ABSTRACT
Processed and ultra‐processed foods have become dietary staples in many developed countries. A major constituent of these foods is a variety of synthetic chemical additives, which are used to improve the texture, preservation, and aesthetics of food. Evidence is mounting that synthetic chemicals used as food additives may have harmful impacts on health. Studies have linked certain additives to health conditions such as attention deficit hyperactivity disorder, cancer, and obesity. In addition, emerging evidence suggests that additives, such as emulsifiers, artificial sweeteners, colorants, and preservatives, may act as potential disruptors of intestinal homeostasis. Indeed, various studies have identified that food additives can impact gut health by modulating gut microbiota and intensifying intestinal inflammation. Considering the lack of known nutritional benefits of these additives and the accumulating evidence on the detrimental effects of these additives on gut health, further experimental, epidemiological, and clinical evaluations are imperative. This will provide significant advances in the prevention and management of gut health, including intestinal inflammation, and in enriching public knowledge on the harmful effects of these additives. In this review, we explore the effects of popular food additives on gut health with a particular focus on intestinal inflammation and examine the broader implications of these impacts on food safety policy and public health.
Keywords: food additives, food dye, food emulsifier, gut health, intestinal inflammation, microbiota, processed/ultra‐processed foods, public health
Chemical food additives are commonly found in ultra‐processed foods. Exposure to these additives has been shown to impact the gastrointestinal tract by altering the gut microbiome, reducing the intestinal mucus layer, impairing barrier function, and triggering abnormal immune responses. This suggests that the consumption of these food additives may have negative implications for gut health, highlighting the need for further exploration of these additives at experimental, epidemiological, and clinical levels.

1. Introduction
The gastrointestinal (GI) tract is a highly sophisticated system responsible for the digestion, absorption, and excretion of ingested liquids and foods. Acting as an interface between internal and external environments, the mucosal layer of the gut, which consists of an epithelial monolayer and the underlying lamina propria and muscularis mucosa, is in large part responsible for sensing and adapting to these exogenous compounds [1]. In the mucosal layer, intestinal epithelial cells (IEC) are linked via adherence and tight junction complexes, forming a highly selective barrier that protects the host from infections, environmental toxins, and allergens [2]. In addition, glycosylated peptides residing adjacent to the apical surface of these epithelial cells constitute the intestinal mucus layer [2], which functions as an additional barrier against luminal contents and potential invaders [3]. This mucosal environment also hosts a large population of immune cells that monitor the intestinal milieu and respond in kind to dietary antigens, invading pathogens, and commensal microorganisms. In order to maintain intestinal homeostasis, tight regulation of intricate biological mechanisms and the integrity of the mucosal barrier within this environment must be preserved [4].
The GI tract is also host to a complex population of commensal bacteria, viruses, fungi, and protozoa, known as the gut microbiota. Along with host‐influencing effects, gut microbes play a significant role in the induction, education, and function of the immune system and are in constant communication with gut‐residing immune cells, including dendritic cells, macrophages, B‐cells, and T‐cells [5]. Ultimately, within the GI tract, components from microbes, exogenous nutrients, and host‐derived compounds form an intricate communication and signaling network to ensure homeostasis is maintained. However, disturbances to this delicate network, including genetic alterations or exposure to particular environmental factors, may lead to pathological consequences and, potentially, in susceptible individuals, the development of intestinal disorders, such as inflammatory bowel disease (IBD) [6].
Categorized largely into Crohn's disease (CD) and ulcerative colitis (UC), IBDs are chronic conditions associated with severe inflammation of the GI tract [7]. To date, a comprehensive understanding of the pathophysiology and pathogenesis of IBD is still lacking; however, evidence suggests that genetics, immune dysregulation, the gut microbiota, as well as environmental factors [8], all play a role in these multifactorial conditions. In recent decades, significant progress has been made in identifying susceptibility genes as well as understanding the role of the immune system and host microbiota in the pathogenesis of IBD. Though several environmental risk factors have been postulated to impact disease development and progression, including exposure to enteric pathogens, antibiotics, smoking, and, increasingly, diet [9], advances in defining specific environmental risk factors that contribute to the pathogenesis of IBD have lagged behind.
Emerging evidence from both epidemiological and experimental studies suggests diet [10], particularly the overconsumption of highly processed foods, is linked with IBD [10, 11]. Indeed, a systematic review published in 2024 identified that higher consumption of ultra‐processed foods is associated with a greater risk of adverse health outcomes, particularly with respect to cardiometabolic health and mortality [12]. Of note, in a 2021 prospective cohort study, Narula et al. found that consuming ultra‐processed food was associated with a higher risk of developing CD, as the use of specific additives in processed foods may have effects at a cellular level that may play a role in the pathogenesis of CD [13]. The consumption of a Western diet (WD), high in processed foods, was also found to promote the colonization of adherent‐invasive Escherichia coli (AIEC), a microbe often elevated in IBD patients [14, 15]. Taken together, these studies support the notion that consuming high levels of processed and ultra‐processed foods may have unfavorable effects on intestinal health and implications for intestinal inflammatory conditions, though the mechanisms of action remain unclear.
With the global rise in processed and ultra‐processed food intake, food additive consumption has also increased drastically [16, 17]. Additives such as dietary emulsifiers, artificial sweeteners, and food colorants are often incorporated into foods and beverages to increase shelf life and enhance attractiveness [18]. Increasingly, evidence suggests that these food additives may, at least in part, be responsible for the disruptions to intestinal physiology attributed to processed food intake. Currently, the European Food Safety Authority (EFSA), the Food and Drug Administration (FDA), and Health Canada regulate the use of food additives in the food and beverage industry in Europe, the United States, and Canada, respectively. However, concerns that current evaluations are outdated have arisen, and given the emerging evidence regarding the impact of these additives on overall health, updated appraisals are needed to ensure that food safety is maintained for the general population [19].
In this review, we explore how various popular food additives, including artificial food colorants, non‐caloric artificial sweeteners, dietary emulsifiers, and antimicrobial preservatives, perturb intestinal homeostasis and influence intestinal inflammation, as well as delve into the implications of these findings for public health and policy.
2. Effects of Food Additives on Gut Health
2.1. Artificial Food Colorants
Artificial food colorants (AFCs) are commonly used in the food and beverage industry to increase the appeal of naturally colorless foods. Products often containing AFCs include breakfast cereals, candy, sports drinks, carbonated soft drinks, and various snack products [20]. Since 1955, the use of AFCs has increased five‐fold, with titanium dioxide (TiO2) [21] and various azo dyes, including Allura Red (AR), Tartrazine (TZ), and Sunset Yellow (SY), being among the most heavily used [20]. In 2007, a seminal study conducted by McCann et al. identified a link between the consumption of these dyes and attention‐deficit/hyperactivity disorder (ADHD) [22]. These findings, which suggested that AFCs are not as innocuous as previously thought, prompted further investigation into the effects of these dyes across other biological contexts.
In the gut, research has indicated that AFCs can have profound effects on GI physiology, particularly with regard to intestinal inflammation (Figure 1) [23, 24, 25, 26]. Recent work from our lab studying the impact of the azo dye, AR, across various murine models of colitis has highlighted these effects. In our work, chronic exposure to AR (at a dose calculated based on the acceptable daily intake set by regulatory agencies) in both drinking water and food induced low‐grade intestinal inflammation in naïve mice and exacerbated colitis in both a CD4 + CD45RBhi T cell‐induced and dextran sodium sulfate (DSS)‐induced mouse model of IBD. This increased susceptibility to colitis was characterized by a significant upregulation in disease activity index (DAI), histopathological scores, and pro‐inflammatory cytokines [24]. Intriguingly, these changes were accompanied by alterations in barrier function and perturbations in the gut microbiome. Further, transplanting AR‐exposed microbiota from naïve into germ‐free mice exacerbated intestinal inflammation upon administration of DSS, indicating that dye‐induced changes to the microbiome can modulate the severity of colitis [24]. In addition, we identified that early‐life exposure to AR enhances susceptibility to DSS‐induced colitis, indicating that AR can prime the colon for inflammatory insults [24]. Most importantly, we found that colonic serotonin is a key mediator and plays an important role in AR‐induced colitis. In parallel, He et al. identified that in mice overexpressing the IBD‐associated cytokine, IL‐23, AR acted as a colitogenic agent and triggered IBD‐like colitis mediated via a CD4+ T‐cell response [23]. He et al. also showed that AR‐induced colitis was dependent on the ability of the commensal microbes, Bacteroides ovatus and Enterococcus faecalis , to convert AR into the metabolite 1‐amino‐2‐naphthol‐6‐sulfonate sodium salt (ANSA‐Na) via azo reduction [23]. Multiple studies have also identified that exposure to AR can induce DNA damage in the colonic tissue of mice, suggesting genetic as well as local environmental changes induced by this dye can disrupt GI homeostasis [27, 28].
FIGURE 1.

The effects of various azo dyes on GI health. (A) He et al. reported that azo reduction of Allura Red (AR) and Sunset Yellow (SY) by B. ovatus and E. faecalis produced the metabolite ANSA‐Na, leading to an IL‐23‐dependent colitis mediated by CD4+ T‐cells and IFN‐γ production [23]. (B) Kwon et al. identified that chronic AR exposure promoted experimental colitis via colonic 5‐HT in both a gut microbiota‐dependent and ‐independent pathway and by decreasing intestinal barrier integrity through MLCK [24]. (C) Zahran and colleagues showed that SY exposure activated the NLRP3 inflammasome, leading to the production of IL‐18 and IL‐1β and inducing microbial dysbiosis, which resulted in increased LPS production and impaired barrier function via altered adherens junction (AJ) complexes [25]. Image created in BioRender.
The azo dye, SY, has also been implicated in altering GI physiology both in vitro and in vivo. SY has been found to alter the growth, differentiation, and proliferation of intestinal organoids [26], as well as increase levels of oxidative‐ and endoplasmic reticulum (ER)‐stress. Similar to studies utilizing AR, in mice, SY administration was linked with exacerbated DSS‐induced inflammation [26]. Recently, Zahran et al. identified that chronic consumption of SY also disrupted the microbial composition, which led to changes in the jejunal adherens junction complex, E‐cadherin/β‐catenin, and Trefoil Factor (TFF)‐3, all implicated in maintaining intestinal barrier function. In addition, SY has been found to increase lipopolysaccharide (LPS) serum levels, an indicator of increased intestinal barrier permeability, and activate the nucleotide‐binding oligomerization domain, leucine‐rich repeat and pyrin domain containing (NLRP) 3 inflammasome pathway [25], which plays a crucial role in innate immune regulation. Outside of the gut, consumption of AR and SY has also been linked with proinflammatory pathways; increased production of reactive oxygen species (ROS) and cyclooxygenase (COX)‐2 in the liver and kidney of rats treated with these dyes was noted along with renal and hepatic toxicity [29].
Along with AR and SY, TZ has also been found to impact GI health. Similar to AR, TZ exposure induced DNA damage in the colon of mice [30] and was shown to alter the intestinal microbiome as well as induce histopathological alterations and oxidative stress in both the liver and intestines of crucian carp [31]. In Wistar rats, TZ treatment led to a significant increase in lymphocytes and eosinophils in the gastric mucosa, suggesting that TZ has implications on the immune system [32]. Rats exposed to high doses of TZ also displayed significantly increased liver and kidney biomarkers compared to controls, indicating that TZ may have negative implications on renal and hepatic function as well [33].
Similar to azo dyes, titanium dioxide (TiO2), a nanoparticle used to enhance and brighten dull colors, has also been linked to altered intestinal physiology and inflammation across in vitro and in vivo studies (Figure 2) [21, 34, 35, 36, 37]. In vitro analysis in the colonic cell lines, HT‐29 and Caco‐2, showed that TiO2 induced the production of ROS and altered permeability in a dose‐dependent manner. Interestingly, in conjunction with this work, Ruiz et al. also found that patients with active UC had higher levels of systemic titanium compared to healthy controls [34], suggesting that the damaged intestinal barrier associated with IBD may lead to the increased transport and circulation of TiO2. Chronic TiO2 exposure has also been linked to perturbations in immune function. In the context of murine DSS colitis, TiO2 administration has been shown to reduce CD4+ T‐cells, regulatory T‐cells, and macrophages in mesenteric lymph nodes [21], as well as impact the NLRP3 inflammasome pathway [34] and exacerbate inflammation [21, 34]. Though drastic alterations in the gut microbiome have not been reported with TiO2 exposure, the diminishment of beneficial taxa such as Bifidobacterium and Lactobacillus [21], decreased production of the microbially produced short‐chain fatty acid (SCFA), acetate. Changes in host‐derived mucin‐2 (Muc2) and beta defensin‐3 expression [37] were also noted, suggesting that TiO2 influences not only gut microbes but also affects, directly or indirectly, host‐derived responses to microbes.
FIGURE 2.
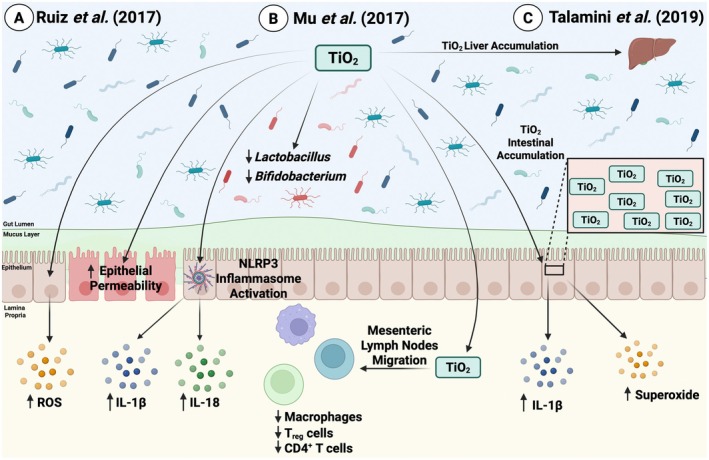
Effects of TiO2 on intestinal homeostasis. (A) Ruiz et al. identified that titanium dioxide (TiO2) administration exacerbated DSS‐induced colitis through increased reactive oxygen species (ROS) production, increased intestinal epithelial permeability, and activated the NLRP3 inflammasome, which subsequently led to increased pro‐inflammatory cytokine (IL‐1β and IL‐18) production [34]. (B) Mu and colleagues showed that TiO2 exposure altered the microbial composition by decreasing Bifidobacterium and Lactobacillus abundance. TiO2 also migrated to the mesenteric lymph nodes and reduced CD4+ T‐cells, regulatory T‐cells, and macrophages [21]. (C) Talamini et al. reported that TiO2 accumulated in both the large intestine and liver, and this accumulation led to increased IL‐1β and superoxide production in the intestines [35]. Image created in BioRender.
Overall, these studies suggest that commonly used AFCs, such as AR, SY, TZ and TiO2, can have negative implications on intestinal health. It should be noted, however, that at present, the effects of the aforementioned AFCs on host physiology have largely been restricted to animal models, and precise evaluation of the impact of these dyes on human health is lacking. Thus, epidemiological studies and randomized controlled trials (RCT) are warranted to truly evaluate the impact of these dyes on intestinal health in both healthy and IBD populations.
2.2. Non‐Caloric Artificial Sweeteners
Non‐caloric artificial sweeteners (NAS), including aspartame, acesulfame potassium (acesulfame K), advantame, saccharin, and sucralose, are commonly used in food and beverages to provide a sweet taste without the associated calories of sugar [38]. Despite claims of these NAS having less of an impact on metabolic health than more traditional sweeteners, a hallmark study conducted by Suez et al. in 2014 identified that consumption of saccharin, sucralose, and aspartame increased the risk of glucose intolerance as characterized by a high glycemic response and elevated blood glucose [39]. Saccharin was found to have the most pronounced effects on glucose intolerance, which, intriguingly, were mediated by alterations in the gut microbial community [39]. In addition to their animal work, Suez et al. found similar results in healthy human subjects; both short‐term and long‐term NAS consumption induced alterations in the gut microbiota and glycemic responses [39]. In Caco‐2 cells, saccharin was also found to disrupt epithelial barrier function via activation of NF‐κB, resulting in the ubiquitination of claudin‐1, a tight junction protein that plays a role in maintaining intestinal barrier integrity (Figure 3) [40].
FIGURE 3.

Effects of non‐caloric artificial sweeteners on gut health. (A) Santos et al. reported that in Caco‐2 cells, saccharin activated NF‐κB, which subsequently led to the ubiquitination of claudin‐1 and disrupted barrier function [40]. (B) Guo et al. identified that sucralose enhanced DSS‐induced colitis in mice via the activation of the TLR5‐MyD88‐NF‐κB signaling pathway, leading to increased IL‐1β, IL‐17A, IL‐18, and TNF‐α production as well as microbial dysbiosis and intestinal barrier damage [41]. (C) Rodriguez‐Palacios and colleagues found that Splenda induced microbial dysbiosis through increased levels of the Proteobacteria phylum and E. coli . Splenda also increased ileal myeloperoxidase (MPO) and increased bacterial infiltration into the ileal lamina propria [42]. Image created in BioRender.
In addition to its effects on metabolic health [39], consumption of sucralose has also been found to have deleterious effects on intestinal physiology and inflammation (Figure 3). Indeed, in a DSS colitis model, mice administered sucralose displayed more severe colitis and damage to the intestinal barrier, along with an altered microbial composition (Figure 3) [41, 43]. In 2, 4, 6‐trinitrobenzenesulfonic acid (TNBS)‐induced colitis, 6 weeks of sucralose administration was shown to exacerbate intestinal inflammation along with alterations in serum D‐lactic acid (a measure of gut permeability), digestive proteases (trypsin and chymotrypsin) and the gut microbiota composition [44]. In addition, sucralose administration has been associated with increased size and numbers of azoxymethane (AOM)/DSS‐induced colorectal tumors in mice, suggesting that sucralose may exacerbate tumorigenesis in vivo [43]. Further, in ileitis, the dextrose‐ and sucralose‐containing NAS, Splenda, was found to promote gut dysbiosis and increase the abundance of the Proteobacteria phylum (Figure 3) [42]. Although Splenda administration did not increase the severity of ileitis compared to controls, increased bacterial infiltration into the lamina propria and increased myeloperoxidase (MPO) activity were present, indicating that this NAS can alter the integrity of the intestinal barrier [42].
Over the years, the body of evidence evaluating the effects of NAS on GI health in both animal models and human studies has grown. However, inconsistencies in the literature with regard to the effect of NAS on the gut microbiome and its role in promoting intestinal inflammation warrant further research, particularly in human subjects [39, 45, 46, 47, 48]. It should also be noted that, at present, the majority of studies investigating NAS's effects on GI health and physiology have been focused on healthy individuals. Therefore, examining the impact of these sweeteners and their roles in intestinal inflammation in individuals with GI diseases like IBD may be a particularly impactful avenue for research in the future.
2.3. Dietary Emulsifiers and Coating/Thickening Agents
Due to their amphiphilic properties, dietary emulsifiers, such as carboxymethylcellulose (CMC) and polysorbate‐80 (P80), are utilized in the food and beverage industry to help stabilize mixtures that contain hydrophilic liquids and hydrophobic lipids [49]. Across various studies, these emulsifiers have been shown to exacerbate intestinal inflammation and have significant effects on the gut microbiota (Figure 4). In a seminal study conducted by Chassaing et al., chronic exposure to CMC and P80 was found to induce low‐grade intestinal inflammation and metabolic syndrome in wild‐type mice as well as promote colitis in genetically susceptible IL‐10−/− mice [50]. Changes in the microbial composition following emulsifier exposure were also present [50]. Intriguingly, the transfer of microbial contents from emulsifier‐treated mice into germ‐free mice was sufficient to induce low‐grade inflammation as well as metabolic syndrome, paralleling the findings with direct emulsifier exposure [50]. Exposure to CMC and P80 has also been reported to predispose mice to tumor development with AOM/DSS, alter the microbial composition, and induce low‐grade inflammation via disturbances in the proliferation and apoptotic balance of epithelial cells [51]. Notably, a direct comparison of CMC to P80 in a humanized mouse model identified that CMC had higher colitogenic potential over the course of 4 weeks than P80 [53]. In human intestinal organoids, administration of P80 was also associated with downregulated mRNA expression of specific junctional proteins as well as upregulated apoptotic, inflammatory, and oxidative stress‐associated genes. This indicates that P80 has implications on intestinal epithelial integrity and inflammatory responses within the GI tract [54]. The impact of CMC consumption on microbial composition has also been reported in humans; a randomized controlled‐feeding study in healthy adults found that relative to controls, subjects who were exposed to CMC had reduced microbial diversity and changes in their metabolome, including decreases in SCFAs [52]. Although this study had a relatively small sample size, it indicates that even short‐term CMC exposure may have detrimental effects on the gut microbiota in healthy humans [52] and provides evidence that limiting emulsifier‐rich foods may be beneficial, especially in individuals who are pre‐disposed to dysbiosis‐associated diseases such as IBD [55].
FIGURE 4.

Effects of dietary emulsifiers on intestinal homeostasis. (A) Chassaing et al. identified that polysorbate‐80 (P80) and carboxymethylcellulose (CMC) exposure led to low‐grade inflammation and metabolic syndrome in wildtype mice and aggravated colitis in genetically susceptible IL‐10−/− mice. CMC and P80 also altered the microbiota composition by reducing levels of Bacteroidales and Akkermansia and increasing the abundance of Ruminococcus gnavus , and these microbial changes were sufficient and necessary to induce metabolic syndrome and low‐grade inflammation [50]. (B) Viennois and colleagues showed that P80 and CMC altered the microbiota composition as well as increased lipopolysaccharide (LPS) and bacterial flagellin levels. Together, emulsifier exposure and the altered microbiome led to low‐grade intestinal inflammation and altered the proliferation and apoptotic balance of epithelial cells, which ultimately predisposed mice to exacerbated tumor development and colon carcinogenesis in an AOM/DSS model [51]. (C) Chassaing and colleagues reported that in a randomized controlled‐feeding study of CMC, patients on a CMC diet had reduced microbial diversity and an altered metabolome indicated by decreased short‐chain fatty acids (SCFAs) and free amino acids [52]. Image created in BioRender.
Similar to emulsifiers, maltodextrin (MDX), an agent used to thicken and stabilize processed foods, has been linked to changes in GI physiology (Figure 5). In 2019, Laudisi et al. reported that a 10‐week MDX diet, but not a 45‐day exposure period, induced low‐grade intestinal inflammation [56], suggesting long‐term consumption can change the inflammatory potential of the gut. Indeed, mice administered an MDX diet also displayed exacerbated DSS‐induced colitis compared to DSS alone. The researchers also found that MDX exposure altered the intestinal mucus barrier, reduced Muc2, and induced ER stress [56]. Further, inhibiting ER stress reduced colitis severity, providing evidence that MDX is, at least in part, acting on ER pathways to modulate colitis susceptibility [56]. The above findings were expanded upon by Zangara and colleagues, who identified that MDX exposure could accelerate colitis onset in IL‐10−/− mice, which spontaneously develop colitis early in life [57]. These mice displayed suppressed mucus production and decreased goblet cell numbers, which, along with changes in microbial diversity and composition, led to increased microbial proximity to the intestinal epithelium [57]. Administration of MDX has also been reported to enhance biofilm formation and adhesion of the CD‐associated AIEC strain LF82 to intestinal epithelial monolayers [59], suggesting MDX may aid the survival and colonization of AIEC, potentially exacerbating intestinal inflammation in an already susceptible patient population [59].
FIGURE 5.

Implications of various coating and thickening agents in GI health. (A) Laudisi and colleagues showed that chronic maltodextrin (MDX) exposure induced low‐grade intestinal inflammation. In addition, an MDX diet followed by DSS administration exacerbated colitis, which was mediated by MDX‐induced endoplasmic reticulum (ER) stress, leading to increased IL‐1β production as well as a reduced intestinal mucus layer [56]. (B) Zangara et al. identified that MDX exposure promoted colitis in genetically susceptible IL‐10−/− mice, which was mediated by a reduced intestinal mucus layer, decreased acetic acid production, and reduced microbial diversity [57]. (C) Wu et al. found that carrageenan (CGN) exacerbated C. rodentium ‐induced colitis and this was mediated by alterations in the overall microbiota composition, increased LPS, decreased SCFAs (acetic acid, butyric acid, isobutyric acid, valeric acid), and increased mucus‐degrading bacteria leading to a reduced intestinal mucus layer [58]. Image created in BioRender.
Similar to MDX, carrageenan (CGN), a commonly used food stabilizer, also has implications on intestinal health, seemingly dependent on changes within the structure and function of the gut microbiota (Figure 5) [58, 60, 61]. This agent has various forms, including ι‐ (iota), κ‐ (kappa) and λ‐ (lambda), which differ in their number of sulfate groups [62]. In a mouse model of TNBS colitis, a 2‐week treatment of κ‐CGN was found to exacerbate intestinal inflammation with increased pro‐inflammatory cytokine levels and decreased proportions of T‐regulatory cells [60]. Additionally, high dietary λ‐CGN intake has been reported to aggravate Citrobacter rodentium ‐induced colitis and alter the gut microbiota in favor of mucus‐degrading bacteria [58]. Interestingly, this compound does not exacerbate Citrobacter rodentium ‐induced colitis in GF mice, which indicates that λ‐CGN acts in a microbiota‐dependent manner to modulate colitis severity [58].
To date, extensive work has been done by various research groups to understand the effects of these emulsifiers and thickening agents on host physiology. Though the number of human RCTs involving dietary emulsifiers is growing, there is still a substantial need for adequately powered RCTs. Additionally, as emulsifier‐focused RCTs continue to be conducted, delving deeper into the effects of these additives via mechanistic studies and studying the roles of these additives in potentially susceptible populations remain of high importance.
2.4. Antimicrobial Preservatives
Inhibiting the growth of microorganisms and prolonging the shelf life of various foods using antimicrobial preservatives (AMPRs) is a staple of modern food preparation. Given the antimicrobial activity of these additives, it is not unreasonable to extrapolate that they may perturb the gut microbiota (Figure 6). In vitro analysis has indicated that the impact of AMPR exposure is highly bacterial strain‐dependent [66], suggesting that the host organisms' microbiota content prior to exposure can greatly impact overall health effects. For instance, exposure to sodium bisulfite and sodium sulfite inhibited the growth of three beneficial strains of bacteria from the Lactobacillus species as well as Streptococcus thermophilus [64]. In vivo, mice colonized with human microbiota samples and exposed to the common preservatives, sodium benzoate, sodium nitrite, and potassium sorbate, were found to have an increased abundance of Proteobacteria and decreased Clostridiales [63].
FIGURE 6.

The effects of antimicrobial preservatives on intestinal homeostasis and microbiota composition. (A) Hrncirova et al. showed that the preservatives sodium benzoate, sodium nitrite, and potassium sorbate altered the microbiome into a composition with increased Proteobacteria and decreased Clostridiales [63]. (B) Irwin and colleagues identified that in vitro, sodium sulfite and sodium bisulfite inhibited the growth of bacteria from the Lactobacillus species and inhibited Streptococcus thermophilus growth [64]. (C) Williams et al. reported that silver nanoparticles (AgNP) could have immunomodulatory effects resulting in decreased gene expression of TLR2, TLR4, GPR43, FOXP3 and MUC3. AgNPs also decreased the abundance of bacteria from the Firmicutes phyla and the Lactobacillus genera [65]. Image created in BioRender.
Effects on the microbial composition have also been reported with exposure to silver nanoparticles (AgNPs), which can also serve as AMPRs. In mice, 28‐day exposure to AgNP increased the Firmicutes to Bacteroidetes ratio as well as altered both α‐diversity and β‐diversity [67]. Javurek and colleagues also illustrated that 2‐week exposure to AgNPs altered the microbial composition in the gut [68]. It should be noted, however, that in these studies, no toxicity or intestinal damage was reported [67, 68]. In addition, in Sprague–Dawley rats, decreases in the Firmicutes phyla and the Lactobacillus genus post‐AgNP exposure have been noted [65], along with decreases in immunomodulatory genes such as TLR2, TLR4, GPR43, FOXP3 and MUC3 within ileal tissue, indicating that AgNP may have implications on host immune function [65].
Together, these data suggest that exposure to AMPRs plays a role in disturbing the gut microbiota. However, despite the growing body of evidence in vitro and in vivo experimental models, there still remains a lack of human studies evaluating the effects of these AMPRs on the human microbiome. This gap in the literature highlights the need for more human‐relevant research to truly elucidate the effects of these AMPRs on human health. Additionally, although no distinct intestinal tissue damage has been reported with these additives, these compounds are rarely consumed in isolation and thus, studying AMPRs in conjunction with other food additives may divulge a deeper role of these compounds in altering gut physiology and intestinal inflammation.
3. Implications for Food Policy Change and Public Health
The use and consumption of food additives have grown drastically in recent decades, and this trend is likely to continue, given the inexpensive nature, easy availability and effectiveness of these agents in prolonging the shelf life and attractiveness of processed foods [69]. However, these additives have no nutritional benefits, and as discussed in this review, evidence is mounting, at least in animal models, that these compounds may have negative implications for GI health. Food safety agencies such as the FDA, EFSA, and Health Canada consider these food additives to be “generally recognized as safe” (GRAS) and have set acceptable daily intake (ADI) recommendations with guidance from the Joint FAO/WHO Expert Committee on Food Additives (JECFA) (Table 1). Nevertheless, given the data present herein, a re‐evaluation of the safety of various food additives needs to be considered [104]. At present, food manufacturers in North America generally only display the food additives used in their products within ingredient lists but rarely indicate the amount that is used. This lack of quantitative information makes it impossible for an individual who consumes a “balanced” or even a “Western” diet to determine if what they are consuming is within currently established acceptable ranges. At present, companies have no obligation to disclose the quantities used, and, thus, it remains unclear if consumers who regularly adhere to a typical “Western” diet exceed the ADI limits. Therefore, going forward, consideration of enacting regulations to disclose the quantity of the additives in food products would be helpful. It would also be of great importance for future studies to use analytical techniques such as liquid‐chromatography‐mass spectrometry to directly quantify these additives in biological samples, such as blood, stool, and urine. This data would help identify real‐world exposure ranges and would help better characterize what an expected food additive exposure might be like for an individual who typically consumes a “Western” diet.
TABLE 1.
Acceptable Daily Intake (ADI) for commonly used food additives.
| Food additive | Acceptable daily intake | Regulatory sources |
|---|---|---|
| Artificial food colorants | ||
| Allura red (E129) | 0–7 mg/kg bw/day [70, 71] | JECFA [70], EFSA [71] |
| Tartrazine (E102) |
0–10 mg/kg of bw/day [72] 0–7.5 mg/kg bw/day [73] |
JECFA [72] EFSA [73] |
| Sunset yellow (E110) | 0–4 mg/kg of bw/day [74, 75] | JECFA [74], EFSA [75] |
| Brilliant blue (E133) | 0–6 mg/kg of bw/day [76, 77] | JECFA [77], EFSA [76] |
| TiO2 (E171) |
No numerical ADI [78] Not used in Europe via EFSA [79] |
JECFA [78] EFSA [79] |
| Artificial sweeteners | ||
| Aspartame (E951) | 0–40 mg/kg bw/day [80, 81] | JECFA [80], EFSA [81] |
| Acesulfame K (E950) | 0–15 mg/kg bw/day [82, 83] | JECFA [82], EFSA [83] |
| Advantame (E969) | 0–5 mg/kg bw/day [84, 85] | JECFA [84], EFSA [85] |
| Neotame (E961) | 0–2 mg/kg bw/day [86, 87] | JECFA [86], EFSA [87] |
| Saccharin (E954) |
0–5 mg/kg bw/day [88] 0–9 mg/kg bw/day [89] |
JECFA [88] EFSA [89] |
| Sucralose (E955) | 0–15 mg/kg bw/day [90, 91] | JECFA [90], EFSA [91] |
| Dietary emulsifiers and coating/thickening agents | ||
| CMC (E466) | No numerical ADI [92, 93] | JECFA [92], EFSA [93] |
| Polysorbate 80 (E433) | 0–25 mg/kg bw/day [94, 95] | JECFA [94], EFSA [95] |
| Maltodextrin | No numerical ADI [96] | FDA [96] |
| Carrageenan (E407) |
No numerical ADI [97] 0–75 mg/kg bw/day [98] |
JECFA [97] EFSA [98] |
| Antimicrobial preservatives | ||
| Sodium nitrite (E250) | 0–0.7 mg/kg bw/day [99] | JECFA [99] |
| Sodium benzoate (E211) |
0–20 mg/kg bw/day [100] 0–5 mg/kg bw/day [101] |
JECFA [100] EFSA [101] |
| Potassium sorbate (E202) |
0–25 mg/kg bw/day [102] 0–11 mg sorbic acid/kg bw/day [103] |
JECFA [102] EFSA [103] |
Given that the emerging evidences indicate that food additives do more harm than good when it comes to GI health, changes to current policy and new guidelines regarding the use of food additives within the food industry need to be considered. Both the FDA and EFSA have begun making strides toward improving food additive safety, which includes conducting re‐evaluations of these additives. In 2025, the FDA amended its food coloring regulations by revoking the authorization for the use of FD&C Red No. 3 (erythrosine) in foods and ingested drugs in the US [105]. This decision was based on animal studies that revealed that high levels of FD&C Red No. 3 were capable of causing cancer in male rats [105, 106]. Additionally, the EFSA has banned the use of TiO2 as genotoxic effects of this additive could not be ruled out [79] and has reduced the ADI of different food dyes such as Sunset Yellow and Brilliant Blue based on toxicity studies [76, 107]. In addition, following a study showing that certain AFCs, as well as the preservative sodium benzoate, can increase hyperactivity in children, the European Union requires foods containing certain additives to come with a warning label that says about potential adverse effects on activity and attention in some children [22, 108].
That being said, similar simple implementations by the FDA and Health Canada, such as indicating the amounts of food additives used in each product or adding “warning” labels to products as in the European Union, can only contribute to increased public awareness, aid consumers in making informed decisions regarding nutrition, and bolster public health. It may be argued that, currently, the majority of findings regarding the safety of these additives are attributed to work done in animal models, and this does not constitute a change in policy; however, these findings, particularly with the rising incidence of inflammatory GI diseases across the globe [109], justify further exploration of the impact of these “GRAS” food additives on the general population.
4. Conclusion & Future Directions
The usage of food additives, staple components within processed foods, has increased drastically given the low cost and high efficacy of these various compounds. Emerging evidence, based on both epidemiological and experimental studies, suggests a potential link between chronic intestinal disorders, such as IBD, and diet. Indeed, various studies have identified that food additives may have detrimental effects on GI health and that they may be a contributing culprit in exacerbating disease symptoms in patients with intestinal disorders (Figure 7). Currently, the majority of this work has been done in animal models, which makes substantial guideline and policy changes difficult. With that being said, future work translating these findings to human subjects is imperative. However, from an ethical perspective, this may be challenging since the literature has identified that certain additives can have detrimental effects in animal models. Nonetheless, it is still crucial and informative to conduct human studies to not only establish the effects of these food additives on human GI health but also to aid in the prevention of potential adverse health outcomes in patients with GI disorders.
FIGURE 7.

Overview of the effects of food additives within the GI tract. (A) In a healthy state, a diverse and robust microbiota composition, an intact intestinal mucus layer and intestinal barrier, as well as a healthy immune response, are all present in the gut [13]. (B) In a state where food additives are consumed, particularly in a chronic fashion, additives including dietary emulsifiers, food colorants, coating and thickening agents, artificial sweeteners, as well as antimicrobial preservatives, have the potential to alter the immune response, disrupt the intestinal mucus layer, impair intestinal barrier function, as well as induce microbial dysbiosis. Image created in BioRender.
Author Contributions
T.S. reviewed the literature, wrote the manuscript as well as designed and created the figures. J.A.G. assisted in writing and editing the manuscript and figures. W.I.K. supervised the project and edited the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to W.I.K. (PJT‐156262). T.S. is a recipient of the Farncombe Studentship Award and CIHR Canadian Graduate Scholarship—Doctoral (CGS‐D). Figures were created using BioRender.com with a full license.
Seto T., Grondin J. A., and Khan W. I., “Food Additives: Emerging Detrimental Roles on Gut Health,” The FASEB Journal 39, no. 13 (2025): e70810, 10.1096/fj.202500737R.
Funding: This work was supported by grants from the Canadian Institutes of Health Research (CIHR) to W.I.K. (PJT‐156262).
Data Availability Statement
The authors have nothing to report.
References
- 1. Zhang K., Hornef M. W., and Dupont A., “The Intestinal Epithelium as Guardian of Gut Barrier Integrity,” Cellular Microbiology 17 (2015): 1561–1569. [DOI] [PubMed] [Google Scholar]
- 2. Peterson L. W. and Artis D., “Intestinal Epithelial Cells: Regulators of Barrier Function and Immune Homeostasis,” Nature Reviews. Immunology 14 (2014): 141–153. [DOI] [PubMed] [Google Scholar]
- 3. Herath M., Hosie S., Bornstein J. C., Franks A. E., and Hill‐Yardin E. L., “The Role of the Gastrointestinal Mucus System in Intestinal Homeostasis: Implications for Neurological Disorders,” Frontiers in Cellular and Infection Microbiology 10 (2020): 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGhee J. R. and Fujihashi K., “Inside the Mucosal Immune System,” PLoS Biology 10 (2012): e1001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thursby E. and Juge N., “Introduction to the Human Gut Microbiota,” Biochemical Journal 474 (2017): 1823–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Maloy K. J. and Powrie F., “Intestinal Homeostasis and Its Breakdown in Inflammatory Bowel Disease,” Nature 474 (2011): 298–306. [DOI] [PubMed] [Google Scholar]
- 7. Fakhoury M., Negrulj R., Mooranian A., and Al‐Salami H., “Inflammatory Bowel Disease: Clinical Aspects and Treatments,” Journal of Inflammation Research 7 (2014): 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chang J. T., “Pathophysiology of Inflammatory Bowel Diseases,” New England Journal of Medicine 383 (2020): 2652–2664. [DOI] [PubMed] [Google Scholar]
- 9. Molodecky N. A. and Kaplan G. G., “Environmental Risk Factors for Inflammatory Bowel Disease,” Gastroenterol Hepatol (N Y) 6 (2010): 339–346. [PMC free article] [PubMed] [Google Scholar]
- 10. Marion‐Letellier R., Savoye G., and Ghosh S., “IBD: In Food we Trust,” Journal of Crohn's & Colitis 10 (2016): 1351–1361. [DOI] [PubMed] [Google Scholar]
- 11. Whelan K., Bancil A. S., Lindsay J. O., and Chassaing B., “Ultra‐Processed Foods and Food Additives in Gut Health and Disease,” Nature Reviews. Gastroenterology & Hepatology 21 (2024): 406–427. [DOI] [PubMed] [Google Scholar]
- 12. Lane M. M., “Ultra‐Processed Food Exposure and Adverse Health Outcomes: Umbrella Review of Epidemiological Meta‐Analyses,” BMJ (Clinical Research Ed.) 384 (2024): e077310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Narula N., Wong E. C. L., Dehghan M., et al., “Association of Ultra‐Processed Food Intake With Risk of Inflammatory Bowel Disease: Prospective Cohort Study,” BMJ (Clinical Research Ed.) 374 (2021): n1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martinez‐Medina M., Denizot J., Dreux N., et al., “Western Diet Induces Dysbiosis With Increased E Coli in CEABAC10 Mice, Alters Host Barrier Function Favouring AIEC Colonisation,” Gut 63 (2014): 116–124. [DOI] [PubMed] [Google Scholar]
- 15. Palmela C., Chevarin C., Xu Z., et al., “Adherent‐Invasive Escherichia coli in Inflammatory Bowel Disease,” Gut 67 (2018): 574–587. [DOI] [PubMed] [Google Scholar]
- 16. Cox S., Sandall A., Smith L., Rossi M., and Whelan K., “Food Additive Emulsifiers: A Review of Their Role in Foods, Legislation and Classifications, Presence in Food Supply, Dietary Exposure, and Safety Assessment,” Nutrition Reviews 79 (2021): 726–741. [DOI] [PubMed] [Google Scholar]
- 17. Marino M., Puppo F., del Bo' C., et al., “A Systematic Review of Worldwide Consumption of Ultra‐Processed Foods: Findings and Criticisms,” Nutrients 13 (2021): 2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhou X., Qiao K., Wu H., and Zhang Y., “The Impact of Food Additives on the Abundance and Composition of Gut Microbiota,” Molecules 28 (2023): 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. FDA , “GRAS Substances (SCOGS) Database, 2024”, https://www.fda.gov/food/generally‐recognized‐safe‐gras/gras‐substances‐scogs‐database.
- 20. Potera C., “Diet and Nutrition: The Artificial Food Dye Blues,” Environmental Health Perspectives 118 (2010): A428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mu W., Wang Y., Huang C., et al., “Effect of Long‐Term Intake of Dietary Titanium Dioxide Nanoparticles on Intestine Inflammation in Mice,” Journal of Agricultural and Food Chemistry 67 (2019): 9382–9389. [DOI] [PubMed] [Google Scholar]
- 22. McCann D., Barrett A., Cooper A., et al., “Food Additives and Hyperactive Behaviour in 3‐Year‐Old and 8/9‐Year‐Old Children in the Community: A Randomised, Double‐Blinded, Placebo‐Controlled Trial,” Lancet 370 (2007): 1560–1567. [DOI] [PubMed] [Google Scholar]
- 23. He Z., Chen L., Catalan‐Dibene J., et al., “Food Colorants Metabolized by Commensal Bacteria Promote Colitis in Mice With Dysregulated Expression of Interleukin‐23,” Cell Metabolism 33 (2021): 1358–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kwon Y. H., Banskota S., Wang H., et al., “Chronic Exposure to Synthetic Food Colorant Allura Red AC Promotes Susceptibility to Experimental Colitis via Intestinal Serotonin in Mice,” Nature Communications 13 (2022): 7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zahran S. A., Mansour S. M., Ali A. E., et al., “Sunset Yellow Dye Effects on Gut Microbiota, Intestinal Integrity, and the Induction of Inflammasomopathy With Pyroptotic Signaling in Male Wistar Rats,” Food and Chemical Toxicology 187 (2024): 114585. [DOI] [PubMed] [Google Scholar]
- 26. Kong X., Wang X., Qin Y., and Han J., “Effects of Sunset Yellow on Proliferation and Differentiation of Intestinal Epithelial Cells in Murine Intestinal Organoids,” Journal of Applied Toxicology 41 (2021): 953–963. [DOI] [PubMed] [Google Scholar]
- 27. Tsuda S., Murakami M., Matsusaka N., Kano K., Taniguchi K., and Sasaki Y. F., “DNA Damage Induced by Red Food Dyes Orally Administered to Pregnant and Male Mice,” Toxicological Sciences 61 (2001): 92–99. [DOI] [PubMed] [Google Scholar]
- 28. Zhang Q., Chumanevich A. A., Nguyen I., et al., “The Synthetic Food Dye, Red 40, Causes DNA Damage, Causes Colonic Inflammation, and Impacts the Microbiome in Mice,” Toxicology Reports 11 (2023): 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khayyat L. I., Essawy A. E., Sorour J. M., and Soffar A., “Sunset Yellow and Allura Red Modulate Bcl2 and COX2 Expression Levels and Confer Oxidative Stress‐Mediated Renal and Hepatic Toxicity in Male Rats,” PeerJ 6 (2018): e5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sasaki Y. F., Kawaguchi S., Kamaya A., et al., “The Comet Assay With 8 Mouse Organs: Results With 39 Currently Used Food Additives,” Mutation Research, Genetic Toxicology and Environmental Mutagenesis 519 (2002): 103–119. [DOI] [PubMed] [Google Scholar]
- 31. Wu L., Xu Y., Lv X., et al., “Impacts of an Azo Food Dye Tartrazine Uptake on Intestinal Barrier, Oxidative Stress, Inflammatory Response and Intestinal Microbiome in Crucian Carp ( Carassius auratus ),” Ecotoxicology and Environmental Safety 223 (2021): 112551. [DOI] [PubMed] [Google Scholar]
- 32. Moutinho I. L. D., Bertges L. C., and Assis R. V. C., “Prolonged Use of the Food Dye Tartrazine (FD&C Yellow no 5) and Its Effects on the Gastric Mucosa of Wistar Rats,” Brazilian Journal of Biology 67 (2007): 141–145. [DOI] [PubMed] [Google Scholar]
- 33. Amin K. A., Abdel Hameid H., and Abd Elsttar A. H., “Effect of Food Azo Dyes Tartrazine and Carmoisine on Biochemical Parameters Related to Renal, Hepatic Function and Oxidative Stress Biomarkers in Young Male Rats,” Food and Chemical Toxicology 48 (2010): 2994–2999. [DOI] [PubMed] [Google Scholar]
- 34. Ruiz P. A., Morón B., Becker H. M., et al., “Titanium Dioxide Nanoparticles Exacerbate DSS‐Induced Colitis: Role of the NLRP3 Inflammasome,” Gut 66 (2017): 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Talamini L., Gimondi S., Violatto M. B., et al., “Repeated Administration of the Food Additive E171 to Mice Results in Accumulation in Intestine and Liver and Promotes an Inflammatory Status,” Nanotoxicology 13 (2019): 1087–1101. [DOI] [PubMed] [Google Scholar]
- 36. Wang S., Kang X., Alenius H., Wong S. H., Karisola P., and el‐Nezami H., “Oral Exposure to ag or TiO2 Nanoparticles Perturbed Gut Transcriptome and Microbiota in a Mouse Model of Ulcerative Colitis,” Food and Chemical Toxicology 169 (2022): 113368. [DOI] [PubMed] [Google Scholar]
- 37. Pinget G., Tan J., Janac B., et al., “Impact of the Food Additive Titanium Dioxide (E171) on Gut Microbiota‐Host Interaction,” Frontiers in Nutrition 6 (2019): 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sylvetsky A. C. and Rother K. I., “Trends in the Consumption of Low‐Calorie Sweeteners,” Physiology & Behavior 164 (2016): 446–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suez J., Korem T., Zeevi D., et al., “Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota,” Nature 514 (2014): 181–186. [DOI] [PubMed] [Google Scholar]
- 40. Santos P. S., Caria C. R. P., Gotardo E. M. F., Ribeiro M. L., Pedrazzoli J., and Gambero A., “Artificial Sweetener Saccharin Disrupts Intestinal Epithelial Cells' Barrier Function In Vitro,” Food & Function 9 (2018): 3815–3822. [DOI] [PubMed] [Google Scholar]
- 41. Guo M., Liu X., Tan Y., et al., “Sucralose Enhances the Susceptibility to Dextran Sulfate Sodium (DSS) Induced Colitis in Mice With Changes in Gut Microbiota,” Food & Function 12 (2021): 9380–9390. [DOI] [PubMed] [Google Scholar]
- 42. Rodriguez‐Palacios A., Harding A., Menghini P., et al., “The Artificial Sweetener Splenda Promotes Gut Proteobacteria, Dysbiosis, and Myeloperoxidase Reactivity in Crohn's Disease–Like Ileitis,” Inflammatory Bowel Diseases 24 (2018): 1005–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X., Liu Y., Wang Y., et al., “Sucralose Promotes Colitis‐Associated Colorectal Cancer Risk in a Murine Model Along With Changes in Microbiota,” Frontiers in Oncology 10 (2020): 710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang X., Guo J., Liu Y., Yu H., and Qin X., “Sucralose Increased Susceptibility to Colitis in Rats,” Inflammatory Bowel Diseases 25 (2019): e3–e4. [DOI] [PubMed] [Google Scholar]
- 45. Suez J., Cohen Y., Valdés‐Mas R., et al., “Personalized Microbiome‐Driven Effects of Non‐Nutritive Sweeteners on Human Glucose Tolerance,” Cell 185 (2022): 3307–3328. [DOI] [PubMed] [Google Scholar]
- 46. Mendoza‐Martínez V. M., Zavala‐Solares M. R., Espinosa‐Flores A. J., et al., “Is a Non‐Caloric Sweetener‐Free Diet Good to Treat Functional Gastrointestinal Disorder Symptoms? A Randomized Controlled Trial,” Nutrients 14 (2022): 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Serrano J., Smith K. R., Crouch A. L., et al., “High‐Dose Saccharin Supplementation Does Not Induce Gut Microbiota Changes or Glucose Intolerance in Healthy Humans and Mice,” Microbiome 9 (2021): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thomson P., Santibañez R., Aguirre C., Galgani J. E., and Garrido D., “Short‐Term Impact of Sucralose Consumption on the Metabolic Response and Gut Microbiome of Healthy Adults,” British Journal of Nutrition 122 (2019): 856–862. [DOI] [PubMed] [Google Scholar]
- 49. Richey Levine A., Picoraro J. A., Dorfzaun S., and LeLeiko N. S., “Emulsifiers and Intestinal Health,” Journal of Pediatric Gastroenterology and Nutrition 74 (2022): 314–319. [DOI] [PubMed] [Google Scholar]
- 50. Chassaing B., Koren O., Goodrich J. K., et al., “Dietary Emulsifiers Impact the Mouse Gut Microbiota Promoting Colitis and Metabolic Syndrome,” Nature 519 (2015): 92–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Viennois E., Merlin D., Gewirtz A. T., and Chassaing B., “Dietary Emulsifier–Induced Low‐Grade Inflammation Promotes Colon Carcinogenesis,” Cancer Research 77 (2017): 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chassaing B., Compher C., Bonhomme B., et al., “Randomized Controlled‐Feeding Study of Dietary Emulsifier Carboxymethylcellulose Reveals Detrimental Impacts on the Gut Microbiota and Metabolome,” Gastroenterology 162 (2022): 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rousta E., Oka A., Liu B., et al., “The Emulsifier Carboxymethylcellulose Induces More Aggressive Colitis in Humanized Mice With Inflammatory Bowel Disease Microbiota Than Polysorbate‐80,” Nutrients 13 (2021): 3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ogulur I., Yazici D., Pat Y., et al., “Mechanisms of Gut Epithelial Barrier Impairment Caused by Food Emulsifiers Polysorbate 20 and Polysorbate 80,” Allergy 78 (2023): 2441–2455. [DOI] [PubMed] [Google Scholar]
- 55. Sandall A. M., Cox S. R., Lindsay J. O., et al., “Emulsifiers Impact Colonic Length in Mice and Emulsifier Restriction Is Feasible in People With Crohn's Disease,” Nutrients 12 (2020): 2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Laudisi F., di Fusco D., Dinallo V., et al., “The Food Additive Maltodextrin Promotes Endoplasmic Reticulum Stress‐Driven Mucus Depletion and Exacerbates Intestinal Inflammation,” Cellular and Molecular Gastroenterology and Hepatology 7 (2019): 457–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zangara M. T., Ponti A. K., Miller N. D., et al., “Maltodextrin Consumption Impairs the Intestinal Mucus Barrier and Accelerates Colitis Through Direct Actions on the Epithelium,” Frontiers in Immunology 13 (2022): 841188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wu W., Zhou D., Xuan R., et al., “λ‐Carrageenan Exacerbates Citrobacter rodentium ‐Induced Infectious Colitis in Mice by Targeting Gut Microbiota and Intestinal Barrier Integrity,” Pharmacological Research 174 (2021): 105940. [DOI] [PubMed] [Google Scholar]
- 59. Nickerson K. P. and McDonald C., “Crohn's Disease‐Associated Adherent‐Invasive Escherichia coli Adhesion Is Enhanced by Exposure to the Ubiquitous Dietary Polysaccharide Maltodextrin,” PLoS One 7 (2012): e52132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wei W., Feng W., Xin G., et al., “Enhanced Effect of κ‐Carrageenan on TNBS‐Induced Inflammation in Mice,” International Immunopharmacology 39 (2016): 218–228. [DOI] [PubMed] [Google Scholar]
- 61. Shang Q., Sun W., Shan X., et al., “Carrageenan‐Induced Colitis Is Associated With Decreased Population of Anti‐Inflammatory Bacterium, Akkermansia muciniphila , in the Gut Microbiota of C57BL/6J Mice,” Toxicology Letters 279 (2017): 87–95. [DOI] [PubMed] [Google Scholar]
- 62. Borsani B., de Santis R., Perico V., et al., “The Role of Carrageenan in Inflammatory Bowel Diseases and Allergic Reactions: Where Do we Stand?,” Nutrients 13 (2021): 3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hrncirova L., Machova V., Trckova E., Krejsek J., and Hrncir T., “Food Preservatives Induce Proteobacteria Dysbiosis in Human‐Microbiota Associated Nod2‐Deficient Mice,” Microorganisms 7 (2019): 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Irwin S. V., Fisher P., Graham E., Malek A., and Robidoux A., “Sulfites Inhibit the Growth of Four Species of Beneficial Gut Bacteria at Concentrations Regarded as Safe for Food,” PLoS One 12 (2017): e0186629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Williams K., Milner J., Boudreau M. D., Gokulan K., Cerniglia C. E., and Khare S., “Effects of Subchronic Exposure of Silver Nanoparticles on Intestinal Microbiota and Gut‐Associated Immune Responses in the Ileum of Sprague‐Dawley Rats,” Nanotoxicology 9 (2015): 279–289. [DOI] [PubMed] [Google Scholar]
- 66. Hrncirova L., Hudcovic T., Sukova E., et al., “Human Gut Microbes Are Susceptible to Antimicrobial Food Additives In Vitro,” Folia Microbiologica 64 (2019): 497–508. [DOI] [PubMed] [Google Scholar]
- 67. van den Brule S., Ambroise J., Lecloux H., et al., “Dietary Silver Nanoparticles Can Disturb the Gut Microbiota in Mice,” Particle and Fibre Toxicology 13 (2016): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Javurek A. B., Suresh D., Spollen W. G., et al., “Gut Dysbiosis and Neurobehavioral Alterations in Rats Exposed to Silver Nanoparticles,” Scientific Reports 7 (2017): 2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dey S. and Nagababu B. H., “Applications of Food Color and Bio‐Preservatives in the Food and Its Effect on the Human Health,” Food Chemistry Advances 1 (2022): 100019. [Google Scholar]
- 70. JECFA , “Allura Red AC,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/2361.
- 71. European Food Safety Authority , “Refined Exposure Assessment for Allura Red AC (E 129),” EFSA Journal 13 (2015): 4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. WHO | JECFA , “Tartrazine,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/3885.
- 73. EFSA Panel on Food Additives and Nutrient Sources Added to Food , “Scientific Opinion on the Re‐Evaluation Tartrazine (E 102),” EFSA Journal 7 (2009): 1331. [Google Scholar]
- 74. WHO | JECFA , “Sunset Yellow FCF,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/2703.
- 75. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Reconsideration of the Temporary ADI and Refined Exposure Assessment for Sunset Yellow FCF (E 110),” EFSA Journal 12 (2014): 3765. [Google Scholar]
- 76. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Scientific Opinion on the Re‐Evaluation of Brilliant Blue FCF (E 133) as a Food Additive,” EFSA Journal 8 (2010): 1853. [Google Scholar]
- 77. WHO | JECFA , “Brilliant Blue FCF,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/3309.
- 78. WHO | JECFA , “Titanium Dioxide,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/2723.
- 79. EFSA Panel on Food Additives and Flavourings (FAF) , EFSA Panel on Food Additives and Flavourings (FAF) , Younes M., et al., “Safety Assessment of Titanium Dioxide (E171) as a Food Additive,” EFSA Journal 19 (2021): e06585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. WHO | JECFA , “Aspartame,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/62.
- 81. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Scientific Opinion on the Re‐Evaluation of Aspartame (E 951) as a Food Additive,” EFSA Journal 11 (2013): 3496. [Google Scholar]
- 82. WHO | JECFA , “ACESULFAME K,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/3613.
- 83. EFSA Panel on Food Additives and Flavourings (FAF) , Castle L., Andreassen M., et al., “Re‐Evaluation of Acesulfame K (E 950) as Food Additive,” EFSA Journal 23 (2025): e9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. WHO | JECFA , “Advantame,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/6181.
- 85. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Scientific Opinion on the Safety of Advantame for the Proposed Uses as a Food Additive,” EFSA Journal 11 (2013): 3301. [Google Scholar]
- 86. WHO | JECFA , “Neotame,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/5107.
- 87. European Food Safety Authority , “Neotame as a Sweetener and Flavour Enhancer—Scientific Opinion of the Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact With Food,” EFSA Journal 5 (2007): 581. [Google Scholar]
- 88. WHO | JECFA , “Saccharin,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/3164.
- 89. EFSA Panel on Food Additives and Flavourings (FAF) , “Re‐Evaluation of Saccharin and Its Sodium, Potassium and Calcium Salts (E 954) as Food Additives,” EFSA Journal 22 (2024): e9044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. WHO | JECFA , “Sucralose,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/2340.
- 91. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Safety of the Proposed Extension of Use of Sucralose (E 955) in Foods for Special Medical Purposes in Young Children,” EFSA Journal 14 (2016): 4361. [Google Scholar]
- 92. WHO | JECFA , “Carboxymethyl Cellulose,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/128.
- 93. EFSA Panel on Food Additives Flavourings (FAF) , “Opinion on the Re‐Evaluation of Sodium Carboxy Methyl Cellulose (E 466) as a Food Additive in Foods for Infants Below 16 Weeks of Age and Follow‐Up of Its Re‐Evaluation as Food Additive for Uses in Foods for All Population Groups,” EFSA Journal 20 (2022): e07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. WHO | JECFA , “Polysorbate 80,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/3735.
- 95. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Scientific Opinion on the Re‐Evaluation of Polyoxyethylene Sorbitan Monolaurate (E 432), Polyoxyethylene Sorbitan Monooleate (E 433), Polyoxyethylene Sorbitan Monopalmitate (E 434), Polyoxyethylene Sorbitan Monostearate (E 435) and Polyoxyethylene Sorbitan Tristearate (E 436) as Food Additives,” EFSA Journal 13 (2015): 4152. [Google Scholar]
- 96. Human Foods Program , “Agency Response Letter GRAS Notice No. GRN 000610, 2024”.
- 97. WHO | JECFA , “Carrageenan,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/377.
- 98. EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS) , “Re‐Evaluation of Carrageenan (E 407) and Processed Eucheuma Seaweed (E 407a) as Food Additives,” EFSA Journal 16 (2018): e05238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. WHO | JECFA , “Sodium Sulfite,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/2993.
- 100. WHO | JECFA , “Sodium Benzoate,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/1098.
- 101. EFSA Panel on Food Additives and Nutrient Sources (ANS) , “Scientific Opinion on the Re‐Evaluation of Benzoic Acid (E 210), Sodium Benzoate (E 211), Potassium Benzoate (E 212) and Calcium Benzoate (E 213) as Food Additives,” EFSA Journal 14 (2016): 4433. [Google Scholar]
- 102. WHO | JECFA , “Potassium Sorbate,” https://apps.who.int/food‐additives‐contaminants‐jecfa‐database/Home/Chemical/2724.
- 103. EFSA Panel on Food Additives and Flavourings (FAF) , “Opinion on the Follow‐Up of the Re‐Evaluation of Sorbic Acid (E200) and Potassium Sorbate (E202) as Food Additives,” EFSA Journal 17 (2019): e05625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. FDA , “Generally Recognized as Safe (GRAS), 2023”, https://www.fda.gov/food/food‐ingredients‐packaging/generally‐recognized‐safe‐gras.
- 105. U.S Food and Drug Administration , “FD&C Red No. 3, 2025”, https://www.fda.gov/industry/color‐additives/fdc‐red‐no‐3.
- 106. Borzelleca J. F., Capen C. C., and Hallagan J. B., “Lifetime Toxicity/Carcinogenicity Study of FD & C Red no. 3 (Erythrosine) in Rats,” Food and Chemical Toxicology 25 (1987): 723–733. [DOI] [PubMed] [Google Scholar]
- 107. EFSA Panel on Food Additives and Nutrient Sources Added to Food , “Scientific Opinion on the Re‐Evaluation of Sunset Yellow FCF (E 110) as a Food Additive,” EFSA Journal 7 (2009): 1330. [Google Scholar]
- 108. Arnold L. E., Lofthouse N., and Hurt E., “Artificial Food Colors and Attention‐Deficit/Hyperactivity Symptoms: Conclusions to Dye for,” Neurotherapeutics 9 (2012): 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ng S. C., Shi H. Y., Hamidi N., et al., “Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population‐Based Studies,” Lancet 390 (2017): 2769–2778. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors have nothing to report.


