Abstract
Background
Ramelteon is the first selective melatonin receptor agonist approved by the FDA, demonstrating significant clinical value in improving sleep latency and sleep quality in patients with insomnia. However, its long-term adverse effects have not been fully evaluated. This study analyzes adverse events associated with ramelteon based on data from the FDA Adverse Event Reporting System (FAERS) database.
Methods
Case reports submitted by physicians and pharmacists were extracted from the FAERS database from the first quarter of 2005 to the third quarter of 2024. Signal detection was performed using Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS) algorithms.
Results
A total of 1,150 reports related to ramelteon were analyzed, covering 26 System Organ Classes (SOC) and 537 preferred terms (PT). The main SOCs included psychiatric disorders, general disorders and administration site conditions, and nervous system disorders. Female patients reported higher instances of insomnia, while hangover, feeling drunk, and decreased blood testosterone were more commonly observed in male patients. At the PT level, hangover, initial insomnia, and glossoptosis exhibited the highest signal strengths. Sleep-related adverse events (AE), such as initial insomnia, somnolence, middle insomnia, poor quality sleep, and hypersomnia, were confirmed. Notably, we report for the first time that ramelteon is associated with parasomnia-related AEs, including sleep talking, sleep terror, screaming, and somnambulism.
Conclusion
Our study reveals a broad spectrum of AEs associated with ramelteon, including unique sensory experiences (e.g., hangover, derealisation, feeling drunk), reproductive system effects (e.g., decreased libido, priapism), hallucination-related AEs (e.g., visual hallucinations), and rare but clinically significant reactions (e.g., glossoptosis, restless legs syndrome, and photopsia). These findings expand the current understanding of ramelteon’s safety and underscore the importance of closely monitoring patients’ responses during treatment. Emphasis should be placed on individualized treatment strategies and strengthened pharmacovigilance.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-025-07127-1.
Keywords: Ramelteon, FAERS, Adverse events, Signal mining, Sleep disorders
Introduction
Insomnia is a common and distressing sleep disorder and a major contributing factor to various health issues, including mood disorders, cardiovascular diseases, and cognitive decline [1]. Globally, insomnia has emerged as a significant public health concern, affecting the quality of life of hundreds of millions of individuals and imposing a substantial socioeconomic burden [2]. The primary goals of insomnia treatment are to improve sleep quality and duration, alleviate associated physical and psychological symptoms, and reduce the risk of chronic diseases [3]. Currently, the most widely used sedative-hypnotic medications in clinical practice primarily act by enhancing gamma-aminobutyric acid (GABA)-mediated neurotransmission. However, these agents are associated with safety concerns such as tolerance, dependence, cognitive impairment, and next-day residual effects [4]. Ramelteon, a selective melatonin MT1 and MT2 receptor agonist, promotes sleep by regulating melatonergic activity in the suprachiasmatic nucleus and pineal gland, thereby modulating circadian rhythms and wakefulness cycles [5]. Its mechanism differs from that of traditional GABAergic sedative-hypnotics. Since 2005, ramelteon has been approved by the U.S. FDA for the treatment of sleep-onset insomnia and has been widely adopted in clinical practice due to its low potential for dependence and favorable efficacy profile [6].
A meta-analysis of 22 studies involving 4,875 patients demonstrated that ramelteon is effective in improving various outcomes, including objective total sleep time, subjective total sleep time, subjective sleep onset latency, and objective sleep onset latency [7]. Moreover, increasing evidence in recent years suggests that ramelteon has promising advantages in managing delirium [8]. Compared to placebo, ramelteon has been shown to reduce the risk of delirium in hospitalized patients [9]. Despite its reasonable efficacy and broad indications in clinical practice, potential adverse effects of ramelteon warrant attention. Existing studies indicate that common adverse events (AE) associated with ramelteon include headache, dizziness, drowsiness, fatigue, and nausea [10]. Additionally, there have been reports of leukopenia [11] and nightmares [12]. However, real-world safety data on ramelteon remains limited, particularly regarding rare AEs, psychiatric and behavioral risks, and potential long-term side effects. In the context of increasing global concerns about prescription drug misuse and AE monitoring, there is a pressing need for a comprehensive evaluation of ramelteon’s safety using real-world data.
The FDA Adverse Event Reporting System (FAERS) is the largest and most comprehensive spontaneous reporting database for drug-related AEs globally. This database provides real-world drug safety data with large sample sizes and long-term dynamic monitoring, aiding in the early identification of rare or severe AE signals. It has been widely used in pharmacovigilance and signal detection research [13]. Currently, studies on the adverse effects of ramelteon mainly focus on meta-analyses and clinical trials, which often have strict inclusion and exclusion criteria and may not fully reflect the real-world medication conditions of patients. Therefore, this study systematically analyzes AE reports related to ramelteon from the FAERS database between 2005 and 2024, with the aim of identifying potential risk signals and providing evidence-based support for clinical rational drug use and safety management.
Methods
Data source
Reports from the FAERS database from the first quarter of 2005 to the third quarter of 2024, in which “ramelteon” was listed as the Primary Suspect Drug (PS), were retrieved. To enhance the accuracy of the analysis, only case reports submitted by physicians and pharmacists were included.
Data extraction and analysis
FAERS quarterly data packages were obtained from the FDA official website, and data cleaning and integration were performed using standardized procedures. Reports with a count of preferred terms (PT) greater than or equal to 3 were selected. Duplicate data were removed. Specifically, for reports with multiple AEs sharing the same case_ID, only the most recent FDA date (fda_dt) was retained, and the higher primary_ID was selected. Records with incomplete report information or missing drug details were excluded. The Medical Dictionary for Regulatory Activities (MedDRA) was used to standardize the coding of System Organ Class (SOC) and PT signals, ensuring terminology consistency.
Signal detection was performed using four mainstream pharmacovigilance signal detection methods, including the Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS) algorithms. Ratio-based methods, such as ROR and PRR, are simple to compute and provide intuitive results, making them suitable for preliminary signal screening in large-scale spontaneous reporting databases. The BCPNN method effectively reduces the impact of data sparsity, making it ideal for stable detection of rare AE signals. The MGPS method offers excellent signal-to-noise ratio control, enhancing the accuracy and sensitivity of signal detection after adjusting for covariates [14].
In this study, the four disproportionate analysis methods were combined to expand the detection scope by leveraging the strengths of each method, allowing for a more comprehensive examination of AE data. The signal detection criteria applied in this study were as follows: for ROR, a signal was considered positive when the lower bound of the 95% confidence interval exceeded 1, and the number of co-occurrences (n) ≥ 3; for PRR, a signal was considered positive when the lower bound of the 95% confidence interval exceeded 1, and the number of co-occurrences (n) ≥ 3; for IC, a positive signal was defined as IC025 > 0; and for EBGM, a signal was considered positive when EBGM05 > 2. These thresholds were established based on standard pharmacovigilance practices [15]. Detailed formulas for the 2 × 2 contingency table, along with the criteria and thresholds for each disproportionality analysis method (PRR, ROR, IC, and EBGM), are provided in Supplementary Table S1. Higher calculated values obtained from these calculations indicate stronger signal strength, reflecting a stronger potential association between the target drug and the AE.
Secondary analysis
To further explore the impact of demographic characteristics on the distribution of AEs to ramelteon, a gender subgroup analysis was conducted based on the overall data analysis. Report data were divided into male and female groups according to the gender information recorded in the FAERS database. The frequency and incidence of each AE were calculated for each group, and the Chi-square test was used to assess gender differences using a 2 × 2 contingency table. A significance level of p < 0.05 was set, and Odds Ratios were calculated to quantify the relative risk between genders. Statistically significant signals were selected.
Statistical analysis
Descriptive statistics were used to present the basic characteristics of AEs. Data processing was performed using MySQL 8.0 and Excel 2016. Statistical analysis and visualization were carried out using R (version 4.2.2) and Python (version 3.10). Specially, the EBGM, EBGM05, and EBGM95 were derived using the MGPS model implemented in the openEBGM package (version 0.9.1).
Human ethics approval and consent to participate declarations
The study was conducted in accordance with the Declaration of Helsinki and was based on data from the publicly available FAERS database. All data were de-identified and contained no personally identifiable information or patient interventions. Therefore, ethical approval and informed consent were not required in accordance with relevant guidelines and regulations.
Clinical trial number
Not applicable.
Results
Basic information on adverse events of Ramelteon
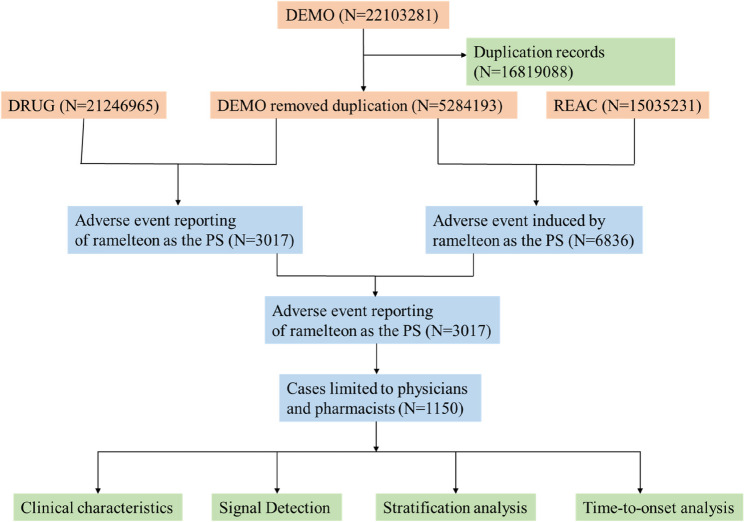
As shown in Fig. 1, a total of 1,150 AE reports related to ramelteon were recorded from the first quarter of 2005 to the third quarter of 2024. Among these, 536 cases (46.6%) involved female patients, significantly more than the 361 cases (31.4%) involving male patients (Table 1). Excluding unknown ages, the majority of patients were aged 18–64 years (20.6%), followed by those aged 65–85 years (12.6%). Notably, 61.7% of the cases did not provide age information, which severely limits further investigation into the impact of age on AEs. The United States accounted for the majority of reports (83.0%), followed by Japan (16.6%). Reports from physicians represented 84.7% of the cases. Regarding clinical outcomes, aside from unspecified AEs, the most commonly reported significant medical AEs were other important medical AEs (124, 10.8%) and hospitalization (104, 9.0%). Nearly three-quarters of AEs occurred within 0–30 days (70.8%), followed by 31–60 days (10.6%). Notably, 13.3% of AEs occurred after 5 months, highlighting the long-term risk of AEs. The median time to AEs was 8 (39) days. Weighted Signal Proportion analysis indicated that the shape parameter and its 95% confidence interval upper limit were both less than 1, suggesting that the clinical signal of ramelteon follows an early failure pattern (Table 2). Time-series analysis showed that the highest number of reports occurred in 2007 (391), accounting for 34.0% of all reports (Fig. 2).
Fig. 1.
The flowchart of the entire study
Table 1.
Basic information on AE reports for Ramelteon
| Factor | Number of events (%) |
|---|---|
| Gender | |
| Female | 536 (46.6%) |
| Male | 361 (31.4%) |
| Unknown | 253 (22.0%) |
| Age | |
| < 18 | 21 (1.8%) |
| 18–64 | 237 (20.6%) |
| 65–85 | 145 (12.6%) |
| > 85 | 37 (3.2%) |
| Unknown | 710 (61.7%) |
| Weight | |
| <50 kg | 27 (2.3%) |
| 50–100 kg | 199 (17.3%) |
| > 100 kg | 22 (1.9%) |
| Unknown | 902 (78.4%) |
| Reported Countries | |
| Unite States | 954 (83.0%) |
| Japan | 191 (16.6%) |
| Brazil | 3 (0.3%) |
| India | 2 (0.2%) |
| Reporter | |
| Physician | 974 (84.7%) |
| Pharmacist | 176 (15.3%) |
| Outcomes | |
| Death | 34 (3.0%) |
| Disability | 1 (0.1%) |
| Hospitalization-Initial or Prolonged | 104 (9.0%) |
| Life-Threatening | 16 (1.4%) |
| Other Serious (Important Medical Event) | 124 (10.8%) |
| Required Intervention to Prevent Permanent Impairment/Damage | 1 (0.1%) |
| Unknown | 870 (75.7%) |
| Induction time | |
| 0–30 days | 160 (70.8%) |
| 31–60 days | 24 (10.6%) |
| 61–90 days | 6 (2.7%) |
| 91–120 days | 4 (1.8%) |
| 121–150 days | 2 (0.9%) |
| 151–180 days | 10 (4.4%) |
| 181–360 days | 12 (5.3%) |
| > 360 days | 8 (3.5%) |
Table 2.
Time-to-onset analysis for signals with Ramelteon
| Prioritization | Weibull distribution | Failure type | ||||||
|---|---|---|---|---|---|---|---|---|
| Case | TTO (days) | Scale parameter | Shape parameter | |||||
| n | Median (IQR) | Min–max | α | 95% CI | β | 95% CI | ||
| Ramelteon | 1150 | 8 (39) | 1-1806 | 27.00 | 20.49–35.57 | 0.50 | 0.46–0.55 | Early failure |
n number of cases with available time-to-onset, TTO time-to-onset, IQR interquartile range
Fig. 2.
Annual reporting of ramelteon-related AEs
Signal mining results
AEs related to ramelteon were screened using the ROR, PRR, IC, and EBGM methods, and were mainly distributed across 26 different System Organ Classes (SOC) (Table 3). The three systems with the highest number of reports were psychiatric disorders (n = 672, ROR 8.04, PRR 6.02, IC 2.59, EBGM 6.02), general disorders and administration site conditions (n = 435, ROR 1.27, PRR 1.22, IC 0.28, EBGM 1.22), and nervous system disorders (n = 400, ROR 2.31, PRR 2.09, IC 1.06, EBGM 2.09), suggesting a need for caution regarding the risk of ramelteon-associated psychiatric symptoms and nervous system AEs. Notably, signals for reproductive system and breast disorders were relatively high (ROR 3.5), which, although not directly related to the characteristics of ramelteon, show some correlation and warrant additional attention.
Table 3.
Signal strength of AEs reports for Ramelteon at the SOC level
| SOC | Case reports | ROR (95% CI) | PRR (95% CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|
| Psychiatric disorders | 672 | 8.04(7.35–8.79) | 6.02(5.96–6.09) | 2953.88 | 2.59(2.46) | 6.02(5.59) |
| General disorders and administration site conditions | 435 | 1.27(1.14–1.4) | 1.22(1.13–1.3) | 19.68 | 0.28(0.13) | 1.22(1.11) |
| Nervous system disorders | 400 | 2.31(2.08–2.58) | 2.09(2-2.18) | 247.1 | 1.06(0.91) | 2.09(1.91) |
| Injury, poisoning and procedural complications | 208 | 1.03(0.89–1.19) | 1.03(0.9–1.16) | 0.16 | 0.04(−0.17) | 1.03(0.91) |
| Gastrointestinal disorders | 121 | 0.6(0.5–0.72) | 0.62(0.44–0.79) | 31.26 | −0.7(−0.96) | 0.62(0.53) |
| Investigations | 93 | 0.54(0.44–0.67) | 0.56(0.36–0.76) | 33.94 | −0.83(−1.13) | 0.56(0.47) |
| Skin and subcutaneous tissue disorders | 63 | 0.48(0.38–0.62) | 0.5(0.26–0.74) | 33.54 | −1(−1.37) | 0.5(0.4) |
| Respiratory, thoracic and mediastinal disorders | 48 | 0.38(0.28–0.5) | 0.39(0.11–0.67) | 48.75 | −1.36(−1.78) | 0.39(0.31) |
| Reproductive system and breast disorders | 47 | 3.5(2.62–4.67) | 3.45(3.16–3.73) | 82.1 | 1.78(1.36) | 3.45(2.71) |
| Musculoskeletal and connective tissue disorders | 39 | 0.33(0.24–0.46) | 0.35(0.03–0.66) | 50.72 | −1.53(−1.99) | 0.35(0.27) |
| Cardiac disorders | 33 | 0.38(0.27–0.54) | 0.39(0.05–0.73) | 32.69 | −1.36(−1.86) | 0.39(0.29) |
| Eye disorders | 27 | 0.59(0.4–0.86) | 0.59(0.22–0.97) | 7.8 | −0.76(−1.31) | 0.59(0.43) |
| Infections and infestations | 26 | 0.18(0.12–0.26) | 0.19(−0.2-0.57) | 97.66 | −2.42(−2.98) | 0.19(0.14) |
| Metabolism and nutrition disorders | 24 | 0.39(0.26–0.58) | 0.39(0-0.79) | 23 | −1.34(−1.92) | 0.39(0.28) |
| Surgical and medical procedures | 20 | 0.7(0.45–1.09) | 0.7(0.27–1.14) | 2.5 | −0.5(−1.14) | 0.7(0.49) |
| Renal and urinary disorders | 18 | 0.38(0.24–0.6) | 0.38(−0.08-0.84) | 18.36 | −1.39(−2.05) | 0.38(0.26) |
| Hepatobiliary disorders | 14 | 0.41(0.24–0.69) | 0.41(−0.11-0.93) | 11.97 | −1.28(−2.03) | 0.41(0.26) |
| Vascular disorders | 14 | 0.24(0.14–0.41) | 0.25(−0.28-0.77) | 32.87 | −2.02(−2.76) | 0.25(0.16) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 9 | 0.11(0.06–0.22) | 0.12(−0.54-0.77) | 62.98 | −3.11(−4.03) | 0.12(0.07) |
| Immune system disorders | 9 | 0.28(0.15–0.55) | 0.29(−0.37-0.94) | 16.26 | −1.81(−2.72) | 0.29(0.17) |
| Ear and labyrinth disorders | 7 | 0.82(0.39–1.72) | 0.82(0.08–1.56) | 0.28 | −0.29(−1.31) | 0.82(0.44) |
| Social circumstances | 6 | 0.84(0.38–1.88) | 0.84(0.05–1.64) | 0.17 | −0.24(−1.34) | 0.84(0.43) |
| Endocrine disorders | 5 | 0.61(0.25–1.47) | 0.61(−0.26-1.49) | 1.24 | −0.71(−1.89) | 0.61(0.29) |
| Blood and lymphatic system disorders | 4 | 0.06(0.02–0.15) | 0.06(−0.92-1.04) | 62.34 | −4.09(−5.39) | 0.06(0.03) |
| Pregnancy, puerperium and perinatal conditions | 3 | 0.26(0.08–0.8) | 0.26(−0.87-1.39) | 6.43 | −1.95(−3.4) | 0.26(0.1) |
| Product issues | 3 | 0.1(0.03–0.31) | 0.1(−1.03-1.23) | 24.43 | −3.31(−4.76) | 0.1(0.04) |
At the PT level, 537 AE signals related to ramelteon were detected. Among them, 80 signals were consistent across all four algorithms (Fig. 3, detailed screening process is shown in Supplementary Figure S1). These scores were ranked according to the most stringent EBGM algorithm, and the top 32 PTs are shown in Table 4. Nearly half of the PTs (13/32) were classified under Psychiatric disorders, indicating positive signals for multiple psychiatric system-related AEs with ramelteon. The top three signals with the highest signal strength were hangover (n = 25, ROR 394.86, PRR 390.67, IC 8.52, EBGM 368.12), initial insomnia (n = 55, ROR 225.11, PRR 219.86, IC 7.73, EBGM 212.55), and glossoptosis (n = 3, ROR 172.11, PRR 171.89, IC 7.39, EBGM 167.4). The top three AEs by report frequency were somnolence (n = 95, ROR 14.69, PRR 14.14, IC 3.82, EBGM 14.11), nightmare (n = 70, ROR 72.29, PRR 70.17, IC 6.12, EBGM 69.41), and abnormal dreams (n = 56, ROR 105.8, PRR 103.3, IC 6.67, EBGM 101.66). In addition to these symptoms, this study also identified other AEs related to parasomnias (sleep talking, sleep terror, screaming, and somnambulism), which warrant further attention. Furthermore, AEs related to sensory experiences, such as hangover, derealisation, feeling drunk, drug screen positive, and prescribed overdose, were identified, with some showing high signal strength, thus requiring adequate attention. Notably, this study not only confirmed the presence of hallucinations as mentioned in the product label but also discovered more specific symptoms, such as visual hallucinations. Beyond the commonly mentioned reproductive system reactions like galactorrhoea, increased blood prolactin, and decreased blood testosterone in the label, our study extended these findings by identifying clinically relevant AEs such as libido decreased and priapism. After excluding PTs unrelated to the drug and symptoms that may occur due to disease progression, our study identified rare potential AE signals such as glossoptosis, restless legs syndrome, and photopsia, which require further mechanistic investigation.
Fig. 3.

Venn diagram under four different algorithms (Among the 537 signal combinations, the ROR method identified 105 related signals, the PRR method identified 114, the MGPS method identified 189, and the BCPNN method identified 254 valid signals.)
Table 4.
The top 32 signal strength of AEs reports for Ramelteon at the PT level
| SOC | PTs | Case reports | ROR (95% CI) | PRR (95% CI) | χ2 | IC (IC025) | EBGM (EBGM05) |
|---|---|---|---|---|---|---|---|
| General disorders and administration site conditions | hangover | 25 | 394.86(263.13-592.55) | 390.67(390.27-391.07) | 9154.69 | 8.52(7.94) | 368.12(262.11) |
| Psychiatric disorders | initial insomnia | 55 | 225.11(171.52-295.46) | 219.86(219.6-220.13) | 11583.64 | 7.73(7.34) | 212.55(169.3) |
| Gastrointestinal disorders | glossoptosis | 3 | 172.11(54.63-542.21) | 171.89(170.75-173.04) | 496.29 | 7.39(5.92) | 167.4(64.08) |
| Psychiatric disorders | middle insomnia | 52 | 119.23(90.36-157.34) | 116.62(116.34-116.89) | 5854.29 | 6.84(6.44) | 114.53(90.82) |
| Psychiatric disorders | abnormal dreams | 56 | 105.8(80.99–138.2) | 103.3(103.03-103.56) | 5583.74 | 6.67(6.28) | 101.66(81.29) |
| Injury, poisoning and procedural complications | labelled drug-disease interaction medication error | 3 | 98.99(31.62-309.84) | 98.86(97.72–100) | 286.17 | 6.61(5.15) | 97.36(37.47) |
| Psychiatric disorders | poor quality sleep | 38 | 80.19(58.08–110.7) | 78.9(78.59–79.22) | 2887.61 | 6.28(5.82) | 77.95(59.51) |
| Injury, poisoning and procedural complications | product administered to patient of inappropriate age | 13 | 72.8(42.08-125.95) | 72.4(71.85–72.95) | 905.15 | 6.16(5.38) | 71.6(45.26) |
| Psychiatric disorders | nightmare | 70 | 72.29(56.92–91.82) | 70.17(69.93–70.4) | 4722.58 | 6.12(5.77) | 69.41(56.83) |
| Psychiatric disorders | sleep talking | 3 | 63.89(20.48-199.38) | 63.81(62.68–64.95) | 183.65 | 5.98(4.53) | 63.19(24.38) |
| Psychiatric disorders | derealisation | 3 | 55.86(17.91-174.19) | 55.79(54.65–56.93) | 160.02 | 5.79(4.34) | 55.31(21.36) |
| General disorders and administration site conditions | paradoxical drug reaction | 7 | 42.08(19.99–88.59) | 41.96(41.22–42.7) | 278.08 | 5.38(4.36) | 41.69(22.36) |
| Nervous system disorders | hypersomnia | 23 | 36.36(24.08–54.89) | 36.01(35.6-36.42) | 778.72 | 5.16(4.57) | 35.82(25.37) |
| Psychiatric disorders | hallucinations, mixed | 6 | 34.68(15.53–77.45) | 34.6(33.8–35.4) | 194.71 | 5.1(4.01) | 34.42(17.57) |
| Reproductive system and breast disorders | galactorrhoea | 7 | 34.56(16.42–72.72) | 34.46(33.72–35.2) | 226.21 | 5.1(4.07) | 34.28(18.39) |
| Psychiatric disorders | sleep terror | 4 | 33.14(12.39–88.59) | 33.08(32.1-34.06) | 123.81 | 5.04(3.74) | 32.92(14.46) |
| General disorders and administration site conditions | feeling jittery | 14 | 32.35(19.11–54.79) | 32.17(31.64–32.69) | 420.74 | 5(4.25) | 32.01(20.6) |
| General disorders and administration site conditions | screaming | 5 | 28.58(11.86–68.86) | 28.52(27.64–29.4) | 132.19 | 4.83(3.65) | 28.4(13.61) |
| Investigations | blood prolactin increased | 7 | 28.55(13.57–60.05) | 28.47(27.72–29.21) | 184.7 | 4.82(3.8) | 28.34(15.21) |
| General disorders and administration site conditions | feeling drunk | 4 | 22.79(8.53–60.89) | 22.76(21.77–23.74) | 82.91 | 4.5(3.21) | 22.68(9.97) |
| Investigations | blood testosterone decreased | 4 | 22.45(8.4-59.97) | 22.41(21.43–23.4) | 81.55 | 4.48(3.19) | 22.34(9.82) |
| Psychiatric disorders | somnambulism | 4 | 19.72(7.38–52.67) | 19.69(18.71–20.67) | 70.75 | 4.3(3) | 19.63(8.63) |
| Nervous system disorders | restless legs syndrome | 10 | 18.89(10.14–35.18) | 18.81(18.19–19.43) | 168.18 | 4.23(3.36) | 18.76(11.15) |
| Psychiatric disorders | libido decreased | 5 | 16.47(6.84–39.65) | 16.43(15.56–17.31) | 72.3 | 4.04(2.85) | 16.39(7.86) |
| Investigations | drug screen positive | 4 | 15.54(5.82–41.48) | 15.51(14.53–16.49) | 54.18 | 3.95(2.66) | 15.48(6.81) |
| Psychiatric disorders | hallucination, visual | 14 | 14.6(8.63–24.7) | 14.52(13.99–15.04) | 175.86 | 3.86(3.11) | 14.49(9.33) |
| Nervous system disorders | somnolence | 95 | 14.69(11.96–18.04) | 14.14(13.94–14.33) | 1160.35 | 3.82(3.52) | 14.11(11.88) |
| Psychiatric disorders | restlessness | 19 | 13.93(8.87–21.89) | 13.83(13.38–14.28) | 225.74 | 3.79(3.14) | 13.8(9.45) |
| Social circumstances | impaired driving ability | 3 | 13.68(4.4-42.51) | 13.67(12.54–14.8) | 35.15 | 3.77(2.32) | 13.64(5.28) |
| Eye disorders | photopsia | 3 | 13.48(4.34–41.88) | 13.47(12.33–14.6) | 34.55 | 3.75(2.3) | 13.44(5.21) |
| Reproductive system and breast disorders | priapism | 3 | 13.25(4.26–41.15) | 13.23(12.1-14.36) | 33.85 | 3.72(2.28) | 13.21(5.12) |
| Injury, poisoning and procedural complications | prescribed overdose | 9 | 11.92(6.19–22.95) | 11.88(11.23–12.53) | 89.52 | 3.57(2.65) | 11.86(6.85) |
Gender subgroup analysis
Overall, the distribution of ramelteon-related AE signals was similar between genders. However, among the top 32 PTs, the proportion of female reports for insomnia was relatively higher, while hangover, feeling drunk, and decreased blood testosterone were more commonly reported in male patients (p < 0.05) (Table 5).
Table 5.
PTs with significant differences between males and females
| PT | Case reports (female) | Case reports(male) | Female rate | Male rate | Female odds | Male odds | OR | p value |
|---|---|---|---|---|---|---|---|---|
| Insomnia | 34 | 7 | 0.027486 | 0.009162 | 0.028263 | 0.009247 | 3.056407 | 0.008085 |
| Hangover | 8 | 13 | 0.006467 | 0.017016 | 0.006509 | 0.01731 | 0.376041 | 0.04299 |
| Feeling drunk | 0 | 4 | 0 | 0.005236 | 0 | 0.005263 | 0 | 0.04212 |
| Blood testosterone decreased | 0 | 4 | 0 | 0.005236 | 0 | 0.005263 | 0 | 0.04212 |
Discussion
As the first selective melatonin receptor agonist sedative-hypnotic approved by the US FDA, ramelteon has demonstrated significant advantages in improving sleep latency due to its unique mechanism of action [6]. By selectively activating the melatonin MT1 and MT2 receptors, ramelteon mimics the physiological effects of melatonin secretion by the pineal gland, regulating the circadian rhythm and thereby enhancing sleep efficiency [10]. Given the clinical application prospects of ramelteon post-market, potential drug-related AEs have remained a key area of concern. However, existing evidence on the safety of ramelteon primarily comes from randomized controlled trials with limited sample sizes and short observation periods, and there is a lack of long-term real-world data supporting its safety profile. To address this gap in research, this study systematically analyzed AE reports related to ramelteon from the FAERS database between 2005 and 2024, aiming to provide a comprehensive depiction of its real-world safety characteristics and offer evidence-based guidance for clinical use.
In terms of baseline information, the proportion of female patients reporting AEs was significantly higher than that of male patients. This finding is consistent with previous studies suggesting that women have differences in drug metabolism enzyme expression, hormone levels, and drug responsiveness [16]. It may also be influenced by higher consultation rates for sleep disorders among female patients [17], as well as higher usage preferences and reporting rates [18]. Further exploration of potential biological and sociological factors is warranted. Regarding age distribution, the largest group was patients aged 18–64 years, followed by those aged 65–85 years, likely reflecting the broader application of ramelteon in these age groups. However, the considerable number of reports lacking age information suggests that there is still room for improvement in the completeness of the FAERS database. In terms of regional distribution, the United States accounted for the majority of reports, followed by Japan. This is consistent with ramelteon being an FDA-approved drug, its earlier market introduction in the United States, and Japan being one of its primary research and development countries [19]. Regional differences may reflect variations in prescribing practices, awareness of AE reporting, and drug accessibility [20], and provide a data foundation for future cross-national drug safety comparison studies. Regarding clinical outcomes, important medical events and hospitalization were more common, suggesting that some AEs have higher clinical severity and require attention. The vast majority of AEs occurred within the first 0–30 days after medication use, aligning with the focus of monitoring during the drug initiation period. However, it is noteworthy that over 13% of AEs occurred more than five months after administration, revealing a certain long-term risk associated with ramelteon. Additionally, the median time to AE occurrence was 8 (39) days, indicating significant individual variability, which may be related to factors such as concomitant medications and underlying diseases [21]. Time series data showed that 2007 was the peak year for AE reports, which may be related to the Weber effect (i.e., a peak in AE reports within two years after drug approval) following ramelteon’s FDA approval in 2005 [22].
As a sedative-hypnotic agent, the AEs associated with ramelteon predominantly cluster within the categories of psychiatric disorders and nervous system disorders, which is closely related to its mechanism of action involving central modulation of circadian rhythms. Notably, this study identified several sleep disorder-related AEs consistent with the product label—such as initial insomnia, somnolence, middle insomnia, poor quality sleep, and hypersomnia—further highlighting ramelteon’s broad impact on sleep architecture. Interestingly, although reproductive effects are mentioned in the prescribing information, detailed AEs are lacking. Our analysis revealed multiple reproductive system and breast disorders not explicitly documented in the label, including libido decreased and priapism, which warrant further clinical attention. Additionally, considering ramelteon’s intended patient population, the AE “Product administered to patient of inappropriate age” may reflect concerns regarding its use in pediatric patients. The identified “Labelled drug-disease interaction medication error” pertains to known contraindications and drug-drug interactions listed in the prescribing information. For ramelteon, co-administration with agents such as fluvoxamine, rifampin, ketoconazole, and donepezil may pose interaction risks, especially given that ramelteon is primarily metabolized via CYP1A2 and CYP2C19 enzyme pathways. These drugs may alter ramelteon plasma concentrations by inducing or inhibiting relevant enzymes [23], thereby increasing the likelihood of AEs. Collectively, these findings underscore the critical role of drug labeling in guiding clinical practice and regulatory decision-making. We recommend that clinicians closely monitor patients receiving ramelteon therapy and promptly intervene should any related AEs arise.
Interestingly, our study identified a notable number of AEs related to parasomnias, including abnormal dreams, nightmares, sleep talking, sleep terror, screaming, and somnambulism, which have not been extensively reported in the current literature. Among these, abnormal dreams exhibited strong signal intensity, while nightmares accounted for a higher number of reported cases. These findings suggest that ramelteon may be associated with pronounced parasomnias symptoms during the process of improving sleep quality, warranting heightened clinical vigilance. The underlying mechanisms remain unclear but may involve ramelteon’s modulation of sleep stage transitions, including rapid eye movement (REM) sleep, non-rapid eye movement (NREM) sleep, and wakefulness [24]. Ramelteon has been shown to increase NREM sleep and enhance primary motor cortex gamma power, leading to localized neuronal firing and modulation of neural oscillations during sleep [25]. Such oscillations may relate to the cyclic rhythm of transitions between NREM and REM sleep [26], potentially resulting in blurred boundaries between these stages and the emergence of dissociated sleep phenomena [27]. Specifically, incomplete separation among wakefulness, REM, and NREM sleep may contribute to repeated cortical arousals and/or heightened sleep inertia, which are implicated in the occurrence of parasomnias. Further research is warranted to elucidate the potential mechanisms linking ramelteon to parasomnias. Additionally, individual patient factors—such as genetic predisposition, concomitant medications, or underlying conditions—may exacerbate these AEs in certain cases [28]. The clinical relevance of parasomnia-related AEs should not be underestimated, considering their potential to disrupt sleep, pose injury risks, and act as markers of sleep instability [29]. Clinicians prescribing ramelteon should carefully monitor patients’ sleep patterns and individual responses, particularly during periods of medication adjustment. When necessary, strategies such as dose modification, timing adjustments, or switching to alternative therapies may help mitigate these adverse effects.
Notably, our study also identified several signals associated with unique sensory experiences, including hangover, derealisation, feeling drunk, drug screen positive, and prescribed overdose. Surprisingly, hangover exhibited the highest signal intensity, and both hangover and feeling drunk were reported more frequently among male patients. As a melatonin receptor agonist, ramelteon primarily improves sleep by regulating circadian rhythms without directly acting on the GABAergic system, theoretically indicating a low potential for dependence [10]. However, the aforementioned signals suggest that certain distinctive sensory effects may still be observed. Specifically, hangover typically manifests as residual effects such as drowsiness, sedation, cognitive impairment, and psychomotor slowing the day after administration [30]. These outcomes may be related to prolonged half-life, metabolic abnormalities, or improper dosing of ramelteon [31]. Derealisation and feeling drunk are characterized by perceptual disturbances and altered consciousness [32], implying potential central nervous system involvement by ramelteon [33], particularly at high doses or in combination with other CNS depressants. The reports of drug screen positive and prescribed overdose further emphasize the need for strict clinical monitoring of patient adherence and self-adjustment of dosages. Although existing evidence supports ramelteon’s minimal risk of dependence [5], the unique sensory experiences identified in our study serve as an important clinical alert. Given ramelteon’s therapeutic potential, these findings warrant careful consideration. Future studies are needed to further elucidate the underlying mechanisms and contributing factors.
We identified reproductive system-related AEs not explicitly listed in the drug label, including libido decreased and priapism. Other reproductive system signals observed were galactorrhoea, blood prolactin increased, and blood testosterone decreased. These findings raise the possibility of an association between sexual dysfunction and alterations in melatonin levels and hormonal regulation involving the hypothalamic-pituitary-gonadal (HPG) axis. Firstly, melatonin has been shown to enhance sexual function via antagonistic properties mediated by the 5-HT2A receptor [34]. As a melatonin receptor agonist, fluctuations in melatonin levels induced by ramelteon may be related to symptoms such as decreased libido and priapism. Secondly, melatonin can modulate sexual maturation and reproductive function through activation of its receptors and binding sites on the HPG axis [35], including changes in gonadotropin levels [36]. Such hormonal changes have been observed in conjunction with symptoms like galactorrhoea, elevated prolactin, and decreased testosterone levels. These effects reflect ramelteon’s broad impact on reproductive endocrinology and suggest the potential for complex alterations in reproductive system function.
Through signal detection techniques, we identified several rare safety concerns associated with ramelteon, such as glossoptosis, restless legs syndrome, and photopsia, some of which exhibited relatively strong signal strength. Glossoptosis is characterized by the relaxation and downward displacement of the tongue, which may lead to breathing difficulties and throat discomfort, particularly noticeable during sleep or when lying down [37]. Some evidence suggests that melatonin can influence muscle tone [38]. Modulation of melatonin levels by ramelteon may be considered to influence neuromuscular coordination, which could be associated with glossoptosis in certain cases. Given that glossoptosis may occur after falling asleep, close monitoring is recommended during ramelteon treatment to prevent potential airway obstruction. Restless legs syndrome, also known as Willis-Ekbom disease, is a common movement disorder characterized by involuntary lower limb movements and an uncontrollable sense of restlessness [39]. This phenomenon might be related to ramelteon’s indirect effect on neurotransmitters, particularly the dopaminergic system. Activation of melatonin receptors may alter neuronal electrical activity, contributing to abnormal limb movements [40]. Photopsia is defined as sudden, brief, flash-like visual disturbances that may impair visual clarity [41]. Melatonin has also been shown to be produced within ocular structures and is implicated in intraocular pressure homeostasis [42]. Several ocular tissues, including the neural retina, retinal pigment epithelium, ciliary body, cornea, sclera, and lens, have been found to express melatonin receptors [43]. Although the exact mechanism remains unclear, photopsia may be related to melatonin homeostasis disruption caused by ramelteon. Such disruptions might be linked to ocular neuronal oscillations and altered electrophysiological signals in the visual cortex [44], which have been hypothesized to underlie complex ocular symptoms, including the visual hallucination identified in our study. These rare AEs highlight ramelteon’s broad action on the nervous system and underscore the need for vigilance in clinical practice. Special attention should be given to monitoring for these rare but potentially serious reactions, particularly during long-term use or in high-risk patients.
Although this study provides valuable insights into the AEs associated with ramelteon, several limitations should be acknowledged. First, this study is based on spontaneous reports from the FAERS database, which are subject to underreporting, reporting biases, and lack of denominator data [45]. Therefore, the findings should be interpreted as exploratory signals, and no causal relationship between ramelteon and the reported AEs can be established. Second, the study only included reports submitted by physicians and pharmacists, excluding feedback from patients and other healthcare professionals, potentially leading to underreporting of certain AEs. Third, the database predominantly comprises data from the United States, lacking sufficient representation from other regions, which may limit the generalizability of the findings due to regional differences. Future research should incorporate other sedative-hypnotic agents as comparators and conduct prospective, multicenter studies. Additionally, exploring racial, genetic, and geographic variations may help to better distinguish the unique adverse effects of ramelteon, thereby providing a more comprehensive safety evaluation to inform clinical practice.
Conclusion
Based on an analysis of the FAERS database, this study reveals the real-world AE profile of ramelteon, with a particular emphasis on psychiatric and nervous system-related AEs. While ramelteon demonstrates significant advantages in improving sleep onset latency and sleep quality, our findings also identified AEs related to parasomnias and sleep disturbances, further highlighting its broad impact on sleep architecture. Notably, this study is the first to report ramelteon-associated sexual dysfunctions, including libido decreased and priapism, as well as unique sensory experiences such as hangover, derealisation, and feeling drunk. Certain signals were reported significantly more frequently in males compared to females. These findings emphasize the need for heightened vigilance regarding the potential safety concerns of this medication in clinical practice. Additionally, new potential AEs, including glossoptosis, restless legs syndrome, and photopsia, were uncovered. Collectively, these findings provide novel evidence contributing to a more comprehensive safety profile of ramelteon and offer important guidance for its clinical use.
Supplementary Information
Acknowledgements
We thank the adverse event reporters and monitoring agencies for their contributions to this field.
Abbreviations
- AEs
adverse events
- SOC
system organ classes
- PT
preferred terms
- ROR
Reporting Odds Ratio
- PRR
Proportional Reporting Ratio
- BCPNN
Bayesian Confidence Propagation Neural Network
- MGPS
Multi-item Gamma Poisson Shrinker
- FAERS
The FDA Adverse Event Reporting System
- PS
Primary Suspect Drug
- TTO
time-to-onset
- IQR
interquartile range
- REM
rapid eye movement
- NREM
non-rapid eye movement
- HPG
hypothalamic-pituitary-gonadal
Authors’ contributions
JW and YZ conceived the study; LQ carried out the data analysis and interpretation of the results; LQ and NL performed literature searching and summary; LL and FW provided funding support; JW contributed to writing the first draft of the manuscript, and LZ and NL edited and revised the manuscript. All authors read and approved the final manuscript.
Funding
The work was supported by the Youth Fund of the National Natural Science Foundation of China (8230082561) and Research Project established by Chinese Pharmaceutical Association Hospital Pharmacy department (No. CPA-Z05-ZC-2023002).
Data availability
The original contributions presented in this study are included in the article material, further inquiries can be directed to the corresponding authors.
Declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki and was based on data from the publicly available FAERS database. All data were de-identified and contained no personally identifiable information or patient interventions. Therefore, ethical approval and informed consent were not required in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jianhong Wu and Yujie Zhou contributed equally to this work.
References
- 1.Buysse DJ. Insomnia Jama. 2013;309(7):706–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benca R, Herring WJ, Khandker R, Qureshi ZP. Burden of insomnia and sleep disturbances and the impact of sleep treatments in patients with probable or possible alzheimer’s disease: A structured literature review. J Alzheimer’s Disease: JAD. 2022;86(1):83–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morin CM, Drake CL, Harvey AG, Krystal AD, Manber R, Riemann D, Spiegelhalder K. Insomnia disorder. Nat Reviews Disease Primers. 2015;1:15026. [DOI] [PubMed] [Google Scholar]
- 4.Madari S, Golebiowski R, Mansukhani MP, Kolla BP. Pharmacological management of insomnia. Neurotherapeutics: J Am Soc Experimental Neurother. 2021;18(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandi-Perumal SR, Srinivasan V, Spence DW, Moscovitch A, Hardeland R, Brown GM, Cardinali DP. Ramelteon: a review of its therapeutic potential in sleep disorders. Adv Therapy. 2009;26(6):613–26. [DOI] [PubMed] [Google Scholar]
- 6.Sateia MJ, Kirby-Long P, Taylor JL. Efficacy and clinical safety of ramelteon: an evidence-based review. Sleep Med Rev. 2008;12(4):319–32. [DOI] [PubMed] [Google Scholar]
- 7.Maruani J, Reynaud E, Chambe J, Palagini L, Bourgin P, Geoffroy PA. Efficacy of melatonin and Ramelteon for the acute and long-term management of insomnia disorder in adults: A systematic review and meta-analysis. J Sleep Res. 2023;32(6):e13939. [DOI] [PubMed] [Google Scholar]
- 8.Beaucage-Charron J, Rinfret J, Coveney R, Williamson D. Melatonin and Ramelteon for the treatment of delirium: A systematic review and meta-analysis. J Psychosom Res. 2023;170:111345. [DOI] [PubMed] [Google Scholar]
- 9.Yu CL, Carvalho AF, Thompson T, Tsai TC, Tseng PT, Tu YK, Yang SN, Yang FC, Chang CH, Hsu CW, et al. Ramelteon for delirium prevention in hospitalized patients: an updated meta-analysis and trial sequential analysis of randomized controlled trials. J Pineal Res. 2023;74(3):e12857. [DOI] [PubMed] [Google Scholar]
- 10.McGechan A, Wellington K, Ramelteon. CNS Drugs. 2005;19(12):1057–65. discussion 1066– 1057. [DOI] [PubMed] [Google Scholar]
- 11.Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly Ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32(3):351–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah C, Kablinger A. Ramelteon-induced nightmares: A case report. Asian J Psychiatry. 2015;18:111–2. [DOI] [PubMed] [Google Scholar]
- 13.Feng Z, Li X, Tong WK, He Q, Zhu X, Xiang X, Tang Z. Real-world safety of PCSK9 inhibitors: A pharmacovigilance study based on spontaneous reports in FAERS. Front Pharmacol. 2022;13:894685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J, Li N, Gu J, Shen Y, Qiu L, Zhu L. Post-marketing safety evaluation of vortioxetine: A decade-long pharmacovigilance study based on the FAERS database. J Affect Disord . 2025;379:586–93. [DOI] [PubMed]
- 15.Jiang Y, Qu Y, Du Z, Ou M, Shen Y, Zhou Q, Tian L, Zhu H. Exploring adverse events of vilazodone: evidence from the FAERS database. BMC Psychiatry. 2024;24(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lacroix C, Maurier A, Largeau B, Destere A, Thillard EM, Drici M, Micallef J, Jonville-Bera AP. Sex differences in adverse drug reactions: are women more impacted? Therapie. 2023;78(2):175–88. [DOI] [PubMed] [Google Scholar]
- 17.Pengo MF, Won CH, Bourjeily G. Sleep in women across the life span. Chest. 2018;154(1):196–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau BHP, Tang CSK, Holroyd E, Wong WCW. Challenges and implications for menopausal health and Help-Seeking behaviors in midlife women from the united States and China in light of the COVID-19 pandemic: Web-Based panel surveys. JMIR Public Health Surveillance. 2024;10:e46538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miyamoto M. Pharmacology of ramelteon, a selective MT1/MT2 receptor agonist: a novel therapeutic drug for sleep disorders. CNS Neurosci Ther. 2009;15(1):32–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schetz D, Scott TE, Waldman W, Sein Anand J, Wiergowski M, Kocić I. Reports analysis of psychotropic drugs related adverse reactions in Australia and Poland during the COVID 19 pandemic. Biomed pharmacotherapy = Biomedecine Pharmacotherapie. 2023;162:114681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Y, Peng H, Zhang X, Zhao Z, Yin J, Li J. Predicting adverse drug reactions of combined medication from heterogeneous Pharmacologic databases. BMC Bioinformatics. 2018;19(Suppl 19):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman KB, Dimbil M, Erdman CB, Tatonetti NP, Overstreet BM. The Weber effect and the united States food and drug administration’s adverse event reporting system (FAERS): analysis of sixty-two drugs approved from 2006 to 2010. Drug Saf. 2014;37(4):283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obach RS, Ryder TF. Metabolism of Ramelteon in human liver microsomes and correlation with the effect of fluvoxamine on Ramelteon pharmacokinetics. Drug Metab Dispos. 2010;38(8):1381–91. [DOI] [PubMed] [Google Scholar]
- 24.Irfan M, Schenck CH, Howell MJ. NonREM disorders of arousal and related parasomnias: an updated review. Neurotherapeutics: J Am Soc Experimental Neurother. 2021;18(1):124–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimoto A, Yamashiro K, Suzuki T, Ikegaya Y, Matsumoto N. Ramelteon modulates gamma oscillations in the rat primary motor cortex during non-REM sleep. J Pharmacol Sci. 2021;145(1):97–104. [DOI] [PubMed] [Google Scholar]
- 26.Weber F. Modeling the mammalian sleep cycle. Curr Opin Neurobiol. 2017;46:68–75. [DOI] [PubMed] [Google Scholar]
- 27.Fleetham JA, Fleming JA, Parasomnias. CMAJ: Can Med Association J = J De l’Association Medicale Canadienne. 2014;186(8):E273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horváth A, Papp A, Szűcs A. Progress in elucidating the pathophysiological basis of nonrapid eye movement parasomnias: not yet informing therapeutic strategies. Nat Sci Sleep. 2016;8:73–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szûcs A, Mutti C, Papp A, Halász P, Parrino L. REM sleep, REM parasomnias, REM sleep behaviour disorder. Ideggyogyaszati Sz. 2022;75(5–06):171–82. [DOI] [PubMed] [Google Scholar]
- 30.Murray JB. Effects of valium and librium on human psychomotor and cognitive functions. Genetic Psychol Monogr. 1984;109(2D Half):167–97. [PubMed] [Google Scholar]
- 31.Srinivasan V, Brzezinski A, Pandi-Perumal SR, Spence DW, Cardinali DP, Brown GM. Melatonin agonists in primary insomnia and depression-associated insomnia: are they superior to sedative-hypnotics? Prog Neuro-psychopharmacol Biol Psychiatry. 2011;35(4):913–23. [DOI] [PubMed] [Google Scholar]
- 32.Baudot J, Soeiro T, Tambon M, Navarro N, Veyrac G, Mezaache S, Micallef J. Safety concerns on the abuse potential of esketamine: multidimensional analysis of a new anti-depressive drug on the market. Fundam Clin Pharmacol. 2022;36(3):572–81. [DOI] [PubMed] [Google Scholar]
- 33.Hunter EC, Charlton J, David AS. Depersonalisation and derealisation: assessment and management. BMJ (Clinical Res ed). 2017;356:j745. [DOI] [PubMed] [Google Scholar]
- 34.Brotto LA, Gorzalka BB. Melatonin enhances sexual behavior in the male rat. Physiol Behav. 2000;68(4):483–6. [DOI] [PubMed] [Google Scholar]
- 35.Shi L, Li N, Bo L, Xu Z. Melatonin and hypothalamic-pituitary-gonadal axis. Curr Med Chem. 2013;20(15):2017–31. [DOI] [PubMed] [Google Scholar]
- 36.Reiter RJ. Melatonin and human reproduction. Ann Med. 1998;30(1):103–8. [DOI] [PubMed] [Google Scholar]
- 37.Donnelly LF, Strife JL, Myer CM 3. Glossoptosis (posterior displacement of the tongue) during sleep: a frequent cause of sleep apnea in pediatric patients referred for dynamic sleep fluoroscopy. AJR Am J Roentgenol. 2000;175(6):1557–60. [DOI] [PubMed] [Google Scholar]
- 38.Hibaoui Y, Reutenauer-Patte J, Patthey-Vuadens O, Ruegg UT, Dorchies OM. Melatonin improves muscle function of the dystrophic mdx5Cv mouse, a model for Duchenne muscular dystrophy. J Pineal Res. 2011;51(2):163–71. [DOI] [PubMed] [Google Scholar]
- 39.Trenkwalder C, Allen R, Högl B, Clemens S, Patton S, Schormair B, Winkelmann J. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. 2018;17(11):994–1005. [DOI] [PubMed] [Google Scholar]
- 40.Zisapel N. Melatonin-dopamine interactions: from basic neurochemistry to a clinical setting. Cell Mol Neurobiol. 2001;21(6):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Virdee J, Mollan SP, Photopsia. Pract Neurol. 2020;20(5):415–9. [DOI] [PubMed] [Google Scholar]
- 42.Alkozi HA, Navarro G, Franco R, Pintor J. Melatonin and the control of intraocular pressure. Prog Retin Eye Res. 2020;75:100798. [DOI] [PubMed] [Google Scholar]
- 43.Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27(2):137–60. [DOI] [PubMed] [Google Scholar]
- 44.Reuss S, Kiefer W. Melatonin administered systemically alters the properties of visual cortex cells in cat: further evidence for a role in visual information processing. Vision Res. 1989;29(9):1089–93. [DOI] [PubMed] [Google Scholar]
- 45.Di Stefano R, Rindi LV, Baldini V, Rossi R, Pacitti F, Jannini EA, Rossi A. Glucagon-Like Peptide-1 receptor agonists, dual GIP/GLP-1 receptor agonist Tirzepatide and suicidal ideation and behavior: A systematic review of clinical studies and pharmacovigilance reports. Diabetes Metabolic Syndrome. 2025;19(4):103238. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in the article material, further inquiries can be directed to the corresponding authors.