Abstract
Neurovascular coupling (NVC) is a vital regulatory mechanism that synchronizes neural activity with vascular responses to support brain function. Although the precise mechanisms of NVC remain incompletely elucidated, its dysfunction is increasingly implicated in the pathogenesis of various neurological disorders. This review synthesizes recent advancements in understanding the vascular cascade, emphasizing key dynamic regulators of NVC, including mechanical forces and diffusible signals mediated by blood flow. We explore the intricate bidirectional interactions between the vasculature and neurons, highlighting their interdependent roles in neurovascular regulation. Using major depressive disorder (MDD) as a case study, we further discuss emerging evidence linking vascular dysfunction and impaired NVC to MDD pathophysiology. These insights position NVC as a promising therapeutic target for emotional disorders, underscoring the pivotal roles of hemodynamic signaling in neurovascular regulation.
Keywords: Neurovascular coupling, Major depressive disorder, Cerebral blood flow, Dynamic regulation
Introduction
The brain maintains one of the body’s most sophisticated vascular networks, with its dense microvasculature playing an indispensable role in supporting neuronal function and metabolic homeostasis [1]. NVC represents a critical physiological process that coordinates bidirectional communication between neural activity and vascular responses, thereby maintaining precise metabolic-vascular homeostasis [2, 3]. During sensory processing or cognitive tasks, localized neuronal activation triggers rapid vasodilation, resulting in increased cerebral blood flow (CBF), blood volume (CBV), and tissue oxygenation [2, 4]. This neurovascular synchrony not only facilitates efficient delivery of oxygen and nutrients but also actively modulates neuronal excitability to maintain cerebral homeostasis. Beyond perfusion, NVC contributes to neuronal signaling [5, 6], stabilizes and optimizes cerebrovascular architecture [7], regulates brain temperature [8, 9], and drives cerebrospinal fluid (CSF) production and circulation [10–12], ultimately preserving brain homeostasis.
In recent years, the vascular system’s involvement in psychiatric disorders has gained increasing attention, offering a unifying framework to explain various pathophysiological abnormalities [13–16]. Perturbations in NVC have been consistently documented across multiple neuropsychiatric conditions, including MDD [17], autism spectrum disorder [18], and related mental illnesses [19, 20]. As the world’s most prevalent psychiatric condition [21, 22], MDD demonstrates particularly strong associations with both cardiovascular/metabolic comorbidities [23] and stress-induced microvascular dysfunction [17]. Notably, preclinical models reveal that cerebrovascular abnormalities often precede neuronal dysfunction in depression pathogenesis, highlighting the need to better understand the mechanistic relationships between vascular regulation, NVC integrity, and mood disorder development. This review explores the mechanisms underlying neurovascular communication and evaluates the role of NVC dysfunction in MDD, emphasizing its potential as a therapeutic target.
Cascade reaction of blood vessels in NVC
The structural diversity of cerebral vasculature implies distinct functional roles for different segments in regulating CBF. Arterioles and capillaries demonstrate the most pronounced relative CBV changes [1], highlighting their central importance in CBF modulation. Neuronal activity triggers vessel dilation via ion channels [24] and receptors [25], maintaining stable cerebral perfusion [26] (Table 1). The spatial pattern of vasodilation—ranging from focal microvascular expansion to widespread blood flow redistribution—emerges from discrete signaling pathways that propagate regulatory signals in a cascading manner.
Table 1.
Functions of different subtypes of channels and receptors in NVC
| Channels | Functional relevance | Cell types | References | |
|---|---|---|---|---|
| Kir2.1 | Sense neuronal activity and propagate hyperpolarization in adjacent ECs | ECs | [147] | |
| NMDAR | Detect synaptic activity and regulate functional hyperemia | ECs | [27, 147] | |
| TRPA1 | Instigate a propagating retrograde signal | ECs | [29, 134] | |
| TRPV4 | Induce vasodilation in upstream arteries | ECs | [148, 149] | |
| IP3R | Mediating Ca2+ release | ECs | [33] | |
| SKCa | Conduce the outward current and modulate CBF | ECs | [150] | |
| IKCa | Conduce the outward current and modulate CBF | ECs | [150] | |
| KATP | Sense neural activity and transform electrical signals | PCs | [32] | |
| Kir | Sensitive to voltage and induce hyperpolarization | PCs | [151] | |
| BKCa | Sensitive to voltage and induce hyperpolarization | PCs, SMCs | [28, 152–154] | |
| SKCa | Sensitive to voltage and induce hyperpolarization | PCs, SMCs | [152, 155, 156] | |
| sGC | Sense NO and open BKCa channel | PCs, SMCs | [152, 157] | |
| P2X7 | Sense ATP and induce depolatization | PCs | [158] | |
| P2X4 | Sense ATP and increase the intracellular Ca2+ | PCs | [159] | |
| Kir2 | Sense neuronal activity and induce hyperpolarization | SMCs | [160] | |
| NMDAR1 | Mediating synaptic-like transmission with axons | SMCs | [25] | |
| Na+, K+-ATPase | Sense the external K+ and induce hyperpolarization | SMCs | [161, 162] | |
| KATP | Modulating intracellular Ca2+ oscillation | SMCs | [163] | |
Beyond mural cell-mediated vasodilation, endothelial cells (ECs) contribute significantly to NVC by integrating neurotransmitter and electrical signals [27–29]. Recent findings have identified an electrochemical (E-Ca) coupling mechanism in brain capillary ECs that facilitates the retrograde transmission of endothelial hyperpolarization signals from capillaries to upstream arterioles, adjusting blood supply to meet downstream demand [30]. Specifically, as sensors of neural activity and generators of hyperpolarized signals, pericytes (PCs) form a distributed electrical communication system that transmits signals upstream to ECs [31, 32], inducing IP3R-mediated Ca2⁺ release and propagating hyperpolarization via Kir 2 activation in adjacent ECs [33]. This mechanism establishes a spatiotemporal framework for cerebral blood flow regulation, integrating diverse signaling pathways across vascular compartments. Notably, perisynaptic capillary-to-arteriole signaling exhibits variability in onset time, directionality, and vessel diameter changes [34], underscoring the complexity of NVC dynamics. The interplay between capillary- and arteriole-level signaling cascades orchestrates cerebral perfusion with high spatiotemporal precision, ensuring optimal neuronal function.
Dynamic regulatory signals from vasculature to neurons
Mechanical forces
The myogenic response and cardiac cycle contribute to the autoregulation of CBF by modulating shear stress, maintaining cerebral perfusion. Arteriole diameter oscillations at ~ 0.1 Hz generate local physiological pressures [35] that elicit transient neuronal responses through mechanosensitive ion channels [36]. Neuronal Piezo-1 receptors respond to vascular pressure fluctuations, interacting bidirectionally with transient receptor potential (TRP) ion channels to modulate neurotransmitter release [37]. Piezo-2 detects rhythmic vascular pulsations, inducing spontaneous spike activity in mitral cells of the olfactory bulb, with similar pulsation-driven neuronal activation observed in the hippocampus and prefrontal cortex [38]. Furthermore, neurons distinguish varying mechanical stimuli from blood flow via voltage-gated channels (e.g., TRPV1, TRPV4), exhibiting transient or sustained responses. Notably, axons demonstrate a higher responsiveness to mechanical stimuli (73%) compared to somata and dendrites [36]. Interestingly, elevated cerebral blood flow/pressure in the rat cortex enhances the firing of somatostatin interneurons while suppressing pyramidal neuron activity [39]. This neuron-specific negative feedback regulates vascular responses to balance energy supply with demand, preventing both hyperperfusion and hypoperfusion damage in brain tissue.
Likewise, astrocytes exhibit the ability to sense mechanical stimuli derived from vascular dynamics. Emerging evidence reveals a sequential vessel-to-astrocyte-to-neuron signaling pathway, where astrocytes serve as critical intermediaries. For example, astrocytic TRPV4 channels respond to PA tone, initiating vascular responses while simultaneously modulating neuronal activity via adenosine release, as shown in vitro [39, 40]. Under physiological conditions, where cerebral autoregulation mechanisms operate efficiently, graded adjustments in neuronal activity—triggered by adenosine release—may encode varying degrees of vascular tone. This could provide a mechanism for the cortex to monitor changes in brain perfusion pressure. This interaction suggests that NVC ensures metabolic homeostasis, allowing neurons to adapt their activity in response to cerebral perfusion. This dynamic equilibrium maintains a balance between energy supply and demand.
Diffusible factors
Diffusible factors play a critical role in vascular-neuronal communication by freely crossing the blood–brain barrier and directly influencing neural activity.
Nitric oxide
NO is a gas neurotransmitter that well-characterized and essential for NVC in humans [41]. Neurophysiologists often focus on neuronal nitric oxide synthase (nNOS)-derived NO [42]. While, endothelial- and blood-derived NO can diffuse up to 100 μm from blood vessels [43, 44] and modulate neuronal function by binding to soluble guanylate cyclase (sGC) and calcium-dependent potassium channels [45]. Endothelial NO has been implicated in synaptic plasticity within the cortex and striatum [46, 47], generated tonic NO signals [48] which are crucial for hippocampal LTP [49]. Experiments with eNOS-/- mice demonstrate endothelial NO’s role in regulating neurogenesis and angiogenesis [50] (Table 2). Insufficient endothelial NO secretion triggers compensatory neuronal mechanisms [51], while nNOS upregulation may offset eNOS deficiency to preserve cognition, emotion, and motor control [52]. Collectively, these findings highlight NO as a key integrator of vascular and neuronal signaling, ensuring the bidirectional communication necessary for maintaining NVC.
Table 2.
Recent advances in eNOS deficiency and phenotype research
| Knockout Strategies | Neuronal phenotype | Behavioural phenotype | References |
|---|---|---|---|
| eNOS± | – | Depressive-like behavior | [17] |
| eNOS− | Impairment of neocortical long-term potentiation | – | [46] |
| eNOS−/− | Reduce the occurrence of LTP in striatal | – | [47] |
| eNOS− | Generate tonic NO signals to induce hippocampal LTP | – | [49] |
| eNOS− | Decrease the CBF responses in the cortex evoked by whisker stimulation and by administration of ATP | – | [164] |
| eNOS− | Involved in an NMDAR-independent form of LTP in the cortex | – | [46] |
| eNOS−/− | Neuroinflammation | Spatial memory impaired | [165] |
| eNOS−/− | Demyelinate of cortical, corpus callosum and hippocampus in middle-ages mice | Gait behavior defect and association recognition memory disorder | [166] |
| eNOS−/− | Influence progenitor cell proliferation, neuronal migration and neurite outgrowth after stroke | Functional recovery disorder after stroke | [50, 167] |
| eNOS−/− | Decrease in retinal neovascularization and the expression of VEGF | – | [168, 169] |
Hormones
Hormones, potent bioactive molecules secreted by endocrine glands, regulate physiological functions across the body and brain [53, 54]. Recent research underscores their role in synaptogenesis, neurogenesis, and NVC [55–57]. Several hormones and their receptors are directly involved in NVC. Angiotensin II (Ang II), a major component of the renin–angiotensin–aldosterone system (RAAS), secreted by renal juxtaglomerular cells, binds to receptors widely distributed in the brain. It regulates vasoconstriction, aldosterone secretion, and sympathetic activity, playing a critical role in fluid balance. Dysregulation of Ang II causes endothelial dysfunction [58, 59] through AT1R- and NADPH oxidase-mediated oxidative stress [60], leading to nNOS expression increased and subsequent NVC impairment [61].
Another key hormone, Insulin-like growth factor-1 (IGF-1), is produced primarily by the liver and exerts protective vascular effects. Age-related IGF-1 decline impairs CBF and NVC responses [62] by disrupting endothelial IGF1R signaling and NO-dependent vasodilation [63, 64]. Additionally, endothelial IGF-1 can also protective neuron [65] and prevent the disruption of BBB [66], maintaining normal NVC responses. Crucially, the disruption of hormone homeostasis triggers neuroprotective adaptations that include the Nrf2/ ARE antioxidant pathway and Trx/ Prx redox buffering system, which collectively counteract oxidative stress [67, 68]. These adaptive responses also regulate endothelial-mediated mitochondrial biogenesis, enhance SOD2 activity, and confer resistance to oxidative damage [69, 70].
Beyond these, signaling molecules such as miRNAs, lncRNAs, and exosomes circulate in body fluids [71] and mediate communication between neurons, ECs, and PCs [72]. These molecules may play a yet-undetermined role in NVC, warranting further investigation.
Caveolae
Caveolae, cholesterol- and lipid-rich membrane invaginations, serve as regulatory hubs for ion channels (e.g., Cav1.2, Nav1.5, NCX1) [73, 74] and mechanotransduction pathways [75]. These structures respond to mechanical stimuli, including membrane stretch, by flattening to provide mechanical protection [76] and modulating signal transduction through protein interactions [77]. While most brain endothelial cells suppress caveolae to control cellular transport and ensure the integrity of the blood–brain barrier [78–80], their role in NVC has been largely unexplored. Recent findings indicate that inhibiting endothelial caveolae in brain arterioles reduces the NVC response by approximately 50%, independent of the eNOS-NO pathway. Simultaneous inhibition of both caveolae and the NO pathway abolishes the NVC response entirely [81]. This suggests that caveolae are essential platforms for clustering ion channels and receptors involved in NVC. Identifying the specific ion channels localized within endothelial caveolae will be critical for elucidating their contribution to cerebrovascular regulation.
Brain temperature
Brain temperature is regulated by a dynamic interplay between metabolic activity, CBF, and systemic body temperature. Even minor fluctuations in brain temperature can disrupt vascular and neuronal homeostasis. Substantial evidence indicates that such changes elicit corresponding vascular responses. For example, as brain temperature decreases, hemoglobin affinity for oxygen increases and reduces cerebral oxygen saturation [82, 83], inducing mild blood–brain barrier leakage in the hypothalamus and piriform cortex, and disrupting cerebral water homeostasis [84]. Conversely, an increase in cortical temperature amplifies vascular oscillations, leading to enhanced power within the 0.05–0.25 Hz frequency range [85]. Positron emission tomography (PET) studies further demonstrate that localized increases in CBF correlate with regional temperature shifts and alterations in oxygen metabolism [86].
Beyond effects on vascular, temperature fluctuations influence synaptic function. Elevated temperature potentiates synaptic transmission via TRPV4-mediated mechanisms [87, 88], which increase presynaptic vesicle release probability and synaptic inhibition, ultimately suppressing spiking activity in thalamocortical neurons [89]. Most importantly, neuronal activity exerts feedback effects on vascular function. For instance, activation of TRPC4 channel in Preoptic region stimulates the hypothalamic warm-sensitive neurons, thereby increasing CBF to facilitate heat dissipation [90]. Recent MRI findings suggest an inverted U-shaped relationship between cortical temperature and evoked neuronal and hemodynamic responses [85], demonstrating that cooling delays neurovascular coupling (NVC). These observations underscore the critical role of brain temperature in modulating neurovascular interactions. Collectively, the evidence underscores the importance of blood flow in neural information processing and its impact on NVC dynamics (Fig. 1).
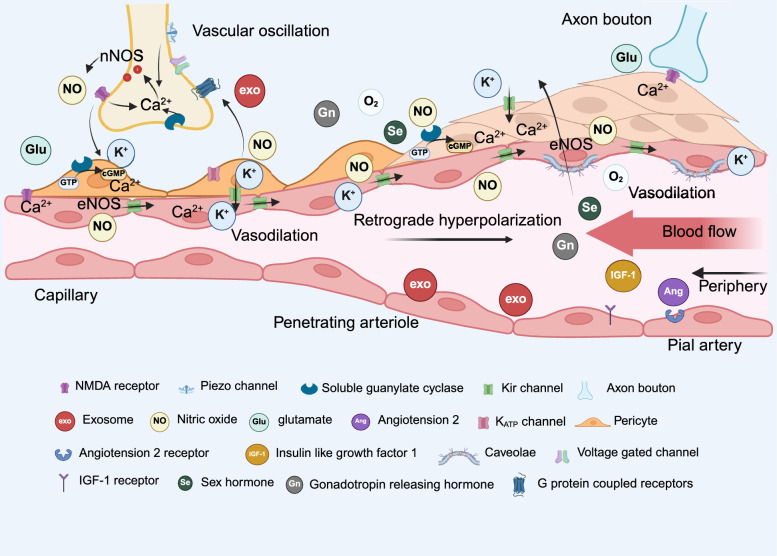
Fig. 1.
Mutual communication in neurovascular coupling. Cascade reactions in NVC. Synaptic activity triggers the release of extracellular K+, NO, and glutamate, which act on ion channels and receptors in PCs, SMCs, and endothelial cells, leading to NO release and hyperpolarization. Additionally, peripheral hormones can interact with endothelial cells via blood circulation or diffuse to neurons, binding to their receptors. Vascular oscillation-induced mechanical forces and blood flow-regulated brain temperature also modulate neuronal activity
Clinical evidence of NVC dysfunction in MDD patients
Imaging study
Mounting evidence links NVC impairment to MDD, with neuroimaging studies consistently reporting altered CBF, reduced arteriolar dilation, and delayed hemodynamic responses [3]. In MDD patients, abnormalities in the fractional amplitude of low-frequency fluctuations (fALFF), disrupted activity in the default mode network (DMN), and shortened time-to-peak (TTP) in hemodynamic response function (HRF) parameters have been documented [91]. MRI studies reveal significant reductions in CBF in key regions implicated in mood regulation, including the prefrontal cortex [92, 93], anterior cingulate cortex [94], thalamus, and left superior temporal gyrus [95]. Notably, alterations in prefrontal-limbic functional connectivity (FC) in severe depression have been linked to suicidal ideation [96]. Additionally, abnormal directional connectivity in the cortical-subcortical-cerebellar network may lead to unbalanced integrating the emotional-related information for MDD, and further exacerbating depressive symptoms [97].
Antidepressant interventions further support the vascular basis of MDD pathophysiology; escitalopram has been shown to enhance CBF in the left temporal lobe, frontal lobe, and anterior cingulate cortex [98], while fluoxetine improves fMRI-BOLD responses in MDD patients [99, 100]. Most neuroimaging studies operate on the fundamental assumption that reduced CBF in MDD reflects neuronal hypometabolism; however, a more direct interplay between cerebrovascular circulation and neuronal activity may underlie these findings. The precise mechanisms underlying NVC impairment in MDD remain elusive, prompting further exploration of potential vascular changes at both structural and functional levels.
Structural changes in the brain vascular network
The cerebral vasculature may function as an interoceptive system, integrating homeostatic reflexes and allostatic responses—including motivational behaviors and emotional states—to maintain physiological equilibrium. Chronic stress induces profound morphological changes in the brain's vascular network, contributing to the pathophysiology of neuropsychiatric disorders. Recent advancements in optical coherence tomography angiography (OCTA) have facilitated clinical assessments of vascular changes in MDD. For instance, MDD patients exhibit abnormal retinal vascular fractal dimensions [101], and symptom severity correlates with retinal vascular diameter [102]. Swept-source optical coherence tomography angiography (SS-OCTA) studies further reveal reduced choroidal vascular density and decreased macular vascular density in the superficial retinal capillary plexus of MDD patients [103].
In elderly individuals with MDD, cortical gray and white matter exhibit increased vascular segment density and perivascular space expansion [104]. Moreover, depression severity correlates with elevated serum levels of angiogenesis inhibitors, such as endostatin [105], suggesting that pathological vascular remodeling plays a central role in mood disorders. Postmortem stereological studies support these findings, reporting increased neurovascular cells and potential vascular alterations in the basolateral amygdala of MDD patients [106]. These findings collectively indicate that MDD is associated with structural cerebrovascular abnormalities, reinforcing that vascular integrity is intricately linked to emotional and cognitive function.
Blood–brain barrier damage
The blood–brain barrier (BBB), a crucial neurovascular interface, maintains central nervous system homeostasis and ensures normal NVC function. Emerging evidence from both MDD patients and animal models indicates BBB disruption [13, 84, 107]. Dynamic contrast-enhanced (DCE) MRI and structural imaging studies have revealed significantly elevated mean volume transfer constants (K trans) in the olfactory region, caudate nucleus, and thalamus of MDD patients compared to healthy controls, suggesting a direct association between BBB leakage and depressive symptom severity [108]. Notably, BBB damage appears more pronounced in female MDD patients, with a ~ 50% reduction in Claudin5 mRNA levels in the prefrontal cortex (PFC) and marked vascular morphological alterations [107]. These findings suggest that chronic stress induces region-specific BBB breakdown, contributing to MDD pathology and potentially explaining gender differences in disease prevalence and severity.
Endothelial cell dysfunction
Endothelial dysfunction, characterized by abnormal endothelial activity, is a key contributor to vascular pathophysiology and has been detected in depression [109]. MRI studies have demonstrated increased relative transit time heterogeneity (RTH) in the capillaries of the bilateral prefrontal cortex, ventral anterior cingulate cortex, and left insular cortex in MDD patients, along with a decreased cerebral metabolic rate of oxygen (nCMRO₂), indicating capillary dysfunction and impaired NVC within the ventral circuit [110]. Additionally, flow-mediated dilation (FMD) assessments in middle-aged and elderly MDD patients have revealed a correlation between microvascular dysfunction and neurocognitive decline, reinforcing the link between endothelial dysfunction and cognitive impairment in MDD [111]. Evidence suggests that early-life stress impairs visceral microcirculation and endothelial function [112], increasing susceptibility to cardiovascular and cerebrovascular diseases later in life [113–115]. These findings underscore the importance of endothelial cell protection and repair as a potential therapeutic strategy for restoring NVC integrity in MDD.
Vascular inflammatory response
Autoimmune diseases exhibit high comorbidity with MDD, suggesting that chronic stress may provoke immune responses targeting the brain [116]. The shared psycho-immune-neuroendocrine (PINE) network model provides a mechanistic framework linking vascular inflammation and depression [117]. MDD patients display elevated plasma levels of pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α, platelet-derived growth factor (PDGF), granulocyte–macrophage colony-stimulating factor (GM-CSF), and IL-8, particularly in response to psychosocial stressors [118]. These inflammatory responses are primarily mediated by serum- and glucocorticoid-regulated kinase 1 (SGK1) [119], autonomic imbalances leading to excessive catecholamine release [120], and disruptions in serotonergic and glutamatergic neurotransmission [91], further exacerbating neurovascular dysfunction. These findings highlight the critical role of chronic stress-induced vascular inflammation in MDD, implicating oxidative stress, neuroinflammation, metabolic dysregulation, and neurotransmitter imbalances in the disorder's pathophysiology.
NVC impairment in models of depression: insights from preclinical studies
The above-mentioned research proves that chronic stress-induced vascular dysfunction disrupts NVC, leading to metabolic deficits and energy shortages in MDD patients. Although the precise mechanisms remain incompletely understood, recent advances in animal models offer promising insights into the intersection of neurovascular and metabolic dysregulation in MDD (Fig. 2).
Fig. 2.
Neurovascular coupling dysfunction in MDD patients. Chronic stress disrupts endothelial ion channels and functions, reduce the area of astrocyte endfoot and vessel, leading to PCs loss and BBB breakdown. The compromised BBB allows inflammatory factors and catecholamines from hyperactive HPA axis signaling to infiltrate the brain parenchyma, inducing ROS accumulation and synaptic loss. Reduced cerebrovascular density and CBF lead to ATP deficiency, impairing action potential firing and circuit dysfunction
Cerebrovascular damage and angiogenesis
A fully developed and functionally intact cerebral vascular network is essential for normal NVC function [121]. However, preclinical studies have revealed region-specific differences in NVC, though the underlying mechanisms remain unclear. Wu et al. mapped the distribution and spatial relationships of cerebral blood vessels, neurons, and PCs in mice, providing insights into these mechanisms [122]. Key brain regions involved in information processing, such as the somatosensory and primary auditory cortices, exhibit higher vascular density and more responsive NVC compared to other regions. In contrast, with its sparse capillary network, the hippocampus demonstrates weaker NVC responses and is more vulnerable to hypoxia [123]. These findings highlight the susceptibility of specific brain regions to stress and suggest potential mechanisms linking cerebrovascular network damage and NVC impairment in MDD.
Single-cell RNA sequencing of brain endothelial cells in mice subjected to chronic social stress revealed upregulation of angiogenesis-related genes and VEGF signaling in capillaries, indicating their potential role in vascular repair following stress exposure [124]. Additionally, human and animal studies have demonstrated that antidepressant treatment promotes endothelial cell proliferation, particularly in the hippocampus and hypothalamus [125–127]. Electroconvulsive therapy [128] and antidepressants promote VEGF- and BDNF-mediated angiogenesis via the SIRT1/FOXO1 pathway, thereby supporting neurogenesis and exerting antidepressant effects [129]. Collectively, these findings underscore the critical role of vascular integrity in maintaining NVC and suggest that targeting angiogenesis may offer a promising therapeutic approach.
BBB damage
Chronic social stress and subchronic variable stress have been shown to alter BBB integrity in emotion-related brain regions in mice [107], suggesting a key pathological link between stress exposure and psychiatric disorders. Chronic stress increases VEGF levels, and VEGF/VEGFR2 signaling compromises the paracellular barrier. Pharmacological inhibition of VEGFR2 mitigates chronic stress-induced BBB damage and anhedonia, further supporting its role in MDD pathophysiology [130]. In addition, aberrant Claudin5 expression and its promoter histone modifications contribute to BBB leakage in stress-exposed mice [131]. Besides, the decrease of the coverage area of the astrocyte endfoot and vessels is involved in the leakage of BBB. Such as the abnormal expression of CB1 receptor in astrocyte endfoot is linked to perivascular endogenous cannabinoid signal, which obstruct the expression of the vascular-related genes and compromised BBB stability. This mechanism has been associated with stress susceptibility and emotional disorders [132].
BBB breakdown permits peripheral inflammatory mediators such as IL-6 and TNF-α to infiltrate the nucleus accumbens (NAc) and hippocampus, exacerbating neuroinflammation. Interestingly, overexpression of Claudin5 or inhibition of histone deacetylase 1 (HDAC1) reverses BBB dysfunction and neuroinflammation, improving depressive-like behaviors in mice [13]. However, these interventions do not ameliorate stress-induced adrenal hypertrophy or weight loss, indicating that while BBB integrity protects against inflammatory neurotoxicity, it remains a downstream response to autonomic nervous system and HPA axis hyperactivation. These findings suggest that targeting BBB stabilization may alleviate MDD symptoms but should be complemented by broader therapeutic strategies addressing systemic stress responses.
Endothelial dysfunction
Cerebrovascular endothelial cells play a central role in NVC by expressing ion channels and receptors that translate neuronal activity into vascular responses (Table 1). Subchronic stress (7 days) has been shown to downregulate Kir 2.1 mRNA in parenchymal arterioles and reduce Kir current density in smooth muscle, impairing Kir channel function and NVC [133]. Endothelial TRPA1, a key sensor of inflammation and oxidative stress, has been implicated in MDD pathophysiology. Pharmacological TRPA1 antagonists reverse despair-like behaviors in mice, suggesting its potential as a therapeutic target [134]. Additionally, endothelial GPR4 (H+ receptor) and Gαq/11 proteins mediate cerebrovascular CO2 sensitivity, and their dysfunction contributes to anxiety-like behaviors and respiratory disturbances in stress-exposed mice [20]. These findings indicate that stress-induced inflammation disrupts endothelial ion channel function, representing a key mechanism underlying vascular and NVC dysfunction in MDD. However, research on endothelial ion channels and receptors under chronic stress remains limited, hindering a full understanding of their role in NVC dysfunction.
Reduced CBF and energy homeostasis imbalance
Chronic stress-induced circulatory dysfunction and energy deficits are well-documented in preclinical models of depression [17]. When vascular dysfunction leads to insufficient energy supply, AMPK acts as a cellular energy sensor, reducing neuronal excitability to adjust local energy availability [135, 136]. However, chronic stress has been shown to reduce AMPK levels and disrupt synaptic integrity in the cortex of depression model mice [137]. While exogenous ATP supplementation has emerged as a potential therapeutic approach for MDD [138, 139], excessive extracellular ATP can cause neuronal hyperactivation and exacerbate metabolic stress [140]. Thus, targeting vascular homeostasis to balance energy supply and demand may offer a more effective treatment strategy.
Neuronal subtypes have distinct energy demands [141], such as glutamatergic neurons (vGlut1+) account for ~ 80% of gray matter energy consumption due to their role in neurotransmission [142]. When mitochondrial respiratory chain inhibitors are applied, hippocampal parvalbumin (PV) neurons—but not pyramidal neurons—exhibit firing deficits [143], suggesting that PV neurons are particularly susceptible to energy depletion. In energy-deficient conditions, which neuronal populations are most vulnerable? Moreover, metabolic pathways differ between neuronal somata (which rely primarily on glycolysis) and synaptic terminals (which depend on oxidative phosphorylation) [144]. In response to stress or energy shortages, do neurons undergo metabolic reprogramming to maintain function? Investigating such adaptive mechanisms may provide critical insights into energy homeostasis and novel treatment strategies for MDD.
Conclusion
NVC embodies the integrated function of neurons and blood vessels in information processing, extending beyond metabolic supply to include dynamic signaling that influences neural activity. Despite its significance, our understanding of NVC remains limited, with several fundamental questions unanswered. Notably, NVC exhibits remarkable regional heterogeneity—manifesting as nonspecific, absent, or even inverted responses in certain brain areas [145, 146]. The underlying causes of this spatial variability warrant systematic investigation. Are these differences solely attributable to vascular architecture, or do they reflect selective vulnerabilities of specific neurovascular unit components or cerebrovascular segments? Resolving these questions will not only advance our understanding of NVC’s physiological role in brain function but also elucidate its pathological alterations in psychiatric disorders, ultimately clarifying the basis of regional and cellular variations in neurovascular interactions.
This review has systematically examined cerebrovascular impairments observed in both MDD patients and animal models. Key findings include structural alterations in the vascular network, BBB disruption, endothelial dysfunction, and neuroinflammatory processes- all of which contribute to NVC deficits. It is well known that depression represents a complex, chronic systemic disorder involving multiple organ systems and interconnected pathological pathways. A comprehensive understanding of its pathophysiology requires interdisciplinary collaboration between researchers and clinicians to elucidate the disorder’s multifactorial risk profile. Through a vascular-focused perspective, we have investigated how cerebrovascular dysfunction serves as a critical mediator of NVC impairment and contributes to MDD pathogenesis. These insights underscore the promising therapeutic potential of vascular-targeted interventions for psychiatric disorders, opening new avenues for treatment development.
Acknowledgements
Not applicable.
Abbreviations
- AMPK
AMP-activated protein kinase
- BBB
Blood–brain barrier
- BDNF
Brain-derived neurotrophic factor
- BKCa
Large conductance, calcium-activated potassium channel
- [Ca2+]i
Intracellular Ca2+ concentration
- CBF
Cerebral blood flow
- CBV
Cerebral blood volume
- ECs
Endothelial cells
- KATP
ATP-sensitive potassium channel
- Kir
Inward rectifier potassium channels
- MDD
Major depressive disorder
- NO
Nitric oxide
- NVC
Neurovascular coupling
- PA
Parenchymal arteriole
- PCs
Pericytes
- P2X
P2X purinoceptor
- sEPSCs
Spontaneous excitatory postsynaptic currents
- sGC
Soluble guanylyl cyclase
- SKCa
Small conductance, calcium-activated potassium channel
- SMCs
Smooth muscle cell
- IGF-1
Insulin-like growth factor-1
- IKCa
Intermediate conductance, calcium-activated potassium channel
- IP3R
Inositol triphosphate 3 receptor
- IP-TNTs
Interpericyte tunneling nanotubes
- TRPA1
Transient receptor potential A1
- TRPV4
Transient receptor potential vanilloid 4
- VEGF
Vascular endothelial growth factor
Author contributions
LY, YC conceived and designed project. XY, MZ, prepared the figures. AH, XH, FG, NW prepared the reference. XY, YZ, JZ, JW wrote the manuscript. XY, LY, YC, JY, YX, TW, MZ helped revise the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported, in part, The National Science Foundation of China (8227142405), The National Science Foundation of China (82374586), the National Comprehensive Traditional Chinese Medicine Reform Demonstration Zone Science and Technology Collaborative Development Project (GZY-KJS-SD-2024-046), The Shanghai institute of Traditional Chinese Medicine for mental health (SZB2024101), the Shandong Traditional Chinese Medicine Technology Development Project (NO. M-2022198), Shandong University of Traditional Chinese Medicine Students’ Innovation and Entrepreneurship Training Program Project (2024059).
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
With the submission of this manuscript, we would like to undertake that all authors of this paper have read and approved the final version submitted.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mester JR, Rozak MW, Dorr A, Goubran M, Sled JG, Stefanovic B. Network response of brain microvasculature to neuronal stimulation. Neuroimage. 2024;287: 120512. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. 2017;96(1):17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drew PJ. Neurovascular coupling: motive unknown. Trends Neurosci. 2022;45(11):809–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Segarra M, Aburto MR, Hefendehl J, Acker-Palmer A. Neurovascular interactions in the nervous system. Annu Rev Cell Dev Biol. 2019;35:615–35. [DOI] [PubMed] [Google Scholar]
- 5.De Miguel Z, Khoury N, Betley MJ, Lehallier B, Willoughby D, Olsson N, et al. Exercise plasma boosts memory and dampens brain inflammation via clusterin. Nature. 2021;600(7889):494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang AC, Stevens MY, Chen MB, Lee DP, Stähli D, Gate D, et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583(7816):425–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katifori E, Szöllosi GJ, Magnasco MO. Damage and fluctuations induce loops in optimal transport networks. Phys Rev Lett. 2010;104(4): 048704. [DOI] [PubMed] [Google Scholar]
- 8.Zhu M, Ackerman JJH, Sukstanskii AL, Yablonskiy DA. How the body controls brain temperature: the temperature shielding effect of cerebral blood flow. J Appl Physiol Bethesda Md 1985. 2006;101(5):1481–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayward JN, Baker MA. Role of cerebral arterial blood in the regulation of brain temperature in the monkey. Am J Physiol. 1968;215(2):389–403. [DOI] [PubMed] [Google Scholar]
- 10.Nedergaard M, Goldman SA. Glymphatic failure as a final common pathway to dementia. Science. 2020;370(6512):50–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fultz NE, Bonmassar G, Setsompop K, Stickgold RA, Rosen BR, Polimeni JR, et al. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366(6465):628–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Veluw SJ, Hou SS, Calvo-Rodriguez M, Arbel-Ornath M, Snyder AC, Frosch MP, et al. Vasomotion as a driving force for paravascular clearance in the awake mouse brain. Neuron. 2020;105(3):549-561.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dudek KA, Dion-Albert L, Lebel M, LeClair K, Labrecque S, Tuck E, et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc Natl Acad Sci U S A. 2020;117(6):3326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsel P, Roussos P, Pletnikov M, Haroutunian V. Microvascular anomaly conditions in psychiatric disease. Schizophrenia—angiogenesis connection. Neurosci Biobehav Rev. 2017;77:327–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hage Z, Madeira MM, Koliatsis D, Tsirka SE. Convergence of endothelial dysfunction, inflammation and glucocorticoid resistance in depression-related cardiovascular diseases. BMC Immunol. 2024;25(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanches ES, Simões D, Baptista FI, Silva AP. Neurovascular dysfunction in psychiatric disorders: underlying mechanisms and therapeutic approaches. Eur J Clin Invest. 2025;55(1): e14319. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Wang Y, Xue S, Gong L, Yan J, Zheng Y, et al. Chronic stress in adulthood results in microvascular dysfunction and subsequent depressive-like behavior. Sci Rep. 2024;14(1):24022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouellette J, Toussay X, Comin CH, da Costa LF, Ho M, Lacalle-Aurioles M, et al. Vascular contributions to 16p11.2 deletion autism syndrome modeled in mice. Nat Neurosci. 2020;23(9):1090–101. [DOI] [PubMed] [Google Scholar]
- 19.Carrier M, Guilbert J, Lévesque JP, Tremblay MÈ, Desjardins M. Structural and functional features of developing brain capillaries, and their alteration in schizophrenia. Front Cell Neurosci. 2020;14: 595002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenzel J, Hansen CE, Bettoni C, Vogt MA, Lembrich B, Natsagdorj R, et al. Impaired endothelium-mediated cerebrovascular reactivity promotes anxiety and respiration disorders in mice. Proc Natl Acad Sci U S A. 2020;117(3):1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uher R, Payne JL, Pavlova B, Perlis RH. Major depressive disorder in DSM-5: implications for clinical practice and research of changes from DSM-IV. Depress Anxiety. 2014;31(6):459–71. [DOI] [PubMed] [Google Scholar]
- 22.Schramm E, Klein DN, Elsaesser M, Furukawa TA, Domschke K. Review of dysthymia and persistent depressive disorder: history, correlates, and clinical implications. Lancet Psychiatry. 2020;7(9):801–12. [DOI] [PubMed] [Google Scholar]
- 23.Simon GE, Moise N, Mohr DC. Management of depression in adults: a review. JAMA. 2024;332(2):141–52. [DOI] [PubMed] [Google Scholar]
- 24.Yamada K. Vascular potassium channels in NVC. Prog Brain Res. 2016;225:63–73. [DOI] [PubMed] [Google Scholar]
- 25.Zhang D, Ruan J, Peng S, Li J, Hu X, Zhang Y, et al. Synaptic-like transmission between neural axons and arteriolar smooth muscle cells drives cerebral neurovascular coupling. Nat Neurosci. 2024;27(2):232–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koller A, Toth P. Contribution of flow-dependent vasomotor mechanisms to the autoregulation of cerebral blood flow. J Vasc Res. 2012;49(5):375–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogan-Cann AD, Lu P, Anderson CM. Endothelial NMDA receptors mediate activity-dependent brain hemodynamic responses in mice. Proc Natl Acad Sci U S A. 2019;116(21):10229–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107(8):3811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thakore P, Alvarado MG, Ali S, Mughal A, Pires PW, Yamasaki E, et al. Brain endothelial cell TRPA1 channels initiate neurovascular coupling. Elife. 2021;10: e63040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Longden TA, Dabertrand F, Koide M, Gonzales AL, Tykocki NR, Brayden JE, et al. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat Neurosci. 2017;20(5):717–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Korte N, Ilkan Z, Pearson CL, Pfeiffer T, Singhal P, Rock JR, et al. The Ca2+-gated channel TMEM16A amplifies capillary pericyte contraction and reduces cerebral blood flow after ischemia. J Clin Invest. 2022;132(9): e154118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Isaacs D, Xiang L, Hariharan A, Longden TA. KATP channel-dependent electrical signaling links capillary pericytes to arterioles during neurovascular coupling. Proc Natl Acad Sci U S A. 2024;121(50): e2405965121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mughal A, Hennig GW, Heppner T, Tsoukias NM, Hill-Eubanks D, Nelson MT. Electrocalcium coupling in brain capillaries: rapidly traveling electrical signals ignite local calcium signals. Proc Natl Acad Sci U S A. 2024;121(51): e2415047121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rungta RL, Chaigneau E, Osmanski BF, Charpak S. Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron. 2018;99(2):362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broggini T, Duckworth J, Ji X, Liu R, Xia X, Mächler P, et al. Long-wavelength traveling waves of vasomotion modulate the perfusion of cortex. Neuron. 2024;112(14):2349–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaub BM, Kasuba KC, Mace E, Strittmatter T, Laskowski PR, Geissler SA, et al. Neurons differentiate magnitude and location of mechanical stimuli. Proc Natl Acad Sci U S A. 2020;117(2):848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin L, He T, Chen S, Yang D, Yi W, Cao H, et al. Roles of mechanosensitive channel Piezo1/2 proteins in skeleton and other tissues. Bone Res. 2021;9(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jammal Salameh L, Bitzenhofer SH, Hanganu-Opatz IL, Dutschmann M, Egger V. Blood pressure pulsations modulate central neuronal activity via mechanosensitive ion channels. Science. 2024;383(6682):eadk8511. [DOI] [PubMed] [Google Scholar]
- 39.Kim KJ, Ramiro Diaz J, Iddings JA, Filosa JA. Vasculo-neuronal coupling: retrograde vascular communication to brain neurons. J Neurosci Off J Soc Neurosci. 2016;36(50):12624–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim KJ, Iddings JA, Stern JE, Blanco VM, Croom D, Kirov SA, et al. Astrocyte contributions to flow/pressure-evoked parenchymal arteriole vasoconstriction. J Neurosci Off J Soc Neurosci. 2015;35(21):8245–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoiland RL, Caldwell HG, Howe CA, Nowak-Flück D, Stacey BS, Bailey DM, et al. Nitric oxide is fundamental to neurovascular coupling in humans. J Physiol. 2020;598(21):4927–39: 8245–57. [DOI] [PubMed]
- 42.O’Gallagher K, Rosentreter RE, Elaine Soriano J, Roomi A, Saleem S, Lam T, et al. The effect of a neuronal nitric oxide synthase inhibitor on neurovascular regulation in humans. Circ Res. 2022;131(12):952–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seidel B, Stanarius A, Wolf G. Differential expression of neuronal and endothelial nitric oxide synthase in blood vessels of the rat brain. Neurosci Lett. 1997;239(2–3):109–12. [DOI] [PubMed] [Google Scholar]
- 44.Stanarius A, Töpel I, Schulz S, Noack H, Wolf G. Immunocytochemistry of endothelial nitric oxide synthase in the rat brain: a light and electron microscopical study using the tyramide signal amplification technique. Acta Histochem. 1997;99(4):411–29. [DOI] [PubMed] [Google Scholar]
- 45.Scarpellino G, Brunetti V, Berra-Romani R, De Sarro G, Guerra G, et al. The unexpected role of the endothelial nitric oxide synthase at the neurovascular unit: beyond the regulation of cerebral blood flow. Int J Mol Sci. 2024;25(16):9071. [DOI] [PMC free article] [PubMed]
- 46.Haul S, Gödecke A, Schrader J, Haas HL, Luhmann HJ. Impairment of neocortical long-term potentiation in mice deficient of endothelial nitric oxide synthase. J Neurophysiol. 1999;81(2):494–7. [DOI] [PubMed] [Google Scholar]
- 47.Doreulee N, Sergeeva OA, Yanovsky Y, Chepkova AN, Selbach O, Gödecke A, et al. Cortico-striatal synaptic plasticity in endothelial nitric oxide synthase deficient mice. Brain Res. 2003;964(1):159–63. [DOI] [PubMed] [Google Scholar]
- 48.Garthwaite G, Bartus K, Malcolm D, Goodwin D, Kollb-Sielecka M, Dooldeniya C, et al. Signaling from blood vessels to CNS axons through nitric oxide. J Neurosci Off J Soc Neurosci. 2006;26(29):7730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci Off J Soc Neurosci. 2006;26(45):11513–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen J, Zacharek A, Zhang C, Jiang H, Li Y, Roberts C, et al. Endothelial nitric oxide synthase regulates brain-derived neurotrophic factor expression and neurogenesis after stroke in mice. J Neurosci Off J Soc Neurosci. 2005;25(9):2366–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S, Kumar TP, Joshee S, Kirschstein T, Subburaju S, Khalili JS, et al. Endothelial cell-derived GABA signaling modulates neuronal migration and postnatal behavior. Cell Res. 2018;28(2):221–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aoki T, Nishimura M, Kataoka H, Ishibashi R, Nozaki K, Miyamoto S. Complementary inhibition of cerebral aneurysm formation by eNOS and nNOS. Lab Investig J Tech Methods Pathol. 2011;91(4):619–26. [DOI] [PubMed] [Google Scholar]
- 53.Catenaccio E, Mu W, Lipton ML. Estrogen- and progesterone-mediated structural neuroplasticity in women: evidence from neuroimaging. Brain Struct Funct. 2016;221(8):3845–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reichlin S. Neuroendocrinology. N Engl J Med. 1963;269:1246–50. [DOI] [PubMed] [Google Scholar]
- 55.McEwen BS, Milner TA. Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res. 2017;95(1–2):24–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yao D, Li R, Hao J, Huang H, Wang X, Ran L, et al. Melatonin alleviates depression-like behaviors and cognitive dysfunction in mice by regulating the circadian rhythm of AQP4 polarization. Transl Psychiatry. 2023;13(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McEwen BS. Hormones and behavior and the integration of brain-body science. Horm Behav. 2020;119: 104619. [DOI] [PubMed] [Google Scholar]
- 58.Ajoolabady A, Pratico D, Ren J. Angiotensin II: role in oxidative stress, endothelial dysfunction, and diseases. Mol Cell Endocrinol. 2024;592: 112309. [DOI] [PubMed] [Google Scholar]
- 59.Nguyen Cat A, Montezano AC, Burger D, Touyz RM. Angiotensin II, NADPH oxidase, and redox signaling in the vasculature. Antioxid Redox Signal. 2013;19(10):1110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bloch S, Obari D, Girouard H. Angiotensin and neurovascular coupling: beyond hypertension. Microcirc N Y N 1994. 2015;22(3):159–67. [DOI] [PubMed] [Google Scholar]
- 61.Jang JH, Chun JN, Godo S, Wu G, Shimokawa H, Jin CZ, et al. ROS and endothelial nitric oxide synthase (eNOS)-dependent trafficking of angiotensin II type 2 receptor begets neuronal NOS in cardiac myocytes. Basic Res Cardiol. 2015;110(3):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toth L, Czigler A, Hegedus E, Komaromy H, Amrein K, Czeiter E, et al. Age-related decline in circulating IGF-1 associates with impaired neurovascular coupling responses in older adults. GeroScience. 2022;44(6):2771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarantini S, Nyúl-Tóth Á, Yabluchanskiy A, Csipo T, Mukli P, Balasubramanian P, et al. Endothelial deficiency of insulin-like growth factor-1 receptor (IGF1R) impairs neurovascular coupling responses in mice, mimicking aspects of the brain aging phenotype. GeroScience. 2021;43(5):2387–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toth P, Tarantini S, Ashpole NM, Tucsek Z, Milne GL, Valcarcel-Ares NM, et al. IGF-1 deficiency impairs neurovascular coupling in mice: implications for cerebromicrovascular aging. Aging Cell. 2015;14(6):1034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang J, Tang Y, Zhang W, Zhao H, Wang R, Yan Y, et al. Insulin-like growth factor-1 secreted by brain microvascular endothelial cells attenuates neuron injury upon ischemia. FEBS J. 2013;280(15):3658–68. [DOI] [PubMed] [Google Scholar]
- 66.Toth P, Tucsek Z, Tarantini S, Sosnowska D, Gautam T, Mitschelen M, et al. IGF-1 deficiency impairs cerebral myogenic autoregulation in hypertensive mice. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2014;34(12):1887–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson JA, Johnson DA, Kraft AD, Calkins MJ, Jakel RJ, Vargas MR, et al. The Nrf2-ARE pathway: an indicator and modulator of oxidative stress in neurodegeneration. Ann N Y Acad Sci. 2008;1147:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lopert P, Patel M. Nicotinamide nucleotide transhydrogenase (Nnt) links the substrate requirement in brain mitochondria for hydrogen peroxide removal to the thioredoxin/peroxiredoxin (Trx/Prx) system. J Biol Chem. 2014;289(22):15611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ma Z, Ma Y, Cao X, Zhang Y, Song T. Avenanthramide-C activates Nrf2/ARE pathway and inhibiting ferroptosis pathway to improve cognitive dysfunction in aging rats. Neurochem Res. 2023;48(2):393–403. [DOI] [PubMed] [Google Scholar]
- 70.Ramprasath T, Freddy AJ, Velmurugan G, Tomar D, Rekha B, Suvekbala V, et al. Context-dependent regulation of Nrf2/ARE axis on vascular cell function during hyperglycemic condition. Curr Diabetes Rev. 2020;16(8):797–806. [DOI] [PubMed] [Google Scholar]
- 71.Krylova SV, Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. 2023;24(2):1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;20(88):487–514. [DOI] [PubMed] [Google Scholar]
- 73.Balijepalli RC, Kamp TJ. Caveolae, ion channels and cardiac arrhythmias. Prog Biophys Mol Biol. 2008;98(2–3):149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sowa G, Pypaert M, Sessa WC. Distinction between signaling mechanisms in lipid rafts vs. caveolae. Proc Natl Acad Sci U S A. 2001;98(24):14072–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sotodosos-Alonso L, Pulgarín-Alfaro M, Del Pozo MA. Caveolae mechanotransduction at the interface between cytoskeleton and extracellular matrix. Cells. 2023;12(6):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Parton RG, Kozlov MM, Ariotti N. Caveolae and lipid sorting: shaping the cellular response to stress. J Cell Biol. 2020;219(4): e201905071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parton RG, Tillu VA, Collins BM. Caveolae. Curr Biol CB. 2018;28(8):R402–5. [DOI] [PubMed] [Google Scholar]
- 78.Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94(3):581-594.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chow BW, Gu C. Gradual suppression of transcytosis governs functional blood-retinal barrier formation. Neuron. 2017;93(6):1325-1333.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chow BW, Nuñez V, Kaplan L, Granger AJ, Bistrong K, Zucker HL, et al. Caveolae in CNS arterioles mediate neurovascular coupling. Nature. 2020;579(7797):106–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Harris SS, Boorman LW, Das D, Kennerley AJ, Sharp PS, Martin C, et al. Physiological and pathological brain activation in the anesthetized rat produces hemodynamic-dependent cortical temperature increases that can confound the BOLD fMRI signal. Front Neurosci. 2018;12:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willford DC, Hill EP, Moores WY. Theoretical analysis of oxygen transport during hypothermia. J Clin Monit. 1986;2(1):30–43. [DOI] [PubMed] [Google Scholar]
- 84.Kiyatkin EA, Sharma HS. Permeability of the blood-brain barrier depends on brain temperature. Neuroscience. 2009;161(3):926–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Boorman LW, Harris SS, Shabir O, Lee L, Eyre B, Howarth C, et al. Bidirectional alterations in brain temperature profoundly modulate spatiotemporal neurovascular responses in-vivo. Commun Biol. 2023;6(1):185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yablonskiy DA, Ackerman JJ, Raichle ME. Coupling between changes in human brain temperature and oxidative metabolism during prolonged visual stimulation. Proc Natl Acad Sci U S A. 2000;97(13):7603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shibasaki K. Regulation of neural functions by brain temperature and thermo-TRP channels. Adv Exp Med Biol. 2024;1461:199–211. [DOI] [PubMed] [Google Scholar]
- 88.Hoshi Y, Okabe K, Shibasaki K, Funatsu T, Matsuki N, Ikegaya Y, et al. Ischemic brain injury leads to brain edema via hyperthermia-induced TRPV4 activation. J Neurosci Off J Soc Neurosci. 2018;38(25):5700–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Hook MJ. Temperature effects on synaptic transmission and neuronal function in the visual thalamus. PLoS ONE. 2020;15(4): e0232451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Q, Fu X, Xu J, Dong S, Liu C, Cheng D, et al. Hypothalamic warm-sensitive neurons require TRPC4 channel for detecting internal warmth and regulating body temperature in mice. Neuron. 2023;111(3):387-404.e8. [DOI] [PubMed] [Google Scholar]
- 91.Bravi B, Verga C, Palladini M, Poletti S, Buticchi C, Stefania S, et al. Effects of kynurenine pathway metabolites on choroid plexus volume, hemodynamic response, and spontaneous neural activity: a new mechanism for disrupted neurovascular communication and impaired cognition in mood disorders. Brain Behav Immun. 2025;125:414–27. [DOI] [PubMed] [Google Scholar]
- 92.Ishizaki J, Yamamoto H, Takahashi T, Takeda M, Yano M, Mimura M. Changes in regional cerebral blood flow following antidepressant treatment in late-life depression. Int J Geriatr Psychiatry. 2008;23(8):805–11. [DOI] [PubMed] [Google Scholar]
- 93.Navarro V, Gastó C, Lomeña F, Mateos JJ, Marcos T. Frontal cerebral perfusion dysfunction in elderly late-onset major depression assessed by 99MTC-HMPAO SPECT. Neuroimage. 2001;14(1 Pt 1):202–5. [DOI] [PubMed] [Google Scholar]
- 94.Nagafusa Y, Okamoto N, Sakamoto K, Yamashita F, Kawaguchi A, Higuchi T, et al. Assessment of cerebral blood flow findings using 99mTc-ECD single-photon emission computed tomography in patients diagnosed with major depressive disorder. J Affect Disord. 2012;140(3):296–9. [DOI] [PubMed] [Google Scholar]
- 95.Massardo T, Quintana JC, Jaimovich R, Sáez CG, Risco L, Liberman C, et al. Regional brain perfusion is associated with endothelial dysfunction markers in major depressive disorder. Neuropsychobiology. 2021;80(3):214–24. [DOI] [PubMed] [Google Scholar]
- 96.Fan D, He C, Liu X, Zang F, Zhu Y, Zhang H, et al. Altered resting-state cerebral blood flow and functional connectivity mediate suicidal ideation in major depressive disorder. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2022;42(9):1603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gao Q, Zou K, He Z, Sun X, Chen H. Causal connectivity alterations of cortical-subcortical circuit anchored on reduced hemodynamic response brain regions in first-episode drug-naïve major depressive disorder. Sci Rep. 2016;6:21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kaichi Y, Okada G, Takamura M, Toki S, Akiyama Y, Higaki T, et al. Changes in the regional cerebral blood flow detected by arterial spin labeling after 6-week escitalopram treatment for major depressive disorder. J Affect Disord. 2016;194:135–43. [DOI] [PubMed] [Google Scholar]
- 99.Harris JJ, Reynell C. How do antidepressants influence the BOLD signal in the developing brain? Dev Cogn Neurosci. 2017;25:45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Smith R, Allen JJB, Thayer JF, Fort C, Lane RD. Increased association over time between regional frontal lobe BOLD change magnitude and cardiac vagal control with sertraline treatment for major depression. Psychiatry Res. 2014;224(3):225–33. [DOI] [PubMed] [Google Scholar]
- 101.Appaji A, Nagendra B, Chako DM, Padmanabha A, Hiremath CV, Jacob A, et al. Retinal vascular fractal dimension in bipolar disorder and schizophrenia. J Affect Disord. 2019;259:98–103. [DOI] [PubMed] [Google Scholar]
- 102.Meier MH, Gillespie NA, Hansell NK, Hewitt AW, Hickie IB, Lu Y, et al. Associations between depression and anxiety symptoms and retinal vessel caliber in adolescents and young adults. Psychosom Med. 2014;76(9):732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Liu L, Li C, Yang Y, He X, Jiang M, et al. Choroidal vessel density in major depressive disorder using swept-source optical coherence tomography angiography. J Affect Disord. 2024;344:79–85. [DOI] [PubMed] [Google Scholar]
- 104.Miguel-Hidalgo JJ, Jiang W, Konick L, Overholser JC, Jurjus GJ, Stockmeier CA, et al. Morphometric analysis of vascular pathology in the orbitofrontal cortex of older subjects with major depression. Int J Geriatr Psychiatry. 2013;28(9):959–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Almeida OP, Ford AH, Flicker L, Hankey GJ, Yeap BB, Clancy P, et al. Angiogenesis inhibition and depression in older men. J Psychiatry Neurosci JPN. 2014;39(3):200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rubinow MJ, Mahajan G, May W, Overholser JC, Jurjus GJ, Dieter L, et al. Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct. 2016;221(1):171–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dion-Albert L, Cadoret A, Doney E, Kaufmann FN, Dudek KA, Daigle B, et al. Vascular and blood-brain barrier-related changes underlie stress responses and resilience in female mice and depression in human tissue. Nat Commun. 2022;13(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shang B, Wang T, Zhao S, Yi S, Zhang T, Yang Y, et al. Higher Blood-brain barrier permeability in patients with major depressive disorder identified by DCE-MRI imaging. Psychiatry Res Neuroimaging. 2024;337: 111761. [DOI] [PubMed] [Google Scholar]
- 109.Halaris A. Inflammation-associated co-morbidity between depression and cardiovascular disease. Curr Top Behav Neurosci. 2017;31:45–70. [DOI] [PubMed] [Google Scholar]
- 110.Dalby RB, Eskildsen SF, Videbech P, Rosenberg R, Østergaard L. Cerebral hemodynamics and capillary dysfunction in late-onset major depressive disorder. Psychiatry Res Neuroimaging. 2021;317: 111383. [DOI] [PubMed] [Google Scholar]
- 111.Smith PJ, Blumenthal JA, Hinderliter AL, Watkins LL, Hoffman BM, Sherwood A. Microvascular endothelial function and neurocognition among adults with major depressive disorder. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry. 2018;26(10):1061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Greaney JL, Koffer RE, Saunders EFH, Almeida DM, Alexander LM. Self-reported everyday psychosocial stressors are associated with greater impairments in endothelial function in young adults with major depressive disorder. J Am Heart Assoc. 2019;8(4): e010825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Greaney JL, Saunders EFH, Santhanam L, Alexander LM. Oxidative stress contributes to microvascular endothelial dysfunction in men and women with major depressive disorder. Circ Res. 2019;124(4):564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Greaney JL, Saunders EFH, Alexander LM. Short-term salicylate treatment improves microvascular endothelium-dependent dilation in young adults with major depressive disorder. Am J Physiol Heart Circ Physiol. 2022;322(5):H880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Şahan E, Güler EM, Tangılntız A, Kırpınar İ. Endocan: a novel biomarker of endothelial dysfunction in depression? J Psychiatr Res. 2023;165:219–24. [DOI] [PubMed] [Google Scholar]
- 116.Shimo Y, Cathomas F, Lin HY, Chan KL, Parise LF, Li L, et al. Social stress induces autoimmune responses against the brain. Proc Natl Acad Sci U S A. 2023;120(49): e2305778120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sapsford TP, Johnson SR, Headrick JP, Branjerdporn G, Adhikary S, Sarfaraz M, et al. Forgetful, sad and old: do vascular cognitive impairment and depression share a common pre-disease network and how is it impacted by ageing? J Psychiatr Res. 2022;156:611–27. [DOI] [PubMed] [Google Scholar]
- 118.Annam J, Galfalvy HC, Keilp JG, Simpson N, Huang YY, Nandakumar R, et al. Plasma cytokine and growth factor response to acute psychosocial stress in major depressive disorder. J Psychiatr Res. 2024;169:224–30. [DOI] [PubMed] [Google Scholar]
- 119.Dattilo V, Amato R, Perrotti N, Gennarelli M. The emerging role of SGK1 (serum- and glucocorticoid-regulated kinase 1) in major depressive disorder: hypothesis and mechanisms. Front Genet. 2020;11:826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tonhajzerova I, Sekaninova N, Bona Olexova L, Visnovcova Z. Novel insight into neuroimmune regulatory mechanisms and biomarkers linking major depression and vascular diseases: the dilemma continues. Int J Mol Sci. 2020;21(7):2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pfau SJ, Langen UH, Fisher TM, Prakash I, Nagpurwala F, Lozoya RA, et al. Characteristics of blood-brain barrier heterogeneity between brain regions revealed by profiling vascular and perivascular cells. Nat Neurosci. 2024;27(10):1892–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wu YT, Bennett HC, Chon U, Vanselow DJ, Zhang Q, Muñoz-Castañeda R, et al. Quantitative relationship between cerebrovascular network and neuronal cell types in mice. Cell Rep. 2022;39(12): 110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shaw K, Bell L, Boyd K, Grijseels DM, Clarke D, Bonnar O, et al. Neurovascular coupling and oxygenation are decreased in hippocampus compared to neocortex because of microvascular differences. Nat Commun. 2021;12(1):3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Samuels JD, Lotstein ML, Lehmann ML, Elkahloun AG, Banerjee S, Herkenham M. Chronic social defeat alters brain vascular-associated cell gene expression patterns leading to vascular dysfunction and immune system activation. J Neuroinflamm. 2023;20(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, et al. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72(7):562–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rotheneichner P, Lange S, O’Sullivan A, Marschallinger J, Zaunmair P, Geretsegger C, et al. Hippocampal neurogenesis and antidepressive therapy: shocking relations. Neural Plast. 2014;2014: 723915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jansson L, Hellsten J, Tingström A. Region specific hypothalamic neuronal activation and endothelial cell proliferation in response to electroconvulsive seizures. Biol Psychiatry. 2006;60(8):874–81. [DOI] [PubMed] [Google Scholar]
- 128.Fournier NM, Duman RS. Role of vascular endothelial growth factor in adult hippocampal neurogenesis: implications for the pathophysiology and treatment of depression. Behav Brain Res. 2012;227(2):440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang S, Lu Y, Shi W, Ren Y, Xiao K, Chen W, et al. SIRT1/FOXO1 axis-mediated hippocampal angiogenesis is involved in the antidepressant effect of Chaihu Shugan San. Drug Des Devel Ther. 2022;16:2783–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Matsuno H, Tsuchimine S, O’Hashi K, Sakai K, Hattori K, Hidese S, et al. Association between vascular endothelial growth factor-mediated blood-brain barrier dysfunction and stress-induced depression. Mol Psychiatry. 2022;27(9):3822–32. [DOI] [PubMed] [Google Scholar]
- 131.Sun ZW, Wang X, Zhao Y, Sun ZX, Wu YH, Hu H, et al. Blood-brain barrier dysfunction mediated by the EZH2-Claudin-5 axis drives stress-induced TNF-α infiltration and depression-like behaviors. Brain Behav Immun. 2024;115:143–56. [DOI] [PubMed] [Google Scholar]
- 132.Dudek KA, Paton SEJ, Binder LB, Collignon A, Dion-Albert L, Cadoret A, et al. Astrocytic cannabinoid receptor 1 promotes resilience by dampening stress-induced blood-brain barrier alterations. Nat Neurosci. 2025;28(4):766–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A. 2014;111(20):7462–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pereira GC, Piton E, Bornholdt J, Dos Santos BM, de Almeida AS, Dalenogare DP, et al. TRPA1 participation in behavioral impairment induced by chronic corticosterone administration. Psychopharmacology. 2023;240(1):157–69. [DOI] [PubMed] [Google Scholar]
- 135.Hardie DG. AMPK and Raptor: matching cell growth to energy supply. Mol Cell. 2008;30(3):263–5. [DOI] [PubMed] [Google Scholar]
- 136.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13(4):251–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhu S, Wang J, Zhang Y, Li V, Kong J, He J, et al. Unpredictable chronic mild stress induces anxiety and depression-like behaviors and inactivates AMP-activated protein kinase in mice. Brain Res. 2014;1576:81–90. [DOI] [PubMed] [Google Scholar]
- 138.Østergaard L, Jørgensen MB, Knudsen GM. Low on energy? An energy supply-demand perspective on stress and depression. Neurosci Biobehav Rev. 2018;94:248–70. [DOI] [PubMed] [Google Scholar]
- 139.Chen YH, Lin S, Jin SY, Gao TM. Extracellular ATP is a homeostatic messenger that mediates cell-cell communication in physiological processes and psychiatric diseases. Biol Psychiatry. 2025;97(1):41–53. [DOI] [PubMed] [Google Scholar]
- 140.Ribeiro DE, Roncalho AL, Glaser T, Ulrich H, Wegener G, Joca S. P2X7 receptor signaling in stress and depression. Int J Mol Sci. 2019;20(11):2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Inan M, Zhao M, Manuszak M, Karakaya C, Rajadhyaksha AM, Pickel VM, et al. Energy deficit in parvalbumin neurons leads to circuit dysfunction, impaired sensory gating and social disability. Neurobiol Dis. 2016;93:35–46. [DOI] [PubMed] [Google Scholar]
- 142.Almeida A, Jimenez-Blasco D, Bolaños JP. Cross-talk between energy and redox metabolism in astrocyte-neuron functional cooperation. Essays Biochem. 2023;67(1):17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Whittaker RG, Turnbull DM, Whittington MA, Cunningham MO. Impaired mitochondrial function abolishes gamma oscillations in the hippocampus through an effect on fast-spiking interneurons. Brain J Neurol. 2011;134(Pt 7):e180; author reply e181. [DOI] [PMC free article] [PubMed]
- 144.Wei Y, Miao Q, Zhang Q, Mao S, Li M, Xu X, et al. Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage. Nat Neurosci. 2023;26(12):2081–9. [DOI] [PubMed] [Google Scholar]
- 145.Boynton GM. Spikes, BOLD, attention, and awareness: a comparison of electrophysiological and fMRI signals in V1. J Vis. 2011;11(5):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shih YYI, Wey HY, De La Garza BH, Duong TQ. Striatal and cortical BOLD, blood flow, blood volume, oxygen consumption, and glucose consumption changes in noxious forepaw electrical stimulation. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2011;31(3):832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Negri S, Faris P, Soda T, Moccia F. Endothelial signaling at the core of neurovascular coupling: the emerging role of endothelial inward-rectifier K+ (Kir2.1) channels and N-methyl-d-aspartate receptors in the regulation of cerebral blood flow. Int J Biochem Cell Biol. 2021;135: 105983. [DOI] [PubMed] [Google Scholar]
- 148.Harraz OF, Longden TA, Hill-Eubanks D, Nelson MT. PIP2 depletion promotes TRPV4 channel activity in mouse brain capillary endothelial cells. Elife. 2018;7: e38689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Moccia F, Negri S, Faris P, Angelone T. Targeting endothelial ion signalling to rescue cerebral blood flow in cerebral disorders. Vascul Pharmacol. 2022;145: 106997. [DOI] [PubMed] [Google Scholar]
- 150.Hannah RM, Dunn KM, Bonev AD, Nelson MT. Endothelial SK(Ca) and IK(Ca) channels regulate brain parenchymal arteriolar diameter and cortical cerebral blood flow. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2011;31(5):1175–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.von Beckerath N, Nees S, Neumann FJ, Krebs B, Juchem G, Schömig A. An inward rectifier and a voltage-dependent K+ current in single, cultured pericytes from bovine heart. Cardiovasc Res. 2000;46(3):569–78. [DOI] [PubMed] [Google Scholar]
- 152.Quignard JF, Harley EA, Duhault J, Vanhoutte PM, Félétou M. K+ channels in cultured bovine retinal pericytes: effects of beta-adrenergic stimulation. J Cardiovasc Pharmacol. 2003;42(3):379–88. [DOI] [PubMed] [Google Scholar]
- 153.Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–403. [DOI] [PubMed] [Google Scholar]
- 154.MacVicar BA, Newman EA. Astrocyte regulation of blood flow in the brain. Cold Spring Harb Perspect Biol. 2015;7(5): a020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Filosa JA, Iddings JA. Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol. 2013;305(5):H609-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Billaud M, Lohman AW, Johnstone SR, Biwer LA, Mutchler S, Isakson BE. Regulation of cellular communication by signaling microdomains in the blood vessel wall. Pharmacol Rev. 2014;66(2):513–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Burnette JO, White RE. PGI2 opens potassium channels in retinal pericytes by cyclic AMP-stimulated, cross-activation of PKG. Exp Eye Res. 2006;83(6):1359–65. [DOI] [PubMed] [Google Scholar]
- 158.Sugiyama T, Kawamura H, Yamanishi S, Kobayashi M, Katsumura K, Puro DG. Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am J Physiol Cell Physiol. 2005;288(3):C568-576. [DOI] [PubMed] [Google Scholar]
- 159.Kawamura H, Sugiyama T, Wu DM, Kobayashi M, Yamanishi S, Katsumura K, et al. ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J Physiol. 2003;551(Pt 3):787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirc N Y N 1994. 2015;22(3):183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R546-552. [DOI] [PubMed] [Google Scholar]
- 162.Horiuchi T, Dietrich HH, Hongo K, Dacey RG. Mechanism of extracellular K+-induced local and conducted responses in cerebral penetrating arterioles. Stroke. 2002;33(11):2692–9. [DOI] [PubMed] [Google Scholar]
- 163.Ando K, Tong L, Peng D, Vázquez-Liébanas E, Chiyoda H, He L, et al. KCNJ8/ABCC9-containing K-ATP channel modulates brain vascular smooth muscle development and neurovascular coupling. Dev Cell. 2022;57(11):1383-1399.e7. [DOI] [PubMed] [Google Scholar]
- 164.Toth P, Tarantini S, Davila A, Valcarcel-Ares MN, Tucsek Z, Varamini B, et al. Purinergic glio-endothelial coupling during neuronal activity: role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am J Physiol Heart Circ Physiol. 2015;309(11):H1837-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Austin SA, Santhanam AV, Hinton DJ, Choi DS, Katusic ZS. Endothelial nitric oxide deficiency promotes Alzheimer’s disease pathology. J Neurochem. 2013;127(5):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chen X, Chen L, Lin G, Wang Z, Kodali MC, Li M, et al. White matter damage as a consequence of vascular dysfunction in a spontaneous mouse model of chronic mild chronic hypoperfusion with eNOS deficiency. Mol Psychiatry. 2022;27(11):4754–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Li ST, Pan J, Hua XM, Liu H, Shen S, Liu JF, et al. Endothelial nitric oxide synthase protects neurons against ischemic injury through regulation of brain-derived neurotrophic factor expression. CNS Neurosci Ther. 2014;20(2):154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ando A, Yang A, Mori K, Yamada H, Yamada E, Takahashi K, et al. Nitric oxide is proangiogenic in the retina and choroid. J Cell Physiol. 2002;191(1):116–24. [DOI] [PubMed] [Google Scholar]
- 169.Ando A, Yang A, Nambu H, Campochiaro PA. Blockade of nitric-oxide synthase reduces choroidal neovascularization. Mol Pharmacol. 2002;62(3):539–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.