Significance
Trichomonas vaginalis, the causative agent of trichomoniasis, is a common sexually transmitted infection. Characterizing the host cell immune response to T. vaginalis and how the parasite subverts these defense mechanisms will be key in attempts to prevent pathogenesis by the parasite. Complicating our understanding of how T. vaginalis causes disease is the symbiosis the parasite establishes with the bacterium Mycoplasma hominis (Mh). Here, we explore how this symbiosis affects the innate immune response of host cells and show that M. hominis triggers an interferon-epsilon (IFNε) response that is protective against T. vaginalis cytoadherence and cytolysis of host cells. To counteract this symbiont-driven response, extracellular vesicles (EVs) produced by the parasite downregulate host cell IFNε to promote infection.
Keywords: Trichomonas vaginalis, extracellular vesicles, interferon, innate immunity, Mycoplasma hominis
Abstract
Trichomonas vaginalis is a common, extracellular, sexually transmitted parasite which is often found in symbiosis with the intracellular bacterium Mycoplasma hominis (Mh), an opportunistic pathogen of the female reproductive tract. How this symbiosis affects infection outcomes and the host cell innate immune response is poorly understood. Here, we show that infection with T. vaginalis in symbiosis with M. hominis or M. hominis alone triggers a noncanonical type I interferon, interferon-epsilon (IFNε), but infection with T. vaginalis alone does not. We also demonstrate that extracellular vesicles (TvEVs) produced by the parasite downregulate host cell IFNε, counteracting this symbiont-driven response and elevating infection. We further demonstrate that IFNε, a hormonally regulated cytokine produced in the human reproductive system, is protective against T. vaginalis cytoadherence and cytolysis of host cells. These studies provide insight into how a parasite and its bacterial symbiont work in concert to regulate host cell innate immune responses to drive infection.
Trichomonas vaginalis is an extracellular, protozoan parasite of the human urogenital tract and causes the most common nonviral sexually transmitted infection (STI) (1–3). Infection can lead to adverse pregnancy outcomes, such as preterm delivery, low birth weight, and infant death, and has been associated with infertility and an increased risk of cervical cancer (4–10). Despite the high prevalence of infection and long-term consequences for reproductive health, the molecular pathogenesis of the disease is poorly understood. Additionally, accumulating epidemiological data indicate an unusually high prevalence of T. vaginalis infection in women at the age of peri-/postmenopause (11–16). Symbiosis of the parasite with Mycoplasma hominis, an obligate intracellular bacterium associated with dysbiosis, bacterial vaginosis, and capable of replicating within parasite and human cells, further complicates our understanding of how T. vaginalis causes disease (17, 18). Although estimates of T. vaginalis clinical isolates that harbor M. hominis can exceed 85% depending on geographical location, very little is known about how this symbiosis affects T. vaginalis pathogenesis (19–28).
T. vaginalis and other eukaryotic pathogens secrete extracellular vesicles (EVs) that contain parasite proteins, nucleic acids, and other small molecules as a form of intracellular communication between pathogens and their host cells (29–43). T. vaginalis EVs (TvEVs) can be internalized by both human ectocervical cells and prostate-derived cells and have been shown to modulate expression and secretion of the cytokines IL-6 and IL-8 (44). Additionally, pretreatment of human cells with TvEVs can increase cytoadherence of the parasite, an important process in its pathogenesis (44, 45). However, we still know very little about the mechanisms underlying the effects of TvEVs on host cell gene expression and immune signaling pathways. Elucidating the full range of effects of TvEVs on recipient host cells will be important not only to the pathogenesis of T. vaginalis but also to outcomes during M. hominis infection as well.
Here, we demonstrate that M. hominis infection alone or in symbiosis with T. vaginalis drives expression of host cell genes involved in the type I interferon (IFN) response that are not triggered by the parasite itself. In turn, TvEVs suppress interferon-epsilon (IFNε) mediated type I IFN responses and are capable of disrupting Signal Transducer and Activator of Transcription 1 and 2 (STAT1/2) nuclear translocation, critical host cell transcription factors that drive a type I IFN response. Furthermore, we show that IFNε is protective against T. vaginalis-mediated cytoadherence and cytolysis of host cells.
Results
Pretreatment with T. vaginalis EVs Renders Host Cells More Susceptible to Parasite-Mediated Killing and Results in Increased Parasite Burden.
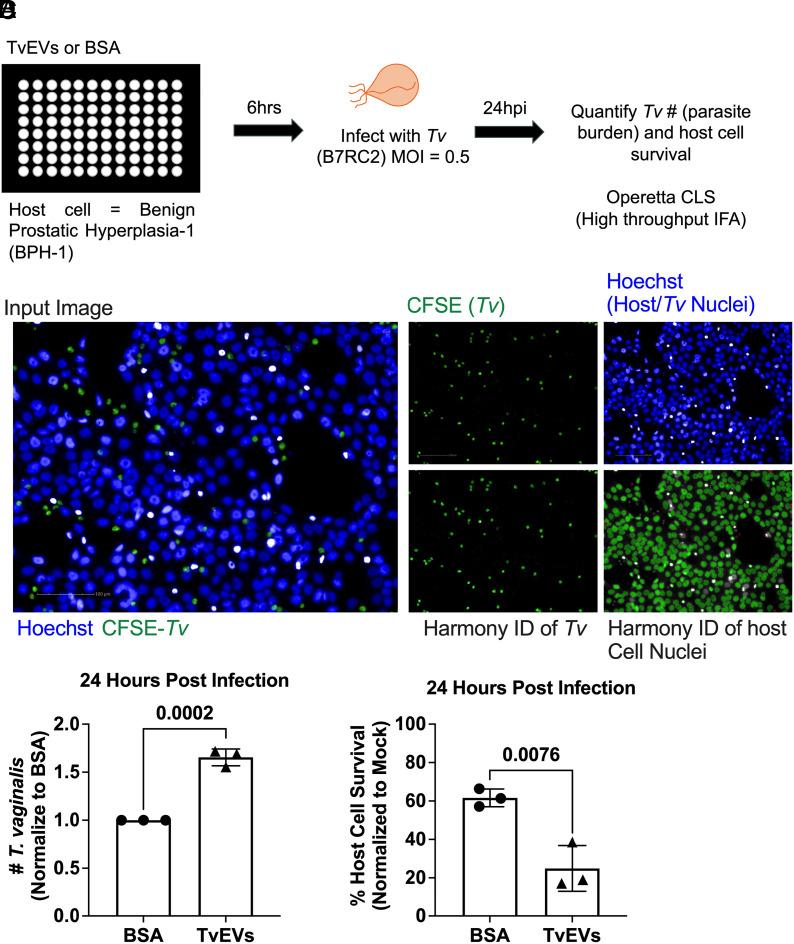
TvEVs, produced by the parasite T. vaginalis, are known to suppress IL-6 and IL-8 secretion and increase parasite adherence to host cells (44, 45). To determine whether TvEVs also alter parasite-mediated cytolysis of host cells and parasite burden, we modified an automated imaging pipeline to track host cell survival and parasite burden (45). Human benign prostatic hyperplasia (BPH-1) cells were pretreated in a 96-well plate format with TvEVs or bovine serum albumin (BSA) prior to infection with fluorescently labeled parasites (Fig. 1 A and B). At 24 h postinfection (hpi), entire wells were imaged and host cell survival and parasite burden were quantified (Fig. 1 C and D). At 24 hpi, we observed an increase in parasite burden when host cells were pretreated with TvEVs compared to BSA control (Fig. 1C). In accordance with increased parasite burden, we also observed a significant decrease in host cell survival when cells were pretreated with TvEVs (Fig. 1D). Together, these data indicate that TvEVs increase susceptibility to T. vaginalis-mediated cytolysis and increase parasite burden.
Fig. 1.
TvEVs increase parasite burden and parasite-mediated host cell cytolysis. (A) Schematic depicting infection of host cells with T. vaginalis. BPH-1 cells were seeded onto 96-well plates to confluency and then treated with 10 µg/mL of TvEVs or BSA for 6 h prior to infection with CFSE-labeled T. vaginalis strain B7RC2 for 24 h. (B) Entire wells, treated with either BSA or TvEVs, were imaged on the Operetta® CLS platform and analyzed using Harmony™ software. (Scale bar, 100 µm.) (C) The number of T. vaginalis Left in the well after 24 h was enumerated using CFSE staining to quantify parasites normalized to BSA control. Data are depicted as fold change in adherence compared to BSA control. Bars, mean ± SD. N = 3 wells/experiment, 51 fields of view/well, 3 experiments total. Numbers above bars indicate P-values for unpaired two-tailed Student’s t test as compared to BSA control. (D) Percent of host cell survival was quantified by enumerating the number of host cells Left in the well after 24 h compared to uninfected controls. Data are depicted as fold change in adherence compared to BSA control. Bars, mean ± SD. N = 3 wells/experiment, 51 fields of view/well, 3 experiments total. Numbers above bars indicate P-values for unpaired two-tailed Student’s t test as compared to BSA control.
Transcriptomic Analyses Indicate that TvEVs Suppress Multiple Host Cell Immune Pathways, Including the Type I IFN Pathway.
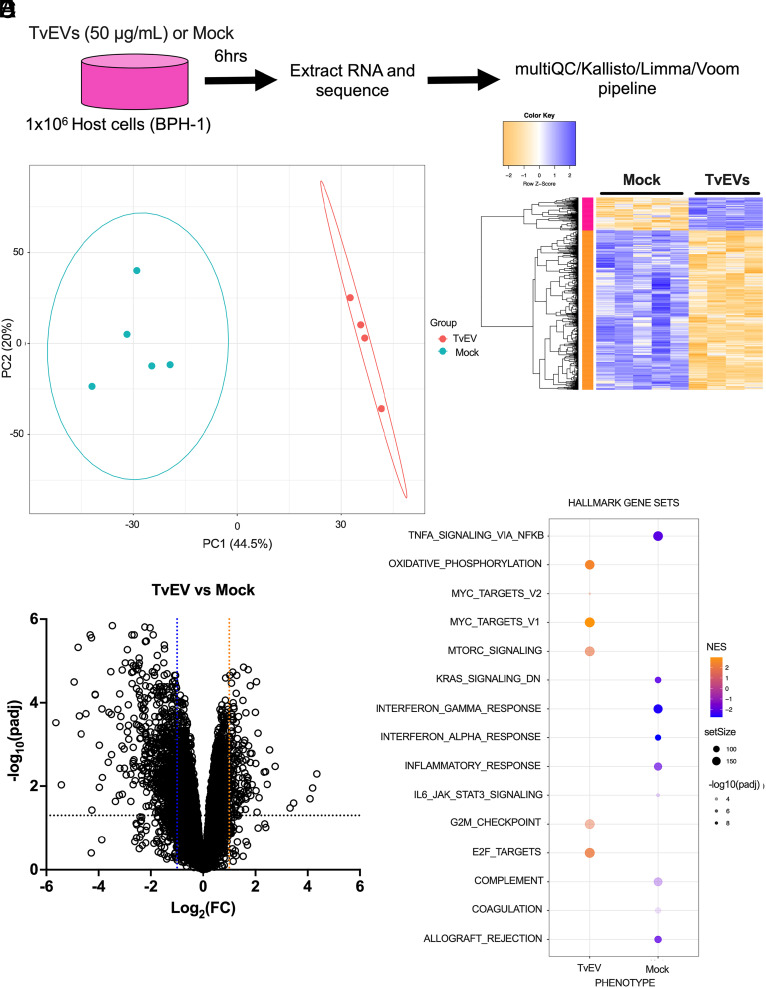
We next sought to identify what effect TvEVs have on host cell gene expression as a way of identifying potential mediators of the observed increased susceptibility of host cells to parasite-mediated killing and increased parasite burden (Fig. 1). We treated cells with TvEVs for 6 h prior to extracting RNA for RNA sequencing (RNA-seq) (Fig. 2A). Sample alignment and quality was assessed using MultiQC (SI Appendix, Fig. S1) (46). Transcriptional profiles of the 5 Mock- and 4 TvEV-treated samples were distinguishable by principal component analysis (PCA) (Fig. 2B), with 833 genes (143 upregulated and 690 downregulated) identified as differentially expressed between the two conditions (Fig. 2 C and D and Datasets 1 and 2). Eighty-three percent (690/833) of the differentially expressed genes (DEGs) were downregulated in response to TvEV treatment, indicating a largely suppressive effect on host cell gene expression (Fig. 2 C and D). Several of the topmost downregulated DEGs included genes involved in innate immunity including inflammatory cytokines and chemokines (TNFSF14, IL36G, IL36G, CCL5, CCL2, CXCL10, and CXCL8) and genes with known antimicrobial properties (NOS2, S100A7, S100A8, and S100A9). We next employed Gene Set Enrichment Analysis (GSEA) to identify host cell pathways that were altered (47). GSEA of the Hallmark dataset indicated that several innate immune signaling pathways (NF-κB signaling via TNFα and interferon alpha and gamma responses) were enriched in mock-treated samples compared to TvEV-treated samples (Fig. 2E and Dataset 3).
Fig. 2.
TvEVs down-regulate innate immune genes and pathways. (A) Schematic depiction of RNA-seq experiment. (B) Principal Component Analysis (PCA) showing distinct clustering of mock-treated BPH-1 cells (Mock; n = 5) and TvEV-treated BPH-1 cells (TvEV; n = 4) from RNA-seq data. (C) Heatmap of row z-score transformed 832 genes (143 upregulated and 689 downregulated; Log2(FC) ≥ 1 or Log2(FC) ≤ −1; padj ≤ 0.05) identified as differentially expressed between mock and TvEV-treated BPH-1 cells. (D) Volcano plot depicting differences in gene expression between TvEVs and mock-treated BPH-1 cells. The x axis corresponds to the log2 fold change in gene expression and the y axis indicates the adjusted P value. Genes with a −log10 P value of 1.3 or greater (P value of ≤ 0.05) and a log2 fold change −1 ≤ or ≥ 1 were deemed significantly differentially expressed. (E) Bubble chart showing results of Gene Set Enrichment Analysis (GSEA) for the Hallmarks dataset in TvEV-treated (Left) and mock-treated BPH-1 cells (Right). Color of bubble represents normalized enrichment score (gold signifies pathway enriched compared to mock and blue signifies pathway is enriched in mock condition). SetSize indicates number of genes in the indicated pathway. −log10(padj) value indicated by intensity of shading.
While previous studies have shown a role for NF-κB signaling in T. vaginalis infection (30, 48–53), we were surprised to observe that GSEA predicted that TvEVs suppressed type I and II IFN pathways (Fig. 2E and SI Appendix, Fig. S2A and Dataset 3) as these innate immune pathways are largely characterized for their role in combating intracellular pathogens (54). However, there is an emerging appreciation for the role of type I IFN responses to certain extracellular pathogens (55). Closer inspection of our data showed that TvEVs resulted in a significant downregulation of multiple components of the type I IFN signaling pathway (SI Appendix, Fig. S2B). These included the host cell transcription factors STAT1/2 and Interferon Regulatory Factor 9 (IRF9), which translocate to the host cell nucleus upon binding of the interferon-α/β receptor (IFNAR) with type I IFNs (SI Appendix, Fig. S2 B and C) (54). The canonical type I IFNs (IFN-α and IFN-β) are secreted in response to stimulation by microbial products and it is possible that TvEVs act as a ligand (29, 56, 57). However, transcripts for both IFNA1 and IFNB1 were not detected in our data; instead, we observed a significant decrease in the expression of a noncanonical type I IFN, IFNε (SI Appendix, Fig. S2 B and C) (58). To determine whether TvEVs were capable of disrupting type I IFN signaling, we tested the ability of TvEV pretreatment to block type I IFN responses in cells expressing a luciferase reporter gene downstream of the interferon-stimulated response element (ISRE) sequence (59). IFNε stimulation resulted in a ~3.8-fold increase in luciferase activity compared to mock- or TvEV-treated cells (SI Appendix, Fig. S2D). However, cells pretreated with TvEVs prior to IFNε stimulation resulted in significant diminishment of luciferase activity indicating that TvEVs disrupt type I IFN signaling (SI Appendix, Fig. S2D).
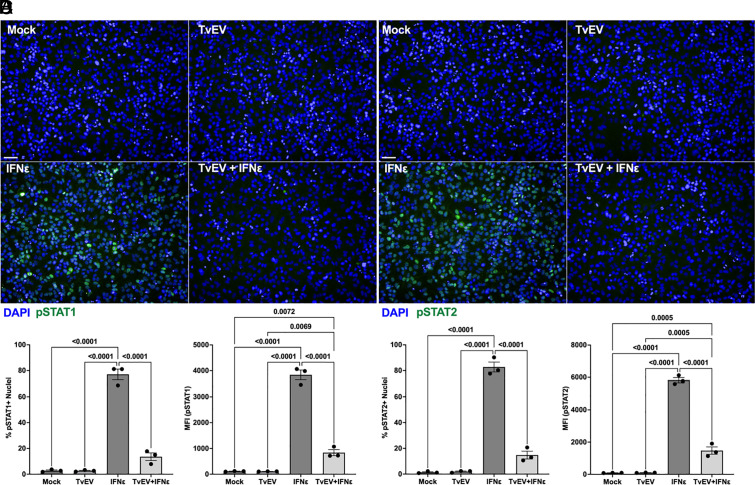
Pretreatment of BPH-1 and Ect1 with TvEVs Suppresses IFNε-Mediated Nuclear Translocation of pSTAT1 and pSTAT2.
Our transcriptomic data indicated that STAT1/2 were significantly downregulated by TvEV treatment (SI Appendix, Fig. S2). To determine whether TvEV treatment also blocked STAT1/2 nuclear translocation, we pretreated BPH-1 with TvEVs prior to stimulating with IFNε and assaying for nuclear translocation of phosphorylated STAT1 and STAT2 (pSTAT1/2). Mock- and TvEV-treated cells displayed little nuclear pSTAT1/2 (Fig. 3 A and B). When we stimulated with IFNε we saw a significant increase in nuclear pSTAT1/2 signal confirming that these pathways are active in BPH-1 s (Fig. 3 C and B). However, pretreatment of host cells with TvEVs prior to IFNε stimulation strongly inhibited pSTAT1/2 nuclear translocation (Fig. 3 C and D). These effects were also observed when an ectocervical cell line (Ect1/E6E7) was pretreated with TvEVs prior to IFNε stimulation. In keeping with our BPH-1 data, Ect1s pretreated with TvEVs prior to IFNε stimulation displayed a significant reduction in pSTAT1/2 positive nuclei (SI Appendix, Fig. S3). Together with our transcriptomic data, these data demonstrate that TvEVs are capable of suppressing type I IFN signaling mediated by IFNε and raises the question of the role of type I IFN responses in T. vaginalis–host interactions.
Fig. 3.
TvEVs block IFNε-mediated nuclear translocation of pSTAT1 and pSTAT2 in prostate cells. (A and B) pSTAT1/2 immunofluorescence in BPH-1 cells treated with either TvEVs (50 µg/mL), IFNε (500 ng/mL), or TvEVs (50 µg/mL) + IFNε (500 ng/mL). Depicted are DAPI staining BPH-1 and parasite nuclei (Blue) and pSTAT1 or pSTAT2 (Green). (Scale bar, 20 µm.) (C) Left, quantification of percent of cells that at positive for nuclear pSTAT1. Right, Mean Fluorescent intensity (MFI) of nuclear pSTAT1. Bars, mean ± SD. N = 3 wells/experiment, 9 fields of view/well, 3 experiments total. Numbers above bars indicate P-values for one-way ANOVA, Dunnett’s multiple comparisons test compared to mock control. (D) Left, quantification of percent of cells that at positive for nuclear pSTAT1. Right, MFI of nuclear pSTAT2. Bars, mean ± SD. N = 3 wells/experiment, 9 fields of view/well, 3 experiments total. Numbers above bars indicate P-values for one-way ANOVA, Dunnett’s multiple comparisons test compared to mock control.
T. vaginalis and Its Bacterial Symbiont, M. hominis, Elicit Distinct and Overlapping Responses in Host Gene Expression During Infection.
While there is an emerging appreciation for the role of type I IFN responses to extracellular pathogens, the type I IFN response has historically been studied for its role in combating intracellular pathogens (54, 55). Interestingly, some strains of T. vaginalis are known to harbor up to five different double-stranded RNA viruses, T. vaginalis virus 1-5 (TVV1-5) (19, 60, 61), as well as a bacterial symbiont M. hominis, which is an opportunistic intracellular pathogen of the female reproductive tract (FRT) (19, 62). The possibility that TVV might be contributing to the type I IFN responses we observed during T. vaginalis infection can be eliminated as the parasite strain used here (B7RC2) is free of TVV1-5 (45). However, T. vaginalis strain B7RC2 does harbor the M. hominis symbiont (SI Appendix, Fig. S4).
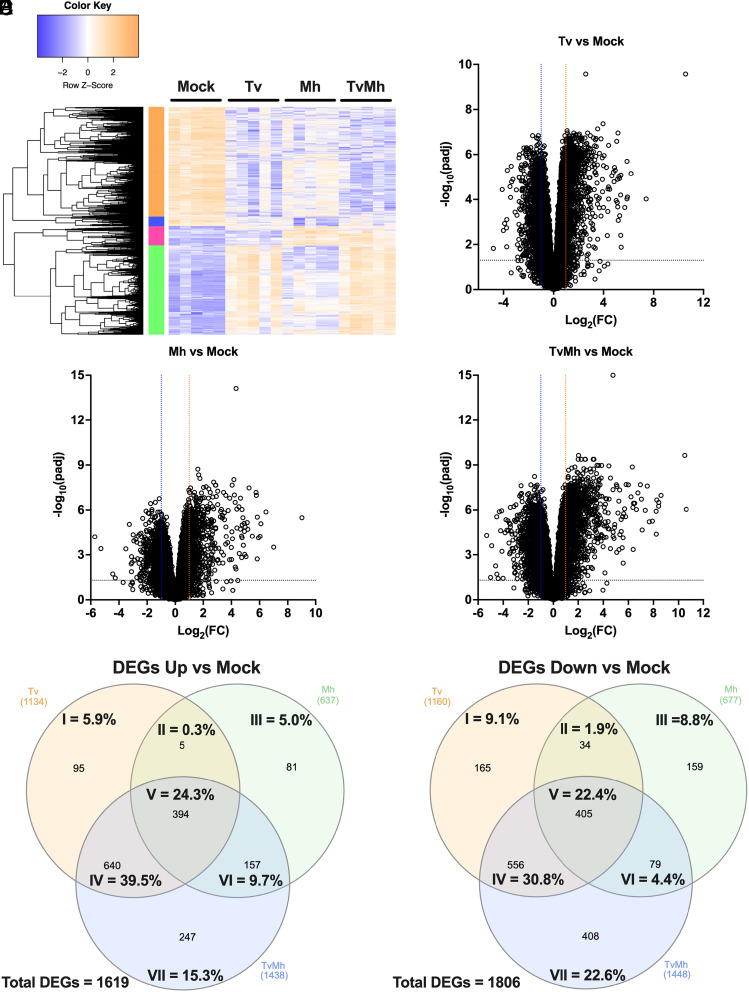
To determine how T. vaginalis, M. hominis, and T. vaginalis in symbiosis with M. hominis affected host cell gene expression, we cocultured BPH-1 with either T. vaginalis that had been cleared of M. hominis (Tv), M. hominis isolated from T. vaginalis (Mh), or T. vaginalis in symbiosis with M. hominis (TvMh) and then extracted RNA from host cells and performed RNA-seq. Tv samples were confirmed clear of M. hominis by PCR (SI Appendix, Fig. S4). Sample alignment and quality was assessed using MultiQC (SI Appendix, Fig. S5) (46). Transcriptional profiles of 5 Mock BPH-1 samples and 5 samples each that had been cocultured with Tv only, Mh only, and both TvMh were distinguishable by PCA, with samples cocultured with TvMh clustering between those cocultured with either Tv or Mh (SI Appendix, Fig. S6 and Datasets 4–11). DEGs fell into distinct modules of expression corresponding to culturing with either Tv (green module), Mh (pink module), or TvMh (Fig. 4A). We identified 2,294 DEGs (1,134 upregulated and 1,160 downregulated) in samples cultured with Tv and 1,314 DEGs (637 upregulated and 677 downregulated) in those cultured with Mh, and 2,886 DEGS (1,438 upregulated and 1,448 downregulated) in those cultured with TvMh, compared to mock controls (Fig. 4 B–D and Datasets 4–11). Of the 1,619 upregulated DEGs, 45.4% (735/1,619; I and IV of Venn diagram) were specific to samples cultured with Tv, 14.7% (238/1,619; III and VI of Venn diagram) to those cultured with Mh, and 15.3% (247/1,619; VII of Venn diagram) to those cultured with TvMh (Fig. 4E and Datasets 4–11). Of the 1,806 downregulated DEGs, 39.9% (721/1,806; I and IV of Venn diagram) were specific to samples cultured with Tv, 13.2% (238/1,806; III and VI of Venn diagram) for those cultured with Mh and 22.6% (408/1,806; VII of Venn diagram) to one cultured with TvMh (Fig. 4F and Datasets 4–11). Of the 1,619 upregulated and 1,806 downregulated DEGs 24.3% (394/1,619) and 22.4% (405/1,806), respectively, were common to all culture conditions (Fig. 4 E and F and Datasets 4–11). Together, these data indicate that while T. vaginalis and M. hominis elicit distinct transcriptional responses from host cells during infection, there is also a large overlap on host cell gene expression.
Fig. 4.
T. vaginalis and M. hominis elicit distinct and overlapping responses in host gene expression during infection. (A) Heatmap of row z-score transformed genes identified as differentially expressed normalized to mock. (B–D) Volcano plot depicting differences in gene expression between (B) Tv infected cells compared to mock-treated BPH-1 cells, (C) Mh infected cells compared to mock-treated BPH-1 cells, and (D) TvMh infected cells compared to mock-treated BPH-1 cells. The x axis corresponds to the log2 fold change in gene expression and the y axis indicates the adjusted P value. Genes with a −log10 P value of 1.3 or greater (P value of ≤ 0.05) and a log2 fold change −1 ≤ or ≥ 1 were deemed significantly differentially expressed. (E) Venn diagram showing overlap in the number upregulated DEGs for each infection compared to mock control cells. (F) Venn diagram showing overlap in of the number downregulated DEGs for each infection compared to mock control cells.
T. vaginalis Association with M. hominis Drives Enrichment of the Type I IFN Pathway.
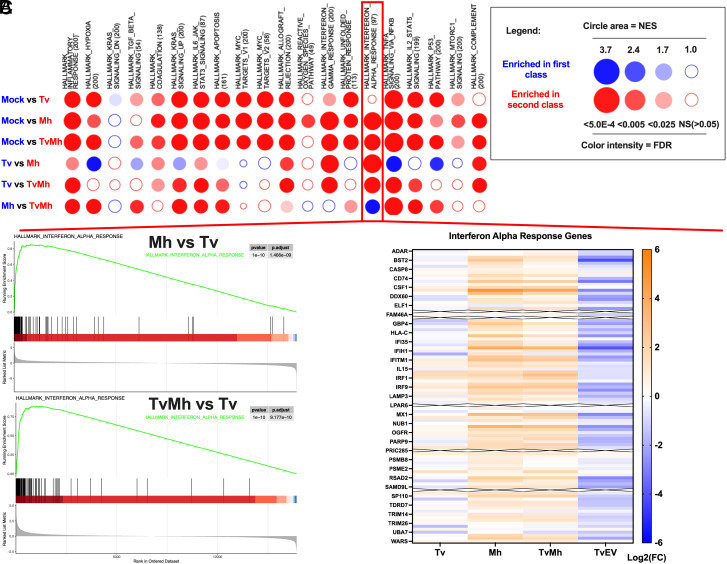
GSEA of host cells cocultured with Tv revealed that, in contrast to the response to TvEVs (Fig. 2), there was an enrichment of several innate immune signaling pathways (NF-κB signaling via TNFα, inflammatory response, complement, and interferon gamma response) (SI Appendix, Fig. S7 and Dataset 3) suggesting that TvEVs may be released by the parasite to suppress innate immune responses induced by the parasite. One exception to this trend was the negative normalized enrichment score for the interferon alpha response pathway upon treatment with TvEVs. In contrast, GSEA of host cells cocultured with Tv only did not have a significant enrichment in the interferon alpha response (Fig. 5A and SI Appendix, Fig. S7). However, host cells cocultured with Mh or TvMh were significantly enriched in the interferon alpha response pathway compared to mock-treated cells (Fig. 5A and Datasets 12–16).
Fig. 5.
GSEA reveals that T. vaginalis association with M. hominis drives enrichment of the IFNα response pathway. (A) BubbleMAP showing results of GSEA for immune pathways in Hallmarks dataset for BPH-1 cells infected with Tv, Mh, or TvMh. Color and area of bubble represents normalized enrichment score (NES). Blue signifies pathway enriched in first class, and red signifies enrichment in second class. Intensity of color indicates false discovery rate (FDR). (B) Top, GSEA enrichment plot showing Hallmark IFN alpha response of Mh infected BPH-1 cells compared to Tv infected BPH-1 cells. Bottom, GSEA enrichment plot showing Hallmark IFN alpha response of TvMh infected BPH-1 cells compared to Tv infected BPH-1 cells. (C) Heatmap of Log2(FC) of individual genes in the interferon alpha response pathway Hallmarks dataset for samples normalized to Mock control. Rows with an X through them represent genes with transcripts below limit of detection in the RNA-seq data.
We reanalyzed our transcriptomic data to focus on differences in gene expression induced when host cells were cocultured with Mh or TvMh compared to coculturing with Tv only. We identified 557 DEGs (213 upregulated and 344 downregulated) in Mh cocultures compared to Tv cocultures and 171 DEGs (170 upregulated and 1 downregulated) when coculturing with TvMh compared to Tv only cocultures (SI Appendix, Fig. S8 and Datasets 12–16). GSEA revealed that host cells cocultured with Mh and TvMh were enriched in interferon alpha and gamma responses compared to coculturing with Tv only (Fig. 5 A and B and Datasets 12–16). Additionally, analysis of the individual genes that comprise the interferon alpha response pathway in the Hallmarks dataset showed a general upregulation of gene expression in cells cultured with either Mh or TvMh compared to mock, while coculturing with Tv or TvEV treatment showed a general suppression of genes in the interferon alpha response pathway (Fig. 5C and Datasets 12–16). Together our transcriptomic datasets indicate that M. hominis is likely the main driver of type I IFN responses during T. vaginalis infection and that these responses may be dampened by secretion of TvEVs.
T. vaginalis Cytoadherence and Host Cell Cytolysis are Inhibited by IFNε-Mediated Type I IFN Responses.
Our data show that TvEVs are capable of dampening type I IFN responses, and while we were unable to detect transcripts for IFN-α and IFN-β, we did detect transcripts for IFNε, and observed a significant decrease in IFNε gene expression in TvEV-treated cells compared to mock controls (SI Appendix, Fig. S2). IFNε has previously been shown to play an important role in protection of the FRT from both bacterial and viral STIs and is expressed by epithelial cells of the FRT and prostate (58, 63–69). Interestingly, IFNε, unlike other interferons, is not regulated by pattern recognition receptor (PRR) stimulation, but rather is hormonally regulated which may explain the high basal level of expression in the BPH-1 cell compared to IFN-α and IFN-β (58). Epithelial cells at mucosal surfaces such as the FRT and male urogenital tract (MUT) are often the first cells to come into contact with invading pathogens and it is thought that tissue-specific expression of cytokines like IFNε may be involved in priming these cells for protection against pathogens (70).
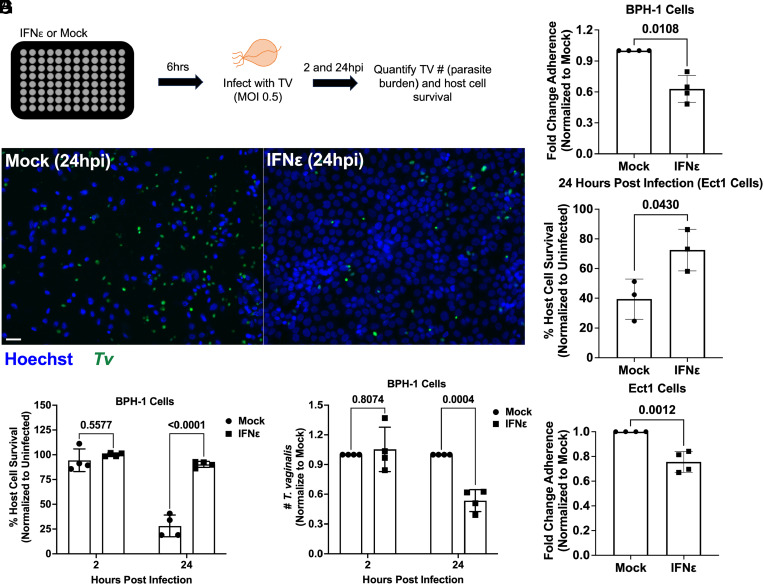
To determine whether IFNε is protective against T. vaginalis, we stimulated BPH-1 s with IFNε prior to infection with parasites (Fig. 6 A and B). At 2 hpi, no differences in host cell survival or parasite burden were observed between stimulated and mock-treated cells (Fig. 6 C and D). However, at 24 hpi, we observed an increase in host cell survival and a decrease in parasite burden for cells pretreated with IFNε (Fig. 6 C and D). We also found that the protective effects of IFNε were inhibited by pretreatment with either TvEVs, an IFNAR1 blocking antibody, or a JAK inhibitor prior to IFNε stimulation and subsequent T. vaginalis infection, indicating that TvEVs or inhibition of proteins involved in the type I IFN pathway disrupts IFNε-mediated protection (SI Appendix, Fig. S9).
Fig. 6.
IFNε is protective against T. vaginalis cytoadherence and cytolysis. (A) Top, Schematic depicting infection of host cells with T. vaginalis. BPH-1 cells were seeded onto 96-well plates to confluency and then treated with 500 ng/mL of IFNε or mock treated for 6 h prior to infection with CFSE-labeled T. vaginalis strain B7RC2 for 2 and 24 h. (B) Representative fluorescence image depicting BPH-1 cells mock or IFNε (500 ng/mL) treated and then infected with T. vaginalis at 2 and 24 h postinfection. Hoechst staining for BPH-1 and parasite nuclei (Blue) and CFSE-labeled T. vaginalis (Green). (Scale bar, 20 µm.) (C) Percent of host cell survival was quantified by enumerating the number of host cells Left in the well after 2 and 24 h compared to uninfected controls. Bars, mean ± SD. N = 3 wells/experiment, 51 fields of view/well, 4 experiments total. Numbers above bars indicate P-values for two-way ANOVA, Dunnett’s multiple comparisons test compared to mock control. (D) The number of T. vaginalis Left in the well after 2 and 24 h was enumerated using CFSE staining to quantify parasites normalized to Mock-treated control. Bars, mean ± SD. N = 3 wells/experiment, 51 fields of view/well, 4 experiments total. Numbers above bars indicate P-values for two-way ANOVA, Dunnett’s multiple comparisons test compared to mock control. (E) Quantification of parasite adherence to BPH-1. Data are depicted as fold change in adherence compared to mock-treated control. Bars, mean ± SD. N = 3 wells/experiment, 4 experiments total. Numbers above bars indicate P-values for Welch’s t test compared to mock control. (F) Percent of host cell survival was quantified by enumerating the number of host cells Left in the well after 24 h compared to uninfected controls. Bars, mean ± SD. N = 3 wells/experiment, 3 experiments total. Numbers above bars indicate P-values for Welch’s t test compared to mock control. (G) Quantification of parasite adherence to Ect1. Data are depicted as fold change in adherence compared to mock-treated control. Bars, mean ± SD. N = 3 wells/experiment, 3 experiments total. Numbers above bars indicate P-values for Welch’s t test compared to mock control.
T. vaginalis-mediated cytolysis has been shown to be contact dependent (71). Thus, we decided to test whether the protective effect of IFNε might be mediated in part by decreased parasite adherence to host cells. When BPH-1 was stimulated with IFNε prior to performing an adherence assay, we observed a ~40% reduction in the number of parasites adhering to IFNε stimulated cells compared to mock controls (Fig. 6E). We then determined whether IFNε would also be protective against parasite-mediated cytolysis and adherence in Ect1s. Consistent with our BPH-1 data (Fig. 6C), IFNε stimulation resulted in a significant increase in Ect1 survival (Fig. 6F) and a decrease in parasite adherence (Fig. 6G). These data demonstrate that IFNε is protective against parasite cytoadherence and cytolysis (Fig. 6) and that the protective effects of IFNε are mediated through signaling of the type I IFN pathway (SI Appendix, Fig. S9) and can be disrupted by TvEVs (SI Appendix, Fig. S9).
Discussion
EVs secreted by many microorganisms have been shown to modulate host cell immune responses during infection (72, 73). Here, we demonstrate a connection between type I IFN responses mediated by IFNε and TvEVs. Specifically, we showed that TvEVs released by the parasite suppress innate immune signaling pathways and can disrupt type I IFN responses resulting in an increase in host cell death. Furthermore, transcriptional analysis of host cells infected with T. vaginalis, M. hominis, or T. vaginalis in symbiosis M. hominis revealed that type I IFN responses are largely elicited in response to M. hominis, but not T. vaginalis. Finally, we demonstrated that IFNε is protective against parasite cytoadherence and cytolysis.
TvEVs are internalized by human cells resulting in increased parasite adherence to host cells and altered cytokine secretion (37, 44). Here, we show that pretreatment of human cells with TvEVs results in an increase in both host cell cytolysis and parasite burden. These observations led us to interrogate the specific effects of TvEVs on host cell gene expression, revealing that TvEVs largely suppressed host cell gene expression and downregulated many immune-related genes and pathways. Multiple genes involved in the type I IFN pathway were significantly downregulated by TvEV treatment. These effects translated into an ability to inhibit IFNε-mediated activation of the type I IFN response and blocked nuclear translocation of pSTAT1/2. These findings suggest that TvEV cargo (i.e., parasite proteins, small RNAs, or other small molecules) inhibit activation of the type I IFN response. Proteomic analyses have identified several TvEV proteins that could mediate downstream host cell signaling (i.e. kinases, phosphatases, and phospho-binding proteins) (44, 57). Alternatively, the immunosuppressive effects of TvEVs may be the result of small RNAs present in TvEVs, as described for EVs from helminthic parasites known to carry micro-RNAs that modulate the expression of host cells genes involved in the immune response (31, 34, 42, 74–76). We can eliminate the possibility that TVV RNAs are involved, as previously reported for TvEV-mediated downregulation of IL-6 and IL-8, as the T. vaginalis strain used for this study does not harbor TVV (45). Future studies will be necessary to identify the exact TvEV cargo that mediate the suppressive effects of TvEVs on the type I IFN response.
Type I IFN responses have primarily been implicated in combating intracellular pathogens and not extracellular parasites such as T. vaginalis (54). Notably, a high percentage of T. vaginalis strains harbor a bacterial symbiont, M. hominis (19). It is unknown whether the symbiont alters T. vaginalis clinical outcomes. In addition to showing that TvEVs suppress type I IFN responses, we have demonstrated that the enrichment of type I IFN response pathway is only seen in host cells infected with T. vaginalis harboring M. hominis or during M. hominis infection alone, but not in infections with the same T. vaginalis strain cleared of M. hominis. These findings indicate that M. hominis activates the type I IFN response observed in infections when the parasite harbors this symbiont. Such a possible negative consequence of this symbiosis is likely balanced by the benefits the bacterium confers to T. vaginalis by increasing parasite growth, hemolytic activity, and adherence to host cells (18, 27, 77–79). We propose that to counter the negative consequence of the symbiont activating the type I IFN pathway, the parasite secretes TvEVs that suppress this innate immune response. Additionally, a second Mycoplasma species, Mycoplasma girerdii, has been identified to be in symbiosis with T. vaginalis (78). While the strain used in this study only harbors M. hominis it remains an important question as to whether these results are specific to symbiosis with M. hominis or a general feature of symbiosis of the parasite with Mycoplasma species in general. Future work elucidating and defining the immune response to T. vaginalis, M. hominis, and M. girerdii will be important in revealing how symbiosis alters the pathogenesis of both microorganisms.
In addition to demonstrating that TvEVs can suppress type I IFN responses, we have shown that activation of the type I IFN response by IFNε prior to infection is protective against T. vaginalis-mediated cytolysis of host cells. This is particularly interesting as IFNε plays an important role in protection of the FRT from both bacterial and viral STIs and is expressed by epithelial cells of the FRT and the MUT. IFNε has a protective role against herpes simplex virus 2, zika virus, HIV, and Chlamydia muridarum and has recently been implicated in restriction of ovarian cancer (58, 63, 65, 69, 80). IFNε is highly expressed in areas of the female reproductive tract including the uterus, cervix, vagina, and ovaries and induces innate immune genes involved in the TNF-α pathway, ROS generation, and phagocyte activation. How IFNε mediates protection of human cells against T. vaginalis-mediated adherence and cytolysis remains to be elucidated.
Our observation that IFNε protects host cells from T. vaginalis infection also sheds light on epidemiological studies showing an unusually high prevalence of T. vaginalis infection in peri/postmenopausal aged women. In contrast to other STIs, the detection rate of T. vaginalis does not decrease with age and reaches maximum rates in women 48-51 years old, raising the possibility that physiological and immunological changes due to menopause may make the FRT more conducive to infection (16, 81). Interestingly, IFNε, unlike other IFNs, is hormonally regulated by estrogen and progesterone (66). In mice, IFNε levels during estrus are 30-fold higher than in diestrus, and in humans, IFNε expression is 10-fold higher during the proliferative phase of the menstrual cycle (late follicular) compared to the secretory phase (luteal) (58). Notably, IFNε levels are ~100-fold decreased in epithelial cells of the FRT from postmenopausal women raising the possibility that loss of IFNε expression may play a role in the increased association of T. vaginalis positivity in aging women (58, 64).
The results presented here have demonstrated that infection with T. vaginalis in symbiosis with M. hominis increases expression of genes in the type I IFN response and show that IFNε, which is greatly diminished in peri- and postmenopausal women, is protective against T. vaginalis infection. Additional distinct and overlapping immune pathways were found to be stimulated by the parasite dependent on the presence and absence of the symbiont. We also identified TvEVs as a means used by the parasite to suppress innate immune responses and enhance host cell killing. Elucidating the mechanisms that drive these dynamic interactions between this human parasite, its symbiotic bacterium, and the host cell will lead the way toward a fuller understanding of this prevalent STI.
Materials and Methods
Parasite Maintenance.
T. vaginalis strain B7RC2 (71) was cultured in Diamond’s modified Trypticase-yeast extract-maltose (TYM) medium supplemented with 10% horse serum (Sigma-Aldrich), 10 U/ml penicillin and 10 μg/ml streptomycin (Gibco), 180 μM ferrous ammonium sulfate, and 2 μM sulfosalicylic acid. To generate M. hominis–free strains, Tv was grown in the presence of 50 μg/ml chloramphenicol and 5 μg/ml tetracycline (Sigma-Aldrich), supplemented daily for at least 5 d (82). M. hominis clearance was confirmed by PCR as described below prior to use in experiments (82, 83). Parasites were grown at 37 °C and passaged daily for no more than 2 wk at a time.
Human Cell Culture.
Human benign prostate hyperplasia 1 (BPH-1) epithelial cells (84) were cultured at 37 °C with 5% CO2 in RPMI 1640, L-glutamine, and HEPES media (Gibco) supplemented with 10 U/ml penicillin, 10 μg/ml streptomycin, and 10% fetal bovine serum (FBS; Gibco) as previously described (44). The human ectocervical cell line Ect1 E6/E7 (85) (CRL 2614) was grown in keratinocyte-SFM (KSFM) (Invitrogen) supplemented with 10 U/ml penicillin, 10 μg/ml streptomycin, and 0.4 M Ca2+ as described previously (44).
EV Isolation.
T. vaginalis was grown to confluency (1 × 106 cells/mL) in 50 mL of complete Diamond’s media. Parasites were spun down at 3,200 rpm for 10 min and washed with 50 mL serum-free Diamond’s media twice. Cells were transferred to 50 mL serum-free Diamond’s media and incubated at 37 °C for 4 h. Cells were spun down at 3,000 rpm at 4 °C for 20 min to remove cell debris. The supernatant was kept and mixed 2:1 with Total Exosome Isolation Reagent (Invitrogen #4478359). The supernatant mixture was incubated overnight at 4 °C. Precipitated TvEVs were recovered by standard centrifugation at 10,000×g for 60 min at 4 °C. The exosome pellet was resuspended in sterile 1× PBS plus 1:100 Halt Protease Inhibitor Cocktail (Thermo Scientific #78428) and stored at −80 °C. The concentration of TvEV protein was determined using the Pierce BCA Kit (Thermo Scientific #23225). To generate the mock control used in our transcriptomic analyses, we used 50 mL serum-free Diamond’s alone (no parasites) subjected to the isolation procedure described above (45).
Host Cell Survival/Parasite Burden Assay.
Host cell survival and parasite burden of BPH-1 and Ect1 cells infected with T. vaginalis was performed using a modified protocol for measuring parasite adherence in a 96-well plate format on the Operetta® CLS™ platform (45). CFSE-labeled parasites were added to confluent monolayers of BPH-1 or Ect1 cells at an MOI of 0.5 at the indicated time points. At the indicated time points, cells and parasites were fixed in 4% formaldehyde in 1× PBS for 20 min at room temperature. Host cells and parasites were stained with Hoechst (1:1,000) (Biotium; Cat. #40044) for 15 min at room temperature. Plates and entire wells were imaged using the Operetta® CLS™ platform, high-throughput microplate imaging system, and analyzed using Harmony™ software. CFSE-positive parasites were identified using the Harmony™ identify cells tool. Fluorescent intensity cutoffs were set at 1,000 and length and width cutoffs were set at 5 μm and 2 μm respectively to filter out any small autofluorescent debris. Host cell nuclei were identified by using the Harmony™ identify nucleus tool. Parasite burden was determined as the number of parasites in the well relative to BSA or mock controls. Host cell survival was quantified by enumerating the number of host cells left in the well after 24 h compared to uninfected controls. For anti-IFNAR blocking assays, cells were preincubated with 1 µg/mL of Anti-interferon alpha/beta receptor 1 antibody (Abcam; Cat # ab124764) or IgG control (Abcam; Cat # ab172730) for 1 h prior to infection with parasites. For JAK inhibitor assays cells were pretreated with 10 µM of Ruxolitinib (Thermo Scientific; Cat # AC469381000) or mock treated (DMSO control) for 1 h prior to infection with parasites.
Transcriptomic Analysis.
For TvEV transcriptomic analysis, BPH-1 cells (1x106 cells per condition per replicate) were treated with 50 µg/mL of TvEVs or mock treated (see EV Isolation methods section) for 6 h in serum-free RPMI prior to RNA isolation. For transcriptomic analysis of infected BPH-1, 1 × 106 cells per condition per replicate were infected (MOI = 1) with T. vaginalis that had been cleared of M. hominis (Tv) using previously established protocols (45, 86), M. hominis isolated from culture with T. vaginalis (Mh), T. vaginalis in symbiosis with M. hominis (TvMh), or mock infected for 6 h in serum-free RPMI media at 37 °C prior to total RNA isolation. Total RNA was isolated using the Direct-zol™ RNA Miniprep Kit (Zymo Research; Cat. #R2071) according to the manufacturer’s instructions. Total RNA was sent to Novogene for RNA quality control (QC) analysis, library prep, and sequencing. RNA QC was analyzed, and quantification of RNA preparations and libraries were carried out using an Agilent 5400. Samples were sequenced on an Illumina NovoSeq 6000 to produce 150–base pair paired end reads with a mean sequencing depth of 20 million reads per sample. After read mapping with Kallisto (87), version 0.46.2, TxImport (88) was used to read Kallisto outputs into the R environment. Annotation data from Ensembl (https://ftp.ensembl.org/pub/release-110/gtf/homo_sapiens/) were used to summarize data from the transcript level to the gene level. All subsequent analyses were carried out using the statistical computing environment R version 4.3.0 in RStudio version 1.1.456, and Bioconductor version 3.8. Briefly, transcript quantification data were summarized to genes using the tximport package and normalized using the trimmed mean of M values (TMM) method in edgeR (89). Genes with <1 CPM in n + 1 of the samples, where n is the size of the smallest group of replicates, were filtered out. Normalized filtered data were variance stabilized using the voom function in limma (90), and differentially expressed genes were identified with linear modeling using limma (FDR ≤ 0.05; absolute Log2FC ≥ 1) after correcting for multiple testing using Benjamini–Hochberg. GSEA was performed using the BubbleGUM package as previously described (91).
PCR Verification of M. hominis.
To assay for the presence of M. hominis in our T. vaginalis strains, total genomic DNA was isolated from 15 mL overnight cultures as previously described. Genomic DNA was then used in a PCR with M. hominis specific primers: 5’-CAATGGCTAATGCCGGATACGC-3’ and 5’-GGTACCGTCAGTCTGCAAT-3’ as previously described (82, 83).
M. hominis Isolation from T. vaginalis culture.
M. hominis was isolated from culture with T. vaginalis for use in RNA-seq experiments as follows. 50 mL of overnight T. vaginalis culture was centrifuged at 3,200 rpm for 10 min to pellet T. vaginalis, while extracellular M. hominis remained in the supernatant. The supernatant was gently transferred to a new 50 mL conical tube and subjected to repeated centrifugation 2 times. After the third centrifugation, 10 µLs of supernatant was visualized under a light microscope to ensure no parasites remained.
Type I IFN-Activated Luciferase Reporter Assay.
HeLa cells stably expressing 11×-ISRE-Gaussia Luciferase reporters were kindly gifted to us by Dr. David Sibley (59). Luciferase reporter cells were treated with 10 µg/mL of B7RC2 TvEVs for 6 h and then stimulated with IFNε (500 ng/mL) for another 6 h. The cells were then washed and assayed for expression of Gaussian Luciferase in cell lysates using a BioLux Gaussia Luciferase Assay Kit (New England BioLabs) as per the manufacturer’s instructions.
pSTAT1 and pSTAT2 Nuclear Translocation by Immunofluorescence.
BPH-1 or Ect1 cells were treated with either TvEVs (50 µg/mL) or mock treated for 6 h and then stimulated with IFNε (500 ng/mL) for 1 h. The cells were fixed with 100% methanol for 15 min and blocked with 5% w/v BSA for 1 h. Fixed cells were stained with 1:200 rabbit anti-phospho-STAT1 (Cell Signaling Technologies; Cat # 9167S) or 1:200 rabbit anti-phospho-STAT2 (Cell Signaling Technologies; Cat # 88410S) overnight at 4 °C, followed by 1:1,000 goat anti-rabbit Alexa 488 (Thermofisher; Cat # A-11094), or 1:1,000 goat anti-mouse Alexa 568 (Thermofisher; Cat # A-11011), and 5 μg/mL DAPI for 1 h. Images were acquired at 20x on an Operetta® CLS™ platform, high-throughput microplate imaging system, and analyzed using Harmony™ software. Briefly, nuclei in each sample were identified as primary objects using DAPI staining and the find nucleus tool, mean pSTAT1/2 intensity in identified nuclei was measured as the nuclear pSTAT1/2 level. Nuclei were deemed positive if they had a pSTAT1/2 signal of MFI ≥ 1,000.
Adherence Assay.
Adherence of T. vaginalis to BPH-1 cells was performed as described previously in a 96-well plate format on the Operetta® CLS™ platform (45). 1 × 106 CFSE-labeled T. vaginalis parasites were incubated with the indicated amount of TvEVs for 30 min and then 5 × 104 parasites were added to confluent monolayers of BPH-1 s for 1 h. Unattached parasites were washed off and cells were fixed in 4% formaldehyde in 1× PBS for 20 min at room temperature. Plates were imaged using the Operetta® CLS™ platform, high-throughput microplate imaging system, and analyzed using Harmony™ software. CFSE-positive parasites were identified using the Harmony™ identify cells tool. Fluorescent intensity cutoffs were set at 1,000 and length and width cutoffs were set at 5 μm and 2 μm respectively to filter out small autofluorescent debris. The % attachment was determined as the number of parasites in the well divided by input (5 × 104) multiplied by 100.
Statistical Analysis.
Graphs were generated, and statistical analyses were performed using Prism 10.0.2 software. All experiments were performed at least three independent times, with at least 3 technical replicates per experiment, and statistical analyses were conducted on the composite data unless reported otherwise.
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Dataset S06 (XLSX)
Dataset S07 (XLSX)
Dataset S08 (XLSX)
Dataset S09 (XLSX)
Dataset S10 (XLSX)
Dataset S11 (XLSX)
Dataset S12 (XLSX)
Dataset S13 (XLSX)
Dataset S14 (XLSX)
Dataset S15 (XLSX)
Dataset S16 (XLSX)
Acknowledgments
We thank our colleagues in the Johnson lab and at UCLA for their helpful discussions and critiques. This research was supported by NIH grants R01AI103182 and R01AI148475 (to P.J.J.). J.A.K. received support from NIH Ruth L. Kirschstein National Research Service Award T32AI007323 and Ruth L. Kirschstein National Research Service Award Individual Fellowship F32AI186416. J.A. and G.E. received support from National Summer Undergraduate Research Project, NSF Grant #: DBI-2149582.
Author contributions
J.A.K., E.L.B., and P.J.J. designed research; J.A.K., E.L.B., G.E., and J.A. performed research; J.A.K., E.L.B., G.E., J.A., and P.J.J. analyzed data; and J.A.K., E.L.B., and P.J.J. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: J.C., Johns Hopkins University Bloomberg School of Public Health; and P.L.F., Universita degli Studi di Sassari.
Data, Materials, and Software Availability
Transcriptomics/RNAseq data have been deposited in Gene Expression Omnibus (GSE278319 and GSE278360) (92, 93).
Supporting Information
References
- 1.Dalby J., Stoner B. P., Sexually transmitted infections: Updates from the 2021 CDC guidelines. Am. Fam. Physician 105, 514–520 (2022). [PubMed] [Google Scholar]
- 2.Rowley J., et al. , Chlamydia, gonorrhoea, trichomoniasis and syphilis: Global prevalence and incidence estimates, 2016. Bull. World Health Organ. 97, 548 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kreisel K. M., et al. , Sexually transmitted infections among US women and men: Prevalence and incidence estimates, 2018. Sex. Transm. Dis. 48, 208–214 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang S., et al. , Trichomonas vaginalis infection-associated risk of cervical cancer: A meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 228, 166–173 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Brotman R. M., et al. , Association between Trichomonas vaginalis and vaginal bacterial community composition among reproductive-age women. Sex. Transm. Dis. 39, 807–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwebke J. R., Burgess D., Trichomoniasis. Clin. Microbiol. Rev. 17, 794–803 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gram I. T., Macaluso M., Churchill J., Stalsberg H., Trichomonas vaginalis (TV) and human papillomavirus (HPV) infection and the incidence of cervical intraepithelial neoplasia (CIN) grade III. Cancer Causes Control 3, 231–236 (1992). [DOI] [PubMed] [Google Scholar]
- 8.Sayed el-Ahl S. A., el-Wakil H. S., Kamel N. M., Mahmoud M. S. E., A preliminary study on the relationship between Trichomonas vaginalis and cervical cancer in Egyptian women. J. Egypt. Soc. Parasitol. 32, 167–178 (2002). [PubMed] [Google Scholar]
- 9.Yap E. H., et al. , Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Sex. Transm. Infect. 71, 402–404 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fichorova R. N., Impact of T. vaginalis infection on innate immune responses and reproductive outcome. J. Reprod. Immunol. 83, 185–189 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton M., et al. , The prevalence of Trichomonas vaginalis infection among reproductive-age women in the United States, 2001–2004. Clin Infect Dis 45, 1319–1326 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Ginocchio C. C., et al. , Prevalence of Trichomonas vaginalis and coinfection with Chlamydia trachomatis and Neisseria gonorrhoeae in the United States as determined by the Aptima Trichomonas vaginalis nucleic acid amplification assay. J. Clin. Microbiol. 50, 2601–2608 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stemmer S. M., Adelson M. E., Trama J. P., Dorak M. T., Mordechai E., Detection rates of trichomonas vaginalis, in different age groups, using real-time polymerase chain reaction. J. Lower Genital Tract Disease 16, 352–357 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Patel E. U., Gaydos C. A., Packman Z. R., Quinn T. C., Tobian A. A. R., Prevalence and correlates of trichomonas vaginalis infection among men and women in the United States. Clin. Infect. Dis. 67, 211–217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicholls J. E., et al. , Cross-sectional study to evaluate Trichomonas vaginalis positivity in women tested for Neisseria gonorrhoeae and Chlamydia trachomatis, attending genitourinary medicine and primary care clinics in Bristol. South West England. Sex. Transm. Infect. 94, 93–99 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stemmer S. M., Mordechai E., Adelson M. E., Gygax S. E., Hilbert D. W., Trichomonas vaginalis is most frequently detected in women at the age of peri-/premenopause: An unusual pattern for a sexually transmitted pathogen. Am. J. Obstet. Gynecol. 218, e1–328.e13 (2018). [DOI] [PubMed] [Google Scholar]
- 17.Fettweis J. M., et al. , An emerging Mycoplasma associated with trichomoniasis, Vaginal Infection and Disease. PLoS ONE 9, e110943 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dessì D., et al. , Trichomonas vaginalis and Mycoplasma hominis: New tales of two old friends. Parasitology 146, 1150–1155 (2019). [DOI] [PubMed] [Google Scholar]
- 19.Fichorova R., Fraga J., Rappelli P., Fiori P. L., Trichomonas vaginalis infection in symbiosis with Trichomonasvirus and Mycoplasma. Res. Microbiol. 168, 882–891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fiori P. L., Rappelli P., Addis M. F., Carta F., Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet 352, 1286 (1998). [DOI] [PubMed] [Google Scholar]
- 21.da Luz Becker D., et al. , High rates of double-stranded RNA viruses and Mycoplasma hominis in Trichomonas vaginalis clinical isolates in South Brazil. Infect. Genet. Evol. 34, 181–187 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Fraga J., et al. , Mycoplasma hominis in Cuban Trichomonas vaginalis isolates: Association with parasite genetic polymorphism. Exp. Parasitol. 131, 393–398 (2012). [DOI] [PubMed] [Google Scholar]
- 23.Butler S. E., Augostini P., Secor W. E., Mycoplasma hominis infection of Trichomonas vaginalis is not associated with metronidazole-resistant trichomoniasis in clinical isolates from the United States. Parasitol. Res. 107, 1023–1027 (2010). [DOI] [PubMed] [Google Scholar]
- 24.Xiao J. C., et al. , Symbiosis of Mycoplasma hominis in Trichomonas vaginalis may link metronidazole resistance in vitro. Parasitol. Res. 100, 123–130 (2006). [DOI] [PubMed] [Google Scholar]
- 25.van der Schee C., et al. , Host and pathogen interaction during vaginal infection by Trichomonas vaginalis and Mycoplasma hominis or Ureaplasma urealyticum. J. Microbiol. Methods 45, 61–67 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Hampl V., Vanácová S., Kulda J., Flegr J., Concordance between genetic relatedness and phenotypic similarities of Trichomonas vaginalis strains. BMC Evol. Biol. 1, 11 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Margarita V., et al. , Symbiotic association with Mycoplasma hominis can influence growth rate, ATP production, cytolysis and inflammatory response of Trichomonas vaginalis. Front. Microbiol. 7, 953 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vancini R. G., Pereira-Neves A., Borojevic R., Benchimol M., Trichomonas vaginalis harboring Mycoplasma hominis increases cytopathogenicity in vitro. Eur. J. Clin. Microbiol. Infect. Dis. 27, 259–267 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Brown Harding H., et al. , Candida albicans extracellular vesicles trigger type I IFN signalling via cGAS and STING. Nat. Microbiol. 9, 95–107 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douanne N., et al. , Leishmania parasites exchange drug-resistance genes through extracellular vesicles Cell Rep. 40, 111121 (2022) 10.1016/j.celrep.2022.111121 [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Silva M. R., et al. , Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 113, 285–304 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Gómez-Chávez F., Murrieta-Coxca J. M., Caballero-Ortega H., Morales-Prieto D. M., Markert U. R., Host-pathogen interactions mediated by extracellular vesicles in Toxoplasma gondii infection during pregnancy J. Reprod. Immunol. 158, 103957 (2023) 10.1016/j.jri.2023.103957 [DOI] [PubMed] [Google Scholar]
- 33.Kioko M., et al. , Extracellular vesicles could be a putative posttranscriptional regulatory mechanism that shapes intracellular RNA levels in Plasmodium falciparum. Nat. Commun. 14, 6447 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J., et al. , Schistosoma japonicum extracellular vesicle miRNA cargo regulates host macrophage functions facilitating parasitism PLoS Pathog. 15, e1007817 (2019) 10.1371/journal.ppat.1007817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu J., et al. , Isolation and characterization of extracellular vesicles from adult Schistosoma japonicum. J. Vis. Exp. 135, 57514 (2018), 10.3791/57514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molgora B. M., et al. , Trichomonas vaginalis adherence phenotypes and extracellular vesicles impact parasite survival in a novel in vivo model of pathogenesis PLoS Negl. Trop. Dis. 17, e0011693 (2023) 10.1371/journal.pntd.0011693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rai A. K., Johnson P. J., Trichomonas vaginalis extracellular vesicles are internalized by host cells using proteoglycans and caveolin-dependent endocytosis. Proc. Natl. Acad. Sci. 116, 21354–21360 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salas N., et al. , Role of cytoneme structures and extracellular vesicles in Trichomonas vaginalis parasite-parasite communication eLife 12, e86067 (2023) 10.7554/eLife.86067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sánchez-López C. M., et al. , Extracellular vesicles from the trematodes Fasciola hepatica and Dicrocoelium dendriticum trigger different responses in human THP-1 macrophages J. Extracell. Vesicles 12, e12317 (2023) 10.1002/jev2.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma M., et al. , Characterization of extracellular vesicles from Entamoeba histolytica identifies roles in intercellular communication that regulates parasite growth and development Infect. Immun. 88, e00349-20 (2020) 10.1128/IAI.00349-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szempruch A. J., et al. , Extracellular vesicles from Trypanosoma brucei mediate virulence factor transfer and cause host anemia. Cell 164, 246–257 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L., et al. , Sja-miR-71a in Schistosome egg-derived extracellular vesicles suppresses liver fibrosis caused by schistosomiasis via targeting semaphorin 4D. J. Extracell. Vesicles 9, 1785738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu S., et al. , Release of extracellular vesicles containing small RNAs from the eggs of Schistosoma japonicum. Parasites Vectors 9, 574 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Twu O., et al. , Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host: Parasite interactions. PLOS Pathogens 9, e1003482 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kochanowsky J. A., et al. , Trichomonas vaginalis extracellular vesicles up-regulate and directly transfer adherence factors promoting host cell colonization Proc. Natl. Acad. Sci. U.S.A. 121, e2401159121 (2024) 10.1073/pnas.2401159121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ewels P., Magnusson M., Lundin S., Käller M., MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Subramanian A., et al. , Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. U.S.A. 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L., et al. , Trichomonas vaginalis Induces production of proinflammatory cytokines in mouse macrophages through activation of MAPK and NF-κB pathways partially mediated by TLR2. Front. Microbiol. 9, 712 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang J.-H., Ryang Y.-S., Morio T., Lee S.-K., Chang E.-J., Trichomonas vaginalis inhibits proinflammatory cytokine production in macrophages by suppressing NF-kappaB activation. Mol. Cells 18, 177–185 (2004). [PubMed] [Google Scholar]
- 50.Chang J.-H., Park J.-Y., Kim S.-K., Dependence on p38 MAPK signalling in the up-regulation of TLR2, TLR4 and TLR9 gene expression in Trichomonas vaginalis-treated HeLa cells. Immunology 118, 164–170 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nam Y. H., et al. , NF-κB and CREB are involved in IL-8 production of human neutrophils induced by Trichomonas vaginalis-derived secretory products. Korean J. Parasitol. 49, 291–294 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gu N.-Y., et al. , Trichomonas vaginalis induces IL-1β production in a human prostate epithelial cell line by activating the NLRP3 inflammasome via reactive oxygen species and potassium ion efflux. Prostate 76, 885–896 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Nam Y. H., et al. , Leukotriene B4 receptor BLT-mediated phosphorylation of NF-κB and CREB is involved in IL-8 production in human mast cells induced by Trichomonas vaginalis-derived secretory products. Microbes Infect. 13, 1211–1220 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Ivashkiv L. B., Donlin L. T., Regulation of type i interferon responses. Nat. Rev. Immunol. 14, 36–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker D., Prince A., Type i interferon response to extracellular bacteria in the airway epithelium. Trends Immunol. 32, 582–588 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nievas Y. R., et al. , Membrane-shed vesicles from the parasite Trichomonas vaginalis: Characterization and their association with cell interaction. Cell. Mol. Life Sci. 75, 2211–2226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olmos-Ortiz L. M., et al. , Trichomonas vaginalis exosome-like vesicles modify the cytokine profile and reduce inflammation in parasite-infected mice Parasite Immunol. 39, (2017) e12426 10.1111/pim.12426 [DOI] [PubMed] [Google Scholar]
- 58.Fung K. Y., et al. , Interferon-ε protects the female reproductive tract from viral and bacterial infection. Science 339, 1088–1092 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Matta S. K., et al. , Toxoplasma gondii effector TgIST blocks type I interferon signaling to promote infection. Proc. Natl. Acad. Sci. U.S.A. 116, 17480–17491 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodman R. P., et al. , Clinical isolates of Trichomonas vaginalis concurrently infected by strains of up to four Trichomonasvirus species (family Totiviridae). J. Virol. 85, 4258–4270 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manny A. R., Hetzel C. A., Mizani A., Nibert M. L., Discovery of a novel species of trichomonasvirus in the human parasite trichomonas vaginalis using transcriptome mining. Viruses 14, 548 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rumyantseva T., Khayrullina G., Guschin A., Donders G., Prevalence of Ureaplasma spp. and Mycoplasma hominis in healthy women and patients with flora alterations. Diagn. Microbiol. Infect. Dis. 93, 227–231 (2019). [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Minambres A., et al. , Interferon epsilon promotes HIV restriction at multiple steps of viral replication. Immunol. Cell Biol. 95, 478–483 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Marks Z. R. C., et al. , Properties and functions of the novel type I interferon epsilon Semin. Immunol. 43, 101328 (2019) 10.1016/j.smim.2019.101328 [DOI] [PubMed] [Google Scholar]
- 65.Mungin J. W., Chen X., Liu B., Interferon epsilon signaling confers attenuated Zika replication in human vaginal epithelial cells. Pathogens 11, 853 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xi Y., Day S. L., Jackson R. J., Ranasinghe C., Role of novel type I interferon epsilon in viral infection and mucosal immunity. Mucosal Immunology 5, 610–622 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao F.-R., Wang W., Zheng Q., Zhang Y.-G., Chen J., The regulation of antiviral activity of interferon epsilon. Front. Microbiol. 13, 1006481 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Filardo S., et al. , Interferon-ε as potential inhibitor of Chlamydia trachomatis infection Microb. Pathog. 185, 106427 (2023) 10.1016/j.micpath.2023.106427 [DOI] [PubMed] [Google Scholar]
- 69.Coldbeck-Shackley R. C., et al. , Constitutive expression and distinct properties of IFN-epsilon protect the female reproductive tract from Zika virus infection PLoS Pathog. 19, e1010843 (2023) 10.1371/journal.ppat.1010843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayall J. R., et al. , Interferon-epsilon is a novel regulator of NK cell responses in the uterus. EMBO Mol. Med. 16, 267–293 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lustig G., Ryan C. M., Secor W. E., Johnson P. J., Trichomonas vaginalis contact-dependent cytolysis of epithelial cells. Infect Immun 81, 1411–1419 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharma M., Lozano-Amado D., Chowdhury D., Singh U., Extracellular vesicles and their impact on the biology of protozoan parasites. Trop. Med. Infect. Dis. 8, 448 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Szempruch A. J., Dennison L., Kieft R., Harrington J. M., Hajduk S. L., Sending a message: Extracellular vesicles of pathogenic protozoan parasites. Nat. Rev. Microbiol. 14, 669–675 (2016). [DOI] [PubMed] [Google Scholar]
- 74.Artuyants A., et al. , Extracellular vesicles produced by the protozoan parasite Trichomonas vaginalis contain a preferential cargo of tRNA-derived small RNAs. Int. J. Parasitol. 50, 1145–1155 (2020). [DOI] [PubMed] [Google Scholar]
- 75.Buck A. H., et al. , Exosomes secreted by nematode parasites transfer small RNAs to mammalian cells and modulate innate immunity. Nat. Commun. 5, 5488 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bernal D., et al. , Surface analysis of Dicrocoelium dendriticum. The molecular characterization of exosomes reveals the presence of miRNAs. J. Proteomics 105, 232–241 (2014). [DOI] [PubMed] [Google Scholar]
- 77.Fiori P. L., Diaz N., Cocco A. R., Rappelli P., Dessì D., Association of Trichomonas vaginalis with its symbiont Mycoplasma hominis synergistically upregulates the in vitro proinflammatory response of human monocytes. Sex. Transm. Infect. 89, 449–454 (2013). [DOI] [PubMed] [Google Scholar]
- 78.Margarita V., et al. , Two different species of Mycoplasma endosymbionts can influence Trichomonas vaginalis pathophysiology. MBio 13, e0091822 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rappelli P., et al. , Mycoplasma hominis and Trichomonas vaginalis symbiosis: Multiplicity of infection and transmissibility of M. hominis to human cells. Arch. Microbiol. 175, 70–74 (2001). [DOI] [PubMed] [Google Scholar]
- 80.Marks Z. R. C., et al. , Interferon-ε is a tumour suppressor and restricts ovarian cancer. Nature 620, 1063–1070 (2023). [DOI] [PubMed] [Google Scholar]
- 81.Shiratori M., Patel A., Gerhold R. W., Sullivan S. A., Carlton J. M., Persistent Trichomonas vaginalis infections and the pseudocyst form. Trends Parasitol 39, 1023–1031 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mercer F., et al. , Leukocyte lysis and cytokine induction by the human sexually transmitted parasite Trichomonas vaginalis PLoS Negl. Trop. Dis. 10, e0004913 (2016) 10.1371/journal.pntd.0004913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Blanchard A., et al. , Evaluation of intraspecies genetic variation within the 16S rRNA gene of Mycoplasma hominis and detection by polymerase chain reaction. J. Clin. Microbiol. 31, 1358–1361 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Q., et al. , Benign prostatic hyperplasia (BPH) epithelial cell line BPH-1 induces aromatase expression in prostatic stromal cells via prostaglandin E2. J. Endocrinol. 195, 89–94 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Fichorova R. N., Rheinwald J. G., Anderson D. J., Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57, 847–855 (1997). [DOI] [PubMed] [Google Scholar]
- 86.Liu S.-Y., Sanchez D. J., Aliyari R., Lu S., Cheng G., Systematic identification of type I and type II interferon-induced antiviral factors. Proc. Natl. Acad. Sci. 109, 4239–4244 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bray N. L., Pimentel H., Melsted P., Pachter L., Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016). [DOI] [PubMed] [Google Scholar]
- 88.Soneson C., Love M. I., Robinson M. D., Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences. F1000Res 4, 1521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Robinson M. D., McCarthy D. J., Smyth G. K., EdgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ritchie M. E., et al. , Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spinelli L., Carpentier S., Montañana Sanchis F., Dalod M., Vu Manh T.-P., Bubblegum: Automatic extraction of phenotype molecular signatures and comprehensive visualization of multiple gene set enrichment analyses. BMC Genomics 16, 814 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kochanowsky J. A., et al. , RNA sequencing Data from “T. vaginalis extracellular vesicles suppress IFNε-mediated responses driven by its intracellular bacterial symbiont Mycoplasma hominis.” Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE278319. Deposited 29 September 2024.
- 93.Kochanowsky J. A., et al. , RNA sequencing Data from “T. vaginalis extracellular vesicles suppress IFNε-mediated responses driven by its intracellular bacterial symbiont Mycoplasma hominis.” Gene Expression Omnibus. https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE278360. Deposited 29 September 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Dataset S03 (XLSX)
Dataset S04 (XLSX)
Dataset S05 (XLSX)
Dataset S06 (XLSX)
Dataset S07 (XLSX)
Dataset S08 (XLSX)
Dataset S09 (XLSX)
Dataset S10 (XLSX)
Dataset S11 (XLSX)
Dataset S12 (XLSX)
Dataset S13 (XLSX)
Dataset S14 (XLSX)
Dataset S15 (XLSX)
Dataset S16 (XLSX)
Data Availability Statement
Transcriptomics/RNAseq data have been deposited in Gene Expression Omnibus (GSE278319 and GSE278360) (92, 93).