Abstract
Bacterial adhesion is an important initial step in biofilm formation, which may cause problems in medical, environmental, and industrial settings. In spite of obvious phenotypic differences between attached and planktonic cells, knowledge about the genetic basis for these differences and how adhesion-induced changes are mediated is limited. The Cpx two-component signal transduction pathway responds specifically to stress caused by disturbances in the cell envelope and activates genes encoding periplasmic protein folding and degrading factors. Here, we address the role of the Cpx-signaling pathway in sensing and responding to the physical change occurring during adhesion of Escherichia coli to surfaces. We present evidence that the expression of Cpx-regulated genes is induced during initial adhesion of E. coli to abiotic surfaces. This induction is specifically observed upon attachment of stationary-phase cells to hydrophobic surfaces. Moreover, surface-induced activity of the Cpx response requires NlpE, an outer membrane lipoprotein, which has previously been shown to induce the Cpx system when overproduced. The importance of a functional Cpx response during adhesion is further supported by the fact that a dramatically lower number of cells attach to the surface and dynamic cell–surface interactions as measured by a quartz crystal microbalance technique are altered when the CpxRA pathway is disrupted. The defects in adhesion exhibited by the cpxR and nlpE mutants were strikingly similar to those of wild-type cells in which protein synthesis was inhibited, suggesting that the Cpx pathway plays a key role in the regulation of adhesion-induced gene expression.
Keywords: biofilm formation|cell envelope stress|NlpE|signal transduction| quartz crystal microbalance
Bacteria often survive within biofilms, which are implicated in environmental problems and numerous infections (1). One important characteristic of biofilms is their high resistance to antibiotics, which makes the treatment of biofilm-related infections difficult. Several surface-related environmental signals, as well as surface-contact mediated by specific cell surface structures, are recognized to trigger changes that allow bacteria to undergo stable cell–surface interactions (2–5). However, it remains a challenge to identify regulatory processes critical for adhesion, the understanding of which will be useful to devise better treatments to combat biofilm formation.
As the interface with the external environment, the bacterial cell envelope is directly exposed to changes in environmental conditions and is particularly sensitive to any perturbations. In Escherichia coli, stressful conditions affecting the cell envelope are perceived by two regulatory pathways, the σE regulatory system and the CpxRA two-component signal transduction pathway (6). Although both pathways similarly respond to general stress conditions in the extracytoplasmic compartment of the cell and partially overlap in their regulons, they can be distinguished by their response to specific signals. For example, σE responds to misfolded outer membrane β-barrel proteins (7), whereas the Cpx system monitors the biogenesis of surface organelles such as pili (8). Activation of the Cpx response proceeds by means of phosphorylation of the cytoplasmic response regulator CpxR by the inner membrane histidine kinase CpxA. This is controlled by autoregulation and feedback inhibition via the negative regulator CpxP, a periplasmic protein (6). DNA binding by CpxR-P upstream of target genes leads to the increased expression of several protein folding and degrading factors in the periplasm, including DegP, DsbA, PpiA, Spy, and CpxP (6).
Because the function of pili is to mediate adhesion through binding to host tissues, another possible role for the Cpx system could be to control cell–surface interactions during the adhesion process. In particular, the Cpx stress response may be induced by initial cell–surface contact. It has previously been shown that cpxA null mutants of E. coli and Salmonella typhi are defective in adherence and invasion (9, 10).
In this study, we show by using gene fusions that the Cpx system is activated upon attachment of E. coli to hydrophobic surfaces. In addition, by using a quartz crystal microbalance (QCM), we also provide evidence that an intact CpxRA-signaling system and the outer membrane lipoprotein NlpE are required for productive cell–surface interactions leading to stable adhesion.
Materials and Methods
Bacterial Strains and Culture Conditions.
All strains were isogenic and were derived by P1 transduction of the respective mutation into strain TR235, which is Escherichia coli K-12 strain MC4100 (F− araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR) containing the cpxR′–lacZ+ fusion inserted at the λatt site in the chromosome. Strains were grown with aeration overnight in LB broth at 37°C. When required, the medium was supplemented with antibiotics at following final concentrations: amikacin, 3 μg/ml−1; ampicillin, 50 μg/ml−1; chloramphenicol, 20 μg/ml−1; kanamycin, 50 μg/ml−1; spectinomycin, 50 μg/ml−1; and tetracycline, 25 μg/ml−1.
For adhesion experiments involving the QCM, bacterial cultures were centrifuged (12,100 × g for 10 min) and cells were resuspended in filter-sterilized conditioned LB medium, i.e., supernatants taken from overnight cultures, at a final concentration of ≈1 × 109 cells per ml.
Adhesion Assay to Study Gene Expression.
Hydrophobic acid-cleaned glass beads (diameter 0.5 mm) contained in glass vials were used as surfaces and were freshly prepared before each experiment as described (11). Glass vials were filled with 3 g of glass beads for each sample. To each vial, 1 ml of overnight culture was added to cover the glass beads. As a control, 1 ml of overnight culture was added to empty vials. These latter samples, either taken at the onset of the experiment or collected as parallels at different time points during the experiments, served as planktonic cells. All samples were incubated at 37°C to allow for surface contact. After the indicated time, cell suspensions were aspirated out of the glass bead-filled samples to remove unattached cells and replaced by 1 ml of Z-buffer containing 0.27% β-mercaptoethanol (12) and 0.04% SDS, which has a surface-active effect (11) but no effect on Cpx activity. Samples were vortexed for 30 sec to remove attached cells from the surfaces. These suspensions were transferred to new tubes, washed once by centrifugation, and resuspended in Z-buffer containing 0.27% β-mercaptoethanol. The cell concentration in the samples was estimated by OD600 measurements before chloroform and SDS (1%) were added to lyse the samples. The β-galactosidase activities were determined as the rate of o-nitrophenol-β-d-galactosidase (ONPG) hydrolysis in the cell lysates by using a microtiter plate assay (12) and were expressed as units per cell.
To confirm that an increased level of Cpx activity per cell is caused by the synthesis of new proteins, tetracycline (100 μg/ml−1) was added to samples. These were subsequently prepared as described above. Exposure of the cells to tetracycline at the concentration applied did not affect their viability as assessed from the number of colony-forming units (data not shown).
To determine whether a change in Cpx activity may be caused by selection of a subpopulation on the surfaces, unattached cells were taken from the supernatant of glass bead-filled samples and repeatedly allowed to attach to fresh surfaces. Samples were taken as described above.
To exclude that a diffusible signal may be released from the surface coating, hydrophobic glass beads were incubated in conditioned medium for up to 4 h. After the respective periods of time, the sterile supernatants of these samples (1 ml) were mixed with 1 ml of an overnight culture of strain TR235 and incubated for 1 h at 37°C. Subsequently, samples were taken as described above.
QCM Experiments.
Hydrophobic, methyl-terminated surfaces were obtained as described (13) by immersing clean quartz crystals (5 MHz, Maxtec, Torrance, CA) in a saturated solution of octadecylmercaptan (Aldrich Chem, Metuchen, NJ) in hexane overnight at 24°C. Because of the temperature sensitivity of the apparatus, the temperature of the detection cell was maintained at 24°C. The quartz crystal was mounted in a detection cell with electrodes connected to the driving unit by means of a relay to a signal generator. An alternating potential field across the crystal induces oscillation in the shear mode at the resonant frequency. Bacterial suspensions were added to a final concentration of ≈109 cells ml−1. Frequency shifts (Δf) and dissipation shifts (ΔD) caused by adhesion of bacteria to the crystal surface were measured continuously for 60 min in filter-sterilized, conditioned LB medium. The pH of all overnight cultures was ≈7.6.
Numbers of attached cells on hydrophobic surfaces were determined after 60 min of adhesion on the quartz crystal surfaces by acridine orange direct counts (14). Crystals were removed from the QCM chamber and stained for 5 min in a filter-sterilized acridine orange solution [AO 100 μg/ml, 2% (vol/vol) formaldehyde in PBS] and surfaces were examined by epifluorescence microscopy at a magnification of ×500. For every sample, all cells in a minimum of 20 fields of view (80 × 80 μm in size) were counted.
Results
Adhesion of E. coli to Hydrophobic Surfaces Activates the Cpx Two-Component Signal Transduction System.
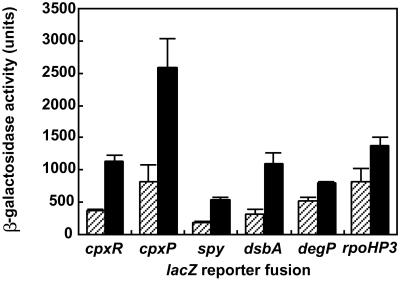
To study whether the Cpx pathway is induced during adhesion, E. coli mutant strains harboring transcriptional fusions of Cpx-regulated promoters to lacZ were allowed to attach to hydrophobic surfaces for 1 h at 37°C. Cells were isolated from the surfaces and the transcriptional activity of the fusions was measured and compared to that of planktonic cells. Upon contact with hydrophobic surfaces, transcriptional activity of all of the Cpx-regulated promoters was increased ≈3-fold as compared to planktonic cells (Fig. 1), except for degP, which was increased only ≈1.5-fold. This gene, encoding a periplasmic protease, differs from the other Cpx-regulated promoters in that its transcription is not only regulated by Cpx but also by the alternative σ factor σE. Likewise, transcription of the rpoHP3 promoter, which depends on σE (15), was only slightly increased during adhesion (Fig. 1). This finding suggests that σE itself is not significantly induced. The surface-induced response was abolished when tetracycline (100 μg/ml−1) was added to the samples, which confirms that the measured induction requires protein synthesis.
Figure 1.
The CpxRA-signaling system is activated during adhesion of E. coli to hydrophobic surfaces. Transcriptional activity of Cpx- and σE-regulated promoters after 1 h of adhesion of E. coli to hydrophobic surfaces (black bars) and in planktonic cells (striped bars). Data shown are means (± SD) from 3–5 experiments.
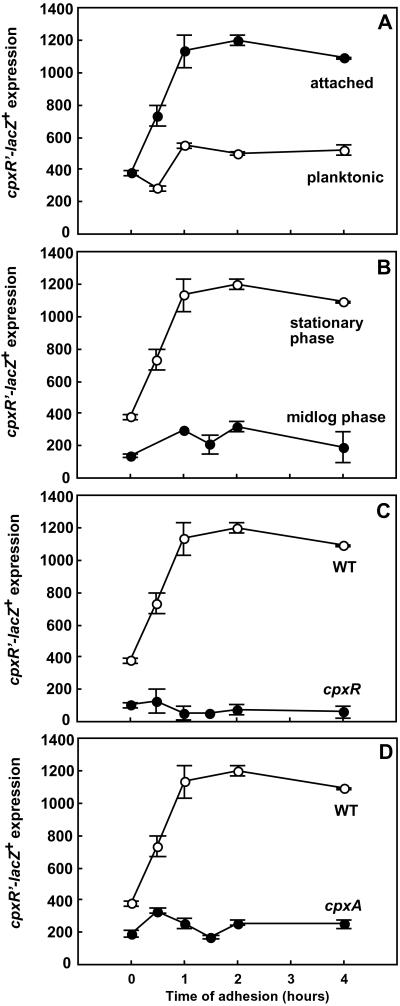
Time-course experiments monitoring the transcriptional activity of cpxR′-lacZ+ over 4 h of adhesion revealed that the Cpx response was activated immediately upon attachment (Fig. 2A). Activity of the cpxR′–lacZ+ fusion was significantly increased after the first 30 min of adhesion and reached a maximum level of 3-fold induction after 1 h of adhesion. In contrast, the cpxR′–lacZ+ expression levels in planktonic cells did not change during the time of the experiment (Fig. 2A). The increased transcription of cpxR′–lacZ+ during adhesion was restricted to cells grown to stationary phase in LB medium, whereas adhesion of cells taken in the midlogarithmic phase of growth did not lead to a higher expression of cpxR′–lacZ+ than in the planktonic state (Fig. 2B), which suggests a growth phase-dependent regulation of the response.
Figure 2.
Induction of cpxR′–lacZ+ during adhesion is growth phase-regulated and requires a functional CpxRA pathway. (A) Time course of induction of cpxR′–lacZ+ during adhesion of strain TR235 in comparison to planktonic cells. (B) Increased transcriptional activity of cpxR′–lacZ+ during adhesion is stationary phase-dependent. Stationary-phase cells were harvested at OD600 ≈ 2.0, and cells in the midlogarithmic phase of growth were harvested at OD600 ≈ 0.5. (C) Time course of induction of cpxR′–lacZ+ during adhesion in the presence or absence of the response regulator CpxR. (D) Time course of induction of cpxR′–lacZ+ during adhesion in the presence or absence of the sensor kinase CpxA in comparison to the wild type. Parallel measurements from one set of experiments are shown (means ± SD), which were representative for three independent experiments.
No elevated induction of Cpx was seen during adhesion of E. coli to hydrophilic surfaces or when cells were grown overnight in M63 minimal medium supplemented either with glucose or glycerol. Cells that remained unattached in the glass bead-filled vials generally showed lower expression levels than attached cells. When the unattached cells were repeatedly isolated from the supernatant of the glass bead-filled samples and exposed to fresh surfaces, induction of Cpx activity occurred upon attachment as described above. Moreover, exposure to supernatants taken from glass beads incubated in conditioned medium did not lead to increased transcriptional activity of cpxR′–lacZ+, demonstrating that no diffusible signal is released from the coated glass beads and that direct surface contact is required for the up-regulation of Cpx activity.
Transcription Activation of cpxR During Adhesion Requires a Functional CpxRA Pathway.
To confirm that the increased transcriptional activity of Cpx-dependent genes during adhesion follows the known molecular mechanism of signal transduction by means of the CpxRA pathway, the induction of cpxR′–lacZ+ was studied in different mutant backgrounds. In the absence of CpxR, the basal level of transcriptional activity of cpxR′–lacZ+ was low and no increase was evident during the course of the adhesion experiment (Fig. 2C), which shows that the adhesion-related induction required a functional response regulator.
Similarly, deletion of cpxA caused a decreased basal level of cpxR expression. During adhesion, no increase in cpxR activity was measured for this mutant as well (Fig. 2D), demonstrating a requirement for the sensor kinase CpxA.
Contact-Dependent Induction of cpxR Requires the Outer Membrane Lipoprotein NlpE.
The close proximity of hydrophobic surfaces causes strong attractive physico-chemical forces, called hydrophobic interactions (16). With respect to bacteria approaching a hydrophobic surface, we reasoned that the forces arising might cause a major perturbation in the outer membrane. This may be perceived by a cell envelope component that mediates an adhesion-specific signal to the Cpx pathway. Initially we thought that adhesive cell surface organelles might be involved. However, under the experimental conditions applied, curli are hardly expressed by E. coli strain MC4100 (17). The production of type 1 fimbriae was also low as assessed by mannose-sensitive yeast agglutination (ref. 11; data not shown). Consistent with this, studies of the transcriptional activity of cpxR′–lacZ+ in the respective csgA and fim mutant backgrounds showed that these adhesive cell surface structures were not required for the adhesion-induced Cpx response (data not shown).
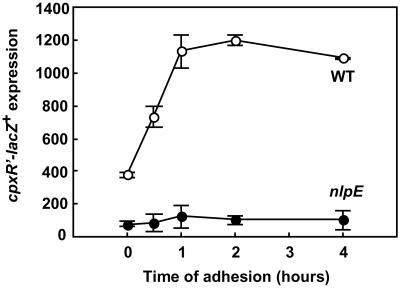
Another potential regulatory factor might be NlpE, an outer membrane lipoprotein that has been shown to activate the Cpx response when overexpressed (18). To test whether NlpE may be involved in mediating an adhesion-specific signal to the Cpx pathway, transcriptional expression of cpxR′–lacZ+ was measured in the absence of nlpE both in planktonic and attached cells. Strikingly, the transcriptional activation of cpxR in response to adhesion was totally abolished in the absence of this protein (Fig. 3). To confirm that the negative effect on Cpx activity during adhesion was caused by the nlpE deletion, we transformed the null mutant with a plasmid carrying nlpE. As expected, the plasmid-containing strain exhibited increased transcriptional activity during adhesion as compared to the vector control (data not shown). Based on these results, we conclude that NlpE is involved in surface sensing and plays an important role in activating the Cpx system specifically in response to adhesion.
Figure 3.
Transcriptional activity of cpxR′–lacZ+ during adhesion requires the outer membrane lipoprotein NlpE. Parallel measurements from one set of experiments are shown (means ± SD), which were representative for three independent experiments.
Adhesion of E. coli to Hydrophobic Surfaces Is Impaired When the CpxRA Pathway Is Disrupted.
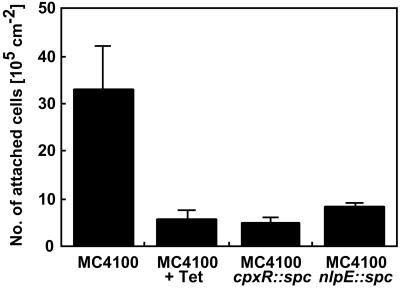
To examine whether a functional CpxRA pathway and NlpE contribute to adhesion of E. coli to hydrophobic surfaces, adhesion of wild-type E. coli MC4100 cells was studied in the presence or absence of tetracycline (100 μg/ml−1) and compared to that of mutant strains lacking either cpxR or nlpE. Cells were allowed to attach for 1 h to hydrophobic quartz crystals, a time point at which a maximum level of Cpx activity was reached as judged by the gene fusion experiments. At the end of each experiment, the number of attached bacteria was determined by acridine orange direct counts. Adhesion of wild-type cells was significantly decreased (6-fold) when protein synthesis was inhibited (Fig. 4). Interestingly, mutants lacking either NlpE or CpxR were equally defective. These results confirm that protein synthesis is necessary for adhesion of certain strains (3, 19) and indicate that the CpxRA pathway is required for efficient adhesion.
Figure 4.
Adhesion of E. coli strain MC4100 to hydrophobic surfaces is impaired when protein synthesis is inhibited and when the CpxRA pathway is disrupted. Numbers of attached cells on hydrophobic surfaces were determined after 60 min of adhesion directly on the quartz crystal surfaces by acridine orange direct counts for strains MC4100, TR51 (cpxR null mutant), and WBS262 (nlpE null mutant). Data shown are means (± SD) from 3–5 experiments.
Dynamic Cell–Surface Interactions Are Altered When cpxR or nlpE Are Absent.
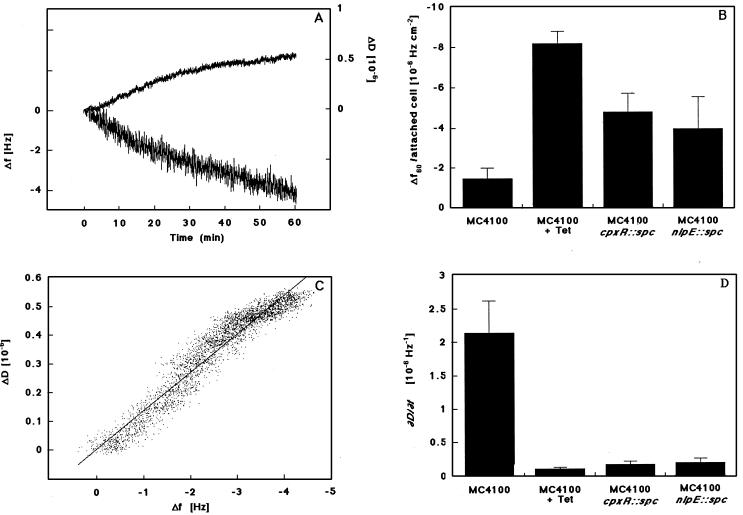
To measure differences in the binding characteristics of E. coli cells in the presence or absence of tetracycline (100 μg/ml−1) as well as in the presence or absence of CpxR or NlpE, we used a QCM. This technique monitors real-time changes in frequency (f), correlated to the increase in mass on the surface, and changes in energy dissipation (D), reflecting the changing viscoelastic properties of the attached mass during the adhesion process (Fig. 5A). To analyze the dynamic character of the adhesion process, two different calculations were made. First, the measured Δf were calculated per attached cell after 1 h of adhesion. These values reflect how much each cell contributes to the mass increase on the surface. Despite the fact that the number of wild-type cells was reduced when protein synthesis was inhibited, the Δf/attached cell was increased >4-fold (Fig. 5B). The cpxR and nlpE null mutants also exhibited a decreased level of adhesion and displayed noticeably higher Δf per attached cell than observed for the wild type.
Figure 5.
Cell–surface interactions of E. coli strain MC4100 with hydrophobic surfaces are altered when cpxR or nlpE are absent and resemble the phenotype of wild-type cells in which protein synthesis is inhibited. (A) Representative graph of a real-time measurement of adhesion for strain MC4100 by using the QCM, where relative Δf (lower line) and ΔD (upper line) are shown as a function of time. (B) Δf/attached cell after 60 min of adhesion shown for strains MC4100 in the presence or absence of tetracycline (100 μg⋅ml−1), TR51 (cpxR-null mutant), and WBS262 (nlpE-null mutant). (C) For the same measurement as in A, the ratio of dissipation per Δf (ΔD/Δf) is shown. The slope of this graph (∂D/∂f), derived from a linear curve fit by using kaleidagraph software (Abelbeck Software, Reading, PA), is thought to reflect the viscoelastic properties of the attached cells. (D) The ∂D/∂f is shown for strains MC4100 in the presence or absence of tetracycline (100 μg⋅ml−1), TR51 (cpxR-null mutant), and WBS262 (nlpE-null mutant). Data shown are means (± SD) from 3–5 experiments.
Second, the ratio of the ΔD to Δf (ΔD/Δf), which is independent of time or the number of attached bacteria, was determined for each time point during the measurement (Fig. 5C). The slopes of these graphs (∂D/∂f) are thought to reflect the viscoelastic properties of the attached mass on the surface (20, 21). The resulting values were significantly lower for cpxR and nlpE mutants and wild-type cells in the presence of tetracycline as compared to the control (Fig. 5D).
Thus, the dynamic cell–surface interactions exhibited by the cpxR and nlpE mutants are strikingly similar to those of wild-type cells in which protein synthesis is inhibited, and they can easily be distinguished from the untreated wild type. These differences can be attributed to profoundly different binding properties of the mutant strains.
Discussion
The initial interactions of bacteria with surfaces or host tissues are an essential step in the formation of biofilms and bacterial infections. Although it is established that bacteria adjust their cell structure and physiology during adhesion, little is known about how this adaptive response is mediated. Here we show that, under specific environmental conditions, the Cpx-signaling system is induced upon cell-surface contact, a response dependent on the presence of the outer membrane lipoprotein NlpE. Moreover, a functional CpxRA pathway and NlpE are required for productive cell–surface interactions, leading to stable adhesion. Our results provide evidence for a specific condition under which NlpE functions to stimulate the Cpx-signaling pathway and clearly demonstrate the critical role of two-component signal transduction in the regulatory control of adhesion.
Induction of the Cpx regulon occurs immediately upon cell-surface contact, suggesting a role for this signal transduction system during the early stage of the adhesion process. When CpxR or NlpE are absent, significantly lower numbers of cells attach to the surface. This may be caused by either a defect in the cellular binding properties required for initial attachment or a defect in an adaptive response required for stabilizing the contact of attached cells with the surface. One possibility is that NlpE might function directly or indirectly to promote adhesion. However, because most outer membrane lipoproteins are thought to be located at the periplasmic face of the outer membrane (22), it is unlikely that NlpE is exposed on the cell surface and that it may act directly as an adhesin. We find it more reasonable to assume that NlpE may play a regulatory role by mediating an adhesion-specific signal to the periplasmic sensing domain of CpxA leading to up-regulation of this pathway upon attachment. According to this idea, the level of adhesion would be a result of cell–surface interactions made after initial attachment, which apparently require both NlpE and a functional Cpx system.
The hypothesis that the Cpx system is required for an adaptive response to adhesion is further supported by the finding that Cpx activity also influences qualitative parameters of the adhesion process. Dynamic cell–surface interactions, as measured by the QCM, are remarkably distinct from those of the wild type when CpxR or NlpE are absent. An increase in mass in direct contact with the surface induces a decrease in the resonant frequency of the quartz crystal (23). Hence, a larger Δf per attached cell has been explained as a larger cell–surface contact contributing to increased surface interactions (20). Because the Cpx system is up-regulated during adhesion and because cells lacking NlpE or CpxR show lower levels of adhesion, one might expect these mutants to have less cell–surface contact and thus to cause a smaller Δf per attached cell. However, mutants lacking cpxR or nlpE exhibit a much larger Δf per attached cell than the wild type. This finding raises the intriguing possibility that specific cell–surface structures may be involved in the adhesion process that actively keep cells at a greater distance from the surface than observed for the mutants lacking NlpE or CpxR. According to this view, attachment would lead to a Cpx-dependent up-regulation of cell envelope components that alter the cell surface and mediate stable adhesion.
Deletion of nlpE or cpxR also causes a lower ΔD per Δf (∂D/∂f), which means that the viscoelastic properties of the cell–surface contact made by wild-type and mutant cells are completely different. Again, this obvious difference between the attached wild-type and mutant cells might be accounted for by different cell surface structures that contact the surface.
It is important to note that adhesion is as low for the nlpE and cpxR mutants as it is for wild-type cells in which protein synthesis is inhibited and that the surface interactions of these mutants closely resemble the surface interactions of “dead” cells. This further indicates that proper interactions of cells with a hydrophobic surface require a factor(s), which apparently is not made in the cpx and nlpE mutants.
Taken together, our results suggest that the Cpx pathway may be required to keep cell–surface interactions in check, i.e., it may combat or prevent damage to the cell envelope caused by excessive interaction of the cell with a hydrophobic surface. The Cpx response during adhesion thus may have two functions. First, it is possible that the Cpx system contributes to adhesion by its role in monitoring the folding or repair of cell envelope proteins. For instance, DsbA, one of the periplasmic factors up-regulated upon attachment, catalyzes the folding of disulfide bond-containing extracytoplasmic proteins and has previously been suggested to be involved in adhesion of E. coli to abiotic surfaces (24). In this case, NlpE may be involved in sensing damage of the outer membrane occurring upon contact of the cell with a hydrophobic surface, which it signals to the Cpx pathway.
Alternatively or in addition, NlpE might sense and generate an adhesion-specific signal to the Cpx pathway, which in turn would regulate additional genes encoding products that play a more direct role in mediating stable cell–surface contact. The fact that NlpE is required for induction of the Cpx pathway specifically during adhesion suggests that this activating signal may be different from those signals responsible for induction of the Cpx response under other cell envelope stress conditions. In any event, our findings provide insight into surface sensing and the regulation of adhesion-induced gene expression and may point to a valuable target for the development of antimicrobial agents to control biofilms.
Acknowledgments
We thank Hans Elwing and Malte Hermansson, Göteborg University, Sweden, for providing space and equipment to perform the QCM experiments. This work was supported by Grant GM34821 from the National Institute of General Medical Sciences to T.J.S. and in part by a fellowship of the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) to K.O., which is gratefully acknowledged.
Abbreviations
- QCM
quartz crystal microbalance
- Δf
frequency shift
- ΔD
dissipation shift
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Costerton J W, Stewart P S, Greenberg E P. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 2.O'Toole G A, Gibbs K A, Hager P W, Phibbs P V J, Kolter R. J Bacteriol. 2000;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otto K, Norbeck J, Larsson T, Karlsson K-A, Hermansson M. J Bacteriol. 2001;183:2445–2453. doi: 10.1128/JB.183.8.2445-2453.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prigent-Combaret C, Vidal O, Dorel C, Lejeune P. J Bacteriol. 1999;181:5993–6002. doi: 10.1128/jb.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang J P, Normark S. Science. 1996;273:1234–1236. doi: 10.1126/science.273.5279.1234. [DOI] [PubMed] [Google Scholar]
- 6.Raivio T L, Silhavy T J. Curr Opin Microbiol. 1999;2:159–165. doi: 10.1016/S1369-5274(99)80028-9. [DOI] [PubMed] [Google Scholar]
- 7.Mecsas J, Rouviere P E, Erickson J W, Donohue T J, Gross C A. Genes Dev. 1993;7:2618–2628. doi: 10.1101/gad.7.12b.2618. [DOI] [PubMed] [Google Scholar]
- 8.Hung D L, Raivio T L, Jones C H, Silhavy T J, Hultgren S J. EMBO J. 2001;20:1508–1518. doi: 10.1093/emboj/20.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dorel C, Vidal O, Prigent-Combaret C, Vallet I, Lejeune P. FEMS Microbiol Lett. 1999;178:169–175. doi: 10.1111/j.1574-6968.1999.tb13774.x. [DOI] [PubMed] [Google Scholar]
- 10.Leclerc G J, Tartera C, Metcalf E S. Infect Immun. 1998;66:682–691. doi: 10.1128/iai.66.2.682-691.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Otto K, Elwing H, Hermansson M. Colloids Surf B. 1999;15:99–111. [Google Scholar]
- 12.Slauch J M, Silhavy T J. J Bacteriol. 1991;173:4039–4048. doi: 10.1128/jb.173.13.4039-4048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prime K L, Whitesides G M. Science. 1991;252:1164–1167. doi: 10.1126/science.252.5009.1164. [DOI] [PubMed] [Google Scholar]
- 14.Kepner R L, Pratt J R. Microbiol Rev. 1994;58:603–615. doi: 10.1128/mr.58.4.603-615.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross C A. In: Escherichia coli and Salmonella: Cellular and Molecular Biology. Neidhardt F C, editor. Washington, DC: Am. Soc. Microbiol.; 1996. pp. 1382–1399. [Google Scholar]
- 16.Israelachvili J N, McGuiggan P M. Science. 1988;241:795–800. doi: 10.1126/science.241.4867.795. [DOI] [PubMed] [Google Scholar]
- 17.Olsén A, Jonsson A, Normark S. Nature (London) 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 18.Snyder W B, Davis L J B, Danese P N, Cosma C L, Silhavy T J. J Bacteriol. 1995;177:4216–4223. doi: 10.1128/jb.177.15.4216-4223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Toole G A, Kolter R. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 20.Otto K, Elwing H, Hermansson M. J Bacteriol. 1999;181:5210–5218. doi: 10.1128/jb.181.17.5210-5218.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodahl M, Höök F, Fredriksson C, Keller C A, Krozer A, Brzezinski P, Voinova M, Kasemo B. Faraday Discuss Chem Soc. 1997;107:229–246. doi: 10.1039/a703137h. [DOI] [PubMed] [Google Scholar]
- 22.Pugsley A P. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodahl M, Höök F, Kasemo B. Anal Chem. 1996;68:2219–2227. doi: 10.1021/ac951203m. [DOI] [PubMed] [Google Scholar]
- 24.Genevaux P, Bauda P, DuBow M S, Oudega B. FEMS Microbiol Lett. 1999;173:403–409. doi: 10.1111/j.1574-6968.1999.tb13532.x. [DOI] [PubMed] [Google Scholar]