Abstract
Accumulation of oxidative damage to mitochondria, protein, and nucleic acid in the brain may lead to neuronal and cognitive dysfunction. The effects on cognitive function, brain mitochondrial structure, and biomarkers of oxidative damage were studied after feeding old rats two mitochondrial metabolites, acetyl-l-carnitine (ALCAR) [0.5% or 0.2% (wt/vol) in drinking water], and/or R-α-lipoic acid (LA) [0.2% or 0.1% (wt/wt) in diet]. Spatial memory was assessed by using the Morris water maze; temporal memory was tested by using the peak procedure (a time-discrimination procedure). Dietary supplementation with ALCAR and/or LA improved memory, the combination being the most effective for two different tests of spatial memory (P < 0.05; P < 0.01) and for temporal memory (P < 0.05). Immunohistochemical analysis showed that oxidative damage to nucleic acids (8-hydroxyguanosine and 8-hydroxy-2′-deoxyguanosine) increased with age in the hippocampus, a region important for memory. Oxidative damage to nucleic acids occurred predominantly in RNA. Dietary administration of ALCAR and/or LA significantly reduced the extent of oxidized RNA, the combination being the most effective. Electron microscopic studies in the hippocampus showed that ALCAR and/or LA reversed age-associated mitochondrial structural decay. These results suggest that feeding ALCAR and LA to old rats improves performance on memory tasks by lowering oxidative damage and improving mitochondrial function.
Memory, i.e., performance on memory tasks, declines with age in animals. In the case of age-related human neurodegenerative diseases, such as Alzheimer's disease (AD), the deficit can be severe (1–4). Memory loss is accompanied but not necessarily caused by accumulation of oxidative damage to lipids, proteins, and nucleic acids, and by mitochondrial decay, all of which can disrupt neuronal function (5–10).
R-α-lipoic acid (LA) is a coenzyme that is involved in carbohydrate utilization necessary for the production of ATP in mitochondria; it is reduced in mitochondria to dihydrolipoic acid (DHLA), a potent antioxidant (11, 12). LA improves long-term memory in aged female NMRI mice (13).
l-Carnitine is a betaine required for the transport of long-chain fatty acids into the mitochondria for β-oxidation, ATP production, and for the removal of excess short- and medium-chain fatty acids (14, 15). Acetyl-l-carnitine (ALCAR) is more widely used than l-carnitine in animal and clinical studies because it enters cells and crosses the blood–brain barrier more efficiently (16). ALCAR improves cognitive function and neuronal bioenergetic mechanisms in rats with both acute and long-term treatments (17–23).
Several clinical studies report the beneficial effects of ALCAR or LA:ALCAR administration in a small group of patients with AD that resulted in improved spatial orientation and short-term memory (24, 25). LA administration in patients with AD for approximately 1 year also resulted in mild cognitive improvements and stabilization of global neuropsychological test scores (26). Thus, as both ALCAR and LA improve mitochondrial decay, their combination may be complementary in decreasing oxidative damage to neurons and cognitive dysfunction.
As our understanding of the importance of mitochondrial decay in aging advances (27–29), the importance of improving mitochondrial function by dietary interventions of mitochondrial metabolites such as ALCAR or LA becomes clearer (30–33). Feeding 0.15–0.5% ALCAR to old rats elevated the levels of carnitine in plasma and brain to that of young rats (34) and 0.1–0.2% LA (T.M.H., unpublished data) was as effective in improving mitochondrial function in the liver as the higher doses originally used (30–33). We have examined the effects of these lower doses of ALCAR, LA, and their combination on spatial memory by using the Morris water maze, on temporal memory by using the peak procedure, decay in mitochondrial structure in the hippocampus, and oxidative damage to nucleic acids in the hippocampus and cortex.
Materials and Methods
Materials.
ALCAR (hydrochloride salt) was a gift of Sigma Tau (Pomezia, Italy), and LA was a gift of Asta Medica (Frankfurt/Main, Germany). All other chemicals were reagent grade or the highest quality available from Sigma.
Animals and Diet.
Fischer 344 male rats were obtained from the National Institute on Aging. Control animals were fed AIN93M diet from Dyets (Bethlehem, PA) and MilliQ water (pH 5.2). The rats in the experimental groups were fed either 0.5% or 0.2% (wt/vol) ALCAR in MilliQ water (pH was adjusted to 5.2 with 1 N NaOH), 0.2% or 0.1% (wt/wt) LA in AIN93M diet, or a combination of (0.5% ALCAR and 0.2% LA) or (0.2% ALCAR and 0.1% LA). The food consumption was determined by weighing the diet and measuring the volume of water weekly; the average daily consumption was then calculated. The weight gain during the course of the experiment was also measured. We did not find any significant differences in diet, water consumption, or weight gain between the unsupplemented old rats (13.4 ± 0.5 g/day; 18.6 ± 1.19 ml/day; body weight from 416.1 ± 14.4 to 409.2 ± 10.1 g mean ± SE) and the old supplemented rats (For example, the ALCAR + LA group 13.1 ± 0.4 g/day; 18.4 ± 0.9 ml/day; body weight from 416.0 ± 19.0 to 414.9 ± 9.4 g; mean ± SE). All animals were acclimatized at the Northwest Animal Facilities on the University of California at Berkeley campus for at least 2 weeks before treatment. Rats were housed individually and provided with ALCAR and/or LA for 7 weeks. The young and old rats were 4.5 and 24.5 months old at the start of the experiment; they were more than 7 weeks older at the time of death. Death, by approved protocol, was with an overdose of ether.
Morris Water Maze Test of Spatial Memory.
The Morris water maze task tests spatial memory by requiring rats to find a submerged platform in a pool of water using external visual cues (35, 36). The time required for individual rats to find the platform and the length of the swim path was measured by using a digital camera and a computer system to record movement (VideoMex-V, Columbus Instruments, Columbus, OH). Trials (4 consecutive days, 4 trials per day) were with the same hidden platform location, but with varied start locations. On day 5, the platform was removed from the pool (transfer trial, 60 sec), and the time spent at the actual site where the platform was located was examined. On day 6, the time required to reach a visible platform was measured to determine visual function and motor ability.
Peak Procedure Test of Temporal Memory.
The peak procedure is a modified fixed-interval schedule commonly used to study temporal memory (37). Rats were tested in 18 identical boxes that contained a light source and a speaker (for delivering light or noise signals) and a lever that dispenses single food pellets (45 mg) when pressed (mix T101, Bioserv, Frenchtown, NJ). The food supply of the rats was decreased to 85% of the free-feeding amount. In this test, the animal is rewarded with one pellet only if the lever is pressed at 40 sec from the signal. In 20% of the tests, no food was given, and an empty trial and the signal lasted 195 sec plus a geometrically distributed duration that averaged 50 sec. The results are presented as a sum of the two types of tests. Peak rate, which is the maximum response rate in a given trial and reflects the rats' choices of what responses to make and their motivation, was measured.
Electron Microscopic Observations.
A subset of rats from each experimental condition was perfused transcardially with 2.5% glutaraldehyde for 2 h. The brain was removed from the skull and the hippocampus was postfixed in 0.1 M PBS with 1% osmium tetroxide. The tissues were block-stained with uranyl acetate and embedded in Epon. Sections were cut at 0.6–0.9-μm thick from the block, stained with uranyl acetate and lead citrate, and examined with a JEOL 100 CX electron microscope.
Immunohistochemical Studies.
A subset of rats from each treatment condition was anesthetized with ether and perfused with 4% paraformaldehyde for 1.5–2 h. The brain was removed and postfixed for preparing paraffin sections. Sections of hippocampus were incubated with monoclonal anti-8-hydroxy-2′-deoxyguanosine/8-hydroxyguanosine (oxo8dG/oxo8G; 1:2000; QED Bioscience, San Diego) and visualized by using standard immunocytochemical methods. Two independent analyses were done on each rat. To determine whether DNA or RNA was oxidatively damaged, sections were pretreated with either 10 units/μl of RNase-free DNase I or 10 mg/ml of DNase-free RNase (Roche Molecular Biochemicals) for 3 h prior to incubation with oxo8dG/oxo8G Ab (38). To quantify the extent of oxo8G/oxo8dG immunolabeling, a 525 × 410 μm area of staining was captured by using a ×2.5 photo eyepiece, a Sony (Tokyo) high-resolution charge-coupled device (CCD) video camera (XC-77), and the built-in video capture capabilities of a Macintosh 8100/80AV. All sections from a given region were captured sequentially during one session and were analyzed blind with respect to treatment condition. Subsequently, public domain image analysis software (IMAGE 1.55, National Institutes of Health) and gray-scale thresholding were used to separate positive staining from background and to calculate the percentage of area occupied by oxo8G/oxo8dG immunoreactivity.
Results
Spatial Memory.
Rats are proficient swimmers and are motivated to escape from water. Once animals learn where the hidden platform is located, they can remember the location and swim rapidly to it from any starting point. Both time taken to reach the platform (Fig. 1) and swimming distance traveled (data not shown) were measured and gave similar results. Fig. 1A shows results obtained on day 4. Young rats spent a significantly shorter time than old rats (P < 0.001) in finding the hidden platform. ALCAR or LA seems to shorten the time in old rats, but the differences were not significant. However, the combination resulted in significantly shorter times (P < 0.05) as compared with old control rats. The tracks of individual rats on successive trials and days have been shown (34).
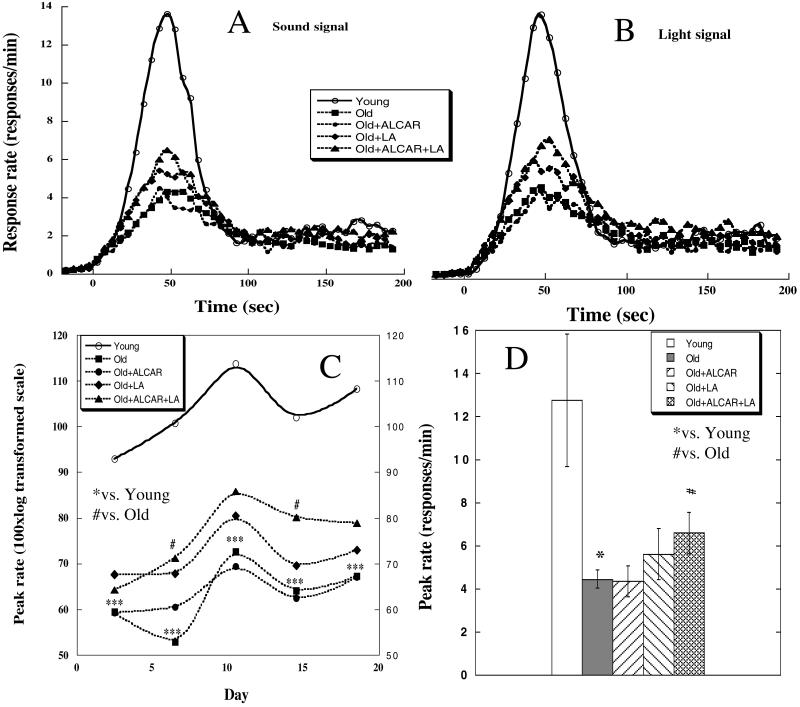
Figure 1.
Morris water maze test in relation to age and treatment. (A) Time on day 4 taken to find the hidden platform. (B) Time spent at the former platform position in the transfer test. (C) Time to find the visible platform. Data are mean ± SEM of 9 rats in young and old, 5 in LA (0.1%), and 6 in ALCAR (0.2%) and ALCAR + LA groups. Higher doses, 0.2% LA and/or 0.5% ALCAR, showed similar results (data not shown). Statistical differences were examined with two-tailed Student t test. ***, P < 0.001 vs. young rats; #, P < 0.05 and ##, P < 0.01 vs. old rats.
A transfer test, in which the platform was removed, was carried out on day 5. The time spent at the previous platform position is a measure of search accuracy and spatial memory. Young rats spent significantly more time at the former platform position (P < 0.001) than old rats did. The ALCAR (P < 0.05) and LA (P < 0.05) significantly restored the lost procedural subcomponent of spatial memory and the combination was even more effective (P < 0.01; Fig. 1B).
A clearly visible platform was used to measure deficits in vision, motivation, motor strength, or coordination on day 6 of the training cycle. The platform protruded 1 cm above the surface of the water. Young rats required less time to find the visible platform than the old animals (Fig. 1C). All three supplementation groups showed improvement, but only the combination treatment group reached statistical significance (Fig. 1C).
Temporal Memory.
The response rate to a sound (Fig 2A) and to a light (Fig. 2B) signal is the same, indicating that the rats responded similarly to both signals. Results from the last 10 days of testing were used, where responses had reached asymptotic levels.
Figure 2.
Peak procedure test related to age and treatment. Response rate functions plotted separately for sound-signaled trials (A) and light-signaled trials (B) obtained during the last 10 days of the test. (C) Peak rate over the 20 days of peak procedure testing. Each data point averages 2 days of testing. (D) Peak rate of the last 10 days averaged. Data are mean ± SEM of 6 in young, 7 in old, 4 in LA (0.2%), and 5 in ALCAR (0.5%) and ALCAR + LA groups. Treatment with lower doses, 0.1% LA and/or 0.2% ALCAR, showed similar results (data not shown). Statistical differences were examined with two-tailed Student t test. *, P < 0.05 and ***, P < 0.001 vs. young rats; #, P < 0.05 vs. old unsupplemented rats.
Peak rate (Fig. 2 C and D) of young animals was significantly higher than that of all other groups: young compared with old (P = 0.001); young compared with old + ALCAR (P = 0.004); young compared with old + LA (P = 0.043); and young compared with old + ALCAR + LA (P = 0.046). Although ALCAR does not show any significant effect (comparing the old + ALCAR group to the old control rats), LA seems to slightly increase peak rate. The old + ALCAR + LA treatment showed a more significant increase (P = 0.033) in peak rate in old animals than treatment with LA alone.
Ultrastructural Observations of Neuronal Mitochondria.
Electron microscope observations of hippocampal neuronal mitochondria indicate that structural abnormalities develop with age. Compared with young rats, old rats showed some disruption and loss of cristae in about half of the mitochondria in the dentate gyrus area, indicating structural decay. Animals treated with 0.5% ALCAR and/or 0.2% LA showed less structural disruption and loss of cristae. In addition, old rats had more lipofuscin in the cytoplasm of granule cells of the dentate gyrus, and the combined treatment rats also seemed to have less lipofuscin. However, these results were obtained from one or two animals per group. Clearly, further quantitative studies with more animals and more fields are needed to confirm these observations.
Oxidative Damage to Nucleic Acids.
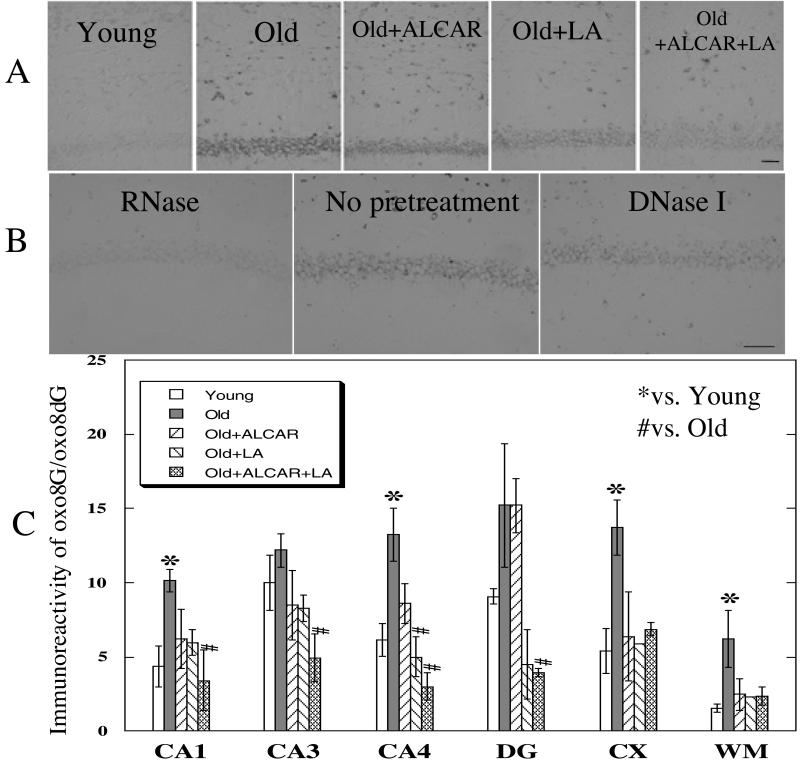
Various regions of the brain were stained with an Ab that recognizes oxidized DNA or RNA (oxo8dG or oxo8G; ref. 39). Fig. 3A shows representative images of the CA1 region of the hippocampus. Fig. 3C shows that old rats without treatment showed significantly higher immunoreactivity than young rats in areas CA1, CA4, cerebral cortex, and in the white matter. Both ALCAR and LA reduced immunoreactivity, but only LA showed a significant effect in the CA4 region. The combination showed a significant effect on lowering immunoreactivity in CA1, CA3, CA4, and dentate granule cells in old rats.
Figure 3.
Immunostaining relative to age and treatment for oxidized nucleic acids in neurons. (A) Representative photographs of oxo8G immunoreactivity in area CA1 of the hippocampus and adjacent white matter, from individual rats selected from young, old, old + ALCAR (0.5%), old + LA (0.2%), and old + ALCAR (0.5%) + LA (0.2%) groups. (B) CA1 sections pretreated with either DNase or RNase before incubation with Ab. (C) Extent of immunoreactivity to oxo8G in the hippocampus [CA1, CA3, CA4, dentate gyrus (DG), cerebral cortex (CX), and white matter (WM) in rat brain]. [Bar = 50 μm.] Values are mean ± SEM of 5 animals for young and old groups, 3 for old + ALCAR and old + LA groups, and 2 for the old + ALCAR + LA group. The Mann–Whitney U test was used to compare values. *, P < 0.005 vs. young rats; #, P < 0.05 vs. old control rats.
Fig. 3B illustrates that pretreatment of sections, including area CA1 with RNase but not DNase, virtually eliminated the immunoreactivity, indicating that the predominant damage to neuronal nucleic acids is to RNA (oxo8G). In CA1, RNase pretreatment reduced the immunoreactivity by 92%, whereas DNase, rather than reducing, enhanced (168%) the immunoreactivity (Fig. 3B).
Discussion
Old rats have increased mitochondrial dysfunction and oxidative damage, which is associated with cognitive deficits in both spatial and temporal memory. Spatial memory relies on intact hippocampal function. Temporal memory may also be associated with the hippocampus, although it may be more closely associated with the striatum and cerebellum. The dietary administration of a combination of ALCAR and LA to old rats improves mitochondrial function in liver (40). The purpose of this study was to determine whether it also improves cognition.
Spatial memory was assayed in the Morris water maze. The Morris water maze has been used extensively to measure cognitive deficits in spatial memory in lesion studies (41–47) and in aging (48–52). Old rats showed decreased spatial memory compared with young rats; ALCAR and/or LA restored some of this function, the combination being more effective than each compound alone. We also observed significant age effects in the transfer test, which measures search accuracy and is considered a procedural (habitual) subcomponent of spatial memory (36). ALCAR and/or LA significantly restored performance in this test, the combination being more effective (P < 0.01) and not significantly different from that of young rats (Fig. 1B). Schenk and Morris (36) have shown that after a retrohippocampal lesion, the procedural component of spatial memory can be partially recovered after training. We also observed significant age effects on the latencies of animals in finding a visible platform, which is a control procedure used to detect sensory motor deficits or motivational differences that impair water maze performance. The dietary interventions have similar effects on the visible platform test as those observed during the hidden platform tests (Fig. 1C).
Many physiological changes occur with age and can have major consequences on cognitive performance (53). We observed age and treatment effects on several noncognitive factors, such as motivation and locomotor activity, which can potentially contribute to the cognitive results. The age-associated decline in the Morris water maze test, therefore, should not be considered solely a test of cognition, but also as revealing a general decline in other systems as a result of aging. Old animals are known to be less sensitive to pain and possibly to temperature, which may affect their motivation to find the hidden platform. ALCAR and LA reduce mitochondrial dysfunction in peripheral systems (31, 33, 54, 55), including sensory systems such as hearing (56). Therefore, improvements shown here in test performances attributable to ALCAR, LA, or their combination, including the visible platform test and ambulatory activity (see ref. 40), suggest that reversing mitochondrial decay might reverse age-associated declines in nervous, cardiovascular, visual, and auditory systems, as well as general effects on motivation and physical strength.
Temporal memory, as assayed by the peak procedure, measures the function of the internal clock, learning processes, attention, and exploratory behavior. The combination of LA with ALCAR showed a significant improvement on peak rate (P < 0.05). The peak procedure is a time-discrimination procedure, which resembles a discrete-trials fixed-interval schedule with catch trials; it has been used to study the timing abilities of animals (37). Several studies have shown that old rats have deficits in time perception (57–61). One advantage of the peak procedure is that it allows for comparison of performance by using different types of signals and sensory modalities. The similarity of performance with light and sound signals suggests that the deficits are the results of deficits in cognition as rats of different ages do not differ in their sensitivity to light and sound at the two levels of light and sound used in this study. Peak rate reflects changes in a response learning mechanism. Old rats had lower peak rates, suggesting that old animals have difficulty learning the relevant response. The combination of ALCAR and LA seems to have a complementary effect on improving the peak procedure performance.
Not all of the old rats tested had cognitive deficits; this resulted in a large SD and the need for larger numbers of rats to achieve statistical significance. In future experiments it would be useful to separate cognitively impaired from unimpaired old rats to show more pronounced effects in old rats that receive treatment (23, 52).
The current study also has tested the hypothesis that cognitive improvements in response to ALCAR and/or LA interventions are linked to reductions in oxidative damage in old brain. To measure oxidative damage to nucleic acids, we used an Ab that detects both oxidized DNA and RNA (39). RNase pretreatment decreased immunoreactivity extensively, whereas DNase had a smaller effect. This result suggests that the oxidized nucleic acid in the aged rat brain is predominantly RNA, which is consistent with studies in human brains with AD (38). It is clear that more than 90% immunoreactivity is from RNA, suggesting that RNA oxidation is a significant biomarker of aging in rat brain. The mechanism of the DNase enhancement of immunoreactivity remains unclear; the digestion of DNA may have unmasked binding sites allowing greater access of the mAb to the RNA. Cytoplasmic punctate staining is consistent with either cellular Rna or mtDNA/RNA. RNA being the predominant oxidized nucleic acid is consistent with the lack of staining of nuclear DNA. The type of RNA oxidized and its subcellular localization remain to be determined, particularly with respect to mitochondria, the most likely oxidant target and the one that is improved by ALCAR and/or LA. RNA oxidation increased significantly as a function of age in rats in areas CA1 and CA4 in the hippocampus, in cortical neurons, and in white matter in the frontoparietal cortex. Feeding old rats LA significantly reduced the levels of oxidized RNA in CA4. The combination of ALCAR and LA was effective in significantly reducing oxidative RNA damage in neurons in CA1, CA3, CA4, and dentate gyrus of the hippocampus to levels not significantly different from young animals.
Poorer performance on memory tasks by old rats could involve, in part, oxidative damage to RNA, with errors in translation (62) compromising protein synthesis critical for the formation of new memories (63, 64). Although oxidative damage to RNA has been shown to be more extensive than damage to DNA in urine and plasma (39), oxidized RNA has not been a focus of interest as an oxidative damage marker for brain aging or cognition, except in some patients with AD sample studies. Neuronal RNA oxidation is a prominent feature of vulnerable neurons in AD, Down's syndrome, and Parkinson's disease, all of which are diseases associated with severe cognitive deficits (38, 65, 66). Neuronal RNA oxidation may thus contribute to memory decline and serve as a sensitive marker for intervention studies. However, oxidant-induced enzyme dysfunction is also an important contributor to neuronal decay and aging (67–69).
The improving effects on performance on memory tasks by ALCAR and/or LA on hippocampal mitochondria are supported by morphological observations. There seems to be a loss of mitochondrial cristae with age. Evidence that ALCAR reversed this loss with a dose-dependent response has been presented (34). Similar to ALCAR, LA also reduced age-dependent cristae loss in the dentate granule cells of the hippocampus. Because ALCAR alone showed a virtually complete reversal of the cristae loss, we cannot say whether the combination has an improving effect or not, but it produced at least as large a reduction as the ALCAR or LA alone.
The loss of memory with age seems to be caused in good part by oxidative mitochondrial decay in neurons. (i) The effectiveness of the mitochondrial metabolites ALCAR and LA suggests that mitochondrial decay is involved. (ii) The oxidation of RNA/DNA in neurons is likely to be mitochondrial (70). (iii) Neuronal mitochondria show structural decay with age.
The cognition-improving effect of ALCAR may also be caused in part by the donation of an acetyl group for the synthesis of the neurotransmitter acetylcholine through choline acetyltransferase and carnitine acetyltransferase (17, 71, 72). Low acetylcholine levels in certain brain regions are associated with age-related cognitive dysfunction, including AD (73). Because of the profound effects of calorie restriction, we have compared dietary intakes carefully and have found no significant differences in food and water consumption or in body weight (see Materials and Methods).
In conclusion, feeding old rats ALCAR and/or LA improved performance on memory tasks, reduced brain mitochondrial structure decay, and reduced oxidative damage in the brain. The combination of ALCAR and LA showed a greater effect than ALCAR or LA alone. These results suggest that feeding a combination of mitochondrial metabolites to old animals may prevent mitochondrial decay in neurons and restore cognitive dysfunction. These results also suggest that consumption of high levels of mitochondrial metabolites may be an efficient intervention in humans for delaying brain aging and age-associated neurodegenerative diseases.
Acknowledgments
We are indebted to Seth Roberts for his stimulation and advice in using the peak procedure; Judith Campisi, John Nides, and Seth Roberts for critical reading of the manuscript; and the Electron Microscope Lab at the University of California at Berkeley for the electron microscopic studies. We thank M. Nistor at the Institute for Brain, Aging, and Dementia for technical assistance. This work was supported by grants from the Ellison Foundation, the National Institute on Aging, the Wheeler Fund of the Dean of Biology, and the National Institute of Environmental Health Sciences Center Grant ES01896 (to B.N.A.), and by National Institute of Aging Grant AG12694 (to C.W.C.).
Abbreviations
- ALCAR
acetyl-l-carnitine
- LA
R-α-lipoic acid
- oxo8dG
8-hydroxy-2′-deoxyguanosine
- oxo8G
8-hydroxyguanosine
- AD
Alzheimer's disease
References
- 1.Grady C L, McIntosh A R, Horwitz B, Maisog J M, Ungerleider L G, Mentis M J, Pietrini P, Schapiro M B, Haxby J V. Science. 1995;269:218–221. doi: 10.1126/science.7618082. [DOI] [PubMed] [Google Scholar]
- 2.Wallace J E, Krauter E E, Campbell B A. J Gerontol. 1980;35:355–363. doi: 10.1093/geronj/35.3.355. [DOI] [PubMed] [Google Scholar]
- 3.Rahner-Welsch S, Frolich L, Stoll S, Hoyer S. Neurosci Lett. 1995;194:121–123. doi: 10.1016/0304-3940(95)11712-6. [DOI] [PubMed] [Google Scholar]
- 4.Head E, Mehta R, Hartley J, Kameka M, Cummings B J, Cotman C W, Ruehl W W, Milgram N W. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- 5.Forster M J, Dubey A, Dawson K M, Stutts W A, Lal H, Sohal R S. Proc Natl Acad Sci USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carney J M, Carney A M. Life Sci. 1994;55:2097–2103. doi: 10.1016/0024-3205(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 7.Carney J M, Starke R P, Oliver C N, Landum R W, Cheng M S, Wu J F, Floyd R A. Proc Natl Acad Sci USA. 1991;88:3633–3636. doi: 10.1073/pnas.88.9.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandekar T. Redox Rep. 1997;3:71–73. doi: 10.1080/13510002.1997.11747093. [DOI] [PubMed] [Google Scholar]
- 9.Shukitt-Hale B, Smith D E, Meydani M, Joseph J A. Exp Gerontol. 1999;34:797–808. doi: 10.1016/s0531-5565(99)00039-x. [DOI] [PubMed] [Google Scholar]
- 10.Guerrero A L, Dorado-Martinez C, Rodriguez A, Pedroza-Rios K, Borgonio-Perez G, Rivas-Arancibia S. NeuroReport. 1999;10:1689–1692. doi: 10.1097/00001756-199906030-00012. [DOI] [PubMed] [Google Scholar]
- 11.Packer L, Roy S, Sen C K. Adv Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 12.Packer L, Tritschler H J, Wessel K. Free Radical Biol Med. 1997;22:359–378. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 13.Stoll S, Hartmann H, Cohen S A, Muller W E. Pharmacol Biochem Behav. 1993;46:799–805. doi: 10.1016/0091-3057(93)90204-7. [DOI] [PubMed] [Google Scholar]
- 14.Bieber L L. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- 15.Rebouche C J. FASEB J. 1992;6:3379–3386. [PubMed] [Google Scholar]
- 16.Kidd P M. Alt Med Rev. 1999;4:144–161. [PubMed] [Google Scholar]
- 17.Ando S, Tadenuma T, Tanaka Y, Fukui F, Kobayashi S, Ohashi Y, Kawabata T. J Neurosci Res. 2001;66:266–271. doi: 10.1002/jnr.1220. [DOI] [PubMed] [Google Scholar]
- 18.Barnes C A, Markowska A L, Ingram D K, Kametani H, Spangler E L, Lemken V J, Olton D S. Neurobiol Aging. 1990;11:499–506. doi: 10.1016/0197-4580(90)90110-l. [DOI] [PubMed] [Google Scholar]
- 19.Caprioli A, Ghirardi O, Ramacci M T, Angelucci L. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14:359–369. doi: 10.1016/0278-5846(90)90024-b. [DOI] [PubMed] [Google Scholar]
- 20.Bertoni-Freddari C, Fattoretti P, Casoli T, Spagna C, Casell U. Brain Res. 1994;656:359–366. doi: 10.1016/0006-8993(94)91480-x. [DOI] [PubMed] [Google Scholar]
- 21.Ghirardi O, Caprioli A, Milano S, Giuliani A, Ramacci M T, Angelucci L. Physiol Behav. 1992;52:185–187. doi: 10.1016/0031-9384(92)90451-7. [DOI] [PubMed] [Google Scholar]
- 22.Ghirardi O, Giuliani A, Caprioli A, Ramacci M T, Angelucci L. J Neurosci Res. 1992;31:375–379. doi: 10.1002/jnr.490310220. [DOI] [PubMed] [Google Scholar]
- 23.Taglialatela G, Caprioli A, Giuliani A, Ghirardi O. Exp Gerontol. 1996;31:577–587. doi: 10.1016/0531-5565(96)00052-6. [DOI] [PubMed] [Google Scholar]
- 24.Bonavita E. Int J Clin Pharmacol Ther Toxicol. 1986;24:511–516. [PubMed] [Google Scholar]
- 25.Rai G, Wright G, Scott L, Beston B, Rest J, Exton-Smith A N. Curr Med Res Opin. 1990;11:638–647. doi: 10.1185/03007999009112690. [DOI] [PubMed] [Google Scholar]
- 26.Hager K, Marahrens A, Kenklies M, Riederer P, Munch G. Arch Gerontol Geriatr. 2001;32:275–282. doi: 10.1016/s0167-4943(01)00104-2. [DOI] [PubMed] [Google Scholar]
- 27.Ames B N, Shigenaga M K, Hagen T M. Proc Natl Acad Sci USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harman D. J Anti-Aging Med. 1999;2:15–36. [Google Scholar]
- 29.Shigenaga M K, Hagen T M, Ames B N. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hagen T M, Wehr C M, Ames B N. Ann NY Acad Sci. 1998;854:214–223. doi: 10.1111/j.1749-6632.1998.tb09904.x. [DOI] [PubMed] [Google Scholar]
- 31.Hagen T M, Ingersoll R T, Wehr C M, Lykkesfeldt J, Vinarsky V, Bartholomew J C, Song M H, Ames B N. Proc Natl Acad Sci USA. 1998;95:9562–9566. doi: 10.1073/pnas.95.16.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagen T M, Ingersoll R T, Lykkesfeldt J, Liu J, Wehr C M, Vinarsky V, Bartholomew J C, Ames A B. FASEB J. 1999;13:411–418. doi: 10.1096/fasebj.13.2.411. [DOI] [PubMed] [Google Scholar]
- 33.Hagen T M, Vinarsky V, Wehr C M, Ames B N. Antioxid Redox Signal. 2000;2:473–483. doi: 10.1089/15230860050192251. [DOI] [PubMed] [Google Scholar]
- 34.Liu, J., Atamna, H., Kuratsyne, H. & Ames, B. N. (2002) Ann. N.Y. Acad. Sci., 959, in press. [DOI] [PubMed]
- 35.Morris R. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 36.Schenk F, Morris R G. Exp Brain Res. 1985;58:11–28. doi: 10.1007/BF00238949. [DOI] [PubMed] [Google Scholar]
- 37.Roberts S. J Exp Psychol Anim Behav Processes. 1981;7:242–268. [PubMed] [Google Scholar]
- 38.Nunomura A, Perry G, Pappolla M A, Wade R, Hirai K, Chiba S, Smith M A. J Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park E M, Shigenaga M K, Degan P, Korn T S, Kitzler J W, Wehr C M, Kolachana P, Ames B N. Proc Natl Acad Sci USA. 1992;89:3375–3379. doi: 10.1073/pnas.89.8.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagen T M, Liu J, Lykkesfeldt J, Wehr C M, Ingersoll R T, Vinarsky V, Bartholomew J C, Ames B N. Proc Natl Acad Sci USA. 2002;99:1870–1875. doi: 10.1073/pnas.261708898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brandeis R, Brandys Y, Yehuda S. Int J Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 42.Dalm S, Grootendorst J, de Kloet E R, Oitzl M S. Behav Res Methods Instrum Comput. 2000;32:134–139. doi: 10.3758/bf03200795. [DOI] [PubMed] [Google Scholar]
- 43.Hatfield T, McGaugh J L. Neurobiol Learn Mem. 1999;71:232–239. doi: 10.1006/nlme.1998.3875. [DOI] [PubMed] [Google Scholar]
- 44.Lai H, Carino M A, Ushijima I. Bioelectromagnetics. 1998;19:117–122. [PubMed] [Google Scholar]
- 45.Moghaddam M, Bures J. Neurobiol Learn Mem. 1997;68:239–251. doi: 10.1006/nlme.1997.3800. [DOI] [PubMed] [Google Scholar]
- 46.McAlonan G M, Dawson G R, Wilkinson L O, Robbins T W, Everitt B J. Eur J Neurosci. 1995;7:1034–1049. doi: 10.1111/j.1460-9568.1995.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 47.Kant G J, Yen M H, D'Angelo P C, Brown A J, Eggleston T. Pharmacol Biochem Behav. 1988;31:487–491. doi: 10.1016/0091-3057(88)90378-4. [DOI] [PubMed] [Google Scholar]
- 48.Joseph J A, Shukitt-Hale B, Denisova N A, Bielinski D, Martin A, McEwen J J, Bickford P C. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shukitt-Hale B, Mouzakis G, Joseph J A. Exp Gerontol. 1998;33:615–624. doi: 10.1016/s0531-5565(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 50.Socci D J, Crandall B M, Arendash G W. Brain Res. 1995;693:88–94. doi: 10.1016/0006-8993(95)00707-w. [DOI] [PubMed] [Google Scholar]
- 51.Sack C A, Socci D J, Crandall B M, Arendash G W. Neurosci Lett. 1996;205:181–184. doi: 10.1016/0304-3940(96)12417-4. [DOI] [PubMed] [Google Scholar]
- 52.Issa A M, Rowe W, Gauthier S, Meaney M J. J Neurosci. 1990;10:3247–3254. doi: 10.1523/JNEUROSCI.10-10-03247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andrews J S. Brain Res Cogn Brain Res. 1996;3:251–267. doi: 10.1016/0926-6410(96)00011-0. [DOI] [PubMed] [Google Scholar]
- 54.Arivazhagan P, Pramanathan K, Panneerselvam C. J Nutr Biochem. 2001;12:2–6. doi: 10.1016/s0955-2863(00)00138-8. [DOI] [PubMed] [Google Scholar]
- 55.Paradies G, Ruggiero F M, Petrosillo G, Gadaleta M N, Quagliariello E. Mech Aging Dev. 1995;84:103–112. doi: 10.1016/0047-6374(95)01636-8. [DOI] [PubMed] [Google Scholar]
- 56.Seidman M D, Khan M J, Bai U, Shirwany N, Quirk W S. Am J Otol. 2000;21:161–167. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- 57.Campbell B A, Haroutunian V. J Gerontol. 1981;36:338–341. doi: 10.1093/geronj/36.3.338. [DOI] [PubMed] [Google Scholar]
- 58.Soffie M, Lejeune H. Neurobiol Aging. 1991;12:25–30. doi: 10.1016/0197-4580(91)90035-i. [DOI] [PubMed] [Google Scholar]
- 59.Meck W H, Church R M, Wenk G L. Eur J Pharmacol. 1986;130:327–331. doi: 10.1016/0014-2999(86)90287-6. [DOI] [PubMed] [Google Scholar]
- 60.Lejeune H, Maquet P, Bonnet M, Casini L, Ferrara A, Macar F, Pouthas V, Timsit-Berthier M, Vidal F. Neurosci Lett. 1997;235:21–24. doi: 10.1016/s0304-3940(97)00698-8. [DOI] [PubMed] [Google Scholar]
- 61.Lejeune H, Ferrara A, Simons F, Wearden J H. J Exp Psychol Anim Behav Processes. 1997;23:211–231. doi: 10.1037//0097-7403.23.2.211. [DOI] [PubMed] [Google Scholar]
- 62.Rhee Y, Valentine M R, Termini J. Nucleic Acids Res. 1995;23:3275–3282. doi: 10.1093/nar/23.16.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meiri N, Rosenblum K. Brain Res. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- 64.Wells D G, Fallon J R. Cell Mol Life Sci. 2000;57:1335–1339. doi: 10.1007/PL00000618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nunomura A, Perry G, Hirai K, Aliev G, Takeda A, Chiba S, Smith M A. Ann NY Acad Sci. 1999;893:362–364. doi: 10.1111/j.1749-6632.1999.tb07855.x. [DOI] [PubMed] [Google Scholar]
- 66.Nunomura A, Perry G, Zhang J, Montine T J, Takeda A, Chiba S, Smith M A. J Anti-Aging Med. 1999;2:227–230. [Google Scholar]
- 67.Fucci L, Oliver C N, Coon M J, Stadtman E R. Proc Natl Acad Sci USA. 1983;80:1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stadtman E R, Levine R L. Ann NY Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu J, Killilea D W, Ames B N. Proc Natl Acad Sci USA. 2002;99:1876–1881. doi: 10.1073/pnas.261709098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirai K, Aliev G, Nunomura A, Fujioka H, Russell R L, Atwood C S, Johnson A B, Kress Y, Vinters H V, Tabaton M, et al. J Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goodman D R, Harbison R D. Biochem Pharmacol. 1981;30:1521–1528. doi: 10.1016/0006-2952(81)90376-2. [DOI] [PubMed] [Google Scholar]
- 72.White H L, Scates P W. Neurochem Res. 1990;15:597–601. doi: 10.1007/BF00973749. [DOI] [PubMed] [Google Scholar]
- 73.Coyle J T, Price D L, DeLong M R. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]