Abstract
Bone marrow is a major homing site for circulating epithelial tumor cells. The present study was aimed to assess the proliferative capacity of occult metastatic cells in bone marrow of patients with operable solid tumors especially with regard to their clinical outcome. We obtained bone marrow aspirates from 153 patients with carcinomas of the prostate (n = 46), breast (n = 45), colon (n = 33), and kidney (n = 29). Most of the patients (87%) had primary disease with no clinical signs of overt metastases [tumor-node-metastasis (TNM)-stage UICC (Union Internationale Contre le Cancer) I-III]. After bone marrow was cultured for 21–102 days under special cell culture conditions, viable epithelial cells were detected by cytokeratin staining in 124 patients (81%). The cultured epithelial cells harbored Ki-ras2 mutations and numerical chromosomal aberrations. The highest median number of expanded tumor cells was observed in prostate cancer (2,619 per flask). There was a significant positive correlation between the number of expanded tumor cells and the UICC-stage of the patients (P = 0.03) or the presence of overt metastases (P = 0.04). Moreover, a strong expansion of tumor cells was correlated to an increased rate of cancer-related deaths (P = 0.007) and a reduced survival of the patients (P = 0.006). In conclusion, the majority of cancer patients have viable tumor cells in their bone marrow at primary tumor diagnosis, and the proliferative potential of these cells determines the clinical outcome.
Even if a primary epithelial tumor can be resected in a curative intention, a significant number of patients will postoperatively develop hematogeneous metastases. There is emerging evidence that epithelial tumor cells are able to disseminate to secondary organs at an early stage of primary tumor development (1). Bone marrow is the most important secondary organ for the detection of these cells, and, even in cancer entities where overt skeletal metastases are rare (e.g., colorectal cancer), bone marrow is a prognostically relevant indicator organ for the presence of hematogeneously disseminated tumor cells (2–4).
Disseminated cancer cells in bone marrow express epithelial cytokeratins (CK) and are therefore immunocytochemically detectable with monoclonal antibodies against these antigens (1). The frequency of CK-positive cells in cytologic bone marrow preparations from cancer patients is about 10−5-10−6 (1). Cytokeratins are part of the cytoskeleton of epithelial cells and therefore represent a unique feature of epithelial cells. Extensive studies on large number of non-carcinoma control patients have shown that an immunocytochemically detectable CK expression in “normal” bone marrow cells is a rare event (5, 6).
The mere detection of CK-positive cells provides, however, no information about the proliferative potential of disseminated cancer cells. Whereas the prognostic significance of the detection of CK-positive cells has been studied in prospective clinical trials in patients afflicted with various types of epithelial tumors (6–12), very little is known about the biologic features and particularly the proliferative potential of these cells. The currently available data suggest that CK-positive cells in bone marrow aspirates of cancer patients represent a selected but still heterogeneous population of dormant (G0-phase) cancer cells (1).
The present study is an attempt to investigate the clinical relevance of the in vitro proliferative potential of CK-positive cells. Our results demonstrate firstly that the majority of cancer patients without overt metastasis harbor viable CK-positive tumor cells in their bone marrow at the time of primary surgery and secondly that the ability of these cells to grow in vitro is a strong indicator of an unfavorable prognosis for these patients.
Patients and Methods
Patients and Clinical Follow-Up.
In total, 153 consecutive patients with primary carcinomas of the colon (n = 33), prostate (n = 46), breast (n = 45), and kidney (n = 29) were admitted to the study between 1991 and 1995 after written, informed consent. The clinicopathological data and the information concerning the therapy modalities of the patients were assessed in the hospital archives of the participating clinics in Munich, Augsburg, and Wiesbaden, Germany. These data are summarized in Table 3, which is published as supporting information on the PNAS web site, www.pnas.org. One hundred eight (83.1%) of 130 patients received a surgical therapy. One hundred four of these patients were treated with curative intention and complete surgical tumor removal (R0), 36 received an adjuvant therapy (chemotherapy, radiation, hormonal therapy) in addition to surgical treatment, and in 4 patients surgical treatment was done with palliative intention. Twenty-two patients (16.9%) did not receive any surgical therapy and were treated with a conservative therapy (chemotherapy, radiation, and hormonal therapy).
The clinical follow-up data were assessed by contacting the general practitioners; the follow-up period ranged from 1 to 108 months postoperatively (median = 39 months). Complete follow-up data were available from 122 patients.
Bone Marrow Preparation.
Between 2–10 ml (median = 4 ml) of bone marrow aspirates were perioperatively obtained from one or both sides of the upper iliac crest or from the sternum (n = 16, only breast cancer patients). The bone marrow cells were washed with Hank‘s medium (Biochrom, Berlin) at 170 × g for 10 min, and mononuclear cells (MNC) were obtained by Ficoll density centrifugation (1.077 g/mol) at 1230 × g for 30 min. MNCs were washed at room temperature at 520 × g for 10 min, and an aliquot of maximal 1 × 106 MNCs was then cytocentrifuged onto a glass slide at room temperature at 130 × g for 5 min.
Primary Screening.
According to our previous work (2, 5, 6, 9, 11–13), mAb CK2 (IgG1; Roche Diagnostics) against CK polypeptide 18 and mAb A45-B/B3 (IgG1; Micromet, Munich), directed to a common epitope of various CK proteins including the heterodimers CK8/18 and CK8/19, were used for tumor cell detection in bone marrow cytospin preparations in an optimal concentration range between 2.5 and 4 μg/ml. A median number of 1.5 × 106 MNCs per aspirate was examined for CK expression (range between 0.4–5.3 × 106 MNCs). As a control for the specificity of the antibody reactions, an additional slide was also incubated with an appropriate dilution of an unrelated mouse-myeloma mAb of the same Ig isotype (IgG1, MOPC21; Sigma). The antibody reaction was developed with the alkaline phosphatase, anti-alkaline phosphatase technique combined with Neufuchsin stain, as described previously (14). Briefly, after incubation with the primary antibody, a polyvalent rabbit anti-mouse Ig antiserum (Z259; Dako) and preformed complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase antibodies (D651; Dako) were used at the dilutions recommended by the manufacturer (Dako).
Cell Culture and Culture Screening.
The bone marrow cells were cultured as previously described (14). Briefly, between 1–6 × 107 MNCs were initially plated in culture flasks coated with an extracellular matrix (Paesel & Lorei, Frankfurt, Germany). The culture medium contained RPMI 1640 supplemented with 10% FCS, 10 μg/ml transferrin, 5 μg/ml insulin, 2 mM glutamine, and 10 ng/ml recombinant human epidermal growth factor (Roche Diagnostics). The cells were cultured under 5% CO2 and reduced oxygen (5–10%). At confluence, the adherent cells (including the epithelial tumor cells) were removed by trypsination and transferred into new culture flasks. To determine the number of CK-positive cells at every passage, an aliquot containing 3 × 104 cells was cytocentrifuged onto glass slides and immunocytochemically stained (culture screening) by using the alkaline phosphatase, anti-alkaline phosphatase method as described above. The actual number of CK-positive cells that were initially placed into cell culture was calculated by multiplying the concentration of CK-positive cells determined at primary screening with the total number of plated bone marrow cells. The number of in vitro expanded CK-positive cells has been calculated by multiplying the concentration of CK-positive cells determined at culture screening with the total number of cells in culture.
PCR-Based Detection of Ki-ras2 Mutations in Laser-Dissected Epithelial Cells.
The cover slips of the archival glycerol gelatin-embedded and CK-immunostained bone marrow cytospins from seven colon cancer patients were removed, and the slides were washed in hot 70°C PBS for 2 h and then air-dried. Laser-isolation was performed with the Robot-MicroBeam system (P.A.L.M., Bernried, Germany; ref. 15 and 16). Individual CK-positive tumor cells were transferred by laser pressure catapulting into a common microfuge cap moistened with a 2-μl droplet of mineral oil (15).
Fifteen to 30 laser-microdissected cells of each sample were pooled and digested with 3.5 mg/ml proteinase K in a volume of 5 μl as previously described (16). As a positive control, we used the SW480 colon cancer cell line, known to carry a mutation at codon 12. The EJ-28 bladder carcinoma cell line was used as a negative control. PCR amplification was performed with Taq polymerase (Thermo Hybaid, Ulm, Germany) in a total of 25 μl by using 10 pmol each of the “outer” primer set ki-ras outF/R. The samples were subjected to 1 cycle of 94°C for 2 min; 30 cycles of 94°C for 30 s, of 52°C for 30 s, and 70°C for 1 min, followed by 1 cycle of 68°C for 15 min. The hemi-nested PCR amplifications in the block cycler were performed in a 25-μl volume with a 5-μl aliquot of the first PCR product containing 10 pmols of the “inner” primer set 12s/ki-ras R by using the conditions mentioned above. Screening for Ki-ras2 mutations was performed by the previously described artificial restriction length polymorphism by using 10 units of the restriction enzyme MspI (15, 17, 18). MspI cuts only within the 140-bp PCR fragment if the Ki-ras2 codon 12 is wild type and creates a 118-bp PCR fragment. The products of the hemi-nested PCR and the MspI digests were fractionated side by side on a 3% agarose gel containing 0.5× Sybr Gold (Molecular Probes).
Multicolor Interphase Fluorescence in Situ Hybridization (FISH).
CK-positive cytospin preparations of bone marrow cultures from a subset of six breast cancer patients were examined by multicolor FISH analysis. The centromere-specific probes (PZ7.6B, chromosome 7, labeled with biotin; PZ8.4, chromosome 8, labeled with digoxigenin; PRB11, chromosome 11, labeled with Cy5; PZ17-14, chromosome 17, labeled with Cy3; 2Xba, chromosome 18, labeled with estradiol) were provided by M. Rocchi (University of Bari, Bari, Italy; http://www.biologia.uniba.it/rmc/index.html). Cells were fixed in 1% formalin diluted in PBS for 10 min at room temperature. After application of the cytokeratin antibody A45-B/B3 labeled with Cy3, an incubation in 2× SSC for 30 min at 37°C, and a dehydration step in ethanol (70%, 90%, and 100%), the cytospins were denatured in 70% formamide in 2× SSC at 72°C for 2 min, 15 s and subsequently dehydrated in cold ethanol. After air drying the slides, the mix containing the centromeric probes was hybridized overnight to the slides without a preannealing step. After the posthybridization washes (3 × 5 min in 4× SSC/Tween 20 at 42°C and 3 × 5 min in 1× SSC) and blocking with 3% BSA, the biotin-labeled probes were detected with avidin-Cy3.5 (1:300; Rockland, Gilbersville, PA), the digoxigenin-labeled probes with anti-digoxigenin FITC (1:200; Vector Laboratories), and the estradiol-labeled probes with IgG rabbit/anti-estradiol (1:200; Roche Diagnostics) in conjugation with anti-rabbit Cy5.5 (1:400; Amersham Pharmacia Biotech). All antibodies were diluted in 4× SSC/Tween 20 with 1% BSA. After final washes (3 × 5 min in 4× SSC/Tween 20 at 42°C), the chromosomes were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) and mounted in p-phenylenediamine dihydrochloride anti-fade solution. Images were acquired by using a motorized epifluorescence microscope (Leica DMRX-RF8, Wetzlar, Germany) equipped with a Sensys CCD (charge-coupled device) camera (Kodak KAF 1400 chip; Photometrics, Tucson, AZ). Microscope and camera were controlled by the Leica qfish software (Leica Microsystems Imaging Solutions, Cambridge, U.K.).
Statistical Analysis.
For statistical analysis, we used the spss 9.1 software package. To compare categorical variables, we used the χ2 test, whereas the Kruskal-Wallis test was used for nonparametric variables. The Kaplan-Meier method was applied to estimate overall survival; these values were compared by the log-rank test. To estimate the prognostic value of the number of in vitro expanded CK-positive cells, patients were divided into two categories. By using the CART-analysis (classification and regression trees), the patient group was analyzed for the parameter with the highest separation and highest log-rank value. The primary end point of our analysis was overall survival, as measured from the date of surgery to the time of the last follow-up or cancer-related death. Differences between groups were considered significant if the P values were less than 0.05 in a two-tailed test.
Results
Expansion of CK-Positive Cells in Various Tumor Types.
Bone marrow was aspirated from the upper iliac crest at the time of primary surgery, and an aliquot of the aspirate was investigated for the presence of CK-positive tumor cells (primary screening). The remaining bone marrow cells were cultured and then screened once more for CK-expression (culture screening). Subsequently, the cultures were screened at different time points (data not shown). The screening result with the highest number of CK-positive cells (i.e., peak of in vitro growth) was used for our present analysis. In 132 of 153 cases (86.8%), this value was reached at the first or second cell culture passage after a median of 21–38 days in culture. In the remaining 20 patients, the peak of in vitro growth was reached at later cell culture passages. There was no correlation between the number of CK-positive cells detected in the bone marrow sample (primary screening) and the number of CK-positive cells in the culture screening (data not shown).
Of the total group of 153 patients with various forms of epithelial tumors, 46 patients (30.1%) showed a CK-positive primary screening result, as compared with 124 patients (81%) who presented with CK-positive cells in the culture screening (Table 1). Similar differences were observed within the subgroups of patients with different tumor types. The most striking increases in the incidence of CK-positive cells were found in patients with prostate cancer and renal cell cancer (Table 1). Only 3 of 46 prostate cancer patients and 1 of 29 renal cell cancer patients failed to present with CK-positive cells in culture.
Table 1.
Incidence of CK-positive tumor cells before and after in vitro expansion
| Tumor type | CK-positive primary screening n patients, % | CK-positive culture screening n patients, % |
|---|---|---|
| Colon cancer (n = 33) | 9 (27.3) | 23 (69.7) |
| Prostate cancer (n = 46) | 11 (23.9) | 43 (93.5) |
| Breast cancer (n = 45) | 16 (35.5) | 30 (66.6) |
| Renal cell cancer (n = 29) | 10 (34.5) | 28 (96.6) |
| Total (n = 153) | 46 (30.1) | 124 (81.0) |
The percentages shown in parentheses indicate the fraction of patients with a CK-positive screening result in each subgroup of patients with a particular tumor type.
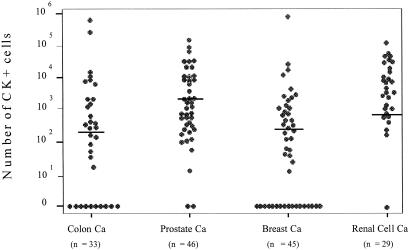
The extent of expansion shown in Figs. 1 and 2 is the total number of cultured epithelial (i.e., CK-positive) cells per flask. This number was calculated by multiplying the concentration of epithelial cells per cytospin, as determined by anti-cytokeratin immunostaining of an aliquot of the cultured cells, with the total number of all cultured cells (i.e., epithelial cells and bone marrow stroma cells). The number of CK-positive cells ranged from 10 to 106 per culture flask among individual patients (Fig. 1). The extent of expansion of CK-positive cells derived from patients with different tumor types revealed some differences. The highest median number of in vitro expanded CK-positive cells (2619) was observed in prostate cancer patients, the lowest in colon cancer patients (275; Fig. 1). To exclude that the high proportion of early stage breast cancer patients, as compared with the other tumor types (Table 3), may bias the outcome of our analysis, we separately analyzed a more homogenous subgroup of patients (only UICC stages I and II). Prostate cancer patients still showed the strongest expansion of CK-positive cells, followed by patients with breast, renal cell, and colon cancer (data not shown). However, none of the observed changes were statistically significant.
Figure 1.
Correlation between the tumor type and the extent of in vitro expansion of CK-positive tumor cells. The y axis shows the total number of cultured CK-positive cells per flask, which was calculated by multiplying the concentration of epithelial cells per cytospin with the total number of all cultured cells. The bars represent the median values in each subgroup of patients.
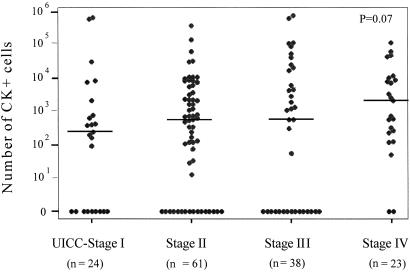
Figure 2.
Correlation between the UICC-stage of the patients and the extent of in vitro expansion of CK-positive cells. The data from all patients with different tumor types and available staging information (n = 146) were pooled. The y axis shows the total number of cultured CK-positive cells per cytospin, which was calculated by multiplying the concentration of epithelial cells per flask with the total number of all cultured cells. The bars represent the median values in each subgroup of patients.
Correlation to Tumor Stage.
For the subsequent analyses, we decided to pool the data from patients with different tumor types. The described differences were observed in the same manner in the individual subgroups of patients with a particular tumor type; however, the number of patients in each subgroup was too small to demonstrate statistical significance.
The tumor staging data were available from 146 patients. Forty-six (31.5%) of these patients had a positive primary screening result, whereas 124 (84.9%) of these patients had a positive culture screening result. This subgroup was therefore representative for the entire group of patients analyzed. There was no significant correlation between the results of the primary screening and the tumor stage (data not shown).
The correlation of the culture screening results and the tumor stage revealed statistically significant results. Among the 124 patients with detectable CK-positive cells in culture, 16 (66.6%) of 24 patients were at UICC (Union Internationale Contre le Cancer)-stage I, 50 (81.9%) of 61 patients at UICC-stage II, 29 (76.3%) of 38 patients at UICC-stage III, and 23 (100%) of 23 patients at UICC-stage IV (P = 0.03, data not shown). As shown in Fig. 2, the extent of in vitro expansion of CK-positive cells tended to be associated with the UICC-stages (P = 0.07). We observed an increase in the number of CK-positive cells per culture comparing patients at UICC-stage I to those at UICC-stage II (236 vs. 593 cells, respectively) and another increase from UICC-stage III to UICC-stage IV (586 vs. 2104 cells, respectively). Interestingly, all cell cultures of the 13 patients with clinical overt metastases harbored CK-positive cells, whereas 105 (78.9%) of 133 patients without overt metastasis (M0) expressed CK-positive cells after in vitro expansion (P = 0.04, data not shown).
Correlation to the Clinical Follow-Up.
The clinical follow-up data of 122 patients were available for statistical analysis; the clinical and histopathological data of this subgroup were representative for the entire cohort of patients.
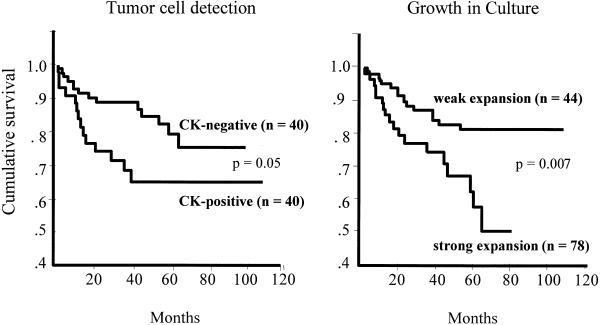
In the primary screening, 40 (32.7%) cases demonstrated CK-positive cells in their bone marrow aspirates. Ten of these patients (25.0%) developed postoperatively a systemic tumor relapse and died. In the CK-negative group, 15 (18.3%) of 82 patients suffered from a systemic relapse, and 14 (17.1%) of these patients died. Statistical analysis (log-rank test) revealed that the primary detection of CK-positive cells was positively correlated with an increased risk of cancer-related death (P = 0.05; Fig. 3). The median survival time was 36 months in the CK-positive group as compared with 43 months in the PS-negative group (P = 0.08).
Figure 3.
Overall survival of all patients depending on the detection (primary screening) and in vitro growth (culture screening) of CK-positive cells. According to the results of the CART analysis, patients were divided into two subgroups: category 1, weak expansion (n CK+ cells per flask < 2,105]; category 2, strong expansion (n CK+ cells per flask ≥ 2,105]
The evaluation of the culture screening results demonstrated that 99 patients (81.1%) harbored CK-positive cells. Nineteen of these patients (19.2%) developed a systemic tumor relapse and died during the median postoperative follow-up period of 36 months (range 1–108 months). The culture screening result was negative in 23 patients (18.9%). Six of these patients developed a systemic tumor relapse, and four of them died during the median postoperative follow-up period of 59.5 months. The difference between the CK-positive and CK-negative group was not statistically significant (P = 0.83).
According to the CART analysis, patients were distributed into two categories. Seventy-eight patients (63.9%) were assigned to category 1 (i.e., “weak” expansion, n CK-positive cells < 2105). Thirteen of these patients (16.7%) suffered from a systemic tumor relapse, and 12 patients (15.3%) died. On the other hand, 44 patients (36.1%) were assigned to category 2 (i.e., “strong” expansion, n CK-positive ≥ 2105). Ten of these patients (22.7%) developed postoperatively a systemic tumor relapse, and all of them died. Kaplan-Meier analysis revealed that the differences in the rates of cancer-related deaths were statistically significant (P = 0.007; Figure 3).
Analysis of Ki-ras2 Mutations at Codon 12 in Cultured CK-Positive Cells.
Ki-ras2 mutations at codon 12 are the most frequent genetic abnormalities in human colon cancers (19). To prove that the cultured CK-positive cells are of neoplastic origin, we investigated these cells from an unselected subgroup of seven colon carcinoma patients for point mutations at codon 12 of the Ki-ras2 gene. Fifteen to 30 CK-positive cells from each patient were isolated by laser-assisted microdissection from archival cytospin preparations, subsequently pooled, and screened for Ki-ras2 mutations at codon 12 by means of MspI-digestion analysis. As shown in Table 2, mutant codon 12 alone was detected in two of the seven patients analyzed, whereas one cell pool (patient Ga) contained only wild-type Ki-ras2. In the remaining four patients, the pooled cells had a mixed genotype with both mutated and wild-type Ki-ras2. This result might reflect the coexistence of either wild-type Ki-ras2 tumor cells or normal CK-positive cells together with Ki-ras2-mutated tumor cells in the cultures.
Table 2.
Ki-ras2 status of cultured CK-positive cells from colon cancer patients
| Patient ID | No. of isolated CK-positive cells | Ki-ras2 status |
|---|---|---|
| Pi | 30 | mt |
| Ma | 21 | mt/wt |
| Ar | 20 | mt/wt |
| Ja | 20 | mt/wt |
| St | 12 | mt/wt |
| Ga | 16 | wt |
| Hu | 15 | mt |
mt, mutation in codon 12 of the Ki-ras2 gene; wt, wild-type Ki-ras2.
Analysis of Numerical Chromosomal Aberrations in Cultured CK-Positive Cells.
We evaluated archival cytospin preparations from bone marrow cultures of a subset of six breast cancer patients for numerical aberrations by using probes for centromeric regions of chromosomes 7, 8, 11, 17, and 18. For each patient, 100 cultured CK-positive cells were analyzed. We used a newly developed interphase FISH assay that allows the simultaneous visualization of the CK-antibody and the detection of multiple FISH signals in an individual interphase nucleus (Fig. 4). As shown in Table 4, which is published as supporting information on the PNAS web site, we observed numerical chromosomal aberrations in 19 to 61% of the cultured cells. This total value, as well as the values for the individual chromosomes, differed from the observed “false-positive” findings in normal blood lymphocytes used as negative control.
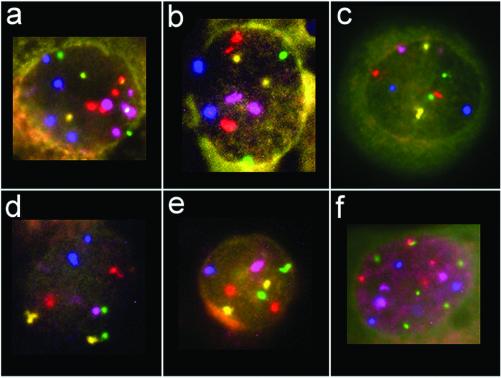
Figure 4.
Representative cell nuclei after hybridization of the five-color probe mix. Centromere 7 is depicted in red, centromere 8 in green, centromere 11 in blue, centromere 17 in yellow, and centromere 18 in purple. (a and b) Interphase cells of patient E.E.: the cell shown in a is in the tetraploid range with loss of one signal for centromere 8 and loss of three centromere 17 signals; the diploid cell in b has three centromere 7 signals. (c–e) Interphase cells of patient M.P.: (c) diploid cell with two copies of each probe; (d and e) interphase cells with loss of one centromere 18 signal (d), and loss of signals for centromeres 11 and 18 (e). (f) Interphase cell of patient K.H. demonstrating a large variety of different signal numbers.
The pattern of observed gains and losses of individual chromosomes varied considerably among different patients (Table 4). Most changes occurred in less than 20% of the cells, and there was no predominant change observed in all or most cultured cells. This finding demonstrates the considerable genetic heterogeneity of these cells, which is probably caused by a significant chromosomal instability. The only common finding was the rare loss of chromosome 8 (i.e., less than 10% of all cultured cells showed this genotype).
Discussion
The present study represents an attempt to investigate the clinical relevance of the extent of in vitro expansion of CK-positive cells present in bone marrow aspirates by using previously established cell culture conditions (14). We have previously excluded the possibility that cultured bone marrow cells may illegitimately express CK detectable with anti-cytokeratin antibodies that we have applied in our present analysis (14). Thus, the CK-positive cells detected in our bone marrow cultures appeared to be epithelial cells.
The number of in vitro expanded CK-positive cells was not correlated to the number of CK-positive cells in the bone marrow aspirates, which is consistent with our former findings demonstrating that the growth kinetics of these tumor cells are variable (14). Thus, the number of CK-positive cells at culture screening is not biased by the different bone marrow tumor loads observed among individual patients but rather reflects the in vitro proliferative potential of these cells. The concentration of tumor cells in culture increases because of the proliferation of tumor cells and the death of nonadherent bone marrow cells (14). A significant number of bone marrow samples became positive after cell culture because we plated, on the average, 10 times more bone marrow cells per culture flask than we examined in the primary screening (on the average 106 cells). Thus, these cultures were grown under almost limiting dilution conditions, i.e., fewer than 10 tumor cells were plated per flask, indicating that micrometastatic cells can inherit a significant growth potential. In contrast, the previous work of other groups demonstrating the growth of metastatic cells in bone marrow has focused on more advanced cancer stages with a higher load of tumor cells in bone marrow (20, 21).
Statistical analysis revealed that the in vitro extent of expansion of CK-positive cells is individually different within one tumor type, and it varies between the different tumor entities analyzed. Comparison of the strength of expansion between patients with different tumor types in comparable tumor stages revealed that the highest median number of in vitro expanded CK-positive cells was observed in bone marrow samples of patients suffering from prostate and breast cancer. This result confirms the clinical finding that these patients suffer more often from bone or bone marrow metastasis than patients with renal cell or colon cancer (1, 2, 13).
Our qualitative analysis of the presence or absence of CK-positive cells in culture revealed a positive correlation between the incidence of CK-positive cells and the tumor stage of the patient. In particular, it is noteworthy that, in patients with advanced disease (stage M1), the detection rate of CK-positive cells at primary screening was only 34%, whereas this rate increased to 100% after culture. Thus, our data suggest that all patients with advanced disease harbor viable tumor cells with a substantial proliferative capacity in their bone marrow but that these cells are missed in many of these patients because of the lower sensitivity of the primary screening. Thus, bone marrow might be an important reservoir for metastatic cells even in patients who present with overt metastases at other secondary sites, such as liver or lung.
To test the clinical relevance of the extent of in vitro expansion of CK-positive cells, we performed a clinical follow-up analysis. By using the CART-analysis, we defined two categories of samples distinguished by a cut-off point of 2,105 CK-positive cells per flask. Patients who were assigned to category 1 samples (i.e., <2,105 tumor cells per flask) had a significantly reduced rate of cancer-related death and an increased overall survival compared with patients with category 2 samples (i.e., ≥2,105 tumor cells per flask). This finding demonstrated a statistically significant relationship between the in vitro extent of expansion of micrometastatic tumor cells and the postoperative clinical outcome of cancer patients. The extent of in vitro expansion of CK-positive cells was a better prognostic indicator than the mere detection of these cells in bone marrow samples. Similar data have been obtained for the expression of HER2/neu and urokinase plasminogen activator receptor, which define a subpopulation of more aggressive CK-positive cells in bone marrow (22–24). A better understanding of the biology of micrometastatic cells should therefore increase the diagnostic precision of the bone marrow assay. Larger prospective clinical trials with separate analysis of different tumor types are now needed to confirm the clinical relevance of the in vitro extent of expansion of CK-positive cells suggested in the present study.
The hypothesis that these cultured CK-positive epithelial cells are of neoplastic nature is also confirmed by the lack of such cells in control cultures of bone marrow from patients who do not have a malignancy (14). Moreover, our present data on tumor-specific alterations in cultured epithelial cells reveal mutations at codon 12 of the Ki-ras2 oncogene in laser-isolated CK-positive cells present in bone marrow cultures from colon cancer patients (Table 2) and numerical changes in chromosomes 7, 8, 11, 17, and 18 in bone marrow cultures from breast cancer patients (Table 4). In addition, we found loss of genomic material at chromosomal regions 6p, 8q, and 8p in bone marrow cultures of prostate cancer patients, by using tri-color interphase FISH analysis (25). Moreover, O’Sullivan et al. recently demonstrated that CK-positive cells grown under the same culture conditions as described in this paper are tumorigenic in athymic nude mice (26). Despite the preliminary character of these data, they already prove the neoplastic nature of the cultured epithelial cells. The variability of their genotypes observed in the present study is consistent with our previous data on the phenotype of CK-positive cells in bone marrow (1, 13, 24), indicating that these cells are a heterogeneous population of cells with different biological properties. Further studies are needed to determine the signaling pathways that stimulate the proliferation of disseminated cancer cells present in bone marrow as a major homing site for epithelial tumor cells. In this context, it might be interesting that breast cancer patients with HER2/neu-positive tumor cells in bone marrow have a greater risk to be afflicted with subsequent metastatic relapse than patients with disseminated tumor cells lacking an immunocytochemically detectable expression of HER2/neu (24). Thus, growth factor receptors of the epidermal growth factor family, which coaggregate with HER2/neu on the tumor cell surface, might be a crucial factor with regard to the observed expansion of CK-positive cells. In this respect, it is noteworthy that our culture medium contained epidermal growth factor.
The analysis of cultured micrometastatic tumor cells will open a new avenue to assess the molecular determinants of potential metastatic stem cells in cancer patients. Cell culture is an efficient way to increase the number of CK-positive cells available for subsequent molecular analyses. Considering the fact that most bone marrow samples contain only very few tumor cells, the expansion of these cells will help to circumvent this bottle neck of micrometastasis research. A common argument against the analysis of cultured cells is that the culture process leads to the selection of those cell clones that adapt best to the culture conditions. The correlation to the clinical outcome observed in the present study, however, indicates that the selection process imposed by our culture conditions mimic to some extent the in vivo situation. The analysis of cultured CK-positive cells may therefore contribute to the identification of the metastatic stem cells responsible for the formation of overt metastases.
Supplementary Material
Acknowledgments
We thank M. Stich (Städtisches Krankenhaus München-Harlaching) and S. Kernstock (Molekulare Onkologie, Universitäts-Klinikum Eppendorf, Hamburg) for helpful technical assistance. This work was supported by the Deutsche Krebshilfe/Dr. Mildred Scheel Stiftung, Bonn, and the Deutsche Forschungsgemeinschaft, Bonn, Germany.
Abbreviations
- MNC
mononuclear cells
- CK
cytokeratin
- FISH
fluorescence in situ hybridization
- UICC
Union Internationale Contre le Cancer
- CART
classification and regression trees
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pantel K, Cote R J, Fodstad Ø. J Natl Cancer Inst. 1999;91:1113–1124. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 2.Lindemann F, Schlimok G, Dirschedl P, Witte J, Riethmüller G. Lancet. 1992;340:685–689. doi: 10.1016/0140-6736(92)92230-d. [DOI] [PubMed] [Google Scholar]
- 3.Soeth E, Vogel I, Röder C, Juhl H, Marxsen J, Krüger U, Henne-Bruns D, Kremer B, Kalthoff H. Cancer Res. 1997;57:3106–3110. [PubMed] [Google Scholar]
- 4.Leinung S, Würl P, Schönfelder A, Weiss C-L, Röder I, Schönfelder M. J Hematother. 2000;9:905–911. doi: 10.1089/152581600750062354. [DOI] [PubMed] [Google Scholar]
- 5.Pantel K, Schlimok G, Angstwurm M, Weckermann C, Schmaus W, Gath H, Passlick B, Izbicki J, Riethmüller G. J Hematother. 1994;3:165–173. doi: 10.1089/scd.1.1994.3.165. [DOI] [PubMed] [Google Scholar]
- 6.Braun S, Pantel K, Janni W, Hepp F, Kentenich C R M, Müller P, Gastroph S, Wischnik A, Dimpfl T, Kindermann G, et al. N Engl J Med. 2000;342:525–533. doi: 10.1056/NEJM200002243420801. [DOI] [PubMed] [Google Scholar]
- 7.Cote R J, Rosen P P, Lesser M L, Old L J, Osborne M P. J Clin Oncol. 1991;9:1749–1756. doi: 10.1200/JCO.1991.9.10.1749. [DOI] [PubMed] [Google Scholar]
- 8.Harbeck N, Untch M, Pache L, Eiermann W. Br J Cancer. 1994;69:566–571. doi: 10.1038/bjc.1994.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantel K, Izbicki J R, Passlick B, Angstwurm M, Häussinger K, Thetter O, Riethmüller G. Lancet. 1996;347:649–653. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 10.Gerber B, Krause A, Müller H, Richter D, Reimer T, Makovitzky J, Herrnring Ch, Jeschke U, Kundt G, Friese K. J Clin Oncol. 2001;19:960–971. doi: 10.1200/JCO.2001.19.4.960. [DOI] [PubMed] [Google Scholar]
- 11.Braun S, Hepp F, Kentenich Ch, Janni W, Blankenstein Th, Schindlbeck Ch, Riethmüller G, Pantel K. J Clin Oncol. 2001;19:368–375. doi: 10.1200/JCO.2001.19.2.368. [DOI] [PubMed] [Google Scholar]
- 12.Schlimok G, Funke I, Pantel K, Strobel F, Lindemann F, Witte J, Riethmüller G. Eur J Cancer. 1991;27:1461–1465. doi: 10.1016/0277-5379(91)90032-9. [DOI] [PubMed] [Google Scholar]
- 13.Pantel K, Schlimok G, Braun S, Kutter D, Schaller G, Funke I, Izbicki J, Riethmüller G. J Natl Cancer Inst. 1993;85:1419–1424. doi: 10.1093/jnci/85.17.1419. [DOI] [PubMed] [Google Scholar]
- 14.Pantel K, Dickmanns A, Zippelius A, Klein C, Shi J, Höchtlen-Vollmar W, Schlimok G, Weckermann D, Oberneder R, Fanning E, Riethmüller G. J Natl Cancer Inst. 1995;87:1162–1168. doi: 10.1093/jnci/87.15.1162. [DOI] [PubMed] [Google Scholar]
- 15.Schütze K, Lahr G. Nat Biotechnol. 1998;16:737–742. doi: 10.1038/nbt0898-737. [DOI] [PubMed] [Google Scholar]
- 16.Lahr G, Stich M, Schütze K, Blümel P, Pösl H, Nathrath W B J. Pathobiology. 2000;68:218–226. doi: 10.1159/000055927. [DOI] [PubMed] [Google Scholar]
- 17.Haliassos A, Chomel J C, Grandjouan S, Kruh J, Kaplan J C, Kitzis A. Nucleic Acids Res. 1989;17:8093–8099. doi: 10.1093/nar/17.20.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lahr G. Lab Invest. 2000;80:1477–1479. doi: 10.1038/labinvest.3780155. [DOI] [PubMed] [Google Scholar]
- 19.Ellis C A, Geoff C. Cell Signal. 2000;12:425–434. doi: 10.1016/s0898-6568(00)00084-x. [DOI] [PubMed] [Google Scholar]
- 20.Ross A A, Cooper B W, Lazarus H M, Mackay W, Moss T J, Ciobanu N, Tallmann M S, Kennedy M J, Davidson N E, Sweet D, et al. Blood. 1993;82:2605–2610. [PubMed] [Google Scholar]
- 21.Carney D N, Gazdar A F, Bepler G, Guccion J G, Marangos P J, Moody T W, Zweig M H, Minna J D. Cancer Res. 1985;45:2913–2923. [PubMed] [Google Scholar]
- 22.Heiss M M, Allgayer H, Gruetzner K U, Funke I, Babic R, Jauch K W, Schildberg F W. Nat Med. 1995;1:1035–1039. doi: 10.1038/nm1095-1035. [DOI] [PubMed] [Google Scholar]
- 23.Allgayer H, Heiss M M, Riesenberg R, Gruetzner K U, Tarabichi A, Babic R, Schildberg F W. Cancer Res. 1997;57:1394–1399. [PubMed] [Google Scholar]
- 24.Braun S, Schlimok G, Henmos I, Schaller G, Riethdorf L, Riethmuller G, Pantel K. Cancer Res. 2001;61:1890–1895. [PubMed] [Google Scholar]
- 25.Reindl C. Ph.D. thesis. Munich: Ludwig-Maximillians-Universität; 2002. [Google Scholar]
- 26.O'Sullivan G C, Sheehan D, Clarke A, Stuart R, Kelly J, Kiely M D, Walsh T, Collins J K, Shanahan F. Gastroenterology. 1999;116:543–548. doi: 10.1016/s0016-5085(99)70175-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.