Abstract
Protein synthesis in neurons is essential for the consolidation of memory and for the stabilization of activity-dependent forms of synaptic plasticity such as long-term potentiation (LTP). Activity-dependent translation of dendritically localized mRNAs has been proposed to be a critical source of new proteins necessary for synaptic change. mRNA for the activity-regulated cytoskeletal protein, Arc, is transcribed during LTP and learning, and disruption of its translation gives rise to deficits in both. We have found that selective translation of Arc in a synaptoneurosomal preparation is induced by the brain-derived neurotrophic factor, a neurotrophin that is released during high-frequency stimulation patterns used to elicit LTP. This effect involves signaling through the TrkB receptor and is blocked by the N-methyl-d-aspartate-type glutamate receptor antagonist, MK801. The results suggest there is a synergy between neurotrophic and ionotropic mechanisms that may influence the specificity and duration of changes in synaptic efficacy at glutamatergic synapses.
The brain-derived neurotrophic factor (BDNF), a member of the nerve growth factor (NGF) superfamily of neurotrophins (1, 2), influences the differentiation and survival of neurons and the maintenance of their arborizations (3–6). Other data suggest that BDNF is also involved in both short- and long-term plasticity of glutamatergic synapses (7, 8). BDNF signaling enhances synaptic maturation and increases synaptic density in the hippocampus (9), and in the adult the synthesis and secretion of BDNF continue to be regulated by activity (10–12). Exogenous application of BDNF induces a rapid and persistent enhancement of synaptic transmission in hippocampal and cortical preparations (13, 14) and facilitates the induction of long-term potentiation (LTP) in slices from young animals (15). Stimulation patterns used to elicit LTP also result in release of the BDNF protein from presynaptic terminals (16, 17).
Local protein synthesis may influence changes in synaptic efficacy induced by BDNF. Early and reversible effects of BDNF involve pre- and postsynaptic changes that do not require protein synthesis. For example, application of BDNF enhances evoked glutamate release (18) and increases the probability that the N-methyl-d-aspartate (NMDA) receptor channel will be open (19). Longer-term influences on synaptic efficacy, however, may require BDNF-induced local protein synthesis. BDNF-induced synaptic potentiation in hippocampal slices is blocked by inhibitors of protein synthesis (20). Potentiation induced by BDNF can be obtained in slices in which synapses have been severed from cell bodies, suggesting that synthesis of the required protein(s) is dendritically localized (20). In accord with this possibility, it has been shown that BDNF specifically induces the expression of transfected c-myc in dissected dendrites (21), and the dendritic synthesis of a reporter protein construct flanked by the 5′ and 3′ untranslated regions of α-calmodulin-dependent protein kinase II [α-CaMKII (22)]. These observations indicate that BDNF can induce local translation of dendritically targeted mRNAs and raise the possibility that the neurotrophin can act in concert with activity-dependent increases in mRNA levels (23) to affect synaptic efficacy.
The activity-regulated cytoskeletal-associated protein, Arc/Arg3.1 (24, 26), is one protein the translation of which may be regulated by BDNF. Arc is an immediate early gene product thought to be involved in the stabilization of synaptic efficacy changes. Both Arc mRNA and protein levels are up-regulated by intense synaptic activity (24–26). Moreover, Arc mRNA is rapidly distributed into dendrites and localized to areas of intense synaptic activation in an NMDA receptor-dependent manner (23, 34). The protein is locally translated and, by virtue of its ability to bind actin, may participate in the reconfiguration of synapses to stabilize efficacy changes. Disruption of the expression of Arc by using antisense oligonucleotides impairs the maintenance phase of LTP without affecting its induction and blocks consolidation of long-term spatial memory without affecting task acquisition or short-term performance (27).
To determine whether BDNF can modulate the dendritic translation of Arc, we quantified BDNF-induced changes in synaptic Arc levels in a synaptoneurosome (SNS) preparation. The data show a selective up-regulation of Arc protein synthesis by BDNF that depends, at least in part, on the activation of NMDA receptors. The results are discussed in terms of their potential significance to changes in synaptic efficacy induced by BDNF and possible synergistic actions between ionotropic and neurotrophic mechanisms controlling plasticity at glutamatergic synapses.
Materials and Methods
Preparation of SNSs.
SNSs were prepared according to reported procedures (28). Briefly, cortex and hippocampus were dissected from 17–22-day postnatal Sprague–Dawley rats that were anesthetized with Metofane or CO2. Tissue was minced with scissors and homogenized with a glass homogenizer in a buffer composed of 33% sucrose, 10 mM Hepes, 0.5 mM EGTA, pH 7.4. After centrifuging for 2 min at 2000 × g, the supernatant was collected and passed through nylon membranes down to 5 μm pore size. The flow-through was centrifuged for 15 min at 1000 × g. The pellet was resuspended in incubation buffer (in mM: 8 KCl/3 CaCl2/5 Na2HPO4/2 MgCl2/33 Tris/72 NaCl/100 sucrose) and diluted to a protein concentration of 0.5–1.0 mg/ml. For each subsequent experiment, aliquots (500–700 μl) from a common preequilibrated stock of SNSs were used.
Reverse Transcription (RT)-PCR.
Total RNA from SNS was extracted with TRIzol (Life Technologies, Rockville, MD). Equal amounts of total RNA were used for first-strand cDNA synthesis with random primers and equal amounts of first-strand cDNA of each sample were amplified further by PCR with specific primers as follows: CaMKIIα, forward sequence: atggctaccatcacctgcaccc; reverse sequence, ggagaggtatccaggtgtccct gcg; Arc: forward sequence, atggagctggaccatatgacgacc; reverse sequence, ctattcaggctgggtcctgt cact. Amplified signals were visualized by ethidium bromide staining after electrophoresis.
Radioisotope Labeling and Scintillation Counting.
SNSs were incubated for 10 min at 37°C before each experiment. MK801 (100 μM) or K252α (200–500 μM) were preincubated for an additional 15–20 min. After preincubation, BDNF (30 ng/ml) was applied for 30 min at 37°C before addition of [35S]methionine and cysteine at a concentration of 100 μCi/ml. After a 10-min labeling followed by a 10-min chase with 200 μM nonradioactive methionine and cysteine, SNSs were spun for 10 min at 6,000 rpm. The supernatant was discarded, the pellet was lysed, and protein was precipitated with 20% trichloroacetic acid (TCA). The precipitate was washed 3 times with 5% TCA followed by 1 wash with cold acetone. Pellets were air-dried and resuspended in 0.5 N NaOH for scintillation counting.
Electrophoresis, Autoradiography, and Immunoblotting.
After treatment, SNS samples were lysed by tip sonication in chilled buffer [1% Nonidet P-40/0.5% TX-100/50 mM DTT/10 mM Tris (pH = 8.0)]. For one-dimensional (1D) separation, proteins were resolved by SDS/PAGE (10–12% gels). For two-dimensional (2D) electrophoresis, manufacturer's protocols were followed (Bio-Rad). In initial experiments, SNS pellets were lysed by tip sonication directly in a buffer composed of 5 M urea, 2 M thiourea, 2% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS), 2% SB3–10, and 2 mM TATA box-binding protein (TBP). In the majority of experiments, samples were solubilized first for 1D electrophoresis and centrifuged (30 min at 60,000 rpm in a Beckman TLA100 rotor) to remove insoluble material; this approach did not result in a detectable loss of Arc, synaptotagmin, or tubulin. After addition of rehydration buffer (8 M urea/2% CHAPS/0.05% IP6, bromophenol blue), the supernatant was used for isoelectric focusing with immobilized pH gradient strips (Amersham Biosciences or Bio-Rad), pH range 3–10, at room temperature overnight. After re-equilibration with buffer (50 mM Tris/6 M urea/30% glycerol/2% SDS, bromophenol blue, pH = 7.5), the second dimension was resolved by SDS/PAGE on 4–12% gradient gels.
Proteins resolved in 1D or 2D were transferred to poly(vinylidene difluoride) (PVDF; Invitrogen) or nitrocellulose membranes (Millipore) for autoradiographic or immunological detection of SNS proteins. For autoradiography, films (Kodak) were scanned on a desktop scanner, and densitometric quantification was performed by using imagequant (Molecular Dynamics). For immunoblotting, primary Abs were Arc (1:1000; Santa Cruz Biotechnology), β-tubulin (1:3000; Roche Diagnostics), and synaptotagmin (1:2000; Santa Cruz Biotechnology). Detection used the enhanced chemiluminescence kit from Amersham Biosciences.
For immunoprecipitation, SNSs were lysed with RIPA buffer (1× PBS/1% Nonidet P-40/0.5% sodium deoxycholate/0.1% SDS) and a complete protease inhibitor tablet (Roche Molecular Biochemicals).
Data Analysis.
Data on Arc levels from immunoblots (2D and 1D) were normalized by using synaptotagmin or β-tubulin as internal references for statistical analyses. The paired t test (graphpad prism, GraphPad , San Diego) was used to statistically evaluate the collected data. P ≤ 0.05 was considered significant.
Results
An SNS preparation (28) was used to study the effects of BDNF on overall dendritic protein synthesis and translation of the immediate early gene Arc. The preparation contains an enriched fraction of resealed and functional pre- and postsynaptic junctions. As such, it can be used for quantitative biochemical analyses of rapid changes in protein expression from existing mRNAs with minimal contributions from transcription.
Dendritically Transported and Translated mRNAs Are Retained in the SNS Preparation.
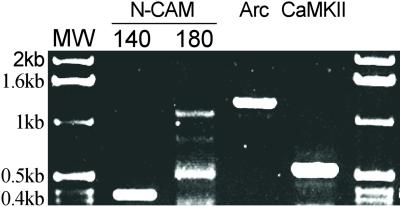
Critical to these experiments is the demonstration that mRNAs known to be targeted to dendrites and locally translated are retained in the SNS preparation. RT-PCR was used to confirm that the mRNAs for Arc and another dendritically localized message, CaMKIIα, are retained in the SNS preparation. Total RNA was directly extracted from SNS, and RT-PCR analysis was performed with specific primers (see Materials and Methods). mRNAs for the dendritically localized Arc and CaMKIIα, as well as for the neural cell adhesion molecule, were detected in the SNS preparation (Fig. 1).
Figure 1.
Electrophoretic gel of RT-PCR-amplified mRNAs in SNS. Total RNA extracted from SNS samples was used for RT-PCR amplification. Primers for specific segments of Arc, CaMKIIα, and neural cell adhesion molecule 140 and -180 isoforms were used for PCR detection.
BDNF Treatment Induces Changes in a Subset of Synaptosomal Proteins Without Altering Total Protein Synthesis.
To study the potential role of BDNF in regulating local translation, we first measured incorporation of [35S]methionine and cysteine into total protein in SNS preparations incubated with or without BDNF. After 10 min of labeling followed by a 10-min chase with nonradioactive amino acids, proteins in SNS samples were precipitated with trichloroacetic acid for scintillation counting or lysed for 2D electrophoresis (see Materials and Methods). Total protein incorporation of radiolabeled amino acids was not significantly affected by BDNF treatment (97.5% ± 1.1% of control). As a positive control, SNSs were treated with 25 mM KCl, which is known to increase protein synthesis in both SNS and cultured cells (29). KCl increased incorporation of label into total proteins to 117% ± 1.4% of the control, and the protein synthesis inhibitor Emetine reduced incorporation to 77% ± 1.2% of control.
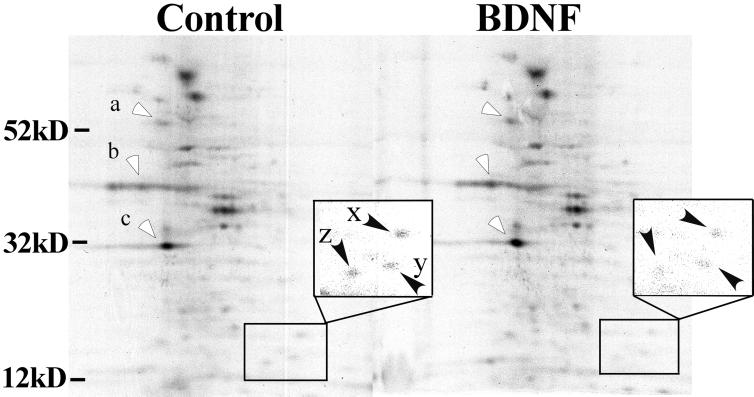
The effects of BDNF were further characterized by 2-D gel electrophoresis. Fig. 2 shows labeled SNS proteins from control and BDNF-treated samples resolved by means of 2D gel electrophoresis and visualized with autoradiography. There was little or no difference in the overall pattern of synaptic protein synthesis, but specific proteins in BDNF-treated samples showed a substantial increase in their intensity (Fig. 2, open arrowheads), whereas others showed little change or even a slight decrease (Inset, closed arrowheads). Densitometric measurements indicated that the average intensity of the signals for proteins at molecular masses of 34, 46, and 56 kDa (labeled a–c, respectively) increased by 30–40% of paired controls. The data suggest that BDNF selectively increases the synthesis of certain proteins in the SNS preparation.
Figure 2.
BDNF increases incorporation of [35S]methionine into a subset of proteins. Total synaptoneurosomal protein was extracted, and proteins were separated by 2D electrophoresis from both control and BDNF-treated samples after radioactive labeling. Proteins with increased 35S label in response to BDNF are marked with open arrowheads. As determined from several independent experiments, the protein at molecular mass 34 kDa (c) was increased by 34% ± 3.9% (n = 8, P < 0 01), at 46 kDa (b) by 43.4% ± 2.4% (n = 6, P < 0.01), and at 56 kDa (a) by 40.4% ± 2.3% (n = 7, P < 0.01). Stated values are means ± SE. Most proteins showed no change and some (marked with filled arrowheads in Inset) were actually reduced in samples treated with BDNF (Right). The intensity ratios (BDNF to control) of spots x, y, and z in the rectangle are 0.78, 0.89, and 0.96, respectively.
BDNF Enhances Synthesis of Arc Protein in SNSs.
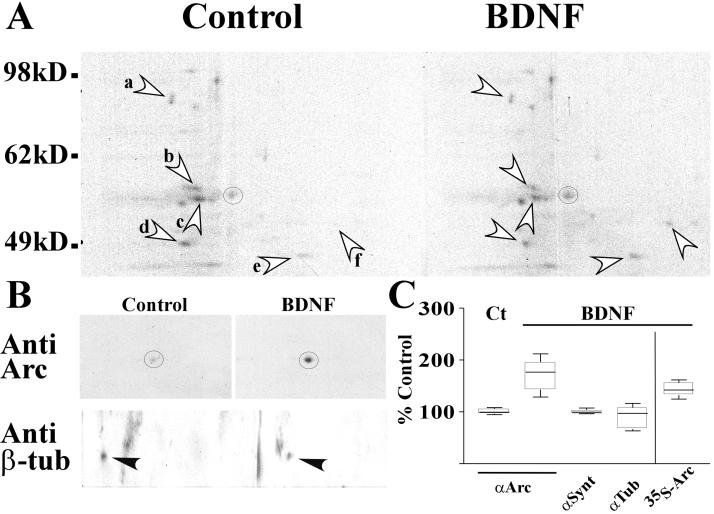
To determine whether Arc synthesis was increased by BDNF treatment, immunoblot analysis was conducted on the same membranes used in the 2D gel studies described previously. After labeling of SNS, equal amounts of total proteins extracted from BDNF-treated and control samples were subjected to 2D electrophoresis and transferred to membranes for autoradiography (Fig. 3A). Probing with a specific anti-Arc Ab (Fig. 3B) demonstrated that net expression of Arc in SNS was increased in response to BDNF stimulation. Incorporation of label into the corresponding spot in the autoradiogram (Fig. 3B, circle) was also increased by treatment with BDNF. Average incorporation of labeled amino acids into Arc and net Arc protein levels measured by immunoblot were increased by 43% and 70% of paired controls, respectively (Fig. 3C). On the same membrane, the level of β-tubulin, a cytoskeletal protein, decreased slightly in response to BDNF (Fig. 3B), and those of other unidentified proteins either increased or decreased (Fig. 3A a–e). The results indicate that Arc can be locally translated and that BDNF treatment can augment this process in a relatively selective manner. The different amounts of Arc detected by incorporation of label (newly synthesized protein) and immunoblot (total Arc) suggest that BDNF may also reduce normal degradation of Arc.
Figure 3.
BDNF selectively enhances Arc label incorporation and expression. Arc protein incorporation and net protein expression are analyzed with autoradiography (A) followed by immunoblot (B) of the same membrane after 2D electrophoresis. Both 35S incorporation and overall Arc protein expression measured on blots, marked with circles, were significantly increased by BDNF treatment. However, BDNF treatment reduced the incorporation of 35S into spots a (0.86), b (0.64), c (0.82), and d (0.91) and increased spots e (1.25) and f (2.74), compared with the control. BDNF did not significantly increase β-tubulin levels overall, and in the experiment represented in B actually decreased β-tubulin by 37% of the control. (C) Summary (range, quartiles, median) of independent experimental results obtained from both autoradiography and immunoblots. The variance of basal Arc protein levels in SNSs was ± 2.1% (SEM) as determined with immunoblots (n = 3) normalized to synaptotagmin levels. On average, BDNF increased Arc 35S incorporation and overall Arc protein levels by 43% ± 4.8% (n = 8, P < 0.01) and 70% ± 9.13% (n = 12, P < 0.01), respectively, relative to paired controls. However, BDNF treatment did not change synaptotagmin (99% ± 1.4%, n = 7, P > 0.05) and β-tubulin (90% ± 7.7%, n = 8, P > 0.05) protein levels compared with controls.
The effect of BDNF on the expression of Arc was verified further by quantitative immunoprecipitation, using a polyclonal anti-Arc Ab followed by immunoblot analysis with a monoclonal anti-Arc Ab. In response to BDNF, the overall level of Arc protein increased to 130% of the control. This increase was reversed by preincubation with the protein synthesis inhibitor Emetine. These data indicate that translation of Arc is up-regulated in a rapid and relatively specific manner by BDNF and that in the absence of BDNF Arc has a short half-life in the SNS preparation.
BDNF-Induced Up-Regulation of Arc Protein Is Attenuated by a Tyrosine Kinase Inhibitor and by the NMDA Receptor Antagonist MK801.
To examine the signaling events associated with BDNF-induced up-regulation of Arc synthesis, we first tested the effects of inhibiting tyrosine kinase activity with a nonspecific inhibitor, K252α, to block activity of the TrkB receptor for BDNF. When SNSs were preincubated with K252α for 20 min before addition of BDNF, Arc levels were reduced by 32% ± 2.5% (n = 9, P < 0.05) as compared with SNS-treated with BDNF alone. Levels of synaptotagmin after similar treatment were not altered (111% ± 8.9% of BDNF alone). Treatment with K252α alone did not significantly change the basal protein levels of Arc (109% ± 15.2% of control, n = 9) or synaptotagmin (110% ± 10.4% of control, n = 5). The data suggest that TrkB receptor kinase activity is at least partially required for BDNF to enhance the expression of Arc protein in the SNS preparation.
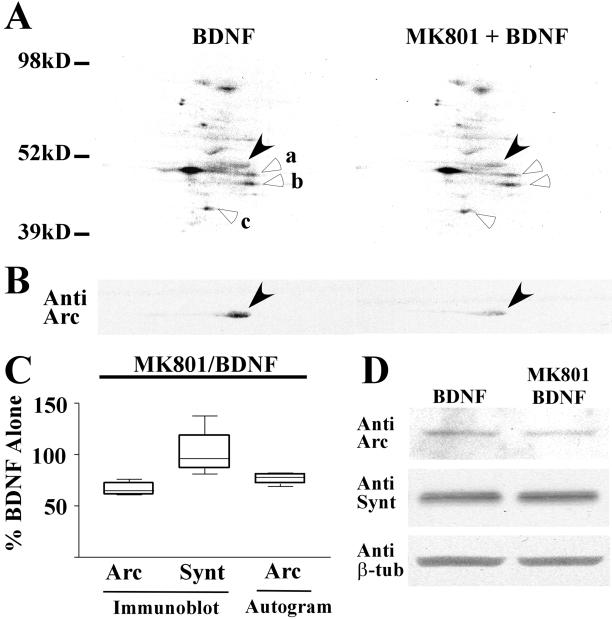
Previous studies have shown that BDNF induces phosphorylation of NMDA receptor subunits 1 and 2b (30, 31) and that application of the neurotrophin increases the open probability of the NMDA receptor channel (19, 32). In addition, recent reports indicate that NMDA receptor activation leads to changes in protein synthesis (33) and is essential for localization of the Arc message (34). We therefore tested the effect of blocking NMDA receptors during BDNF application. After a 20-min preincubation with the specific noncompetitive NMDA receptor antagonist, MK801, BDNF-induced increases of Arc synthesis were significantly reduced, both in autoradiograms (Fig. 4A) and immunoblots (Fig. 4B) of treated SNS samples resolved by 2D electrophoresis. On average (Fig. 4C), in the presence of MK801, the incorporation of radiolabeled amino acids into Arc protein as well as total Arc protein levels were reduced by 24% ± 4.7% in autoradiograms (n = 6) and by 34% ± 2.5% in immunoblots (n = 6). Preincubation with MK801 alone did not affect the basal level of Arc (97 ± 10.3 of control, n = 6). In addition, treatment with MK801 did not alter the level of synaptotagmin in SNSs as assessed by counterstaining Arc immunoblots with Abs to the presynaptic vesicle protein (Fig. 4C; n = 6). The attenuating effects of MK801 were also observed in 1D immunoblot experiments (Fig. 4D). These data suggest that changes in Arc protein expression levels after BDNF treatment depend on the status of NMDA receptors.
Figure 4.
The NMDA receptor antagonist MK801 prevents BDNF-induced increases in Arc protein synthesis. (A) The effect of the NMDA receptor antagonist MK801 on Arc protein incorporation was monitored with autoradiography (A) and immunoblot (B) after 2D electrophoresis. BDNF-induced Arc 35S incorporation (A) and overall Arc protein levels (B) were blocked by incubation with MK801 (filled arrowheads). Meanwhile, MK801 did not decrease the incorporation of radiolabeled amino acids into spots a (1.14), b (1.14), and c (1.15) marked with blank arrowheads. (C) Summary chart (range, quartiles, and median) displaying the results of six independent experiments performed as in A and analyzed for Arc and synaptotagmin protein levels. On average, MK801 suppressed BDNF-induced up-regulation of Arc 35S incorporation by 24% ± 4.7% (P < 0.01) and overall Arc protein levels by 34% ± 2.5% (P < 0.01) relative to BDNF alone. MK801 treatment did not significantly affect synaptotagmin levels (101% ± 7.6%, P > 0.05). MK801 alone (not shown) did not significantly affect the basal level of Arc protein (97% ± 10.26% compared with untreated controls). (D) A typical 1D SDS/PAGE Western blot in which BDNF and BDNF + MK801-treated samples were compared by using anti-Arc, anti-synaptotagmin, and anti-β-tubulin Abs. Preincubation with MK801 suppressed the BDNF-induced Arc protein increase by 35%; little or no change was evident for synaptotagmin (93% of BDNF alone) or β-tubulin (95% of BDNF alone).
Discussion
Previous work has shown that BDNF modulates transmission at glutamatergic synapses through multiple mechanisms (7, 35, 36) and that lasting effects on synaptic efficacy may involve local protein synthesis at postsynaptic sites (20). To gain further insight into the latter possibility, we used radiolabeled amino acid incorporation and Western blotting to measure BDNF-induced changes in proteins in an SNS preparation. We found that BDNF treatment led to increased Arc expression in a rapid and relatively selective manner without increasing overall protein synthesis. The BDNF-induced increase in Arc levels was attenuated by preincubation of SNSs with the NMDA receptor antagonist MK801 or with the tyrosine kinase inhibitor K252α.
Under our conditions, brief treatment with BDNF selectively increased the synthesis of only a subset of proteins in the SNS preparation, in contrast to the effects of KCl treatment, which increased total protein synthesis. Differential synthesis of synaptic proteins has been reported for chronic treatments with BDNF (37–41). For example, prolonged treatment of cultured neurons with the neurotrophin-increased α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor levels without changing those of NMDA receptors (37). This treatment up-regulated the expression of synaptic vesicle proteins synaptophysin, synaptotagmin, synaptobrevin, and neuregulin without changing levels of the presynaptic cytoskeletal proteins, synapsin1, syntaxin, and SNAP25 (38, 39). Selective regulation of non-L-type Ca2+ channels and type 3 dopamine receptors in vivo has also been reported (40, 41). However, in these chronic experiments in intact neurons, changes in protein levels may predominantly reflect differences in rates of somatic synthesis (transcription and translation), degradation, or transport to the synapse. Our data confirm that BDNF differentially modifies the expression of synaptic proteins, and we extend these observations to reveal that changes are rapid and a product of increased local translation.
The effects of BDNF on Arc may be similar to those controlling local CaMKIIα expression. In accord with this notion, preliminary data suggest that levels of CaMKIIα protein are increased by BDNF treatment in SNS preparations (Y.Y., unpublished observations). Arc and CaMKIIα mRNAs are found in association with polyribosomes in sucrose-gradient purified synaptosomes (42) and both messages are present in the SNS preparation used in this study. Translation of CaMKIIα is triggered rapidly by BDNF (22) and by NMDA (33) even without an increase in overall translation (43, 44). In the present study, incubation of SNSs with MK801 attenuated Arc synthesis triggered by BDNF, but did not alter baseline levels of protein synthesis. The basis of selective regulation of protein synthesis in dendrites is unknown, but it may reflect mechanisms for controlling translation that are unique to mRNAs encoding synaptic proteins involved in synaptic efficacy. It is of interest that both Arc and CaMKIIα mRNAs contain internal ribosomal entry sites within their 5′ leader sequences (45), which may give them a competitive advantage for translational machinery (33).
Although the data presented here support a role for BDNF in the regulation of local synthesis of Arc, there are certain considerations about the use of SNS preparations that must be addressed. First, although the preparation used is highly enriched in resealed synaptic junctions (29, 33, 46), we cannot exclude the possibility that some of the changes were the results of synthesis occurring in resealed fragments of cell bodies, neuronal or nonneuronal. However, this possibility is unlikely in that significant somatic contamination should increase the effect of BDNF on overall synthesis (22), which was not seen. This study did not address directly the possibility that BDNF also attenuates Arc turnover. However, reduced turnover is suggested by the differences in the magnitude of the BDNF effect observed by using Western blot vs. 35S incorporation.
BDNF-induced up-regulation of Arc expression may play a role in synaptic potentiation induced by the neurotrophin. Local protein synthesis triggered by BDNF may stabilize efficacy changes by stimulating more activity-dependent phosphorylation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and assisting in remodeling of the postsynaptic element into a more efficacious configuration. In accord with the latter idea, stabilization of LTP requires expression of Arc during a period (27) when the structural modifications associated with LTP are beginning to emerge (47), and BDNF signaling has been shown to have profound effects on synaptic density (48, 49), the thickness of the postsynaptic density (50), and the length of the active zone at asymmetric synapses (38).
The present results suggest a potentially important synergy between neurotrophic and ionotropic mechanisms for inducing synaptic plasticity. Protein synthesis is required in order for LTP expression to persist for hours or more, and new proteins may be specifically targeted to synapses undergoing plasticity. Arc mRNA is rapidly synthesized in response to LTP-inducing stimulation and spatial learning (24, 26) and is subsequently transported into activated regions of dendrites over hours (26, 51). Inhibition of Arc synthesis by infusion of antisense oligonucleotides blocks LTP stabilization and results in spatial learning deficits (27). In a recent report, Arc mRNA induced in dentate gyrus by electroconvulsive seizure was restricted to the middle molecular dendritic layer by subsequent high-frequency stimulation of the medial perforant path (34). This localizing effect was blocked by NMDA receptor antagonists applied in dentate gyrus after induction. Moreover, BDNF is transported in an anterograde fashion (52) and released in an activity-dependent manner in response to patterns of stimulation optimal for triggering LTP (53). BDNF is known to increase the open probability of NMDA receptors and to enhance glutamate release from presynaptic terminals, which may also augment NMDA receptor currents. Combined with our finding that BDNF-induced Arc synthesis requires NMDA receptor activation, these findings raise the possibility that BDNF can promote synapse-specific translation during LTP production or memory formation. BDNF released during intense synaptic activation could provide a mechanism for prolonged augmentation (32, 54) of NMDA currents or other factors regulating synaptic translation (55) in the absence of repeated episodes of high-frequency activity. Such a role for BDNF would fit well with several synaptic and behavioral models of memory consolidation.
Acknowledgments
We thank Drs. Kathryn L. Crossin, Bruce A. Cunningham, and Joseph Gally for their constructive criticisms. This work was supported by U.S. Public Health Service Grants NS39837 (to G.M.E.) and MH64036 (to P.W.V.), as well as by a grant from the G. Harold and Leila Y. Mathers Foundation. P.W.V. is partly supported by The Skaggs Institute of Chemical Biology.
Abbreviations
- BDNF
brain-derived neurotrophic factor
- LTP
long-term potentiation
- NMDA
N-methyl-d-aspartate
- CaMKIIα
α-calmodulin-dependent protein kinase II
- SNS
synaptoneurosome
- RT
reverse transcription
- 2D
two-dimensional
- 1D
one-dimensional
References
- 1.Barbacid M. J Neurobiol. 1994;25:1386–1403. doi: 10.1002/neu.480251107. [DOI] [PubMed] [Google Scholar]
- 2.Patapoutian A, Reichardt L F. Curr Opin Neurobiol. 2001;11:272–280. doi: 10.1016/s0959-4388(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh A, Carnahan J, Greenberg M E. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 4.Davies A M. Curr Biol. 2000;10:R198–R200. doi: 10.1016/s0960-9822(00)00351-1. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Cory S, Fraser S E. Nature (London) 1995;378:192–196. doi: 10.1038/378192a0. [DOI] [PubMed] [Google Scholar]
- 6.Alcantara S, Frisen J, del Rio J A, Soriano E, Barbacid M, Silos-Santiago I. J Neurosci. 1997;17:3623–3633. doi: 10.1523/JNEUROSCI.17-10-03623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuman E M. Curr Opin Neurobiol. 1999;9:105–109. doi: 10.1016/s0959-4388(99)80013-0. [DOI] [PubMed] [Google Scholar]
- 8.Lu B, Gottschalk W. Prog Brain Res. 2000;128:231–241. doi: 10.1016/S0079-6123(00)28020-5. [DOI] [PubMed] [Google Scholar]
- 9.Martinez A, Alcantara S, Borrell V, Del Rio J A, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, Soriano E. J Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isackson P J, Huntsman M M, Murray K D, Gall C M. Neuron. 1991;6:937–948. doi: 10.1016/0896-6273(91)90234-q. [DOI] [PubMed] [Google Scholar]
- 11.Nawa H, Carnahan J, Gall C. Eur J Neurosci. 1995;7:1527–1535. doi: 10.1111/j.1460-9568.1995.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 12.Kohara K, Kitamura A, Morishima M, Tsumoto T. Science. 2001;291:2419–2423. doi: 10.1126/science.1057415. [DOI] [PubMed] [Google Scholar]
- 13.Kang H, Schuman E M. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- 14.Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figurov A, Pozzo-Miller L D, Olafsson P, Wang T, Lu B. Nature (London) 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 16.Gooney M, Lynch M A. J Neurochem. 2001;77:1198–1207. doi: 10.1046/j.1471-4159.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- 17.Balkowiec A, Katz D M. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumoto T, Numakawa T, Adachi N, Yokomaku D, Yamagishi S, Takei N, Hatanaka H. J Neurochem. 2001;79:522–530. doi: 10.1046/j.1471-4159.2001.00591.x. [DOI] [PubMed] [Google Scholar]
- 19.Levine E S, Kolb J E. J Neurosci Res. 2000;62:357–362. doi: 10.1002/1097-4547(20001101)62:3<357::AID-JNR5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Kang H, Schuman E M. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- 21.Crino P B, Eberwine J. Neuron. 1996;17:1173–1187. doi: 10.1016/s0896-6273(00)80248-2. [DOI] [PubMed] [Google Scholar]
- 22.Aakalu G, Smith W B, Nguyen N, Jiang C, Schuman E M. Neuron. 2001;30:489–502. doi: 10.1016/s0896-6273(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 23.Steward O, Schuman E M. Annu Rev Neurosci. 2001;24:299–325. doi: 10.1146/annurev.neuro.24.1.299. [DOI] [PubMed] [Google Scholar]
- 24.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steward O, Wallace C S, Lyford G L, Worley P F. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 26.Lyford G L, Yamagata K, Kaufman W E, Barnes C A, Sanders L K, Copeland N G, Gilbert D J, Jenkins N A, Lanahan A A, Worley P F. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 27.Guzowski J F, Lyford G L, Stevenson G D, Houston F P, McGaugh J L, Worley P F, Barnes C A. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollingsworth E B, McNeal E T, Burton J L, Williams R J, Daly J W, Creveling C R. J Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weiler I J, Greenough W T. Mol Cell Neurosci. 1991;2:305–314. doi: 10.1016/1044-7431(91)90060-2. [DOI] [PubMed] [Google Scholar]
- 30.Suen P C, Wu K, Levine E S, Mount H T, Xu J L, Lin S Y, Black I B. Proc Natl Acad Sci USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin S Y, Wu K, Levine E S, Mount H T, Suen P C, Black I B. Brain Res Mol Brain Res. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- 32.Levine E S, Crozier R A, Black I B, Plummer M R. Proc Natl Acad Sci USA. 1998;95:10235–10239. doi: 10.1073/pnas.95.17.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheetz A J, Nairn A C, Constantine-Paton M. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- 34.Steward O, Worley P F. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 35.Black I B. J Neurobiol. 1999;41:108–118. [PubMed] [Google Scholar]
- 36.Lessmann V. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- 37.Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Neuroscience. 1999;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- 38.Tartaglia N, Du J, Tyler W J, Neale E, Pozzo-Miller L, Lu B. J Biol Chem. 2001;276:37585–37593. doi: 10.1074/jbc.M101683200. [DOI] [PubMed] [Google Scholar]
- 39.Loeb J A, Fischbach G D. J Neurosci. 1997;17:1416–1424. doi: 10.1523/JNEUROSCI.17-04-01416.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baldelli P, Forni P E, Carbone E. Eur J Neurosci. 2000;12:4017–4032. doi: 10.1046/j.1460-9568.2000.00305.x. [DOI] [PubMed] [Google Scholar]
- 41.Guillin O, Diaz J, Carroll P, Griffon N, Schwartz J C, Sokoloff P. Nature (London) 2001;411:86–89. doi: 10.1038/35075076. [DOI] [PubMed] [Google Scholar]
- 42.Bagni C, Mannucci L, Dotti C G, Amaldi F. J Neurosci. 2000;20:RC76. doi: 10.1523/JNEUROSCI.20-10-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galabru J, Hovanessian A. J Biol Chem. 1987;262:15538–15544. [PubMed] [Google Scholar]
- 44.Nairn A C, Palfrey H C. J Biol Chem. 1987;262:17299–17303. [PubMed] [Google Scholar]
- 45.Pinkstaff J K, Chappell S A, Mauro V P, Edelman G M, Krushel L A. Proc Natl Acad Sci USA. 2001;98:2770–2775. doi: 10.1073/pnas.051623398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu L, Wells D, Tay J, Mendis D, Abbott M A, Barnitt A, Quinlan E, Heynen A, Fallon J R, Richter J D. Neuron. 1998;21:1129–1139. doi: 10.1016/s0896-6273(00)80630-3. [DOI] [PubMed] [Google Scholar]
- 47.Yuste R, Bonhoeffer T. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 48.Causing C G, Gloster A, Aloyz R, Bamji S X, Chang E, Fawcett J, Kuchel G, Miller F D. Neuron. 1997;18:257–267. doi: 10.1016/s0896-6273(00)80266-4. [DOI] [PubMed] [Google Scholar]
- 49.Shimada A, Mason C A, Morrison M E. J Neurosci. 1998;18:8559–8570. doi: 10.1523/JNEUROSCI.18-21-08559.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu Y S, Zhou H, Kartsogiannis V, Eisman J A, Martin T J, Ng K W. Mol Endocrinol. 1998;12:1721–1732. doi: 10.1210/mend.12.11.0195. [DOI] [PubMed] [Google Scholar]
- 51.Waltereit R, Dammermann B, Wulff P, Scafidi J, Staubli U, Kauselmann G, Bundman M, Kuhl D. J Neurosci. 2001;21:5484–5493. doi: 10.1523/JNEUROSCI.21-15-05484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yurek D M, Hipkens S B, Wiegand S J, Altar C A. J Neurosci. 1998;18:6040–6047. doi: 10.1523/JNEUROSCI.18-15-06040.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korte M, Kang H, Bonhoeffer T, Schuman E. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- 54.Kang H, Welcher A A, Shelton D, Schuman E M. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- 55.Takei N, Kawamura M, Hara K, Yonezawa K, Nawa H. J Biol Chem. 2001;276:42818–42825. doi: 10.1074/jbc.M103237200. [DOI] [PubMed] [Google Scholar]