Abstract
Purpose
Previous studies have suggested that milk consumption can promote growth in children. However, limited studies have been performed on the effects of cow milk varieties, especially β-casein A2 milk. This study aims to investigate the effect of β-casein A2 cow milk supplementation on physical growth, inflammation, and growth-related hormone and nutritional biomarker profiles in growth-stunted children.
Methods
This is a quasi-experimental study with only one group and a pre-and posttest design. This research is divided into 3 stages: allele testing in the β-casein gene, processing into ready-to-drink milk, and a clinical trial. The participants were children aged 12–36 months who were given 200-mL β-casein A2 cow milk supplementation once a day for 3 months. The outcome assessments were physical growth (body weight and height), inflammation (tumor necrosis factor-alpha [TNF-α] and cortisol levels), and biological markers related to growth and nutrition (insulin-like growth factor-1 [IGF-1], growth hormone [GH], and transferrin) that were measured during pre-, mid (week 6)-, and post (week 12)-intervention periods.
Results
This study included 30 study participants. Significant body weight and height improvements were observed at week 6 and postintervention (week 12) compared to preintervention. There were significant reductions in the inflammation markers TNF-α and cortisol levels postintervention. Additionally, IGF-1 and GH levels increased significantly, and transferrin levels also rose, potentially reflecting improved nutritional status.
Conclusions
This study suggests that β-casein A2 milk supplementation was associated with improvements in physical growth and related biomarkers in stunted children. Additionally, β-casein A2 milk may produce fewer BCM-7 metabolites compared to β-casein A1 milk, which has been hypothesized to be associated with certain adverse health outcomes. However, further controlled studies are needed to confirm its efficacy as a dietary intervention.
Keywords: Caseins, Growth, Growth regulation, Hormones, Inflammation, Milk, Nutrition, Stunting
GRAPHICAL ABSTRACT
Highlights
· β-casein A2 milk supplementation was associated with improvements in physical growth and a reduction in inflammation in stunted children.
· Significant increases in body weight, height, insulin-like growth factor-1, growth hormone, and transferrin, as well as reductions in tumor necrosis factor-alpha and cortisol levels, were observed, suggesting potential benefits.
· β-casein A2 milk may offer a safer alternative to A1 milk, with fewer potential health risks, though further studies are needed for confirmation.
Introduction
Stunting remains a significant global health problem. Worldwide, there are more than 149 million children, with 21% under the age of 5 suffering from stunted growth. The majority (91%) of children with stunted growth reside in low- and middle-income countries [1]. Of such children, more than 50% are from Asia, while more than 33% are from Africa [2]. From 2005 to 2017, an average of 36.4% of children younger than 5 years in Indonesia experienced growth stunting [3,4]. Stunting also has wide ranging social, psychological, and economic effects throughout a person's life. These effects include limited physical growth and height, inferior educational achievements, cognitive impairments, and decreased productivity in the workforce [5].
Several supplements have been used to prevent stunting. Previous studies have found a relationship between linear growth of children and protein, especially from animal sources in the form of cow milk [6-9]. Milk is commonly advised to be included in the daily diet of individuals in numerous countries, with a recommended intake of 2-3 cups (473-709 mL) per day [10]. Several studies have identified beneficial correlations between milk intake and body weight and fat mass levels [11,12]. Additionally, milk consumption has been associated with appropriate skeletal growth and development [10]. Milk components, such as casein and its fractions, have also been reported to act as immunoregulators [13,14]. Although cow milk and dairy products have numerous positive impacts, there is limited research validating the benefits of various types of cow milk on children's growth and immunological and hormonal levels.
β-Casein is a major protein in milk that varies by genetic makeup; the most common forms are β-casein A1 and A2 [15]. These 2 forms have different amino acid compositions of milk protein and metabolite results of enzymatic hydrolysis in the digestive tract. Milk with β-casein A1 produces more of the metabolite β-casomorphins-7 (BCM-7) than does milk containing β-casein A2 [16]. The BCM-7 is reported to be related to the risk of diseases associated with opioid receptors in the nervous system, endocrine glands, and immune system including type 1 diabetes, coronary heart disease, and arteriosclerosis [16-18].
Given the importance of milk in preventing stunting, its potential immunomodulatory effects, and the need to understand the specific impacts of β-casein A2 milk, this study aims to investigate the effect of β-casein A2 cow milk supplementation on physical growth (body weight and body height), inflammation (tumor necrosis factor-alpha [TNF-α] and cortisol levels), and biological markers associated with growth and nutrition (insulin-like growth factor-1 [IGF-1], growth hormone [GH], and transferrin levels).
Materials and methods
1. Study design and participants
This was a quasi-experimental study with a single group and a pre- and posttest design.
This research was divided into 3 stages. The first stage was allele testing to differentiate the A1 and A2 alleles in the β-casein gene. In the second stage, milk from the population of dairy cows whose allelic variants had been identified, especially the A2 allele, were processed into ready-to-drink milk. The third stage was a clinical trial evaluating the effects of milk on physical growth, inflammation, growth-related hormones, and nutritional biomarkers in children with stunting.
This study used a formula to calculate the sample size for a paired sample t-test; assuming a moderate effect size (d=0.5), an alpha of 0.05, and a power of 0.75, 28 participants were needed. To account for potential dropouts, we employed a quota sampling method targeting 30 participants. The inclusion criteria in the study were (1) children aged 12–36 months with (2) a World Health Organization z-score between -3 and -2 standard deviation (stunted). The exclusion criteria were (1) children with chronic diseases, (2) children with milk allergies, (3) children currently undergoing other nutritional interventions, and (4) parents or guardians who withdrew consent. Dropout criteria comprised (1) children who missed more than 3 consecutive intervention days and (2) children who developed acute illnesses requiring hospitalization during the study period.
2. Assessment of alleles in cow milk
This process was carried out by determining the location of DNA sampling and conducting a series of variation analysis (genotyping) activities, including DNA extraction, DNA fragment amplification by polymerase chain reaction (PCR), and sequencing. DNA samples for allele analysis were extracted from the blood of Holstein Friesian cows that had produced milk in the previous one to 3 months. Ten blood samples (3–5 mL) were collected from local farms and companies from the cow jugular vein using a venoject and heparin vacutainer tube and then stored at -20℃. Next, DNA extraction was performed using the DNeasy Blood and Tissue Kits (Qiagen GmbH). The work procedure followed the protocol recommended by the manufacturer. Finally, 50-μL DNA samples were obtained for genotyping based on PCR analysis. Next, the DNA fragment of the β-casein gene was amplified from total cow DNA using the PCR method. The PCR product samples for each primer were sequenced to ensure that the amplified DNA fragment was the β-casein gene (CSN2 casein beta bos Taurus, Gene ID: 281099). The resulting sequence was then compared with the sequence in the National Center for Biotechnology Information database and used for chromatogram analysis of DNA sequence variations. The sequencing results were analyzed using Unipro Ugene v33.0 software (Unipro) for sequence trimming and multiple alignment. The sequencing trimming results were compared using the Basic Local Alignment Searching Tool Nucleotide (https://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the similarity of the sample sequence and to obtain a reference sequence.
3. Preparation of ready-to-drink milk and intervention process
Ready-to-drink cow milk was produced through pasteurization, packaging, labelling, and distribution. Pasteurization aims to kill germs for safe consumption. To extend its shelf life, cow milk is processed into various products, such as ultra-high temperature and powdered milk. In this research, powdered milk was chosen considering its practicality in packaging and distribution to research participants. Next, milk was packaged in 2 forms. The first packaging was an Alufoil three-sided sealed pouch with dimensions of 8 × 12 cm and that could hold 25 g of product. The second type of packaging comprised a folding box wrapped around an aluminum foil packaging containing the product or a cardboard box containing cans and sachets. Labelling was carried out in accordance with the Republic of Indonesia Law No. 7 of 1996 and the Processed Food Labeling Regulations of 2020. Product was administered to research participants as a liquid 200-mL β-casein A2 milk supplement once a day for 3 months.
4. Outcome assessment
The assessed outcomes were characteristics, physical growth, inflammation, and growth-related hormones and nutritional biomarkers. Mothers were interviewed to determine demographic characteristics, including maternal age, child age and sex, family education level, and monthly income. Subsequently, the children’s body weight and height were measured before the intervention, in the middle of the intervention (week 6), and after the intervention (week 12). We placed the electronic scale on a flat surface and adjusted the weight reading to zero before each measurement. We used a measuring board or height meter to measure each child. Further, inflammation biomarkers (TNF-α and cortisol) and biomarkers related to growth and nutritional status (IGF-1, GH, and transferrin) were investigated before and after intervention. A 3-mL blood sample was drawn from the children's veins, and biomarker levels were measured as follows: General Cortisol, COR ELISA Kit; Human Tumor Necrosis Factor Αlpha, TNF-A ELISA Kit; Human Growth Hormone Variant, GH2 ELISA Kit; Human Transferrin, TRF ELISA Kit; and Human Insulin-like Growth Factor 1, IGF-1 ELISA Kit (Bioassay Technology Laboratory, Shanghai Crystal Day Biotech Co., Ltd.).
5. Statistical analysis
The statistical analysis was conducted using IBM SPSS Statistics 23.0 (IBM Co.), and the results were graphically represented using GraphPad Prism 9 (GraphPad Software Inc.). A descriptive analysis determined the frequency (and percentage) or mean (and standard deviation) of each variable. The Shapiro-Wilk test was used to investigate the distribution of the data. The homogeneity of the data between groups was assessed using Levene's test. The differences within groups were examined utilizing repeated measures analysis of variance + Bonferroni post hoc test for body weight and height or the paired samples Wilcoxon test for TNF-α, cortisol, IGF-1, GH, and transferrin levels. All statistical tests were conducted using a 2-sided approach, and P-values less than 0.05 were considered statistically significant.
6. Ethical statement
The study adhered to the Helsinki Declaration and was approved by the Health Research Ethics Committee of Dr. Moewardi Hospital, Surakarta, Central Java, Indonesia (reference number 524/III/HREC/2023). The study procedures and aims were conveyed to the participants, as was their right to voluntarily terminate their participation in the study at any time. Parents signed written consent forms for their children to participate in this study following discussion with one of the principal investigators (RGHN).
Results
This study included 30 study participants; none met the dropout criteria. The participant characteristics are listed in Table 1. Most of the study participants were boys (56.7%), with a mean age of 24.89±7.35 months. In addition, most mothers were aged 20–35 years (66.6%), with an average of 30.30±7.10 years. Most mothers had only a middle school education (50%), and their monthly income was less than Rupiah 500,000 (43.3%).
Table 1.
Participant characteristics
| Characteristic | Value |
|---|---|
| Child | |
| Sex | |
| Boy | 17 (56.7) |
| Girl | 13 (43.3) |
| Age (mo) | 24.89±7.35 |
| 12–24 | 15 (50.0) |
| 25–36 | 15 (50.0) |
| Mothers | |
| Age (yr) | 30.30±7.10 |
| <20 | 0 (0) |
| 20–35 | 20 (66.6) |
| >35 | 10 (33.3) |
| Education level | |
| Elementary school | 9 (30.0) |
| Middle school | 15 (50.0) |
| High school | 6 (20.0) |
| College | 0 (0) |
| Monthly income (Rupiah) | |
| <500,0000 | 13 (43.3) |
| 500,000–1,000,000 | 10 (33.3) |
| 1,000,000–2,000,000 | 6 (20.0) |
| >2,000,000 | 1 (3.3) |
Values are presented as number (%) or mean±standard deviation.
There were significant differences in physical characteristics, including body weight (P<0.001) and height (P<0.001), from pre- to postintervention. The body weight and height at week 6 (P<0.001) and at postintervention (week 12) (P<0.001) were significantly higher than those preintervention. This study also showed significant differences in inflammation parameters, including TNF-α (P<0.001) and cortisol (P<0.001) levels, which were significantly lower after intervention. Additionally, significant increases were observed in the levels of IGF-1 (P<0.001), GH (P<0.001), and transferrin (P<0.001). The physical growth, inflammation, and biomarker trends during the study period are visualized in Fig. 1. These findings represent associations observed within the study period and should be interpreted cautiously in the absence of a control group.
Fig. 1.

Physical growth, inflammation markers, and growth-related and nutritional biomarker profiles during the study period. Data are presented as mean (±standard deviation) and individual values. Differences in body weight and height were analyzed using repeated measures analysis of variance with Bonferroni post hoc test (data were normally distributed; Shapiro-Wilk test, P≥0.05). TNF-α, cortisol, IGF-1, transferrin, and GH levels were analyzed using the paired samples Wilcoxon test (data not normally distributed; Shapiro-Wilk test, P<0.05). TNF, tumor necrosis factor; IGF, insulin-like growth factor; GH, growth hormone. *P<0.05, statistically significant difference.
Discussion
The existing studies on milk products acknowledge the presence of several bioactive components, such as lactate, whey protein, and β-casein protein [19-24]. Specifically, cow milk contains distinct subvariants of the β-casein protein, A1 and A2, which differ by a single nucleotide that alters the codon at position 67 [25-27] (Fig. 2). The A1 type contains a histidine amino acid at this location, while the A2 type contains a proline. A histidine residue at position 67, such as in the β-casein A1 protein, results in enzymatic cleavage of the following 7 amino acids, forming the peptide BCM-7, which is a μ-opioid receptor agonist that exerts its effects on various systems in the body, including the cardiovascular, neurological, and endocrine systems [20,28-30]. If a proline residue occupies position 67, such as in the β-casein A2 protein, the potential for enzymatic cleavage resulting in BCM-7 is reduced [20,27,31,32]. Studies have linked the opioid peptides produced from β-casein A1 in cow milk to non-communicable diseases in humans [20,28-30], although such associations remain inconclusive and require further validation in well-controlled human trials.
Fig. 2.
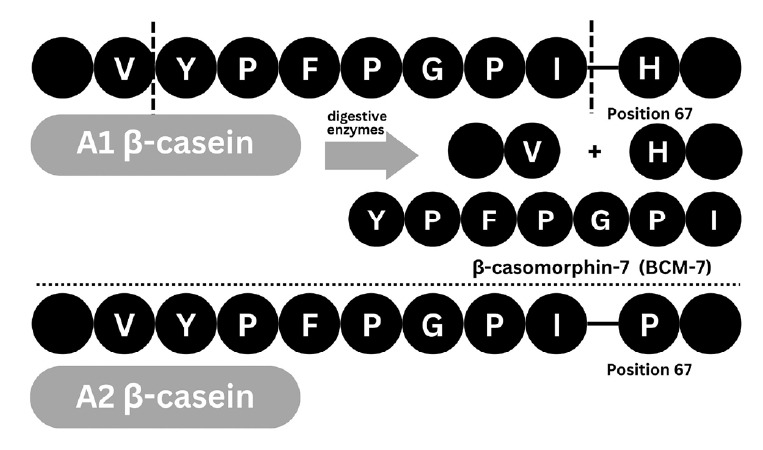
Differences in protein hydrolysis products between β-casein A1 and β-casein A2. A1 β-casein contains histidine (H) at position 67, which permits enzymatic cleavage and release of β-casomorphin-7 (BCM-7, YPFPGPI), a bioactive peptide. In contrast, A2 β-casein contains proline (P) at this position, which limits enzymatic cleavage and BCM-7 release.
The present study showed supplementation with β-casein A2 cow milk was associated with improvements in physical growth, inflammation, and growth-related biomarkers in stunted children aged 12–36 months. The findings indicate significant improvements in body weight and height, as well as favorable changes in inflammation markers, including reduced TNF-α and cortisol levels. Moreover, supplementation was associated with increased levels of the growth-related hormones IGF-1 and GH, along with elevated transferrin levels, a nutritional biomarker reflecting iron transport and systemic nutritional status. A previous study showed that milk intake stimulates the somatotropic axis at multiple levels by increasing GH and IGF-1 secretion [33]. Fig. 3 illustrates the mechanism by which β-casein A2 cow milk supplementation affects physical growth, inflammation, growth, and biomarker regulation.
Fig. 3.
Proposed biological pathway of β-casein A2 cow milk supplementation on growth, inflammation, and nutritional biomarkers. This schematic illustrates a hypothesized pathway whereby β-casein A2 milk intake may influence physical growth and health markers. β-casein A2 milk potentially stimulates increased secretion of GH and IGF-1, which contribute to enhanced bone mass, growth, and maturation. Concurrently, β-casein A2 milk may elevate transferrin levels, reflecting improved iron status and nutritional condition, while reducing inflammation through decreased TNF-α and cortisol levels. This model is based on associative evidence from the present study and existing literature and has not been experimentally confirmed. GH, growth hormone; IGF, insulin-like growth factor; TNF, tumor necrosis factor.
IGF-1 is a crucial hormone synthesized by the liver in response to GH stimulation. It is involved in the regulation of cell development and replication, bone growth, bone maturation, and increases in bone mass and muscle strength [34-37]. Subsequently, GH plays several crucial roles in stimulating the production and secretion of IGF-I in the liver and other tissues, which are essential for somatic and brain growth [38,39]. It also helps regulate protein, lipid, and carbohydrate metabolism, all of which are essential for maintaining normal bodily functions [38]. In addition, transferrin is a protein found in milk, particularly in the whey fraction, which plays several crucial roles in infants, including iron transport, iron regulation, immune function, and nutrition delivery [40,41]. While transferrin itself is not a GH, improved transferrin levels may reflect better iron status or recovery from malnutrition, indirectly supporting growth.
Last, almost all components of milk can function as immunoregulators [13,42]. These components include fat in the form of conjugated linoleic acid; protein in the form of colostrinin and peptides rich in proline; and milk peptides in the form of casein, β-lactoglobulin, α-lactalbumin, immunoglobulin, lactoferrin, transferrin, and serum albumin. Casein and its fractions can function as anti-inflammatory agents and immunoregulators by modulating the innate immune response [43,44]. No specific studies directly address the effect of β-casein A2 cow milk supplementation on cortisol levels. Furthermore, our results regarding TNF-α differ from those of previous studies, which showed no effect of β-casein A2 on TNF-α levels [45]. Subsequently, a previous study revealed that β-casein A2 milk can reduce inflammation by reducing the contents of interleukin-4; immunoglobulins G, E, and G1; and BCM-7, as well as increasing blood serum glutathione levels compared to milk containing β-casein A1 [46]. This decrease in inflammation is also possibly related to the reduction of cortisol and TNF-α levels in this study. There are other hypotheses, such as the possibility of a gut-brain axis mechanism, which may also have an influence.
Despite its findings, this study also has several limitations that need to be acknowledged. First, the quasi-experimental design with a single group and no control group limits the ability to attribute the observed changes directly to the β-casein A2 milk intervention. Second, the study was conducted in a specific region of Central Java, Indonesia, and may not represent other regions or countries with different environmental, dietary, and genetic backgrounds. The study duration of 3 months may not be long enough to observe the long-term effects of β-casein A2 milk supplementation. Future studies should consider a randomized controlled trial design, a more extended follow-up period, and include different environmental, dietary, and genetic backgrounds and multiple time points to better understand the intervention effects. Last, studies that compare β-casein A2 milk and other forms of milk are necessary to clarify their effects on physical growth, inflammation, and biological markers of growth and nutrition.
In conclusion, this study shows that β-casein A2 cow milk supplementation was associated with improvements in physical growth, inflammation, and growth-related and nutritional biomarkers in stunted children aged 12–36 months. The findings indicate significant improvements in body weight and height, as well as favorable changes in inflammation markers. Moreover, the supplementation was associated with increased levels of biological markers associated with growth and nutrition (IGF-1, GH, and transferrin). Additionally, β-casein A2 milk produces less BCM-7 than A1 milk, which has hypothetically been linked to various health risks based on previous studies. However, controlled trials are needed to determine its effectiveness as a dietary intervention.
Footnotes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study can be provided by the corresponding author upon reasonable request.
Acknowledgments
This study is a collaboration between the Research and Innovation Funding Directorate, National Research and Innovation Agency, Indonesia, and the Research and Community Service Institute, Universitas Sebelas Maret, with collaboration agreement number 123/IV/KS/2022.
Author contribution
Conceptualization: RN, AS, SN, RM, LM, NW, LS, SP; Data curation: RN, AS, SN, RM, LM, NW, LS, SP; Formal analysis: RN, AS, SN, RM, LM, NW, MI, SP; Funding acquisition: LS; Methodology: RN, AS, SN, RM, LM, NW, MI, SP; Project administration: RN, AS, SN, RM, LM, NW, SP; Visualization: MI; Writing - original draft: RN, MI; Writing - review & editing: RN, AS, SN, RM, LM, NW, LS, SP, CMJ
References
- 1. United Nations Children's Fund (UNICEF). UNICEF nutrition strategy 2020–2030: nutrition, for every child [Internet]. New York: UNICEF; 2024 [cited 2024 Jun 12]. Available from https://knowledge.unicef.org/childnutrition-and-development/resource/unicef-nutritionstrategy-2020-2030.
- 2. World Health Organization. Joint child malnutrition estimates [Internet]. Geneva (Switzerland): World Health Organization; 2014 [cited 2024 Jun 12]. Available from https://www.who.int/data/gho/data/themes/topics/jointchild-malnutrition-estimates-unicef-who-wb.
- 3.Laksono AD, Wulandari RD, Amaliah N, Wisnuwardani RW. Stunting among children under two years in Indonesia: Does maternal education matter? PLoS One. 2022;17:e0271509. doi: 10.1371/journal.pone.0271509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Data and Information Center of Ministry of Health of The Republic of Indonesia. Situation of Stunted Toddler in Indonesia [Internet]. Jakarta (Indonesia): Ministry of Health of The Republic of Indonesia; 2018 [cited 2024 Jun 12]. Available from https://independen.id/upload/DATA/Laporan%20Situasi%20Balita%20Pendek%202016.pdf.
- 5.Akseer N, Tasic H, Nnachebe Onah M, Wigle J, Rajakumar R, Sanchez-Hernandez D, et al. Economic costs of childhood stunting to the private sector in low- and middle-income countries. EClinicalMedicine. 2022;45:101320. doi: 10.1016/j.eclinm.2022.101320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mbabazi J, Pesu H, Mutumba R, Filteau S, Lewis JI, Wells JC, et al. Effect of milk protein and whey permeate in large quantity lipid-based nutrient supplement on linear growth and body composition among stunted children: a randomized 2 × 2 factorial trial in Uganda. PLoS Med. 2023;20:e1004227. doi: 10.1371/journal.pmed.1004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan Y, Pang X, Yang Z, Wang J, Jiang S, Bi Y, et al. Association between dairy intake and linear growth in Chinese pre-school children. Nutrients. 2020;12:2576. doi: 10.3390/nu12092576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parikh P, Semba R, Manar y M, Swaminathan S, Udomkesmalee E, Bos R, et al. Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low- and middle-income countries. Matern Child Nutr. 2022;18:e13264. doi: 10.1111/mcn.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grenov B, Larnkjær A, Mølgaard C, Michaelsen KF. Role of milk and dairy products in growth of the child. Nestle Nutr Inst Workshop Ser. 2020;93:77–90. doi: 10.1159/000503357. [DOI] [PubMed] [Google Scholar]
- 10.Yoshiike N, Hayashi F. New food guides in Japan and the US: Japanese Food Guide Spinning Top and MyPyramid. Jpn J Nutr Dietetics. 2006;64:1–11. [Google Scholar]
- 11.López-Sobaler AM, Aparicio A, López Díaz-Ufano ML, Ortega RM, Álvarez-Bueno C. Effect of dairy intake with or without energy restriction on body composition of adults: overview of systematic reviews and meta-analyses of randomized controlled trials. Nutr Rev. 2020;78:901–13. doi: 10.1093/nutrit/nuaa003. [DOI] [PubMed] [Google Scholar]
- 12.Stonehouse W, Wycherley T, Luscombe-Marsh N, Taylor P, Brinkworth G, Riley M. Dairy intake enhances body weight and composition changes during energy restriction in 18–50-year-old adults—a meta-analysis of randomized controlled trials. Nutrients. 2016;8:394. doi: 10.3390/nu8070394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perdijk O, van Splunter M, Savelkoul HFJ, Brugman S, van Neerven RJJ. Cow’s milk and immune function in the respiratory tract: potential mechanisms. Front Immunol. 2018;9:143. doi: 10.3389/fimmu.2018.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieters BC, Arntz OJ, Bennink MB, Broeren MG, van Caam AP, Koenders MI, et al. Commercial cow milk contains physically stable extracellular vesicles expressing immunoregulatory TGF-β. PLoS One. 2015;10:e0121123. doi: 10.1371/journal.pone.0121123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jianqin S, Leiming X, Lu X, Yelland GW, Ni J, Clarke AJ. Effects of milk containing only A2 beta casein versus milk containing both A1 and A2 beta casein proteins on gastrointestinal physiology, symptoms of discomfort, and cognitive behavior of people with self-reported intolerance to traditional cows’ milk. Nutr J. 2015;15:35. doi: 10.1186/s12937-016-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bolat E, Eker F, Yılmaz S, Karav S, Oz E, Brennan C, et al. BCM-7: opioid-like peptide with potential role in disease mechanisms. Molecules. 2024;29:2161. doi: 10.3390/molecules29092161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho S, Woodford K, Kukuljan S, Pal S. Comparative effects of A1 versus A2 beta-casein on gastrointestinal measures: a blinded randomised cross-over pilot study. Eur J Clin Nutr. 2014;68:994–1000. doi: 10.1038/ejcn.2014.127. [DOI] [PubMed] [Google Scholar]
- 18.de Vasconcelos ML, Oliveira LMFS, Hill JP, Vidal AMC. Difficulties in establishing the adverse effects of β-casomorphin-7 released from β-casein variants—a review. Foods. 2023;12:3151. doi: 10.3390/foods12173151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pelmuş RŞ, Lazăr C, Palade ML, Stancu M, Rotar CM, Gras MA. Study on milk composition and milk protein distribution in Romanian Holstein cattle. Archiva Zootechnica. 2020;23:13–21. [Google Scholar]
- 20.Kay SS, Delgado S, Mittal J, Eshraghi RS, Mittal R, Eshraghi AA. Beneficial effects of milk having A2 β-casein protein: myth or reality? J Nutr. 2021;151:1061–72. doi: 10.1093/jn/nxaa454. [DOI] [PubMed] [Google Scholar]
- 21.Ni H, Raikos V. Lactic-acid bacteria fermentation-induced effects on microstructure and interfacial properties of oil-in-water emulsions stabilized by goat-milk proteins. LWT. 2019;109:70–6. [Google Scholar]
- 22.de Vitte K, Kerziene S, Klementavičiūtė J, de Vitte M, Mišeikienė R, Kudlinskienė I, et al. Relationship of β-casein genotypes (A1A1, A1A2 and A2A2) to the physicochemical composition and sensory characteristics of cows’ milk. J Appl Anim Res. 2022;50:161–6. [Google Scholar]
- 23.Abbring S, Hols G, Garssen J, van Esch BCAM. Raw cow’s milk consumption and allergic diseases – The potential role of bioactive whey proteins. Eur J Pharmacol. 2019;843:55–65. doi: 10.1016/j.ejphar.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 24.Ng SW, Lu P, Rulikowska A, Boehm D, O’Neill G, Bourke P. The effect of atmospheric cold plasma treatment on the antigenic properties of bovine milk casein and whey proteins. Food Chem. 2021;342:128283. doi: 10.1016/j.foodchem.2020.128283. [DOI] [PubMed] [Google Scholar]
- 25.De Poi R, De Dominicis E, Gritti E, Fiorese F, Saner S, Polverino de Laureto P. Development of an LC-MS method for the identification of β-casein genetic variants in bovine milk. Food Anal Methods. 2020;13:2177–87. [Google Scholar]
- 26.Sebastiani C, Arcangeli C, Ciullo M, Torricelli M, Cinti G, Fisichella S, et al. Frequencies evaluation of β-casein gene polymorphisms in dairy cows reared in central Italy. Animals. 2020;10:252. doi: 10.3390/ani10020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asledottir T, Le TT, Petrat-Melin B, Devold TG, Larsen LB, Vegarud GE. Identification of bioactive peptides and quantification of β-casomorphin-7 from bovine β-casein A1, A2 and I after ex vivo gastrointestinal digestion. Int Dairy J. 2017;71:98–106. [Google Scholar]
- 28.Cieślińska A, Sienkiewicz-Szłapka E, Wasilewska J, Fiedorowicz E, Chwała B, Moszyńska-Dumara M, et al. Influence of candidate polymorphisms on the dipeptidyl peptidase IV and μ-opioid receptor genes expression in aspect of the β-casomorphin-7 modulation functions in autism. Peptides (NY) 2015;65:6–11. doi: 10.1016/j.peptides.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Kaskous S. A1- and A2-milk and their effect on human health. J Food Eng Technol. 2020;9:15–21. [Google Scholar]
- 30.Chitra P. Bovine milk: A1 and A2 beta casein milk proteins and their impact on human health: a review. Agric Rev. 2021;43:374–8. [Google Scholar]
- 31.Pal S, Woodford K, Kukuljan S, Ho S. Milk intolerance, beta-casein and lactose. Nutrients. 2015;7:7285–97. doi: 10.3390/nu7095339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chia J, McRae J, Enjapoori A, Lefèvre C, Kukuljan S, Dwyer K. Dietary cows’ milk protein A1 beta-casein increases the incidence of T1D in NOD mice. Nutrients. 2018;10:1291. doi: 10.3390/nu10091291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barrea L, Di Somma C, Macchia PE, Falco A, Savanelli MC, Orio F, et al. Influence of nutrition on somatotropic axis: Milk consumption in adult individuals with moderate-severe obesity. Clin Nutr. 2017;36:293–301. doi: 10.1016/j.clnu.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 34.Yakar S, Isaksson O. Regulation of skeletal growth and mineral acquisition by the GH/IGF-1 axis: Lessons from mouse models. Growth Horm IGF Res. 2016;28:26–42. doi: 10.1016/j.ghir.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bikle DD, Tahimic C, Chang W, Wang Y, Philippou A, Barton ER. Role of IGF-I signaling in muscle bone interactions. Bone. 2015;80:79–88. doi: 10.1016/j.bone.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Locatelli V, Bianchi VE. Effect of GH/IGF-1 on bone metabolism and osteoporosis. Int J Endocrinol. 2014;2014:235060. doi: 10.1155/2014/235060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wolfe A, Divall S, Wu S. The regulation of reproductive neuroendocrine function by insulin and insulin-like growth factor-1 (IGF-1) Front Neuroendocrinol. 2014;35:558–72. doi: 10.1016/j.yfrne.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Samerria S, Radovick S. The role of insulin-like growth factor-1 (IGF-1) in the control of neuroendocrine regulation of growth. Cells. 2021;10:2664. doi: 10.3390/cells10102664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wrigley S, Arafa D, Tropea D. Insulin-like growth factor 1: at the crossroads of brain development and aging. Front Cell Neurosci. 2017;11:14. doi: 10.3389/fncel.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross JH, Prentice AM, Cerami C. Hepcidin, serum iron, and transferrin saturation in full-term and premature infants during the first month of life: a state-of-the-art review of existing evidence in humans. Curr Dev Nutr. 2020;4:nzaa104. doi: 10.1093/cdn/nzaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerami C. Iron nutriture of the fetus, neonate, infant, and child. Ann Nutr Metab. 2017;71 Suppl 3(Suppl 3):8–14. doi: 10.1159/000481447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vlasova AN, Saif LJ. Bovine immunology: implications for dairy cattle. Front Immunol. 2021;12:643206. doi: 10.3389/fimmu.2021.643206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Wu X, Zhao J, Ma X, Murad MS, Mu G. Peptidome comparison on the immune regulation effects of different casein fractions in a cyclophosphamide mouse model. J Dairy Sci. 2024;107:40–61. doi: 10.3168/jds.2023-23761. [DOI] [PubMed] [Google Scholar]
- 44.Bamdad F, Shin SH, Suh JW, Nimalaratne C, Sunwoo H. Anti-inflammatory and antioxidant properties of casein hydrolysate produced using high hydrostatic pressure combined with proteolytic enzymes. Molecules. 2017;22:609. doi: 10.3390/molecules22040609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung TH, Hwang HJ, Yun SS, Lee WJ, Kim JW, Ahn JY, et al. Hypoallergenic and physicochemical properties of the A2 β-casein fractionof goat milk. Korean J Food Sci Anim Resour. 2017;37:940–7. doi: 10.5851/kosfa.2017.37.6.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheng X, Li Z, Ni J, Yelland G. Effects of conventional milk versus milk containing only A2 β-casein on digestion in chinese children. J Pediatr Gastroenterol Nutr. 2019;69:375–82. doi: 10.1097/MPG.0000000000002437. [DOI] [PMC free article] [PubMed] [Google Scholar]




