Abstract
In recent years, new treatments have become available which have improved survival rates in lung cancer patients. One promising treatment option is the rapidly growing field of oral targeted therapies, which employs drugs that interfere with specific molecules involved in the growth, progression, and spread of cancer. However, these therapies can cause a variety of symptoms and adverse events that can impair quality of life. mHealth technologies may help individuals with lung cancer better track their side effects and manage medications on a day-to-day basis. However, understanding patients’ attitudes toward smart devices such as smartphones, smartwatches, and smart pill bottles, as well as their specific needs when using these devices, is critical before design and deployment studies of medication adherence can be carried out. In this study, we conducted interviews with 9 individuals with stage III-IV lung cancer at a National Cancer Institute-designated comprehensive cancer center in the Mid-Atlantic region of the United States to assess the feasibility of using such devices for managing medication and medication related side-effects. We evaluated patients’ attitudes towards the design and function of smart devices and how these devices fit into their daily life. Our results may help clinicians and researchers to co-develop effective mHealth system deployments for side effect and medication management in oncology populations.
Keywords: mHealth, smartphone, medication adherence, side effect, cancer
1. Introduction
Lung cancer is the second most common type of cancer and the leading cause of cancer death worldwide, with over 2 million cases newly diagnosed each year [14]. The significant impact of lung cancer on the global population has led to the development of new targeted oral anticancer medications, which patients tend to prefer for their convenience over intravenous chemotherapy [6, 26]. While promising for survival outcomes, these new therapies are commonly associated with adverse events (AEs) such as rashes or edema. AEs can lead to worsening symptoms, dose reductions, and even medication discontinuation if left undetected or untreated [4]. Researchers and clinicians are increasingly seeking more accurate ways to track medication-taking, monitor side effects, and detect possible AEs among patients taking oral anti-cancer medications at home, such as individuals with lung cancer.
Devices, such as smartphones, wearable sensors (e.g., smartwatches) and medication event monitoring systems (MEMS), enable direct, unobtrusive collection of clinically relevant behaviors in-situ. Mobile health (mHealth) and human-computer interaction (HCI) studies have shown that these “smart” devices are less prone to errors than traditional self-reports [2] and have established the usefulness of smart devices for medication and symptom tracking in daily life [3,19]. At the intersection of HCI, mhealth, and oncology, smart devices have been shown to encourage medication adherence to oral chemotherapy [15], help patients feel more in control and informed about their care [11, 20, 21], and help clinicians feel better able to monitor patients’ symptoms and tailor treatment accordingly [21]. However, the use of smart technologies for medication and symptom tracking in practice is not without its challenges. A 2017 study of medication adherence technologies such as smartphone apps among older adults showed that adherence was impacted both by participants’ schedules and the symptoms they experienced [19]. Further, a study of medication tracking among patients with atrial fibrillation uncovered issues such as the inability of smart pill bottles to integrate into patients’ existing routines [25]. These challenges highlight the importance of further investigation into patients’ perceptions of smart device use during treatment.
Despite the promise of smart devices for symptom and medication management, the majority of studies in mHealth and oncology have focused on physical activity tracking for breast cancer patients [13, 16]. Comparatively few studies to date have focused medication and symptom tracking. Perhaps even fewer have focused exclusively on lung cancer patients, with a handful of notable exceptions. LuCApp is a mobile application for patients with lung cancer that sends automated reminders to complete symptom logs as well as questionnaires related to quality of life and support needs [5]. A proposed randomized controlled trial for the app will examine the impact of side-effect tracking on quality of life. A randomized controlled trial has also been proposed for SYMPRO-Lung, a web application for lung cancer patients that leverages patient-reported outcomes (PROs) for symptom monitoring [1]. We draw inspiration from these and other works as we address the following research question (RQ): What attitudes do patients with lung cancer have toward smart device use for managing their medications and tracking their symptoms? In this paper, we present the results of a cross-sectional, qualitative study in which we conducted semi-structured usability interviews with 9 individuals with stage III-IV lung cancer receiving treatment at a large university cancer center in the Mid-Atlantic region of the United States. Our results give insight into patients’ preferences and priorities regarding the use of smart devices as part of their self-management routines during cancer treatment.
2. Methods
This study was approved by the Institutional Review Board for Health Sciences Research (IRB-HSR) at our university, and the study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice standards. Patients provided written informed consent prior to enrollment and participation.
2.1. Recruitment
Using purposive sampling, we recruited patients at a National Cancer Institute (NCI)-designated comprehensive cancer center in the Southeastern United States. All patients were 18 years of age or older and were being treated for advanced stage non-small cell lung cancer (NSCLC) with EGFR mutations or ALK gene rearrangements and were receiving oral targeted therapies (tyrosine kinase inhibitors [TKIs]) as part of their treatment. Patients were first identified for inclusion by the sixth and seventh authors, who are practicing oncologists. The first author attempted to contact prospective participants both in clinic and via telephone calls, and provided interested individuals with a secure, electronic consent form to sign. We approached 40 patients in total, 23 of whom either explicitly declined prescreening or were unreachable after one or more attempts to contact them. 17 patients agreed to prescreening, and 11 ultimately consented to participate in the study. Two participants did not respond to study coordinators’ efforts to schedule the study interviews after consenting, bringing the final number of participants to 9.
2.2. Data Collection
Using a standardized interview guide, we conducted semi-structured interviews with 9 participants between September 2020 and July 2021. Out of an abundance of caution during the COVID-19 pandemic, interviews were conducted by one interviewer remotely via a HIPPA-compliant version of Webex1 using a standardized semi-structured interview guide. We administered a secure, online demographics survey via Qualtrics at the end of each interview. The first interview (with P1) focused on smartphone use and an interactive demonstration (demo) of a smartphone app emulator. P1’s interview informed the design of subsequent interviews, which included an interactive demo of a smartwatch app emulator and a researcher-guided demo of a smart pill bottle cap in addition to the original smartphone app demo. In this section, we describe our process for each device demo in detail.
Smartphone.
We created a high-fidelity prototype of Sensus [27], a smartphone application that gathers passively sensed indicators of human health and behavior (e.g., location, heart rate, and skin temperature). In deployment studies, Sensus can also be used to gather real-time participant feedback using ecological momentary assessment (EMA), a method of gathering data in which participants are polled in real time in order to avoid recall bias [22]. EMAs are commonly delivered via digital methods such as text messages or push notifications from mobile apps. We created a Sensus study protype with EMA surveys about participants’ quality of life (e.g., sleep, symptoms, and side effects) and day-to-day activities (e.g., location and socialization). We then loaded the Sensus protocol via Appetize.io2, an app demo platform for the web; the prototype is shown in Fig. 1. During the interview, we used screen sharing to show participants how to scroll and select questions within the prototype; this was necessary, as using a mouse for these tasks is very different from swiping and tapping with one’s finger on a real smartphone. We then asked participants to use the prototype via screen sharing to practice answering the survey questions. Finally, we asked participants 15 follow-up questions that covered their perceptions about the experience of filling out surveys on a smartphone, the relevance of the smartphone to their current medication management routine, and their willingness to use Sensus on a smartphone for a long period of time.
Fig. 1.
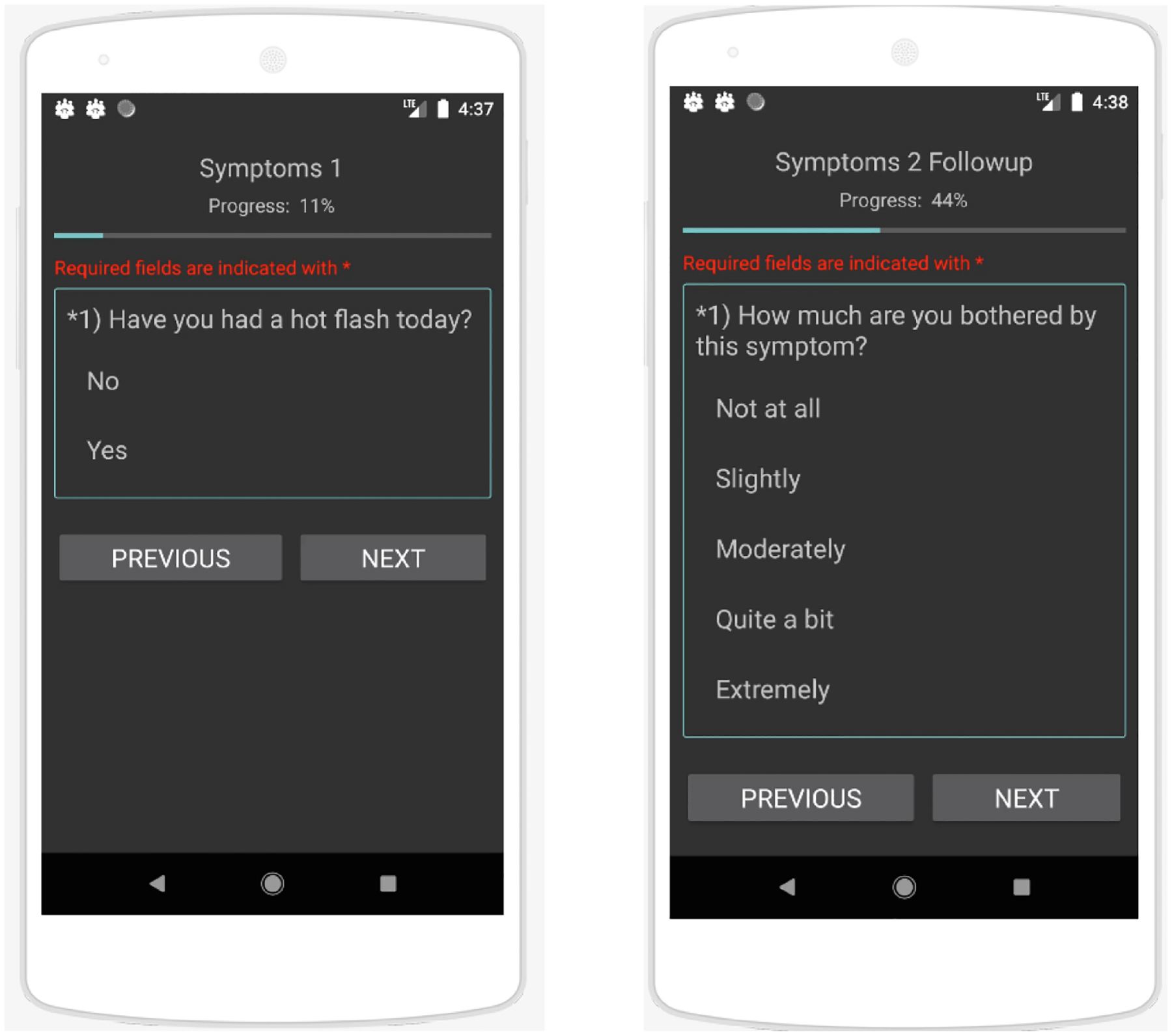
Sensus prototype showing EMA survey about symptoms.
Smartwatch.
We used a web-based prototype of a smartwatch application (shown in Fig. 2) that was preloaded with quality-of-life questions, as well as questions regarding activities (e.g., “Are you exercising right now?”). We explained to participants how the smartphone could be used to send EMAs and notifications to the smartwatch. We then asked participants to use the smartwatch app prototype via screen sharing to practice filling out EMAs. Finally, we asked participants 15 follow-up questions that covered their perceptions about the experience of filling out EMAs on a smartwatch, the relevance of the smartwatch to their current medication-taking routine, and their willingness to use the app on a smartwatch for a long period of time.
Fig. 2.

Smartwatch app prototype showing EMA survey about symptoms.
Smart MEMS Cap.
For the final segment of the interview, we used screen sharing to demonstrate the use of the RxCap3, a bluetooth-enabled MEMS cap that records each time the cap is unscrewed as a medication-taking event. Since most participants had not seen or used a smart pill bottle before, we first explained the purpose and function of the cap. We then showed participants how one would remove the cap to take medication, and how the cap would blink and beep upon removal. We also explained how the cap could be connected to an application on the user’s smartphone to help keep track of when they took their medication. In order to gain a better understanding of participants’ medication-related needs, we asked questions about the types of medications participants were currently taking, the frequency with which they took them, their preferred storage method (e.g., pill box; original bottle) and location (e.g., in the bathroom; on a nightstand), and what kinds of alerts or reminders they used to help them remember to take their medications (e.g., app; phone alarm) We then asked 15 follow-up questions about the relevance of the cap to their current medication management routine and their willingness to use a smart pill bottle to store their medications for a long period of time.
2.3. Data Analysis
Interviews lasted between 30 min and 1.5 h and were audio recorded. The first and second author transcribed the interviews verbatim. The first author then applied initial codes from the interview transcripts to develop a preliminary code-book. She then worked with the second, third, and fourth authors use an iterative, inductive approach to refine the initial codes, develop new codes, and extract the overall themes. Iterations continued until all coders reached consensus.
Demographic data were gathered and analyzed by the first and second author, who extracted the data from the secure Qualtrics survey and ran descriptive statistics (e.g., means, standard deviation, frequencies) using Microsoft Excel.
3. Results
3.1. Participant Demographics
Participants’ ages ranged from 33 to 86 years (μ= 60.2 years; σ= 15.8 years), with a gender distribution of 6:3 (female: male). Most participants self-identified as White (8/9; 88.9%), with one participant indicating they were Asian (1/9; 11.1%). Among those who reported their ethnicity (8/9; 88.9%), one participant reported being of Hispanic ethnicity (1/8, 12.5%). All participants (9/9; 100%) had been diagnosed with lung cancer at least 6 months prior to the study. Among those who reported their lung cancer stage (8/9; 88.9%), the majority were diagnosed with stage IV (7/8; 87.5%), followed by stage III (1/8; 12.5%). One-third were former smokers (3/9; 33%). Among the former smokers, the mean number of years of tobacco use was μ= 21.7 years (σ= 2.4 years).
3.2. Interview Study Findings
Participants’ attitudes, concerns, and needs regarding smart device use spanned four key thematic areas: device and application design, lifestyle, and abilities, and obligations. In this section, we delve into each of these themes in detail to answer our original research question (RQ): What attitudes do patients with lung cancer have toward smart device use for managing their medications and tracking their symptoms? In this section, we present participants’ attitudes and concerns toward the individual devices as well as their needs and preferences for notifications they might receive from any device. We also describe how participants’ personal and social obligations affect their willingness to use smart devices. Finally, we highlight the roles of self-efficacy and obligation to self and others in motivating smart device use.
Device and Application Design.
Smartphone and Smartwatch.
Participants appreciated the smartphone and smartwatch for their compact size and ease of use. Both P1 and P11, for instance, expressed a preference for the smartphone over bulkier technologies. In P11’s words, “It doesn’t force me to have to go to the computer.” While the smartphone was familiar to most participants, the smartwatch was not, and participant opinions on the smartwatch were divided. Some were drawn to the smartwatch because they could input data directly on their wrist in a discreet way. P4, for instance, described how his personal smartwatch was useful for discreetly checking messages while at work. P11, however, worried that smartwatch notifications in particular were a privacy risk. Given that the watch must be worn at all times rather than kept aside in a purse or pocket, the watch has the potential to draw unwanted attention to private messages in social settings: “Notifications will bother me more on my watch than on my phone…I’m not sure if I would like going out for dinner, and all of a sudden noticing that my wrist is lighting up with a message and somebody across the table says, oh, you have a message in your wrist… [Or] what if I’m having an important meeting with somebody?” P4 also found the smartwatch somewhat intrusive (despite regularly using his own), and preferred to keep the device in the background as much as possible: “It requires an answer right then and there. Personally I don’t like inputting [data]. I see [the watch] as more of a way to receive information.”
Participants also mentioned several design-related needs and concerns, with regard to the smartwatch and smartphone. Some worried the surveys were too long and would become cumbersome, or that the device’s battery might drain too quickly due to running an app. Attitudes about device size were divided; one participant found the smartwatch screen too small and hard to navigate, while another, P6, found the watch to be too big for regular use: “I’m not a big fan of wearing much on my wrist..I would be inclined to forget wearing it, I’m afraid, because it’s bulky… I do not like intrusive technology.” Several participants wanted changes to the surveys, including more aesthetically-pleasing color schemes and the ability to comment on the frequency of their symptoms. Participants also wanted to complete surveys at their own time and pace, with several wanting to set their own notification schedule.
Pill Bottle.
Of the three smart devices we studied, the smart pill bottle received the least support from participants. Most participants were taking multiple prescription medications in addition to multiple vitamins and supplements, and disliked the smart pill bottle because it could only hold one type of pill at a time. As P11 described, they tended to prefer divided pill boxes for everyday use: “I think the box that has the separated days would be more useful because it would be recording not only that you took it, but [when] you took it…So the data that you would record would be more complete.”
Notifications.
In general, participants valued their privacy and peace. They wanted notifications to be unobtrusive and discreet, especially in public settings such as the workplace. Participants’ preferences, in this regard, were very personal. Some participants preferred to leave most notifications off and found them “annoying”. P8 was willing to receive vibrations only, in keeping with his work obligations: “A vibration is best, because a lot of times I’m in management meetings. Obviously, we all have our phones turned down.” Others, such as P7, were willing to receive audible “dings” on any device, provided they were not overly loud or repetitive: “I would want it to be quieter and more subtle,…and also not persistent. So one notification is fine, [but] five notifications would not be fine… I would not want to have to keep seeing it. I’m [also] notorious for clearing out my notifications, and in fact I turn off a lot of my notifications because it’s a privacy issue to me.” P6 expressed a similar preference: “I set my alarms for my meds so I would definitely [want notifications]…I would probably have it be a single ding…I don’t want anything irritating like ‘DA, DA, DA, DA!’ Just like a single ding would work.”
Several participants also expressed a desire for survey notifications to be integrated with their electronic health record apps, so that all their health-related notifications showed up in one place. For example, P4 described how he would be more likely to take surveys when checking for appointments in MyChart4, a popular electronic health record (EHR) application. Importantly, participants wanted to receive notifications only when absolutely needed, given the burden of time their cancer treatment already placed on them. For instance, P11 described how her view of time had changed since her diagnosis and re-emphasized that notifications should be minimally disruptive, especially during family and personal time: “When you have cancer, too many things seem too trivial, and you want to concentrate every day on using your time in the best possible manner. So it’s funny, I’m now quite bothered by all these notifications that come to me about celebrities…but I do want to get notifications if my sons do something. So I think it depends. I would say not too many; enough notifications that we can do this [study], but not unnecessary notifications.”
Lifestyle.
Participants’ lifestyles heavily influenced their attitudes towards smart device use. P6, echoing many other participants, cited her familiarity and current use of smartphones as a reason they would be willing to use the Sensus app as part of a future study: “Like a lot of people, I use my phone more than any other device.” Participants were less familiar with the smartwatches. Even P4, who owned a smartwatch, was concerned about learning to use a different type of smartwatch when he already owned one that worked well for him: “I wouldn’t like it. I prefer my watch and the features my watch has, I’ve already gotten used to it. I wouldn’t want to learn a whole new system, and I’m assuming if it’s a research watch I wouldn’t be able to install any of my own apps on the watch anyways.”
Participants’ schedules and levels of flexibility varied, though most concluded that they were more available on weekdays than on weekends. Weekends were often reserved for family time and other social activities (e.g., hosting friends or attending church services). For instance, P1 described the importance of time with her husband: “We’re out hiking or doing projects. I’m less likely to think about doing something like a survey.” Similarly, P6 did not want to be interrupted during her valued social time: “Generally we are busier on the weekends with catching up with friends and family…I set an alarm on my phone for taking my medication, and then honestly if we’re out socializing, I end up snoozing the alarm and snoozing the alarm… I take it within an hour or two. But… [at] nine o’clock on Saturday night I don’t generally want to be interrupted with a reminder about something, and I think I’ll feel the same way about the surveys.” Several participants also mentioned that their activities could put them out of range for receiving push notifications from a study device (e.g., alarms or reminders to take a medication dose). For instance, before the COVID-19 pandemic forced many people to stay at home, P8 liked to go hiking on the weekends in remote areas with little-to-no cellular service: “Right now we’re all hunkered down [during the pandemic]. I used to be out of range of any technology if I was skiing or backpacking. I could be gone for 13 days, out of range.”
Throughout the interviews, participants repeated their commitment to habits and routines as a major influencing factor on their attitudes toward smart devices. Several participants, such as P6, reported that using the devices would “just become a part of a daily routine”. P9 even likened smart device use to taking medicine regularly: “What’s the difference of using the app every day and then using the medicine every day? I don’t know if there would be a difference.” While participants felt the smartwatch and smartphone could fit into their existing routines, they did not feel the same way about the pill bottle. The reasons participants gave for not wanting to use the pill bottle were as much of a lifestyle concern as a design concern. Namely, managing multiple pills had driven participants to establish longstanding, personalized medication-management routines that already worked well for them. P2 put it simply: “I don’t need [the smart pill bottle] – “I have no problem with what I’m doing now.”
Abilities.
Participants exhibited varying degrees of self-efficacy with regard to using smart devices as part of their medication management routine. P2 noted that, while she was confident, she could use the real, physical devices, the screen size and difficult scrolling in the online prototype were challenging for her. Others, such as P1, expressed confidence in their own technical skills, but were doubtful that other people, especially in older age groups, would be able to use the devices: “I don’t think I would have any problem at all using it. I could see if it was my mother, I would have to train her how to use it. But the way it’s set up, for anyone that uses apps, it’s pretty obvious what to do.” Still others believed they would need significant support from the study team or another support person. For instance, P7 asked, “Are you going to train me really well in how to use that smartwatch?” P3 believed she could use the smartphone if she received outside help from her grandchildren or a tutor: “I might even hire a tutor to help me…I’m not that great on a smartphone for sure.”
Obligations.
Besides force of habit, participants cited obligations to their doctors and to themselves as a factor influencing their willingness to use devices. Participants took great pride and responsibility in managing their health as best they could during treatment. They valued smart devices for their ability to track symptoms over time and wanted the ability to share this information with their providers. For instance, P1 described how an app could help her keep an accurate record of her symptoms and side effects to present to her oncologist: “I’m not going to call my doctor and say, oh I had a mouth sore today. But if I had an app, if I was supposed to use it every day, I would put it into the app.” Likewise, P7 would be willing to fill out surveys more frequently if it helped with symptom management: “If [there were] questions that I felt like were important for me and my doctor to know, I would do it five times a day… I would do it… if I thought it would help manage my symptoms.”
4. Discussion
Our study highlights a number of considerations and challenges for designers at the intersection of mHealth, medication adherence, and oncology. Participants’ openness to using certain devices may be mitigated by their familiarity with the device, confidence in their technical abilities, work and social obligations, and even their fashion preferences. Moreover, fundamental challenges, such as whether participants will be available and within range for receiving push notifications, will necessarily influence the design and deployment of medication management technologies. Based on these findings, we present several practical design considerations in the following sections.
4.1. Give participants agency over their notifications
Notifications and reminders to take one’s medication play an important role in interventions for individuals with cancer. Prior work has demonstrated the feasibility of using information from smart devices to inform the delivery of missed dose messages via EHRs, such as MyChart [25]. This approach is a promising step towards more personalized notifications and interventions. Prior research with cancer patients taking oral chemotherapy has emphasized the importance of taking the user’s schedule into account when delivering reminders [23]. In a similar vein, participants in our study overwhelmingly expressed a desire to control the timing and format of notifications as a condition of using the smart devices in their regular medication-taking routine. We recommend that designers of smart device applications for medication management give participants a range of options for customizing their notification frequency from within the app. Designers might consider setting a default schedule based on times of day most commonly associated with medication taking and alertness, then enabling participants to customize this schedule as needed. For instance, 8 AM and 8 PM often correspond with morning mealtimes and evening bedtime routines, respectively. Ideally, users would be presented with a screen that allows them to set the exact day(s) and time(s) they would like to receive notifications, as well as the type of notification (e.g., vibration, beep, or banner) for each day and time. Giving participants agency over their notifications in this manner is a small cost for designers, but a major step toward protecting participant privacy and ensuring notifications are well-integrated into participants’ daily routines (rather than intruding on them).
4.2. Advocate for Better Avenues for Secure Sharing of Patient-Entered Data with Clinical Care Providers
Prior works have established patients’ openness to sharing information such treatment satisfaction and adverse effects with their clinicians via apps, provided that the information can be used to complement their treatment [12]. Our participants shared this attitude. Given the significant physical and emotional toll of their lung cancer treatment, participants had a vested interest in being able to review and share as much of their passively-and actively-sensed health data as possible with their doctors. Researchers have called for better infrastructure for secure information sharing between patients and providers in the context of mHealth for cancer care, given the limitations of current modalities [18]. Yet, this remains an open challenge. Electronic Health Records (EHRs) are the gold standard for health information and records management in our digital world, yet they are primarily designed for displaying information entered by clinical care providers (e.g., patient lab values and test results) and for facilitating basic secure messaging between patients and providers. EHRs in their current form are not equipped to receive and process information from consumer devices, such as smartwatches, or from custom medication and symptom tracking apps, in part due to strict requirements imposed by laws such as HIPAA [9] and the HITECH Act [8]. Moreover, building a standalone application that securely transmits patient-entered data to the patient’s healthcare provider via an existing EHR platform would be an enormous challenge. Such an application would not only need to comply with current market standards for secure health information sharing, such as Health Level 7 (HL7) [10], but would require direct collaboration with leading EHR vendors. Additionally, such an application would need to navigate the gaps left by HIPAA with regard to digital healthcare tools [24].
Designers seeking a short-term solution for patients who wish to share information with their doctors could provide in-app visualizations at different timescales, such as daily charts of the times of day a patient took their medication, or weekly and monthly graphs of adherence percentages over time. These should be easily-exportable to images that participants could save to their smartphone and share with their doctors manually in-clinic during routine appointments. Designers taking this approach should consult with both patients and clinicians when designing such visualizations, to ensure they are clear and concise for both parties. As a long-term solution, designers should consider advocating for improved guidance on patient-to-clinician information sharing via digital technologies, at the national level, and should seek out long-term collaborations with EHR vendors and smart device manufacturers where possible.
4.3. Ensure Participants have Access to Adequate Support Resources During Deployment Studies
Prior work in mHealth has shown that patients with lung cancer may feel they are lacking sufficient support and self-management skills, in regards to their disease [17]. Several participants echoed these concerns specifically with regard to using smart devices. Indeed, many expressed a hesitancy to use smart devices due to their perceived lack of technical proficiency. Participants also expressed a need for extensive support from the study team, should they choose to use the smart devices in a future deployment study. To increase participants’ confidence, we suggest providing a comprehensive technology use manual and other written educational materials that describe how to use each study device. We also recommend that study team members review these materials with participants, and provide in-depth demonstrations of each device. We note that technological concerns are likely to arise during deployment. To adequately address these concerns, we also recommend providing the participant with a specific study contact designated to addressing and supporting individual technology needs.
5. Limitations
This study is novel given its focus in evaluating perceptions of smart technology among individuals living with advanced lung cancer. However, it does have limitations, including the small sample size. We faced several recruitment challenges during this study. Cold-calling potential participants was largely unsuccessful. Among those who responded to cold calls or were willing to speak to us in clinic, most declined. Their reasons included disease burden (e.g., fatigue from treatment), busyness due to participating in other research studies, and lacking a computer for the study interview. Whether these challenges were unique to our study population is outside the scope of this paper; however, we recommend that future studies cast a broad recruitment net across multiple treatment facilities if possible.
Like most studies conducted during the COVID-19 pandemic, we also faced challenges in adapting our study activities to be fully remote. While we were able to conduct recruitment in clinic by taking many precautions such as masking, we opted to conduct the interviews remotely to reduce the risk of transmission of COVID-19 to participants. This decision fundamentally altered who we enrolled in the study. Our remotely conducted interviews required a personal computer (PC) with a mouse and microphone, preventing those without a PC from participating. Moreover, those who did participate did not get the in-person experience of physically interacting with the study devices. Additionally, we struggled to recruit a racially diverse sample. We also urge researchers in the field to continue to increase efforts to recruit diverse participants. Given the significant racial and social disparities present in the incidence of lung cancer [7] and other diseases, diverse samples are necessary for designing mHealth tools that serve as many patients as possible.
6. Conclusion
In this study, we presented finding from interviews with 9 individuals with lung cancer on patients’ attitudes and needs towards mHealth devices for side effect and medication management. Our findings shows that patients’ motivations for using these devices are dependent on a number of important factors, including the devices’ design, patients’ lifestyles and existing habits, their unique abilities and feelings of self-efficacy, and their preexisting obligations. These findings may help clinicians and researchers to co-develop effective deployments of mHealth systems for side effect and medication management in oncology populations. Specifically, our results will help study developers to identify which features patients find most valuable for specific “smart” devices, such as the ability to view and download one’s data from a mobile app. More work is needed to see if our results and implications for design overlap with findings for other oncology populations.
Acknowledgements.
This research was supported by the following sources: the National Cancer Institute Cancer Center Support Grant 2P30CA044579-31 (to A. N. Baglione, R. Hall, and R. Gentzler); the National Cancer Institute, under award number R01CA239246 (to Dr. L. E. Barnes, Dr. J. Gong, and Dr. K. J. Wells); and the Lung Cancer Research Foundation (LCRF), supported by Pfizer (to Dr. R. Gentzler). The content in this paper is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned sources.
We thank Cristian Garcia Alcaraz and Manuel Gonzales for contributing to the development of the interview questions and demographic surveys. We also thank Xishi (Ethan) Zhu and Xue (Shirley) Wu for their work on the smartwatch mobile app, and Dr. Jackey Gong for his supervision of the app’s development.
Footnotes
WebEx; https://www.webex.com/.
Appetize.IO; https://appetize.io/.
RxCap; https://rxcap.com/.
MyChart; https://www.mychart.com/.
References
- 1.Billingy NE, et al. : SYMptom monitoring with Patient-Reported Outcomes using a web application among patients with Lung cancer in the Netherlands (SYMPRO-Lung): study protocol for a stepped-wedge randomised controlled trial. BMJ Open 11, e052494 (2021). 10.1136/bmjopen-2021-052494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burney KD, Krishnan K, Ruffin MT, Zhang D, Brenner DE: Adherence to single daily dose of aspirin in a chemoprevention trial: an evaluation of self-report and microelectronic monitoring. Arch. Fam. Med 5, 297 (1996) [DOI] [PubMed] [Google Scholar]
- 3.Caldeira C, Bhowmick P, Komarlingam P, Siek KA: A state-based medication routine framework. In: CHI Conference on Human Factors in Computing Systems, pp. 1–16. ACM, New Orleans LA USA: (2022). 10.1145/3491102.3517519 [DOI] [Google Scholar]
- 4.Califano R, et al. : Expert consensus on the management of adverse events from EGFR tyrosine kinase inhibitors in the UK. Drugs 75(12), 1335–1348 (2015). 10.1007/s40265-015-0434-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciani O, et al. : Lung Cancer App (LuCApp) study protocol: a randomised controlled trial to evaluate a mobile supportive care app for patients with metastatic lung cancer. BMJ Open 9, e025483 (2019). 10.1136/bmjopen-2018-025483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geynisman DM, Wickersham KE: Adherence to targeted oral anticancer medications. Discov. Med 15, 231–241 (2013) [PMC free article] [PubMed] [Google Scholar]
- 7.Harper S, Lynch J, Meersman SC, Breen N, Davis WW, Reichman ME: An overview of methods for monitoring social disparities in cancer with an example using trends in lung cancer incidence by area-socioeconomic position and race-ethnicity, 1992–2004. Am. J. Epidemiol 167, 889–899 (2008). 10.1093/aje/kwn016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Health Information Technology for Economic and Clinical Health (HITECH) Act, Title XIII of Division A and Title IV of Division B of the American Recovery and Reinvestment Act of 2009 (ARRA), Pub. L. No. 111–5, 123 Stat. 226 (Feb. 17, 2009). (full-text), codified at 42 U.S.C. §§300jj et seq.; §§17901 et seq
- 9.Health Insurance Portability and Accountability Act. Pub. L. No. 104–191, § 264, 110 Stat.1936
- 10.Health Level 7 Standards. Health Level 7 International; (2022). http://www.hl7.org/ [Google Scholar]
- 11.Jacobs ML, Clawson J, Mynatt ED: My journey compass: a preliminary investigation of a mobile tool for cancer patients. In: Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, pp. 663–672. Association for Computing Machinery, New York, NY, USA: (2014). 10.1145/2556288.2557194 [DOI] [Google Scholar]
- 12.Kessel KA, et al. : Mobile health in oncology: a patient survey about app-assisted cancer care. JMIR mHealth uHealth. 5, e81 (2017). 10.2196/mhealth.7689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Low CA: Harnessing consumer smartphone and wearable sensors for clinical cancer research. NPJ Digit. Med 3, 1–7 (2020). 10.1038/s41746-020-00351-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lung cancer statistics: How common is lung cancer? American Cancer Society; (2022). https://www.cancer.org/cancer/lung-cancer/about/key-statistics.html [Google Scholar]
- 15.Mauro J, Mathews KB, Sredzinski ES: Effect of a smart pill bottle and pharmacist intervention on medication adherence in patients with multiple myeloma new to Lenalidomide therapy. JMCP 25, 1244–1254 (2019). 10.18553/jmcp.2019.25.11.1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGregor BA, Vidal GA, Shah SA, Mitchell JD, Hendifar AE: Remote oncology care: review of current technology and future directions. Cureus. 12, e10156 (2020). 10.7759/cureus.10156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni X, et al. : Development of mobile health–based self-management support for patients with lung cancer: a stepwise approach. Nurs. Open 9, 1612–1624 (2022). 10.1002/nop2.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Panayi ND, Mars MM, Burd R: The promise of digital (mobile) health in cancer prevention and treatment. Future Oncol. 9,613–617(2013). 10.2217/fon.13.42 [DOI] [PubMed] [Google Scholar]
- 19.Pater J, Owens S, Farmer S, Mynatt E, Fain B: Addressing medication adherence technology needs in an aging population. In: Proceedings of the 11th EAI International Conference on Pervasive Computing Technologies for Healthcare, pp. 58–67. ACM, Barcelona Spain: (2017). 10.1145/3154862.3154872 [DOI] [Google Scholar]
- 20.Pereira-Salgado A, et al. : Mobile health intervention to increase oral cancer therapy adherence in patients with chronic myeloid leukemia (the REMIND system): clinical feasibility and acceptability assessment. JMIR mMhealth uUhealth 5, e184 (2017). 10.2196/mhealth.8349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmalz O, et al. : Digital monitoring and management of patients with advanced or metastatic non-small cell lung cancer treated with cancer immunotherapy and its impact on quality of clinical care: interview and survey study among health care professionals and patients. J. Med. Internet Res 22, e18655 (2020). 10.2196/18655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiffman S, Stone AA, Hufford MR: Ecological momentary assessment. Annu. Rev. Clin. Psychol 4, 1–32 (2008). 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- 23.Skrabal Ross X, Gunn KM, Patterson P, Olver I: Development of a smartphone program to support adherence to oral chemotherapy in people with cancer. Patient Prefer. Adherence 13, 2207–2215 (2019). 10.2147/PPA.S225175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Theodos K, Sittig S: Health information privacy laws in the digital age: HIPAA doesn’t apply. Perspect. Health Inf. Manag 18, 1l (2021) [PMC free article] [PubMed] [Google Scholar]
- 25.Toscos T, et al. : Medication adherence for atrial fibrillation patients: triangulating measures from a smart pill bottle, e-prescribing software, and patient communication through the electronic health record. JAMIA Open 3, 233–242 (2020). 10.1093/jamiaopen/ooaa007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood L: A review on adherence management in patients on oral cancer therapies. Eur. J. Oncol. Nurs 16, 432–438 (2012). 10.1016/j.ejon.2011.10.002 [DOI] [PubMed] [Google Scholar]
- 27.Xiong H, Huang Y, Barnes LE, Gerber MS: Sensus: a cross-platform, general-purpose system for mobile crowdsensing in human-subject studies. In: Proceedings of the 2016 ACM International Joint Conference on Pervasive and Ubiquitous Computing - UbiComp 2016, pp. 415–426. ACM Press, Heidelberg, Germany: (2016). 10.1145/2971648.2971711 [DOI] [Google Scholar]


