Abstract
Mismatch repair genes are important in maintaining the fidelity of DNA replication. To determine the function of the Caenorhabditis elegans homologue of the MSH2 mismatch repair gene (msh-2), we isolated a strain of C. elegans with an insertion of the transposable element Tc1 within msh-2. Early-passage msh-2 mutants were similar to wild-type worms with regard to lifespan and meiotic chromosome segregation but had slightly reduced fertility. The mutant worms had reduced DNA damage-induced germ-line apoptosis after genotoxic stress. The msh-2 mutants also had elevated levels of microsatellite instability and increased rates of reversion of the dominant unc-58(e665) mutation. In addition, serially passaged cultures of msh-2 worms died out much more quickly than those of wild-type worms. These results demonstrate that msh-2 function in C. elegans is important in regulating both short- and long-term genomic stability.
Keywords: apoptosis‖mutation‖mutator‖fertility
The low mutation rate observed in wild-type organisms is not a consequence of intrinsically accurate DNA replication but, instead, reflects the existence of mechanisms that are capable of removing DNA polymerase errors (1). One of these mechanisms is postreplication DNA mismatch repair (MMR) (2). In Escherichia coli, MMR requires MutS, MutL, MutH, as well as a number of other proteins (3). In the yeast Saccharomyces cerevisiae, MMR requires proteins homologous to MutS (which binds the mismatch) and MutL, although all of the components required for strand-specific repair have not been identified (4). In yeast, three complexes have a role in MMR: a complex with Msh2p, Msh6p, Pms1p, and Mlh1p, primarily involved in the repair of base–base mismatches, a complex with Msh2p, Msh3p, Pms1p, and Mlh1p, involved in the repair of small DNA loops, and a complex with Msh2p, Msh3p, Mlh1p, and Mlh3p that has a minor role in DNA loop repair (4). Because the Msh2p is a component of all three complexes, msh2 mutants lack MMR.
In yeast, msh2 strains have a mutator phenotype (5). Although the frequency of point mutations is elevated in msh2 strains, there is a greater effect on the stability of simple, repetitive DNA sequences (microsatellites) (6). In addition to their role in the correction of DNA polymerase errors, the MutS homologues have other roles: repair of mismatches during recombination, removal of branched DNA structures formed during mitotic recombination of nontandemly arranged repeated genes, transcription-coupled repair of UV damage, recognition of damaged DNA bases, and prevention of recombination between homologous DNA sequences (4). In mammals, loss of mismatch repair is also associated with reduction of apoptosis associated with certain mutagens (7).
Although most of the MutS and MutL homologues function in DNA repair, in both yeast and Caenorhabditis elegans, Msh4p and Msh5p are involved only in meiotic crossing-over (4). Although mutations in MLH1, MSH5, and PMS2 result in male and/or female infertility in mice, no short-term effect on fertility is observed in mice homozygous for the msh2 mutation (7). In humans and mice, mutations in MSH2 result in a greatly increased likelihood of developing certain types of tumors and tumors derived from MMR-deficient cells have increased rates of microsatellite instability (7).
Analysis of the genome sequence of C. elegans indicates the existence of homologues of MSH2, MSH4, MSH5, MSH6, PMS1, MLH1, and MLH3, but not MSH3 and MLH2 (4). To determine the role of msh-2 in C. elegans, we isolated msh-2 mutant worms. The mutant worms had elevated rates of microsatellite instability and reduced DNA damage-induced germ-line apoptosis. Serially passaged msh-2 mutant lines died out much more rapidly than wild-type lines, suggesting that msh-2 is required for long-term genome stability.
Materials and Methods
Identification of Worms with the msh2(ev679∷Tc1) Allele.
Strains of C. elegans were grown at 18–20°C and handled by standard procedures (8). Wild-type strains were N2 Bristol. The Tc1-induced msh-2 mutation was isolated in a worm line (MT-3126) with increased levels of germ-line Tc1 transposition (9) by using a PCR-based screen with primers from the msh-2 locus and Tc1 (10, 11). The H26D21.2 ORF (C. elegans WormPeptide database) is closely (E value = e−96) related to the yeast Msh2p. We designed eight primers for this gene and four Tc1-specific primers (Tc1-L1, -L2, -R1, -R2) (Table 1 and Fig. 1).
Table 1.
Primers used in this study
| Name | 5′→3′ sequence |
|---|---|
| msh-2-A | gcgttgttcttgcgtagactcc |
| msh-2-B | aaggctgagaatggcttgtcgg |
| msh-2-C | gcaagcatgttcacgagaatgg |
| msh-2-D | acattgtgcgcttgggatgctgg |
| msh-2-J1 | ccaagttcatcaattaccacga |
| msh-2-J2 | cgtcttcttaaatcagaaatcaccg |
| msh-2-P3 | accaactcgtgtgaaaatccc |
| msh-2-P4 | gctatggctcagctcatcgc |
| Tc1-L1 | cgtgggtattccttgttcgaagccagctac |
| Tc1-L2 | tcaagtcaaatggatgcttgag |
| Tc1-R1 | tcacaagctgatcgactcgatgccacgtcg |
| Tc1-R2 | gattttgtgaacactgtggtgaag |
| (GT)14-F | aagggcatgaggagaagtcaacgg |
| (GT)14-R | gagcagctctcgttctgattgc |
| (GT)26-F | gctccacttttcaacatccttcc |
| (GT)26-R | ttgtccccgctatttcgctc |
| (GT)59-F | accccaatggactacgttgtgc |
| (GT)59-R | acgtgacgcctttggttcatag |
| (AAT)28-F | aacaaaaatgtggcagggag |
| (AAT)28-R | gggttacggtagtggtactgtagg |
| (AAAT)43-F | gaccattagatgaatttcc |
| (AAAT)43-R | cgagcaacagaatttttac |
Primers msh-2-A to msh-2-P4 represent sequences within msh-2 gene (Fig. 1). Primers Tc1-L1 to Tc1-R2 represent sequences within the Tc1 element. Primers (GT)14-F to (AAAT)43-R are derived from sequences that flank microsatellites in the C. elegans genome (L. Frisse, L. Vassilieva, M. Lynch, and W. K. Thomas, personal communication).
Figure 1.
Structure of the msh-2 C. elegans gene. The msh-2 gene corresponds to the sequence of ORF H26D21.2 (Wormbase) and is flanked by ORFs H26D21.1 and D1037.5; black arrows show the transcriptional orientation of the flanking genes. Putative exons and introns are shown as black and white rectangles, respectively. The positions of the primers used in the study are shown by gray arrows.
Pools from a library derived from about 600 individual cultures of MT-3126 worms were screened with four pairs of primers (A/R1, A/L1, D/R1, and D/L1). With one of these pairs (msh-2-A and Tc1-R1), we found a strain (NW 1270 ev679∷Tc1) that had a PCR fragment of about 1.1 kb. To determine the precise position of the insertion, we used PCR amplification with two pairs of primers (msh-2-J2 and L2, and msh-2-B and R2) to generate DNA fragments containing the two Tc1-msh-2 junctions. Sequence analysis showed that Tc1 was inserted at amino acid 648 (Fig. 1); this allele is msh-2(ev679∷Tc1).
Ten backcrosses of the msh-2 mutant strain with a wild-type N2 strain were done to generate msh-2 strains with low levels of transposition. The segregation of the mutant msh-2 allele was followed by PCR by using the primers P3, P4, and R2 (Fig. 1). Amplification of DNA containing the msh-2(ev679∷Tc1) allele with R2 and P3 resulted in an ≈200-bp DNA fragment, whereas amplification of the wild-type msh-2 allele with P4 and P3 resulted in an ≈400-bp fragment. After the last backcross, we allowed self-fertilization, resulting in five homozygous msh-2 lines: M1–M5.
Construction of msh-2(ev679∷Tc1), unc-58(e665) Strains.
The unc-58(e665) allele is a dominant mutation resulting in paralysis. Intragenic and extragenic suppressor mutations in unc-58 restore movement (ref. 12; M. Tzoneva and J. Thomas, personal communication). We crossed hermaphrodites homozygous for the unc-58(e665) mutation (strain CB665 backcrossed five times to N2; S. Ahmed, personal communication) to males homozygous for msh-2(ev679∷Tc1), line M2. F1 progeny from this cross were allowed to self-fertilize, and F2 worms were screened for those homozygous for unc-58(e665) and msh-2(ev679∷Tc1).
Measurements of Egg Laying and Egg Hatching.
Single L4 adults were placed on plates containing bacteria. Twice a day, the adults were moved to new plates. After egg hatching, but before the newly hatched worms were laying their own eggs, the worms were counted. L4 worms were counted and removed during the counting.
Lifespan Measurements.
Fifteen wild-type or msh-2 worms (passage 15 of the M2 line) were placed on single plates and allowed to lay eggs for 2 h. The adults were then removed. After the resulting eggs had hatched, the worms were allowed to develop to the L4 stage and then were counted and transferred to new plates. These transfers were repeated every day until the worms stopped laying eggs. The numbers of dead worms on the plates were counted daily.
Analysis of Microsatellite Alterations.
DNA was purified (13) from worms grown on 60-mm plates. We did PCR amplification by using DNA primers that flanked the microsatellites (Table 1) and one labeled nucleotide (dATP) (14). The resulting DNA fragments were analyzed on 6% polyacrylamide DNA sequencing gels. Five different microsatellites were examined.
Analysis of DNA Damage-Induced Apoptosis in the Germ Line.
The methods of Gartner et al. (15) were used. In brief, synchronized worms (24 h after the L4/adult molt) were exposed to gamma rays derived from a 137Cs source and mounted onto glass slides by using standard conditions. Apoptotic cell corpses in one gonad arm were scored by using Nomarski optics (15).
Statistical Analysis.
Statistical comparisons were done by using the instat 1.12 and prism 3.0 programs for Macintosh.
Results
Identification of msh-2 Mutant Worms.
To identify a worm strain with a Tc1-induced msh-2 mutation, we used a PCR screen of a library of worms with random insertions of Tc1 (9–11). After 10 backcrosses of the original mutant strain to the wild type, we isolated five lines of worms, M1–M5, homozygous for the msh-2(ev679∷Tc1) allele. These strains were used in subsequent studies.
The Msh2 protein has four domains involved in ATP binding (16). The insertion in msh-2(ev679∷Tc1) is within domain I (Fig. 1). In yeast, point mutations within the ATP-binding domains result in the same mutator phenotype as the null msh2 mutation (17). The primers P3 and P4 produce an ≈400-bp fragment if DNA with the wild-type allele is amplified, and primers P3 and R2 produce a 200-bp fragment if DNA with the msh-2(ev679∷Tc1) allele is amplified (Fig. 1). Amplification of DNA derived from the M1–M5 homozygous lines with the P3 and P4 primers results in a small amount of the 400-bp product, whereas amplification of this DNA by P3 and R2 produces a large amount of the 200-bp product. Because the M1–M5 lines never give rise to homozygous wild-type offspring, the small amount of the 400-bp fragment likely represents somatic excision of the Tc1 element. The high frequency of Tc1 excision from the somatic cells, but retention of the insertion in the germ line, is expected (18).
Somatic excision of a Tc1 element does not usually restore the wild-type sequence (19). DNA was isolated from small pools of worms from each of the five homozygous msh-2 lines. This DNA was amplified with the primers described above, and the 400-bp fragment characteristic of the msh-2 gene lacking the Tc1 insertion was cloned and sequenced. Two individual clones were sequenced for each line, except for M4. In none of the nine events (at least seven of which were independent) was the wild-type sequence restored. Additions of ACAT (six events), AGTT (one event), AGT (one event), and AT (one event) were observed. Because only one of these excision events resulted in an in-frame insertion, most of the cells in worms in the homozygous msh-2 lines, even those in which the Tc1 element was excised somatically, lack MSH-2 function. This conclusion is supported by the analysis of the microsatellite instability described below.
msh-2(ev679∷Tc1) Worms Have Reduced Fertility.
Fertility of mutant msh-2 strains relative to wild type was examined. A total of 25 msh-2 mutant worms were examined from three different lines (M1, M2, and M3). All three lines were examined at early passages after their initial characterization (P1); the M2 line was also examined after 15 passages (P15). The average numbers of hatched (indicated before the + sign) and unhatched eggs (indicated after the + sign) in the msh-2 lines were: M1(P1), 122 + 33; M2(P1), 188 + 38; M2(P15), 222 + 12; and M3(P1), 94 + 22. The average numbers of hatched and unhatched eggs from all of the msh-2 mutant lines (95% confidence limits shown in parentheses) were 170 (±33) and 23 (±10), respectively.
We examined 15 wild-type worms, 10 of which were subcultured for 15 generations in parallel with the M2 line. The average number of hatched and unhatched eggs in these experiments were (P indicating passage number): N2(P1), 233 + 4; N2(P15), 247 + 5. The average numbers of hatched and unhatched eggs from all of the wild-type worms (95% confidence limits shown in parentheses) were 242 (±47) and 5 (±4), respectively. The differences in the number of hatched eggs and the number of unhatched eggs in msh-2 and wild-type worms were both significant (P < 0.01 by Mann–Whitney nonparametric test).
One interpretation of this result is that the msh-2 mutation directly affects fertility. For example, if the mutation reduced meiotic crossing-over, one would expect increased levels of chromosome nondisjunction, leading to a decrease in the level of viable progeny. Alternatively, the msh-2-associated mutator phenotype might elevate the rate of mutations that reduce fertility. We favor the second explanation because, as described below, the msh-2 mutation does not appear to increase meiotic chromosome nondisjunction.
Meiotic Chromosome Nondisjunction Is Normal in msh-2(ev679∷Tc1) Worms.
Mutations in several of the MutS and MutL homologues reduce gamete viability (7). In wild-type hermaphrodites of C. elegans, males arise as a consequence of nondisjunction of the X chromosome at a frequency of about 0.2% (20). Worms with a mutation in him-14 (a MutS homologue related to MSH4) have reduced crossing-over (21), and 45% of the surviving progeny are male (22). We found only a single male in the 1,445 progeny of five wild-type adults, a frequency of 0.07%. Two males were detected in the 1,741 progeny of 10 mutant msh-2 adults, a frequency of 0.1%. Thus, the msh-2 mutation does not significantly elevate meiotic chromosome nondisjunction. Because mutations that reduce crossovers increase nondisjunction in C. elegans (23), as they do in other organisms, the msh-2 mutation does not significantly reduce meiotic crossing-over.
msh-2(ev679∷Tc1) Worms Have a Normal Lifespan.
Different wild-type strains of C. elegans have different lifespans, varying between 12 and 18 days at 20° (24). We measured lifespan for wild-type and msh-2(ev679∷Tc1) worms maintained on plates at 18°. The average lifespans for wild type (75 worms) and msh-2(ev679∷Tc1) (107 worms) were 13.1 ± 1.6 and 13.8 ± 1.0 days, respectively. These results indicate that the msh-2(ev679∷Tc1) mutation has no significant effect on the lifespan of individual worms. Because we began the lifespan measurements with L4 worms (as described in Materials and Methods), influences of the msh-2 mutation on fertility or survival of early larval stages were not examined in these experiments. As described below, the long-term survival of serially passaged mutant lines is reduced significantly relative to wild-type lines.
Reduced Long-Term Survival of msh-2 Worm Cultures.
To determine the effects of the msh-2 mutation on the long-term survival of worm lines and on microsatellite stability, we set up subcultures from 40 homozygous msh-2 mutant (10 each from M1–M4) and 40 wild-type worms. At each passage, a single L4 hermaphrodite from each subculture was transferred to a new plate. After eggs were laid, the adult was removed. When the L4 hermaphrodites developed from the eggs, one was transferred to a new plate and allowed to lay eggs. Every five generations, worms from each line were frozen in two aliquots, one providing a worm stock and one as a source of DNA for analysis of microsatellite instability.
A graph of the number of surviving worm lines as a function of the number of generations is shown in Fig. 2. For the wild-type worms, we terminated the experiment at passage 20. We continued the experiment with the msh-2 worms until the last line died out (at passage 38). After 20 generations, only 20% of the wild-type lines had become extinct compared with 95% of the msh-2 lines. Of the seven wild-type lines that became extinct, in four lines, the transferred worm died before laying eggs (class 1); in one line, the worm was viable, but failed to lay eggs (class 2); and in two lines, the transferred worms could not be detected on the plate and no eggs were laid (class 3). The extinctions observed with the msh-2 worm lines included 10 class 1 events, nine class 2 events, and eight class 3 events. There were also nine lines in which eggs were laid but did not hatch (class 4) and four lines in which the eggs hatched but the worms failed to develop to the L4 stage (class 5). The simplest interpretation of these results is that the mutant msh-2 worms have a mutator phenotype and that the level of mutations affecting fertility or viability accumulate with passing generations.
Figure 2.
Reduced survival of serially passaged cultures of msh-2 mutant worms. Forty subcultures each of wild-type (indicated by squares) and msh-2 mutant (indicated by circles) worms were initiated. Single L4 hermaphrodites were transferred to new plates at each passage. For the wild-type worms, the experiment was ended after 20 such passages.
msh-2 Is Required for Microsatellite Stability.
Because mutations in MSH2 are associated with microsatellite instability in yeast, Drosophila, and mammals (4), we examined microsatellite stability in the wild-type and mutant msh-2 lines. DNA was isolated from small pools of worms derived from each viable line every five generations. We measured the lengths of microsatellites by using DNA-sequencing gels to examine the sizes of DNA fragments generated by PCR amplification, using primers flanking the microsatellites. Five microsatellite loci, a subset of those examined in wild-type worm lines by Frisse et al. (L. Frisse, L. Vassilieva, M. Lynch, and W. K. Thomas, personal communication), were examined. The microsatellites (GenBank accession numbers indicated in Table 2) were: (GT)14, (GT)26, (GT)59, (AAT)28, and (AAAT)43. The (GT)14, (GT)26, (AAT)28, and (AAAT)43 microsatellites were the same size at the first passage in both the mutant and wild-type worm lines. Because of the extreme instability of the poly(GT)59 microsatellite in the mutant msh-2 strains, even at the beginning of the lineage experiment, this microsatellite had lengths that varied between 57 and 61 repeats. At the beginning of the experiment, the N2 wild-type worm used to initiate the wild-type lineages was heterozygous for alleles of 55 and 63 repeats.
Table 2.
Microsatellite instability in msh-2(ev679∷Tc1) and wild-type worms
| Microsatellite | GenBank accession number | Number of microsatellite alterations/total samples tested
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Passage 1–5
|
Passage 5–10
|
Passage 10–15
|
Passage 15–20
|
Total
|
|||||||
| Wild type | msh-2 (ev679) | Wild type | msh-2 (ev679) | Wild type | msh-2 (ev679) | Wild type | msh-2 (ev679) | Wild type | msh-2 (ev679) | ||
| (GT)14 | U50067 | 0/37 | 1/29 | 0/35 | 2/18 | 0/33 | 0/7 | 0/31 | 0/3 | 0/136 | 3/57 |
| (GT)26 | Z81497 | 0/35 | 3/29 | 0/33 | 7/18 | 0/33 | 3/8 | 0/31 | 0/3 | 0/132 | 13/58 |
| (GT)55 | Z81558 | 0/23 | NA | 0/21 | NA | 0/24 | NA | 0/23 | NA | 0/91 | NA |
| (GT)“59” | Z81558 | NA | 7/13 | NA | 9/16 | NA | 4/7 | NA | 2/3 | NA | 22/39 |
| (GT)63 | Z81558 | 0/14 | NA | 0/3 | NA | 0/9 | NA | 0/8 | NA | 0/34 | NA |
| (AAT)28 | AF016674 | 0/36 | 3/29 | 0/35 | 1/18 | 0/34 | 1/7 | 0/32 | 0/3 | 0/134 | 5/56 |
| (AAAT)43 | Z73422 | 0/33 | 2/27 | 0/29 | 3/15 | 0/31 | 0/6 | 0/30 | 0/3 | 0/126 | 5/52 |
| Total | 0/178 | 16/127 | 0/156 | 22/85 | 0/164 | 8/35 | 0/155 | 2/15 | 0/653 | 48/262 | |
NA, not available.
A summary of the frequencies of alterations detected is shown in Table 2. No alterations were detected in the wild-type worm lines (0 alterations in 653 samples tested at five passage intervals for 20 passages). In contrast, in the mutant lines, we found 48 alterations in 262 samples tested. A line was scored as having an alteration if it had a different number of repeats from the number scored in the same line five passages previously. The difference in the frequency of alterations between wild-type and mutant lines is significant at a P value of <0.0001 (Fisher exact test).
The frequencies in the total column in Table 2 represent the number of observed alterations divided by the number of worm lines examined at intervals of five passages. We assume about 15 cell divisions per worm generation, five divisions to produce germ cell precursors, and about 10 divisions of the germ cell precursors to produce the gametes (25); the latter number is only approximate, because there is a switch from exponential growth of germ cells to stem cell-like divisions during development of the gonad (26). Using these numbers, we can convert the frequencies of alterations to rates of alterations per cell division. For example, the rate of alterations for the (GT)14 microsatellite in the mutant msh-2 strain is about 3/(57 × 5 × 15) or 7 × 10−4/cell division. Rates for the other microsatellites in the msh-2 mutant lineages are: 3 × 10−3 ([GT]26), 8 × 10−3 ([GT]“59”), 1 × 10−3 ([AAT]28), and 1 × 10−3 ([AAAT]43). As expected from previous studies in yeast (27), the rate of instability increases as the number of (GT) repeats is increased. Our calculated rates are estimates that do not compensate for the fluctuations caused by the stochastic nature of mutations (28). Nonetheless, the rates of alterations for the mutant worms are remarkably similar to those estimated more directly in msh2 mutant yeast strains with poly(GT) tracts of similar sizes (27): 9 × 10−4 ([GT]16.5), 5 × 10−3 ([GT]26.5), and 6.3 × 10−3 ([GT]49.5).
Although we did not observe microsatellite alterations in the wild-type strain, in a more extensive analysis involving 80 wild-type worm lines propagated for 140 generations, Frisse et al. (L. Frisse, L. Vassilieva, M. Lynch, and W. K. Thomas, personal communication) found alterations in four of five of these same microsatellites. From these data, they calculated approximate mutation rates of: 1.8 × 10−4/worm generation and 1.8 × 10−5/cell division for the (GT)26 tract, and 9.5 × 10−5/cell division for the (GT)59 tract; no alterations were detected in the (GT)14 tract. It was noted that these rates of microsatellite instability are similar to those observed in wild-type yeast cells. We estimate that dinucleotide microsatellites were destabilized about 100-fold in msh-2 mutant worms, a destabilization similar to that observed in msh2 yeast (6) and mammalian cells (29).
The numbers and types of microsatellite alterations (indicated in number of repeats; additions by + and deletions by −) for all microsatellites were: (GT)14 (3, +1); (GT)26 (7, +1; 1, +2; 4, −1; 1, −2); (GT)“59” (9, +1; 3, +2; 2, +4; 6, −1, 1, −2; 1, −3); (AAT)28 (3, +1; 2, −1); and (AAAT)48 (5, +1). As observed previously in MMR-deficient yeast and mammalian cells (6, 14, 29), most (39 of 48) of the alterations were single repeat changes. There is also a significant (P of 0.01 by χ2 test) bias in favor of insertions over deletions. Similar biases have been observed in mismatch repair-deficient mammalian cells (30) and Drosophila (B. Harr, J. Todorova, and C. Schlotterer, personal communication) and for long microsatellites in msh2 yeast (27).
Worm Strains with the msh-2 Mutation Have Elevated Frequencies of Reversion for the unc-58(e665) Mutation.
Many microsatellites are located in noncoding genomic regions. To determine whether the msh-2 mutation also affected mutation rates in coding regions of the genome, we examined the effects of the mutation on reversion of the paralysis phenotype associated with unc-58(e665). The unc-58(e665) allele is a dominant point mutation leading to paralysis (ref. 8; M. Tzoneva and J. Thomas, personal communication). Because worms that have null unc-58 mutations are not paralyzed, worms that have reverted the paralysis phenotype of unc-58(e665) often have a second inactivating mutation within unc-58 (ref. 12; M. Tzoneva and J. Thomas, personal communication).
We used two worm strains, one containing only the unc-58(e665) mutation and one with defects in both msh-2 and unc-58. For each strain, we transferred 3–5 hermaphrodites to each of many (200 or more) small (60-mm) Petri dishes. After 3 weeks at 20°, the starved worms were transferred to large (90-mm) Petri dishes and, 2 days later, were scored for mobile revertants. Only one of 200 dishes had revertants for the wild-type strain, whereas 67 of 371 dishes had revertants for the msh-2 strain; this difference is significant at a P value of <0.0001 (Fisher exact test). Forty independent revertants from the mutant strain were crossed to wild-type males, and progeny derived from the offspring of this mating were examined. In 35 of the 40 revertants, no paralyzed offspring were detected, indicating that the reversion event was intragenic. Using unc58-specific primers in a PCR analysis, we found no alterations in the size of the unc-58 gene in five of five intragenic revertants, indicating that the inactivating mutations were not large deletions or insertions of transposable elements. The elevated mutation rates, therefore, do not reflect residual Tc1 transposition events.
Reduced DNA Damage-Induced Apoptosis in the Germ Line of msh-2(ev679∷Tc1) Worms.
Both mouse and human msh2 cells have defective DNA damage-induced apoptosis (7). Apoptosis can be induced in the germ line of hermaphrodites by DNA-damaging agents such as x-rays (15). The damage-induced apoptosis requires some of the same gene functions needed for programmed cell death as well as some checkpoint proteins that do not affect developmental programmed cell death (15).
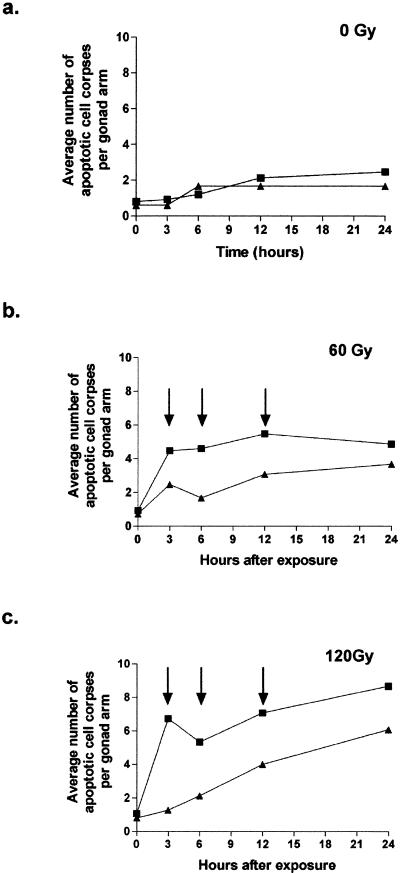
We examined DNA damage-induced apoptosis by using microscopy to count cell corpses in the gonad arms of synchronized worms (24 h after the L4/adult molt) treated with various doses of γ-rays. Fifteen wild-type and 15 msh-2 mutant (M2 line) worms were analyzed for each dose; similar results were also obtained with the M1 line (data not shown). In unirradiated control wild-type and msh-2 mutant worms, the number of corpses was low for all time points and was not different for the two genotypes (Fig. 3a). In contrast, the msh-2 mutant worms had significantly fewer corpses than wild-type worms 3, 6, and 12 h after radiation with either 60 (Fig. 3b) or 120 (Fig. 3c) Gy. Twenty-four hours after radiation, the difference between msh-2 and wild-type worms was no longer statistically significant, although there were still fewer corpses in the msh-2 worms. Thus, DNA damage-induced apoptosis is reduced and/or delayed in the absence of msh-2, but is not eliminated.
Figure 3.
Reduced response of msh-2 mutant worms to DNA damage-induced apoptosis in the germ line. Twenty-four hours after the L4/adult molt, wild-type (▪) and msh-2 (▴) mutant worms were either irradiated (b and c) or left unirradiated (a). Worms were mounted for microscopy at time intervals of 3 h and examined for the number of apoptotic cell corpses in one arm of the gonad. Fifteen worms of each genotype were used in these experiments. For those time points indicated with arrows, there is a significant difference (P < 0.05 by Mann–Whitney nonparametric test) in the number of corpses.
Discussion
The Msh2 protein, in association with the Msh3 or Msh6 proteins, binds mismatched bases and certain unusual DNA structures (2, 3). The msh2 mutation is extremely pleiotropic in its effects in yeast, mammals, and worms (4, 7). In C. elegans, the msh-2(ev679∷Tc1) allele results in a mutator phenotype, a reduction in fertility and DNA damage-induced apoptosis in the germ line, and a dramatic loss of long-term survival of worm cultures. No effects on lifespan or meiotic chromosome disjunction were observed.
Microsatellite instability is a phenotype observed in msh2 mutants of yeast, mammalian cells, and Drosophila (4). The interpretation of this instability is that simple, repetitive DNA sequences have high rates of DNA polymerase slippage, producing mismatches involving one or more repeats (31). In a wild-type strain, these mismatches are corrected, restoring the original number of repeat units per microsatellite. In a mismatch repair-deficient strain, however, the failure to correct the mismatch results in either an addition of repeats (if the displaced repeats are on the primer strand) or a deletion of repeats (if the displaced repeats are on the template strand) (6). The msh-2 mutation also stimulates the reversion of unc-58(e665). Although the unc-58 gene in these revertants has not yet been sequenced, based on studies done in yeast (32, 33), one would predict that most of the alterations will be a loss or gain of single bases in small, homopolymeric tracts.
The msh-2 mutation also reduced fertility, although this reduction was modest. Although part of this reduction might be a direct effect of the msh-2 mutation on fertility, because different msh-2 lines had different levels of fertility, at least part of the reduction is likely to reflect mutations accumulated as a consequence of the mutator phenotype of these strains. This conclusion is also supported by the increased rate of extinction of serially passaged msh-2 lines. Our results argue that the mutation rates in msh-2 mutant worms are too high for the long-term survival of these populations.
Ahmed and Hodgkin (34) isolated C. elegans mutants that became sterile as they were passaged from one generation to the next. One of these mutants, mrt-2 (mortal germ line), results in a telomere-shortening phenotype. Our results argue that some mrt mutants are likely to contain mutations that result in increased levels of genomic damage. It also should be pointed out that the recent evidence that human cells with mismatch repair mutations have elevated rates of gene amplification (35) suggests the possibility that msh-2 worms may accumulate multiple types of genomic damage.
The msh-2 mutant worms have a diminished germ-line apoptotic response to DNA damage. One possibility is that the msh-2 protein affects the efficiency of this apoptotic pathway. Alternatively, there may be two discrete pathways affecting DNA damage-induced apoptosis in the germ line, one dependent and one independent of msh-2. Although the role of the Msh2 protein in apoptosis is not clear in any system investigated thus far, it is likely that binding of lesions in damaged DNA by mismatch repair proteins acts as an early signal for both the repair of DNA (if there are low levels of DNA damage) and the destruction of the cell by apoptosis (if there are high levels of DNA damage) (36). Although mutations in spellchecker1 (the Drosophila msh-2 homologue) lead to increased microsatellite instability, flies homozygous for this mutation are not sensitive to methyl methanesulfonate or to γ-irradiation (37).
In conclusion, although the effect of msh2 mutations is likely to be similar at the cellular level in unicellular and multicellular organisms (global elevation of mutation rates), the “readout” at the level of the organism will be quite different.
Acknowledgments
We thank P. Anderson, S. Ahmed, L. Frisse, L. Vassilieva, M. Lynch, M. Tzoneva, J. Thomas, B. Harr, J. Todorova, C. Schlotterer, R. Goldstein, J.-C. Labbe, and W. K. Thomas for communicating unpublished information and/or useful discussions. The research was supported by grants from the National Institutes of Health (GM24110 and GM52319 to T.P.; GM52540 to M.H.; and F32 GM20801 to E.R.H.), the Canadian Institutes of Health Research (to J.C.), and the Canadian Centre of Genetic Disease Network (to L.Z.).
Abbreviation
- MMR
DNA mismatch repair
References
- 1.Umar A, Kunkel T A. Eur J Biochem. 1996;238:297–307. doi: 10.1111/j.1432-1033.1996.0297z.x. [DOI] [PubMed] [Google Scholar]
- 2.Kolodner R D, Marsischky G. Curr Opin Genet Dev. 1999;9:89–96. doi: 10.1016/s0959-437x(99)80013-6. [DOI] [PubMed] [Google Scholar]
- 3.Modrich P, Lahue R. Annu Rev Biochem. 1996;65:101–133. doi: 10.1146/annurev.bi.65.070196.000533. [DOI] [PubMed] [Google Scholar]
- 4.Harfe B D, Jinks-Robertson S. Annu Rev Genet. 2000;34:359–399. doi: 10.1146/annurev.genet.34.1.359. [DOI] [PubMed] [Google Scholar]
- 5.Reenan R A, Kolodner R D. Genetics. 1992;132:975–985. doi: 10.1093/genetics/132.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strand M, Prolla T A, Liskay R M, Petes T D. Nature (London) 1993;365:274–276. doi: 10.1038/365274a0. [DOI] [PubMed] [Google Scholar]
- 7.Buermeyer A B, Deschenes S M, Baker S M, Liskay R M. Annu Rev Genet. 1999;33:359–399. doi: 10.1146/annurev.genet.33.1.533. [DOI] [PubMed] [Google Scholar]
- 8.Brenner S. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rushforth A M, Saari B, Anderson P. Mol Cell Biol. 1993;13:902–910. doi: 10.1128/mcb.13.2.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plasterk R H. Methods Cell Biol. 1995;48:59–80. doi: 10.1016/s0091-679x(08)61383-7. [DOI] [PubMed] [Google Scholar]
- 11.Roy P J, Zheng H, Warren C E, Culotti J G. Development (Cambridge, UK) 1999;127:755–767. doi: 10.1242/dev.127.4.755. [DOI] [PubMed] [Google Scholar]
- 12.Park E-C, Horvitz H R. Genetics. 1986;113:821–852. doi: 10.1093/genetics/113.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sulston J, Hodgkin J. In: The Nematode C. elegans. Wood W B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 587–606. [Google Scholar]
- 14.Sia E A, Kokoska R J, Dominska M, Greenwell P, Petes T D. Mol Cell Biol. 1997;17:2851–2858. doi: 10.1128/mcb.17.5.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gartner A, Milstein S, Ahmed S, Hodgkin J, Hengartner M O. Mol Cell. 2000;5:435–443. doi: 10.1016/s1097-2765(00)80438-4. [DOI] [PubMed] [Google Scholar]
- 16.New L, Liu K, Crouse G F. Mol Gen Genet. 1993;239:97–108. doi: 10.1007/BF00281607. [DOI] [PubMed] [Google Scholar]
- 17.Alani E, Sokolsky T, Studamire B, Miret J J, Lahue R S. Mol Cell Biol. 1997;17:2436–2447. doi: 10.1128/mcb.17.5.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emmons S W, Yesner L. Cell. 1984;36:599–605. doi: 10.1016/0092-8674(84)90339-8. [DOI] [PubMed] [Google Scholar]
- 19.Plasterk R H, van Luenen H. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 97–116. [PubMed] [Google Scholar]
- 20.Hodgkin J, Horvitz H R, Brenner S. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zalevsky J, MacQueen A J, Duffy J B, Kemphues K J, Villeneuve A M. Genetics. 1999;153:1271–1283. doi: 10.1093/genetics/153.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kemphues K J, Kusch M, Wolf N. Genetics. 1988;120:977–986. doi: 10.1093/genetics/120.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dernburg A F, McDonald K, Moulder G, Barstead R, Dresser M, Villeneuve A M. Cell. 1998;94:387–398. doi: 10.1016/s0092-8674(00)81481-6. [DOI] [PubMed] [Google Scholar]
- 24.Kenyon C. In: C. elegans II. Riddle D L, Blumenthal T, Meyer B J, Priess J R, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1997. pp. 791–813. [Google Scholar]
- 25.Kimble J, Ward S. In: The Nematode C. elegans. Wood W B, editor. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. pp. 191–213. [Google Scholar]
- 26.Greenstein D. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Wierdl M, Dominska M, Petes T D. Genetics. 1997;146:769–779. doi: 10.1093/genetics/146.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lea D E, Coulson C A. J Genet. 1949;49:264–285. doi: 10.1007/BF02986080. [DOI] [PubMed] [Google Scholar]
- 29.Hanford M G, Rushton B C, Gowen L C, Farber R A. Oncogene. 1998;16:2389–2393. doi: 10.1038/sj.onc.1201751. [DOI] [PubMed] [Google Scholar]
- 30.Twerdi C D, Boyer J C, Farber R A. Proc Natl Acad Sci USA. 1999;96:2875–2879. doi: 10.1073/pnas.96.6.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Streisinger G, Okada Y, Emrich J, Newton J, Tsugita A, Terzaghi E, Inouye M. Cold Spring Harbor Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- 32.Marsischky G, Filosi N, Kane M F, Kolodner R. Genes Dev. 1996;10:407–420. doi: 10.1101/gad.10.4.407. [DOI] [PubMed] [Google Scholar]
- 33.Hadjimarcou M I, Kokoska R J, Petes T D, Reha-Krantz L J. Genetics. 2001;158:177–186. doi: 10.1093/genetics/158.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed S, Hodgkin J. Nature (London) 2000;403:159–166. doi: 10.1038/35003120. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Bigner S H, Modrich P. Proc Natl Acad Sci USA. 1999;98:13802–13807. doi: 10.1073/pnas.241508098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G M. Oncol Res. 1999;11:393–400. [PubMed] [Google Scholar]
- 37.Flores C, Engels W. Proc Natl Acad Sci USA. 1999;96:2964–2969. doi: 10.1073/pnas.96.6.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]