Abstract
Although most retroviruses require activated cells as their targets for infection, it is not known how this is achieved in vivo. A candidate protein for the activation of B cells by either mouse mammary tumor virus (MMTV) or murine leukemia virus is the toll-like receptor 4 (TLR4), a component of the innate immune system. MMTV caused B cell activation in C3H/HeN mice but not in C3H/HeJ or BALB/c (C.C3H Tlr4lps-d) congenic mice, both of which have a mutant TLR4 gene. This activation was independent of viral gene expression, because it occurred after treatment of MMTV with ultraviolet light or 2,2′-dithiodipyridine and in azidothymidine-treated mice. Nuclear extracts prepared from the lymphocytes of MMTV-injected C3H/HeN but not C3H/HeJ mice showed increased nuclear factor κB activity. Additionally, the MMTV- and Moloney murine leukemia virus envelope proteins coimmunoprecipitated with TLR4 when expressed in 293T cells. The MMTV receptor failed to coimmunoprecipitate with TLR4, suggesting that MMTV/TLR4 interaction is independent of virus attachment and fusion. These results identify retroviral proteins that interact with a mammalian toll receptor and show that direct activation by such viruses may initiate in vivo infection pathways.
Retroviral infection of cells is a multistep process beginning with the binding of the viral envelope (Env) protein to one or more cell surface receptors and terminating with the migration of reverse-transcribed viral DNA into the nucleus and integration into the chromosomes. For most retroviruses, this latter step is thought to depend on the nuclear membrane breakdown that occurs during cell division, because the reverse-transcribed replication complex cannot cross the nuclear membrane pores (1–3). Although it has been well documented in tissue culture cells that this process requires cell division, it currently not known how most retroviruses infect primary cells, given that these targets generally are quiescent.
Mouse mammary tumor virus (MMTV), a milk-acquired virus that first infects B cells in the Peyer's patches of the gut (4, 5), is thought also to require cell division to complete its replication. After the initial infection of B cells, a virus-encoded superantigen (Sag) is expressed and presented to cognate T cells bearing particular Vβ chains of the T cell receptor (6). The Sag-stimulated T cells produce cytokines that both amplify the pool of already-infected B cells and activate additional targets for viral infection including T cells (7–9). T cell activation is critical for in vivo infection, because mice lacking Sag-cognate T cells are immune to milk-borne infection, and mutant viruses lacking sag are not propagated (9, 10).
Several mechanisms have been proposed for the activation and infection of quiescent B cells by MMTV. One possibility is that B cells activated by newly acquired gut flora and other ingested antigens are infected by MMTV. This hypothesis is supported by the observation that only small numbers of virus-infected B cells are found in the Peyer's patch during milk-borne transmission (4). However, it has been reported recently that preactivation of B cells with bacterial lipopolysaccharide (LPS) and other mitogens resulted in decreased virus load compared with quiescent B cells, suggesting that naive or unactivated B cells are the targets for MMTV (11). A second possibility is that the virus itself activates B cells by binding to a cell surface receptor. Indeed, it has been shown that s.c. injection of MMTV results in rapid activation of B cells in the draining lymph node at a time when very few cells are virus-infected (12). Similar B cell activation was seen after injection of murine leukemia virus (MuLV; ref. 12). These results suggested that MMTV and MuLV both initiate a signal transduction pathway leading to B cell activation, perhaps by binding of the virus to a cell surface receptor.
One candidate cell surface molecule for the activation of B cells by MMTV is the toll-like receptor (TLR) 4. This molecule belongs to a family of receptors, the first of which was identified as a developmental gene in Drosophila and later shown to be required for antimicrobial immune responses in this organism (13, 14). Similarly, the mammalian family members are part of the innate immune system, initiating a signal transduction pathway after binding to ligand that ultimately results, at least in part, in nuclear factor (NF)-κB activation (15, 16). TLR4 functions as the receptor for LPS from Gram-negative bacteria, TLR2 is activated by peptidoglycan from Gram-positive bacteria, and TLR9 recognizes unmethylated CpG DNA; the ligands for the remaining mammalian TLRs have not been determined yet (17). Recent evidence also indicates that heterodimerization of different TLR molecules might extend the range of molecules that these receptors recognize (17).
We tested whether TLR4 interacted with MMTV because of an early observation that C3H/HeJ mice have a reduced incidence and increased latency of MMTV-induced mammary tumors compared with the C3H/HeN strain from which they were derived (18). C3H/HeJ mice, in contrast to C3H/HeN mice, have a defect in LPS responsiveness caused by a missense mutation in the cytoplasmic domain of one member of the TLR gene family, TLR4 (19, 20). The point mutation in C3H/HeJ mice results in the loss of this signaling and moreover causes the TLR4 molecule from C3H/HeJ mice to function as a codominant negative receptor (21).
Here we demonstrate that MMTV activates B cells in a TLR4-dependent mechanism at an early stage of infection and that this activation is independent of viral gene expression. Additionally, we show that the MMTV and Moloney (Mo)-MuLV Envs coimmunoprecipitate with TLR4. We propose that activation of lymphocytes through the innate immune system represents a common mechanism used by mammalian retroviruses.
Materials and Methods
Mice.
C3H/HeN and C3H/HeJ mice were obtained from the National Cancer Institute (Fredericksburg, MD). BALB/cJ and congenic C.C3H Tlr4 lps-dmice were obtained from The Jackson Laboratory. The mice were housed according to University of Pennsylvania policies.
Virus Injections.
Serial dilutions of MMTV (LA) (22) or MMTV (FM) (23) [isolated from mammary tumors or lactating mammary gland as described (24)] were made in PBS, and the highest dilution giving the maximum B cell response at 20 h in C3H/HeN mice was used for all subsequent injections (usually 1:5–1:10). An aliquot of each preparation also was analyzed by Western blot analysis with polyclonal anti-MMTV antisera to determine the relative amount of viral proteins. Fifty microliters of diluted MMTV (LA) or MMTV (FM) virus stock or LPS (10 μg, Sigma) was injected into the right hind footpad of mice; the left hind footpad served as the uninjected control. For antibody-blocking experiments, virus was mixed with a 1:15 dilution of goat anti-MMTV antiserum (Quality Biotech, Camden, NJ) or normal goat serum (Jackson ImmunoResearch) before injection. For virus-inactivation studies, MMTV (FM) was UV-inactivated for 30 min on ice with a hand-held short-wave UV lamp, diluted 1:5, and injected as above. For 2,2′-dithiodipyridine (AT-2) treatment, MMTV (FM) was diluted 1:3, treated with 5 mM AT-2 (Sigma) for 60 min at 37°C, and used immediately for injections; this concentration of AT-2 was determined by titration to inhibit 100% of Sag-mediated stimulation of B and T cells at 4 days postinjection (Fig. 2A and data not shown). MMTV (FM) injections also were performed in the presence of 3 mg of azidothymidine (AZT, Sigma), which was administered intravenously into the tail vein before injection as described for in vivo inhibition of MMTV infection (25).
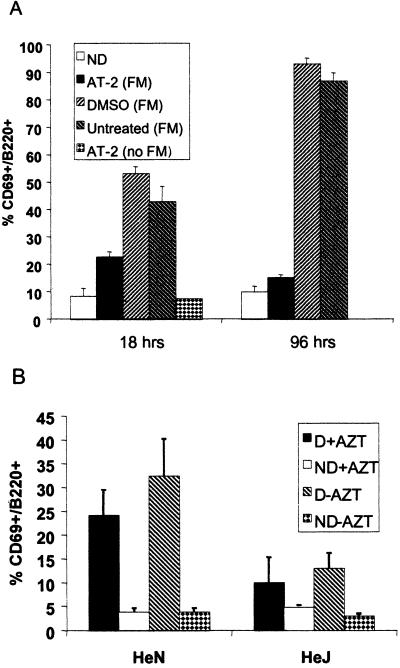
Figure 2.
MMTV-induced B cell activation is independent of viral gene expression. (A) MMTV (FM) was treated with 5 mM AT-2, DMSO vehicle, or untreated and injected into C3H/HeN mice (n = 3). Draining and nondraining lymph nodes were isolated at 18 h and 4 days postinjection, stained for CD69 and B220 expression, and analyzed by FACS. (B) Percentage of CD69/B220+ lymphocytes in AZT-treated mice injected with MMTV (LA). D, draining lymph node; ND, nondraining lymph node.
Fluorescence-Activated Cell Sorter (FACS) Analysis.
At either 18–20 h or 4 days after injection as indicated, the mice were killed, and lymphocytes were isolated from the popliteal lymph nodes and stained with FITC-conjugated anti-B220 and phycoerythrin-conjugated anti-CD69 antibodies (PharMingen). The cells were fixed in 2% paraformaldehyde and analyzed with a FACScan cytometer (Becton Dickinson). Data were analyzed by using CELLQUEST software (Becton Dickinson Immunocytometry Systems). The percentage of CD69/B220+ cells was calculated based on analysis of the total lymphocyte population in the draining and nondraining lymph nodes.
Electromobility Shift Analysis.
MMTV (FM) was injected into the right footpads of eight C3H/HeN and eight C3H/HeJ mice, and the draining and nondraining contralateral footpads were harvested and pooled at 20 h after injection. Nuclear extracts were prepared (26), and electromobility shift analyses were carried out with an NF-κB probe (5′-AGTTGAGGGGACTTTCCCAGGC, Santa Cruz Biotechnology). Ten micrograms of nuclear extract were used in each reaction. For the antibody supershift reactions, 0.4 μg of anti-NF-κB p50 or p65 was added to the incubation as recommended by the manufacturer (Santa Cruz Biotechnology). For the cold-competition experiments, a 1,000-fold excess of cold oligonucleotide was added to the reaction.
Cotransfection/Coimmunoprecipitations.
All plasmids have been described previously (27–30) with the exception of the MMTV surface (SU) construct. The SU-encoding sequences from MMTV C3H Env were PCR-amplified by using the primers 5′-GTGACAGATCTCCACCATGCCGAATCACCAATCT and 5′-GCGAATTCCTATCGCTTGGCTCGAATTAAATC (terminating at the junction between SU and transmembrane). The resulting PCR product was cloned into pcDNA 3 (Invitrogen). 293T cells grown in DMEM supplemented with 10% FBS were transiently transfected with each plasmid by using the calcium phosphate method. At 24 h after transfection, the cells were washed with PBS and lysed in 1 ml of RIPA/Triton buffer (25 mM Tris, pH 7.8/150 mM NaCl/2 mM EDTA/2% Triton X-100/1 mM PMSF/5 μg/leupeptin/5 μg/ml aprotinin). The lysates were immunoprecipitated with 10 μl of anti-FLAG M5 monoclonal antibody (Sigma) or 5 μl of anti-MMTV sera (Quality Biotech) and protein A or G agarose beads (Life Technologies, Rockville, MD). Approximately 1/20th of the total lysate was saved for electrophoresis. Samples were analyzed by electrophoresis on 8% polyacrylamide gels followed by Western blotting using the antibodies indicated in the figure legends and detected by chemiluminescence using ECL (Amersham Pharmacia). The immunoprecipitated samples and the total lysates were exposed to film for equal times. Cells were treated with tunicamycin (10 μg/ml, Sigma) 6 h posttransfection and incubated for ≈18 h before harvest. For the endoglycosidase digestions, cell extracts were prepared, brought to pH 6.0 with phosphate buffer, incubated with or without endoglycosidase H (0.25 units/0.3 ml extract, Sigma) overnight at 37°C, and immunoprecipitated.
Results
MMTV B Lymphocyte Activation Is TLR4-Dependent.
The TLR4 mutation in C3H/HeJ mice prevents bacterial LPS from activating macrophages and B cells. To determine whether the TLR4 protein was involved also in MMTV-induced B cell activation, C3H/HeN and C3H/HeJ mice received s.c. footpad inoculations of MMTV (FM), virus preincubated with anti-MMTV antisera, or LPS. At 18–20 h postinfection, lymphocytes from the draining popliteal lymph nodes were analyzed by FACS for expression of the B220 B cell and CD69 lymphocyte activation markers (Fig. 1A). Lymphocytes isolated from the contralateral nondraining lymph nodes served as controls. As reported previously, there was a dramatic increase (≈4-fold) in the percentage of activated B cells in the draining lymph nodes from C3H/HeN mice that received MMTV injections (12). In contrast, B cell activation was at background levels in C3H/HeJ mice that were inoculated with MMTV. Similar results were obtained when injections of MMTV (LA) were performed (Fig. 7, which is published as supporting information on the PNAS web site, www.pnas.org). Because the FM and LA viruses encode Sags that interact with T cells with different T cell receptor Vβ chains, the MMTV-induced B cell activation was not Sag-specific. C3H/HeJ mice also showed very low levels of B cell activation in response to LPS treatment compared with C3H/HeN mice (Fig. 1A); the small response seen in C3H/HeJ mice was probably caused by contaminating lipoprotein in the crude LPS preparation that was used (31).
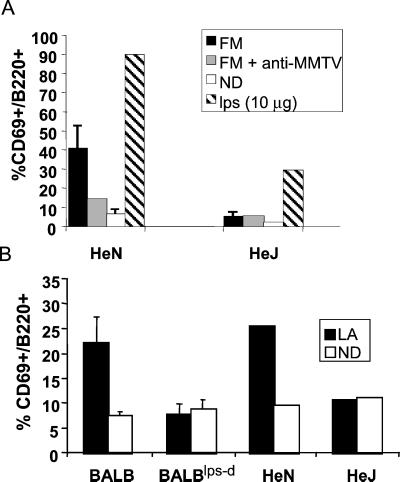
Figure 1.
MMTV-induced activation of B cells in draining lymph nodes of C3H/HeN and C3H/HeJ mice. (A) Percentage of CD69/B220+ lymphocytes isolated from the draining popliteal lymph nodes of C3H/HeN (HeN) or C3H/HeJ (HeJ) 18–20 h after footpad injection of MMTV (FM) (average of 5–15 mice). Virus was injected with or without anti-MMTV antiserum. In some cases, virus was preincubated with normal goat serum, which had no effect on the ability of virus to stimulate B cells. LPS (10 μg) was injected into a separate set of mice. (B) Percentage of CD69/B220+ lymphocytes isolated from the draining popliteal lymph nodes of BALB/cJ (BALB) or congenic C.C3H-Tlr4lps−d (BALBlps−d) 18–20 h after footpad injection of MMTV (LA). D, draining lymph node; ND, nondraining lymph node.
B cell activation was reduced to background levels when virus was pretreated with polyclonal anti-MMTV antisera but not normal goat sera (Fig. 1A), demonstrating that it depended on virus interaction with B cells. The antibody treatment also completely blocked infection with virus, because at 4 days no Sag-mediated stimulation of T cells was detected (data not shown). Although there was no MMTV-mediated B cell activation at 18–20 h postinjection in C3H/HeJ mice, Sag-mediated B and T cell activation was observed at 4 days (data not shown), indicating that they were infected.
To determine whether the defective MMTV-induced B cell activation phenotype cosegregated with the TLR4 gene, BALB/cJ mice congenic for the defective LPS response allele from C3H/HeJ mice (C.C3H-Tlr4lps−d; ref. 32) were also tested in this assay. In response to MMTV (LA) injection, wild-type BALB/cJ mice exhibited B cell activation levels comparable to those seen in C3H/HeN mice (>3-fold activation; Fig. 1B). In contrast, congenic BALB/cJ mice containing the TLR4 gene from C3H/HeJ mice (C.C3H-Tlr4Lps−d) exhibited only background levels of B cell activation, similar to C3H/HeJ mice. From these data, it appeared that activation of B cells at the early stages of MMTV infection occurred via a TLR4-dependent mechanism.
MMTV-Induced B Cell Activation Is Independent of Viral Gene Expression.
Our hypothesis was that the MMTV-induced B cell activation resulted from the direct interaction of virus particles with B lymphocytes. However, it was possible that B cell activation at 18 h postinfection was caused by expression of Sag or other viral gene product in infected cells. To determine whether the observed B cell activation depended on viral gene expression, three different treatments were used: AT-2, UV light, and the reverse transcriptase inhibitor AZT. Each treatment blocks infection immediately postentry. First, MMTV (FM) was incubated with 5 mM AT-2; AT-2 interacts with the zinc finger domains of the nucleocapsid and has been shown previously to inactivate HIV-1 in vitro (33). AT-2-treated MMTV induced a 3-fold increase in the level of activated B cells at 18 h postinjection in the footpad assay compared with the uninjected control (Fig. 2A). However, this level was reduced ≈2-fold from the activation levels seen with untreated- or DMSO-treated MMTV (FM). AT-2 itself had no effect on B cell activation (Fig. 2A, AT-2, no MMTV). AT-2 resulted in the complete inactivation of virus, because there was no Sag-dependent B cell (Fig. 2A) or cognate T cell (data not shown) activation at 4 days postinjection when B cell stimulation depends on Sag presentation and T cell help, resulting in very high percentages of CD69+ B cells.
Similar results were obtained with UV-inactivated MMTV (FM). Injection of the inactivated virus resulted in an ≈3-fold increase in the percentage of activated B cells compared with the uninjected control, although this was also reduced compared with the untreated inoculums (Fig. 8, which is published as supporting information on the PNAS web site). The UV treatment also was sufficient to block B and T cell activation at 4 days postinjection (Fig. 8).
To demonstrate further that the MMTV-induced B cell activation was independent of Sag function, C3H/HeN and C3H/HeJ mice were treated with the reverse transcriptase inhibitor AZT before injection with MMTV (LA). AZT treatment of C3H/HeN mice resulted in a 5-fold increase in the level of activated B cells over the uninjected controls (Fig. 2B). In contrast to the AT-2 and UV-inactivated virus preparations, the activation levels seen in the AZT-treated mice was not significantly different from that seen in untreated mice. Thus, although a small part of the early B cell activation may be the result of Sag expression in infected cells, these data show that the early MMTV-induced B cell activation at 18 h postinjection is largely independent of viral gene expression. Supporting this observation, Ardavin and colleagues showed that early B cell stimulation by virus occurs in mice that are unable to present or respond to the MMTV Sag (12).
NF-κB Activation in Lymphocytes in Response to MMTV Is TLR4-Dependent.
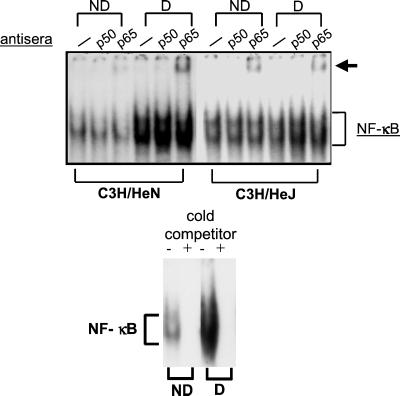
Because activation of the TLR4 pathway ultimately results in the release of NF-κB and its translocation into the nucleus, we determined whether lymphocytes isolated from mice injected with MMTV exhibited changes in NF-κB activity. Nuclear extracts were prepared from lymphocytes harvested from the draining and nondraining lymph nodes of C3H/HeN and C3H/HeJ mice 20 h after injection of MMTV (LA). The extracts were used in electromobility shift assays with a radiolabeled NF-κB probe. As shown in Fig. 3, extracts prepared from the draining lymph nodes of C3H/HeN but not C3H/HeJ mice showed increased binding to this probe. The NF-κB complexes could be competed away with a 1,000-fold excess of unlabeled probe indicating that this complex was the result of a specific interaction (Fig. 3). In addition, the complex that formed was supershifted with anti-p65 antibody but not anti-p50 subunit antibody (Fig. 3). The p65 subunit of the NF-κB complex is characteristically activated after TLR4 signaling (34).
Figure 3.
MMTV-induces NF-κB in the draining lymph nodes of C3H/HeN but not C3H/HeJ mice. C3H/HeN and C3H/HeJ mice received footpad injections of MMTV (FM), and an aliquot of cells from the draining (D) and nondraining (ND) contralateral footpads was taken for FACS analysis at 20 h postinjection. The remaining cells were used to prepare nuclear extracts. The C3H/HeN B cells showed activation (CD69+/B220+ cells, 45.9% draining, 15.0% control), whereas the C3H/HeJ B cells did not (17.9 vs. 16.4% draining vs. control). Electromobility shift analyses were carried out with an NF-κB probe in the absence or presence of anti-p50 or anti-p65 antibody. The arrow indicates the supershifted complex. (Lower) A 1,000-fold excess of cold NF-κB oligonucleotide was added to the C3H/HeN B cell extracts.
Retroviral Envelopes Bind to TLR4.
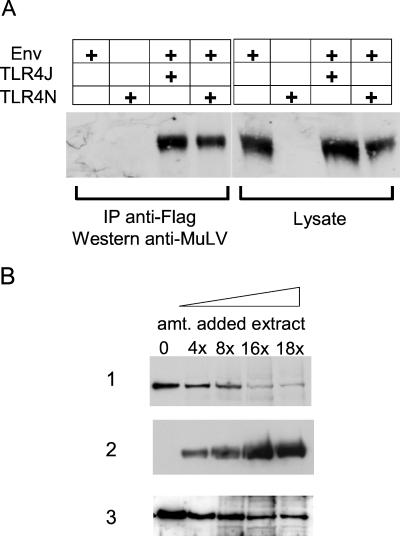
Retroviruses bind to cells via Env proteins found on their surface. Thus, the MMTV Env protein was a likely candidate for the interaction with TLR4. We performed cotransfection/coimmunoprecipitation assays to determine whether these two proteins bound each other. When plasmids encoding a FLAG epitope-tagged TLR4 and the MMTV Env polyprotein cell SU/transmembrane proteins were cotransfected into 293T cells, anti-FLAG antibody coimmunoprecipitated a significant percentage of the unprocessed Env precursor, gp70 expressed from transfected cells (Fig. 4A). Both the wild-type TLR4 protein derived from TLR4N mice and that from TLR4J mice (Fig. 4A) were able to coimmunoprecipitate with the Env protein. Immunoprecipitation with anti-MMTV antisera followed by Western blotting with anti-FLAG antibodies also demonstrated binding of the two proteins. Similar results were obtained when cell extracts were mixed from separately transfected 293T cell populations (see below), suggesting that the interaction between Env and TLR4 could occur in trans, as would be expected when virus binds to cells. Because the mutation in the TLR4J molecule is in the cytoplasmic signaling domain and not the extracellular, ligand-binding domain, we expected no difference in binding of the MMTV Env protein to the wild-type and mutant TLR4 proteins. We did observe slightly reduced binding of the MMTV Env to TLR4J; however, this reduction likely was the result of differences in the expression levels of the two TLR4 proteins.
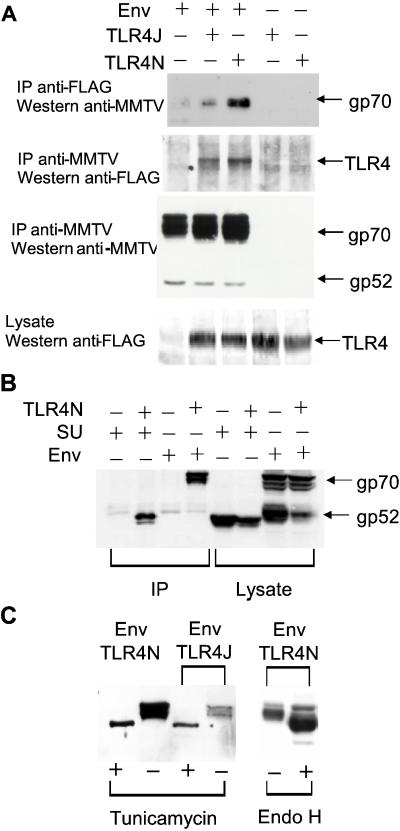
Figure 4.
The MMTV Env interacts with TLR4. (A) 293T cells were transfected with expression vectors encoding the MMTV Env SU/transmembrane polyprotein (lanes 1–3), FLAG-tagged C3H/HeJ TLR4 (TLR4J, lanes 2 and 4), or C3H/HeN TLR4 (TLR4N, lanes 3 and 5) proteins. Extracts were made from the transfected cells, immunoprecipitated, and subjected to Western blot analysis with either goat anti-MMTV or mouse anti-FLAG antisera as indicated. (B) The MMTV SU or Env plasmids were cotransfected with TLR4N, and coimmunoprecipitation with an anti-FLAG antibody followed by Western blot analysis with goat anti-MMTV antisera was performed. (C) After cotransfection of the MMTV Env and C3H/HeN and TLR4J plasmids, cells were treated with tunicamycin or extracts were prepared and subjected to endoglycosidase H (Endo H) treatment. After immunoprecipitation with anti-FLAG antibody, the blots were probed with anti-MMTV antisera.
293T cells transfected with the MMTV Env plasmid predominantly expressed the unprocessed precursor gp70 and very small amounts of the processed products gp52 and gp36 (Fig. 4 A, third panel, and B). Thus, only small amounts of gp52 and gp36 were found to be associated with the TLR4 receptors (data not shown). The SU proteins of retroviruses are exposed at the surface of the virus and encode the receptor binding domains, thus we also transfected a plasmid encoding the SU protein alone with the TLR4N and TLR4J constructs and subjected extracts from these cells to immunoprecipitation with anti-FLAG antisera. The SU protein bound both of the TLR4 molecules (Fig. 4B and data not shown for TLR4J), indicating that it is most likely this domain of Env that interacts with the receptor.
Ardavin et al. also showed that footpad injection of MuLV resulted in B cell activation (12). We tested whether ecotropic Mo-MuLV Env bound TLR4. Cotransfection of a plasmid encoding the Mo-MuLV Env (30) was carried out with both the TLR4N and TLR4J plasmids. By using the same cotransfection/coimmunoprecipitation assay described above, we found that the Mo-MuLV Env polyprotein bound both TLR4 receptors (Fig. 5A).
Figure 5.
The Mo-MuLV Env interacts with TLR4. (A) Plasmid pHIT123 encoding the Mo-MuLV Env protein was cotransfected with TLR4N and TLR4J, and extracts were prepared and immunoprecipitated with anti-FLAG antibody. Western blots were performed with goat anti-MuLV antisera. (B) One plate of 293T cells was cotransfected with pHIT 123 and the TLR4N expression plasmid; another plate was transfected with the MMTV Env expression plasmid. Extracts were prepared from both transfections, and increasing amounts of the MMTV Env-containing extract was added to the MuLV/TLR4N extract as indicated in rows 1 and 2. As controls, extract from untransfected 293T cells was added in the same increasing increments to the MuLV/TLR4N extract (row 3). The extracts were precipitated with mouse anti-FLAG antiserum, and then Western blots were performed with anti-MuLV antisera (rows 1 and 3). Blot A was stripped and reprobed with anti-MMTV antisera (row 2).
We also tested whether the MuLV and MMTV Env proteins competed for the same site on TLR4. 293T cells were cotransfected with the Mo-MuLV Env and TLR4N expression plasmids; another plate was transfected with the MMTV Env expression plasmid. Extracts were prepared from both transfections, and increasing amounts of the MMTV Env-containing extract were added to the MuLV/TLR4N extract. As a control, extract from untransfected 293T cells was added in the same increasing increments to the MuLV/TLR4N extract. The MMTV Env-containing extract was able to compete efficiently with the MuLV Env for binding to TLR4 (Fig. 5B). These results demonstrate that the MMTV Env is able to bind to TLR4 when added in trans and indicate that two different retroviral Envs bind to the same or similar sites on this receptor.
Env Binding Is Independent of N-Linked Glycosylation.
The known ligands for the TLR4 protein are glycolipids consisting of acyl chains on a polysaccharide backbone (27), although in Drosophila there is genetic evidence that the ligand for the toll receptor is a protein ligand, the product of the snake locus (35). All retroviral Env proteins are N-glycosylated. Although there are no structural homologies between the carbohydrate residues found on eukaryotic proteins and the glycan structures on bacteria, we tested whether N-linked glycosylation played a role in the interaction of Env with TLR4. Neither endoglycosidase H treatment of extracts nor treatment of the transfected cells with tunicamycin had an effect on the MMTV (Fig. 4C) or Mo-MuLV (data not shown) Env/TLR4 binding, demonstrating that neither protein requires N-linked glycosylation for this interaction. The decrease in the amount of tunicamycin-treated Env bound to TLR4 was caused by a reduction in the total amount of Env expressed in these conditions (data not shown). Thus, it is most likely that the SU and TLR4 proteins interact at the amino acid level.
The MMTV Receptor Does Not Bind TLR4.
Recently, we identified a cellular entry receptor for MMTV termed MTVR (29). To determine whether this receptor played a role in the Env/TLR4 interaction, perhaps through binding to TLR4, transfection/coimmunoprecipitation assays were performed with plasmids encoding TLR4N and the MTVR (29). The MTVR protein was expressed as an immunoadhesin fusion protein with a C-terminal rabbit Ig Fc domain that can be immunoprecipitated with protein A agarose beads (29). As seen in Fig. 6, the TLR4N protein did not coimmunoprecipitate with MTVR (lane 4) (because the MTVR is expressed as an immunoadhesin, it is brought down by the protein A beads). In contrast, the MTVR immunoadhesin did coimmunoprecipitate with Env (lane 6, immunoprecipitated protein A, Western anti-MMTV). These data indicate that the interaction between Env and TLR4 is specific and suggests that the interaction with TLR4 is independent of virus attachment and fusion with the host cell. Moreover, transfection of the TLR4N plasmid into MMTV-resistant cells did not make them permissive for infection by MMTV Env-pseudotyped viruses (data not shown).
Figure 6.
The MTVR does not interact with TLR4. 293T cells were transfected with expression plasmids MMTV Env, TLR4N, and an MTVR/Ig fusion construct. The MTVR/Ig protein binds directly to protein A. Cell lysates were immunoprecipitated (IP) with or without anti-MMTV in the presence of protein A. Immunoprecipitations were analyzed by Western blot analysis with anti-FLAG, anti-MMTV, or anti-rabbit Ig.
Discussion
It is well established that MMTV subverts the immune system in its infection pathway because of Sag-mediated stimulation of T cells. Thus, the use of a receptor involved in innate immunity represents a second way that this virus has evolved to take advantage of the immune system. The interaction of the MMTV Env protein with TLR4 accomplishes several steps in the virus-infection pathway. First, it allows MMTV to activate cells directly, thereby allowing the completion of the initial round of infection in B cells. These infected B cells then express and present Sag, resulting in amplification of the population of infected cells.
A second consequence of the activation of the TLR4 pathway is cytokine production. At least one of the cytokines produced, IL-1, the receptor of which also transduces a signal through the NF-κB pathway, induces MMTV provirus transcription (data not shown). Indeed, treatment of B cells with bacterial LPS itself increases the transcription of endogenous MMTV loci in B cells (36, 37), and C3H/HeJ B cells treated with LPS in vitro show no induction of endogenous MMTV transcription (Fig. 9, which is published as supporting information on the PNAS web site). Thus, C3H/HeJ mice may have several alterations in their MMTV-infection pathway, all related to the TLR4 mutation.
Activation of the TLR4 pathway by binding of Env also may alter virus production; binding of Env to TLR4 on already infected cells could cause increased viral transcription and subsequent particle formation. Cellular activation through the TLR4 pathway may function in other cell types infected by MMTV. For example, we have found that TLR4 is transcribed in mammary epithelial cells and lactating mammary glands as well as lymphoid tissue (Fig. 10, which is published as supporting information on the PNAS web site).
Previously it was shown that preactivation of B cells with LPS resulted in a decreased virus load compared with quiescent cells (11), which could be because MMTV only infects naive B cells efficiently. However, LPS treatment results in the loss of functional cell surface TLR4, resulting in cells that are resistant to further activation by LPS, a phenomenon termed tolerance (38). Thus, the decreased infection of LPS-pretreated B cells could result from the loss of cell surface TLR4 that can bind MMTV, thereby preventing activation by the virus.
In addition to showing decreased mammary tumor incidence, C3H/HeJ (MMTV+) mice have been recently shown to carry a recombinant exogenous virus containing the endogenous Mtv-1 gag, pol, and env genes and the MMTV (C3H) sag gene (39). This recombinant virus termed MMTV (HeJ) was not tumorigenic in C3H/HeN mice. The relationship between the Tlr4 mutation and the selection for a less tumorigenic virus requires further investigation but could be the result of altered activation of innate immunity in this strain.
A number of MuLVs also infect B cells, but it has not been demonstrated that this is required for in vivo infection (40). Taken together with the previous observation that MuLV binding activates B cells (12), these results indicate a role for TLR4 in the infection pathways of at least two murine retroviruses. Recently it has been shown also that respiratory syncytial virus proteins are capable of activating cells via the TLR4 protein, inducing an innate immune response (41). Our data indicate that in contrast to respiratory syncytial virus, retroviruses may use this host antipathogen response to activate cells and thereby establish in vivo infection of cells that are normally quiescent.
Supplementary Material
Acknowledgments
We thank Drs. Bruce Beutler for the TLR4N and TLR4J expression plasmids, Akio Matsuzawa for the FM virus, and Glen Gaulton for plasmid pHIT123 and helpful discussions. This work was supported by National Cancer Institute Grants R01-CA45954 and R01-CA73746. J.C.R. was supported by the University of Pennsylvania Virology Training Grant T32-AI 7324, and R.K. was supported by Department of Defense Breast Cancer Grant BC971653.
Abbreviations
- Env
envelope
- MMTV
mouse mammary tumor virus
- Sag
superantigen
- LPS
lipopolysaccharide
- MuLV
murine leukemia virus
- TLR
toll-like receptor
- NF-κB
nuclear factor κB, Mo, Moloney
- AT-2
2,2′-dithiodipyridine
- AZT
azidothymidine
- FACS
fluorescence-activated cell sorter
- SU
surface
- TLR4N
C3H/HeN TLR4
- TLR4J
C3H/HeJ TLR4
- MTVR
MMTV receptor
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Fritsch E F, Temin H M. J Virol. 1977;24:461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Varmus H E, Padgett T, Heasley S, Simon G, Bishop J M. Cell. 1977;11:307–319. doi: 10.1016/0092-8674(77)90047-2. [DOI] [PubMed] [Google Scholar]
- 3.Harel J, Rassart E, Jolicoeur P. Virology. 1981;110:202–207. doi: 10.1016/0042-6822(81)90022-2. [DOI] [PubMed] [Google Scholar]
- 4.Karapetian O, Shakhov A N, Kraehenbuhl J P, Acha-Orbea H. J Exp Med. 1994;180:1511–1516. doi: 10.1084/jem.180.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beutner U, Draus E, Kitamura D, Rajewsky K, Huber B T. J Exp Med. 1994;179:1457–1466. doi: 10.1084/jem.179.5.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ross S R. Microbes Infect. 2000;2:1215–1223. doi: 10.1016/s1286-4579(00)01275-2. [DOI] [PubMed] [Google Scholar]
- 7.Dzuris J L, Golovkina T V, Ross S R. JVirol. 1997;71:6044–6048. doi: 10.1128/jvi.71.8.6044-6048.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Upragarin N, Nishimura H, Wajjwalku W, Ando Y, Nagafuchi S, Watanabe T, Yoshikai Y. J Immunol. 1997;159:2189–2195. [PubMed] [Google Scholar]
- 9.Golovkina T V, Dudley J P, Ross S R. J Immunol. 1998;161:2375–2382. [PubMed] [Google Scholar]
- 10.Golovkina T V, Chervonsky A, Dudley J P, Ross S R. Cell. 1992;69:637–645. doi: 10.1016/0092-8674(92)90227-4. [DOI] [PubMed] [Google Scholar]
- 11.Finke D, Mortezavi L, Acha-Orbea H. J Virol. 1998;72:7688–7691. doi: 10.1128/jvi.72.9.7688-7691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ardavin C, Luthi F, Andersson M, Scarpellino L, Martin P, Diggelmann H, Acha-Orbea H. J Virol. 1997;71:7295–7299. doi: 10.1128/jvi.71.10.7295-7299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belvin M P, Anderson K V. Annu Rev Cell Dev Biol. 1996;12:393–416. doi: 10.1146/annurev.cellbio.12.1.393. [DOI] [PubMed] [Google Scholar]
- 14.Kopp E B, Medzhitov R. Curr Opin Immunol. 1999;11:13–18. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 15.Qureshi S T, Gros P, Malo D. Trends Genet. 1999;15:291–294. doi: 10.1016/s0168-9525(99)01782-5. [DOI] [PubMed] [Google Scholar]
- 16.Beutler B. Curr Opin Immunol. 2000;12:20–26. doi: 10.1016/s0952-7915(99)00046-1. [DOI] [PubMed] [Google Scholar]
- 17.Imler J-L, Hoffmann J A. Trends Cell Biol. 2001;11:304–311. doi: 10.1016/s0962-8924(01)02004-9. [DOI] [PubMed] [Google Scholar]
- 18.Outzen H C, Morrow D, Shultz L D. J Natl Cancer Inst. 1985;75:917–923. doi: 10.1093/jnci/75.5.917. [DOI] [PubMed] [Google Scholar]
- 19.Poltorak A, He X, Smirnova I, Liu M-Y, van Huffel C, Ku X, Birdwell D, Alejos E, Silva M, Galanos C. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi S T, Lariviere L, Leveque G, Clermont S, Moore K J, Gros P, Malo D. J Exp Med. 1999;189:615–625. doi: 10.1084/jem.189.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel S N, Johnson D, Perera P, Medvedev A, Lariviere L, Qureshi S T, Malo D. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- 22.Golovkina T V, Piazzon I, Nepomnaschy I, Buggiano V, de Olano Vela M, Ross S R. J Virol. 1997;71:3895–3903. doi: 10.1128/jvi.71.5.3895-3903.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto T, Nagase H, Nakano H, Matsuzawa A, Nariuchi H. Eur J Immunol. 1994;24:1612–1619. doi: 10.1002/eji.1830240724. [DOI] [PubMed] [Google Scholar]
- 24.Le Bon A, Wache A-C, Papiernik M. Int Immunol. 1999;11:373–382. doi: 10.1093/intimm/11.3.373. [DOI] [PubMed] [Google Scholar]
- 25.Held W, Waanders G A, Acha-Orbea H, MacDonald H R. J Exp Med. 1994;180:2347–2351. doi: 10.1084/jem.180.6.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dignam J D, Lebovitz R M, Roeder R. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poltorak A, Ricciardi-Castagnoli P, Beutler B. Proc Natl Acad Sci USA. 2000;97:2163–2167. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dzuris J L, Zhu W, Golovkina T V, Ross S R. Virology. 1999;263:418–426. doi: 10.1006/viro.1999.9967. [DOI] [PubMed] [Google Scholar]
- 29.Golovkina T V, Dzuris J L, van den Hoogen B, Jaffe A B, Wright P C, Cofer S M, Ross S R. J Virol. 1998;72:3066–3071. doi: 10.1128/jvi.72.4.3066-3071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hirschfeld M, Ma Y, Weis J H, Vogel S N, Weis J J. J Immunol. 2000;165:618–622. doi: 10.4049/jimmunol.165.2.618. [DOI] [PubMed] [Google Scholar]
- 32.Vogel S N, Wax J S, Perera P Y, Padlan C, Potter M, Mock B A. Infect Immun. 1994;62:4454–4459. doi: 10.1128/iai.62.10.4454-4459.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossio J L, Esser M T, Suryanarayana K, Schneider D K, Bess J W, Jr, Vasquez G M, Wiltrout T A, Chertova E, Grimes M K, Sattentau Q. J Virol. 1998;72:7992–8001. doi: 10.1128/jvi.72.10.7992-8001.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweet M J, Hume D A. J Leukocyte Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 35.Mizuguchi K, Parker J S, Blundell T L, Gay N J. Trends Biochem Sci. 1998;23:239–242. doi: 10.1016/s0968-0004(98)01216-x. [DOI] [PubMed] [Google Scholar]
- 36.Carr J K, Traina-George V L, Cohen J C. Virology. 1985;147:210–213. doi: 10.1016/0042-6822(85)90241-7. [DOI] [PubMed] [Google Scholar]
- 37.King L B, Corley R B. Mol Cell Biol. 1990;10:4211–4220. doi: 10.1128/mcb.10.8.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Medvedev A E, Kopydlowski K M, Vogel S N. J Immunol. 2000;164:5564–5574. doi: 10.4049/jimmunol.164.11.5564. [DOI] [PubMed] [Google Scholar]
- 39.Hook L M, Agafonova Y, Ross S R, Turner S J, Golovkina T V. J Virol. 2000;74:8876–8883. doi: 10.1128/jvi.74.19.8876-8883.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss R, Teich N, Varmus H, Coffin J, editors. RNA Tumor Viruses. Plainview, NY: Cold Spring Harbor Lab. Press; 1984. [Google Scholar]
- 41.Kurt-Jones E A, Popova L, Kwinn L, Haynes L M, Jones L P, Tripp R A, Walsh E E, Freeman M W, Golenbock D T, Anderson L J, et al. Nat Immunol. 2000;1:398–401. doi: 10.1038/80833. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.