Abstract
Two isoforms of estrogen receptor (ER) have been described: ERα and ERβ. The initial gene targeting of ERα, consisting in the introduction of a Neo cassette in exon 1 [αERKO, hereafter called ERα-Neo KO (knockout)], was reported in 1993. More recently, another mouse deficient in ERα because of the deletion of exon 2 (ERαKO, hereafter called ERα-Δ2 KO) was generated. In ovariectomized ERα-wild-type mice, estradiol (E2) increases uterine weight and basal production of endothelial nitric oxide (NO). Both of these effects are abolished in ERα-Δ2 KO mice. In contrast, we show here that both of these effects of E2 are partially (uterine weight) or totally (endothelial NO production) preserved in ERα-Neo KO. We also confirm the presence of two ERα mRNA splice variants in uterus and aorta from ERα-Neo KO mice. One of them encodes a chimeric ERα protein (ERα55), partially deleted in the A/B domain, that was detected in both uterus and aorta by Western blot analysis. The other ERα mRNA splice variant codes for an isoform deleted for the A/B domain (ERα46), which was detected in uterus of ERα-Neo KO, and wild-type mice. This protein isoform was not detected in aorta. The identification of these two N-terminal modified isoforms in uterus, and at least one of them in aorta, probably explains the persistence of the E2 effects in ERα-Neo KO mice. Furthermore, ERα-Neo KO mice may help in the elucidation of the specific functions of full-length ERα (ERα66) and ERα46, both shown to be physiologically generated in vivo.
Cardiovascular diseases resulting from atherosclerosis are a leading cause of death in Western societies (1). Epidemiological studies show men and postmenopausal women are at a higher risk for such diseases than premenopausal women, suggesting that estrogen may be cardioprotective (2, 3). Although lipoprotein changes occur in premenopausal women and postmenopausal women receiving hormone replacement therapy, in large-scale studies with adjustments for multiple risk factors, only 25–50% of the beneficial effects of estrogen seem to be caused by lipoprotein effects (4). Several large animal species including non-human primate (5), swine (6), and rabbits (7) and, more recently, apolipoprotein E-deficient mice (8, 9) have been used to assess these nonlipid effects of estrogen. They showed that effects on endothelium (10, 11) may contribute to the cardioprotection observed in humans.
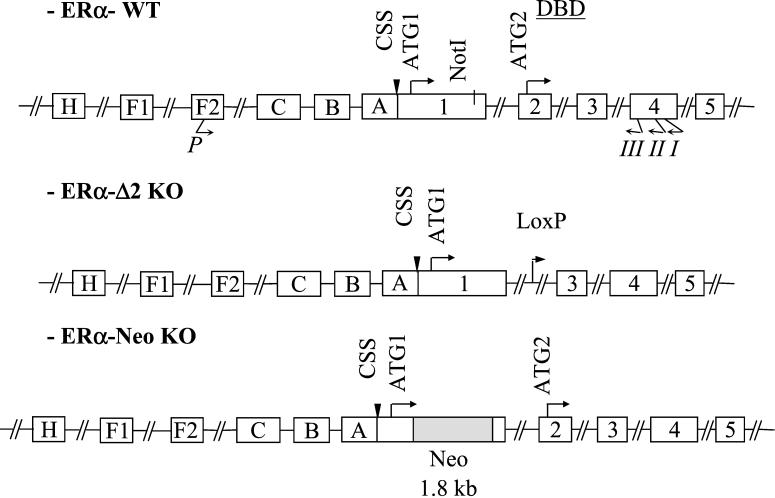
To date, two estrogen receptor (ER) isoforms, encoded by two different genes, have been described: ERα and ERβ. Lubahn et al. (12) first reported the gene targeting of ERα (αERKO), consisting in the introduction of a Neo cassette in the NotI site of exon 1, hereafter designated as the ERα-Neo knockout (KO) mouse (Fig. 1). Although the reproductive function is abolished in these mice, several effects of estradiol (E2) persist, such as a certain degree of uterine hypertrophy or the prevention of carotid media hyperplasia in response to endovascular artery injury (13). In addition, Couse et al. (14) reported that ERα-Neo KO mice encode, through alternative splicing, a chimeric ERα protein of 55 kDa (ERα55), in which 64 aa residues, belonging mainly to the B region (see Fig. 3B), have been partially deleted from the N-terminal A/B regions of ERα and replaced by 7 aa encoded by a small portion of the Neo insert. Although they failed to demonstrate directly the immunoreactive presence of this altered isoform in tissues, they have overexpressed and characterized this mutant in vitro and showed that it possesses a residual estrogen-dependent transcriptional activity. It has also been reported that human umbilical cultured endothelial cells contain two prominent ERα immunoreactive species, one of the expected 66-kDa mass corresponding to full-length ERα and the other of 46 kDa, which may separately trigger genomic or rapid signaling responses (15). Tissue-specific expression of such ERα mRNA variants, including aorta, has previously been described in the mouse (16) and human (17).
Figure 1.
Schematic representation of ER gene structure. The drawing is done according to Kos and colleagues (16) and White et al. (21). The position of the primers used successively for reverse transcription (primer I), PCR (nested downstream primer II, in association with the upstream primer P), and sequencing (nested primer III) are shown. The sequences of these primers are given in Materials and Methods. Structures of ERα-Δ2 KO (consisting in the deletion of exon 2, see ERαKO in ref. 18) and ERα-Neo KO [consisting of the introduction of a Neo insert in the site NotI (nucleotide 337) of exon 1, see αERKO in refs. 12 and 14] are also drawn.
Figure 3.
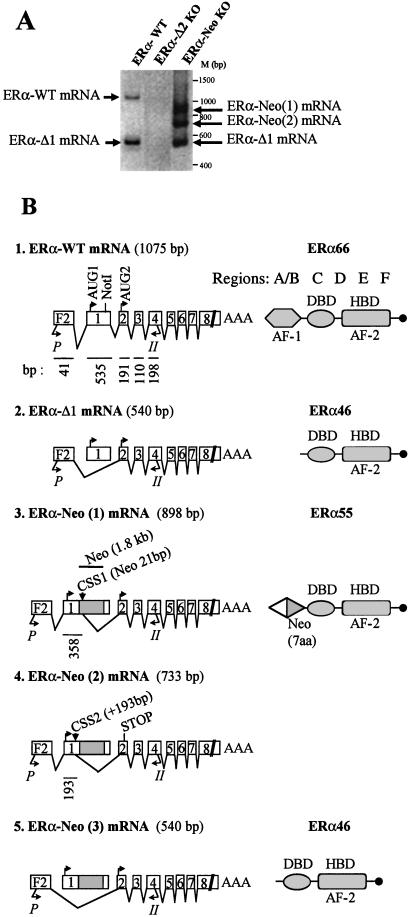
RT-PCR analysis of ERα mRNA expressed in the aorta from ovariectomized ERα-WT, ERα-Δ2 KO, or ERα-Neo KO mice. (A) ERα mRNA splice variants isolated in thoracic aorta homogenates. (B) Structures of ERα mRNA splice variants as evidenced by sequencing of the RT-PCR products and corresponding ERα protein isoforms (ERα66, ERα46, and ERα55) from ERα-WT and ERα-Neo KO mice. There is no mRNA expressed in ERα-Δ2 KO mice. CSS, consensus splice site.
More recently, Dupont et al. (18) generated mice deficient in either ERα (ERαKO, designated ERα-Δ2 KO mouse hereafter) or ERβ (designated ERβKO). ERα-Δ2 KO mouse has the deletion of exon 2 (Fig. 1) and corresponds to a complete and unambiguous inactivation of ERα (18). The use of these two ERα and ERβ KOs allowed us to unambiguously demonstrate that ERα mediates the endothelial effects of E2. Whereas E2 accelerates re-endothelialization and increases endothelial production of endothelium-derived relaxing factor (EDRF)/NO in ovariectomized wild-type (WT) and ERβKO mice, these effects of E2 are completely lost in ERα-Δ2 KO mouse (19, 20).
To evaluate the possible activities of ERα lacking the B region, we sought to compare the effects of E2 on the endothelial production of EDRF/NO in WT ERα (ERα-WT), ERα-Δ2 KO, and ERα-Neo KO mice.
Materials and Methods
Animals.
All experimental protocols were performed in accordance with the recommendations of the French Accreditation of Laboratory Animal Care. Targeted disruption (KO) of mouse ERα was performed by homologous recombination, resulting in ERα-Neo KO (αERKO in ref. 12) and ERα-Δ2 KO (ERαKO in ref. 18) mice as described (12, 18). Only 4-week-old female homozygous deficient (−/−), and homozygous WT (+/+) mice were used in the present study and maintained in our animal facilities under pathogen-free conditions. For estrogen hormone administration, 3-mm pellets containing 0.1 mg of 17β-E2 were implanted s.c. on the animal's back at 4 weeks of age after bilateral ovariectomy. These pellets (Innovative Research of America) provide controlled continuous release of a constant level of hormone over a period of 60 days (i.e., 80 μg/kg per day). This E2 dose has previously been defined as adequate for a maximal effect on vascular functions in female mice (9, 19). Mice were killed by an overdose of ketamine after 2 weeks of treatment. To measure serum E2 concentrations, RIA kits for E2 were used according to the manufacturer's instructions (Sorin Biomedica, Saluggia, Italy).
Endothelial EDRF/NO Production in Isolated Vascular Ring Experiments.
Endothelial EDRF/NO production was measured as described (20). Briefly, the two distal ring segments of 3 mm were obtained from the descending thoracic aorta. They were suspended in individual organ chambers filled with Krebs buffer (5 ml) with the following composition: 118.3 mM NaCl/4.69 mM KCl/1.25 mM CaCl2/1.17 mM MgSO4/1.18 mM K2HPO4/25.0 mM NaHCO3/11.1 mM glucose, pH 7.4. The solution was aerated continuously with 95% O2-5% CO2 and maintained at 37°C. Care was taken not to injure the endothelium during ring preparation. Tension was recorded with a linear force transducer. The resting tension was gradually increased to 1 g over a period of 45 min, and the vessels were left at this resting tension throughout the remainder of the study. One gram was determined as the optimal resting tension of aortic rings to develop the maximal contraction in response to 80 mM KCl (20). The vessels were contracted with L-phenylephrine (3 μM) to determine maximal contraction, then they were precontracted to 80% of that value (phenylephrine, 0.25 μM). When a stable contraction plateau had been reached, the rings were exposed cumulatively to either acetylcholine (1 nM-30 μM), A23187 (1 nM-1 μM), or sodium nitroprusside (0.1 nM-0.3 μM). Experiments were performed in the presence of the cyclooxygenase inhibitor diclofenac (2 μM).
Basal NO production by aortic ring was evaluated from the contraction elicited after 30 min with NG-nitro-L-arginine (final concentration, 100 μM) added to rings preconstricted for 30 min with the thromboxane A2 mimetic U46619 (7.5 nM). Data were collected by ACKNOWLEDGE software (Biopac System, Santa Barbara, CA), and relaxation was expressed as the percentage tension decrease below the tension elicited by precontracting the aortic rings with phenylephrine.
RNA Isolation and Reverse Transcriptase (RT)-PCR.
After killing, tissues were frozen in liquid nitrogen before homogenization. Total RNA was extracted from uterine and aortic tissues by using RNAble (Eurobio, Paris). The absorbance ratio of RNA/proteins at 260/280 nm was between 1.8 and 1.9. In the present study, we used the description of the oligonucleotides and the terminology recently described by Kos et al. (16). All positions were according to the cDNA sequence cloned by White et al. (21) (Fig. 1). Total RNA was reverse-transcribed by using the SuperScript First-Strand Synthesis System (Life Technologies, Rockville, MD; GIBCO/BRL) as described by the manufacturer with a gene-specific primer I (5′-ATAAACAGAATAGATCATGG-3′), which is complementary to nucleotides 219–239 of exon 4 of mouse ERα. The product was then used as a template for PCR by using a nested downstream primer II specific for exon 4 (5′-GCACTGACCATCTGGTCAGC-3′) and an upstream primer P specific for exon leader F2 (5′-GCTGTCTCCTCAAACACATCC-3′). Amplified products were separated by agarose gel electrophoresis. After recuperation, fragments were automatically sequenced by using AmpliTaq FS (Perkin–Elmer) with a nested primer III (5′-CCAAGTCATCTCTCTGACGCTTG-3′) specific for exon 4.
Western Blot Analysis.
One hundred micrograms of whole aortic or uterus tissue extracts was subjected to SDS/PAGE, after protein denaturation at 95°C for 3 min, on a 10% SDS/polyacrylamide gel with prestained size markers (BenchMark, GIBCO/BRL, Life Technologies). After electrotransfer onto a nitrocellulose membrane (Protran BA83, Schleicher & Schuell), the membrane was blocked in TBST-3% milk (25 mM Tris/140 mM NaCl/0.1% Tween, pH 8/3% nonfat milk powder). The membrane was then incubated with either a rabbit polyclonal antibody (MC20, directed toward the common C-terminal domain of the different ERα isoforms, Santa Cruz Biotechnology) or a mAb (1D5, which recognizes a sequence localized around the 119th amino acid of the A/B domain, Dako), 0.3 μg/ml in TBST-1% nonfat milk powder. After incubation with a peroxidase-coupled goat anti-rabbit antibody, ERα proteins were visualized by using the ECL system (Amersham Pharmacia) according to the manufacturer's instructions.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded blocks of uterus and aorta were cut as 6-μm sections (microtome Leica CM 1900). Immunohistochemical assays (on Tech Mate Horizon, Dako) with MC20 antibody (dilution of 1:600) were performed by using a modification of the antigen-retrieval technique based on microwave exposure (22). Endogenous peroxidase activity was quenched by using a 3% hydrogen peroxide/methanol solution. The sections were incubated with the horseradish peroxidase-conjugated rabbit anti-mouse IgG (EnVision, Dako). To visualize MC20 antibody binding, tissue sections were incubated in 3,3′-diaminobenzydine, rinsed in distilled water, and counterstained in Gill's hematoxylin. Slides were prepared for analysis by standard light microscopy.
Statistical Analysis.
Data were expressed as mean ± SE. Comparisons of concentration-response relaxations between the various aortic segments studied were made by one-factor ANOVA. Comparisons of data between different groups were made with repeated measures and a Scheffe's post hoc test used when differences were indicated. When an interaction was observed between the two factors, the effect of E2 treatment was studied in each genotype by using a t test. P < 0.05 was considered statistically significant.
Results
Effect of E2 on Uterus Weight.
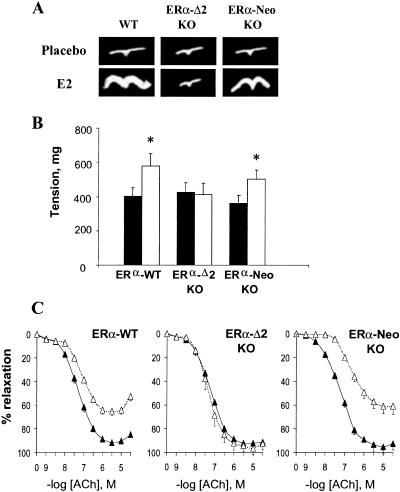
Ovariectomized mice implanted with a placebo pellet showed nondetectable (< 5 pg/ml, i.e., < 20 × 10−12 M) circulating levels of E2 and atrophied uterus (< 20 mg). The ovariectomized mice implanted with a pellet releasing 0.1 mg of E2 for 60 days (i.e., 80 μg/kg per day) showed serum E2 concentrations averaging 0.5 × 10−9 M, irrespective of the genotype. As expected, ovariectomized ERα-WT mice treated with E2 presented a significant increase in uterine weight. ERα-Δ2 KO mice showed no increase under E2 treatment. ERα-Neo KO mice showed a significant increase in uterine weight, although only partial when compared with WT mice (Table 1 and Fig. 2A).
Table 1.
Effect of E2 treatment on body and uterine weights, serum estradiol, aortic contraction (in response to KCl, phenylephrine, and U-46619), basal endothelial NO release, and aortic relaxation in response to acetylcholine from ovariectomized ERα-WT, ERα-Δ2 KO, and ERα-Neo KO mice given either placebo or E2
| Phenotype treatment | ERα-WT
|
ERα-Neo KO
|
ERα-Δ2 KO
|
|||
|---|---|---|---|---|---|---|
| Placebo | E2 | Placebo | E2 | Placebo | E2 | |
| Body weight, g | 28.1 ± 2.7 | 24.1 ± 2.1 | 23.2 ± 1.9 | 22.7 ± 1.2 | 20.6 ± 2.6 | 23.3 ± 1.6 |
| Uterine weight, mg | <20 | 151 ± 26 | <20 | 42 ± 16 | <20 | <20 |
| Plasma E2, pg/ml | <5 | 162 ± 26 | <5 | 151 ± 25 | <5 | 158 ± 40 |
| Maximal contraction, mg | ||||||
| KCl, 80 mM | 691 ± 31 | 678 ± 28 | 740 ± 21 | 773 ± 66 | 694 ± 35 | 830 ± 57 |
| Phe, 3 μM | 1177 ± 83 | 897 ± 94 | 714 ± 83 | 583 ± 61 | 700 ± 41 | 842 ± 78 |
| U46619, 7.5 nM | 739 ± 58 | 801 ± 77 | 690 ± 64 | 695 ± 97 | 783 ± 155 | 750 ± 139 |
| Basal NO, mg | 403 ± 51 | 579 ± 72* | 361 ± 46 | 503 ± 53* | 427 ± 55 | 413 ± 67 |
| ACh-elicited relaxation | ||||||
| EC50, 10−8 M | 5.87 ± 0.88 | 13.79 ± 2.60* | 6.99 ± 4.9 | 27.48 ± 3.87* | 10.23 ± 6.52 | 6.23 ± 2.06 |
| Emax, % relaxation | 90.3 ± 2.3 | 63.8 ± 3.5* | 87.3 ± 1.9 | 60.7 ± 7* | 89.6 ± 4.0 | 96.5 ± 5.6 |
Data for ERα-Δ2 KO mice have been published (20). Data are means ± SE from the two distal rings of the thoracic aorta from five separate mice in each group.
, P < 0.05 vs. respective placebo.
Figure 2.
(A) Effect of E2 on uterine hypertrophy in ovariectomized ERα-WT, ERα-Δ2 KO, and ERα-Neo KO mice. (B) Basal NO release. The basal NO release of aortic rings from ovariectomized ERα-WT, ERα-Δ2 KO, and ERα-Neo KO mice treated either with placebo (filled bars) or E2 (empty bars) was evaluated from the l-nitroarginine (10−4 M) induced contraction in rings precontracted with U-46619. *, P < 0.05 vs. placebo group. (C) Acetylcholine-stimulated NO release. Concentration response curves in response to acetylcholine of aortic rings precontracted with phenylephrine, from ovariectomized ERα-WT, ERα-Δ2 KO, and ERα-Neo KO mice treated either with placebo (▴) or E2 (▵). For B and C, data are means ± SE from n = 5 rings from five separate mice for each point. Data of ERα-Δ2 KO mice have been published (20).
Effect of E2 on Endothelial EDRF/NO Production.
As previously reported in C57BL/6 mice (20), E2 did not significantly alter the contraction of aortic rings in response to 80 mM KCl or to the α1-adrenergic agonist phenylephrine in ERα-WT mice (Table 1). The basal NO release (evaluated from the NG-nitro-L-arginine-induced contraction obtained in U-46619 precontracted rings) was significantly enhanced by E2 (Fig. 2B). The vessels were precontracted to 80% of the maximal contraction by phenylephrine, and stimulated EDRF activity was estimated from the relaxation in response to acetylcholine. The EC50 was significantly increased (about 2.3-fold), and the maximal relaxation was significantly decreased (29%) by E2 in comparison to the placebo group (Fig. 2C and Table 1). The relaxation in response to the NO-releasing agent sodium nitroprusside was not influenced by E2 (not shown).
Different responses were observed in ERα-Neo KO and ERα-Δ2 KO mice. Contraction and relaxation in mice of both KO genotypes, implanted with a placebo pellet, were similar to untreated ERα-WT mice (Table 1). In ERα-Δ2 KO mice, E2 treatment did not alter basal NO release or acetylcholine-elicited relaxation (Fig. 2 B and C and Table 1). In contrast, in ERα-Neo KO mice, E2 treatment significantly increased basal NO release (Fig. 2B) and decreased acetylcholine-elicited relaxation (increasing EC50 about 3.9-fold, P < 0.01, and decreasing the maximal relaxation by 30%, P < 0.01; Fig. 2C and Table 1). Thus, the effect of E2 on aortic NO production of ERα-Neo KO (αERKO in ref. 12) mouse was the same as in ERα-WT mice.
Identification of ERα mRNA Expressed in the Aorta of ERα-WT, ERα-Δ2 KO, and ERα-Neo KO Mice.
In the aorta of an ovariectomized WT mouse, two ERα mRNA variants were observed (Fig. 3A). As shown by sequencing, the first one corresponded to the 1,075-bp PCR product (1: ERα-WT mRNA), including the exon leader F2 but not A, B, or C exon leaders (see refs. 16 and 17) and encoding for the “classical” full-length ERα 66 protein through initiation of translation from AUG1 of exon 1 (Fig. 3B). The second mRNA corresponded to the 540-bp PCR product (2: ERα-Δ1 mRNA) resulting from the splicing of exon 1 (Fig. 3B). This ERα mRNA would encode an ERα 46 isoform deleted for the A/B domain. E2 treatment did not alter the expression profile of ERα mRNA (not shown).
No RT-PCR products were generated from mRNA obtained in the aorta of ERα-Δ2 KO mutant mouse. In contrast, expression of three different RT-PCR products (Fig. 3 A and B) of 898 bp [3: ERα-Neo(1)], 733 bp [4: ERα-Neo(2)], and 540 bp [5: ERα-Neo(3) identical to ERα-Δ1] were obtained from aorta of the ERα-Neo KO mouse. As shown by sequencing, ERα-Neo(1) mRNA was generated through a remodeling of exon 1 with a partial splicing out of 192 nts in the 3′ part of exon 1 and of 1,779 nts in the C terminal of the Neo insert, leading to the persistence of an ORF able to encode for a chimeric ERα55 protein through initiation of translation from the AUG1 in exon 1 (Fig. 3B). This was similar to the observations reported by Couse et al. (14). ERα-Neo(2) mRNA was generated through the remodeling of exon 1 with a splicing out of 342 nts in the 3′ part of exon 1 and the whole Neo insert, creating a frameshift and a stop codon in the 5′ end of exon 2. Fragment 5, which had not been reported so far in ERα-Neo KO mice, was indeed identical to the fragment 2 (ERα-Δ1) obtained in ERα-WT mice. Similar RT-PCR profiles were obtained in uterine tissues (data not shown).
Immunodetection of ERα Isoforms in the Aorta and the Uterus of WT, ERα-Δ2 KO, and ERα-Neo KO Mice.
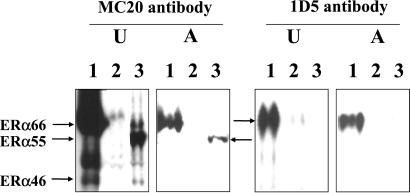
Using the MC20 antibody directed toward the C-terminal domain (Fig. 4), Western blot of uterus and aorta homogenates of ERα-WT mouse revealed not only ERα66, the classical full-length isoform of ERα, but also another species in the uterus migrating at 46 kDa, which was undetectable in the aorta. The immunoreactivity was not altered by treatment with E2 (data not shown). In ERα-Neo KO mice, a species corresponding to ERα55 was evidenced both in the uterus and the aorta. In addition, a 46-kDa species was detected in the uterus but was, again, undetectable in the aorta. After washing, the same membranes were blotted again with 1D5 antibody, directed toward the A/B domain. Like the MC20 antibody, this antibody detected ERα66 in WT homogenates but did not reveal any 55-kDa or 46-kDa species, as expected from the absence or modification of the A/B domain. In marked contrast, in homogenates from ERα-Δ2 KO mice, no immunoreactivity could be detected, providing a control for these experiments.
Figure 4.
Western blot analysis of ERα isoform abundance in the uteri (U) and the thoracic aorta (A) from ovariectomized ERα-WT (1), ERα-Δ2 KO (2), or ERα-Neo KO (3) mice. Antibody MC20 is directed toward the C-terminal domain and thus reveals all three isoforms (ERα66, ERα55, ERα46), and antibody 1D5 recognizes a sequence around the 119th amino acid of A/B domain and thus detects only ERα66. Each Western blot is representative of three separate experiments.
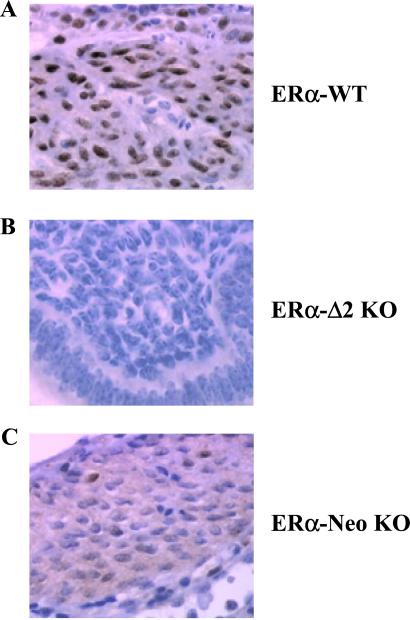
MC20 antibody was also used to localize ERα in paraffin-embedded blocks of uterus and aorta of the three castrated strains treated with E2. As expected, the nuclei of the uterine smooth muscle of ERα-WT were intensively stained (Fig. 5A), whereas no immunoreactivity could be detected in ERα-Δ2 KO (Fig. 5B). Interestingly, staining of several nuclei and diffuse staining of the cytoplasm was noted in ERα-Neo KO mice (Fig. 5C). However, we were unable to detect ERα immunostaining in aorta from WT or ERα-Neo KO and, of course, from ERα-Δ2 KO mice (not shown).
Figure 5.
Immunohistochemical analysis of ERα expression in the uterus from ERα-WT (A), ERα-Δ2 KO (B), and ERα-Neo KO mice (C). MC20 antibody was used to localize ERα in paraffin-embedded blocks of uteri from the three castrated strains to which E2 was given. Nuclei of uterus smooth muscle cells of ERα-WT were intensively stained. No immunoreactivity was detected in ERα-Δ2 KO mice. Nuclei and diffuse staining of the cytoplasm were observed in ERα-Neo KO mice.
Discussion
The cardiovascular protective effects of estrogen are well established, and the direct effects of the hormone on vascular tissues are now well recognized (23). Using a mouse model of carotid arterial injury, Mendelsohn and his collaborators have shown that E2 administration completely inhibits the vascular injury response in ovariectomized WT and ERα-Neo KO (αERKO in ref. 12) mice (13, 24). At the time, these observations were interpreted as indicating that estrogen inhibits the vascular injury response by an ERα-independent pathway and therefore suggested that ERβ could mediate the protective effects of E2 on the vasculature. Studies demonstrating that ERβ is expressed in vascular cells and tissues, and, in contrast to ERα, that ERβ mRNA expression increases in vascular endothelial and smooth muscle cells after vascular injury (25–27), also suggested that ERβ could play a central role in mediating the cardiovascular effects of E2 (28, 29). However, in subsequent studies, Karas et al. (30) showed that the effect of E2 is similar in WT and in βERKO mice, ruling out an essential role for ERβ in the arterial wall. Finally, by using ERα-Δ2 KO mice (ERαKO mice in ref. 18), which represent a complete and unambiguous inactivation of ERα, as well as ERβKO mice (18), we demonstrated that, on its own, ERα mediates the effect of E2 on re-endothelialization, endothelial NO production, and uterine hypertrophy (present work and refs. 19 and 20).
In initial studies, several effects of E2 in ERα-Neo KO (αERKO) mice were investigated (14). These studies showed some degree of uterine hypertrophy in response to E2 under high dose or continuous treatment, as well as the preservation of an ERα reading frame that could encode for a smaller mutant ERα, as a possible source for the residual uterus E2 binding (14). In the present study, we have characterized the presence of three ERα mRNA splice variants of the targeted ERα-Neo gene: ERα-Neo(1), ERα-Neo(2), and ERα-Neo(3) mRNA (Fig. 3) by using an upstream primer specific for exon leader F2 in the PCR step. Similar data were obtained by using a primer specific for exon leader C (data not shown), in agreement with Kos and colleagues (16). ERα-Neo(2) mRNA would encode for a truncated nonsense protein, but ERα−Neo(1) mRNA encodes for a chimeric ERα protein (ERα55) deleted for 64 aa of the B domain (amino acids 92–155), which are replaced by 7 aa encoded by a small portion of the Neo gene. ERα-Neo(3) mRNA, which is identical to ERα-Δ1 mRNA, would code for an isoform of ERα deleted for the A/B domain (ERα46). This latter isoform had been characterized at the mRNA and protein level in rat uterus and human cells (16, 17, 31, 32). The N-terminal region of the full-length ERα harbors a ligand-independent transcriptional activation function AF-1 (33–36). AF-1 has been considered to act synergistically with the ligand-dependent activation function AF-2, contained in the C-terminal region, for full activity of the ER (34, 35, 37, 38). As ERα55 and the ERα46 isoform share an alteration and absence of the AF-1 domain, respectively, all other domains being intact, disappearance of AF-1 function but maintenance of AF-2 function is expected (34). Indeed, Couse et al. (14) and Chambon et al. (33, 34) have overexpressed and characterized in vitro the ERα55 and ERα46 proteins, respectively, and showed that they possesses estrogen-dependent transcriptional activity, although reduced when compared with the WT full-length ERα66.
Our present data indicate that the effects of E2 on NO production are likely to be mediated by ERα55, as this isoform was the only one detected in aorta homogenates (Fig. 4). The role of ERα46 itself could not be established, as its abundance in the aorta was below the detection threshold of Western blot analysis, even after prolonged exposure. As the two ERα molecular isoforms, ERα66 and ERα46, are physiologically generated, the question arises as to whether they have distinct functions in vivo. It seems that the full-length ERα66 (which harbors the transactivation function AF-1) mediates fertility in both males and females (39), some vascular effects of E2, such as the angiogenic effect (40), as well as inhibition of the constitution of fatty streak in apolipoprotein E-deficient mice (41). Conversely, AF-1 of ERα (absent and altered in ERα46 and ERα55, respectively) could be at least in part dispensable to mediate the effect of E2 on the uterine response (present study), the inhibition of smooth muscle hyperplasia in response to endoluminal carotid injury (13, 42), and the endothelial production of NO (present study). Thus, the former hypotheses (30, 42) of a reciprocal functional redundancy between ERα and ERβ, or of the existence of a putative third isoform “ERγ,” seem to be ruled out. Moreover, our data further demonstrate that the αERKO (designated here ERα-Neo) mutation (12) that may lack the AF-1, but not the AF-2 activation function, is not an ERα null mutation, in contrast to the more recent ERαKO (designated here ERα-Δ2 KO) null mutation (18). In this respect αERKO mice might help in studies aimed at defining the role of ERα AF-1 in physiology and pathology.
Acknowledgments
We are grateful to Dr. Béatrice Desvergnes and Dr. Geoff Richards for a critical reading of the manuscript and Dr. Jacques Rami and Mrs. Marie-José Fouque for their invaluable help. The work at Institut National de la Santé et de la Recherche Médicale U397 was supported in part by Institut National de la Santé et de la Recherche Médicale, the Ministère de la Recherche et de la Technologie, the Fondation de France, The Fondation de l'Avenir, the Fondation pour la Recherche Médicale, and the Conseil Régional Midi-Pyrénées. The work at Institut de Génétique et de Biologie Moléculaire et Cellulaire was supported by a grant from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Collège de France, the Hôpital Universitaire de Strasbourg, the Association pour la Recherche Médicale, the Fondation pour la Recherche Médicale, and by Groupement d'Intérêt Public/Hoechst Marion Roussel.
Abbreviations
- ER
estrogen receptor
- KO
knockout
- EDRF
endothelium-derived relaxing factor
- RT
reverse transcriptase
- WT
wild type
- E2
estradiol
References
- 1.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Lobo R A, Speroff L. Fertil Steril. 1994;61:592–595. doi: 10.1016/s0015-0282(16)56630-8. [DOI] [PubMed] [Google Scholar]
- 3.Stampfer M, Grodstein F. Br Med J. 1994;309:808–809. doi: 10.1136/bmj.309.6957.808b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush T L, Barrett-Connor E, Cowan L D, Criqui M H, Wallace R B, Suchindran C M, Tyroler H A, Rifkind B M. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- 5.Adams M R, Kaplan J R, Manuck S B, Koritnik D R, Parks J S, Wolfe M S, Clarkson T B. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 6.Keaney J F, Shwaery G T, Xu A M, Nicolosi R J, Loscalzo J, Foxall T L, Vita J A. Circulation. 1994;89:2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- 7.Hough J L, Zilversmit D B. Arteriosclerosis. 1986;6:57–63. doi: 10.1161/01.atv.6.1.57. [DOI] [PubMed] [Google Scholar]
- 8.Bourassa P-A, Milos P M, Gaynor B J, Breslow J L, Aiello R J. Proc Natl Acad Sci USA. 1996;93:10022–10027. doi: 10.1073/pnas.93.19.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elhage R, Arnal J F, Pierragi M-T, Duverger N, Fiévet C, Faye J C, Bayard F. Arterioscler Thromb Vasc Biol. 1997;17:2679–2684. doi: 10.1161/01.atv.17.11.2679. [DOI] [PubMed] [Google Scholar]
- 10.Miller V M, Vanhoutte P M. Am J Physiol. 1991;261:R1022–R1027. doi: 10.1152/ajpregu.1991.261.4.R1022. [DOI] [PubMed] [Google Scholar]
- 11.Gisclard V, Miller V M, Vanhoutte P M. J Pharmacol Exp Ther. 1988;244:19–22. [PubMed] [Google Scholar]
- 12.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iafrati M D, Karas R H, Aronovitz M, Kim S, Sullivan T R, Jr, Lubahn D B, O'Donnell T F, Jr, Korach K S, Mendelsohn M E. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 14.Couse J F, Curtis S W, Washburn T F, Lindzey J, Golding T S, Lubahn D B, Smithies O, Korach K S. Mol Endocrinol. 1995;9:1441–1454. doi: 10.1210/mend.9.11.8584021. [DOI] [PubMed] [Google Scholar]
- 15.Russell K S, Haynes M P, Sinha D, Clerisme E, Bender J R. Proc Natl Acad Sci USA. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kos M, O'Brien S, Flouriot G, Gannon F. FEBS Lett. 2000;477:15–20. doi: 10.1016/s0014-5793(00)01750-6. [DOI] [PubMed] [Google Scholar]
- 17.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, Sonntag-Buck V, Gannon F. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Development (Cambridge, UK) 2000;127:4277–4291. doi: 10.1242/dev.127.19.4277. [DOI] [PubMed] [Google Scholar]
- 19.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal J F. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 20.Darblade, B., Pendaries, C., Krust, A., Dupont, S., Fouque, M.-J., Rami, J., Chambon, P., Bayard, F. & Arnal, J.-F. (2002) Circ. Res.90, in press. [DOI] [PubMed]
- 21.White R, Lees J A, Needham M, Ham J, Parker M. Mol Endocrinol. 1987;1:735–744. doi: 10.1210/mend-1-10-735. [DOI] [PubMed] [Google Scholar]
- 22.Shi S R, Key M E, Kalra K L. J Histochem Cytochem. 1991;39:741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- 23.Mendelsohn M E, Karas R H. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan T R, Jr, Karas R H, Aronovitz M, Faller G T, Ziar J P, Smith J J, O'Donnell T F, Jr, Mendelsohn M E. J Clin Invest. 1995;96:2482–2488. doi: 10.1172/JCI118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindner V, Kim S K, Karas R H, Kuiper G G, Gustafsson J A, Mendelsohn M E. Circ Res. 1998;83:224–229. doi: 10.1161/01.res.83.2.224. [DOI] [PubMed] [Google Scholar]
- 26.Register T C, Adams M R. J Steroid Biochem Mol Biol. 1998;64:187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 27.Makela S, Savolainen H, Aavik E, Myllarniemi M, Strauss L, Taskinen E, Gustafsson J A, Hayry P. Proc Natl Acad Sci USA. 1999;96:7077–7082. doi: 10.1073/pnas.96.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gustafsson J A. Nat Med. 1997;3:493–494. doi: 10.1038/nm0597-493. [DOI] [PubMed] [Google Scholar]
- 29.Katzenellenbogen B S, Korach K S. Endocrinology. 1997;138:861–862. doi: 10.1210/endo.138.3.5080. [DOI] [PubMed] [Google Scholar]
- 30.Karas R H, Hodgin J B, Kwoun M, Krege J H, Aronovitz M, Mackey W, Gustafsson J A, Korach K S, Smithies O, Mendelsohn M E. Proc Natl Acad Sci USA. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faye J C, Fargin A, Bayard F. Endocrinology. 1986;118:2276–2283. doi: 10.1210/endo-118-6-2276. [DOI] [PubMed] [Google Scholar]
- 32.Barraille P, Chinestra P, Bayard F, Faye J C. Biochem Biophys Res Commun. 1999;257:84–88. doi: 10.1006/bbrc.1999.0334. [DOI] [PubMed] [Google Scholar]
- 33.Kumar V, Green S, Stack G, Berry M, Jin J R, Chambon P. Cell. 1987;51:941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 34.Berry M, Metzger D, Chambon P. EMBO J. 1990;9:2811–2818. doi: 10.1002/j.1460-2075.1990.tb07469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pham T A, Hwung Y P, Santiso-Mere D, McDonnell D P, O'Malley B W. Mol Endocrinol. 1992;6:1043–1050. doi: 10.1210/mend.6.7.1508220. [DOI] [PubMed] [Google Scholar]
- 36.Metzger D, Ali S, Bornert J M, Chambon P. J Biol Chem. 1995;270:9535–9542. doi: 10.1074/jbc.270.16.9535. [DOI] [PubMed] [Google Scholar]
- 37.Tzukerman M T, Esty A, Santiso-Mere D, Danielian P, Parker M G, Stein R B, Pike J W, McDonnell D P. Mol Endocrinol. 1994;8:21–30. doi: 10.1210/mend.8.1.8152428. [DOI] [PubMed] [Google Scholar]
- 38.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. Cell. 1989;59:477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 39.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 40.Johns A, Freay A D, Fraser W, Korach K S, Rubanyi G M. Endocrinology. 1996;137:4511–4513. doi: 10.1210/endo.137.10.8828515. [DOI] [PubMed] [Google Scholar]
- 41.Hodgin J B, Krege J H, Reddick R L, Korach K S, Smithies O, Maeda N. J Clin Invest. 2001;107:333–340. doi: 10.1172/JCI11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karas R H, Schulten H, Pare G, Aronovitz M J, Ohlsson C, Gustafsson J A, Mendelsohn M E. Circ Res. 2001;89:534–539. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]