Abstract
Circadian coordination of life functions is believed to contribute to an organism's fitness; however, such contributions have not been convincingly demonstrated in any animal. The most significant measure of fitness is the reproductive output of the individual and species. Here we examined the consequences of loss of clock function on reproductive fitness in Drosophila melanogaster with mutated period (per0), timeless (tim0), cycle (cyc0), and Clock (ClkJrk) genes. Single mating among couples with clock-deficient phenotypes resulted in ≈40% fewer progeny compared with wild-type flies, because of a decreased number of eggs laid and a greater rate of unfertilized eggs. Male contribution to this phenotype was demonstrated by a decrease in reproductive capacity among per0 and tim0 males mated with wild-type females. The important role of clock genes for reproductive fitness was confirmed by reversal of the low-fertility phenotype in flies with rescued per or tim function. Males lacking a functional clock showed a significant decline in the quantity of sperm released from the testes to seminal vesicles, and these tissues displayed rhythmic and autonomous expression of clock genes. By combining molecular and physiological approaches, we identified a circadian clock in the reproductive system and defined its role in the sperm release that promotes reproductive fitness in D. melanogaster.
Many life functions, from cellular activities to behavior, display daily (circadian) rhythms. These rhythms are generated by cell-autonomous circadian clocks and involve several genes encoding transcriptional regulators, which are substantially conserved among animals ranging from fruit flies to humans (1). The core clock mechanism in Drosophila melanogaster involves rhythmic transcription of the period (per) and timeless (tim) genes followed by nuclear accumulation of their proteins, PER and TIM. Another gene, Clock (Clk), shows mRNA oscillations that are out of phase with the oscillations of per and tim mRNAs. Protein encoded by Clk, together with protein encoded by the gene cycle (cyc), activates transcription of per and tim. These four genes are essential for the function of the brain clock, and a null mutation in any of them renders the fly behaviorally arrhythmic (2).
Although the importance of circadian clocks in controlling behavior is well documented, their functional significance for physiology is less understood. Clock genes are rhythmically and autonomously expressed in peripheral tissues of D. melanogaster (3–5), zebrafish (6), and mammals (7), suggesting that peripheral clocks may coordinate physiological processes ultimately affecting fitness. Few studies have compared physiological parameters between individuals with a normal or disrupted circadian clock in any species. A study in cyanobacteria demonstrated that colonies in which free-running circadian periods are in resonance with environmental 12-h light/12-h dark cycles (LD) out-compete colonies in which internal and external periodicities are out of synchrony (8). In D. melanogaster, mutations in the per gene and photoperiodic conditions affect developmental time and longevity (9–11).
The role of the circadian clock in fitness, defined as a measure of reproductive success, has not been addressed in animals with a genetically disrupted pacemaker. However, physiological data indicated an important role of circadian timing in reproduction of moths. In these insects, a circadian clock controls rhythms associated with the release and posttesticular maturation of sperm (12, 13). Disruption of circadian rhythms by rearing male moths in constant light reduces sperm release and male fertility (14). These experiments provided indirect evidence that the circadian system is important for reproduction.
We tested genetically the links between the circadian system and reproductive fitness in D. melanogaster, in which both spermatogenesis and reproductive behavior are relatively well understood (15, 16). We report here that flies with loss-of-function mutations in the per, tim, Clk, or cyc genes produce significantly fewer progeny than wild-type flies. Clock-mutant males contribute to this phenotype by releasing smaller quantities of sperm than their wild-type counterparts. Both fertility and sperm release are restored to wild-type levels in flies with rescued clock function. Finally, we demonstrate that clock genes are rhythmically and autonomously expressed in testes and seminal vesicles (SV) of male flies, suggesting that these tissues harbor a circadian system important for optimal sperm output and fertility.
Materials and Methods
Fly Rearing and Strains.
Flies were raised on cornmeal/yeast medium in LD at 25°C. By convention, the time of lights-on is denoted as Zeitgeber time (ZT) 0 and time of lights-off as ZT 12. Wild-type flies used were Canton-S. The following loss-of-function alleles of the clock genes per, tim, Clk, and cyc were applied: per01 (17), per04 (18), tim01 (19), ClkJrk (20), and cyc01 (21). Additionally, a timeless null-allele, tim03, was analyzed (22).
Rescue of per function in a per01 genetic background was performed by crossing per01 virgins to transgenic males carrying a P element containing a 13.2-kb genomic per DNA fragment. This transgene restores behavioral rhythmicity when crossed to per01 flies (23). Similarly, tim function was rescued by introducing a transgene expressing the tim cDNA under the control of the tim promoter into the background of tim-null alleles. The transgene used restores behavioral rhythmicity in tim01 and tim03 mutant flies (22, 24). For the rescue, tim01 males carrying two copies of the transgene were crossed to tim03 females. The resulting transheterozygous tim01/tim03 F1 males, carrying one copy of the rescue transgene, were analyzed for their reproduction phenotype. tim01/tim03 flies were used as a control to exclude the possibility of rescue by complementation.
To elucidate expression patterns of per and tim in the reproductive tract, the following reporter strains were used: (i) “BG” flies carrying per–lacZ fusion gene (encoding two-thirds of PER fused to β-galactosidase) in a per+ genetic background (25); (ii) doubly transgenic flies carrying tim–gal4 combined with UAS–gfp (green fluorescent protein; ref. 26); (iii) transgenic flies in which luciferase (luc) cDNA was fused to upstream flanking material of either per (BG-luc) or tim (tim-luc) gene (4, 27).
Mating and Fertility Assessments.
Males and females of the appropriate strain were collected 1 day before eclosion and isolated in individual vials with 1 ml of diet. Flies of appropriate age, sex, and genotype were paired in mating wheels (28) ≈3 h after lights-on (29), and their copulation was observed. After a single mating was completed, individual females were transferred to 50-mm Petri dishes with 2 ml of diet, where they oviposited for 4 days. Flies that were allowed to mate ad libitum were aspirated directly into Petri dishes, where both sexes remained together for 4 days. Flies were then removed, and larvae were allowed to hatch for 24 h. The number of larvae, dark unhatched embryos and white unfertilized eggs were counted. All data were analyzed for statistical significance by using ANOVA and Fischer's least significant difference procedure (LSD intervals) at 95% confidence intervals.
Quantification of Sperm.
To determine the quantity of sperm released from testes into SVs, individual spermatozoa were counted in SVs dissected from 1- and 2-day-old males at 12-h intervals. Individual SVs were placed in a drop of distilled water and ruptured to facilitate dispersion of spermatozoa. After water evaporated, dispersed spermatozoa were mounted in Vectashield supplemented with 4′,6-diamidino-2-phenylindole (DAPI) to stain sperm nuclei. Digital images were acquired by using a SPOT charge-coupled device camera and software (Diagnostic Instruments) and printed. Thin and elongated sperm nuclei contained in individual SVs were counted, and data were averaged for each strain. Data were analyzed by using ANOVA and Fischer's LSD intervals at 95% confidence intervals.
Assessments of Clock Gene Expression.
Reproductive systems (RSs) from doubly transgenic tim–gal4, UAS–gfp flies were dissected in PBS and observed live under a Zeiss Axiovert microscope. RSs from flies carrying per–lacZ were fixed and stained with 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) as described (30). For whole-mount immunocytochemistry and in situ hybridizations, RSs were fixed in 4% paraformaldehyde for 15 min. Immunodetection of PER and TIM was performed on RSs dissected and fixed at ZT 8 and ZT 20. Anti-TIM (diluted 1:4,000) or anti-PER (diluted 1:15,000) primary antisera were used, followed by application of anti-rat or anti-rabbit (for TIM and PER detection, respectively) secondary antibodies conjugated to Alexa Fluor 594 (Molecular Probes) diluted 1:1,000. In situ hybridizations were performed as described (31). Digoxigenin-4-labeled per and Clk antisense RNA probes were hybridized to whole RSs dissected and fixed at ZT 4 and ZT16. Tissues were whole-mounted in Vectashield-DAPI medium (Vector Laboratories) to allow localization of clock proteins and mRNAs relative to cell nuclei.
To determine luciferase-reported activity of per and tim genes in vitro, testes–SV complexes were dissected and cultured individually in wells of 96-well plates (4). The bioluminescence from each well was measured once per hour in a Packard Topcount Multiplate Scintillation Counter (27); the resulting counts were analyzed for phase, period, and amplitude (32).
Results
Mutations in Clock Genes Decrease Reproductive Capacity.
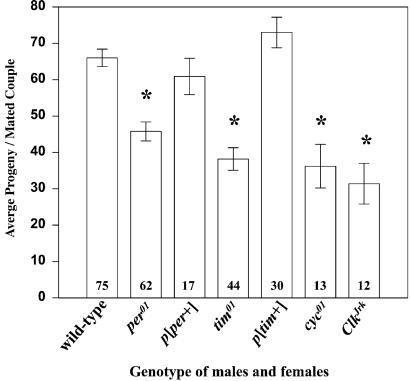
To investigate the effects of loss-of-clock-function on reproductive capacity, we compared offspring produced by wild-type and clock-mutant flies after matings between males and females of a given genotype. Null mutations in clock genes, per01, tim01, ClkJrk, and cyc01, invariably led to a significant decline in average progeny as compared with wild-type flies (Fig. 1). Flies carrying alternative null genotypes, per04 and tim03, as well as tim01/tim03, mutations also showed statistically significant decreases in progeny compared with wild-type flies (Table 1). The decline in progeny was caused by a lower number of eggs laid per couple, and a higher than normal production of unfertilized eggs; these two factors contributed to a varying degree to the lower fertility (Table 1). This phenotype was rescued to wild-type in per01 males and females carrying a per+ construct and in tim01/tim03 flies rescued with a tim+ construct. Thus, the reduction in progeny observed in flies with a disrupted circadian clock maps to the per and tim loci.
Figure 1.
Mutations in four clock genes dramatically reduce fertility in D. melanogaster. Bars represent average number (±SEM) of progeny produced by couples of a given genotype (numbers of couples tested are shown in bars). *, Strains that produce significantly less progeny then wild-type flies as calculated by ANOVA and Fisher's LSD intervals (P < 0.05). Males and females were 4 days old when mated.
Table 1.
Effects of clock genes on reproductive output in D. melanogaster
| Strain | n | Eggs laid | Unfertilized, % | Progeny |
|---|---|---|---|---|
| Wild type | 75 | 71.2 | 6.2 | 65.7 |
| per01 | 62 | 49.7 | 10.3 | 45.7 |
| per04 | 7 | 71 | 40.2 | 42.3 |
| per01; p[per+] | 17 | 65.7 | 4.6 | 60.9 |
| tim01 | 44 | 50.9 | 24.9 | 38.2 |
| tim03 | 14 | 67.9 | 25 | 43.6 |
| tim01/tim03 | 7 | 64.2 | 7.8 | 50.6 |
| tim01/tim03; p[tim+] | 30 | 89 | 4.2 | 73.1 |
| ClkJrk | 12 | 40.5 | 9.5 | 36.2 |
| cyc01 | 13 | 40.2 | 12.4 | 31.4 |
Numbers in bold are significantly different from wild type (P < 0.05). Males and females were of the same strain and were 4 days old when mated. n = Number of pairs tested. The unfertilized column shows the unfertilized eggs as a percentage of total eggs laid. Statistical analysis showed nonnormal distribution of this parameter. The Kruskal–Wallis test confirmed statistically significant differences between the medians (P < 0.05). The progeny column shows average offspring per mated pair.
The significant declines in fertility observed with singly mated flies are not as readily detected in routine laboratory cultures, where many males and females are housed in one vial for several days, such that flies can potentially mate multiple times. We investigated whether leaving pairs of mutant flies together for 4 days, thus allowing them to mate ad libitum, would affect their fertility. per01 couples mated ad libitum produced an average of 57.6 larvae (n = 15), a 20.5% increase over single mating. tim01 couples mated ad libitum produced on average 55.6 larvae (n = 7), a 31.3% increase over singly mated flies. These increases were statistically significant (P < 0.05) in both mutant strains. In contrast, ad libitum conditions (n = 23) did not significantly increase the average number of progeny over single matings (n = 16) in wild-type flies. These data suggest that clock mutants may compensate for their lower reproductive fitness by re-mating.
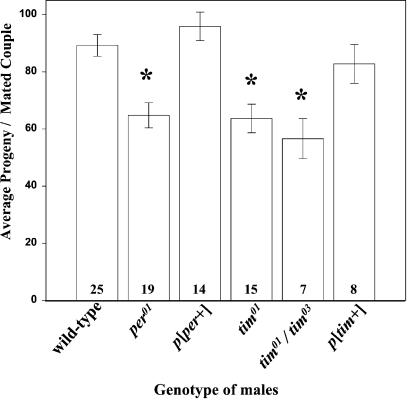
Crosses between per01 or tim01 mutant flies of either sex with wild-type flies of another sex indicated that both sexes contribute to the low fertility phenotype. It this paper, we examined in detail the male contribution to the low fertility phenotype (the results describing female contribution will be reported elsewhere). Single matings of per01, tim01, or tim01/tim03 males with wild-type females resulted in a significant reduction in progeny over wild-type pairs (Fig. 2). The average number of progeny was raised to normal levels in females mated with per01 mutants carrying a per+ construct or with tim01/tim03 heterozygotes carrying a tim+ construct (Fig. 2). The decline in progeny produced by per01, tim01, and tim01/tim03 males was caused by a significantly lower number of eggs laid per couple (76.2, 67.5, and 63.4, respectively, compared with 95.5 in wild type) and by an increased percentage of unfertilized eggs (data not shown). Both parameters reverted to wild-type levels in flies with rescued clock function (Fig. 2).
Figure 2.
Effects of per and tim mutations on male fertility. Two-day-old males of the indicated genotypes were mated with 5-day-old wild-type females. Bars represent the average number (±SEM) of progeny produced by couples of given genotype (n shown in bars). *, Genotypes that produced significantly fewer progeny compared with wild-type, as calculated by ANOVA and LSD intervals (P < 0.05).
Mutations in Clock Genes Decreases Male Fecundity.
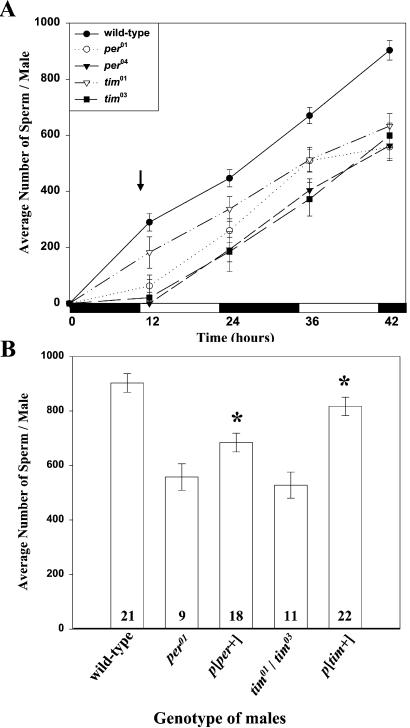
A factor affecting oviposition rate in D. melanogaster is the amount of sperm transferred to a female during copulation. To analyze the causes of lower fertility in clock-mutant males, we compared the quantity of spermatozoa released from testes and stored in SVs in wild-type and clock-deficient males. The number of spermatozoa released during the first 2 days after adult emergence was significantly lower in males expressing either of two null mutations of the per or tim gene (Fig. 3A). The buildup of sperm was most impeded at the last time point; therefore, we chose this time to assess rescue of the mutant phenotype by per+ or tim+ transgenes. per01 and tim01/tim03 males, each carrying the appropriate construct, accumulated a significantly higher quantity of sperm in the SV than their respective clock-deficient genetic variants (Fig. 3B; P > 0.05). The mean number of spermatozoa accumulated in tim-rescued males was similar to that in wild-type males (P < 0.05), whereas in per-rescued males it was lower than in wild type.
Figure 3.
Effects of per and tim mutations on male fecundity. (A) Time course of sperm accumulation in SVs of young virgin males. Each point represents the average number (±SEM) of spermatozoa from 5–30 SVs dissected at 2 h after lights-off and lights-on. Clock mutants accumulated significantly fewer sperm at all time points tested as calculated by ANOVA and LSD intervals (P < 0.05). The arrow indicates approximate time of adult emergence. Black and white bars indicate times when lights were on and off, respectively. (B) Reversal of low-sperm phenotype in males with rescued clock function. Bars represent average number (±SEM) of spermatozoa from the of SVs of given genotype (n shown in bars) dissected at 42 h. Asterisks denote per-rescue and tim-rescue flies that accumulated significantly more sperm than did their corresponding clock-deficient males.
Expression of Clock Genes in the Male Reproductive System.
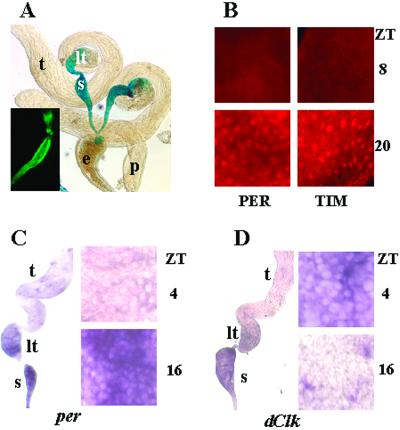
To determine whether the low-sperm phenotype is correlated with clock function in the reproductive system, we studied the activity of clock genes in these tissues. Spatial expression of per and tim was evaluated in flies that carry a per–lacZ reporter construct or express green fluorescent protein under control of the tim promoter. Both reporters exhibited strong activity in the lower testes and SVs, weak activity in the ejaculatory duct and the upper testes, and no activity in the paragonial (accessory) glands (Fig. 4A). Immunocytochemical analysis showed rhythmic expression of PER and TIM proteins limited to the lower testes and the SVs. PER and TIM were not detected at ZT 8, whereas both proteins were ubiquitously expressed in the nuclei of the epithelial cells forming the lower testes and the SV at ZT 20 (Fig. 4B). PER and TIM proteins were absent in per01, per04, and tim01 flies. However, similar to wild-type, distribution of both proteins in the SV and lower testes was observed in the reproductive system of per01 mutants rescued with a per+ construct and tim01 mutants transformed with a tim+ construct. Nuclear localization of PER and TIM was evident in those flies at ZT 20 (data not shown).
Figure 4.
Spatial and temporal expression of clock genes in the reproductive system of D. melanogaster. (A) 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining demonstrating strong per–lacZ expression in the SV (s) and the lower testes (lt), weak signal in the ejaculatory duct (e) and in the upper testes (t), and no signal in the paragonial glands (p). (Inset) Expression of tim-driven green fluorescent protein reporter in the lower testes–SV. (B) Immunofluorescence detecting PER and TIM proteins in the nuclei of SV epithelial cells at ZT 20 but not at ZT 8. (C) In situ hybridization with antisense probes detecting per mRNA in the SV and lower testis. Higher magnification of SV showing that epithelial cells are per-negative at ZT 4 and per-positive at ZT 16. (D) In situ hybridization at ZT 4 with antisense Clk mRNA show colocalization with per in the SV and lower testis. Higher magnification of SV showing that Clk is expressed in epithelial cells at ZT 4 but not at ZT 16. Control hybridization with respective sense probes yielded no signal. Images represent the prevailing pattern observed among at least five specimens used to assess staining with each reagent at a given time point.
Because the functional clock of the fruit fly involves out-of-phase cycling of per and Clk mRNAs (2), we did in situ hybridization of the male RS with antisense probes for both genes. Both per and Clk mRNA were detected in the lower-testes–SV epithelium. The level of per mRNA was low at ZT 4 and high at ZT 16 (Fig. 4C), whereas Clk mRNA showed cycling in the opposite phase with high levels at ZT 4 and low levels at ZT 16 (Fig. 4D). Taken together, these results demonstrate cycling of clock components that is similar to patterns observed in fly brains (2) and is consistent with the existence of a circadian clock in the male RS.
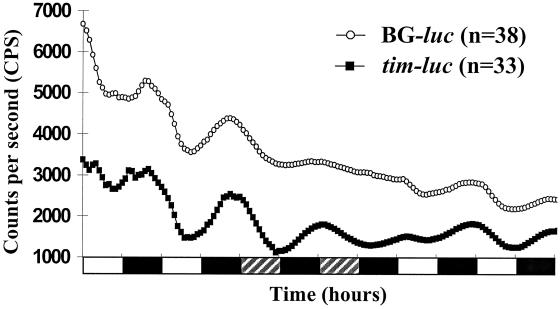
To elucidate the autonomy of the testes–SV circadian system, we used BG–luc (reporting per) or tim–luc transgenic flies. Testes–SV complexes were dissected from these flies and individually cultured in vitro in LD followed by dark/dark (DD) cycles and a return to LD. Isolated organs showed clear, high-amplitude cycling of BG-luc and tim-luc activity during the initial LD cycles with peak expression during the night (Fig. 5). Quantitative analysis of the data revealed that 59% of testes–SVs from BG–luc flies (n = 49) and 76% of same organs from tim–luc flies (n = 41) were rhythmic in vitro in the circadian range. On transfer to DD, cycling continued in 36% of both BG–luc and tim–luc organs with reduced amplitude. When the free-running cultures were returned to LD cycles, the amplitude increased for both constructs, demonstrating direct light responsiveness of the testes–SV circadian system (Fig. 5). When both constructs were crossed into genetic backgrounds carrying a loss-of-function mutation for the respective clock gene (BG–luc into per01 and tim–luc into tim01), circadian oscillations were eliminated (data not shown), indicating that wild-type alleles of the two clock genes are needed to support reporter-gene cycling.
Figure 5.
Real-time expression of BG–luc (per–luc) and tim–luc reporter genes in testes–SVs from individual flies. Plots show average bioluminescence from BG–luc- and tim–luc-expressing tissues, which were determined as rhythmic in LD and dark/dark cycles by quantitative analysis. The average LD oscillation period of 23.2 h for BG–luc was not significantly different from the average period of 23.7 h for tim–luc. Black and white bars indicate 12-h periods where lights were on and off, respectively; shaded bars indicate subjective day, where the lights would be on in LD.
Discussion
Our results identify a previously uncharacterized circadian clock in the reproductive systems of male flies with known clock components detected in the lower testes and SV. Three lines of evidence confirm the earlier suggestion (33) that a clock mechanism is located in these tissues. First, per and Clk mRNA appear to cycle out of phase, such that per mRNA levels are high in the early night whereas Clk mRNA levels are high in the morning. This pattern is consistent with the current model of brain clock function (2) and is demonstrated here for a peripheral clock in flies. Second, PER and TIM proteins were detected in the SV cell nuclei late at night, as has been reported for other clock-containing cells (2). Third, cultures of testes–SVs taken from flies transformed with per–luc or tim–luc showed that reporter-gene activity in the RS free-runs in constant darkness and is autonomously light sensitive. Thus, the testes–SV complex appears to contain a bona fide circadian clock that is self-sustained and brain-independent. Such clocks were reported in the fly excretory system (4) and antennae (5).
Our study suggests a link between the molecular clockworks in the reproductive tissues and normal sperm output in D. melanogaster. We showed that null mutations in the per or tim genes lead to a significant decline in the number of spermatozoa released from the testes into the SV. In flies with rescued per and tim function, this low-sperm phenotype is reversed and PER and TIM expression is restored in the testes–SV complex. The significance of the RS clock for male fecundity has been shown in moths. The release of sperm from moth testes follows a robust circadian rhythm, which persists in the RSs isolated in vitro (12, 13). Disruption of the moth circadian system by constant light leads to a dramatic reduction in the amount of released sperm (13, 14). Thus, physiological disruption of the circadian mechanism in moths and genetic disruption of clock genes in flies both cause a decline in male fecundity. These data suggest that sperm release from testes may also be rhythmic in flies; however, our experiments monitoring sperm accumulation in SVs at every 12 h (Fig. 3A) and in more frequent intervals (J.M.G., unpublished data), did not uncover a rhythmic component in this process. Although the principles of sperm release are shared in moths and flies (34, 35), fly spermatozoa are extremely long (up to 2 mm) and their slow translocation into the SV via a narrow testis neck (see Fig. 4A) may mask a putative release rhythm in the lower testes. The expression of clock genes in the sperm release zone in both moths (31) and flies further implies that the circadian clock may aid sperm release in both groups of insects. Clock genes are also expressed in testes of zebrafish, mouse, rat, and humans (36), suggesting a possibility that circadian clocks may be involved in fecundity across animal phyla.
Our finding that clock-mutant males have fewer sperm available for mating provides an explanation for the low fertility phenotype of such males. Males lacking per or tim function sire 30–50% fewer larvae because females inseminated by them deposit fewer eggs than those inseminated by wild-type males (Fig. 2). The rate of oogenesis and oviposition in D. melanogaster depends on transfer of sperm during mating (37, 38). Clock-mutant males, having about 40% less sperm stored in the SV than wild-type males, are likely to transfer proportionally fewer spermatozoa to females; this may adversely affect egg output. Proteins produced by male accessory glands also stimulate egg production in mated females (39); however, clock genes are not expressed in accessory glands, therefore their contribution to mating should not be affected directly by mutations in those genes.
The second factor contributing to low offspring in females inseminated by clock-mutant males is a higher incidence of unfertilized egg deposition. The lower number of stored sperm may underlie the female phenotype; however, it is also possible that the loss of circadian clock function adversely affects quality or viability of sperm. It is known that disruption of the circadian system in moths leads to production of defective sperm that fail to fertilize eggs (14). It has been proposed that the circadian system orchestrate the physiological processes involved in posttesticular maturation of sperm in moths (36, 40). Rhythmic expression of clock genes in the sperm-storing SVs suggests that clock-controlled processes may also aid sperm maturation in flies. Although we surmise that lowered reproductive potential of clock-mutant males is attributable to the loss of clock function in the RS, the lack of clock function in other organs may adversely affect the physiological and metabolic state of the fly (5, 41). These conditions could lead to diminished resources available for gamete production.
In conclusion, we demonstrated a low-fertility phenotype in clock-deficient mutants of D. melanogaster that correlates with decreased sperm release. Our results provide direct evidence that genes essential for the operation of the circadian clock are important for reproductive fitness. Although these genes may also have clock-unrelated functions, the low-fertility phenotype consistently observed in flies defective in any of the four clock genes tested (per, tim, Clk, and cyc) suggests that they aid reproduction by means of their respective roles in clock mechanism. Working at the molecular and physiological level, we identified an autonomous clock in the reproductive system and its role at the end of the clock-output pathway. To dissect molecular components of this pathway, we initiated microarray screening of expressed sequence tags for testis-specific genes differentially expressed during day and night (J. Lü, B. Oliver, and J.M.G., unpublished data, and ref. 42).
Acknowledgments
We thank J. Hall for anti-PER antibody and helpful discussions, A. Vilella for gift of mating wheels, M. Young for anti-TIM antibody, and B. Taylor for suggestions on experimental design. This work was supported by National Science Foundation and U.S. Department of Agriculture grants (to J.M.G.) and Deutsche Forschungsgemeinschaft Grant STA421/3 (to R.S.).
Abbreviations
- RS
reproductive system
- SV
seminal vesicle
- ZT
Zeitgeber time
- LSD
least significant difference
- LD
12-h light/12-h dark cycles
- DD
24-h dark period
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dunlap J C. Cell. 1999;96:271–290. doi: 10.1016/s0092-8674(00)80566-8. [DOI] [PubMed] [Google Scholar]
- 2.Williams J A, Sehgal A. Annu Rev Physiol. 2001;63:729–755. doi: 10.1146/annurev.physiol.63.1.729. [DOI] [PubMed] [Google Scholar]
- 3.Plautz J D, Kaneko M, Hall J C, Kay S A. Science. 1997;278:1632–1635. doi: 10.1126/science.278.5343.1632. [DOI] [PubMed] [Google Scholar]
- 4.Giebultowicz J M, Stanewsky R, Hall J C, Hege D M. Curr Biol. 2000;10:107–110. doi: 10.1016/s0960-9822(00)00299-2. [DOI] [PubMed] [Google Scholar]
- 5.Krishnan B, Levine J D, Lynch M K, Dowse H B, Funes P, Hall J C, Hardin P E, Dryer S E. Nature (London) 2001;411:313–317. doi: 10.1038/35077094. [DOI] [PubMed] [Google Scholar]
- 6.Whitmore D, Foulkes N S, Strahle U, Sassone-Corsi P. Nat Neurosci. 1998;1:701–708. doi: 10.1038/3703. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block G D, Sakaki Y, Menaker M, Tei H. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 8.Ouyang Y, Andersson C R, Kondo T, Golden S S, Johnson C H. Proc Natl Acad Sci USA. 1998;95:8660–8664. doi: 10.1073/pnas.95.15.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kyriacou C P, Oldroyd M, Wood J, Sharp M, Hill M. Heredity. 1990;64:395–401. doi: 10.1038/hdy.1990.50. [DOI] [PubMed] [Google Scholar]
- 10.Klarsfeld A, Rouyer F. J Biol Rhythms. 1998;13:471–478. doi: 10.1177/074873098129000309. [DOI] [PubMed] [Google Scholar]
- 11.Sheeba V, Sharma V K, Shubha K, Chandrashekaran M K, Joshi A. J Biol Rhythms. 2000;15:380–392. doi: 10.1177/074873000129001477. [DOI] [PubMed] [Google Scholar]
- 12.Giebultowicz J M, Riemann J G, Raina A K, Ridgway R L. Science. 1989;245:1098–1100. doi: 10.1126/science.245.4922.1098. [DOI] [PubMed] [Google Scholar]
- 13.Bebas P, Cymborowski B, Giebultowicz J M. J Insect Physiol. 2001;47:859–866. [Google Scholar]
- 14.Giebultowicz J M, Ridgway R L, Imberski R B. Physiol Entomol. 1990;15:149–156. [Google Scholar]
- 15.Fuller M T. In: The Development of Drosophila melanogaster. Bate M, Martinez Arias A, editors. Vol. 1. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 271–147. [Google Scholar]
- 16.Hall J C. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 17.Konopka R J, Benzer S. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamblen-Coyle M, Konopka R J, Zwiebel L J, Colot H V, Dowse H B, Rosbash M, Hall J C. J Neurogenet. 1989;5:229–256. doi: 10.3109/01677068909066210. [DOI] [PubMed] [Google Scholar]
- 19.Sehgal A, Price J, Man B, Youngs M. Science. 1994;263:1603–1606. doi: 10.1126/science.8128246. [DOI] [PubMed] [Google Scholar]
- 20.Allada R, White N E, So W V, Hall J C, Rosbash M. Cell. 1998;93:791–804. doi: 10.1016/s0092-8674(00)81440-3. [DOI] [PubMed] [Google Scholar]
- 21.Rutila J E, Suri V, Le M, So V, Rosbash M, Hall J C. Cell. 1998;93:805–814. doi: 10.1016/s0092-8674(00)81441-5. [DOI] [PubMed] [Google Scholar]
- 22.Stempfl, T., Vogel, M., Szabo, G., Wülbeck, C., Liu, J., Hall, J. C. & Stanewsky, R. (2002) Genetics160, in press. [DOI] [PMC free article] [PubMed]
- 23.Citri Y, Colot H V, Jacquier A C, Yu Q, Hall J C, Baltimore D, Rosbash M. Nature (London) 1987;326:42–47. doi: 10.1038/326042a0. [DOI] [PubMed] [Google Scholar]
- 24.Rutila J E, Maltseva O, Rosbash M. J Biol Rhythms. 1998;13:380–392. doi: 10.1177/074873098129000200. [DOI] [PubMed] [Google Scholar]
- 25.Stanewsky R, Frisch B, Brandes C, Hamblen-Coyle M J, Rosbash M, Hall J C. J Neurosci. 1997;17:676–696. doi: 10.1523/JNEUROSCI.17-02-00676.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko M, Hall J C. J Comp Neurol. 2000;422:66–94. doi: 10.1002/(sici)1096-9861(20000619)422:1<66::aid-cne5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 27.Stanewsky R, Jamison C F, Plautz J, D, Kay S A, Hall J C. EMBO J. 1997;16:5006–5018. doi: 10.1093/emboj/16.16.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall J C. Genetics. 1979;92:437–457. doi: 10.1093/genetics/92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai T, Ishida N. Proc Natl Acad Sci USA. 2001;98:9221–9225. doi: 10.1073/pnas.151443298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hege D M, Stanewsky R, Hall J C, Giebultowicz J M. J Biol Rhythms. 1997;12:300–308. doi: 10.1177/074873049701200402. [DOI] [PubMed] [Google Scholar]
- 31.Gvakharia B O, Kilgore J A, Bebas P, Giebultowicz J M. J Biol Rhythms. 2000;15:27–35. doi: 10.1177/074873040001500102. [DOI] [PubMed] [Google Scholar]
- 32.Plautz J D, Straume M, Stanewsky R, Creston F J, Brandes C, Dowse H B, Hall J C, Kay S A. J Biol Rhythms. 1997;12:204–217. doi: 10.1177/074873049701200302. [DOI] [PubMed] [Google Scholar]
- 33.Hall J C. Trends Neurosci. 1995;18:230–240. doi: 10.1016/0166-2236(95)93908-g. [DOI] [PubMed] [Google Scholar]
- 34.Giebultowicz J M, Weyda F, Erbe E F, Wergin W P. J Insect Physiol. 1997;43:1133–1147. doi: 10.1016/s0022-1910(97)00061-9. [DOI] [PubMed] [Google Scholar]
- 35.Tokuyasu K T, Peacock W J, Hardy R W. Z Zellforsch Mikrosk Anat. 1972;127:492–525. doi: 10.1007/BF00306868. [DOI] [PubMed] [Google Scholar]
- 36.Giebultowicz J M. Phil Trans R Soc London B. 2001;356:1791–1799. doi: 10.1098/rstb.2001.0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heifetz Y, Tram U, Wolfner M F. Proc R Soc London B. 2001;268:175–180. doi: 10.1098/rspb.2000.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xue L, Noll M. Proc Natl Acad Sci USA. 2000;97:3272–3275. doi: 10.1073/pnas.060018897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfner M F. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 40.Bebas P, Cymborowski B, Giebultowicz J M. J Exp Biol. 2002;205:37–44. doi: 10.1242/jeb.205.1.37. [DOI] [PubMed] [Google Scholar]
- 41.Sarov-Blat L, So W V, Liu L, Rosbash M. Cell. 2000;101:647–656. doi: 10.1016/s0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 42.Andrews J, Bouffard G G, Cheadle C, L, Becker K G, Oliver B. Genome Res. 2000;10:2030–2043. doi: 10.1101/gr.10.12.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]