Abstract
Nodes of Ranvier are excitable regions of axonal membranes highly enriched in voltage-gated sodium channels that propagate action potentials. The mechanism of protein clustering at nodes has been a source of controversy. In this study, developmental analysis of nodes of Ranvier in optic nerve axons reveals that early node intermediates are defined by ankyrin-G. Other node components, including beta IV spectrin, voltage-gated sodium channels, and the L1 cell adhesion molecule neurofascin, are subsequently recruited to sites of ankyrin-G clustering. The role of intact paranodes in protein clustering was examined in the dysmyelinating mouse mutant jimpy. Jimpy mice do not have intact paranodal axoglial contacts, which is indicated by a complete lack of neurexin/contactin-associated protein/paranodin clustering in paranodes. In the absence of intact paranodes, ankyrin-G was still able to cluster, although fewer ankyrin clusters were seen in jimpy optic nerves than in wild-type optic nerves. Recruitment of Nav1.2, Nav1.6, beta IV spectrin, and neurofascin to sites of ankyrin-G clustering is unimpaired in jimpy mice, indicating that node formation occurs independent of intact paranodal axoglial contacts.
Nodes of Ranvier, axon initial segments, and postsynaptic folds of the neuromuscular junction contain high densities of voltage-gated sodium channels crucial for generating sufficient local current to initiate a self-regenerating action potential. The mechanism for targeting and restricting sodium channels to these excitable membranes has been proposed to require one or more of the proteins that colocalize with sodium channels, including beta IV spectrin (1), ankyrin-G (ankyrin-3) (2, 3), and the L1 cell adhesion molecules (L1 CAMs) neurofascin and NrCAM (4, 5). Sodium channel clustering has also been hypothesized to require intact paranodal axoglial contacts that physically restrict sodium channels to a progressively smaller zone during node development (6–9).
Spectrins are a family of extended molecules comprising α and β subunits that assemble with actin to form a subplasmalemmal lattice required for mechanical support of plasma membranes of metazoan cells (ref. 10; reviewed in ref. 11). Spectrins are linked to the plasma membrane through ankyrins and through ankyrin-independent interactions with several molecules, including membrane lipids, the N-methyl-D-aspartate receptor, the α subunit of the epithelial sodium channel, and the Na+/H+ exchanger (11). A recently identified beta spectrin isoform, beta IV spectrin, is specifically enriched at nodes of Ranvier and axon initial segments and has been proposed to be involved in targeting and/or stabilizing the other proteins at these sites (1). Beta IV spectrin is present early in development at Purkinje neuron initial segments and depends on ankyrin-G expression for its localization to initial segments (12). A reciprocal requirement for beta IV spectrin in ankyrin-G localization and/or stabilization was not ruled out by these studies.
Ankyrins are a family of adaptor proteins that link the spectrin-based membrane skeleton to several integral membrane proteins, including the anion exchanger, the Na+/Ca2+ exchanger, the Na+/K+ ATPase, members of the L1 CAM family, and voltage-gated sodium channels (11). Ankyrin-G, along with beta IV spectrin, is present at developing Purkinje neuron initial segments before the voltage-gated sodium channel Nav1.6 and the L1 CAMs neurofascin and NrCAM (12). Elimination of ankyrin-G expression in Purkinje neuron initial segments results in an impaired ability to generate action potentials and mislocalization of the voltage-gated sodium channel Nav1.6, the L1 CAMs neurofascin and NrCAM, and beta IV spectrin (3, 12), suggesting a central role for ankyrin-G in the formation of this excitable membrane domain.
In contrast to axon initial segments, initiation of protein clustering at nodes of Ranvier has been hypothesized to depend on the L1 CAMs neurofascin and/or NrCAM (4, 5). L1 CAM clusters that do not contain ankyrin-G or sodium channels have been described during early sciatic nerve development (4). Moreover, neurofascin binds directly to the voltage-gated sodium channel β1 and β3 subunits (5), suggesting that L1 CAMs could recruit sodium channels to developing nodes. L1 CAMs are composed of variable extracellular domains that can contribute to both homophilic and heterophilic binding and a relatively conserved cytoplasmic domain containing the conserved sequence QFNEDGSFIGQY that mediates an association with ankyrin when the FIGQY tyrosine is dephosphorylated (13–15). FIGQY-dephosphorylated neurofascin and NrCAM are present in sciatic nerve at mature nodes of Ranvier (16), suggesting that an unphosphorylated FIGQY tyrosine may mediate assembly of L1 CAM–ankyrin-G complexes.
Protein clustering at nodes of Ranvier has also been proposed to depend on intact paranodal axoglial contacts (6–9). Proteins restricted to paranodes include the contactin-associated protein neurexin/contactin-associated protein (CASPR)/paranodin 1 (NCP1) and contactin in axons and neurofascin 155 in glial paranodal loops (17–20). During node development, early paranodal axoglial contacts, as indicated by NCP1 clustering, overlap with, or are adjacent to, developing nodes (6). As development proceeds, NCP1 is excluded from nodes and restricted to paranodes. The presence of axoglial contacts early in node development has led to the hypothesis that these contacts form a molecular sieve that clusters nodal protein complexes while allowing smaller juxtaparanodal complexes to pass through (9). This hypothesis is supported by work with hypomyelinating shiverer mutant mice, which have decreased numbers of nodes, and nodes that do form are invariably adjacent to intact paranodes, as indicated by clustered NCP1 labeling (6–8).
In this study, developmental analysis of nodes of Ranvier revealed that beta IV spectrin, the voltage-gated sodium channels Nav1.2 and Nav1.6, and the L1 CAM neurofascin are recruited to sites of ankyrin-G clustering. Recruitment of Nav1.2, Nav1.6, beta IV spectrin, and neurofascin to sites of ankyrin-G clustering is unimpaired in the dysmyelinating mutant jimpy mouse, which does not contain intact paranodal axoglial contacts.
Materials and Methods
Immunocytochemistry.
Tissue sections were prepared as described (4) from postnatal day 22 (P22) rats that were perfused with 3.0 mM NaEDTA in PBS followed by 2.0% paraformaldehyde. P9 rats, P14 rats, P11 mice, and P20 mice were decapitated after carbon dioxide anesthesia, and optic nerves were immersion fixed in cold 2.0% paraformaldehyde. Fixed tissue was incubated in a sucrose gradient overnight and sectioned into 10-μm sections on a cryostat. Antibodies used include a mouse monoclonal antibody against the ankyrin-G spectrin-binding domain (16); a mouse monoclonal pan sodium channel antibody (Sigma); a chicken polyclonal antibody against the ankyrin-G spectrin-binding domain (21); affinity-purified rabbit polyclonal antibodies against neurofascin (22), Nav1.2 (Upstate Biotechnology, Lake Placid, NY), the peptide CIANHTGVDIHRNGDFQKNG corresponding to residues 1042–1061 of mouse or rat Nav1.6 (Alomone Labs, Jerusalem); a chicken polyclonal antibody against the beta IV spectrin unique domain that has been adsorbed against brain lysate from a beta IV spectrin knockout mouse (generous gift of M. Komada, Tokyo Institute of Technology) (12); and a guinea pig polyclonal antibody against NCP1 (generous gift of M. Bhat, Mt. Sinai School of Medicine, New York). Secondary antibodies were donkey anti-mouse, -rabbit, -goat, and -chicken labeled with rhodamine, fluorescein, or Cy-5 (Jackson ImmunoResearch).
Jimpy Mice.
Mice carrying the X-linked jimpy mutation in the myelin proteolipid protein were obtained from The Jackson Laboratory. Heterozygous females were mated to wild-type males, and hemizygous male jimpy offspring were analyzed.
Results
We examined protein clustering at optic nerve nodes of Ranvier early in development (P9) and late in development (P22). By P22, ankyrin-G is clustered at nodes (arrowheads) and to a lesser extent in paranodal regions (arrows) (Fig. 1 A, D, G, and J). Paranodal ankyrin-G is lost in adult animals (data not shown). Nodal ankyrin-G overlaps with voltage-gated sodium channels (Fig. 1B), neurofascin (Fig. 1E), and beta IV spectrin (Fig. 1H). Both Nav1.2 and Nav1.6 are present in nodes at this time (see below). Paranodal ankyrin-G overlaps with neurofascin in some paranodes (Fig. 1 D and E, arrows) and with the axonal paranodal protein NCP1 (Fig. 1 J and K, arrows). Paranodal neurofascin is glial and may bind in trans to NCP1 (19). We could not determine whether the paranodal ankyrin-G is glial or axonal. Composite images are shown in Fig. 1 C, F, I, and L.
Figure 1.
Ankyrin-G colocalizes with other nodal and paranodal proteins in P22 optic nerve. Optic nerve from P22 rats was double-labeled with antibodies against ankyrin-G (A, D, G, J) and either voltage-gated sodium channels (B), neurofascin (E), beta IV spectrin (H), or NCP1 (K). The ankyrin-G chicken polyclonal antibody was used in A–C, whereas the ankyrin-G mouse monoclonal antibody was used in D–L. Composite images are shown in C, F, I, and L. Nodes of Ranvier are indicated by arrowheads, and paranodes are indicated by arrows. (Bars = 5 μm.)
Developing Nodes of Ranvier Are Defined by Ankyrin-G Clustering.
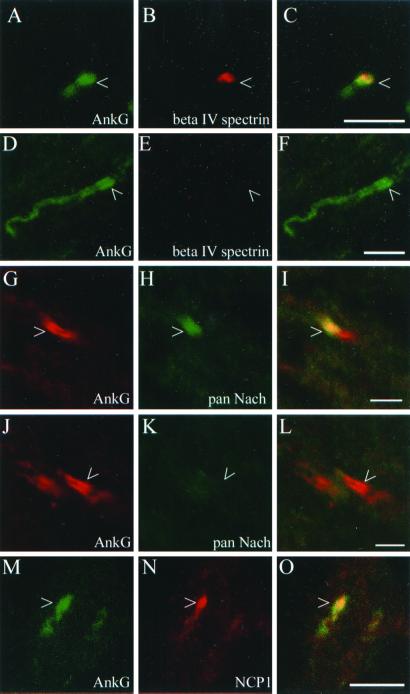
We examined the relative clustering of ankyrin-G and beta IV spectrin at developing nodes of Ranvier in optic nerve. At P9, a time when ankyrin clusters are first observed, 62% (50 of 80) of the ankyrin clusters examined contained overlapping beta IV spectrin (for example, see Fig. 2 A–C). The remainder, 38%, did not contain overlapping beta IV spectrin (Fig. 2 D–F). Many of the ankyrin-G clusters at P9 were broad suggesting a relatively immature stage of node development. The extended region of ankyrin-G clustering in Fig. 2D was seen early in development and could represent an initial stage of ankyrin clustering during node formation. Clusters of beta IV spectrin resembling mature or developing nodes were not observed in the absence of ankyrin-G. Thus, ankyrin clustering precedes that of beta IV spectrin during node of Ranvier development.
Figure 2.
Ankyrin-G clusters define early node intermediates. Optic nerve from P9 rats was labeled with ankyrin-G (A, D, G, J, M) and either beta IV spectrin (B, E), voltage-gated sodium channels (H, K), or NCP1 (N). The ankyrin-G mouse monoclonal antibody was used in A–F and M–O, whereas the ankyrin-G chicken polyclonal antibody was used in G–L. Composite images are shown in C, F, I, L, and O with areas of overlap indicated in yellow. Arrowheads indicate developing nodes of Ranvier. [Scale bars = 5 μm (A–F and M–O) or 2.5 μm (G–L).]
Approximately 59% (27 of 46) of ankyrin clusters at P9 overlapped with voltage-gated sodium channels (for example, see Fig. 2 G–I). The remainder of the ankyrin clusters examined, 41%, did not overlap with clustered voltage-gated sodium channels (Fig. 2 J–L) suggesting that voltage-gated sodium channels are recruited to ankyrin-G clusters at developing nodes of Ranvier. A similar pattern was also seen with an isoform-specific antibody against the voltage-gated sodium channel Nav1.2 (data not shown). The voltage-gated sodium channel present at mature nodes of Ranvier, Nav1.6, is not yet present in the P9 optic nerve (data not shown).
The formation of NCP1-mediated paranodal axoglial contacts has been hypothesized to be required for appropriate protein clustering at nodes of Ranvier (6–9). Ankyrin-G clusters at P9 generally either overlap NCP1 (Fig. 2 M–O) or are bordered by NCP1 (data not shown).
The relative clustering of ankyrin-G and neurofascin was more complicated than the other node components. P9 optic nerve contained three different patterns of ankyrin-G and neurofascin clustering. First, some clusters of ankyrin-G and neurofascin overlapped. Second, clusters of neurofascin were observed in the absence of overlapping ankyrin-G. Third, clusters of ankyrin-G were observed in the absence of overlapping neurofascin (data not shown). These results indicate that early in development ankyrin-G and neurofascin can cluster independently of each other. We hypothesized that some of these protein clusters were not destined to become nodes of Ranvier and might disappear during later stages of development. At P14, 89% (132 of 149) of ankyrin-G clusters contained overlapping neurofascin whereas the remaining 11% did not. Neurofascin clusters that resembled nodes of Ranvier always contained overlapping ankyrin-G (100 of 100). Figure 3 A–C shows several nodes of Ranvier containing both ankyrin-G (Fig. 3A) and neurofascin (Fig. 3B). A composite image is shown in Fig. 3C. Fig. 3 A–C Insets show a developing node containing ankyrin-G without overlapping neurofascin.
Figure 3.
Ankyrin-G clustering at developing nodes of Ranvier precedes clustering of neurofascin. Optic nerve from P14 rats was double-labeled with the mouse monoclonal antibody against ankyrin-G (A) and the rabbit polyclonal antibody against neurofascin (B). A composite image is shown in C. Inset shows ankyrin-G clustering in the absence of neurofascin clustering. Arrowheads indicate developing nodes of Ranvier. (Bar = 5 μm.)
Recruitment of Nav1.2, Beta IV Spectrin, and Neurofascin to Ankyrin-G Clusters Is Independent of NCP1-Mediated Axoglial Contacts.
The hypothesis that intact paranodes are necessary for protein clustering during node of Ranvier development implies that mutations that disrupt paranodal contact should eliminate protein clustering at nodes. To examine this question, we used the dysmyelinating mouse mutant Jimpy which has a mutation in the glial proteolipid protein causing an increase in oligodendrocyte cell death and dramatic hypomyelination (see refs. 23 and 24 for reviews).
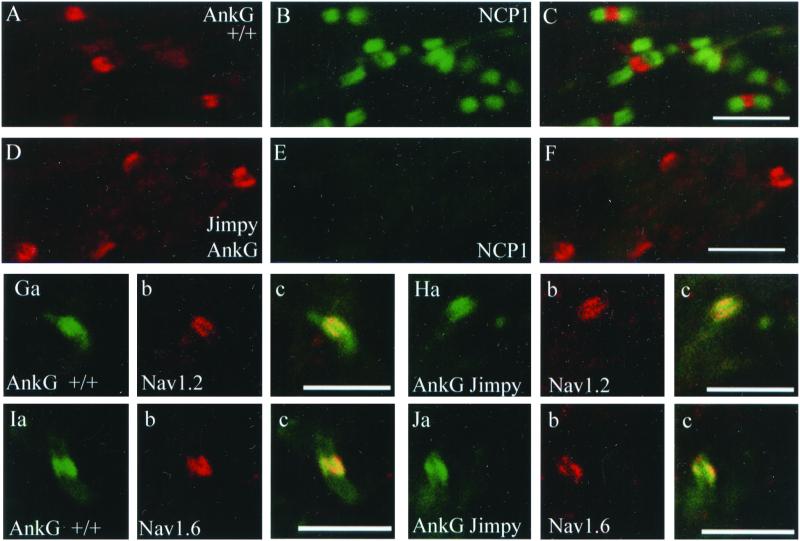
Optic nerves from wild-type mice at P11 contain clusters of ankyrin-G (Fig. 4A) that either overlap (white arrowheads) or border (arrow) sites of axoglial contact containing NCP1 (Fig. 4B). Fig. 4C shows a composite image with areas of ankyrin-G and NCP1 overlap in yellow. A rare ankyrin-G cluster that does not border or overlap NCP1 is indicated by the yellow arrowhead.
Figure 4.
Recruitment of voltage-gated sodium channels, beta IV spectrin, and neurofascin, to clusters of ankyrin-G during node of Ranvier development is unimpaired in the absence of intact paranodes. Optic nerves from P11 wild-type mice (A–C, G, I, K) or Jimpy mice (D–F, H, J, L) were labeled with the indicated antibodies. In A–C, clusters of ankyrin-G that overlap with NCP1 are indicated by white arrowheads, and a cluster that borders NCP1 is indicated by an arrow. A rare ankyrin-G cluster that neither overlaps with nor borders NCP1 is indicated by the yellow arrowhead. In D–F, ankyrin-G clusters that form in the absence of normal paranodal contacts are indicated by arrowheads. Composite images are shown in C and F. In Ga, Ia, and Ka developing nodes in wild-type mice that contain ankyrin-G also contain the voltage-gated sodium channel Nav1.2 (Gb), beta IV spectrin (Ib), and neurofascin (Kb). Composite images are shown in Gc, Ic, and Kc with areas of overlap indicated in yellow. In Ha, Ja, and La clusters of ankyrin-G also contain the voltage-gated sodium channel Nav1.2 (Hb), beta IV spectrin (Jb), and neurofascin (Lb). Composite images are shown in Hc, Jc, and Lc with areas of overlap indicated in yellow. (Bars = 5 μm.)
NCP1 does not cluster at sites of axoglial contact in jimpy mutant mice (Fig. 4E) indicating that intact paranodes do not form in mutants. In the absence of intact paranodes, ankyrin-G clusters are still seen (Fig. 4D, arrowheads). Paranodal ankyrin-G seen in wild-type optic nerve (Fig. 4 G, I, and K) is not present in jimpy optic nerve (Fig. 4 H, J, and L) providing further evidence for disrupted paranodes in jimpy mutants.
Recruitment of the voltage-gated sodium channel Nav1.2, beta IV spectrin, and neurofascin to ankyrin-G clusters is unimpaired in jimpy mutants. In P11 wild-type mice, approximately 73% (27 of 37) of ankyrin-G clusters examined contained Nav1.2 (for example, see Fig. 4G). In jimpy littermates, approximately 93% (25 of 27) of ankyrin-G clusters examined contained Nav1.2 (Fig. 4H) indicating that recruitment of Nav1.2 to ankyrin clusters is unimpaired in the absence of intact paranodes. Recruitment of beta IV spectrin (compare Fig. 4 I and J) and neurofascin (compare Fig. 4 K and L) to ankyrin-G clusters was also unimpaired in jimpy mutants.
The Developmental Switch from Nav1.2 to Nav1.6 Is Independent of Intact NCP1-Mediated Axoglial Contacts.
Nodes of Ranvier in P20 wild-type mouse optic nerve, marked by ankyrin-G (Fig. 5A), are flanked by paranodal NCP1 (Fig. 5B). A composite image is shown in Fig. 5C. In jimpy mutants, ankyrin clusters are present (Fig. 5D) in the absence of paranodal specializations as determined by the lack of NCP1 staining (Fig. 5E). The number of ankyrin-G clusters per field of view in P20 jimpy mice is approximately 35% of that in wild-type mice.
Figure 5.
Recruitment of the voltage-gated sodium channels Nav1.2 and Nav1.6 to developing nodes of Ranvier is normal in the absence of normal paranodal axoglial contacts. Optic nerves from P20 wild-type (A–C) or jimpy (D–F) mice were double labeled with ankyrin-G (A, D) and NCP1 (B, E). Composite images are shown in C and F. In G and I, wild-type optic nerve was double labeled for ankyrin-G (Ga and Ia) and either Nav1.2 (Gb) or Nav1.6 (Ib). Composite images are shown in Gc and Ic. In H and J, Jimpy optic nerve was double labeled for ankyrin-G (Ha and Ja) and either Nav1.2 (Hb) or Nav1.6 (Jb). Composite images are shown in Hc and Jc. [Scale bars = 2.5 μm (A–F) or 5 μm (G–J).]
Recruitment of the voltage-gated sodium channels Nav1.2 and Nav1.6 to clusters of ankyrin-G is unimpaired in jimpy mutants. The voltage-gated sodium channel Nav1.2 overlaps with ankyrin-G at approximately 86% (49 of 57) of wild-type nodes examined. An example of this overlap is shown in Fig. 5G. In jimpy mutants, the voltage-gated sodium channel Nav1.2 overlaps with approximately 92% (22 of 24) of ankyrin-G clusters examined. An example of this overlap in jimpy mutants is seen in Fig. 5H. By P20, some nodes of Ranvier should express the voltage-gated sodium channel Nav1.6 as well as Nav1.2. Nav1.6 overlaps with ankyrin-G at approximately 87% (40 of 46) of nodes of Ranvier in wild type P20 optic nerves. An example of this overlap can be seen in Fig. 5I. Nav1.6 overlaps with approximately 82% (28 of 34) of examined ankyrin-G clusters in P20 jimpy optic nerves. An example of this overlap can be seen in Fig. 5J. These results demonstrate that, although ankyrin-G clustering is impaired in jimpy mutants, it does still occur in the absence of intact paranodes, and recruitment of the voltage-gated sodium channels Nav1.2 and Nav1.6 to ankyrin clusters is unimpaired in the absence of intact paranodes. Beta IV spectrin and neurofascin are also present at ankyrin-G clusters in P20 optic nerve from both wild-type and jimpy mice (data not shown).
Discussion
This study has demonstrated that clusters of ankyrin-G in developing optic nerve define early intermediates in the morphogenesis of nodes of Ranvier, and suggests that other node components including voltage-gated sodium channels, beta IV spectrin, and neurofascin are recruited to ankyrin clusters. These results do not rule out the possibility that other node components will be reciprocally required for stabilization of ankyrin-G clusters. Lack of intact axoglial contacts at paranodes in jimpy mutant mice does not adversely affect recruitment and stabilization of the voltage-gated sodium channels Nav1.2 and Nav1.6, beta IV spectrin, and neurofascin at ankyrin-G clusters.
The finding that ankyrin-G clustering at developing nodes of Ranvier precedes clustering of the L1 CAM neurofascin is in contrast to reports from our laboratory (4). It is possible that this difference reflects a difference between sciatic nerve and optic nerve. However, when we looked at P9 optic nerve, a time when ankyrin and sodium channel clusters are just becoming visible, we did see both neurofascin clustering in the absence of ankyrin-G and ankyrin-G clustering in the absence of neurofascin (data not shown). We hypothesized that all of these clusters were not destined to become nodes of Ranvier. When we examined optic nerves later in development (P14) we found that a small percentage of ankyrin-G clusters did not contain detectable clusters of neurofascin. We did not find node-like neurofascin clusters in the absence of detectable ankyrin-G at P14, which suggests that although ankyrin-G and neurofascin can cluster independently of each other early in development, ankyrin-G clusters represent the earliest node of Ranvier developmental intermediates. It could be argued that the developing nodes observed without clustered neurofascin contain NrCAM instead, which is an unlikely because all nodes of Ranvier examined in adult optic nerve contained neurofascin (data not shown). Thus, a division between nodes containing neurofascin and nodes containing NrCAM seems unlikely.
Our results imply the existence of an as yet undiscovered ankyrin-G receptor that recruits ankyrin to developing nodes. The proposed ankyrin-G receptor likely clusters at developing nodes of Ranvier of central nervous system axons in response to a soluble factor secreted by oligodendrocytes (25, 26). A requirement for multiple ankyrin domains to restrict ankyrin-G to initial segments in cultured dorsal root ganglia neurons has been demonstrated (14). Thus, the ankyrin-G receptor may interact with multiple ankyrin domains or it may recruit other ankyrin-binding proteins to sites of future nodes of Ranvier.
It has been hypothesized that paranodal axoglial contacts are necessary for clustering of nodal proteins (6–9). According to this hypothesis, intact paranodes act as a rake forcing nodal protein complexes into a progressively smaller area. This hypothesis has empirical support from studies demonstrating that paranodal contacts, as defined by NCP1 clustering, are present at the earliest nodal developmental intermediates (6). Additionally, in the hypomyelinating mouse mutant shiverer, “normal” node-like sodium channel clusters are rare. The node-like clusters that do form are invariably adjacent to intact axoglial contacts as defined by clustered NCP1 suggesting a necessary role for such contacts in protein clustering at nodes (6–8). In addition, in these mutants the normal developmental transition from Nav1.2 to Nav1.6 does not occur except in clusters directly adjacent to NCP1-positive paranodes (8).
This hypothesis has not been supported universally. Early studies hypothesized that nodes develop before formation of well-defined paranodal axoglial specializations (27, 28). Recent work demonstrates that disruption of individual proteins involved in axoglial contact leaves nodes of Ranvier seemingly intact (20, 29), although these studies did not look specifically at the transition from Nav1.2 to Nav1.6. Additionally, previous work with cocultures of peripheral neurons and schwann cells has yielded mixed results with regard to the role of axoglial contact in node formation (30, 31).
Jimpy is a spontaneously occurring mutation in the glial proteolipid protein that results in hypomyelination and causes oligodendrocyte cell death (23, 24). Unlike the shiverer mutants used in previous studies, which do retain some intact paranodal axoglial contacts, jimpy mice do not contain intact paranodes as indicated by elimination of paranodal NCP1 and paranodal ankyrin-G. Consistent with oligodendrocyte cell death, we observed a dramatic decrease in MAG labeling in jimpy optic nerves compared with wild type (data not shown).
Recruitment of node proteins including neurofascin, beta IV spectrin, Nav1.2, and Nav1.6 to ankyrin clusters is unimpaired in jimpy mutants, suggesting that the mechanism responsible for recruiting neurofascin, beta IV spectrin, Nav1.2, and Nav1.6 to developing nodes is unimpaired in the absence of intact paranodal contacts. It could be argued that jimpy mutants initially form normal paranodes with accompanying nodal structures, and that paranodes disappear with death of oligodendrocytes but leave behind clusters of nodal proteins. However, this occurrence is unlikely because we did not see any NCP1 clustering in jimpy mice at the earliest times examined.
The most likely explanation for the difference between our results and previous results with shiverer mice in terms of number of protein clusters that form (6) is a difference in data interpretation. Rasband et al. (6) demonstrated that the number of “normal” node-like sodium channel clusters in shiverer optic nerve was ≈6% of that in wild type. However, inclusion of “abnormal” sodium channel clusters raised this number to approximately 25% (our own approximation based on graph in figure 8A in ref. 6). This number is similar to our own data that jimpy optic nerve contains approximately 35% of the wild-type number of ankyrin-G clusters.
Sodium channel and ankyrin-G clustering in response to a soluble factor released by differentiated oligodendrocytes has been demonstrated (25, 26). The lack of normal oligodendrocytes in jimpy and shiverer mice could decrease the amount of this diffusable factor. Such a decrease could explain the decrease in the number of nodes of Ranvier seen in jimpy and shiverer mice. Alternatively, we cannot rule out the possibility that this decrease in node of Ranvier number is caused by an effect of axoglial contact on node stabilization.
This study demonstrates that clustering of ankyrin-G defines early developmental intermediates in node of Ranvier formation. Similarities between axon initial segment (12) and node of Ranvier development suggest that ankyrin-G-dependent targeting of ion channels, and membrane-skeleton components might be a shared feature of these excitable membrane domains. Although our results demonstrate that intact NCP-1-mediated axoglial contacts are not necessary for nodal protein clustering, they do not rule out a role for NCP1-independent axoglial contact in development or maintenance of nodes.
Acknowledgments
We thank Dr. M. Komada, Department of Biological Sciences, Tokyo Institute of Technology, for the anti-beta IV spectrin polyclonal antibody; we also thank Dr. M. Bhat, Mt. Sinai School of Medicine, for the anti-NCP1 antibody. Funding for this work was provided by the Howard Hughes Medical Institute.
Abbreviations
- CASPR
contactin-associated protein
- NCP1
neurexin/CASPR/paranodin
- L1 CAM
L1 family of cell adhesion molecules
- AnkG
ankyrin-G
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel J M, Zhang J P, Philbrick W, Slepnev V, Ort T, Solimena M. J Cell Biol. 2000;151:985–1001. doi: 10.1083/jcb.151.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kordeli E, Lambert S, Bennett V. J Biol Chem. 1995;270:2352–2359. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 3.Zhou D, Lambert S, Malen P L, Carpenter S, Boland L M, Bennett V. J Cell Biol. 1998;143:1295–1304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert S, Davis J Q, Bennett V. J Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ratcliffe C F, Westenbroek R E, Curtis R, Catterall W A. J Cell Biol. 2001;154:427–434. doi: 10.1083/jcb.200102086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasband M N, Peles E, Trimmer J S, Levinson R, Lux S E, Shrager P. J Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasband M N, Schrager P. J Physiol (London) 2000;525:63–73. doi: 10.1111/j.1469-7793.2000.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boiko T, Rasband M N, Levinson S R, Caldwell J H, Mandel G, Trimmer J S, Matthews G. Neuron. 2001;30:91–104. doi: 10.1016/s0896-6273(01)00265-3. [DOI] [PubMed] [Google Scholar]
- 9.Pedraza L, Huang J K, Colman D R. Neuron. 2001;30:335–344. doi: 10.1016/s0896-6273(01)00306-3. [DOI] [PubMed] [Google Scholar]
- 10.Moorthy S, Chen L, Bennett V. J Cell Biol. 2000;149:915–930. doi: 10.1083/jcb.149.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett V, Baines A J. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins S M, Bennett V. J Cell Biol. 2001;155:739–746. doi: 10.1083/jcb.200109026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis J Q, McLaughlin T, Bennett V. J Cell Biol. 1993;121:121–133. doi: 10.1083/jcb.121.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Bennett V. J Cell Biol. 1998;142:1571–1581. doi: 10.1083/jcb.142.6.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hortsch M. Mol Cell Neurosci. 2000;15:1–10. doi: 10.1006/mcne.1999.0809. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins S M, Kizhatil K, Kramarcy N R, Sen A, Sealock R, Bennett V. J Cell Sci. 2001;114:3823–3835. doi: 10.1242/jcs.114.21.3823. [DOI] [PubMed] [Google Scholar]
- 17.Einheber S, Zanazzi G, Ching W, Scherer S, Milner T A, Peles E, Salzer J L. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rios J C, Melendez-Vasquez C V, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer J L. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tait S, Gunn-Moore F, Collinson J M, Huang J, Lubetzki C, Pedraza L, Sherman D L, Colman D R, Brophy P J. J Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhat M A, Rios J C, Lu Y, Garcia-Fresco G P, Ching W, St. Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, et al. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X, Bennett V. J Biol Chem. 1996;271:31391–31398. doi: 10.1074/jbc.271.49.31391. [DOI] [PubMed] [Google Scholar]
- 22.Davis J Q, Lambert S, Bennett V. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miguel Vela J, Gonzalez B, Castellano B. Brain Res Rev. 1998;26:29–42. doi: 10.1016/s0165-0173(97)00055-6. [DOI] [PubMed] [Google Scholar]
- 24.Baumann N, Pham-Dinh D. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan M R, Meyer-Franke A, Lambert S, Bennett V, Duncan I D, Levinson S R, Barres B A. Nature (London) 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan M R, Cho M-H, Ullian E M, Isom L L, Levinson S R, Barres B A. Neuron. 2001;30:105–119. doi: 10.1016/s0896-6273(01)00266-5. [DOI] [PubMed] [Google Scholar]
- 27.Waxman S G, Foster R E. Proc R Soc London Ser B. 1980;209:441–446. doi: 10.1098/rspb.1980.0105. [DOI] [PubMed] [Google Scholar]
- 28.Waxman S G, Anderson M J. Cell Tissue Res. 1980;208:343–352. doi: 10.1007/BF00233869. [DOI] [PubMed] [Google Scholar]
- 29.Boyle M E, Berglund E O, Murai K K, Weber L, Peles E, Ranscht B. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 30.Melendez-Vasquez C V, Rios J C, Zanazzi G, Lambert S, Bretscher A, Salzer J L. Proc Natl Acad Sci USA. 2001;98:1235–1240. doi: 10.1073/pnas.98.3.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ching W, Zanazzi G, Levinson S R, Salzer J L. J Neurocytol. 1999;28:295–301. doi: 10.1023/a:1007053411667. [DOI] [PubMed] [Google Scholar]