Abstract
Immune tolerance to β-amyloid (Aβ) was broken in NORBA transgenic mice presenting Aβ plaques on their pancreases. Vaccination of Black C57, BALB/c, and NORBA mice with the synthetic Aβ1–16 sequence modified by covalently attaching two palmitoyl residues at each end of the peptide, subsequently reconstituted in liposomes–Lipid A elicited titers of 1:5,000 of anti-Aβ1–16 antibodies within 10 weeks after the first inoculation. On direct interaction, sera with antibody titers of 1:5,000 solubilized in vitro up to 80% of preformed Aβ1–42 aggregates. Cryosections of pancreases of unvaccinated NORBA mice show, on staining with Thioflavin T, extensive areas of high-intensity fluorescence in the acinar cell fields. Quantitation of the average fluorescence intensity in each section indicated that: (i) whereas nonvaccinated NORBA mice develop plaques within 45–60 days after birth, vaccinated 8-week-old NORBA mice did not develop amyloid plaques on their pancreases over a period of 7 months; (ii) cryosections from pancreases of 9- and 15-month-old vaccinated NORBA mice showed less than 50% of the fluorescence shown by cryosections from unvaccinated animals of the same age. The results indicate that palmitoylated Aβ peptides, reconstituted in liposomes–lipid A, are highly immunogenic, eliciting “therapeutic” antibody titers within 3 months of the first inoculation and preventing amyloid plaque formation in young animals or significantly reducing existing plaques in older transgenic mice. Possible implications for the therapy of Alzheimer's disease are discussed.
Keywords: immune tolerance‖anti-Aβ-antibodies‖lipid A‖Aβ–fiber solubilization
Alzheimer's disease (AD) is a progressive degenerative disorder of insidious onset characterized by memory loss, confusion, and a variety of cognitive disabilities. The major neuropathological change in the brains of AD patients is neuronal death, particularly in regions related to memory and cognition (1). One of the major pathological features of AD is the abundant presence of amyloid plaques in the brain of affected individuals (2). Intracellular bundles of paired helical filaments, composed largely of phosphorylated tau protein, accumulate in large amounts in dying neurons (3). On the neuron surfaces, insoluble aggregates of proteinaceous debris, termed amyloid, appear in the form of neuritic plaques and vascular amyloid deposits (1). The frequency and distribution of the neurofibrillar tangles and of the neuritic plaques appear to correlate well with the extent of cognitive impairment and other characteristic symptoms of AD (3).
Amyloid plaques are formed by the β-amyloid peptide (Aβ), a 39- to 43-aa-long polypeptide that is mostly coiled and slightly α-helical in its benign soluble form and, on conformational transition into a mainly β-sheet secondary structure, spontaneously aggregates into insoluble deposits. Aβ is a physiological metabolite of the much larger amyloid precursor protein (APP), 695–770 aa long, which undergoes sequential proteolysis (4). The peptide may remain in solution as a random coil or an α-helix (5).
A mAb, raised against the sequence Aβ1–16 of the amyloid protein, was shown in vitro to have a solubilizing effect on fibrils formed by the Aβ1–42 amyloidogenic peptide (6). The amyloid filaments obtained were similar to those found in amyloid plaques and cerebrovascular amyloid, assembled from chemically synthesized amyloid sequences under defined experimental conditions (6).
Previous studies in our laboratory had shown that palmitoylated peptide sequences of the multidrug-resistance (MDR)1 protein, reconstituted in the bilayer of liposomes, when injected into mice elicited strong immune responses, breaking the immune tolerance to this “self” protein (7).
In the present study, we report that the palmytoilated Aβ1–16 sequence reconstituted in liposomes-lipid A, when injected i.p. into mice, including transgenic NORBA mice, which overexpress human APP resulting in amyloid plaque deposits on their pancreases, elicited significant titers of antiamyloid antibodies displaying therapeutic as well as prophylactic action in NORBA transgenic mice.
Materials and Methods
Chemicals.
Dichloromethane from Carlo Erba (Milan) was distilled freshly from calcium hydride under nitrogen before use. Acetic anhydride, 4-dimethylaminopyridine, and dicyclohexylcarbodiimide were purchased from Sigma–Aldrich; α,ɛ-dipalmitoyllysine was synthesized according to literature methods (8); α-fluorenylmethoxycarbonyl (Fmoc)-ɛ -palmitoyllisine [FmocLys(Pal)OH], the 4-alkoxybenzyl alcohol resin for automated solid-phase synthesis, as well as Aβ1–42, was purchased from Bachem; chromatography solvents were HPLC grade; the goat-anti-mouse-antibody (alkaline phosphatase conjugated) and para-nitrophenylphosphate were obtained from Sigma–Aldrich; the murine anti-His5–IgG antibody from Qiagen (Courtaboeuf, France); and the monoclonal anti-Aβ1–16 antibody 6C6 was a gift from Dale Schenk (Elan Pharma, San Francisco). Dimyristoylphosphatidyl–choline, dimyristoylphosphatidyl–glycerol, and cholesterol were purchased from Avanti Polar Lipids, monophosphoryl lipid A from List Biological Laboratories (Campbell, CA), aluminium hydroxide (Alum) (Rehydrogel, HPA) was a gift from Reheis.
Animals.
BALB/c and C57BL/6 mice were obtained from Centre Elevage JANVIER (Le Genest Saint Isle, France); transgenic NORBA mice were a gift from Hoechst–Roussel. The mice were maintained in a facility that was tested routinely and found to be free of transmissible agents that cause disease in mice.
Instrumentation.
Solid-phase synthesis of the two different tetrapalmitoyl peptides was carried out on an Applied Biosystems 431A peptide synthesizer by using Fmoc (Sheppard) chemistry. Electrospray ionization mass spectra were recorded on a Micromass BIO Q Instrument (Micromass, Manchester, U.K.). HPLC separations were effected on a Hewlett–Packard HP 1090 chromatograph equipped with a VYDAC-C18 reverse-phase column that was thermostated at 50°C and eluted with a gradient of two solvent mixtures [A, 0.1% trifluoroacetic acid (TFA) in water and B, 70% acetonitrile, 30% of 0.09% TFA in water]. Fourier transform–IR Spectra were recorded in potassium bromide pellets on a Perkin–Elmer 1600 spectrometer.
Synthesis of Tetrapalmitoyl-Tris-Lysine-Aβ-Peptide and of Tetrapalmitoyl-tetrakis-Lysine-Aβ-Peptide.
Two sequential ɛ-palmitoylated lysines were introduced at the peptide's C terminus. The first ɛ-palmitoylated lysine was anchored onto the 4-alkoxylbenzyl alcohol resin by reacting 335 mg of resin with 388 mg of FmocLys(Pal)OH in the presence of dicyclohexylcarbodiimide (DCC) (185 mg) and 4-dimethylaminopyridine (DMAP) (20 mg) in dry freshly distilled dichloromethane according to Merrifield (9). After stirring for 3 hours at room temperature, the reaction mixture was filtered and washed thoroughly 10 times with dry methylene chloride. To ensure complete reaction, the obtained resin was reacted once more with a fresh portion of FmocLys(Pal)OH in the presence of DCC and DMAP in dry methylene chloride, suspended in 45 ml of dry methylene chloride, and 5 ml of acetic anhydride was added for capping any unreacted resin. After washing with methylene chloride, the resin was dried in vacuum. The Fourier transform (FT)–IR spectrum of the product shows the expected bands for the ester linkage at 1,720 cm−1, for the NH group at 3,327 cm−1, and for the amide carbonyl group at 1,626 cm−1. All these bands are absent in the FT-IR spectrum of the starting 4-alkoxybenzyl alcohol resin. The final quantity was 302 mg with a loading of about 0.5 mmol/g. The second ɛ-palmitoylated lysine was anchored on the peptide synthesizer by using for coupling also FmocLys(Pal)OH. Subsequently, 16 cycles of conventional automated solid-phase peptide synthesis were performed, resulting in the sequence Aβ1–16 appended to the first two palmitoylated lysines. At this point, a small amount of peptide was cleaved from the resin and analyzed by electrospray mass spectrometry and HPLC, showing that, in addition to the desired lipopeptide, minor components of peptides lacking either one or two palmitoylated lysines were also present.
Two further couplings, each repeated twice, were effected with Fmoc protected ɛ-palmitoyllysine [Fmoc-Lys(Pal)-OH]. Final deprotection and cleavage from the resin afforded a mixture of lipopeptides in which the desired tetrapalmitoylpeptide, shown in Fig. 1, amounted to 35% (peaks at 1140.6, 855.4, and 685.4 Da for the +3, +4, +5 molecular ions, respectively, lead to an inferred mass of 3421.65 ± 0.39 as compared to the theoretical value of 3421.05 Da). As the other components lacked palmitoylated lysines, their insertion into liposomes was less probable, so that a purification at this stage could be performed.
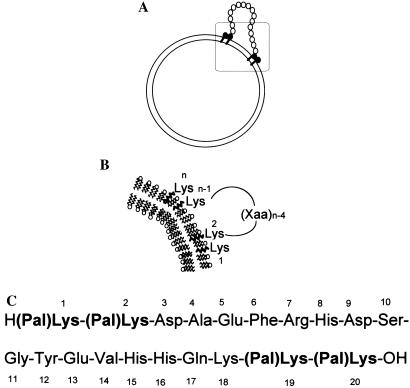
Figure 1.
Reconstitution of Aβ1–16 in the liposome bilayer. (A) Liposome with anchored Aβ1–16. (B) Magnification of the boxed region in the upper part. (C) Two palmitoylated lysine residues (in the sequence shown with bold characters) are covalently attached at each end of the Aβ1–16 sequence.
Preparation of the Antigen.
Liposomes with lipid A and Alum were used as adjuvants to prepare the antiamyloid vaccine (7). Dimyristoylphosphatidyl–choline, dimyristoylphosphatidyl–glycerol, and cholesterol were mixed in the molar ratios of 0.9:0.1:0.7. Monophosphoryl lipid A, a strong immunomodulator, was added at a concentration of 40 mg per mmol of phospholipids. The palmitoylated peptides were added at a molar ratio peptide to phospholipids of 1:100. Solvents were evaporated, and the resultant film was hydrated with sterile PBS (pH 7.3) to a final phospholipid concentration of 4 mmol and was further homogenized. The liposome suspension was mixed with sterile Alum 15 min before injection (9:1 vol/vol).
Immunization Protocol.
Three groups of animals were immunized: (i) eight BALB/c mice were immunized by six i.p. inoculations at 2-week intervals with 200 μl of the palmitoylated peptide–liposome–lipid A/Alum suspension. Six additional mice were injected with PBS and Alum or with phospholipids and Alum (three animals each); (ii) in a group of 12 C57BL/6-mice, three received liposomes-lipid A and palmitoylated Aβ1–16, three received liposomes-lipid A mixed with the scrambled sequence Aβ42–1, and six were injected with lipids only or left untreated (each three animals); (iii) the final group consisting of 19 NORBA transgenic mice of different ages, constitutively presenting Aβ plaques on the pancreas, were immunized by using the protocol described. Four animals were left untreated as controls. Blood was collected from the tail vein 4 days after injection. The collected blood (10–30 μl) was diluted immediately with 10 μl of PBS and 5 μl of heparin. The samples were centrifuged, and the serum was tested for anti-Aβ1–42 antibodies by ELISA.
ELISA.
Microtiter plates were coated with 50 μl of Aβ1–42 solution (1 mg of Aβ1–42/5 ml PBS) and left overnight at 4°C. Wells were blocked with 200 μl of BSA/PBS (0.5% BSA) for 2 hours at 37°C and washed with 200 μl of PBS/0.005% Triton X-100. Dilutions of sera (1:10–1:100,000) were incubated for 90 min at 37°C. The plates were washed twice with 200 μl of PBS/0.005% Triton X-100 before 50 μl of a goat-anti-mouse antibody (alkaline phosphatase conjugated) was added in a 1:30,000 dilution. After 90 min at 37°C, the wells were washed as described. One hundred microliters of the paranitrophenyl phosphate substrate (1 tablet in 5 ml of deionized water) was added, and optical absorption was measured at 405 nm in an ELISA reader 20–60 min later (10).
Disaggregation Assay.
Reaction tubes containing 30 μg of Aβ1–42/10 μl of PBS, pH 7.3, were incubated for 1 week at 37°C. Aggregation was measured by the thioflavin T (ThT)-binding assay in which the dye's fluorescence emission intensity reflects the degree of Aβ fibrillar aggregation. Disaggregation was followed after addition of various undiluted sera of immunized mice to the preformed fibers (10 μl each). The reaction mixtures were incubated for 2 days at 37°C. The mAb, 6C6, and an irrelevant control antibody (mouse IgG, anti-His5) were used at a final concentration of 0.4–3.5 mg/ml. Fluorescence (excitation: 450 nm; emission: 482 nm) was measured after addition of 1 ml of ThT (3 μM in 50 mM sodium phosphate buffer, pH 6.0) (4).
Histochemistry
Quantitative Fluorescence Analysis.
Vaccinated and nonvaccinated NORBA mice of different age having received seven immunizations were killed 7 months after the first inoculation, and their pancreases were collected and preserved in formalin. The formalin-preserved pancreas pieces were soaked in sucrose solution to prepare them for cryosectioning. The sections obtained were analyzed by ThT staining (11) to detect Aβ on the surface of the pancreas. Washed sections were stained with a 1% ThT aqueous solution for 3 min. To remove excess fluorochrome from the background, sections were rinsed with water and incubated in 1% acetic acid for 20 min. Sections were washed with water extensively before analysis by fluorescence microscopy. Thin sections stained with ThT were analyzed with a Nikon Labophot Microscope by using a 100-watt mercury source and a Lucifer Yellow Filter Set (Chroma Technology, Brattleboro, VT). Images were captured with a Cool Snap Pro Digital Capture kit (Media Cybernetics, Silver Spring, MD) by using a ×40 objective and an exposure time of 600 msec. Images were analyzed with image pro plus v. 4.1 (Media Cybernetics). Three sections were used per treatment group, and treatments were unknown to the analyst. A uniform threshold setting of 567,000 pixels per image was maintained for all groups. Measurements included the total area of the fluorescent region and the mean intensity within the region. The person conducting these studies did not know the history of the cryosections analyzed.
Results
To anchor a synthetic peptide of desired sequence in the liposome bilayer, thus enhancing its immunogenicity, we used lysines that are acylated at the ɛ-amino group with a fatty acid residue capable of insertion in the liposomal bilayer. Palmitoyl residues (16 carbon atoms) have proven to have the appropriate length for the stable insertion into the lipid bilayer (7). Two such palmitoyl residues at each end of the peptide make it sufficiently hydrophobic so that insertion is thermodynamically favored, a stable supramolecular assembly being formed, as shown in Fig. 1.
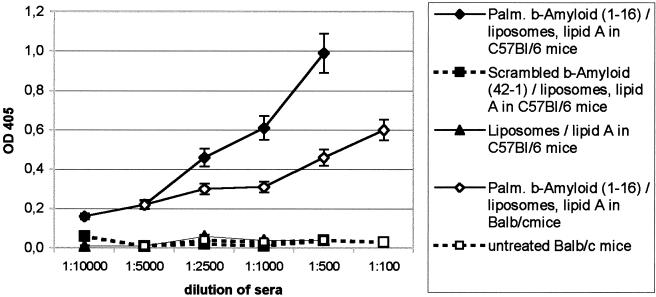
A significant immune response was observed in the liposomes/Aβ1–16 vaccinated BALB/c mice after the third inoculation. The titers of the elicited anti Aβ1–42 antibodies were around 1:5,000 10 weeks after the first inoculation. Control animals receiving phospholipids/Alum or Alum alone had negligible titers to Aβ1–42. Sera of C57BL/6 mice having received palmitoylated Aβ1–16 reached titers up to 1:10,000 against Aβ1–42. No antibodies against Aβ1–42 were found in sera from mice having received scrambled Aβ42–1 or lipids only.
Figs. 2 and 3 a and b show the evolutions of the antiamyloid antibody titers in time, first after three inoculations with a variety of antigens and then after six inoculations by using only the palmitoylated peptide in liposomes.
Figure 2.
Antibody response in mice to different antigens reconstituted in liposomes −lipid + Alum, 6 weeks after the first inoculation. Three groups of animals were immunized: (i) eight BALB/c mice were immunized by six i.p. inoculations at 2-week intervals with 200 μl of the palmitoylated peptide–liposome–lipid A/Alum suspension. Six additional mice were injected with PBS and Alum or with phospholipids and Alum (three animals each). (ii) In a group of 12 C57BL/6-mice, three received liposomes-lipid A and palmitoylated Aβ1–16, three received liposomes-lipid A mixed with the scrambled sequence Aβ42–1, and six were injected with lipids only or left untreated (each three animals). Four animals were left untreated as controls. Blood was collected from the tail vein 4 days after injection. The collected blood (10–30 μl) was diluted immediately with 10 μl of PBS and 5 μl of heparin. The samples were centrifuged, and the serum was tested for anti-Aβ1–42 antibodies by ELISA (see Materials and Methods). (♦) Means of sera from 12 C57BL/6 mice; (⋄) means of sera from 8 BALB/c mice; (▴, □, ▪) means of sera from three animals each and SDs are shown.
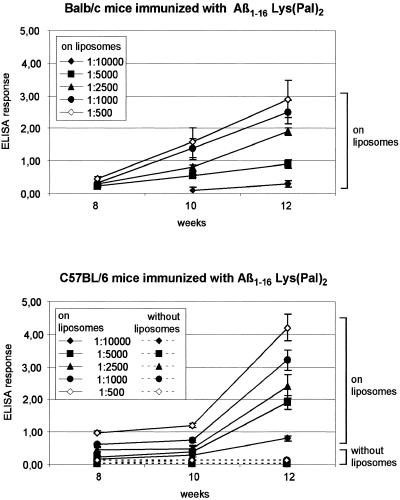
Figure 3.
Time dependence of anti-Aβ1–16 antibody titers in animals vaccinated with the liposomal, palmitoylated Aβ1–16 antigen. Antibody response was assayed by ELISA. Microtiter plates were coated with 50 μl of Aβ1–42 solution (1 mg of Aβ1–42/5 ml of PBS) and left overnight at 4°C. Wells were blocked with 200 μl of BSA/PBS (0.5% BSA) for 2 hours at 37°C and washed with 200 μl of PBS/0.005% Triton X-100. Dilutions of sera (1:10–1:100,000) were incubated for 90 min at 37°C. The plates were washed twice with 200 μl of PBS/0.005% Triton X-100 before 50 μl of a goat-anti-mouse antibody (alkaline phosphatase conjugated) was added in a 1:30,000 dilution. After 90 min at 37°C, the wells were washed as described. One hundred microliters of the paranitrophenyl phosphate substrate (one tablet in 5 ml of deionized water) was added, and optical absorption was measured at 405 nm in an ELISA reader 20–60 min later (8). Means of sera from 8 BALB/c mice and means of sera from 12 C57Bl/6 mice and SDs are shown.
Disaggregation of Aβ1–42 Fibrils.
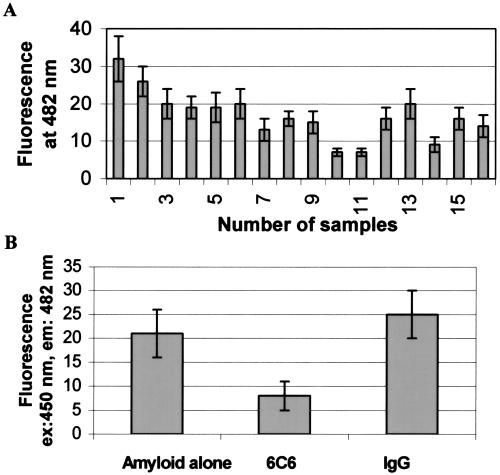
Because the mAb 6C6 raised against Aβ1–16 was shown to solubilize Aβ fibers in vitro, the antisera of the vaccinated mice showing titers of at least 1:5,000 were tested for their capacity to solubilize preformed Aβ1–42 fibers. A disaggregation assay of Aβ1–42 aggregates was performed as described by Solomon et al. (6). A significant solubilization of Aβ1–42 fibers by 15 different antisera was observed with an incubation time of 2 days (Fig. 4A). The sera induced disaggregation at an extent up to 80%, exceeding the solubilization of Aβ1–42 fibers by the Elan mAb (6C6, 60–70%) (Fig. 4B) used in comparison. The irrelevant IgG had no significant effect on disaggregation of the Aβ1–42 fibrils (Fig. 4B).
Figure 4.
Disaggregation of Aβ1–42 fibers by sera of immunized C57BL/6 mice. (A) ThT fluorescence emission intensity correlates with the amount of fibrillar amyloid present in solution. Aβ fiber formation during 7 days at 37°C in PBS, pH = 7.1 Antisera were added after 7 days and incubated for 24 h. Bar no. 1, serum of a nonvaccinated animal; bars nos. 2–16, sera of vaccinated animals. (B) Anti-Aβ1–16 mAb 6C6 and irrelevant IgG were incubated at a final concentration of 30 μg/20 μl with Aβ1–42 aggregates for 24 h. Average of three measurements with three different serum samples.
Histochemistry Studies.
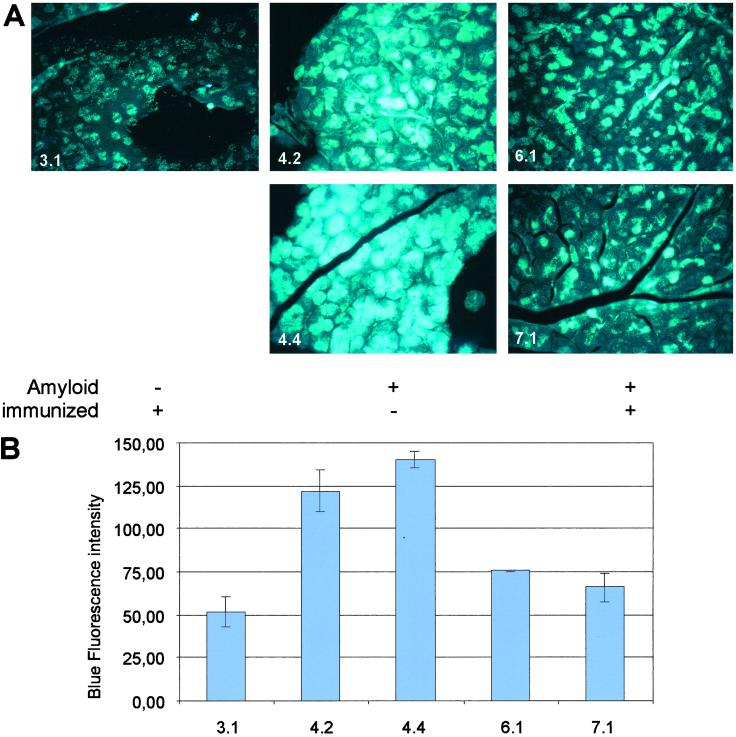
Pancreases of vaccinated and nonvaccinated NORBA mice were collected for histology studies. Thin sections were analyzed by ThT staining to assess the Aβ burden on the pancreas. Fig. 4A shows the fluorescence of thioflavin-stained pancreatic tissue of a 2-month-old Aβ negative animal (3.1) that had been vaccinated 7 weeks after birth. There is diffuse weak background fluorescence, and the acinar cells are dark. By contrast, a ThT-stained pancreas section of 14-month-old animals (4.4, 4.2; Fig. 5A) with fully developed Aβ plaques shows intense fluorescence throughout the acinar cell fields. There were also very bright fluorescent areas suggesting larger blood vessels. Tissues of animals 6.1 and 7.1 show the fluorescence of a ThT-stained section of the pancreas of 9- and 15-month-old mice, with fully developed Aβ plaques 7 months after vaccination. There are some focal (patchy) areas of fluorescence among acinar cells but predominantly many patches of nonfluorescent acinar cells. It appears from these images that the vaccination either had disintegrated Aβ plaques in animals having already developed such plaques or had prevented their deposition on the pancreas when young transgenic animals were vaccinated before plaque formation.
Figure 5.
Histological study and quantitation of Aβ in thin sections of pancreases from vaccinated and unvaccinated NORBA transgenic mice, stained with ThT. (A) (3.1) 9-month-old mouse vaccinated 7 weeks after birth, without Aβ plaques; (4.4, 4.2) 14-month-old mice with fully developed Aβ plaques, unvaccinated; (9- and 15-month-old mice with fully developed Aβ plaques, vaccinated. (B) Quantitation of fluorescence in images in A. Three sections were used per treatment group, and treatments were unknown to the analyst. Washed sections were stained with a 1% ThT aqueous solution for 3 min. To remove excess fluorochrome from the background, sections were rinsed with water and incubated in 1% acetic acid for 20 min. Sections were washed with water extensively before analysis by fluorescence microscopy. Thin sections stained with ThT were analyzed with a Nikon Labophot Microscope by using a 100-watt mercury source and a Lucifer Yellow Filter Set (Chroma Technology). Images were captured with a Cool Snap Pro Digital Capture Kit (Media Cybernetics) by using a ×40 objective and an exposure time of 600 msec. Images were analyzed with image pro plus v. 4.1 (Media Cybernetics). A uniform threshold setting of 567,000 pixels per image was maintained for all groups. Measurements included the total area of the fluorescent region and the mean intensity within the region.
With individual variations, this is the pattern observed throughout the experiment performed on 19 NORBA mice. Quantitative evaluation of the average fluorescence intensity in each section stained with ThT by using the luminosity analysis software described indicated that pancreas sections from 9- and 15-month-old vaccinated NORBA mice showed less than 50% the fluorescence of unvaccinated animals of similar age (Fig. 5B).
Discussion
The palmitoylated Aβ1–16 sequence reconstituted in liposomes containing lipid A and Alum injected i.p. to mice proved to be a strong antigen capable of eliciting a significant immune response. These results extend earlier observations made with the murine multidrug-resistance (MDR)1 sequences (7) by using also palmitoylated peptides reconstituted in liposomes–lipid A.
Antibody titers of 1:5,000–1:10,000 were detected about 12 weeks after the first inoculation and subsequent boostings at 2-week intervals. Schenk et al. (12) obtained similar titers of anti-Aβ1–42 antibodies over an 11-month period, when the antigen used was the Aβ1–42 sequence emulsified with the complete and subsequently incomplete Freund adjuvant. The titers of antiamyloid antibodies in mice inoculated with the palmitoylated Aβ1–16 sequence reconstituted in liposomes/lipid A were measured by ELISA by using Aβ1–42 as antigen. The antibodies proved effective in solubilizing in vitro Aβ fibers (Fig. 4). As can be seen in Fig. 4A, all sera from immunized mice solubilized Aβ fibers, albeit with different efficacies. Their activity was comparable with that of the most efficient antiamyloid mAb reported, 6C6 (6). Mouse sera containing irrelevant IgGs had no solubilizing effect on the Aβ fibers in vitro. This observation may be significant for the mechanism of action of these antibodies in vivo, because the amyloid fibers assembled in solution under the condition described are similar to those found in amyloid plaques (6).
Deposition of amyloid plaques was observed in pancreas of transgenic NORBA mice (13). Study of the Aβ production, accumulation, and recycling in the pancreas of transgenic NORBA mice indicated that Aβ deposits are formed in four types of pancreatic cells: acinar cells, macrophages infiltrating stroma, epithelial cells of pancreatic ducts, and blood monocytes/macrophages in the lumen of pancreatic cells. All these types of cells produce fibrillar amyloids. Amyloid production in acinar pancreatic cells starts in mice younger than 45 days, progresses in 2- to 7-month-old mice, and plateaus in the second year of life (14).
Schenk et al. (12) reported that immunization with Aβ1–42 with complete or/and incomplete Freund adjuvant reduced the development of AD-like pathology in PDAPP mice. In 18-month-old PDAPP mice, reduction of neuritic plaque burden was between 50 and 60% on vaccination (12), although the burden was relatively low in absolute terms. The pancreas burden in the 16- and 17-month-old NORBA mice was very high (Fig. 4A), so that its reduction by 50% appears to be significant and correlates well with the in vitro solubilization of the preformed Aβ1–42 fibers by the antisera (Fig. 4A).
Recently, Janus et al. (15) and Morgan et al. (16) indicated that, by using the Schenk immunization technique (12), reduction of behavioral impairment was observed in transgenic (Tg) CNRD8 mice and of memory loss in Tg 2576 APP Tg mice, confirming the involvement of Aβ in memory loss. However, as Morgan et al. (16) point out, prevention of memory loss by vaccination occurs in the presence of still substantial Aβ deposits.
Breaking the immune tolerance to Aβ by using palmitoylated Aβ1–16 peptide reconstituted in liposomes appears to be quite efficient, as “therapeutic titers” are obtained rapidly, only 12 weeks after the first inoculation. Moreover, the amount of plaque removal/solubilization is high after immunization with this system: in the 9- and 15-month-old mice, the reduction of plaque burden is ≈50% compared with controls. Moreover, liposomes of the composition used in this work have been used in for a number of clinical studies (17–19).
An extensive pathology study carried out with the vaccinated mice did not find any autoimmune lesions in lung, kidney, liver, adrenals, and pancreas of the NORBA mice 7 months after vaccination. The absence of the blood–brain barrier hurdle to be crossed by the antibodies to reach the pancreatic plaques in the NORBA mice may reduce the value of our animal model, though.
The mechanism by which the elicited antibodies reduce the plaque burden in all of the transgenic mice used until now is not yet clearly understood. In an ex vivo assay, Bard et al. (20) showed that antibodies against Aβ1–42 triggered microglial cells to clear plaques through F0 receptor-mediated phagocytosis and subsequent peptide degradation.
Recently, DeMattos et al. (21) showed that a mAb directed against the central domain of Aβ was able to bind and completely sequester plasma Aβ. They postulated that the presence of this mAb in the peripheral compartment alters the transport and dynamic equilibrium of Aβ between brain and plasma, favoring the peripheral clearing and catabolism instead of deposition within the brain.
Finally, our own in vitro data (Fig. 4A) suggest that direct interaction of anti-Aβ antibodies with Aβ aggregates induces extensive solubilization of the latter. It appears thus that three mechanisms may be implicated in plaque destruction by anti-Aβ antibodies: (i) opsonization of the plaques and their subsequent destruction by microglia macrophages, (ii) alteration of the transport and dynamic equilibrium of Aβ between brain and plasma, and (iii) direct interaction of the antiamyloid antibodies with the plaques.
On the basis of available data, it is difficult to select which is the principal mechanism, but all three mechanisms might be involved.
The results described herein suggest a possible therapeutic and a prophylactic role for vaccination with a chemically modified Aβ fragment reconstituted in liposomes.
Acknowledgments
We thank Pascal Eberling (Institut de Génétique et de Biologie Moléculaire et Cellulaire, Illkirch, France) for the solid-phase peptide syntheses, Dr. Vincent Ball (Laboratoire de Spectrométrie de Masse, Université Louis Pasteur, Strasbourg, France) for the electrospray–MS spectra, and Professor Jean-Marie Lehn for support and laboratory facilities. We thank Dr. D. Schenk (Elan Pharmaceuticals, South San Francisco, CA) for generously supplying the mAb 6C6. It is a pleasure to thank Prof. J. M. Vetter (Institut de Pathologie, Université Louis Pasteur, Strasbourg, France) for his help with the pathology studies. This work was funded by Grant No. RP 98084 (to C.N.) from the Ministère de l'Education Nationale et la Recherche Scientifique, Groupement d'Intérêt Public Hoechst Marion Roussel (Paris).
Abbreviations
- Aβ
β-amyloid
- AD
Alzheimer's disease
- Alum
aluminium hydroxide
- APP
amyloid precursor protein
- Fmoc
9-fluorenylmethoxycarbonyl ester
- ThT
thioflavin T
References
- 1.Soto C. Mol Med Today. 1999;5:343–350. doi: 10.1016/s1357-4310(99)01508-7. [DOI] [PubMed] [Google Scholar]
- 2.Selkoe D J. Trends Neurochem Sci. 1995;16:403–409. [Google Scholar]
- 3.Terry R D. Prog Brain Res. 1994;101:383–390. doi: 10.1016/s0079-6123(08)61964-0. [DOI] [PubMed] [Google Scholar]
- 4.Baumeister R D, Eimer S. Angew Chem Int Ed Engl. 1998;37:2978–2982. doi: 10.1002/(SICI)1521-3773(19981116)37:21<2978::AID-ANIE2978>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 5.Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Schlossmacher M, Whaley J, Swindlehurst C, et al. Nature (London) 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- 6.Solomon B, Koppel R, Frankel D, Hanan-Aharon E. Proc Natl Acad Sci USA. 1997;94:109–112. doi: 10.1073/pnas.94.8.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tosi P-F, Radu D, Nicolau C. Biochem Biophys Res Com. 1995;212:494–500. doi: 10.1006/bbrc.1995.1997. [DOI] [PubMed] [Google Scholar]
- 8.Lapidot Y, Rappoport S, Wolman Y. J Lipid Res. 1967;8:142–145. [PubMed] [Google Scholar]
- 9.Lu G, Mojsov S, Tam J P, Merrifield R B. J Org Chem. 1981;46:3433–3436. [Google Scholar]
- 10.Frenkel D, Katz O, Solomon B. Proc Natl Acad Sci USA. 2000;97:11455–11459. doi: 10.1073/pnas.97.21.11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassar P S, Culling C F A. Arch Pathol. 1959;68:487–489. [PubMed] [Google Scholar]
- 12.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T H K, Huang J, Johnson-Wood K, Kholodenko D, Lee M, et al. Nature (London) 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 13.Shoji M, Kawarabayashi T, Sato M, Sasaki A, Matsubara E, Iizuka T, Harigaya Y, Hirai S. Gerontology. 1996;42, Suppl. 1:48–56. doi: 10.1159/000213824. [DOI] [PubMed] [Google Scholar]
- 14.Wegiel J, Wisniewski H M, Muzylak M, Tarnawski M, Badmajew E, Nowakowski J, Wang K C, Shoji M, Mondadori C, Giovanni A. Amyloid. 2000;7:95–104. doi: 10.3109/13506120009146245. [DOI] [PubMed] [Google Scholar]
- 15.Janus C, Pearson J, McLaurin J, Mathews P M, Jiang Y, Schmidt S D, Chrishti M A, Horne P, Heslin D, French J, et al. Nature (London) 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 16.Morgan D, Diamond D M, Gottschall P E, Ugen K E, Dickey C, Hard J, Duff K, Jantzen P, DiCarlo G, Wilcock D, et al. Nature (London) 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 17.Fries L F, Gordon D M, Richards R L, Egan J E, Hollingdale M R, Gross M, Silverman C, Alving C R. Proc Natl Acad Sci USA. 1992;89:358–362. doi: 10.1073/pnas.89.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris D T, Matyas G R, Gomella L G, Talor E, Winship M D, Spitler L E, Mastrangelo M J. Semin Oncol. 1999;26:439–447. [PubMed] [Google Scholar]
- 19.Heppner D G, Gordon D M, Gross M, Wellde B, Leitner W, Krzych U, Schneider I, Wirtz R A, Richards R L, Trofa A, et al. J Infect Dis. 1996;174:361–366. doi: 10.1093/infdis/174.2.361. [DOI] [PubMed] [Google Scholar]
- 20.Bard F, Cannon C, Barbour R, Burke R L, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, et al. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 21.DeMattos R, Bales K R, Cumins D J, Dobart J-C, Paul S M, Holtzmann D M. Proc Natl Acad Sci USA. 2001;98:8850–5855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]