Abstract
An 8-mer peptide (EMTOVNOG) derived from α-fetoprotein was compared with tamoxifen for activity against growth of human breast cancer xenografts implanted in immune-deficient mice. Both peptide and tamoxifen prevented growth of estrogen-receptor-positive MCF-7 and T47D human breast cancer xenografts. A subline of MCF-7, made resistant to tamoxifen by a 6-month exposure to this drug in culture, was found to be resistant to tamoxifen in vivo. Peptide completely prevented the xenograft growth of this tamoxifen-resistant subline of MCF-7. Neither peptide nor tamoxifen was effective in slowing the xenograft growth of the estrogen-receptor-negative MDA-MB-231 human breast cancer. A worrisome side effect of tamoxifen is its hypertrophic effect on the uterus. In this study, tamoxifen was shown to stimulate the growth of the immature mouse uterus in vivo, and the peptide significantly inhibited tamoxifen's uterotrophic effect. The mechanism of action of peptide is different from that of tamoxifen in that the peptide does not interfere with the binding of [3H]estradiol to the estrogen receptor. In conclusion, α-fetoprotein-derived peptide appears to be a novel agent that interferes with the growth of tamoxifen-sensitive as well as tamoxifen-resistant estrogen-receptor-positive human breast cancers; it inhibits the uterotrophic side effect of tamoxifen and, thus, it may be useful in combination with or in place of tamoxifen for treatment of estrogen-receptor-positive human breast cancers.
Several population studies as well as laboratory studies have indicated that α-fetoprotein (AFP) interferes with estrogen-dependent responses, including the growth-promoting effects of estrogen on breast cancer (1). For example, Couinaud et al. (2) have reported that women with AFP-secreting hepatomas develop amenorrhea, which self-corrects after removal of the hepatoma, and Mizejewski et al. (3) have shown that AFP inhibits the responsiveness of the uterus to estrogen. Jacobson et al. (4) and Richardson et al. (5) have shown that elevated levels of AFP during pregnancy are associated with a subsequent reduction in lifetime risk for breast cancer, and Jacobson has hypothesized that this should be caused by a diminution in estrogen-dependent breast cancers (6). Sonnenschein et al. (7) have shown in rats that an AFP-secreting hepatoma prevents the growth of an estrogen-dependent breast cancer in the same rat. Finally, we have shown that AFP purified from a human hepatoma culture and then injected into tumor-bearing, immune-deficient mice stopped the growth of estrogen-receptor-positive (ER+), but not estrogen-receptor-negative (ER−), human breast cancer xenografts in these mice and did so by a mechanism different from that of tamoxifen (1).
More recently, we have identified the active site of AFP responsible for its antiestrotrophic activity (8). It consists of amino acids 472–479 (EMTPVNPG), an 8-mer sequence in the 580-aa AFP molecule. We have synthesized this 8-mer peptide, modified it by substituting hydroxyproline (O) for proline (P) for the purpose of stabilization, and have shown that this new analog (EMTOVNOG) is stable during long-term storage and, like AFP, has the ability to inhibit estrogen-stimulated growth of breast cancer cells in culture and estrogen-stimulated growth of the uterus in immature mice (8, 9). Having the stable analog in hand, a purpose of the study described herein was to determine whether this peptide has anti-breast cancer activity in vivo like its parent protein, AFP. A second purpose of the study was to compare the activities of this peptide with those of tamoxifen. Currently, tamoxifen is the most widely used agent for the treatment of estrogen-responsive breast cancers and has provided significant benefits to women with this disease (10, 11). However, a vexatious problem connected with its clinical use is that not all ER+ breast cancers are sensitive to this drug. About one-third to one-sixth (depending on the lab cutoff for ER positivity) of the ER+, newly diagnosed breast cancers do not respond to tamoxifen (Elwood Jensen, personal communication; ref. 12). Moreover, it is not uncommon that women whose disease is being managed successfully by tamoxifen therapy in time will experience recurrence during treatment apparently because their tumor has acquired resistance to the drug. Because these two groups constitute a substantial number of women whose disease fails to respond to tamoxifen therapy, we considered it important to determine whether AFP-derived peptide would be active against ER+ breast cancer that had become resistant to tamoxifen. We therefore undertook a study of human breast cancers sensitive and resistant to tamoxifen that were grown as xenografts in immune-deficient mice and tested for sensitivity to AFP-derived peptide (EMTOVNOG), hereafter referred to as AFPep.
Materials and Methods
Cell Lines.
T47D and MDA-MB-231 human breast cancer cell lines were purchased from the American Type Culture Collection (Manassas, VA). Growth medium for T47D cells consisted of RPMI 1640 (Life Technologies, Gaithersburg, MD) supplemented with 10% (vol/vol) FBS (Life Technologies) and 8 μg/ml bovine insulin (Sigma). Growth medium for MDA-MB-231 consisted of DMEM (Life Technologies) supplemented with l-glutamine (2 mM), nonessential amino acids (1%; Life Technologies), and bovine insulin (1 μg/ml). The MCF-7 cell line was obtained from Alberto C. Baldi (Institute of Experimental Biology and Medicine, Buenos Aires), and was maintained as described by Gierthy et al. (13). This strain of MCF-7 demonstrated 17β-estradiol (E2) sensitivity in regard to induction of tissue plasminogen activator, cell proliferation, and in vivo tumor growth and was sensitive to the suppression of these effects by tamoxifen (13–15). Continuous exposure of these cells to 1 μM tamoxifen citrate during routine culture conditions (1:10 subculture ratio once a week) resulted after 6 months in a strain that was resistant to the suppressive effects of tamoxifen in vitro.
Peptide Synthesis and Purification.
AFPep (EMTOVNOG) was generated by using solid-phase peptide synthesis, as described (8, 9). Amino acids with their amino group protected by the 9-fluorenylmethoxycarbonyl (Fmoc) group were Fmoc-Asn(Trt), Fmoc-Glu(OtBu), Fmoc-Met, Fmoc-Pro, Fmoc-hydroxyproline(tBu), Fmoc-Thr(tBu), Fmoc-Gly, and Fmoc-Val. The carboxyl groups on incoming amino acids were activated by [O-(7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate], obtained from PerSeptive Biosystems (Framingham, MA). After synthesis, the resin was washed with propanol and partially dried, and peptides were cleaved from the solid support and deprotected simultaneously with 10 ml of trifluoroacetic acid/thioanisole/anisole/ethanedithiol (90:5:2:3) per 0.5 g of resin for 5 h. Peptide was recovered from the liquid phase after repeated extraction, first with ether and then with ethyl acetate/ether (1.5:1). The peptide was dissolved in water, purified by reverse-phase HPLC, and then lyophilized. Peptide quality was ascertained by amino acid analysis and mass spectroscopy.
Human Breast Cancer Xenograft Assay.
A bioassay for anti-breast cancer activity was performed according to Bennett et al. (1, 16, 17). Confluent human breast cancer cells of several different cell lines were trypsinized into suspension and pelleted by centrifugation at 200 × g. The pellet of each cell line then was solidified into a fibrin clot by exposing it to 10 μl of fibrinogen (50 mg/ml) and 10 μl of thrombin (50 units/ml). The solid mass of tumor cells then was cut into segments 1.5 mm in diameter. A tumor segment was implanted under the kidney capsule of an immunodeficient Institute for Cancer Research (ICR)-severe combined immunodeficient male mouse (Taconic Farms) that weighed about 25 g. Estrogen supplementation was accomplished by s.c. implantation of a silastic tubing capsule containing solid E2 inserted on the day of tumor implantation. Peptide was injected i.p. every 12 h at a dose of 1 μg per mouse. Tumor growth was monitored during survival laparotomy at 15-day intervals by measurement of the diameters of the short (d) and long axes (D) of each tumor by using a dissecting microscope equipped with an ocular micrometer. Tumor volumes were calculated by using the formula (Π/6)(d) (2)D, assuming the tumor shape to be an ellipsoid of revolution around its long axis (D). There were five to seven replicate mice included in each treatment group. Mean tumor volume ± SE in each group was calculated for display of growth curves. Significance of differences between groups was tested by using the one-sided Wilcoxon Sum of Ranks Test.
Immature Mouse Uterine Growth Assay.
A bioassay for antiestrotrophic activity was performed by using an immature mouse uterine growth assay based on previous studies, which demonstrated that i.p. administration of 0.5 μg E2 to these mice doubled their uterine weights with a corresponding increase in mitotic figures by 24 h after E2 (3, 18). Swiss/Webster female mice, 6–8 g in body weight (13–15 days old), were obtained from Taconic Farms. Mice were weighed and distributed into treatment groups (typically five mice per group) such that each group contained the same range of body weights. In a typical experiment, each group received two sequential i.p. injections spaced 1 h apart. Test material or vehicle control for that material was contained in the first injectant. E2 or vehicle control for E2 was contained in the second injectant. Twenty-two hours after the second injection, uteri were dissected, trimmed free of mesenteries, and weighed immediately. The uterine weights were normalized to mouse body weights (mg uterine weight per g of body weight) to compensate for differences in body weight among litters of the same age. Experiments used a minimum of five mice per group, and the mean normalized uterine weight ± SE for each group was calculated. Significance of differences between groups was evaluated, employing the nonparametric Wilcoxon Sum of Ranks test.
Assessment of Estrogen Receptor Antagonism.
Commercially obtained rabbit uteri (Pel-Freez Biologicals) were used as a source of estrogen receptor (ER). Uteri were pulverized in a stainless steel impact mortar under liquid nitrogen and homogenized (20% wt/vol) in assay buffer (10 mM Tris, pH 7.4/1.5 mM EDTA/10% glycerol/10 mM monothioglycerol/10 mM sodium molybdate) on ice. Centrifugation (50,000 × g) for 1 h yielded a supernatant containing cytosol, which was adjusted with assay buffer to 2.5 mg protein/ml. All incubations were carried out in triplicate, each containing 100 μl of cytosol, 20 μl of 10 mM 6,7-[3H]estradiol [50 Ci/mmol (1 Ci = 37 GBq); NEN], and 80 μl of putative antagonist in assay buffer. Total count tubes received 20 μl of [3H]estradiol and 180 μl of assay buffer. After incubation overnight at 4°C, all but the total count tubes received 300 μl of dextran-coated charcoal suspension; tubes were agitated for 15 min and then centrifuged (1,000 × g) for 15 min. Supernatants were decanted into counting vials, scintillant was added, and protein-bound tritium was determined by liquid scintillation counting.
Results
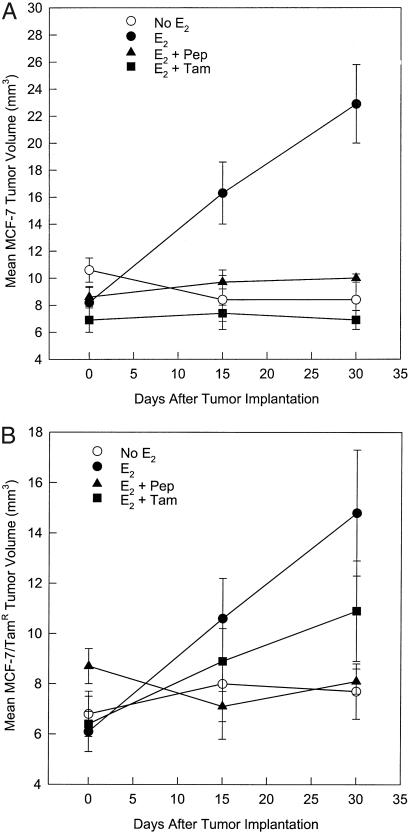
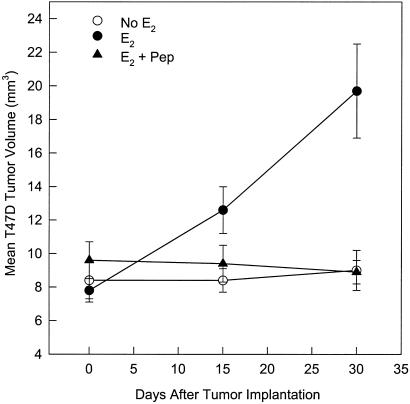
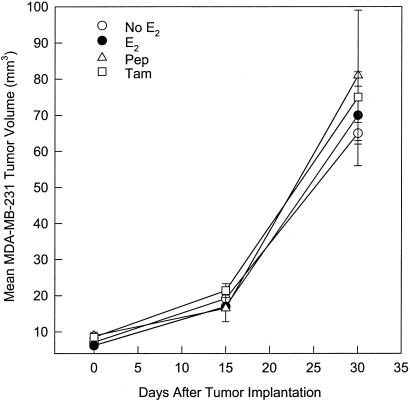
It was determined in a screening assay of the inhibition of E2-stimulated growth of immature mouse uterus by AFPep that an effective antiestrotrophic dose of AFPep was 0.1–1.0 μg per mouse. Also, preliminary pharmacokinetic studies suggested that the biological half-life of this peptide in these mice was 2–3 h. Therefore, for the breast cancer xenograft studies, it was deemed reasonable to administer this peptide twice a day at a dose of 1.0 μg per i.p. injection into tumor-bearing severe combined immunodeficient mice. The ER+ MCF-7 human breast cancer was used as a first step in evaluating the effectiveness of AFPep against human breast cancer. As shown in Fig. 1A, MCF-7 xenografts were completely dependent on estrogen for growth in severe combined immunodeficient mice. They underwent an approximate 3-fold increase in tumor volume in the presence of a slow-release E2 implant during the 30-day observation. Without E2 supplementation, there was no tumor growth. When E2-supplemented mice were given twice-daily injections of 1 μg of AFPep, tumor growth was prevented over the 30-day observation period. Similarly, when E2-supplemented tumors were given once-daily injections of 50 μg of tamoxifen, there was no increase in tumor volume. When a subline of MCF-7 that had been made resistant to tamoxifen in cell culture was used, a rather provocative outcome was obtained; that is, AFPep completely prevented the in vivo xenograft growth of this tumor, whereas tamoxifen was only marginally effective in slowing the growth of this tumor (Fig. 1B). In fact, at day 30 after tumor implantation, the tumor volume in the E2 plus tamoxifen group was not significantly different from that found in the group receiving E2 only (Fig. 1B). In contrast, AFPep completely stopped the growth of this tamoxifen-resistant MCF-7 subline, and at day 30 after tumor implantation, tumor volumes in the E2 plus peptide group were dramatically different from those found in the group receiving E2 only. The peptide was also tested on ER+ T47D human breast cancer. Like the MCF-7, T47D xenografts were completely dependent on E2 supplementation for growth (Fig. 2) and more than doubled in tumor volume over the 30-day observation period. Daily treatment with AFPep during this time interval also completely prevented the growth of this tumor (Fig. 2). An ER− human breast cancer, MDA-MB-231, then was tested for sensitivity to peptide. This tumor grew independent of estrogen supplementation and demonstrated a rather aggressive growth rate during the second 2 weeks of the observation period (Fig. 3). Daily treatment with AFPep had no effect on the growth of this tumor at any time during the 30-day observation period (Fig. 3). Similarly, tamoxifen did not affect the growth of this ER− tumor.
Figure 1.
Effect of AFP-derived peptide on growth of ER-positive MCF-7 and MCF-7/Tam human breast cancer xenografts. Tumors were implanted as described in Materials and Methods. Estrogen (solid symbols) was provided by means of a slow-release pellet of E2 implanted s.c. ▴, Peptide (Pep) was given twice a day i.p. at a dose of 1 μg per injection. ■, Tamoxifen (Tam) was given once a day s.c. at a dose of 50 μg per mouse. Tumor volumes in each mouse were measured at the time of tumor implantation, again at day 15 after tumor implantation during survival laparotomy, and again at day 30 after tumor implantation during necropsy. There was a minimum of five mice per group. (A) MCF-7 tumors. At day 30 after tumor implantation, tumor volumes in the E2 + Pep group and in the E2 + Tam group were significantly different from tumor volumes in the E2 alone group, P < 0.05. (B) MCF-7 subline made resistant to tamoxifen in culture. At day 30 after tumor implantation, tumor volumes in the E2 + Pep group but not in the E2 + Tam group were significantly different from tumor volumes in the E2 alone group, P < 0.05.
Figure 2.
Effect of AFP-derived peptide on growth of estrogen-receptor-positive T47D human breast cancer xenografts. See legend to Fig. 1 for experimental protocol. At day 30 after tumor implantation, tumor volumes in the E2 + Pep group were significantly different from tumor volumes in the E2 alone group, P < 0.05.
Figure 3.
Effect of AFP-derived peptide on growth of estrogen-receptor-negative MDA-MB-231 human breast cancer xenografts. See legend to Fig. 1 for experimental protocol. There were no differences in tumor volumes between any of the groups.
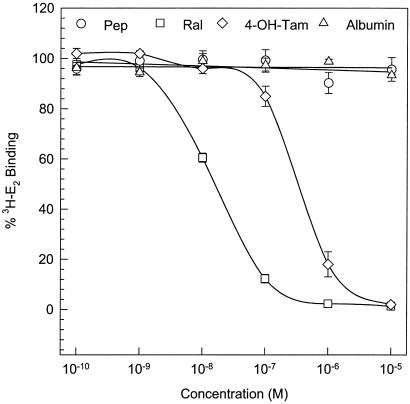
Although the spectrum of tumors that have been tested for sensitivity to AFPep is somewhat limited thus far, it appears that this peptide interferes with E-dependent, but not E-independent, breast cancer growth. As a first step in evaluating the mechanism of action of this peptide, it was compared with 4-hydroxytamoxifen and raloxifene as a competitor of E2 for binding to ER. As shown in Fig. 4, both 4-hydroxytamoxifen and raloxifene exhibit their well documented interference with E2 binding to ER. In contrast, AFPep produced no interference with E2 binding to ER over a peptide concentration range of 10−10 M to 10−5 M. Thus, the mechanism by which AFPep interferes with response to estrogen is clearly different from that of tamoxifen and other agents that directly compete with E2 for binding to ER.
Figure 4.
Effect of AFP-derived peptide on binding of E2 to its receptor. Rabbit uterine cytosol was used as a source of estrogen receptor. All incubations were performed in triplicate, each containing 100 μl of cytosol, 20 μl of 10 nM 6,7-[3H]estradiol (50 Ci/mmol), and 80 μl of test agent at the final concentrations indicated on the abscissa. Concentration of [3H]E2-complex with receptor in the presence of different concentrations of test agent is expressed as a percentage of the amount of complex formed in the absence of test agent.
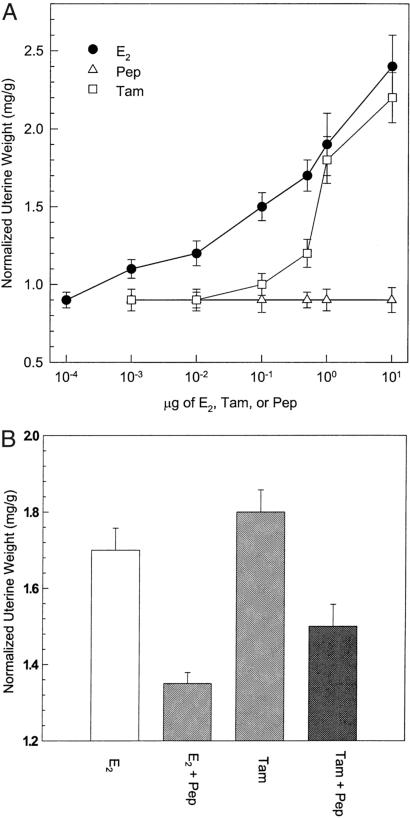
A troublesome side effect of tamoxifen in women has been its hypertrophic effect on the uterus (19). It is likewise an estrogen agonist in the murine uterus. As shown in Fig. 5A, tamoxifen stimulated the growth of immature mouse uterus by 50% at a dose of 1 μg/mouse. Tamoxifen's potency was approximately one-tenth that of E2, but nevertheless, Fig. 5A reaffirms that tamoxifen acts as an estrogen agonist on the murine uterus, even though it antagonizes the effect of estrogen on cancer of the breast. AFPep, on the other hand, had no uterotrophic effect whatsoever (Fig. 5A), even at a dose of 10 μg/mouse, which is 10-fold greater than the dose used to prevent breast cancer growth (Figs. 1 and 2). Moreover, peptide inhibited the uterotrophic effect of tamoxifen as well as that of estradiol (Fig. 5B).
Figure 5.
(A) Effect of E2, AFP-derived peptide (Pep) and tamoxifen (Tam) on the growth of the immature mouse uterus. The assay procedure is described in Materials and Methods. Various doses indicated on the abscissa of each test agent were injected i.p. Twenty-two hours later, uteri were harvested and weighed. Mean normalized uterine weights (mg uterine weight/g mouse body weight) for each group are shown on the ordinate. (B) Pep (1 μg) or vehicle (saline, 0.2 ml) were injected i.p. One hour later, E2 (0.5 μg) or Tam (1.0 μg) was injected i.p. Twenty-two hours later, uteri were harvested and weighed. Normalized uterine weights in the E2 + Pep and Tam + Pep groups were significantly different, respectively, from normalized uterine weights in the E2 group and Tam group, P < 0.05.
Discussion
The results of this study demonstrate that a synthetic 8-mer peptide derived from AFP prevented the E2-stimulated growth of human breast cancer xenografts, including an ER+ breast cancer line that had become resistant to tamoxifen. Although tamoxifen has been the mainstay of medical treatment for ER+ breast cancers and has provided significant clinical benefit (10, 11, 20), additional drugs are needed for the treatment of ER+ breast cancer, especially when these cancers are found to be refractory to tamoxifen. AFPep has the potential to fill this niche. There are other drugs further along in development that also could fill this niche. Letrozole, which blocks estrogen synthesis by inhibiting aromatase, and goserelin, which stifles ovarian release of estrogen by inhibiting gonadotropin release, are both being tested for this purpose (21, 22). AFPep, on the other hand, seems to represent a new class of agents able to inhibit the growth of ER+ breast cancer. How AFPep effects this inhibition is not clear at this time. It is clear from the data shown in Fig. 4, however, that its mechanism is different from that of tamoxifen in that it does not compete with E2 for binding to ER. Furthermore, in earlier studies we have shown that AFP administered to rodents did not reduce serum E2 levels (1), and, in preliminary studies, AFPep also did not reduce serum E2 levels, making its mechanism different from that of both letrozole and goserelin. In preliminary studies, we have found that this peptide reduces the level of MAPK kinase. This would restrict the phosphorylation of ER, which is MAPK kinase-dependent (23), and phosphorylation of ER is needed to fully operationalize this receptor. We are continuing to investigate this signal transduction pathway for additional information that would elucidate further the mechanism by which this peptide acts. There is no agent currently in use for the treatment of breast cancer that utilizes this signal transduction pathway for interfering with estrogen response, so this peptide truly represents a novel breast cancer therapeutic both in structure and in the biological mechanism through which it operates.
There is still a great deal of developmental work that remains with regard to this peptide. Optimization of its structure, as well as its dose, route, and schedule, and understanding its mechanism all remain to be worked out; its toxicology also needs to be explored. With regard to toxicology, we have observed no adverse effects of this peptide in the mice used for either the xenograft assays or the uterine growth assays. The cancer xenograft assays are important from a toxicological perspective because AFPep was given twice a day for 30 days in these assays, during which we observed no change in mouse body weight, cage activity, fur texture, or body temperature. Furthermore, on necropsy, there was no change in size or appearance of major organs relative to mice in the control group. Similarly, the uterine growth studies were important from a toxicological perspective because treatment with AFPep, unlike tamoxifen, did not stimulate murine uterine growth and interfered with the uterine growth stimulated by tamoxifen. This is significant because tamoxifen treatment of breast cancer patients induces uterine hypertrophy in ≈30% of the women who receive this drug (19). Moreover, it has been reported that uterine cancer develops in ≈0.2% of the women who are treated with tamoxifen (24). Although there are newer drugs in preclinical development that compete at the ER and have minimal agonist activity on the uterus (25), that AFPep acts at a different site than these drugs to mediate its antiestrotrophic activity and actually interferes with the uterotrophic effect of tamoxifen suggests that it may have use not only alone, but also in combination with ER competitors, even if these agents have partial agonist activity on the uterus. For example, the tamoxifen-stimulated uterine growth curve shown in Fig. 5A indicates that a slight lowering of tamoxifen dose substantially blunts uterine stimulation. Although we do not know the tamoxifen dose-response curve for uterine hypertrophy in women, this murine data suggest that AFPep in combination with lower doses of tamoxifen might well maintain full anti-breast cancer activity while reducing tamoxifen-induced uterine hypertrophy.
In summary, AFP is known to inhibit estrogen-dependent responses. We have isolated the active site in AFP responsible for this activity and have synthesized it as a biologically active peptide. This 8-mer peptide prevented E2-dependent human breast cancer xenograft growth, including breast cancers that had become resistant to tamoxifen. Its mechanism is different from that of tamoxifen and appears to be well tolerated in mice. Thus, this 8-mer peptide or peptidomimetics derived therefrom warrant further development as novel agents for the treatment of human breast cancer.
Acknowledgments
This work was supported by National Institutes of Health Grant CA 87434 and U.S. Army Grant DAMD17-99-1-9370.
Abbreviations
- AFP
α-fetoprotein
- ER
estrogen receptor
- E2
17β-estradiol
- AFPep
AFP-derived peptide
References
- 1.Bennett J A, Zhu S, Pagano-Mirarchi A, Kellom T A, Jacobson H I. Clin Cancer Res. 1998;4:2877–2884. [PubMed] [Google Scholar]
- 2.Couinaud C, Schwarzmann V, Cevara B, Orengo P, Fitterer R. Ann Chir. 1973;27:151–156. [PubMed] [Google Scholar]
- 3.Mizejewski G J, Vonnegut M, Jacobson H I. Proc Natl Acad Sci USA. 1983;80:2733–2737. doi: 10.1073/pnas.80.9.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson H I, Thompson W D, Janerich D T. Am J Epidemiol. 1989;129:865–873. doi: 10.1093/oxfordjournals.aje.a115220. [DOI] [PubMed] [Google Scholar]
- 5.Richardson B E, Hulka B S, David-Peck J L, Hughes C L, Van Den Berg B J, Christianson R E, Calvin J A. Am J Epidemiol. 1998;148:719–727. doi: 10.1093/oxfordjournals.aje.a009691. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson H I, Janerich D T. In: Biological Activities of Alpha-Fetoprotein. Mizejewski G J, Jacobson H I, editors. Boca Raton, FL: CRC; 1989. pp. 94–100. [Google Scholar]
- 7.Sonnenschein C, Ucci A A, Soto A M. J Natl Cancer Inst. 1980;64:1147–1152. [PubMed] [Google Scholar]
- 8.Mesfin F B, Bennett J A, Jacobson H I, Zhu S, Andersen T T. Biochim Biophys Acta. 2000;1501:33–43. doi: 10.1016/s0925-4439(00)00008-9. [DOI] [PubMed] [Google Scholar]
- 9.Mesfin F B, Andersen T T, Jacobson H I, Zhu S, Bennett J A. J Pep Res. 2001;58:246–256. doi: 10.1034/j.1399-3011.2001.00922.x. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, Dimitrov N V, Wolmark N, Wickerham D L, et al. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Costantino J, Wickerham D L. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 12.Jensen E V, DeSombre E R. In: Cancer Medicine. Holland J F, Frei E, Bast R C, Kufe D W, Morton D L, Weichselbaum R R, editors. Baltimore: Williams & Wilkins; 1996. pp. 1049–1060. [Google Scholar]
- 13.Gierthy J F, Lincoln D W, Roth K E, Bowser S S, Bennett J A, Bradley L, Dickerman H W. J Cell Biochem. 1991;45:177–187. doi: 10.1002/jcb.240450209. [DOI] [PubMed] [Google Scholar]
- 14.Gierthy J F, Lincoln D W, Gillespie M B, Seeger J I, Martinez H, Dickerman H W, Kumar S A. Cancer Res. 1987;47:6198–6203. [PubMed] [Google Scholar]
- 15.Gierthy J F, Bennett J A, Bradley L M, Cutler D S. Cancer Res. 1993;53:3149–3153. [PubMed] [Google Scholar]
- 16.Bennett J A, Pilon V A, MacDowell R T. Cancer Res. 1985;45:4963–4969. [PubMed] [Google Scholar]
- 17.Bennett J A, Pilon V A, Briggs D R, McKneally M F. J Natl Cancer Inst. 1985;75:925–936. doi: 10.1093/jnci/75.5.925. [DOI] [PubMed] [Google Scholar]
- 18.Bennett J A, Semeniuk D J, Jacobson H I, Murgita R A. Breast Cancer Res Treat. 1997;45:169–179. doi: 10.1023/a:1005841032371. [DOI] [PubMed] [Google Scholar]
- 19.Assikis V J, Jordan V C. Int J Gynecol Obstet. 1995;49:241–257. doi: 10.1016/0020-7292(95)02387-r. [DOI] [PubMed] [Google Scholar]
- 20.Jordan V C. In: Tamoxifen for the Treatment and Prevention of Breast Cancer. Jordan V C, editor. Melville, NY: PRR; 1999. pp. 53–96. [Google Scholar]
- 21.Goss P E, Strasser K. J Clin Oncol. 2001;19:881–894. doi: 10.1200/JCO.2001.19.3.881. [DOI] [PubMed] [Google Scholar]
- 22.Nystedt M, Berglund G, Bolund C, Brandberg Y, Fornander T, Rutqvist L E. Acta Oncol. 2000;39:959–968. doi: 10.1080/02841860050215945. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masuhige S, Gotoh Y, Nishida E, Kawashima H, et al. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Costantino J, Redmond C K. J Natl Cancer Inst. 1994;86:527–537. doi: 10.1093/jnci/86.7.527. [DOI] [PubMed] [Google Scholar]
- 25.Greenberger L M, Annable T, Collins K I, Komm B S, Lyttle C R, Miller C P, Satyaswaroop P G, Zhang Y, Frost P. Clin Cancer Res. 2001;7:3166–3177. [PubMed] [Google Scholar]