K for 24 h. After this, biochar promptly moved to a 200 ml solution of 1% (w/v) glutaraldehyde for cross linking. The solution stirred at 160 rpm. and 303 K for 30 min. Subsequently, modified biochar was rinsed by using deionized water.
The Co-composted biochar (COMBI) was prepared by mixing 1:2 mass ratio (w/w) of normal biochar and compost respectively during composting process at the compost production unit of ISES. The composting of plant litters (Green Waste) and animal manure was done by using aboveground piles. The plant litters (62%), animals manure (31%) and finished compost material (7%) were firstly placed above ground. The plant litters were laid down initially and animal manure placed on it, then finished compost material spread on it. Then sprayed the pile with water and turned it for 6 times. Now, Biochar was added above pile and turned it for 2 times further. The composts were then aerated by passing plastic perforated pipes through pile. Finally, the piles were covered with plastic sheets. The materials were mixed and sprayed with water at regular intervals and composting process was continued for 2.5 months [53, 54]. The basic characteristics of normal, modified as well as co-composted biochar (COMBI) shown in Table 1.
Table 1.
The physico-chemical characteristics of Biochar, co-composted Biochar and soil used in present study
| Characteristics of biochar | Soil | Normal biochar | Modified biochar | Co-composted biochar |
|---|---|---|---|---|
| Soil Texture | Sandy clay loam | - | - | - |
| pH | 7.74 | 7.61 | 7.45 | 7.26 |
| Electrical conductivity (dS m−1) | 1.46 | 3.4 | 3.1 | 3.7 |
| Cation exchange capacity (cmolc kg−1) | 14.2 | 78.5 | 68.3 | 92.7 |
| Organic matter/organic carbon (%) | 0.67 | 52.35 | 51.48 | 50.62 |
| Lime content (%) | 2.98 | - | - | - |
| Nitrogen (%) | 0.047 | 1.75 | 1.64 | 2.06 |
| Phosphorous (g kg−1) | 0.38 | 2.14 | 2.01 | 2.36 |
| Potassium (g kg−1) | 1.19 | 12.4 | 9.2 | 15.7 |
| Calcium (g kg−1) | 1.62 | 9.6 | 8.4 | 11.7 |
| Magnesium (g kg−1) | - | 5.1 | 4.7 | 6.8 |
| Sulfur (g kg−1) | - | 2.7 | 2.5 | 3.1 |
| Zinc (mg kg−1) | - | 84.21 | 65.20 | 122 |
| Iron (mg kg−1) | - | 88.34 | 68.32 | 162 |
| Manganese (mg kg−1) | - | 81.22 | 59.89 | 145 |
| Cadmium (mg kg−1) | ND | - | - | - |
ND Not detected
Pot experiment and treatment plan
A pot experiment was carried out in a wire-house at the Institute of Soil and Environmental Sciences (ISES), University of Agriculture Faisalabad (UAF), located at 31°26′17″N 73°04′09″E. The heavy metals uncontaminated soil (0–20 cm) collected from long term research farm area of ISES. The air-dried, ground and sieved (2 mm mesh size) soil of about 8 kg filled in plastic pots of 10-liter capacity each. The pre-analysis of sieved soil sub-sample was carried out to characterize basic physico-chemical properties (Table 1) through standard methods [55]. Cadmium (Cd) at a rate of 0, 30 and 60 mg kg−1 by using salt of cadmium chloride (CdCl2) before sowing of sunflower seeds was added to soil. Prepared normal, modified, and co-composted biochar (COMBI) were supplemented (1% w/w). Sunflower variety (Hysun-33) was sown, after thinning, 2 plants pot−1 were retained in each pot. Data concerning growth, physiology and yield of plants were recorded at various stages during crop growth.
Measurement of growth and yield attributes
At harvesting stage, meter rod(cm) was used to measure the shoot and root length of plants after 90 days of sowing. A digital electric balance was used to measure fresh and dry weight of shoot and root. The stem diameter of each plant was measured by using vernier caliper. The head diameter of each plant was taken by measuring the head from one point to the opposite end using a measuring rod. The number of achenes were calculated by counting the achene head−1 of each plant and weight of 1000 grains was measured by an electrical balance.
Physiological parameters
Plant physiological parameters were taken with fully expanded top 2nd leaf at 45th day of plant growth. Portable infrared gas analyzer (IRGA), (Model LCA-4, Germany) at photosynthetic photon flux density of 1200–1400 µmolm−2s−1 was used for this purpose [56]. Chlorophyll content, i.e., SPAD-502 m (Konica-Minolta, Japan) was used to measure the SPAD (Soil Plant Analysis Development) value [57].The relative water contents (RWC) of sunflower leaves were measured by previous method [58]. For this purpose, fresh, dry, and turgid weight of plant leaves (1 cm2) were determined and RWC was calculated by using the formula given below:
Relative water content (%) = 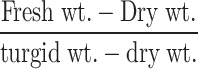 × 100
× 100
For determination of electrolyte leakage (EL), cork borer with sharp edge was used to cut leaves into identical discs (1 cm2) kept in test tubes (10 ml.) containing distilled water and electrical conductivity (EC1) was recorded. The EC2 was noted by placing these tubes on a mechanical shaker for 2 hours. Afterwards, the tubes were subjected to autoclaving at 120 °C and EC3 was observed upon cooling [59]. Following formula was used to determine EL.
EL (%) =  × 100
× 100
Following equation was used to calculate water use efficiency (WUE) [60].
WUE = 
Antioxidant activities
The previously given reduction method by [61] was used to measure superoxide dismutase (SOD) activity and the peroxidase (POD) activity was measured by using the protocol given by [62]. The catalase (CAT) enzyme was estimated by calculating the reduction in H2O2 absorption at 240 nm [63]. Ascorbate peroxidase (APX) activity was observed by method given below by Ahmad et al. [30] and glutathione reductase (GR) was determined by following [64] method.
AB-DTPA extractable cd analysis
Available cadmium (Cd) was determined by AB-DTPA method. For this purpose, AB-DTPA solution was prepared by dissolving diethylenetriamine penta acetic acid (DTPA) (1.97 gm) and ammonium bicarbonate (NH4)2HCO3 (79.06 g) and then adjusting the pH to 7.6. After taking 10 g soil in Erlenmeyer flasks, 20 mL of formerly prepared solution was added in it, centrifuged, and transparent extract was filtered, that afterwards was run on atomic absorption spectrophotometer [65].
Metal Cd (mg kg−1) = 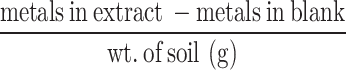 × Total volume of extract (ml)
× Total volume of extract (ml)
The soil immobilized Cd was calculated by using difference method of soil spiked with Cd (30 and 60 mg kg−1) and AB-DTPA extractable Cd.
For Cd analysis in soil and plant tissues, soil/ground plant samples (1 g) were taken in Erlenmeyer flasks and di-acid solution (HNO3-HClO4) (10 mL) was added in conical flasks that were retained for 24 h and placed on hot plate until the solution turned cleared. After cooling, the samples were filtered and readings were noted using an atomic absorption spectrophotometer [31].
Remediation indices of cd by sunflower plants
Several indices of remediation efficiency of sunflower plants against Cd were measured such as;
The enrichment factor of Cd metal was calculated as following [66];
 |
The EF > 1 shows the ability of sunflower plants to store cadmium metal while EF < 1 shows Cd absorption in sunflower plant.
The translocation factor of the Cd metal by sunflower plants was calculated by [67];
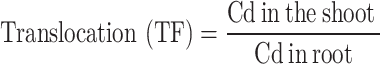 |
Bio accumulation factor (BAF) of sunflower plants was analyzed by [68];
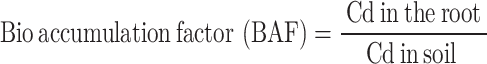 |
Bio accumulation coefficient (BAC) was calculated as described by [69];
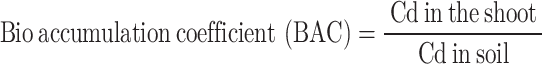 |
Cd health risk assessment parameters
Several indices for health risk assessment were calculated as mentioned below;
The average daily intake (ADI) index of cadmium (Cd) metal determined by using method given below;
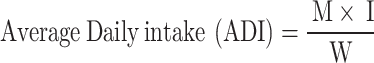 |
The Cd concentration in plant, daily intake of sunflower and average body weight (BW) denoted by M, I and W respectively in above equation. The adults have an average BW of 60 kg with average daily intakes of sunflower were considered 0.345 kg person−1 day−1 [70].
The Cd non-cancer risk (NCR) Cd was due to consumption of contaminated sunflower, calculated as [71];
 |
Oral reference doses (RFD) for Cd, is 0.5 [72].
Following formula to calculate integrated lifetime cancer risk (ILTCR), through ingestion of Cd contaminated grain [73];
 |
Where “CSF” represents the oral cancer slope factor for metal. For this study, cadmium, has CSF of 0.38 [74].
Statistical analysis
The Analysis of variance (ANOVA) test was used to analyzed the data obtained and means were compared with Tukey’s honestly significant difference (HSD) test for significant differences among different applied treatments at 5% probability level using Statistix 8.1 (Analytical software, 2005) [75]. The correlation plot was plotted in Origin Pro software and PCA plot was constructed with R studio software.
Results
Impact on plant growth and yield
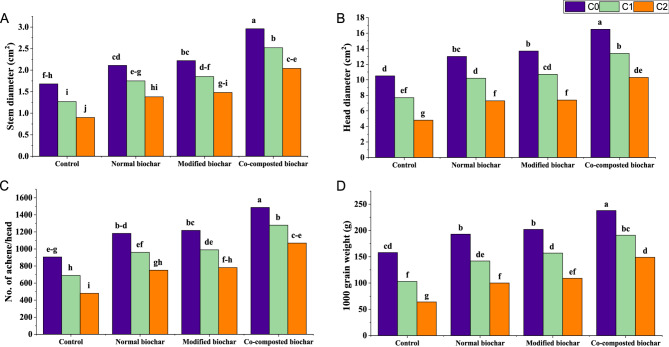
Plant growth and yield parameters were remarkably affected by Cadmium (Cd). Cd concentration at 60 mg kg−1 negatively affected growth of plant (Table 2) and yield (Fig. 1) attributes. However, application of both normal and modified biochar significantly improved the plant growth attributes at elevated Cd toxicity (30 and 60 mg kg−1), compared to control treatment. Furthermore, co-composted biochar (COMBI) addition significantly improved plant height (128%), root length (120%), fresh weight of shoot (127%) and root (121%), dry weight (DW) of shoot (126%) and root (118%), stem diameter (126%), head diameter (115%), no. of achene head−1 (123%), and 1000 grain weight (132%) at 60 mg kg−1 Cd over treatment set as control.
Table 2.
Effect of normal, modified, co-composted Biochar on growth attributes of sunflower under cadmium contaminated soil
| Cadmium | Amendment | Plant height (cm) | Fresh weight (g) | Dry weight (g) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | Shoot | Root | Shoot | Root | Shoot | Root | |||||||||||||||||||
| 0 | Control | 75 ± 3.66 | de | 47.6 ± 1.58 | d | 95 ± 4.23 | de | 58.7 ± 4.33 | d-f | 12.4 ± 0.63 | d-f | 9.4 ± 0.35 | de | ||||||||||||
| Normal biochar | 91 ± 1.92 | bc | 57.9 ± 1.37 | bc | 120 ± 4.81 | bc | 76.2 ± 3.03 | bc | 15.9 ± 0.78 | bc | 12.1 ± 0.41 | bc | |||||||||||||
| Modified biochar | 99 ± 3.65 | b | 60.3 ± 1.30 | bc | 130 ± 4.23 | B | 83.1 ± 2.87 | ab | 17.0 ± 0.66 | b | 13.4 ± 0.47 | b | |||||||||||||
| Co-composted biochar | 115 ± 3.87 | a | 69.3 ± 1.56 | a | 151 ± 6.46 | A | 99.9 ± 4.54 | a | 21.3 ± 0.52 | a | 16.0 ± 0.59 | a | |||||||||||||
| 30 | Control | 54 ± 2.41 | f | 37.7 ± 2.26 | e | 76 ± 3.99 | ef | 42.9 ± 3.12 | f | 9.6 ± 0.43 | f | 7.2 ± 0.47 | e-g | ||||||||||||
| Normal biochar | 75 ± 3.76 | de | 49.5 ± 2.01 | d | 98 ± 2.44 | D | 61.0 ± 3.19 | c-e | 12.9 ± 0.48 | c-e | 9.2 ± 0.48 | d | |||||||||||||
| Modified biochar | 83 ± 1.29 | cd | 53.0 ± 0.93 | cd | 103 ± 2.97 | cd | 65.8 ± 2.58 | cd | 13.8 ± 0.55 | cd | 10.7 ± 0.54 | cd | |||||||||||||
| Co-composted biochar | 98 ± 1.93 | b | 62.2 ± 1.83 | ab | 124 ± 2.44 | B | 83.9 ± 3.27 | ab | 17.1 ± 0.57 | b | 13.2 ± 0.58 | b | |||||||||||||
| 60 | Control | 34 ± 2.26 | g | 21.8 ± 1.82 | f | 41 ± 4.23 | G | 29.8 ± 2.42 | g | 6.0 ± 0.65 | g | 4.7 ± 0.45 | h | ||||||||||||
| Normal biochar | 51 ± 1.25 | f | 33.1 ± 1.05 | e | 62 ± 3.99 | F | 43.5 ± 1.98 | f | 9.5 ± 0.62 | f | 6.9 ± 0.40 | fg | |||||||||||||
| Modified biochar | 62 ± 3.23 | ef | 38.6 ± 1.13 | e | 72 ± 4.23 | F | 47.8 ± 3.44 | ef | 10.1 ± 0.76 | e-f | 7.6 ± 0.51 | ef | |||||||||||||
| Co-composted biochar | 77 ± 4.02 | cd | 48.0 ± 2.06 | d | 93 ± 2.44 | de | 65.9 ± 4.10 | cd | 13.6 ± 0.69 | cd | 10.1 ± 0.59 | cd | |||||||||||||
The values are mean ± S.E. (n = 3). Means sharing similar letter(s) in a column for each parameter do not differ significantly at P = 0.05
Fig. 1.
Effect of normal, modified, and co-composted biochar on yield attributes i.e., (A) stem diameter, (B) head diameter, (C) No. of achene/head, (D) 1000 grain weight of sunflower in Cd spiked soil. Here, C0, C1, and C2 indicate Cd 0, 30 and 60 mg kg−1, respectively
Impact on crop physiology
Cadmium (Cd) at 60 mg kg−1 extremely decreased chlorophyll content (16.4 mg cm−2), A (8.02 µmol m−2 s−1), E (1.79 mmol m−2 s−1), gs (60 mmol m-2 s-1), Ci (107 µmol mol−1) as shown in Table 3. However, the application of both Normal and modified biochar expressively improved the plant physiological parameters under increased Cd toxicity levels of 30 and 60 mg kg−1 compared to the control treatment. Nevertheless, co-composted biochar (COMBI) application considerably better enhanced the chlorophyll content (122%), A (126%), E (133%), gs (128%), and Ci (131%) at 60 mg kg−1 Cd.
Table 3.
Effect of normal, modified, co-composted Biochar on physiological attributes of sunflower under cadmium contaminated soil
| Cadmium | Amendment | Photosynthesis | Transpiration | Stomatal | Sub-stomatal | Relative water | Electrolyte | Chlorophyll Contents |
Water Use |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rate (A) | rate (E) | conductance (gs) | conductance (Ci) | content (RWC) | leakage (EL) | Efficiency (WUE) |
|||||||||||||||||||||||
| (mg kg−1) | (µmol m−2 s−1) | (mmol m−2 s−1) | (mmol m−2 s−1) | (µmol mol−1) | (%) | (%) | (mg cm−2) | (%) | |||||||||||||||||||||
| 0 | Control | 15.3 ± 0.87 | De | 3.91 ± 0.19 | de | 122 ± 3.33 | de | 210 ± 8.83 | Ef | 50.1 ± 2.32 | d | 5.83 ± 0.23 | Cd | 35 ± 1.9 | d | 2.11 ± 0.05 | ef | ||||||||||||
| Normal biochar | 19.9 ± 0.58 | Bc | 5.01 ± 0.2 | bc | 157 ± 4.54 | bc | 284 ± 9.91 | b-d | 61.5 ± 2.34 | bc | 3.36 ± 0.17 | Fg | 42 ± 1.27 | bc | 2.75 ± 0.08 | bc | |||||||||||||
| Modified biochar | 21.2 ± 0.98 | bc | 5.43 ± 0.24 | b | 174 ± 5.90 | b | 290 ± 11 | Bc | 65.1 ± 2.50 | b | 3.25 ± 0.15 | Fg | 44 ± 1.21 | b | 2.88 ± 0.06 | b | |||||||||||||
| Co-composted biochar | 26.1 ± 0.7 | A | 6.51 ± 0.25 | a | 202 ± 7.34 | a | 373 ± 13.1 | A | 76.6 ± 1.15 | a | 1.56 ± 0.12 | H | 53 ± 1.21 | a | 3.46 ± 0.07 | a | |||||||||||||
| 30 | Control | 11.7 ± 0.53 | F | 2.84 ± 0.1 | F | 91 ± 3.40 | f | 159 ± 7.51 | G | 36.5 ± 2.09 | e | 7.54 ± 0.22 | B | 28 ± 1.54 | ef | 1.6 ± 0.07 | g | ||||||||||||
| Normal biochar | 16 ± 0.54 | D | 3.9 ± 0.12 | de | 123 ± 4.34 | de | 227 ± 8.66 | Ef | 49.5 ± 2.38 | d | 4.90 ± 0.20 | De | 34 ± 1.33 | de | 2.24 ± 0.07 | de | |||||||||||||
| Modified biochar | 16.5 ± 0.54 | D | 4.24 ± 0.2 | cd | 140 ± 5.34 | cd | 235 ± 9.24 | De | 51.4 ± 2.41 | cd | 4.39 ± 0.17 | E | 37 ± 0.86 | cd | 2.45 ± 0.06 | cd | |||||||||||||
| Co-composted biochar | 21.9 ± 0.58 | B | 5.35 ± 0.2 | b | 170 ± 5.65 | b | 310 ± 9.82 | B | 62.6 ± 2.26 | b | 2.78 ± 0.12 | G | 45 ± 1.32 | b | 3.01 ± 0.04 | b | |||||||||||||
| 60 | Control | 8.02 ± 0.35 | G | 1.79 ± 0.16 | g | 60 ± 4.18 | g | 108 ± 6.14 | H | 22.3 ± 2.31 | f | 9.14 ± 0.25 | A | 16 ± 1.02 | g | 1.15 ± 0.06 | h | ||||||||||||
| Normal biochar | 11.8 ± 0.58 | F | 2.81 ± 0.2 | f | 91 ± 5.46 | f | 180 ±9.05 | Fg | 35.3 ± 1.25 | e | 6.40 ± 0.23 | C | 24 ± 1.37 | f | 1.73 ± 0.04 | g | |||||||||||||
| Modified biochar | 12.7 ± 0.64 | ef | 3.12 ± 0.25 | ef | 107 ± 6.77 | ef | 185 ± 10.1 | Fg | 37.2 ± 1.48 | e | 5.99 ± 0.16 | C | 26 ± 1.16 | f | 1.87 ± 0.08 | fg | |||||||||||||
| Co-composted biochar | 18.1 ± 0.45 | cd | 4.17 ± 0.22 | cd | 138 ± 7.13 | cd | 250 ± 12.1 | c-e | 49.7 ± 2.08 | d | 4.14 ± 0.12 | Ef | 36 ± 1.62 | cd | 2.5 ± 0.06 | cd | |||||||||||||
The values are mean ± S.E. (n = 3). Means sharing similar letter(s) in a column for each parameter do not differ significantly at P = 0.05
Water relation parameters (Table 3) were also affected by toxicity of Cd at elevated level (30 and 60 mg kg−1). In control treatment, Cd at 60 mg kg−1 level, decreased the WUE (1.15%), RWC (22.3%), and EL (9.14%). Application of normal and modified biochar efficiently enhanced the plant physiological parameters under Cd toxicity (30 and 60 mg kg−1) over control treatment. Here, application of COMBI further enhanced WUE (117%), RWC (123%), and decreased the EL (121%) under Cd stressed soil.
Antioxidant activities
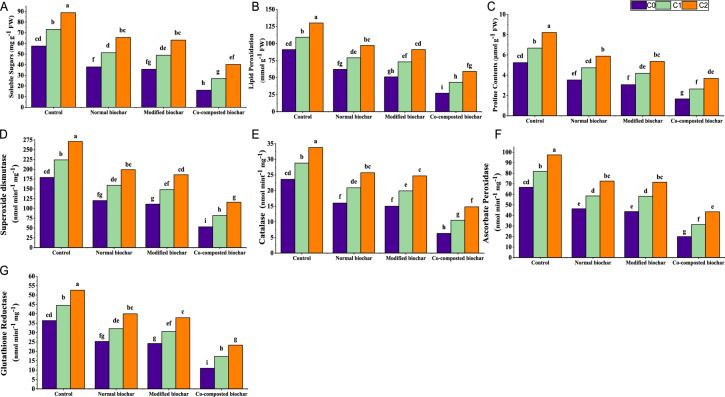
Under Cadmium (Cd) contaminated soil, the stress-related metabolites including, proline content, soluble sugar, lipid peroxidation and activity of antioxidant enzymes (CAT, APX, SOD and GR) significantly increased as shown in Fig. 2. However, stress-related metabolites and antioxidant activities were significantly declined by addition of normal and modified biochar. While, application of co-composted biochar (COMBI) showed substantial better results and decreased the proline content (123%), soluble sugars (121%), lipid peroxidation (118%), CAT (128%), APX (124%), SOD (133%), and GR (126%) over control treatment in Cd contaminated soils.
Fig. 2.
Effect of normal, modified, and co-composted biochar on sunflower’s antioxidant activities i.e., (A) soluble sugar, (B) lipid peroxidation, (C) proline contents, (D) superoxide dismutase, (E) catalase (F) ascorbate peroxidase, (G) Glutathione reductase, in Cd spiked soil. Here, C0, C1, and C2 indicate Cd 0, 30 and 60 mg kg−1, respectively
Cd content in soil and plant tissues
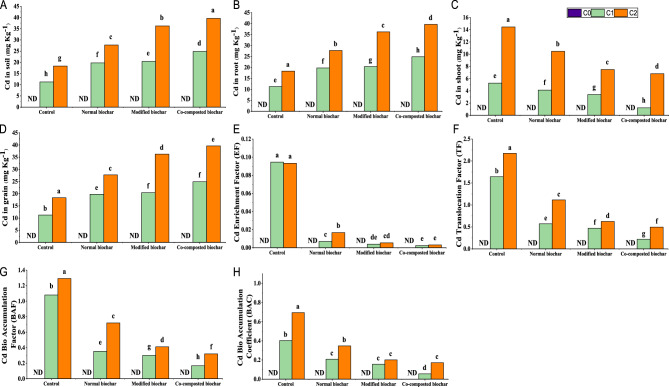
Concentration of cadmium (Cd), (Fig. 3) in the soil was observed at 11.2 and 18.3 µg g−1 and most of the Cd was accumulated in root (12.1 and 23.7 µg g−1), shoot (5.2 and 14.5 µg g−1) and grain (1.06 and 1.7 µg g−1) at 30, and 60 mg kg−1 Cd, respectively in control. However, application of both normal and modified biochar significantly immobilized Cd in soil and decreased its accumulation in aerial parts (root and shoot) of the plant. Whereas co-composted biochar (COMBI) significantly increased the Cd presence in soil (121%) and decreased Cd concentrations in root (66%), shoot (77%) and grains (94%) over control under 30 mg kg−1 Cd toxicity.
Fig. 3.
Effect of normal, modified, and co-composted biochar on Cd concentration in sunflower i.e., (A) Cd in soil, (B) Cd in root, (C) Cd in shoot, (D) Cd in grain, (E) Cd enrichment factor, (F) Cd translocation factor, (G) Cd bio accumulation factor, (H) Cd bio accumulation coefficient, in Cd spiked soil. Here, C0, C1, and C2 indicate Cd 0, 30 and 60 mg kg−1, respectively
Cd phytoremediation
The data obtained from the cadmium (Cd) phytoremediation by analyzing parameters of bio accumulation factor, translocation factor, and bio accumulation coefficient for Cd (Fig. 3E, F, G, H). The least values of 0.002 for EF, 0.29 for TF, 0.17 for BAF, and 0.055 for BAC were observed in presence of co-composted biochar in sunflower supplemented with 30 mg kg−1 cadmium.
Health risk assessment
Health risk assessment parameters of sunflower plants showed minimum values 0.003 for ADI, 0.30 for NCR, and 0.00011 for CR in plants treated with co-composted biochar cultivated with 30 mg kg−1 cadmium contaminated soil (Table 4).
Table 4.
Normal, modified, co-composted biochar’s effects on health risk assessment of sunflower under cadmium contaminated soil
| Cadmium | Amendment | Health Risk Assessment | |||||
|---|---|---|---|---|---|---|---|
| (mg kg−1) | ADI | NCR | CR | ||||
| 0 | Control | 0 | 0 | 0 | |||
| Normal biochar | 0 | 0 | 0 | ||||
| Modified biochar | 0 | 0 | 0 | ||||
| Co-composted biochar | 0 | 0 | 0 | ||||
| 30 | Control | 0.0052 b | 5.24 b | 0.00199 b | |||
| Normal biochar | 0.0007 e | 0.68 e | 0.00026 e | ||||
| Modified biochar | 0.0004 f | 0.39 f | 0.00015 f | ||||
| Co-composted biochar | 0.0003 f | 0.30 f | 0.00011 f | ||||
| 60 | Control | 0.0084 a | 8.42 a | 0.00320 a | |||
| Normal biochar | 0.0023 c | 2.2 c | 0.00087 c | ||||
| Modified biochar | 0.0010 d | 0.96 d | 0.00037 d | ||||
| Co-composted biochar | 0.0006 e | 0.61 e | 0.00023 e | ||||
The values are mean ± S.E. (n = 3). Means sharing similar letter(s) in a column for each parameter do not differ significantly at P = 0.05
Pearson correlation and principal component analysis (PCA.)
Significant positive as well as negative correlations (Fig. 4) were analyzed among the plant growth (length, fresh and dry weight of root and shoot), yield (head and stem diameter, no. of achene per head and 1000 grain weight), physiology (relative water contents, chlorophyll contents, water use efficiency), biochemical (soluble sugars, lipid peroxidation, proline, sodium dismutase, catalase, ascorbate peroxidase and glutathione), and heavy metal contents (soil, root, shoot, grain, EF, TF, BAF, BAC) of treatments under cadmium (Cd) stress and application of amendments. The SL, RL, SFWT, RFWT, SDWT, RDWT, SD, HD, APH, TGWT, RWC, CC, WUE, showed positive correlation with each other indicating that these parameters showed improved activity in presence of amendments under Cd stress. However, growth, physiology and yield parameters showed negative correlation with biochemical and heavy metals related parameters like EL, SS, H2O2, PC, SOD, CAT, APX, GR, S.Cd, R.Cd, Sh.Cd, and G.Cd.
Fig. 4.
Pearson correlation between parameters of sunflower plants under application of normal, modified, and co-composted biochar grown in Cd spiked soil. Here parameters are indicated as SL = shoot length, RL = root length, SFWT = shoot fresh weight, RFWT = root fresh weight, SDWT = shoot dry weight, RDWT = root dry weight, SD = stem diameter, HD = head diameter, APH = number of achene per head, TGWT = thousand grain weight, RWC = relative water contents, EL = electrolyte leakage, CC = chlorophyll contents, WUE = water use efficiency, SS = soluble sugars, H2O2 = lipid peroxidation, P.C = proline contents, SOD = superoxide dismutase, CAT = catalase, APX = ascorbate peroxidase, GR = glutathione reductase, S.Cd = Cd in soil, RCd = Cd in root, Sh.Cd = Cd in shoot, GCd = Cd in gain
The principal component analysis (PCA) revealed (Fig. 5) that shoot length (SL), root length (RL), fresh weight of shoot (SFWT) and root (RFWT), dry weight of shoot (SDWT) and root (RDWT), stem diameter (SD), head diameter (HD), achene per head (APH), thousand grain weight (TGWT), relative water contents (RWC), chlorophyll contents (CC) and water use efficiency (WUE), indicated strong correlated among parameters due to application of amendments under Cd toxicity. Whereas, growth, physiology and yield parameters showed negative correlation with biochemical and heavy metals related parameters like electrolyte leakage (EL), soluble sugars (SS), lipid peroxidation (H2O2), proline (PC), sodium dismutase (SOD), catalase (CAT), (ascorbate peroxidase (APX), glutathione (GR), Cd in soil (S.Cd), root Cd (RCd), shoot Cd (SCd) and Cd in grain (GCd).
Fig. 5.

Principal component analysis (PCA) between parameters of sunflower plants under application of normal, modified, and co-composted biochar grown in Cd spiked soil. Here parameters are indicated as SL = shoot length, RL = root length, SFWT = shoot fresh weight, RFWT = root fresh weight, SDWT = shoot dry weight, RDWT = root dry weight, SD = stem diameter, HD = head diameter, APH = number of achene per head, TGWT = thousand grain weight, RWC = relative water contents, EL = electrolyte leakage, CC = chlorophyll contents, WUE = water use efficiency, SS = soluble sugars, H2O2 = lipid peroxidation, P.C = proline contents, SOD = superoxide dismutase, CAT = catalase, APX = ascorbate peroxidase, GR = glutathione reductase, S.Cd = Cd in soil, RCd = Cd in root, Sh.Cd = Cd in shoot, GCd = Cd in grain
Discussion
Heavy metals have gained a significant attention from researchers due to their persistent and hazardous nature, having capacity to localized in plant parts and therefore disturbing agroecosystem and risking health of living organisms [30]. The crop production limited by number of factors, drought, salinity, chilling stress and heavy metals toxicity as described by researchers [9, 76]. Among heavy metals, cadmium (Cd) is more harmful for human, plant, and animal health due to its mobility in soil [6, 77].
The growth of sunflower plants was limited due to contamination of soil with cadmium at both 30 and 60 mg kg−1 levels. Declined in the length of root and shoot as well as fresh and dry weight, were observed with application of 30 and 60 mg kg−1 cadmium. The presence of Cd in soil interrupts the nutrient uptake by plants, therefore, causing nutrients deficiency. Similar to this study previously, with increased application of metal contaminated textile wastewater, plant growth in tomato plants was declined [78].
However, cadmium (Cd) stress was remediated effectively by applying normal, modified, and co-composted biochar (COMBI) to improve the growth of sunflower in Cd spiked soil. The current study showed significant increase in lengths, fresh and dry weight of root and shoot of sunflower plants with addition of normal, modified, and co-composted biochar, compared to control (without amendments) grown in soil contaminated with Cd. Biochar is a source of various micro and macro nutrients and hence beneficial in soils with nutrients deficiency. Hence, improves the fertility of soil as reported by [79]. Large surface area of biochar was also beneficial in holding Cd metal in soil. Therefore, the increase growth was attributed to the provision of nutrients, as well as immobilization and retention of soluble Cd by biochar, coalesce with the findings of [80]. However, overall significant growth was noticed when COMBI was supplied to sunflower in contrast to normal and modified biochar under normal and Cd stressed soils [81].
The presence of cadmium (Cd) resulted in reduced diameter of stem and head, number of achenes per head and thousand grain weight, therefore, causing reduction in yield of sunflower plants. Declined yield of sunflower plants exposed to Cd, might be linked with harmful effects of Cd [82]. High concentration of Cd in soil probably has disrupted soil characteristics and have replaced the essential nutrient required for proper growth of plant and eventually resulted in limiting yield [83]. Whereas application of amendments most importantly co-composted biochar (COMBI) provided the desired nutrients to the normal functioning of plants while remediating cadmium metal [9]. Overall comparison revealed that yield parameters were increased maximum by using COMBI than modified and normal biochar. Therefore, it might be considered as an effective methodology to be applied for sustainable agricultural practices in Cd stress [76, 84].
Cadmium decreased the physiological parameters in sunflower plant, which ultimately declined plant growth [16, 85, 86]. Furthermore, declined CO2 assimilation is associated with decreases production of carboxylic enzymes [87] and Rubisco activity [88]. Therefore, data of the current study correlates with previous studies, indicating that Cd causes a reduction in photosynthetic pigments, the number of stomata, and other physiological traits, thereby exerting a negative effect [19, 20]. The study results revealed a decline in sunflower efficiency regarding physiological processes, when exposed to Cd.
When plant was exposed to cadmium (Cd) stress, parameters like WUE and RWC decreased. Reduction in chlorophyll pigment decreased the photosynthetic and transpiration rate in plants which may lead to decrease in WUE. According to [89], Cd retards the physiological mechanisms like photosynthesis and transpiration in plants. Whereas, by the application of biochar, increase in WUE and RWC parameters is associated with the improved growth as well as crop yield. Moreover, EL was also increased due to membrane damage Cd phytotoxicity [90]. The supplementation of normal and surface modified biochar caused significant decrease in Cd levels in soil, while use of co-composted biochar (COMBI) led to an even more substantial reduction. This effect might enhance Cd immobilization in soil and reduced uptake by plants. Conversely, prior research has reported a significant increase in these physiological attributes through the utilization of biochar [91, 92]. The physiological traits were more significantly increased by addition of modified biochar, but application of COMBI showed much better and significant results over all the applied treatments including control. The biochar and compost application synergistically immobilized Cd in the soil and reduced harmful effects because of their ability to make metals complex, and decreased Cd bioavailability resulting in improved physiological attributes of crops [93].
Sunflower plants exposed to the cadmium (Cd), showed significant reduction in antioxidant activities. Increased metal levels in plants leads to disruption in DNA, producing reactive oxygen species, and hence damaging the biomolecules [11]. Moreover, translocation of Cd metal in plant tissues causes altered function of essential nutrients and therefore, generating reactive oxygen species (ROS) [90]. However, application of normal, modified, and co-composted biochar significantly improved the production of antioxidants in sunflower in contrast to control under the toxicity of Cd that showed their progressive role in reducing toxicity of Cd [11].
Increased immobilization of cadmium metal was found in soil polluted with different cadmium (Cd) concentrations (30, 60 mg Kg−1), in presence of co-composted biochar (COMBI). Whereas increased Cd level was reported in roots as compared to other plant parts. Plants highly differ in their biogeochemical behavior to buildup Cd in underground and above ground parts as well as in transportation of metal to root through soil that largely depends upon accessible soil portion of metal and in concern of roots to shoots vary with species and genotype of the tested plant [94–96]. In this study, Cd buildup into various plant parts was observed due to intentional addition of Cd to the soil as an exogenous source. These findings are in accordance with the previously reported literature [97]. Moreover, large quantity of root exudates production which binds Cd by cell wall and extracellular medium led to buildup of their larger concentration in root [98]. The Cd bioaccumulation depends upon its binding capacity, intercellular complex as well as its transportation within various plant parts [99–101]. Furthermore, concentration of the Cd in plant highly differs with its capacity for intercellular buildup of CO2 and transpiration. The findings of current study elucidated that exposure to the Cd exerted substantial detrimental effects on the stomatal conductance, transpiration rate, and also sub-stomatal conductance of the investigated crop. This phenomenon likely facilitated the enhanced accumulation of Cd in sunflower plants via soil contamination. Nevertheless, the utilization of co-composted, modified, and normal biochar proved efficacious in mitigating these adverse effects by instigating a mechanism of Cd immobilization within the soil, thereby reducing its concentration in the soil-plant continuum. Notably, stomatal conductance and transpiration rate are integral factors governing the dynamic transport and uptake of both essential nutrients and contaminants, including Cd, within the intricate soil-plant-atmosphere interface [60]. However, reduced Cd was transported and stored in other plant parts shoot and grains respectively. The declined cadmium uptake was also indicated by least values of EF, TF, BAF, and BAC along with reduced ADI, NCR, and CR for sunflower grown in Cd contaminated soils in presence of co-composted biochar (COMBI). The reduction of Cd uptake and translocation to upper parts of plant is also a part of adaptive strategy in plants growing in soil contaminated with metals [77].
Use of amendments especially co-composted biochar (COMBI) showed increased cadmium (Cd) concentration in soil while declined metal uptake was observed in root, shoot and also grains of sunflower plant sown in soil contaminated with Cd. The biochar has the unique remediation properties like presence of large surface area, macro and micro pores, binding sites and presence of various functional groups that are key factors in holding Cd in soil and hence reducing uptake and translocation of the Cd within plant tissue. The increased immobilization of metals like Cd is reported previously by researchers [102, 103]. This study revealed, addition of normal, modified, and COMBI significantly stabilized Cd in soil and reduced their bioavailability to crop which may lead to significant improvement in the growth as well as physiological factors. However, efficiency of these factors more significantly improved by use of COMBI in contrast to modified and normal biochar. Because, in addition to biochar, compost also contains different functional group on its surface and an immediate as well as rich source of nutrients which increases the effectiveness of biochar in regard to growth, physiology as well as Cd immobilization in soil. Furthermore, COMBI use resulted in significantly more decline in bioavailable fractions of soil’s Cd and decreased its concentration in plant [104] as compared to normal and modified biochar.
From the above discussion, it reveals that Cadmium (Cd) toxicity cause serious threats to soil-plant system. Whereas co-composted biochar (COMBI) has significant potential for crop growth and amelioration of Cd polluted soil. Cd bioavailability in the soil significantly reduced by the application of (COMBI) and ultimately decreased its accumulation in sunflower plant tissues. The capacity of soil to hold water and nutrients increased by the application of COMBI which improves root development along with plant health which eventually results in higher plant biomass and yield. It restricts the entering of Cd into food chain as well as improve crop safety along with marketability. By Using COMBI as an amendment in the Cd polluted soil, farmers can grow crops safely which are susceptible to stress of heavy metals. It promotes improvement in structure, organic matter contents as well as soil microbial properties of soil, leading to sustainability in agriculture. In this way, farmers operating on heavy metals polluted fields can get good economics outcomes from higher yields. This approach is adaptable and farmers can use COMBI as an effective organic soil amendment for sustainable agricultural practices.
Conclusion
The study demonstrates that co-composted biochar is a highly effective amendment for mitigating cadmium (Cd) stress in sunflower, significantly improving plant growth, physiological performance, and antioxidant responses. Under Cd-contaminated soil conditions, co-composted biochar outperformed normal and modified biochar by enhancing key agronomic parameters, such as root and shoot growth, yield, and physiological traits like chlorophyll content, water use efficiency, and photosynthetic rate. It also boosted antioxidant enzyme activities (CAT, APX, SOD, GR) and stress-related metabolites, reducing oxidative damage caused by Cd toxicity. Importantly, co-composted biochar immobilized Cd in the soil, reducing its uptake in roots, shoots, and grains by up to 94%, thereby minimizing health risks associated with Cd accumulation. The synergistic effects of biochar and compost in co-composted biochar not only improved soil fertility and nutrient availability but also enhanced Cd immobilization through its unique physicochemical properties, such as large surface area and functional groups. This study highlights the potential of co-composted biochar as a sustainable and eco-friendly strategy for the remediation of heavy metal-contaminated soils, promoting crop productivity, and ensuring food safety.
Acknowledgements
The Institute of Soil and Environmental Sciences (ISES) at University of Agriculture Faisalabad (UAF), Pakistan has provided facilities to conduct this research, and the authors are very thankful. This work has been financially supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. KFU252288).
Authors’ contributions
Conceptualization, M.N. and A.M.; methodology, M.R. and M.A.B.; software, M.R. and A.G.; formal analysis, A.M., M.M. and M.N.; investigation, M.A.B. and M.R.; resources, M.N., M.M., H.A.D. and H.A.M.; data curation, M.R. and M.A.; writing-original draft preparation, M.R. and M.N.; writing-review and editing, A. M., M.N.S. and A.G.; visualization, A.M., supervision, M.N.
Funding
This work has been financially supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia (Project No. KFU252288).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/28/2025
The original online version of this article was revised: the authors would like to update the Authors' contribution section
Change history
7/29/2025
A Correction to this paper has been published: 10.1186/s12870-025-07098-1
Contributor Information
Muhammad Naveed, Email: muhammad.naveed@uaf.edu.pk.
Abdul Ghafoor, Email: aghafoor@kfu.edu.sa.
Adnan Mustafa, Email: adnanmustafa780@gmail.com.
References
- 1.Li X, Li L, Zhou Z, Li T, An J, Zhang S, Xu X, Pu Y, Wang G, Jia Y, Liu X, Li Y. Soil potentially toxic element pollution at different urbanization intensities: quantitative source apportionment and source-oriented health risk assessment. Ecotoxicol Environ Saf. 2023;251:114550. [DOI] [PubMed] [Google Scholar]
- 2.Saeed Q, Xiukang W, Haider FU, Kučerik J, Mumtaz MZ, Holatko J, Naseem M, Kintl A, Ejaz M, Naveed M. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int J Mol Sci. 2021;22:10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mustafa A, Zulfiqar U, Mumtaz MZ, Radziemska M, Haider FU, Holatko J, Hammershmiedt T, Naveed M, Ali H, Kintl A, Saeed Q. Nickel (Ni) phytotoxicity and detoxification mechanisms: A review. Chemosphere. 2023;328:138574. [DOI] [PubMed] [Google Scholar]
- 4.Saleh HM, Hassan AI. Environmental impact and remediation of heavy metals. In: IntechOpen; 2022. 10.5772/intechopen.97895.
- 5.Li HJ, Ming LL, Zhang WS. Uptake, translocation and tolerance mechanism of cadmium in plants: a review. Asian J Ecotoxicol. 2022;17(2):86–95. [Google Scholar]
- 6.Zulfiqar U, Jiang W, Xiukang W, Hussain S, Ahmad M, Maqsood MF, Ali N, Ishfaq M, Kaleem M, Haider FU. Cadmium phytotoxicity, tolerance, and advanced remediation approaches in agricultural soils: a comprehensive review. Front Plant Sci. 2022;13:773815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan Z, Elahi A, Bukhari DA, Rehman A. Cadmium sources, toxicity, resistance and removal by microorganisms - a potential strategy for cadmium eradication. J Saudi Chem Soc. 2022;26:101569. [Google Scholar]
- 8.Shahabivand S, Parvaneh A, Aliloo AA. Root endophytic fungus Piriformospora indica affected growth, cadmium partitioning and chlorophyll fluorescence of sunflower under cadmium toxicity. Ecotoxicol Environ Saf. 2017;145:496–502. [DOI] [PubMed]
- 9.Naveed M, Mustafa A, Majeed S, Naseem Z, Saeed Q, Khan A, et al. Enhancing cadmium tolerance and pea plant health through Enterobacter sp. MN17 inoculation together with Biochar and gravel sand. Plants. 2020;9:530. [DOI] [PMC free article] [PubMed]
- 10.Bashir S, Hussain Q, Jun Z, Qingling F, Houben D, Hongqing H. Efficiency of KOH-modified rice straw-derived Biochar for reducing cadmium mobility, bioaccessibility and bioavailability risk index in red soil. Pedosphere. 2020;30:874–82.
- 11.Sabir A, Naveed M, Bashir MA, Hussain A, Mustafa A, Zahir ZA, Kamran M, Ditta A, Núñez-Delgado A, Saeed Q. Cadmium mediated phytotoxic impacts in Brassica napus: managing growth, physiological and oxidative disturbances through combined use of Biochar and Enterobacter sp. MN17. J Environ Manage. 2020;265:110522. [DOI] [PubMed]
- 12.Hussain A, Murtaza G, Ghafoor A, Basra SMA, Qadir M, Sabir M. Cadmium contamination of soils and crops by long term use of Raw effluent, ground and Canal waters in agricultural lands. Int J Agric Biol. 2010;12:851–6.
- 13.Paunov M, Koleva L, Vassilev A, Vangronsveld J, Goltsev V. Effects of different metals on photosynthesis: cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int J Mol Sci. 2018;19:787. [DOI] [PMC free article] [PubMed]
- 14.Charkiewicz AE, Omeljaniuk WJ, Nowak K, Garley M, Niklinski J. Cadmium toxicity and health Effects-A brief summary. Molecules. 2023;28:6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sibuar AA, Zulkafflee NS, Selamat J, Ismail MR, Lee SY, Abdull Razis AF. Quantitative analysis and human health risk assessment of heavy metals in paddy plants collected from perak. Malaysia. Int J Environ Res Public Health. 2022;19:731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zea M, Souza A, Yang Y, Lee L, Nemali K, Hoagland L. Leveraging highthroughput hyperspectral imaging technology to detect cadmium stress in two leafy green crops and accelerate soil remediation efforts. Environ Pollut. 2022;292:118405. [DOI] [PubMed] [Google Scholar]
- 17.Rizwan M, Ali S, Abbas T, Zia-ur-Rehman M, Hannan F, Keller C, Al-Wabel MI, Ok YS. Cadmium minimization in wheat: a critical review. Ecotoxicol Environ Saf. 2016;130:43–53. [DOI] [PubMed] [Google Scholar]
- 18.Naveed M, Tanvir B, Xiukang W, Brtnicky M, Ditta A, Kucerik J, Subhani Z, Nazir MZ, Radziemska M, Saeed Q. Co-composted Biochar enhances growth, physiological, and phytostabilization efficiency of brassica napus and reduces associated health risks under chromium stress. Front Plant Sci. 2021;12:775785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu Y, Wang H, Lv X, Zhang Y, Wang W. Effects of Biochar and biofertilizer on cadmium-contaminated cotton growth and the antioxidative defense system. Sci Rep. 2020;10:20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbas A, Azeem M, Naveed M, Latif A, Bashir S, Ali A, Bilal M, Ali L. Synergistic use of Biochar and acidified manure for improving growth of maize in chromium contaminated soil. Int J Phytorem. 2020;22:52–61. [DOI] [PubMed] [Google Scholar]
- 21.Cui L, Pan G, Li L, Bian R, Liu X, Yan J, Quan G, Ding C, Chen T, Liu Y. Continuous immobilization of cadmium and lead in Biochar amended contaminated paddy soil: a five-year field experiment. Ecol Eng. 2016;93:1–8. [Google Scholar]
- 22.Murtaza G, Ahmed Z, Usman M, Tariq W, Ullah Z, Shareef M, Iqbal H, Waqas M, Tariq A, Wu Y. Biochar induced modifications in soil properties and its impacts on crop growth and production. J Plant Nutr. 2021;44:1677–91. [Google Scholar]
- 23.Fischer D, Glaser B. Synergisms between compost and Biochar for sustainable soil amelioration. Manage Org Waste. 2012;1:167–98. [Google Scholar]
- 24.Qi X, Gou J, Chen X, Xiao S, Ali I, Shang R, Wang D, Wu Y, Han M, Luo X. Application of mixed bacteria-loaded Biochar to enhance uranium and cadmium immobilization in a co-contaminated soil. J Hazard Mater. 2021;401:123823. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Sale PW, Tang C. Cadmium uptake by Carpobrotus Rossii (Haw.) schwantes under different saline conditions. Environ Sci Pollut Res. 2016;23:13480–8. [DOI] [PubMed] [Google Scholar]
- 26.Ullah N, Ditta A, Khalid A, Mehmood S, Rizwan MS, Ashraf M, Mubeen F, Imtiaz M, Iqbal MM. Integrated effect of algal Biochar and plant growth promoting rhizobacteria on physiology and growth of maize under deficit irrigations. J Soil Sci Plant Nutr. 2020;20:346–56. [Google Scholar]
- 27.Han J, Wu D, Yang J, Shi Y, Abid G, Wang L, Li Z. A biochar-based amendment improved cadmium (Cd) immobilization, reduced its bioaccumulation, and increased rice yield. Front Environ Sci. 2024;12:1487190. [Google Scholar]
- 28.Mustafa A, Brtnicky M, Hammerschmiedt T, Kucerik J, Kintl A, Chorazy T, Naveed M, Skarpa P, Baltazar T, Malicek O, Holatko J. Food and agricultural wastes-derived biochars in combination with mineral fertilizer as sustainable soil amendments to enhance soil Microbiological activity, nutrient cycling and crop production. Front Plant Sci. 2022;13:1028101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mustafa A, Holatko J, Hammerschmiedt T, Kucerik J, Skarpa P, Kintl A, Racek J, Baltazar T, Malicek O, Brtnicky M. Comparison of the responses of soil enzymes, microbial respiration and plant growth characteristics under the application of agricultural and food waste-derived biochars. Agronomy. 2022;12(10):2428. [Google Scholar]
- 30.Ahmad S, Wang GY, Muhammad I, Zeeshan M, Zhou XB. Melatonin and KNO3 application improves growth, physiological and biochemical characteristics of maize seedlings under waterlogging stress conditions. Biology. 2022;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X, Feng J, Cai Y, Fang M, Kong M, Alsaedi A, Hayat T, Tan X. Porous Biochar modified with polyethyleneimine (PEI) for effective enrichment of U (VI) in aqueous solution. Sci Total Environ. 2020;708:134575. [DOI] [PubMed] [Google Scholar]
- 32.Pang Y, Zeng G, Tang L, Zhang Y, Liu Y, Lei X, Li Z, Zhang J, Liu Z, Xiong Y. Preparation and application of stability enhanced magnetic nanoparticles for rapid removal of cr (VI). Chem Eng J. 2011;175:222–7. [Google Scholar]
- 33.Rajapaksha AU, Chen SS, Tsang DC, Zhang M, Vithanage M, Mandal S, Gao B, Bolan NS, Ok YS. Engineered/designer Biochar for contaminant removal/immobilization from soil and water: potential and implication of Biochar modification. Chemosphere. 2016;148:276–91. [DOI] [PubMed] [Google Scholar]
- 34.Inyang M, Gao B, Zimmerman A, Zhou Y, Cao X. Sorption and cosorption of lead and Sulfapyridine on carbon nanotube-modified biochars. Environ Sci Pollut Res. 2015;22:1868–76. [DOI] [PubMed] [Google Scholar]
- 35.Abbas HMM, Rais U, Sultan H, Tahir A, Bahadur S, Shah A, Iqbal A, Li Y, Khan MN, Nie L. Residual effect of Microbial-Inoculated Biochar with nitrogen on rice growth and salinity reduction in paddy soil. Plants. 2024b;13:2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regmi P, Moscoso JLG, Kumar S, Cao X, Mao J, Schafran G. Removal of copper and cadmium from aqueous solution using Switchgrass Biochar produced via hydrothermal carbonization process. J Environ Manage. 2012;109:61–9. [DOI] [PubMed] [Google Scholar]
- 37.Abbas HMM, Rais U, Altaf MM, Rasul F, Shah A, Tahir A, Rehman MNU, Shaukat M, Sultan H, Zou R, Khan MN, Nie L. Microbial-inoculated Biochar for remediation of salt and heavy metal contaminated soils. Sci Total Environ. 2024a;954:176104. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Li H, Lin S. Advances in the study of heavy metal adsorption from water and soil by modified Biochar. Water. 2022;14:3894. [Google Scholar]
- 39.Wiedner K, Fischer D, Walther S, Criscuoli I, Favilli F, Nelle O, Glaser B. Acceleration of Biochar surface oxidation during composting. J Agric Food Chem. 2015;63:3830–7. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Lü F, Luo C, Shao L, He P. Humification characterization of Biochar and its potential as a composting amendment. J Environ Sci. 2014;26:390–7. [DOI] [PubMed] [Google Scholar]
- 41.Lin C, Cheruiyot NK, Buic XT, Ngo HH. Composting and its application in bioremediation of organic contaminants. Bioengineered. 2022;13:1073–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gruda NS, Bragg N. Developments in alternative organic materials for growing media in soilless culture systems’, Advances in horticultural soilless culture. Burleigh Dodds Sci Publishing. 2021:73–106. 10.19103/AS.2020.0076.03.
- 43.Awasthi MK, Wang Q, Huang H, Li R, Shen F, Lahori AH, Wang P, Guo D, Guo Z, Jiang S. Effect of Biochar amendment on greenhouse gas emission and bio-availability of heavy metals during sewage sludge co-composting. J Clean Prod. 2016;135:829–35. [Google Scholar]
- 44.Schulz H, Dunst G, Glaser B. No effect level of co-composted Biochar on plant growth and soil properties in a greenhouse experiment. Agronomy. 2014;4:34–51. [Google Scholar]
- 45.Mikajlo I, Lerch TZ, Louvel B, Hynšt J, Zahora J, Pourrut B. Composted Biochar versus compost with Biochar: effects on soil properties and plant growth. Biochar. 2024;6:85. [Google Scholar]
- 46.Antonangelo JA, Sun X, Zhang H. The roles of co-composted Biochar (COMBI) in improving soil quality, crop productivity, and toxic metal amelioration. J Environ Manage. 2021;277:111443. [DOI] [PubMed] [Google Scholar]
- 47.Tessfaw ZA, Beyene A, Nebiyu A, Pikoń K, Landrat M. Co-composting of khat-derived Biochar with municipal solid waste: a sustainable practice of waste management. Sustainability. 2020;12:10668. [Google Scholar]
- 48.Gao S, Harrison BP, Thao T, Gonzales ML, An D, Ghezzehei TA, Diaz G, Ryals RA. Biochar co-compost improves nitrogen retention and reduces carbon emissions in a winter wheat cropping system. GCB Bioenergy. 2023;15(4):462–77. [Google Scholar]
- 49.Jiang W, Liu Y, Zhou J, Tang H, Meng G, Tang X, Ma Y, Yi T, Elsaid FG. Biochar co-compost increases the productivity of Brassica napus by improving antioxidant activities and soil health and reducing lead uptake. J Hazard Mater. 2023;443:130273. [DOI] [PMC free article] [PubMed]
- 50.Sánchez M, Lindao E, Margaleff D, Martínez O, Morán A. Pyrolysis of agricultural residues from rape and sunflowers: production and characterization of bio-fuels and Biochar soil management. J Anal Appl Pyrol. 2009;85:142–4. [Google Scholar]
- 51.Jing XR, Wang YY, Liu WJ, Wang YK, Jiang H. Enhanced adsorption performance of Tetracycline in aqueous solutions by methanol-modified Biochar. Chem Eng J. 2014;248:168–74.
- 52.Ma Y, Liu WJ, Zhang N, Li YS, Jiang H, Sheng GP. Polyethyleneimine modified Biochar adsorbent for hexavalent chromium removal from the aqueous solution. Bioresour Technol. 2014;169:403–8. [DOI] [PubMed]
- 53.Agegnehu G, Bass AM, Nelson PN, Bird MI. Benefits of biochar, compost and biochar–compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci Total Environ. 2016;543:295–306. [DOI] [PubMed]
- 54.Qayyum MF, Liaquat F, Rehman RA, Gul M, ul Hye MZ, Rizwan M, Rehman MZ. Effects of co-composting of farm manure and Biochar on plant growth and carbon mineralization in an alkaline soil. Environ Sci Pollut Res. 2017;24:26060e26068. [DOI] [PubMed] [Google Scholar]
- 55.Estefan G, Sommer R, Ryan J. Methods of Soil, Plant, and Water Analysis: A Manual for the West Asia and North Africa Region. Third Edition, International Center for Agricultural Research in the Dry Areas (ICARDA). Beirut. 2013. pp. 84–105.
- 56.Ben-Asher J, Tsuyuki I, Bravdo BA, Sagih M. Irrigation of grapevines with saline water: I. Leaf area index, stomatal conductance, transpiration and photosynthesis. Agric Water Manag. 2006;83:13–21. [Google Scholar]
- 57.Agüero M, Barg M, Yommi A, Camelo A, Roura S. Postharvest changes in water status and chlorophyll content of lettuce (Lactuca sativa L.) and their relationship with overall visual quality. J Food Sci. 2008;73:S47–55. [DOI] [PubMed]
- 58.Mayak S, Tirosh T, Glick BR. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Sci. 2004b;166:525–30. [Google Scholar]
- 59.Lutts S, Kinet J, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot. 1996;78:389–98.
- 60.Farquhar G, Richards R. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Aust J Plant Physiol. 1984;11(6):539.
- 61.Mobin M, Khan NA. Photosynthetic activity, pigment composition and antioxidative response of two mustard (Brassica juncea) cultivars differing in photosynthetic capacity subjected to cadmium stress. J Plant Physiol. 2007;164:601–10. [DOI] [PubMed]
- 62.Liang Y, Bai T, Liu B, Yu W, Teng W. Different antioxidant regulation mechanisms in response to aluminum-induced oxidative stress in Eucalyptus species. Ecotoxicol Environ Saf. 2022;241:113748. [DOI] [PubMed]
- 63.Bilal S, Khan AL, Shahzad R, Kim YH, Imran M, Khan MJ, Al-Harrasi A, Kim TH, Lee IJ. Mechanisms of cr (VI) resistance by endophytic Sphingomonas sp. LK11 and its cr (VI) phytotoxic mitigating effects in soybean (Glycine max L). Ecotoxicol Environ Saf. 2018;164:648–58. [DOI] [PubMed] [Google Scholar]
- 64.Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem. 1988;175:408–13. [DOI] [PubMed]
- 65.Soltanpour P. Use of ammonium bicarbonate DTPA soil test to evaluate elemental availability and toxicity. Commun Soil Sci Plant Anal. 1985;16:323–38.
- 66.Lorestani B, Cheraghi M, Yousefi N. Accumulation of pb, fe, mn, Cu and Zn in plants and choice of hyperaccumulator plant in the industrial town of vian, Iran. Arch Biol Sci. 2011;63:739–45. [Google Scholar]
- 67.Cui S, Zhou Q, Chao L. Potential hyperaccumulation of pb, zn, Cu and cd in endurant plants distributed in an old smeltery, Northeast China. Environ Geol. 2007;51:1043–8. [Google Scholar]
- 68.Yoon J, Cao X, Zhou Q, Ma LQ. Accumulation of pb, cu, and Zn in native plants growing on a contaminated Florida site. Sci Total Environ. 2006;368:456–64. [DOI] [PubMed] [Google Scholar]
- 69.Rizova V. Assessment of phytoremediation potential of Indigenous plants growing around the solid waste open dumpsite. Environ Eng Res. 2020;24:234. [Google Scholar]
- 70.Latif A, Bilal M, Asghar W, Azeem M, Ahmad MI, Abbas A, Ahmad MZ, Shahzad T. Heavy metal accumulation in vegetables and assessment of their potential health risk. J Environ Anal Chem. 2018;5:2380–91. [Google Scholar]
- 71.Jan FA, Ishaq M, Khan S, Ihsanullah I, Ahmad I, Shakirullah M. A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J Hazard Mater. 2010;179:612–21. [DOI] [PubMed] [Google Scholar]
- 72.Yahaya TO, Oladele EO, Fatodu IA, Abdulazeez A, Yeldu YI. The concentration and health risk assessment of heavy metals and microorganisms in the groundwater of lagos, Southwest Nigeria. J Adv Environ Health Res. 2020;8:234–42. [Google Scholar]
- 73.Rehman ZU, Khan S, Qin K, Brusseau ML, Shah MT, Din I. Quantification of inorganic arsenic exposure and cancer risk via consumption of vegetables in Southern selected districts of Pakistan. Sci Total Environ. 2016;550:321–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fontaine A, Straka W, Meyer R, Jonson M, Young S, Neary VS. Performance and wake flow characterization of a 1: 8.7-scale reference USDOE MHKF1 hydrokinetic turbine to Establish a verification and validation test database. J Renew Energy. 2020;159:451–67. [Google Scholar]
- 75.Steel RGD, Torrie JH. Principles and procedures of statistics. New York, Toronto, London: McGraw-Hill Book Company, Inc; 1960. p. xvi + 481.
- 76.Abrar MM, Saqib M, Abbas G, Atiq-ur-Rahman M, Mustafa A, Shah SAA, Mehmood K, Maitlo AA, Sun N, Xu M. Evaluating the contribution of growth, physiological, and ionic components towards salinity and drought stress tolerance in Jatropha curcas. Plants. 2020;9:1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moradi N, Karimi A. Fe-Modified common Reed Biochar reduced cadmium (Cd) mobility and enhanced microbial activity in a contaminated calcareous soil. J Soil Sci Plant Nutr. 2021;21:329–40. [Google Scholar]
- 78.Naseem Z, Naveed M, Asghar HN, Hameed M. Metal resistant Enterobacter cloacae ZA14 enhanced seedling Vigor and metal tolerance through improved growth, physiology and antioxidants in tomato (Solanum lycopersicum) irrigated with textile effluents. Sustainability. 2022;14:13619.
- 79.Lehmann J. Bio-energy in the black. Front Ecol Environ. 2007;5:381–7. [Google Scholar]
- 80.Jun L, Wei H, Aili M, Juan N, Hongyan X, Jingsong H, Yunhua Z, Cuiying P. Effect of lychee Biochar on the remediation of heavy metal-contaminated soil using sunflower: A field experiment. Environ Res. 2020;188:109886. [DOI] [PubMed] [Google Scholar]
- 81.Teodoro M, Trakal L, Gallagher BN, Šimek P, Soudek P, Pohořelý M, Beesley L, Jačka L, Kovář M, Seyedsadr S. Application of co-composted Biochar significantly improved plant-growth relevant physical/chemical properties of a metal contaminated soil. Chemosphere. 2020;242:125255. [DOI] [PubMed] [Google Scholar]
- 82.Ahmad I, Akhtar MJ, Asghar HN, Zahir ZA. Comparative efficacy of growth media in causing cadmium toxicity to wheat at seed germination stage. Int J Agric Biol. 2013;15(3):1560–8530.
- 83.Haider FU, Liqun C, Coulter JA, Cheema SA, Wu J, Zhang R, Wenjun M, Farooq M. Cadmium toxicity in plants: impacts and remediation strategies. Ecotoxicol Environ Saf. 2021;211:111887. [DOI] [PubMed]
- 84.Ahmad I, Akhtar MJ, Zahir ZA, Jamil A. Effect of cadmium on seed germination and seedling growth of four wheat (Triticum aestivum L.) cultivars. Pak J Bot. 2012;44:1569–74.
- 85.Liu C, Guo J, Cui Y, Lü T, Zhang X, Shi G. Effects of cadmium and Salicylic acid on growth, spectral reflectance and photosynthesis of castor bean seedlings. Plant Soil. 2011;344:131–41. [Google Scholar]
- 86.Shi G, Cai Q. Photosynthetic and anatomic responses of peanut leaves to cadmium stress. Photosynthetica. 2008;46:627–30. [Google Scholar]
- 87.Krantev A, Yordanova R, Janda T, Szalai G, Popova L. Treatment with Salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J Plant Physiol. 2008;165:920–31. [DOI] [PubMed] [Google Scholar]
- 88.Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Gonçalves B, Santos C. Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant. 2013;35:1281–9. [Google Scholar]
- 89.Choppala G, Bolan NS, Bibi S, Iqbal M, Rengel Z, Kunhikrishnan A, Ashwath N, Ok YS. Cellular mechanisms in higher plants governing tolerance to cadmium toxicity. Crit Rev Plant Sci. 2014;33:374–91. [Google Scholar]
- 90.Quan R, Shang M, Zhang H, Zhao Y, Zhang J. Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J. 2004;2:477–86. [DOI] [PubMed]
- 91.Bashir S, Qayyum MA, Husain A, Bakhsh A, Ahmed N, Hussain MB, Elshikh MS, Alwahibi MS, Almunqedhi BM, Hussain R. Efficiency of different types of biochars to mitigate cd stress and growth of sunflower (Helianthus annuus; L.) in wastewater irrigated agricultural soil. Saudi J Biol Sci. 2021;28:2453–9. [DOI] [PMC free article] [PubMed]
- 92.Seleiman MF, Alotaibi MA, Alhammad BA, Alharbi BM, Refay Y, Badawy SA. Effects of ZnO nanoparticles and Biochar of rice straw and cow manure on characteristics of contaminated soil and sunflower productivity, oil quality, and heavy metals uptake. Agronomy. 2020;10:790. [Google Scholar]
- 93.Abbas T, Rizwan M, Ali S, Adrees M, Mahmood A, Zia-ur-Rehman M, Ibrahim M, Arshad M, Qayyum MF. Biochar application increased the growth and yield and reduced cadmium in drought stressed wheat grown in an aged contaminated soil. Ecotoxicol Environ Saf. 2018;148:825–33. [DOI] [PubMed] [Google Scholar]
- 94.Shahid M, Dumat C, Khalid S, Niazi NK, Antunes PM. Cadmium bioavailability, uptake, toxicity and detoxification in soil-plant system. Rev Environ Contam Toxicol. 2017;241:73–137. [DOI] [PubMed] [Google Scholar]
- 95.Gonçalves J, Tabaldi L, Cargnelutti D, Pereira L, Maldaner J, Becker A, Rossato L, Rauber R, Bagatini M, Bisognin D. Cadmium-induced oxidative stress in two potato cultivars. Biometals. 2009;22:779–92. [DOI] [PubMed] [Google Scholar]
- 96.Singh R, Agrawal M. Effects of sewage sludge amendment on heavy metal accumulation and consequent responses of Beta vulgaris plants. Chemosphere. 2007;67:2229–40. [DOI] [PubMed] [Google Scholar]
- 97.Rascio N, Navari-Izzo F. Heavy metal hyperaccumulating plants: how and why do they do it? And what makes them so interesting? Plant Sci. 2011;180:169–81. [DOI] [PubMed] [Google Scholar]
- 98.Mishra S, Srivastava S, Tripathi R, Govindarajan R, Kuriakose S, Prasad M. Phytochelatin synthesis and response of antioxidants during cadmium stress in Bacopa monnieri L. Plant Physiol Biochem. 2006;44:25–37. [DOI] [PubMed] [Google Scholar]
- 99.Cobbett CS, May MJ, Howden R, Rolls B. The glutathione-deficient, cadmium‐sensitive mutant, cad2–1, of Arabidopsis thaliana is deficient in γ‐glutamylcysteine synthetase. Plant J. 1998;16:73–8. [DOI] [PubMed] [Google Scholar]
- 100.Marchiol L, Leita L, Martin M, Peressotti A, Zerbi G. Physiological responses of two soybean cultivars to cadmium. American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America. 1996;25(3):562–66.
- 101.Horst WJ. The role of the Apoplast in aluminium toxicity and resistance of higher plants: a review. J Plant Nutr Soil Sci. 1995;158:419–28. [Google Scholar]
- 102.Rafique MI, Ahmad M, Al-Wabel MI, Ahmad J, Al-Farraj AS. Mitigating the toxic effects of chromium on wheat (Triticum aestivum L.) seed germination and seedling growth by using Biochar and Polymer-Modified Biochar in contaminated soil. Sustainability. 2022;14:16093. [Google Scholar]
- 103.Uchimiya M, Bannon DI, Wartelle LH. Retention of heavy metals by carboxyl functional groups of biochars in small arms range soil. J Agric Food Chem. 2012;60:1798–809. [DOI] [PubMed] [Google Scholar]
- 104.Namgay T, Singh B, Singh BP. Influence of Biochar application to soil on the availability of as, cd, cu, pb, and Zn to maize (Zea mays L). Soil Res. 2010;48:638–47. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript or supplementary information files.