Abstract
The effects of the growth temperature on the lipids and carotenoids of a filamentous cyanobacterium, Cylindrospermopsis raciborskii, were studied., The relative amounts of polyunsaturated glycerolipids and myxoxanthophylls in the thylakoid membranes increased markedly when this cyanobacterium was grown at 25°C instead of 35°C. Fourier transform infrared spectroscopy was used to analyze the low-temperature-induced structural alterations in the thylakoid membranes. Despite the higher amount of unsaturated lipids there, conventional analysis of the νsymCH2 band (characteristic of the lipid disorder) revealed more tightly arranged fatty-acyl chains for the thylakoids in the cells grown at 25°C as compared with those grown at 35°C. This apparent controversy was resolved by a two-component analysis of the νsymCH2 band, which demonstrated very rigid, myxoxanthophyll-related lipids in the thylakoid membranes. When this rigid component was excluded from the analysis of the thermotropic responses of the νsymCH2 bands, the expected higher fatty-acyl disorder was observed for the thylakoids prepared from cells grown at 25°C as compared with those grown at 35°C. Both the carotenoid composition and this rigid component in the thylakoid membranes were only growth temperature-dependent; the intensity of the illuminating light during cultivation had no apparent effect on these parameters. We propose that, besides their well-known protective functions, the polar carotenoids in particular may have structural effects on the thylakoid membranes. These effects should be exerted locally—by forming protective patches, in-membrane barriers of low dynamics—to prevent the access of reactive radicals generated in either enzymatic or photosynthetic processes to sensitive spots of the membranes.
Photosynthetic organisms are subject to regulatory mechanisms that afford protection against environmental stresses. One of these mechanisms is the desaturation of glycerolipids, which plays an important role in the modulation of the membrane dynamics and structure (1, 2). This adaptation strategy is especially effective against low-temperature stresses, because a higher number of unsaturated fatty-acyl chains can more easily maintain the high membrane dynamics required even at low temperatures by the functioning of the given membrane (3).
In model systems, it has been demonstrated that carotenoids, as integrated membrane constituents, may provide an alternative for the modification of the membrane structure and for the regulation of the membrane dynamics (4). These apolar compounds serve as protective pigments against high-light stress in photosynthetic organisms (5). A carotenoid molecule has a rod-like structure, which often terminates with polar groups. The length of a typical carotenoid matches the thickness of the hydrophobic membrane core (6). The molecular architecture of these pigments is responsible for their localization and orientation in the membranes and for their effects on the membrane properties (7–10). Studies on a mixture of liposomes and carotenoids demonstrated that formation of the hydroxy derivatives of these compounds not only increased the rigidity of the lipid phase, but also blocked the penetration of protons through the membrane (11) and significantly decreased the oxygen diffusion-concentration product in the hydrocarbon region of model membranes (12).
However, the question of whether carotenoid molecules can be present separately in the lipid phase of photosynthetic membranes or whether they are exclusively attached to protein complexes of the photosynthetic apparatus has not been clarified, although indirect evidence points to their existence in lipid bilayers. Havaux and Niyogi (13) found that, besides their known roles in protection against high-light stress, the xanthophylls in higher plants can also protect the highly unsaturated glycerolipids of the photosynthetic membranes against peroxidation. A similar protective effect was found in a model system (14). It has also been demonstrated that the relative abundance of xanthophylls in the thylakoid membranes of higher plants leads to an enhanced thermostability of the lipid phase in photosynthetic membranes by changing their fluidity (15, 16). The use of xanthophyll molecules as internal markers of phase properties in biomembranes likewise suggests the presence of xanthophyll molecules in the lipid phases of these membranes (17).
To address the problem of the structural aspects of carotenoids in the protection against heat and light stresses, we chose cyanobacteria for our investigations. The cyanobacteria have a well-studied lipid desaturase system (18), their carotenoid composition is rather simple (19), and their carotenoid content responds to high-light stress. The induction of carotenoid synthesis by low temperature has also been detected in some cyanobacterial strains (20, 21).
The present study was performed on Cylindrospermopsis raciborskii, a nitrogen-fixing filamentous cyanobacterial strain (22), which inhabits subtropical/tropical waters, with an optimal growth temperature between 25–35°C. This strain displays a considerably enhanced tolerance against low-temperature stress as compared with that of Synechocystis PCC 6803 (23).
To reveal the structural aspects, both the lipid and the carotenoid changes in isolated thylakoid membranes were followed by chemical methods and by characterizing the membrane dynamics. For the latter, noninvasive Fourier transform infrared (FTIR) spectroscopy was used. FTIR spectroscopy has been applied successfully to study lipid conformational order in both model (24, 25) and biological membranes (26, 27). Mostly the νsymCH2 band of the lipid fatty-acyl chains has been used in this respect. Its shift toward higher frequencies is associated with more disordered lipid fatty-acyl chains in the membranes. We have shown that this band can be decomposed into two components characteristic of the disordered (gauche) or the ordered (trans) segments of the fatty-acyl chains (28). Applying this approach to understand the membrane dynamics of Synechocystis PCC 6803 and of its mutant strains defective in lipid desaturation, we have shown that the cells strive to maintain a standard level of membrane dynamics at different growth temperatures (29).
We have shown that in C. raciborskii the level of polar carotenoids increases strongly with low-temperature stress. We suspect that these additional polar carotenoids, which are forced to span the membrane bilayer, form rigid “protective” patches that affect the local dynamics considerably but leave the large-scale membrane dynamics relatively unaffected. Thus, for the membrane as a whole, lipid desaturation can maintain the required dynamics in response to low-temperature stress.
Materials and Methods
Cells, Culturing Conditions, and Preparation of Thylakoid Membranes.
C. raciborskii was cultivated in a modified BG-11 medium that contained only 10% of the microelement concentrations of the original BG-11 medium and was supplemented with 4 mM Hepes-NaOH (pH 7.5) as described by Zsiros et al. (30). The cells were illuminated with incandescent lamps of 100 or 400 μEm−2s−1. The thylakoid membranes were isolated by the method of Murata and Omata (31).
Sample Naming.
C. raciborskii thylakoid membranes are indicated as RT, and the temperature and light conditions are indicated as temperature/light (e.g., RT35 indicates thylakoid membranes prepared from cells grown at 35°C, whereas RT25/100 indicates thylakoid membranes prepared from cells grown at 25°C and illuminated with 100 μEm−2s−1 during cultivation).
Fatty Acid Analysis.
The fatty acid analyses were carried out according to Wada and Murata (32). The extracted lipids were transmethylated in the presence of absolute methanol containing 5% HCl at 80°C for 3 h. Fatty acid methyl esters were separated with gas chromatography on an FFAP (Supelco) capillary column (30 m × 0.25 mm i.d.).
Protein Determination.
Protein concentrations in the thylakoids were determined by the method of Bradford (33), using a Bio-Rad protein assay kit with BSA as standard.
Pigment Analysis.
Chl a and the known carotenoids were identified from their absorption spectra and their retention times on an HPLC equipped with a μBondapak C18 column (8 × 100 mm, RCM type; Waters), eluted with methanol, as described (34).
FTIR Spectroscopy.
Membrane suspensions were centrifuged in a Beckman TL100 centrifuge at 75,000 rpm for 20 min at 4°C and then resuspended in a D2O-based 10 mM TES-NaOH pD 7.0 buffer. (The 0.4 unit shift between pH and pD was taken into account.) This membrane suspension was placed between CaF2 windows separated by a 25-μm thick alumina spacer. FTIR measurements were carried out on a Philips (Cambridge, U.K.) PU9800 FTIR spectrometer at 2 cm−1 spectral resolution. For each background and sample spectrum 128 interferograms were accumulated. Temperature scans were carried out by repeating the following measurement cycle: recording the sample and background spectra, waiting 7 min, recording the sample and background spectra again, setting of new temperature, and waiting for 7 min for the new thermal equilibrium to be established. During the evaluation of the measurements, the data obtained from the two spectra recorded at the same temperature were averaged. The temperature was increased in 2–3°C steps by using a water-thermostated sample holder. The whole measurement cycle was computer-controlled. The accuracy of the temperature setting was better than 0.5°C.
Data were analyzed with spserv software (Cs. Bagyinka, Szeged, Hungary). Before data analysis, a linear baseline was subtracted in the 2800–3050 cm−1 region of the spectra. No other data manipulation (smoothing, resolution enhancement, etc.) was carried out. The C-H stretching region of the spectrum was fitted with Lorentzian component bands as described earlier (28), using two components for the fit of the νsymCH2 band. All of the component parameters (frequency, bandwidth, and intensity) were initially freely optimized by the program. For the subsequent analysis of the temperature scans, the frequency and the bandwidth of the rigid component were fixed; the details are explained in the text. The accuracy of the component band frequency determination was better than 0.1 cm−1.
Results
Lipid and Fatty Acid Compositions of C. raciborskii Thylakoid Membranes.
The fatty acid compositions of the lipids obtained from the total lipid extracts of thylakoid membranes prepared from cells grown at 25°C and 35°C at a light intensity of 100 μEm−2s−1 are listed in Table 1.
Table 1.
Mass percentages of fatty acids in lipids of isolated thylakoid membranes prepared from C. raciborskii cells grown at 35°C or 25°C and at a light intensity of 100 μEm−2s−1
| Fatty acid | TL
|
MGDG
|
DGDG
|
SQDG
|
PG
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 35°C | 25°C | 35°C | 25°C | 35°C | 25°C | 35°C | 25°C | 35°C | 25°C | |
| 16:0 | 44.9 | 40.8 | 45.6 | 38.8 | 43.3 | 36.2 | 49.4 | 50.5 | 24.8 | 39.4 |
| 16:1 | 2.5 | 5.6 | 1.7 | 5.6 | 2.9 | 7.0 | 2.0 | 3.8 | 10.8 | 5.2 |
| 18:0 | 5.3 | 3.4 | 2.6 | 2.2 | 12.5 | 4.2 | 8.2 | 4.9 | 7.5 | 8.0 |
| 18:1 | 8.1 | 2.2 | 4.9 | 1.8 | 6.0 | 1.6 | 8.6 | 4.3 | 43.8 | 4.0 |
| 18:2 | 23.0 | 1.7 | 26.6 | 1.7 | 17.6 | 1.3 | 20.0 | 1.8 | 7.5 | 1.5 |
| γ18:3 | 3.3 | 1.5 | 4.0 | 1.5 | 4.8 | 2.6 | 0.8 | 0.7 | 0.5 | 1.6 |
| α18:3 | 10.1 | 24.8 | 12.0 | 22.6 | 9.5 | 23.6 | 7.6 | 32.8 | 2.5 | 28.2 |
| 18:4 | 2.9 | 20.0 | 2.6 | 25.9 | 3.4 | 23.6 | 3.5 | 1.2 | 2.6 | 12.1 |
| S/U* | 1.01 | 0.79 | 0.93 | 0.69 | 1.26 | 0.68 | 1.36 | 1.24 | 0.48 | 0.90 |
| DBI† | 1.08 | 1.70 | 1.18 | 1.87 | 1.01 | 1.82 | 0.90 | 1.17 | 0.89 | 1.49 |
The results are the averages for two independent cell cultures for each condition. The SD was not more than ±1% for any individual fatty acid. TL, total lipid extract; PG, phosphatidylglycerol; MGDG, monogalactosyldiacylglycerol; DGDG, digalactosyldiacylglycerol.
This number is the ratio of unsaturated and saturated fatty acids.
The double bond index (DBI), calculated by dividing by 100 the sum of the percentages of the unsaturated fatty acids, each multiplied by the number of its double bonds.
The major fatty acid species (in the sequence of decreasing order) were, palmitic (16:0), linoleic [18:2(9, 12)], α-linolenic [18:3(9, 12, 15)], oleic [18:1(9)], γ-linolenic [18:3(6, 9, 12)], stearic (18:0), and octadecatetraenoic [18:4(6, 9, 12, 15)] acids. The numbers in parentheses indicate the positions of the double bonds. As expected, decrease of the growth temperature caused a marked increase in the amounts of polyunsaturated fatty acids. The changes were most pronounced for the C18 polyunsaturated fatty acids. The α-18:3 and 18:4 fatty acid contents of the different lipid classes increased by 2–10-fold at the expense of oleic and linoleic acids after decrease of the growth temperature from 35°C to 25°C.
In monogalactosyldiacylglycerol and digalactosyldiacyglycerol, the relative amounts of α-linolenic and the 18:4 acids were increased dramatically, whereas the linoleic acid content was decreased at 25°C. In sulfoquinovosyldiacylglycerol (SQDG), the levels of fatty acids with a double bond at the Δ6 position (i.e., γ-linolenic and the 18:4 fatty acids), remained very low at 25°C. The lack of a significant increase in the level of the SQDG 18:4 fatty acids in the cells grown at 25°C indicates that in C. raciborskii, as in other cyanobacterial strains (35), Δ6-desaturase cannot accept SQDG as substrate. The Δ6 desaturation was also suppressed in phosphatidylglycerol, but not as strictly as in SQDG. The data further show that in C. raciborskii cells Δ6-desaturase cannot accept the usual 18:2(9,12) fatty acids (only the α-18:3 species as substrates), and therefore the level of γ-18:3 is very low in all lipid classes at 25°C.
The saturated to unsaturated fatty acid ratios of the different lipid classes were not uniformly affected by the decrease of the growth temperature. The saturated to unsaturated fatty acid ratio (S/U in Table 1) strongly decreased in monogalactosyldiacylglycerol and digalactosyldiacyglycerol. In SQDG, S/U remained practically unchanged, and in phosphatidylglycerol, albeit at a very low level, it increased.
We also determined the double bond index (DBI), a more precise indicator of changes in the level of fatty acid unsaturation. Decrease of the growth temperature from 35°C to 25°C increased DBI in all lipid classes. The increase in DBI was more pronounced in monogalactosyldiacylglycerol and digalactosyldiacyglycerol than in SQDG or phosphatidylglycerol.
Concerning the relative amounts of the constituting lipid classes and their fatty acid compositions, we did not observe significant differences between thylakoid membranes prepared from cells grown at various light intensities (40–1000 μEm−2s−1; data not shown).
Pigment Content As a Function of Growth Temperature and Light Intensity.
We determined the chlorophyll and carotenoid composition of isolated thylakoids prepared from cells grown at 25 or 35°C under light intensities of 100 and 400 μEm−2s−1 (Table 2). Carotenoids were separated with an HPLC system. Four carotenoid species were identified according to their retention times. C. raciborskii cells contain β-carotene, echinenone (4-oxo-β-carotene), zeaxanthin, and myxoxanthophyll as the most abundant carotenoid species in their thylakoid membranes.
Table 2.
Carotenoid and chlorophyll contents of the thylakoids isolated from cells grown at 25°C or 35°C and a light intensity of 100 or 400 μEm−2s−1
| Thylakoid, temperature/light | Σcarotenoid, nmol/mg of protein | Chlorophyll, nmol/mg of protein | Σcar/Chl (ratio) |
|---|---|---|---|
| RT35/100 | 42.7 ± 5.2 | 114.2 ± 23.1 | 0.37 |
| RT35/400 | 42.9 ± 5.3 | 87.4 ± 20.4 | 0.49 |
| RT25/100 | 36.1 ± 2.8 | 64.5 ± 9.1 | 0.56 |
| RT25/400 | 42.1 ± 3.9 | 48.6 ± 7.8 | 0.87 |
The results are the averages of three independent measurements. The error in the ratio Σcarotenoid/Chl is about 20%.
The chlorophyll content in C. raciborskii thylakoids was affected by both the growth temperature and the illuminating light intensity (Table 2). It was lower at the lower growth temperature, and at a given temperature it was lower at higher light intensity.
Neither the growth temperature nor the light intensity appreciably affected the total amount of carotenoids as calculated vs. the protein content of the thylakoid membranes (Table 2). However, there were large changes in the relative amounts of the major carotenoid constituents, but these changes depended on only the growth temperature (Table 3). The intensity of the illuminating light during cultivation apparently did not affect the carotenoid composition of the C. raciborskii thylakoids.
Table 3.
Relative amounts of carotenoids in thylakoids isolated from cells grown at 25°C or 35°C and a light intensity of 100 or 400 μEm−2s−1
| Thylakoid, temperature/light | β-Carotene (neut), relative % | Echinenone, relative % | Zeaxanthin, relative % | Myxoxanthophyll, (pol.), relative % | Pol/neut (ratio) |
|---|---|---|---|---|---|
| RT35/100 | 32 | 40 | 2 | 26 | 1.2 |
| RT35/400 | 30 | 43 | 2 | 25 | 1.2 |
| RT25/100 | 16 | 39 | 2 | 43 | 2.7 |
| RT25/400 | 18 | 40 | 1 | 41 | 2.8 |
The error in the relative amounts of carotenoids is about 15%, and that in the polar/neutral ratio (pol/neut) is about 20%. Relative amounts are calculated from the measurements listed in Table 2.
Regarding the carotenoid composition as a function of temperature, opposite changes appeared in the amounts of βcarotene, a neutral carotenoid, and myxoxanthophyll, a carotenoid in which the isoprene chain is terminated by two polar hydroxy groups at both ends and additionally there is one sugar molecule. The relative amount of β-carotene was high in the RT35 membranes, whereas myxoxanthophyll was more abundant in the RT25 membranes (Table 3). Carotenoid compositions were calculated according to Takaichi et al. (36). Concerning carotenoid integration into the membrane, it may be expected that β-carotene, searching for an apolar environment, can adopt any orientation in the membrane bilayer; in contrast, myxoxanthophylls have to span the bilayer because their polar groups at the ends of the polyene chain prefer a polar environment. These spanning carotenoids may rigidify the membranes. Thus, the ratio of polar to neutral carotenoids is a good measure of the rigidifying capacity of a given carotenoid composition (Table 3). When it is high, one can expect a stronger carotenoid influence on the membrane structure and dynamics. However, the polar/neutral carotenoid ratio does not reveal whether this influence is an overall one or is manifested in localized membrane domains.
Analysis of the C-H Stretching Region of the FTIR Spectra of Thylakoid Membranes.
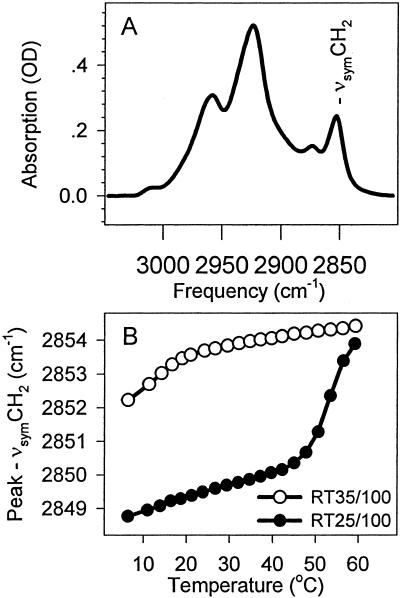
The C-H stretching region of the infrared spectra of C. raciborskii membranes is shown in Fig. 1A. The frequency of the νsymCH2 band is generally used to characterize the phase properties of the membrane lipids. Its frequency is higher when the fatty-acyl chains in the membrane bilayer are more disordered. Curve-fitting to the data points around the maximum of the νsymCH2 band, a method widely used to determine its characteristic frequency, leads to a surprising result (Fig. 1B). The thermotropic response of the νsymCH2 band determined from a series of spectra recorded between 5 and 60°C exhibited higher frequencies, i.e., higher lipid disorder, over the whole temperature range for thylakoids prepared from cells grown at 35°C as compared with thylakoids prepared from cells grown at 25°C. This result is apparently in contradiction with other cases where cold acclimation was studied. It has always been found previously that, by alarming their desaturase enzyme systems, cells can increase the level of unsaturation in their membrane lipids, thereby extending the temperature range of high lipid disorder toward lower temperatures, as required by the physiological demand for the maintenance of appropriate membrane dynamics (e.g., ref. 29). Because the level of lipid unsaturation did change considerably, and in the expected direction, when C. raciborskii cells were grown at lower temperature (Table 1), we had to find an explanation for this contradiction.
Figure 1.
The C-H stretching region and the thermotropic response of the νsymCH2 band of thylakoid membranes prepared from C. raciborskii cells. (A) The C-H stretching region of RT35/100 thylakoids. (B) The thermotropic responses of the νsymCH2 band in RT35/100 and RT25/100 membranes. Peak frequencies were determined by fitting a Lorentzian curve to the 10 most intensive data points.
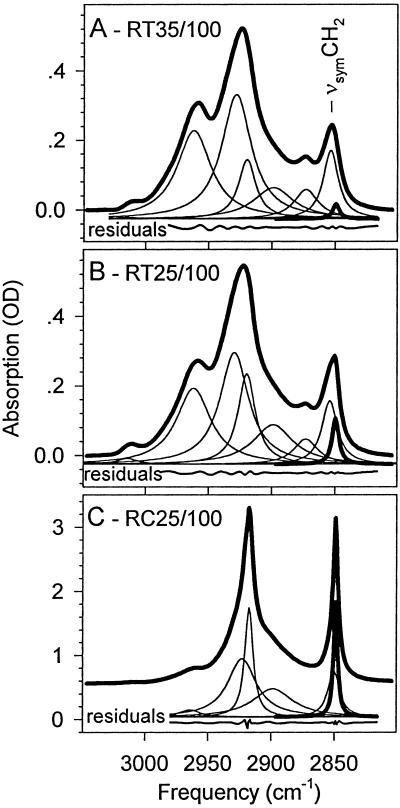
A thorough curve decomposition that involved the fitting of two components to the νsymCH2 band, as described earlier (29), revealed (Fig. 2) that there is a narrow component, corresponding to very rigid fatty-acyl chains, under the contour of the νsymCH2 band. The intensity of this component band increases with increasing polar carotenoid content in the thylakoids, i.e., it is higher for RT25/100 (Fig. 2B) than for RT35/100 (Fig. 2A). Fig. 2C presents the C-H stretching region of the cytoplasmic membrane (RC25/100) of C. raciborskii cells grown at 25°C at 100 μEm−2s−1; it illustrates that at extremely high carotenoid concentrations the rigid component predominates in the νsymCH2 band. [Details of cytoplasmic membrane spectra are not discussed in this communication. We merely mention that the carotenoid content was about 10 times higher here as compared with thylakoid membranes prepared from the same cells, similarly as for the carotenoid composition of the membranes isolated from Synechococcus cells (37).]
Figure 2.
Two-component analysis of the νsymCH2 band in the infrared spectra of the thylakoid membranes RT35/100 (A), RT25/100 (B), and a cytoplasmic membrane RC35/100 (C) to illustrate the case of extremely high polar carotenoid concentration in a membrane. Thick solid lines indicate the measured spectra. The fitted component bands are indicated with thinner lines, except for the component assigned to the rigid fatty acid population, which is drawn with a thicker line. It should be noted that the relative intensity of this component band increases with the amount of polar carotenoids in the membranes. For details, see the text. Both the fitted component bands and the residual curves have been displaced for clarity.
The C-H stretching region of thylakoid membranes of C. raciborskii grown at 35 or 25°C at 100 or 400 μEm−2s−1 were analyzed with the following strategy. The C-H stretching region of the thylakoid membranes recorded at 5°C were freely fitted with the components shown in Fig. 2 A and B. It should be noted that the νsymCH2 band was fitted with two components. For this band, the fit resulted in a low-frequency (“rigid”) component reflecting ordered (“trans”) fatty-acyl chain segments (28). The frequency and the bandwidth of this component agreed well with those of the same component in the cytoplasmic membrane spectrum (Fig. 2C). These rigid components are drawn with thicker lines in Fig. 2.
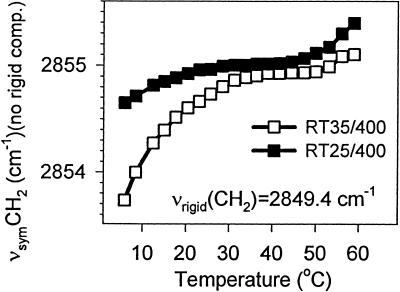
To analyze the thermotropic response of the νsymCH2 band, the parameters (frequency and bandwidth) of the rigid component of the νsymCH2 band were fixed (as obtained from the 5°C spectra) during the fitting of other spectra in the same series. These other spectra were recorded at higher temperatures, which increased up to 60°C. The reasoning behind this restriction was the assumption that something can be ordered in only one way, whereas it can be disordered over a wide range of conformations. We therefore left all parameters of the higher-frequency component νsymCH2 band to be optimized freely when the spectra of a temperature scan were fitted. We observed similar results for the two light intensities under which the cells were grown. Fig. 3 depicts the thermotropic response curves of the higher-frequency component of the νsymCH2 band for RT25/400 and RT35/400. Very similar results were obtained for the RT25/100 and RT35/100 membranes (data not shown). These thermotropic responses are similar to those found for another cyanobacterium, Synechocystis PCC 6803 (29), with the difference that the frequency values obtained from a one-component fit were there somewhat lower.
Figure 3.
Thermotropic response curves of the higher-frequency component of the νsymCH2 band. The frequency of the rigid component was found to be the same for both thylakoids and is given as νrigid(CH2) in the figure. For details, see the text.
The higher frequencies seen here may be because of the fact that we are limited when fitting two components to the νsymCH2 band, but the spectral resolution and the signal to noise ratio would make the fitting of more components illusory. In this situation, we may handle the really rigid lipid components (apparently related somehow to the carotenoid constituents) of the membranes together with the ordered segments of the unaffected fatty-acyl chains. Therefore, the other component that we regard at present as a true representative of the functional membrane lipids, which participate in the maintenance of the membrane dynamics, may lack the contribution of some of the ordered segments. Thus, its frequency may be somewhat higher than in other cyanobacteria. Additionally, the frequency of the νsymCH2 band depends on the protein to lipid ratio and on the level of unsaturation of the fatty-acyl chains as well (29). Nevertheless, the shapes of the curves in Fig. 3 show that the cells grown at 25°C have a membrane which can preserve its high dynamics until lower temperatures as compared with the membranes prepared from cells grown at 35°C. Moreover, the level of membrane disorder (dynamics) is very similar in the two types of membranes at around the temperatures at which their mother cells were grown.
Discussion
The prevailing light and temperature conditions determine the life of photosynthetic organisms. Both may have beneficial or destructive effects, which may depend on their combination too. Light and temperature can also interplay in several ways in the responses of organisms to stresses.
Lipids are mostly involved in the responses to temperature stresses by the altered level of unsaturation of their fatty acids (38), but the induction of the desaturase system responsible for the altered unsaturation is additionally light-regulated (39). Unsaturated lipids are further needed in the repair of the PSII reaction centers after photoinhibitory destruction (40).
Xanthophylls have been found to be important during the defense against light-generated stresses, by quenching triplet chlorophylls in the reaction centers and intercepting reactive oxygen species in the membranes. Their conditions for interception, however, depend on the strongly temperature-related dynamics of the membrane.
We have studied the cooperation of lipid desaturation and the induction of xanthophyll production in the adaptation of a cyanobacterium, C. raciborskii, to different growth temperatures and light conditions, by analyzing the lipid and pigment compositions, and the thylakoid membrane structure and dynamics.
In C. raciborskii, the desaturase system responsible for the in situ desaturation of membrane lipid fatty-acyl chains seems to be more active (Table 1) than in other cyanobacteria (30). Thus, the cells should be able to adapt the dynamics of their thylakoid membranes to lower than optimal growth temperatures.
The absolute amount of the carotenoids did not really change in response to variation of either the growth temperature or the illuminating light intensity (Table 2). The distribution of the carotenoid species, however, did change substantially as a function of the growth temperature, although it too was insensitive to the light intensity (Table 3). This finding does not mean that the light-protective function of the carotenoids is not light-dependent, because the carotenoid/chlorophyll ratio of the thylakoid membranes was light-dependent (Table 2). The carotenoid/chlorophyll ratio increased with increasing light intensity, and the increment was larger at 25°C than at 35°C. This result correlates with the assumption that, in consequence of the decreased dynamics of the thylakoid membrane at the lower temperature, which restricts the movements of both the quenchers and the reactive species, more carotenoids are needed for protection.
The changes in the distribution of the carotenoid species mostly involve the neutral β-carotene and the polar myxoxanthophylls. β-Carotene may assume any orientation in the interior of the membrane, the only restriction being that the environment around it should be apolar. Therefore, the rigidity of the β-carotene molecule will decrease the order in the gel phase and decrease the disorder in the liquid-crystalline phase of the lipid membranes, similarly to the situation with cholesterin. In contrast with β-carotene, myxoxanthophylls contain polar groups (one hydroxy group and one sugar molecule at the ends of their isoprene backbone) and have to span the lipid bilayer, because the polar groups prefer a polar environment. Such an orientation may increase the lipid order in both the gel and the liquid-crystalline phases.
The conventional analysis of the thermotropic responses of the νsymCH2 band (Fig. 1) has demonstrated that, despite their high level of lipid unsaturation, the RT25 membranes are so rigid that their functioning should be impossible. A detailed two-component analysis of the thermotropic responses of the νsymCH2 band, however, has revealed the presence of a very rigid lipidic component in the thylakoid membranes (Fig. 2). The relative intensity of this rigid component was higher where there were more myxoxanthophylls in the membrane. When the rigid component was not considered in the analysis, a usual thermotropic response was obtained for the frequency of the νsymCH2 band (Fig. 3), which means that thylakoids prepared from cells grown at 25°C preserved their high dynamics until lower temperatures than those grown at 35°C (29). Like the polar carotenoid content, these thermotropic response curves were also virtually only growth temperature-sensitive; they were practically insensitive to the intensity of the illuminating light during the cultivation of the cells.
This duality in the lipid dynamics of the thylakoids, which contain high amounts of polar carotenoids, may indicate that polar carotenoids can indeed rigidify certain lipids, but this rigidification does not affect the whole membrane. Carotenoids in the membranes should necessarily have a structural effect. In our interpretation of our data, this effect is not uniform throughout the whole membrane. Accordingly, we believe that the carotenoids are arranged in local patches, which can be very rigid, as they are seen in the analysis of the νsymCH2 band. The existence of such patches is in agreement with the different changes in the absorption of xanthophylls, assigned to their aggregation-dissociation and used to measure membrane-phase properties (41).
It remains an open question, however, why the neutral/polar carotenoid equilibrium is so greatly affected by the growth temperature. This phenomenon may indicate that the forming carotenoid patches have a protective role in the thylakoid membrane; they might be formed at loci where rigidity, despite the lower temperature, can provide advantages. One reason may be that, at higher temperatures, because of the high activity of the photosynthetic apparatus, the cells need β-carotene to protect against direct photochemical damage. Despite the lower photosynthetic activity at lower temperatures, there is an enhanced production of free radicals (42). These free radicals can have longer lifetimes in consequence of the low membrane dynamics, which decreases the probability of their quenching. Thus, it might be more “economical” for the cells to protect locally certain “expensive” and vital components of the membrane, repair/reproduction of which would be difficult under energy-constraint low-temperature conditions. Polar carotenoids may be suitable for such a role by virtue of their strong rigidifying effect, which can make it impossible for reactive agents to reach the protected spots.
Acknowledgments
Z.V. thanks Profs. C. Wilhelm and G. Garab for their help in her HPLC measurements within the frame of a German–Hungarian bilateral cooperation (TÉT: D-44/96). This work was supported by Hungarian Scientific Research Fund Grants OTKA T025804, -T030631, -T034174 (to Z.V. and Z.G.), and T031973 (to B.S.).
Abbreviations
- FTIR
Fourier transform infrared
- SQDG
sulfoquinovosyldiacylglycerol
References
- 1.Nishida I, Murata N. Annu Rev Plant Mol Biol. 1996;47:541–568. doi: 10.1146/annurev.arplant.47.1.541. [DOI] [PubMed] [Google Scholar]
- 2.Gombos Z, Murata N. In: Lipids in Photosynthesis. Siegenthaler P-A, Murata N, editors. Dordrecht, The Netherlands: Kluwer Academic; 1998. pp. 249–262. [Google Scholar]
- 3.Murata N, Wada H. Biochem J. 1995;308:1–8. doi: 10.1042/bj3080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Havaux M. Trends Plant Sci. 1998;3:147–151. [Google Scholar]
- 5.Demmig-Adams B, Adams W W. Trends Plant Sci. 1996;1:21–26. [Google Scholar]
- 6.Guszecki W I. In: The Photochemistry of Carotenoids. Frank H A, Young A J, Britton G, Cogdell R J, editors. Dordrecht, The Netherlands: Kluwer Academic; 1999. pp. 363–379. [Google Scholar]
- 7.Gruszecki W I, Grudzinski W, Banaszek-Glos A, Matula M, Kernen P, Krupa Z, Sielewiesiuk J. Biochim Biophys Acta. 1999;1412:173–183. doi: 10.1016/s0005-2728(99)00055-9. [DOI] [PubMed] [Google Scholar]
- 8.Gruszecki W I, Sielewiesiuk J. Biochim Biophys Acta. 1991;1069:21–26. doi: 10.1016/0005-2736(91)90099-t. [DOI] [PubMed] [Google Scholar]
- 9.Strzalka K, Gruszecki W I. Biochim Biophys Acta. 1994;1194:138–142. doi: 10.1016/0005-2736(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 10.Van de Ven M, Kattenberg M, Van Ginkel G, Levine Y K. Biophys J. 1984;45:1203–1210. doi: 10.1016/S0006-3495(84)84269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berglund A H, Nilsson R, Liljenberg C. Plant Physiol Biochem. 1999;37:179–186. [Google Scholar]
- 12.Subczynski W K, Markowska E, Sielewiesiuk J. Biochim Biophys Acta. 1991;1068:68–72. doi: 10.1016/0005-2736(91)90061-c. [DOI] [PubMed] [Google Scholar]
- 13.Havaux M, Niyogi K. Proc Natl Acad Sci USA. 1999;96:8762–8767. doi: 10.1073/pnas.96.15.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woodall A A, Britton G, Jackson M J. Biochim Biophys Acta. 1997;1336:575–686. doi: 10.1016/s0304-4165(97)00007-x. [DOI] [PubMed] [Google Scholar]
- 15.Tardy T, Havaux M. Biochim Biophys Acta. 1997;1330:179–193. doi: 10.1016/s0005-2736(97)00168-5. [DOI] [PubMed] [Google Scholar]
- 16.Havaux M, Gruszecki W I. Photochem Photobiol. 1993;58:607–614. [Google Scholar]
- 17.Gombos Z, Vigh L. Plant Physiol. 1986;80:415–419. doi: 10.1104/pp.80.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wada H, Murata N. In: Lipids in Photosynthesis. Siegenthaler P-A, Murata N, editors. Dordrecht, The Netherlands: Kluwer Academic; 1998. pp. 65–81. [Google Scholar]
- 19.Hirschberg J, Chamovitz D. In: The Molecular Biology of Cyanobacteria. Bryant D A, editor. Dordrecht, The Netherlands: Kluwer Academic; 1994. pp. 559–579. [Google Scholar]
- 20.Celerin M, Gilpin A A, Schisler N J, Ivanov A G, Miskiewicz E, Krol M, Laudenbach D E. J Bacteriol. 1998;180:5173–5182. doi: 10.1128/jb.180.19.5173-5182.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miskiewicz E, Ivanov A G, Williams J P, Khan M U, Falk S, Huner N P. Plant Cell Physiol. 2000;41:767–775. doi: 10.1093/pcp/41.6.767. [DOI] [PubMed] [Google Scholar]
- 22.Vinogradska T A. Ukr Bot Rev. 1974;31:733–739. [Google Scholar]
- 23.Várkonyi Z, Zsiros O, Farkas T, Garab G, Gombos Z. Biochem Soc Trans. 2000;28:892–894. [PubMed] [Google Scholar]
- 24.Mantsch H H, McElhaney R N. Chem Phys Lipids. 1991;57:213–226. doi: 10.1016/0009-3084(91)90077-o. [DOI] [PubMed] [Google Scholar]
- 25.Mendelsohn R, Senak L. In: Biomolecular Spectroscopy, Part A. Clark R J H, Hester R E, editors. Chichester, U.K.: Wiley; 1993. pp. 339–380. [Google Scholar]
- 26.Moore D J, Mendelsohn R. Biochemistry. 1994;33:4080–4085. doi: 10.1021/bi00179a037. [DOI] [PubMed] [Google Scholar]
- 27.Moore D J, Sills R H, Mendelsohn R. Biospectroscopy. 1995;1:133–140. [Google Scholar]
- 28.Kóta Z, Debreczeny M, Szalontai B. Biospectroscopy. 1999;5:169–178. doi: 10.1002/(SICI)1520-6343(1999)5:3<169::AID-BSPY6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 29.Szalontai B, Nishiyama Y, Gombos Z, Murata N. Biochim Biophys Acta. 2000;1509:409–419. doi: 10.1016/s0005-2736(00)00323-0. [DOI] [PubMed] [Google Scholar]
- 30.Zsiros O, Várkonyi Z, Kovács A, Farkas T, Gombos Z, Garab G. Indian J Biochem Biophys. 2000;37:470–476. [PubMed] [Google Scholar]
- 31.Murata N, Omata T. Methods Enzymol. 1988;167:245–251. [Google Scholar]
- 32.Wada H, Murata N. Plant Cell Physiol. 1989;30:971–978. [Google Scholar]
- 33.Bradford M M. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 34.Takaichi S, Shimada K. Methods Enzymol. 1992;213:374–385. [Google Scholar]
- 35.Murata N, Wada H, Gombos Z. Plant Cell Physiol. 1992;33:933–941. [Google Scholar]
- 36.Takaichi S, Maoka T, Masamoto K. Plant Cell Physiol. 2001;42:756–762. doi: 10.1093/pcp/pce098. [DOI] [PubMed] [Google Scholar]
- 37.Masamoto K, Zsiros O, Gombos Z. J Plant Physiol. 1999;155:136–138. [Google Scholar]
- 38.Tasaka Y, Gombos Z, Nishiyama Y, Mohanty P, Ohba T, Ohki K, Murata N. EMBO J. 1996;15:6416–6425. [PMC free article] [PubMed] [Google Scholar]
- 39.Kis M, Zsíros O, Farkas T, Wada H, Nagy F, Gombos Z. Proc Natl Acad Sci USA. 1998;95:4209–4214. doi: 10.1073/pnas.95.8.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gombos Z, Wada H, Murata N. Proc Natl Acad Sci USA. 1994;91:8787–8791. doi: 10.1073/pnas.91.19.8787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada H, Gombos Z, Murata N. Nature (London) 1990;347:200–203. doi: 10.1038/347200a0. [DOI] [PubMed] [Google Scholar]
- 42.Demmig-Adams B, Adams W W. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:599–626. [Google Scholar]