ABSTRACT
Introduction:
Myocardial stunning, a temporary reduction in cardiac contractility, is well-recognized in chronic hemodialysis patients but less studied in critically ill patients undergoing continuous renal replacement therapy (CRRT).
Methods:
A prospective observational 11-month study included 38 intensive care unit (ICU) patients with acute kidney injury at Mohammed V University Hospital. Myocardial stunning was evaluated using echocardiographic parameters such as global longitudinal strain and left ventricular ejection fraction.
Results:
Myocardial stunning was observed in 60.5% of patients. It was strongly associated with increased vasopressor requirements, longer ICU stays, and higher mortality (47.8% vs. 6.7%).
Conclusion:
The high incidence of myocardial stunning in critically ill patients undergoing CRRT highlights the need for vigilant cardiac monitoring and targeted interventions to improve patient outcomes.
Keywords: Acute kidney injury, echocardiography, mortality, myocardial stunning, renal replacement therapy
INTRODUCTION
Acute kidney injury (AKI) is a common and serious complication in critically ill patients, leading to significant morbidity and mortality and often requiring renal replacement therapy (RRT).[1,2,3] Continuous RRT (CRRT) is typically preferred for patients with unstable hemodynamics because it allows for gradual fluid and solute removal, which is intended to reduce cardiac stress.[4] However, this potential benefit has not led to improved clinical outcomes, as mortality and morbidity rates between intermittent and continuous renal support therapies remain comparable.[5]
Myocardial stunning is defined as a temporary and reversible impairment of cardiac contractility that occurs despite the absence of irreversible myocardial damage and the restoration of normal coronary blood flow.[6] This phenomenon has been extensively documented in chronic hemodialysis (HD) patients, where recurrent episodes of myocardial stunning contribute to progressive cardiac dysfunction and increased mortality.[7,8,9] The underlying mechanisms of myocardial stunning include ischemia-reperfusion injury, hemodynamic instability, and intradialytic hypotension, all of which can precipitate transient myocardial ischemia.[6,7,8]
In critically ill patients with AKI, the initiation of CRRT is associated with significant hemodynamic challenges. Although CRRT is designed to offer more stable hemodynamics compared to intermittent modalities,[10] the prevalence and impact of myocardial stunning in this setting remain inadequately studied.
The objective of this study is to investigate the prevalence of myocardial stunning in critically ill patients undergoing CRRT, to explore its association with various clinical parameters, and to assess its impact on patient outcomes. By understanding the prevalence and determinants of myocardial stunning in this population, we aim to identify potential strategies for mitigating this risk and improving the overall management of patients requiring CRRT in the intensive care unit (ICU).
METHODS
Study design and setting
This study involved a retrospective data collection but was designed prospectively to assess specific parameters over 11 months, from August 2023 to June 2024. It was conducted in the Department of Anesthesia and Intensive Care at Mohammed V University Hospital in Oujda, Morocco. All procedures were carried out in accordance with institutional ethical standards.
Patient population
The study included critically ill patients who were admitted to the ICU and required CRRT due to AKI. Patients were excluded if they had preexisting end-stage renal disease on maintenance HD, known cardiomyopathy with reduced left ventricular ejection fraction (LVEF), significant valvular heart disease, cardiac arrhythmias, or poor echogenicity. In addition, patients without a known history of cardiomyopathy but who demonstrated reduced LVEF on the initial echocardiography before the initiation of CRRT were also excluded. Patients under the age of 18 years were excluded as well.
Definition and inclusion criteria for acute kidney injury
AKI was defined according to the kidney disease: improving global outcomes criteria.[11] The decision to initiate CRRT was made by the attending ICU physician based on the presence of urgent dialysis indications, which included severe metabolic acidosis, hyperkalemia, nephrogenic pulmonary edema, uremic encephalopathy, or anuria lasting more than 12 h. Pre-CRRT bloodwork and dialysis indications are summarized in Table 1.
Table 1.
Baseline patient characteristics, admission diagnoses, and clinical outcomes
| Patient ID | Age | Sex | BMI | Baseline comorbidities | Admission diagnosis | SOFA | Survival outcome |
|---|---|---|---|---|---|---|---|
| 1 | 24 | Female | 23,1 | Diabetes | Septic shock | 5 | Alive, off RRT |
| 2 | 19 | Female | 22,1 | diabetes | Septic shock | 9 | Alive, off RRT |
| 3 | 21 | Male | 23,8 | Diabetes | Septic shock | 7 | Alive, off RRT |
| 4 | 35 | Female | 29,4 | Hypertension, diabetes, dyslipidaemia | Septic shock | 11 | Alive, on RRT |
| 5 | 20 | Male | 26,5 | diabetes | Septic shock | 10 | Alive, off RRT |
| 6 | 64 | Female | 31,5 | Hypertension, diabetes, dyslipidaemia | Septic shock | 13 | Died in the ICU |
| 7 | 84 | Male | 28,4 | Hypertension, diabetes, dyslipidaemia, stroke, chronic kidney disease | Septic shock | 12 | Died in the ICU |
| 8 | 77 | Male | 23,1 | diabetes | Haemorrhagic shock | 11 | Died in the ICU |
| 9 | 62 | Male | 32,4 | Hypertension, diabetes, dyslipidaemia, stroke, chronic kidney disease | Septic shock | 12 | Alive, on RRT |
| 10 | 85 | Male | 26,8 | Hypertension, diabetes, dyslipidaemia, stroke | Haemorrhagic stroke | 6 | Died in the ICU |
| 11 | 54 | Female | 31,1 | Hypertension, dyslipidaemia | toxicoderma | 9 | Alive, off RRT |
| 12 | 30 | Female | 21,5 | Chronic kidney disease | Septic shock | 10 | Died in the ICU |
| 13 | 55 | Male | 29,4 | Diabetes, dyslipidaemia | Septic shock | 8 | Alive, on RRT |
| 14 | 50 | Male | 35,1 | Hypertension, diabetes, dyslipidaemia | Septic shock | 12 | Died in the ICU |
| 15 | 75 | Male | 26,5 | Hypertension ; diabetes, dyslipidaemia, chronic kidney disease, COPD | Septic shock | 10 | Alive, off RRT |
| 16 | 19 | Female | 21,5 | diabetes | Septic shock | 8 | Alive, off RRT |
| 17 | 25 | Female | 22,3 | Diabetes | Septic shock | 7 | Alive, off RRT |
| 18 | 35 | Female | 28,4 | Hypertension, dyslipidaemia | eclampsia | 6 | Alive, on RRT |
| 19 | 26 | Male | 23,5 | No comorbidities | poisoning | 12 | Alive, on RRT |
| 20 | 84 | Male | 31,5 | Hypertension, diabetes, dyslipidaemia, chronic kidney disease | Septic shock | 14 | Alive, on RRT |
| 21 | 75 | Female | 32,5 | Hypertension, diabetes, dyslipidaemia, stroke, chronic kidney disease | Septic shock | 14 | Died in the ICU |
| 22 | 20 | Female | 26,4 | diabetes | Septic shock | 6 | Alive, off RRT |
| 23 | 19 | Male | 21,6 | Diabetes, chronic kidney disease | Septic shock | 12 | Alive, on RRT |
| 24 | 91 | Male | 23,1 | Hypertension, diabetes, stroke, dyslipidaemia, chronic kidney disease | Haemorrhage stroke | 8 | Alive, on RRT |
| 25 | 50 | Male | 29,8 | Hypertension, diabetes, dyslipidaemia | toxicoderma | 10 | Alive, off RRT |
| 26 | 35 | Male | 24,5 | No comorbidities | Viper envenomation | 12 | Died in the ICU |
| 27 | 29 | Female | 23,5 | Hypertension | Acute fatty liver of pregnancy | 10 | Alive, on RRT |
| 28 | 65 | Male | 32,5 | Hypertension, diabetes, dyslipidaemia, stroke | Septic shock | 12 | Alive, on RRT |
| 29 | 80 | Female | 28,4 | Hypertension, diabetes, dyslipidaemia, chronic kidney disease, COPD | Septic shock | 14 | Died in the ICU |
| 30 | 24 | Male | 23,5 | No comorbidities | poisoning | 11 | Alive, off RRT |
| 31 | 45 | Female | 32,4 | Hypertension, diabetes, dyslipidaemia, chronic kidney disease | Septic shock | 9 | Alive, off RRT |
| 32 | 29 | Female | 23,7 | No comorbidities | poisoning | 12 | Died in the ICU |
| 33 | 66 | Male | 28,4 | Diabetes, dyslipidaemia, chronic kidney disease, COPD | Septic shock | 11 | Alive, on RRT |
| 34 | 30 | Male | 24,1 | No comorbidities | Viper envenomation | 9 | Alive, off RRT |
| 35 | 73 | Male | 31,4 | Hypertension, diabetes, dyslipidaemia, stroke, chronic kidney disease | Haemorrhage stroke | 6 | Died in the ICU |
| 36 | 28 | Female | 23,2 | No comorbidities | Severe pre-eclampsia | 8 | Alive, off RRT |
| 37 | 43 | Male | 29,1 | Hypertension, diabetes, dyslipidaemia, chronic kidney disease | Septic shock | 6 | Alive, on RRT |
| 38 | 79 | Male | 26,4 | Hypertension, diabetes, dyslipidaemia, stroke, chronic kidney disease | Haemorrhage stroke | 12 | Died in the ICU |
Data collection
Clinical parameters, dialysis treatment details, and laboratory tests were obtained before and after dialysis. Baseline demographic details, comorbidities, and renal function before the AKI episode were obtained from patients’ medical charts. Hemodynamic and echocardiographic parameters were recorded at three-time points: immediately before the initiation of CRRT, at 8 h after initiation, and at 24 h.
Echocardiographic assessments focused on the left ventricular function, with particular attention to segmental longitudinal strain, global longitudinal strain (GLS), and LVEF. Myocardial stunning was indicated by changes in segmental longitudinal strain with or without accompanying changes in GLS and LVEF. Standardized protocols were employed to ensure consistency in measurements across all time points.
Study population
A total of 53 patients were initially enrolled in the study. However, nine patients died before the 24-h echocardiographic assessment, and 6 had poor echogenicity, leaving 38 patients in the final analysis [Figure 1]. The primary outcomes of interest were the incidence of myocardial stunning as indicated by changes in segmental longitudinal strain or in GLS and LVEF and the association of myocardial stunning with clinical outcomes such as ICU mortality and length of stay.
Figure 1.

Patient flow diagram
Continuous renal replacement therapy details
All patients included in the study were treated with continuous venovenous hemodiafiltration. The dialysis procedures were conducted using either Nikkiso or Multifiltrate dialysis machines. The ultrafiltration volume was carefully adjusted based on the specific dialysis indication, the patient’s hemodynamic status, and overall fluid balance. Blood flow rates were maintained between 100 and 160 mL/min, and the dialysate flow rate was set at 25 mL/kg/h to ensure adequate solute clearance and fluid removal while minimizing hemodynamic instability.[12] Anticoagulation was administered using either unfractionated heparin or systemic anticoagulation with low molecular weight heparin. The prescribed settings aimed to balance the need for effective dialysis with the prevention of additional cardiovascular stress, consistent with best practices in CRRT management.
Cardiac imaging
Cardiac imaging was performed using the Philips EPIQ Elite ultrasound system, with a transthoracic echography (TTE) probe, which is equipped with advanced speckle-tracking echocardiography capabilities. This high-resolution imaging technology allowed for precise assessment of left ventricle (LV) function, focusing on both global and segmental myocardial performance. Several operators performed different TTE examinations between patients and even within the same patient.
Standard gray-scale echocardiography was used to acquire apical two-, three-, and four-chamber views of the left ventricle (LV). The images were captured at three key time points: Before the initiation of CRRT (baseline), 8 h after CRRT initiation (H8), and 24 h after CRRT initiation (H24). The frame rate for image acquisition was maintained between 60 and 90 frames per second to ensure adequate temporal resolution for speckle-tracking analysis.
LVEF was calculated using the biplane Simpson’s method, a widely accepted technique for estimating global systolic function from apical two- and four-chamber images.[13] The threshold used to specify reduced EF in this study was <50%, as per established echocardiographic criteria for left ventricular systolic dysfunction.[14] GLS was calculated by averaging the peak systolic strains from all myocardial segments, providing a comprehensive assessment of global myocardial function.[15] Normal GLS values typically range between 16% and 19%, with less negative values indicating reduced myocardial contractility.[16]
Myocardial stunning was defined as a decrease in segmental longitudinal strain by more than 20% from baseline values, in accordance with the guidelines established by the American Society of Echocardiography (ASE).[14] Segmental longitudinal strain was specifically monitored to capture early and regional myocardial dysfunction that might not be reflected in global metrics such as GLS or LVEF.
Statistical analysis
Data were analyzed using SPSS (IBM Corp., Armonk United States). Continuous variables were summarized as mean ± standard deviation (SD) or median (interquartile range [IQR]), and categorical variables as frequencies. Myocardial stunning was assessed with paired tests comparing strain values at baseline, H8, and H24. Logistic regression analyzed the association with clinical outcomes, adjusting for confounders. Correlations were examined using Spearman’s or Pearson’s coefficients. Kaplan–Meier and Cox models were used for survival analysis. Statistical significance was set at P < 0.05.
RESULTS
Baseline characteristics and patient details
The study included 38 critically ill patients, with a mean age of 48.03 years (SD: 23.92), ranging from 19 to 91 years. The cohort was predominantly male, comprising 22 males and 16 females. The average body mass index was 26.91 kg/m² (SD: 3.90). Hypertension (HTN) was prevalent in 52.6% of the patients, while 73.7% had a history of diabetes mellitus. Other common comorbidities included dyslipidemia in 55.3% of the patients and a history of cerebrovascular accident in 23.7%. The most common reason for admission was septic shock, accounting for 60.52% of cases. The median sequential organ failure assessment (SOFA) score at admission was 10.0, indicating severe organ dysfunction in this population. The median partial pressure of carbon dioxid (PCO2) gap at baseline was 4.0 mmHg (SD: 2.77, IQR: 5.25). The median creatinine level at admission was 91.1 mg/L, and the median urea level was 4.04 mg/L. The primary indications for dialysis included severe metabolic acidosis, along with combinations of hyperkalemia and uremic encephalopathy. The CRRT was initiated within a median of 3 days, and the median ultrafiltration rate was at 1 ml/kg/h. These baseline characteristics reflect a population with a high burden of comorbidities, particularly cardiovascular and metabolic disorders, which could influence their clinical outcomes during CRRT and the incidence of myocardial stunning. Baseline patient characteristics, admission diagnoses, and clinical outcomes are summarized in Table 2. Pre-CRRT bloodwork and dialysis indications are summarized in Table 1.
Table 2.
pre-CRRT bloodwork and dialysis indication
| ID | Hb | CRP | PCT | Creat | Urea | pH | HCO3 | Lactate | PCO2 gap | Diuresis | K+ | Na+ | Dialysis indication |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 12,5 | 250 | 3,5 | 22 | 0,98 | 6,85 | 3,5 | 5,6 | 2 | 0,6 | 4,5 | 142 | Severe metabolic acidosis |
| 2 | 11,3 | 150 | 4,9 | 49 | 1,84 | 6,79 | 2,1 | 6,4 | 3 | 0,54 | 3,6 | 135 | Severe metabolic acidosis |
| 3 | 16,4 | 109 | 4,5 | 74 | 1,9 | 6,81 | 2,6 | 4,6 | 2 | 0,41 | 5,1 | 132 | Severe metabolic acidosis |
| 4 | 10,2 | 326 | 6,4 | 69,8 | 2,3 | 6,75 | 2,9 | 8,12 | 4 | 0,3 | 5,9 | 136 | Severe metabolic acidosis |
| 5 | 15,4 | 239 | 10,2 | 46,3 | 1,79 | 6,94 | 4,5 | 4,12 | 2 | 0,58 | 4,1 | 146 | Severe metabolic acidosis |
| 6 | 8,5 | 399 | 23,1 | 88,1 | 3,8 | 7,12 | 9,5 | 10,4 | 4 | 0,1 | 4,9 | 149 | Severe metabolic acidosis + Acute pulmonary oedema |
| 7 | 9,5 | 450 | 9,98 | 64,1 | 3,32 | 7,21 | 11,2 | 3,1 | 8 | 0,41 | 7,1 | 126 | hyperkalaemia + Acute pulmonary oedema |
| 8 | 5,4 | 85 | 0,14 | 49,5 | 1,54 | 6,89 | 6,1 | 5,8 | 2 | 0,2 | 6,9 | 131 | Severe metabolic acidosis + hyperkalaemia |
| 9 | 9,5 | 462 | 154 | 112 | 4,8 | 7,04 | 8,7 | 10,74 | 7,5 | 0,34 | 5,8 | 129 | Severe metabolic acidosis + Acute pulmonary oedema |
| 10 | 13,5 | 143 | 2,5 | 115 | 5,2 | 7,14 | 10,9 | 4,71 | 2 | 0,45 | 7,4 | 122 | Severe metabolic acidosis + hyperkalaemia |
| 11 | 11,4 | 255 | 4,15 | 98 | 4,1 | 7,11 | 11,4 | 6,21 | 4 | 0,22 | 6,8 | 125 | Severe metabolic acidosis + hyperkalaemia + Uremic encephalopathy |
| 12 | 9,4 | 458 | 5,6 | 79 | 3,84 | 7,13 | 10,4 | 5,91 | 4 | 0,12 | 5,9 | 120 | Severe metabolic acidosis + hyperkalaemia + Uremic encephalopathy |
| 13 | 10,5 | 384 | 25,4 | 91 | 4,1 | 7,21 | 11,5 | 6,7 | 8 | 0,1 | 6,1 | 110 | Acute pulmonary oedema + hyperkalaemia + Uremic encephalopathy |
| 14 | 10,5 | 425 | 106 | 108 | 4,65 | 7,14 | 10,4 | 7,14 | 3 | 0,25 | 6,2 | 120 | Acute pulmonary oedema + hyperkalaemia + Uremic encephalopathy |
| 15 | 9,5 | 520 | 185,1 | 122 | 5,02 | 7,09 | 9,5 | 8,41 | 7 | 0,21 | 3,54 | 124 | Severe metabolic acidosis + Acute pulmonary oedema+ Uremic encephalopathy |
| 16 | 12,5 | 266 | 16,5 | 83,4 | 3,41 | 6,85 | 2,1 | 5,55 | 1 | 0,45 | 4,5 | 134 | Severe metabolic acidosis + Uremic encephalopathy |
| 17 | 13,5 | 356 | 18,4 | 76,9 | 3,12 | 6,81 | 2,2 | 6,8 | 2 | 0,38 | 4,2 | 134 | Severe metabolic acidosis + Uremic encephalopathy |
| 18 | 9,54 | 125 | 3,2 | 95,4 | 3,65 | 7,06 | 4,6 | 9,8 | 4 | 0,41 | 5,78 | 124 | Severe metabolic acidosis + Acute pulmonary oedema |
| 19 | 13,5 | 250 | 2,5 | 95,4 | 4,58 | 6,95 | 3,2 | 10,5 | 8 | 0,12 | 7,8 | 132 | Severe metabolic acidosis + hyperkalaemia + Uremic encephalopathy |
| 20 | 9,84 | 429 | 98,5 | 105,4 | 5,6 | 7,11 | 9,5 | 11,2 | 8 | 0,2 | 6,34 | 120 | Severe metabolic acidosis + Acute pulmonary oedema+ Uremic encephalopathy |
| 21 | 10,2 | 580 | 124,1 | 125,6 | 5,9 | 7,02 | 8,4 | 12,4 | 10 | 0,1 | 7,1 | 121 | Severe metabolic acidosis + hyperkalaemia + Uremic encephalopathy |
| 22 | 14,5 | 368 | 48,1 | 56 | 3,1 | 6,87 | 2,1 | 6,7 | 2 | 0,55 | 3,4 | 134 | Severe metabolic acidosis |
| 23 | 7,5 | 600 | 83,1 | 111 | 5,12 | 6,98 | 4,1 | 10,2 | 8 | 0,2 | 6,1 | 124 | Severe metabolic acidosis + Acute pulmonary oedema+ Uremic encephalopathy + hyperkalaemia |
| 24 | 8,4 | 85 | 1,25 | 98,5 | 4,52 | 7,1 | 6,4 | 5,84 | 3 | 0,4 | 6,8 | 120 | Severe metabolic acidosis + hyperkalaemia |
| 25 | 10,8 | 495 | 50,7 | 90,4 | 4,1 | 7,12 | 9,5 | 6,75 | 4 | 0,45 | 6,1 | 149 | Severe metabolic acidosis + hyperkalaemia |
| 26 | 14,5 | 380 | 6,4 | 85,4 | 3,98 | 6,84 | 4,2 | 10,5 | 3 | 0,32 | 7,6 | 148 | Severe metabolic acidosis + hyperkalaemia |
| 27 | 9,1 | 125 | 2,5 | 83,9 | 4,12 | 7,26 | 11,2 | 8,7 | 4 | 0,23 | 6,3 | 120 | Uremic encephalopathy + hyperkalaemia |
| 28 | 10,5 | 680 | 125,4 | 125 | 5,6 | 7,2 | 10,3 | 9,5 | 10 | 0,12 | 5,9 | 119 | Acute pulmonary oedema+ Uremic encephalopathy + hyperkalaemia |
| 29 | 9,2 | 550 | 150 | 110 | 5,1 | 7,1 | 9,2 | 10,2 | 10 | 0,4 | 6,8 | 121 | Severe metabolic acidosis + Acute pulmonary oedema+ Uremic encephalopathy + hyperkalaemia |
| 30 | 14,5 | 120 | 1,5 | 80,2 | 3,5 | 6,8 | 3,5 | 9,5 | 9,5 | 0,24 | 7 | 145 | Severe metabolic acidosis + hyperkalaemia |
| 31 | 10,5 | 391 | 50,6 | 98,4 | 4,9 | 6,95 | 4,1 | 10,2 | 4,2 | 0,34 | 4,5 | 121 | Severe metabolic acidosis + Uremic encephalopathy |
| 32 | 11,5 | 50 | 1,2 | 57,6 | 2,86 | 6,83 | 3,1 | 10,2 | 2 | 0,29 | 7,4 | 146 | Severe metabolic acidosis + hyperkalaemia |
| 33 | 9,3 | 425 | 69,8 | 109 | 5,1 | 7,13 | 6,1 | 7,9 | 3 | 0,4 | 5,9 | 130 | Severe metabolic acidosis + Uremic encephalopathy |
| 34 | 14,5 | 80 | 2,1 | 87,1 | 2,6 | 6,9 | 3,2 | 6,8 | 3,2 | 0,35 | 7,1 | 149 | Severe metabolic acidosis + hyperkalaemia |
| 35 | 13,2 | 95 | 2,98 | 103 | 4,88 | 7,21 | 10,1 | 4,2 | 4 | 0,2 | 6,4 | 120 | Uremic encephalopathy + hyperkalaemia |
| 36 | 8,5 | 49 | 0,95 | 92,1 | 3,4 | 7,22 | 10,9 | 7,8 | 2,5 | 0,3 | 7,4 | 122 | Uremic encephalopathy + hyperkalaemia |
| 37 | 10,1 | 319 | 48,1 | 91,2 | 3,87 | 7,12 | 9,2 | 8,1 | 5 | 0,4 | 6,7 | 118 | Uremic encephalopathy + hyperkalaemia |
| 38 | 8,2 | 409 | 98,1 | 107 | 5,3 | 7,14 | 9,8 | 11,5 | 8,2 | 0,41 | 6 | 121 | Acute pulmonary oedema+ Uremic encephalopathy + hyperkalaemia |
Echocardiographic findings
Echocardiographic assessments were conducted at three key time points: Baseline (H0), 8 h after CRRT initiation (H8), and 24 h after CRRT initiation (H24). The median LVEF at H0 was 57.0% (SD: 3.16%), which progressively decreased to 55.0% (SD: 4.02%) at H8 and further to 54.0% (SD: 5.64%) at H24. The decrease in LVEF was statistically significant (P < 0.001) across all time points, indicating a progressive decline in global systolic function during CRRT. This is summarized in Figure 2.
Figure 2.

Evolution of the left ventricular ejection fraction at baseline, H8 and H24
Similarly, the median GLS at H0 was −17.6% (SD: 0.86%), indicating relatively preserved myocardial function at baseline. However, GLS showed a significant decline to −17.15% (SD: 1.13%) at H8 and further to −16.65% (SD: 1.67%) at H24 (P < 0.001). This decline in GLS over time was also statistically significant, reflecting a worsening of myocardial strain during CRRT. These findings are summarized in Figure 3.
Figure 3.

Evolution of the global longitudinal strain at baseline, H8 and H24
The number of myocardial segments affected by stunning increased noticeably over time, with a median of 0 segments at H0, increasing to 5 segments at H8 (SD: 2.05), and remaining at 5 segments at H24 (SD: 3.78). These findings underscore the progressive impact of CRRT on myocardial function, marked by deterioration in both global and regional myocardial performance. These findings are summarized in Figure 4.
Figure 4.

Evolution of the number of segments affected at baseline, H8 and H24
Hemodynamic parameters
Myocardial stunning was observed in 23 patients, representing 60.5% of the cohort. The median mean arterial pressure (MAP) at initiation was 70.0 mmHg, with no significant change observed at 24 h (median MAP remained 70.0 mmHg; P = 0.178). However, there was a significant increase in the median dose of vasopressor agents from 0.3 μg/kg/min at initiation to 0.5 μg/kg/min at 24 h (P < 0.001). In addition, a strong positive correlation was found between the increase in vasopressor dose and the incidence of myocardial stunning (r = 0.77, P < 0.001). No significant correlation was observed between changes in MAP and myocardial stunning (r = −0.25, P = 0.132).
Impact of myocardial stunning: Left ventricular ejection fraction, global longitudinal strain, and segmental longitudinal strain changes on mortality and length of stay
The mortality rate was 31.6%. The median length of stay in the ICU was 7.5 days, and the median hospital stay was 10 days.
The study findings reveal a significant association between changes in LVEF, GLS, and the occurrence of myocardial stunning during CRRT with key patient outcomes, including mortality and length of stay in the ICU and hospital.
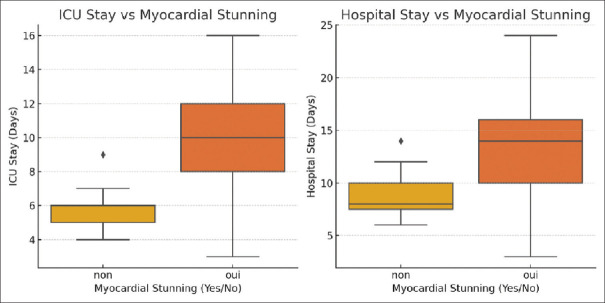
Myocardial stunning, characterized by temporary but significant impairment of cardiac contractility during CRRT, with or without changes in the LVEF and the GLS, was identified in 60.5% of the patients and had profound effects on outcomes. Myocardial stunning was strongly correlated with longer ICU stays (r = 0.587, P < 0.001) and extended hospital stays (r = 0.445, P = 0.005), highlighting its critical impact on patient prognosis and the need for targeted interventions to manage these high-risk patients. Patients with myocardial stunning had a mortality rate of 47.8%, compared to just 6.7% in those without stunning. These patients also had significantly longer ICU stays (median of 10 days) and hospital stays (median of 14 days) and were more likely to require continued renal support post-CRRT (43.5% vs. 13.3%). These findings are summarized in Figure 5.
Figure 5.
The correlation between myocardial stunning and the intensive care unit and hospital stay. ICU: Intensive care unit
Survival analysis using Kaplan–Meier curves revealed significant differences in survival probabilities between patients with and without myocardial stunning. The survival probability was significantly lower in patients with myocardial stunning, as indicated by a log-rank test (P < 0.001).
Potentiel factors predictive of myocardial stunning
In this study, we investigated the relationship between various clinical factors and the incidence of myocardial stunning in critically ill patients undergoing CRRT. The prevalence of myocardial stunning in our cohort, particularly in those with a history of stroke (P = 0.017) and chronic kidney disease not requiring HD (P = 0.037), underscores the vulnerability of these patients to cardiac injury during CRRT.
A significant positive correlation was found between the increase in vasopressor dose at 24 h and the incidence of myocardial stunning (r = 0.77, P < 0.001). This indicates that higher vasopressor requirements were strongly associated with the likelihood of experiencing myocardial stunning during CRRT. This is summarized in Figure 6.
Figure 6.

The difference of vasopressor dose at H24 between patients who did and did not experienced myocardial stunning, with the correlation between the vasopressor dosage and the incidence of myocardial stunning
In this study, a PCO2 gap >6 mmHg, which suggests cardiac involvement in shock, was found to be moderately correlated with myocardial stunning (r = 0.43, P = 0.007) and increased segmental involvement (r = 0.49, P = 0.002). These results indicate that an elevated PCO2 gap may be an important marker of cardiac complications, including myocardial stunning and segmental myocardial involvement, in patients undergoing continuous HD in the ICU. The moderate correlation with the PCO2 gap further emphasizes the role of cardiac involvement in the pathophysiology of myocardial stunning.
However, no significant correlation was found between changes in MAP and myocardial stunning (r = −0.25, P = 0.132).
The significant association between septic shock and myocardial stunning (P = 0.019) aligns with the well-documented impact of sepsis on cardiac function. Septic shock induces a hyperinflammatory state, leading to myocardial depression and an increased risk of ischemic injury, which likely contributes to the high incidence of myocardial stunning observed in our study. The moderate positive correlation between SOFA scores and myocardial stunning (correlation coefficient = 0.51, P = 0.036) further emphasizes the role of systemic inflammation and multiorgan dysfunction in exacerbating cardiac vulnerability during CRRT.
Interestingly, while diabetes is a well-known risk factor for cardiovascular disease, it did not emerge as a significant predictor of myocardial stunning in our cohort (P = 0.677). This finding contrasts with studies in chronic dialysis populations, where diabetes has been linked to increased susceptibility to dialysis-induced cardiac injury. The absence of a significant correlation in our study may reflect the acute nature of the patient population or the specific pathophysiological mechanisms at play in critically ill patients undergoing CRRT.
HTN, pulmonary disease, and smoking/alcohol use also did not show significant correlations with myocardial stunning, suggesting that these factors, while important in chronic cardiovascular disease, may not independently contribute to the risk of stunning in the acute setting of CRRT. The borderline significance of dyslipidemia (P = 0.063) suggests a potential role in predisposing patients to myocardial stunning, warranting further investigation.
DISCUSSION
The results of our study highlight the intricate relationship between CRRT and myocardial stunning in critically ill patients. This phenomenon, observed in a significant portion of our cohort, aligns with the growing body of evidence suggesting that both intermittent and CRRTs can precipitate acute cardiac injury. Our findings, while aligned with previous research on myocardial stunning during RRT, should not be overgeneralized. The observed association with clinical outcomes is significant but not definitive, given the constraints of our study design.
Mechanisms of myocardial stunning
Myocardial stunning, a reversible reduction in myocardial contractility, has been extensively documented in chronic HD patients and is now recognized as a critical complication in AKI patients undergoing RRT.[10,17] Previous studies, such as those by Burton et al., have demonstrated that intermittent HD can lead to repeated episodes of myocardial stunning, contributing to progressive left ventricular dysfunction and increased mortality.[8,10] The repeated exposure to cardiac stunning, particularly in patients undergoing HD three to five times weekly, can lead to a progressive and irreversible decline in LV systolic function,[8] which correlates with an increased mortality risk.[9] These findings are mirrored in our study, where a significant number of patients developed myocardial stunning within the first 24 h of CRRT initiation, despite the modality’s reputation for being more hemodynamically stable.
This phenomenon occurs despite preserved coronary blood flow, as indicated by cardiac magnetic resonance imaging and positron emission tomograph scans,[7,18] suggesting that the injury is driven by microcirculatory disruptions rather than global hemodynamic changes.[19] Interventions such as dialysate cooling have demonstrated potential in reducing RRT-induced cardiac stunning, and these strategies are currently being explored further to enhance clinical outcomes.[20,21]
The pathophysiology of myocardial stunning in the setting of CRRT is complex and likely multifactorial. One of the key mechanisms is the ischemia-reperfusion injury that occurs during hemodynamic fluctuations,[6,7] even in the context of continuous modalities like CRRT. While CRRT is designed to provide more stable fluid and solute removal compared to intermittent HD,[10] our findings suggest that it does not entirely prevent the hemodynamic disturbances that can precipitate myocardial stunning.
The significant increase in vasopressor doses observed in patients with myocardial stunning points to another potential mechanism: The increased myocardial oxygen demand coupled with coronary vasoconstriction induced by vasopressors.[22,23] This scenario creates a mismatch between oxygen supply and demand, leading to transient myocardial ischemia and subsequent stunning. Furthermore, patients with higher SOFA scores, indicative of greater overall organ dysfunction, are likely more susceptible to the compounded effects of systemic inflammation, oxidative stress, and metabolic disturbances, all of which contribute to myocardial injury.[24]
The role of systemic inflammation, particularly in the context of septic shock, cannot be overlooked. Septic shock is characterized by profound vasodilation, myocardial depression, and a hyperinflammatory state, all of which may exacerbate the myocardial stress experienced during CRRT.[25,26] The moderate correlation between the PCO2 gap and myocardial stunning observed in this study further suggests that patients with significant cardiac involvement in shock are at increased risk of myocardial dysfunction.
However, we did not assess the progression of myocardial function outside of RRT, leaving the possibility that the worsening of the underlying acute condition, such as sepsis for the majority of our patients, may have influenced our findings.
Previous studies in patients with septic shock have indicated that global left ventricular systolic function can decline within 24–48 h of ICU admission[27,28] which aligns with the timeframe of our current CRRT study. In our study, patients experienced regional LV dysfunction shortly after CRRT began, suggesting the myocardial injury was ischemic rather than septic in nature.
Comparative insights
Our study further supports the observations by Slessarev et al., who reported that CRRT, despite its lower ultrafiltration rates and improved hemodynamic stability, does not fully protect against myocardial stunning.[29]
In their study, the majority of patients developed myocardial stunning within the first 4 h of CRRT initiation despite stable hemodynamics, highlighting that even continuous modalities with more stable fluid removal rates do not completely mitigate the risk of cardiac injury.[29] Our study extends these findings by identifying specific predictors of stunning, such as vasopressor dose increases and SOFA scores, which could inform more targeted monitoring and intervention strategies.
Study limitations
While this study provides valuable insights into the incidence and impact of myocardial stunning in critically ill patients undergoing CRRT, several limitations should be acknowledged. First, the sample size of 38 patients, although sufficient to detect significant associations, limits the generalizability of the findings. A larger sample size would provide greater statistical power and allow for more robust subgroup analyses, particularly in identifying specific patient characteristics that may predispose them to myocardial stunning.
Second, the study was conducted in a single center, which may introduce selection bias and limit the applicability of the results to different populations or clinical settings. The specific practices and protocols in place at our institution may differ from those in other regions, potentially influencing the outcomes observed. Future studies should aim to address these gaps by including larger, more diverse populations and incorporating more comprehensive hemodynamic monitoring.
Furthermore, myocardial stunning is a transient and reversible impairment of cardiac contractility in the absence of coronary perfusion abnormalities. Moreover, coronary perfusion abnormalities were not systematically excluded in patients with myocardial stunning, which is particularly relevant given the high prevalence of cardiovascular comorbidities and potential coronary artery disease in our population. Future studies should include follow-up TTE assessments post-CRRT and incorporate diagnostic methods to evaluate and exclude coronary artery disease, thereby enhancing the understanding of myocardial stunning mechanisms in this patient group.
Significance and future directions
The findings of this study open several avenues for future research. One important area of investigation is the development of predictive models that can accurately identify patients at high risk of myocardial stunning during CRRT. Such models could incorporate a combination of clinical, hemodynamic, and echocardiographic parameters to guide early interventions aimed at mitigating cardiac injury.
Future studies should also explore the potential benefits of adjunctive therapies, such as dialysate cooling or remote ischemic preconditioning, in reducing the incidence of myocardial stunning.[20,21,30] Randomized controlled trials comparing different CRRT protocols or modalities could provide valuable insights into optimizing RRT to minimize cardiac stress.
Finally, it would be beneficial to investigate the long-term outcomes of patients who experience myocardial stunning during CRRT, including their cardiac function and quality of life after discharge. Understanding the lasting effects of myocardial stunning could inform strategies to improve post-ICU care and rehabilitation.
CONCLUSION
This study highlights the significant prevalence of myocardial stunning in critically ill patients undergoing CRRT and its association with adverse clinical outcomes, including increased mortality and prolonged ICU stays. Our findings underscore the importance of vigilant cardiac monitoring and proactive management in this high-risk population. The strong correlation between vasopressor dose increases and myocardial stunning suggests that optimizing hemodynamic support may play a critical role in reducing the incidence of this phenomenon.
Research quality and ethics statement
This study was approved by the Ethics Committee for Biomedical Research of Oujda (Approval #2023/CERBO/101; Approval date July 15, 2023). The authors followed applicable EQUATOR Network (https://www.equator-network.org/) guidelines during the conduct of this research project.
Conflicts of interest
There are no conflicts of interest.
Funding Statement
Nil.
REFERENCES
- 1.Bellomo R, Ronco C, Mehta RL, Asfar P, Boisramé-Helms J, Darmon M. Acute kidney injury in the ICU: From injury to recovery: Reports from the 5th Paris International Conference. Ann Intensive Care. 2017;7:49. doi: 10.1186/s13613-017-0260-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AY, Bellomo R, Cass A, Finfer S, Gattas D, Myburgh J. Health-related quality of life in survivors of acute kidney injury: The Prolonged outcomes study of the randomized evaluation of normal versus augmented level replacement therapy study outcomes. Nephrology (Carlton) 2015;20:492–8. doi: 10.1111/nep.12488. [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Lamontagne F, Joannidis M, Wald R. When to start renal replacement therapy in critically ill patients with acute kidney injury: Comment on AKIKI and ELAIN. Crit Care. 2016;20:245. doi: 10.1186/s13054-016-1424-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagshaw SM, Darmon M, Ostermann M, Finkelstein FO, Wald R, Tolwani AJ, et al. Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med. 2017;43:841–54. doi: 10.1007/s00134-017-4762-8. [DOI] [PubMed] [Google Scholar]
- 5.Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: A meta-analysis. Crit Care Med. 2008;36:610–7. doi: 10.1097/01.CCM.0B013E3181611F552. [DOI] [PubMed] [Google Scholar]
- 6.Heusch G. Myocardial stunning and hibernation revisited. Nat Rev Cardiol. 2021;18:522–36. doi: 10.1038/s41569-021-00506-7. [DOI] [PubMed] [Google Scholar]
- 7.Buchanan C, Mohammed A, Cox E, Köhler K, Canaud B, Taal MW. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol. 2017;28:1269–77. doi: 10.1681/ASN.2016060686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol. 2009;4:1925–31. doi: 10.2215/CJN.04470709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang SH, Crowley LE, Jefferies HJ, Eldehni MT, Odudu A, McIntyre CW. The impact of hemodialysis on segmental and global longitudinal myocardial strain. Can J Cardiol. 2014;30:1422–8. doi: 10.1016/j.cjca.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: Determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–20. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024;105:S117–314. doi: 10.1016/j.kint.2023.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Claure-Del Granado R, Clark WR. Continuous renal replacement therapy principles. Semin Dial. 2021;34:398–405. doi: 10.1111/sdi.12967. [DOI] [PubMed] [Google Scholar]
- 13.Otterstad JE. Measuring left ventricular volume and ejection fraction with the biplane Simpson's method. Heart. 2002;88:559–60. doi: 10.1136/heart.88.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 15.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 16.Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, et al. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: The HUNT study in Norway. Eur J Echocardiogr. 2010;11:176–83. doi: 10.1093/ejechocard/jep194. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud H, Forni LG, McIntyre CW, Selby NM. Myocardial stunning occurs during intermittent haemodialysis for acute kidney injury. Intensive Care Med. 2017;43:942–4. doi: 10.1007/s00134-017-4768-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasselaar JJ, Slart RH, Knip M, Pruim J, Tio RA, McIntyre CW, et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2009;24:604–10. doi: 10.1093/ndt/gfn501. [DOI] [PubMed] [Google Scholar]
- 19.Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin Dial. 2007;20:220–8. doi: 10.1111/j.1525-139X.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 20.Odudu A, Eldehni MT, McCann GP, McIntyre CW. Randomized controlled trial of individualized dialysate cooling for cardiac protection in hemodialysis patients. Clin J Am Soc Nephrol. 2015;10:1408–17. doi: 10.2215/CJN.00200115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson Health Research Institute. Major Cardiovascular and Other Patient-Important Outcomes with Personalized Dialysate Temperature (MY TEMP) [[Last accessed on 2018 Sep 07]]. Available from: https://clinicaltrials.gov/ct2/show/NCT02628366 .
- 22.Overgaard CB, Dzavík V. Inotropes and vasopressors: Review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118:1047–56. doi: 10.1161/CIRCULATIONAHA.107.728840. [DOI] [PubMed] [Google Scholar]
- 23.Follath F. Vasopressors and inotropes: Do they increase myocardial oxygen consumption? Intensive Care Med. 2000;26(Suppl 2):S275–8. [Google Scholar]
- 24.Vincent JL, Moreno R. Clinical review: Scoring systems in the critically ill. Crit Care. 2010;14:207. doi: 10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy RJ. Mitochondrial dysfunction, bioenergetic impairment, and metabolic down-regulation in sepsis. Shock. 2007;28:24–8. doi: 10.1097/01.shk.0000235089.30550.2d. [DOI] [PubMed] [Google Scholar]
- 26.Teboul JL, Monnet X. Myocardial dysfunction in sepsis. Crit Care Clin. 2009;25:859–75. [Google Scholar]
- 27.Shahul S, Gulati G, Hacker MR, Mahmood F, Canelli R, Nizamuddin J, et al. Detection of myocardial dysfunction in septic shock: A speckle-tracking echocardiography study. Anesth Analg. 2015;121:1547–54. doi: 10.1213/ANE.0000000000000943. [DOI] [PubMed] [Google Scholar]
- 28.Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008;36:1701–6. doi: 10.1097/CCM.0b013e318174db05. [DOI] [PubMed] [Google Scholar]
- 29.Slessarev M, Salerno F, Ball IM, McIntyre CW. Continuous renal replacement therapy is associated with acute cardiac stunning in critically ill patients. Hemodial Int. 2019;23:325–32. doi: 10.1111/hdi.12760. [DOI] [PubMed] [Google Scholar]
- 30.Selby NM, McIntyre CW. How is the heart best protected in chronic dialysis patients?Protecting the heart in dialysis patients –Intra-dialytic issues. Semin Dial. 2014;27:332–5. doi: 10.1111/sdi.12180. [DOI] [PubMed] [Google Scholar]