Abstract
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related mortality, associated with viral hepatitis, alcohol consumption, and non-alcoholic fatty liver disease. Hepatocyte nuclear factor 4 alpha (HNF4α), a crucial transcription factor for liver function (glucose and lipid metabolism, bile acid homeostasis, and cellular differentiation), is often dysregulated in HCC progression. This review provides a comprehensive overview of the role of HNF4α in hepatic oncogenesis, providing novel inshight into its regulatory effects on epithelial-mesenchymal transition (EMT), metabolic alterations (including the Warburg effect), cell cycle control, and tumor microenvironment. We also discuss therapeutic strategies targeting HNF4α focusing on restoring metabolic balance and inducing apoptosis. This integrated analysis advances our understanding of HNF4α’s contribution to HCC and may pave the way for the development of targeted therapies (Fig. 1).
Keywords: HNF4α, Hepatocellular carcinoma, Epithelial-mesenchymal transition, Progression
Introduction
Hepatocellular carcinoma (HCC) has emerged as a leading cause of cancer-related mortality worldwide, with an increasing incidence driven by a combination of risk factors, including chronic viral hepatitis, excessive alcohol consumption, and non-alcoholic fatty liver disease (NAFLD) [1–4]. The complex and multifactorial etiology of HCC underscores the critical need to elucidate the underlying molecular mechanisms governing tumor initiation and progression.
Among the key players in liver cancer biology, hepatocyte nuclear factor 4 alpha (HNF4α) is a critical transcription factor in maintaining hepatocyte function and regulating key metabolic processes [5]. HNF4α exists as two primary isoforms: P1 and P2. These isoforms, generated through alternative promoter usage, while these isoforms share a common C-terminal ligand-binding domain, they exhibit significant differences in their N-terminal regions, leading to distinct transcriptional activities and functional roles, particularly in cancer progression [6]. The P1 isoform, predominantly expressed in differentiated tissues, regulates genes vital for normal tissue homeostasis and differentiation. In contrast, the P2 isoform, often expressed at lower levels, exhibits a more complex and context-dependent role; capable of both inhibiting P1 activity and exerting independent transcriptional functions [6].
In HCC, downregulation of the P1 isoform generally correlates with disease progression, reflecting its compromised ability to maintain normal tissue function and suppress oncogenic pathways. This loss of P1 function can lead to uncontrolled cell growth, invasion, and metastasis [7, 8]. The role of the P2 isoform is more nuanced; in some cancers, its upregulation may contribute to tumorigenesis by promoting cell proliferation and survival by inhibiting tumor suppressor genes; in other contexts, P2 may act as a tumor suppressor [7, 9]. The intricate interplay between these isoforms warrants further investigation.
Within hepatocytes, HNF4α is indispensable for maintaining normal liver function, orchestrating glucose and lipid metabolism, bile acid homeostasis, and cellular differentiation- processes integral to liver physiology [10]. Disruptions in HNF4α function impair homeostasis and contribute to the development of various liver diseases, including HCC [11]. While HNF4α tightly regulated under normal conditions to maintain metabolic balance, its dysregulation is has been implicated in the progression of liver diseases, such as steatosis and fibrosis, which can ultimately lead to HCC [12, 13]. Extensive research has highlighted the multifaceted role of HNF4α in hepatic oncogenesis, impacting HCC aggressiveness, cellular metabolism, and tumor progression [14, 15].
Beyond HCC, HNF4α’s influence extends to other liver cancers. While it often acts as a tumor suppressor in HCC [16], reduced HNF4α expression contributes to cholangiocarcinoma (CCA) development through altered hepatoblast differentiation and interactions with β-catenin [17]. The interplay between HNF4α and C/EBPα in regulating FBP1 [18] also warrants further investigation, particularly in the context of combined HCC-CCA (cHCC-CCA). Further research is clearly needed to fully elucidate the diverse roles of HNF4α and its therapeutic potential across the spectrum of liver cancer.
This review aims to consolidate the current understanding of HNF4α’s essential functions in hepatic oncogenesis, with a specific focus on its regulatory roles in the EMT, metabolic reprogramming, cell death, cell proliferation, and immune response modulation. By elucidating the complex interplay between HNF4α and the various cellular processes implicated in HCC development, we hope stimulate future research aimed at developing innovative therapeutic interventions. A comprehensive understanding of HNF4α’s role in liver cancer progression may pave the way for the development of targeted therapies, ultimately improving patient management and outcomes for this challenging disease.
HNF4α loss drives EMT and metabolic reprogramming in liver cancer
HNF4α is essential for maintaining hepatocyte identity and liver homeostasis, and its reduced HNF4α expression is strongly linked to HCC progression. HNF4α exerts its tumor-suppressive effects primarily by regulating EMT, a process critical for tissue development and repair but frequently co-opted in cancer progression. HNF4α inhibits EMT by repressing key EMT regulators such as Snail and Slug, and by modulating chromatin structure to downregulate EMT-associated gene expression [19–21].
Recent findings identify HNF4α as a key node connecting EMT and altered cancer metabolism, demonstrating that its downregulation - a frequent occurrence in HCC- contributes to enhanced cellular fitness in a competitive environment [22]. Significantly, augmentation of HNF4α expression via a synthetic antisense long non-coding RNA profoundly modulates both EMT and onco-metabolic pathways. Specifically, HNF4α overexpression suppresses mesenchymal transcription factors, and restores hepatocytic function. These effects translate to demonstrably reduced cell migration, invasion, and proliferation, all of which contribute to tumor progression. This strongly suggests that HNF4α’s broad transcriptional influence on genes implicated in EMT and the Warburg effect positions it as a compelling therapeutic target for HCC [23]. Disruption of the HNF4α regulatory network contributes to inflammation and metabolic dysregulation, accelerating HCC development [24]. HNF4α downregulation promotes both EMT and metabolic reprogramming, including the Warburg effect, leading to increased cancer cell proliferation and invasiveness [23, 24]. The complexity of metabolic dysregulation in HCC presents a significant clinical challenge [23]. Current therapeutic approaches are often inadequate, highlighting the urgent need for innovative strategies targeting the underlying mechanisms of HCC pathogenesis, particularly those involving metabolic reprogramming [25]. This need is further emphasized by the strong association between metabolic dysregulation and aggressive HCC. Thus, HNF4α loss promotes tumorigenesis via EMT and metabolic reprogramming, highlighting its pivotal role in HCC pathogenesis (Figs. 1 and 2).
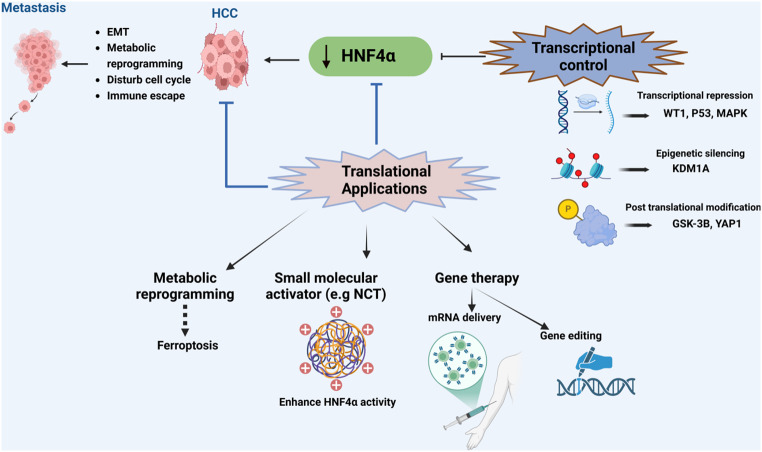
Fig. 1.
Hepatocyte Nuclear Factor 4α (HNF4α) in Hepatocellular Carcinoma (HCC). Downregulation of HNF4α promotes HCC progression through Epithelial-Mesenchymal Transition (EMT), metabolic reprogramming, and immune dysregulation. Transcriptional control of HNF4α is impacted by Wilms Tumor 1 (WT1), tumor protein P53 (p53), mitogen-activated protein kinase (MAPK) signaling, lysine-specific demethylase 1 A (KDM1A), glycogen synthase kinase 3 beta (GSK-3β), and Yes-associated protein 1 (YAP1). Potential therapeutic strategies targeting HNF4α include small molecule activation and gene therapy. Created with BioRender (BioRender.com)
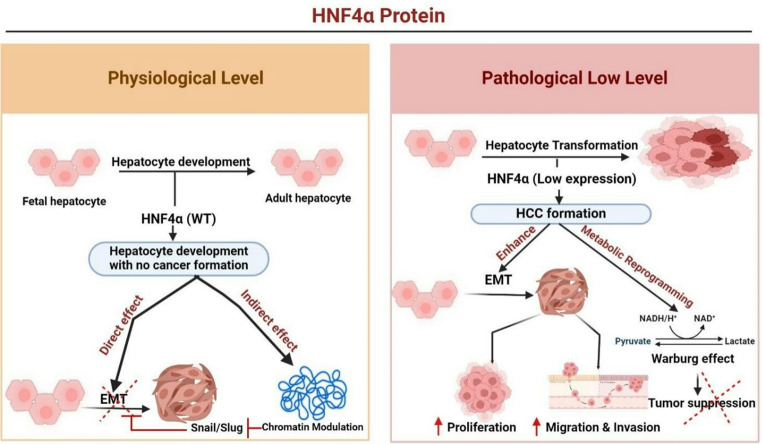
Fig. 2.
HNF4α’s Role in Hepatocyte Development and HCC Progression: Under normal conditions, HNF4α supports healthy hepatocyte development by exerting direct (EMT via Snail/Slug) and indirect (chromatin modulation) effects. In HCC, HNF4α downregulation drives hepatocyte transformation, promoting EMT, metabolic reprogramming, and the Warburg effect, resulting in increased cancer cell proliferation, migration, and invasion. Created using BioRender (BioRender.com)
-
2.
HNF4α as a target for metabolic dysregulation in liver cancer
The liver plays a crucial role in maintaining energy homeostasis and regulating various metabolic processes, including glycolysis, gluconeogenesis, the tricarboxylic acid (TCA) cycle, and lipid metabolism. Disruptions in these pathways, whether due to mutations, toxins, or inflammation, can lead to metabolic imbalances, that significantly contribute to liver diseases, including HCC. HNF4α is a central regulator of hepatic metabolism, and its downregulation has been linked to metabolic shifts that favor cancer-promoting metabolic profiles.
HNF4α is essential for maintaining liver-specific metabolic functions, particularly glucose homeostasis. Working in concert with the glucocorticoid receptor, HNF4α loss disrupts these metabolic networks, leading to increased glycolysis and impaired glucose metabolism, both of which enhance cancer cell invasiveness [10]. Furthermore, HNF4α modulates the glucocorticoid response by decreasing glycolytic activity and promoting mitochondrial oxidative phosphorylation. This metabolic shift counteracts the Warburg effect, a characteristic of cancer metabolism, where cells preferentially rely on glycolysis for energy production even in the presence of oxygen to support rapid growth [26]. Given its pivotal role in regulating these metabolic processes, targeting HNF4α in HCC could offer a promising therapeutic strategy to counteract the metabolic reprogramming that drives cancer progression.
In liver cancer models, upregulation of HNF4α expression reverses several key metabolic alterations. Specifically, elevated HNF4α levels attenuate glycolysis, promote gluconeogenesis, and improve cholesterol metabolism, collectively contributing to the inhibition of tumor growth, metastasis, and cancer cell proliferation [23]. Furthermore, HNF4α plays a critical role in regulating sulfur amino acid metabolism, thereby influencing liver cancer cell responses to methionine restriction. These findings highlight the extensive regulatory capacity of HNF4α over metabolic processes and its potential as a therapeutic target of metabolic dysregulation associated with HCC [27]. HNF4α downregulation disturbs not only glucose metabolism but also cholesterol and lipid metabolism, both of which are crucial for liver homeostasis. These metabolic alterations contribute to liver cancer progression, with HNF4α’s influence on cholesterol metabolism specifically impacting key HCC development pathways [28]. Disruptions in these metabolic pathways are a key drivers of liver cancer progression, highlighting the importance of targeting metabolic pathways in HCC treatment [29–32].Taken together, these findings underscore the potential of HNF4α-targeted therapies as a promising strategy for improving patient outcomes in liver cancer (Fig. 3).
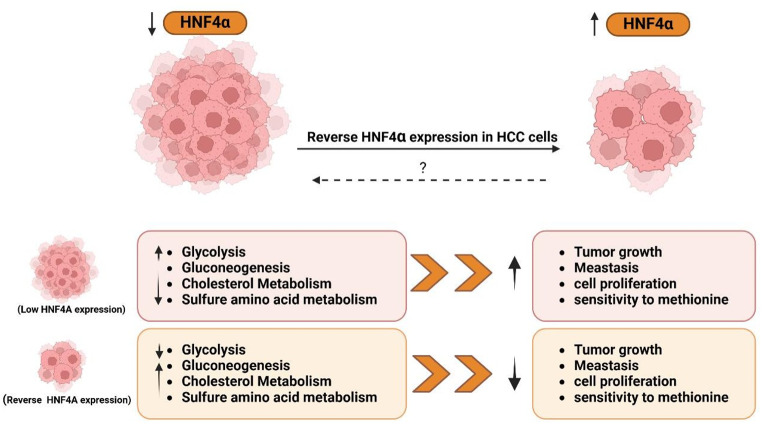
Fig. 3.
HNF4α Restoration Reverses HCC Metabolism and Growth: Low level of HNF4α in HCC correlates with increased glycolysis, gluconeogenesis, cholesterol metabolism, and sulfur amino acid metabolism, leading to enhanced tumor growth, metastasis, and proliferation. Restoring HNF4α reverses these metabolic changes, resulting in smaller tumors with reduced metastatic potential. The therapeutic potential of HNF4α restoration warrants further investigation. Created with BioRender (BioRender.com)
-
3.
HNF4α regulates tumor suppression and ferroptosis in HCC
HNF4α plays a multifaceted role in maintaining liver homeostasis and preventing HCC progression [23, 28, 33]. Its crucial function in preserving hepatocyte differentiation and functionality is underscored by the observation that HNF4a disruption frequently leads to tumorigenesis. HNF4α regulates key cellular processes, including proliferation, apoptosis, and metastasis, and interacts with vital signaling pathways such as Wnt/β-catenin, NF-κB, and TGF-β, all implicated in tumor development [34, 35].
A significant aspect of HNF4α’s function involves modulating ferroptosis, an iron-dependent form of cell death characterized by lipid peroxidation and oxidative stress. Given the liver’s iron-rich environment, which can exacerbate oxidative stress, the regulation of ferroptosis is particularly relevant in HCC. HNF4α acts as a suppressor of ferroptosis by regulating factors such as glutathione peroxidase 4 (GPX4) that inhibit lipid peroxidation, thereby maintaining cellular integrity. By preventing ferroptosis, HNF4α allows cancer cells to evade death, potentially contributing to a poor prognosis. Therefore, understanding HNF4α’s influence on ferroptosis is crucial for developing novel therapeutic strategies aimed at inducing cancer cell death and improving outcomes in liver cancer therapy.
Furthermore, HNF4α’s regulatory influence extends to lipid and iron metabolism. It directly controls genes involved in fatty acid desaturation and lipid synthesis, maintaining hepatic lipid homeostasis [36]. Its overexpression reduces intracellular lipid content and mitigates lipotoxicity in non-alcoholic fatty liver disease (NAFLD) models [37]. However, oxidative stress, often associated with high-fat diets, impairs HNF4α function, leading to hepatic steatosis [38]. Increased levels of reactive oxygen species (ROS) can stimulate HNF4α expression via the ASK1-CREB pathway, which may inadvertently exacerbate lipid peroxidation [39]. This complex interplay highlights HNF4α’s role in metabolic diseases [40]. Additionally, in iron metabolism, HNF4α regulates transferrin receptor 1 (TfR1), ferritin, and ferroportin, which are essential for controlling iron uptake, storage, and export. HNF4α downregulation leads to iron overload, increased ROS production, and heightened susceptibility to ferroptosis.
HNF4α’s activity is intricately linked to oxidative stress, and antioxidants exert a dual regulatory effect. Some antioxidants, such as 4’-nitro-6-hydroxyflavone (NOHF), directly inhibit HNF4α via AMPK activation, causing degradation and reduced activity. Others counteract ROS-mediated HNF4α upregulation, maintaining metabolic balance [41]. This dual modulation highlights the complexity of HNF4α regulation. The HNF4α-BAP31 axis is crucial in cancer cell survival; BAP31, by activating the PI3K/AKT pathway, promotes HCC cell survival and invasion [42]. BAP31 silencing enhances ferroptosis, reducing tumor growth [42], and regulates cell cycle progression and invasion in other cancer types [43]. HNF4α also maintains mitochondrial function, chromatin accessibility, and stress response; mutations lead to dysfunction and cell death [44, 45]. Furthermore, HNF4α influences cancer stem cell (CSC) stemness, reducing CD133-expressing CSC populations and promoting differentiation [46], although its role in tumorigenesis is complex [47]. In summary, HNF4α is a crucial regulator for tumor suppression, maintaining hepatocyte differentiation and preventing malignant transformation, through its multifaceted roles in metabolism and cancer cell regulation (Fig. 4).
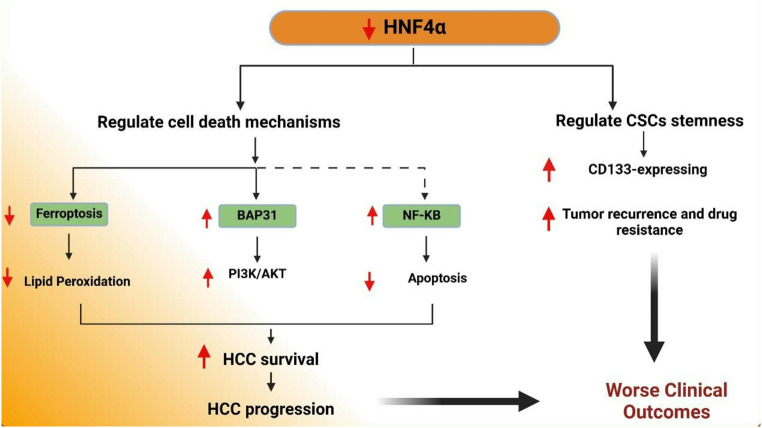
Fig. 4.
HNF4α regulates various pathways involved in HCC progression. HNF4α modulates cell death mechanisms, including ferroptosis, in which it decreases lipid peroxidation, and apoptosis via interactions with BAP31 and NF-κB. These pathways contribute to enhanced HCC survival and progression. Additionally, HNF4α influences the stemness CSC, as indicated by increased expression of CD133, promoting tumor recurrence and drug resistance. The combined effects enhance HCC survival, progression, and worsen clinical outcomes. Created with BioRender (BioRender.com)
-
4.
HNF4α regulates proliferation drivers in HCC
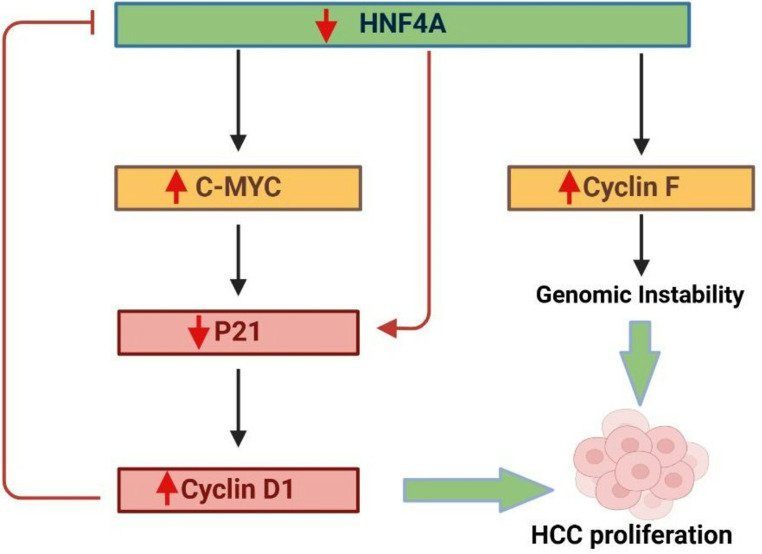
The interplay between proliferative drivers and tumor suppressors is critical in maintaining normal liver cell function and preventing cancer progression. In HCC, c-Myc and Cyclin D1 are key regulators of cell proliferation, with their activities tightly linked to tumorigenesis [48]. The loss of HNF4α, a pivotal transcription factor in liver function, leads to an upregulation of these drivers. c-Myc accelerates cell cycle progression, while Cyclin D1 facilitates cell cycle entry by activating cyclin-dependent kinases. Conversely, HNF4α mitigates this proliferative surge by inducing cell cycle arrest through the upregulation of p21. The delicate balance between HNF4α’s growth-suppressive actions and the proliferative influence of c-Myc and Cyclin D1 is crucial for regulating liver cell proliferation [40]. Disruption of HNF4α’s function tips this balance, enabling uncontrolled proliferation and driving liver carcinogenesis [49–51]. The interplay among HNF4α, c-Myc, and Cyclin D1 is crucial for HCC development. When HNF4α is depleted, the resulting elevated levels of c-Myc and Cyclin D1 promote tumor proliferation [52, 53]. Consequently, HNF4α acts to reduce HCC proliferation by regulating key metabolic genes and counteracting the effects of oncogenes such as c-Myc. The overexpression of Cyclin D1 intensifies this reaction, fostering a feedback loop that enhances cancer progression [54–57].
As a tumor suppressor, HNF4α regulates key metabolic genes, opposing oncogenes such as c-Myc, whose activation drives malignant transformation. Cyclin F maintains dNTP levels during DNA replication, and disruptions in dNTP homeostasis can lead to genomic instability, a key feature of uncontrolled cell proliferation. Reduced Cyclin F expression leads to increased RRM2 expression, promoting HCC growth [53, 58]. This dNTP imbalance elevates mutation rates, further enhancing the malignant phenotype. The deletion of HNF4α activates a c-Myc-regulated gene network, correlating with increased Cyclin D1 in tumors, thereby supporting the proposed regulatory loop involving c-Myc, Cyclin D1, and HNF4α [59]. In addition to its role in tumorigenesis, HNF4α is critical for liver regeneration, particularly following partial hepatectomy. It negatively regulates proto-mitogenic genes like c-Myc, which are essential for timely liver recovery. In knockout models, the absence of HNF4α delays hepatocyte proliferation, underscoring its essential function in liver regeneration [60]. Furthermore, HNF4α upregulates the expression of pregnane X receptor, an important regulator of HCC cellular resistance to antitumor drugs, promoting hepatocyte proliferation and reducing apoptosis during liver recovery [61, 62].
In summary, the interplay between proliferative drivers (c-Myc and Cyclin D1) and the tumor suppressor HNF4α is crucial for liver function and preventing HCC. Loss of HNF4α leads to increased c-Myc and Cyclin D1 activity, promoting uncontrolled cell proliferation and tumor growth. HNF4α counteracts this by inducing cell cycle arrest and regulating metabolic genes. Additionally, HNF4α is essential for liver regeneration, as its absence delays hepatocyte proliferation and increases resistance to antitumor drugs (Fig. 5).
Fig. 5.
HNF4α Downregulation Drives Hepatocellular Carcinoma Proliferation and Genomic Instability: Downregulation of HNF4α promotes HCC progression through key molecular pathways. Reduced HNF4α leads to increased c-MYC activation, which suppresses p21 and subsequently elevates Cyclin D1, driving cell cycle progression and HCC proliferation. Additionally, increased Cyclin F expression due to HNF4α loss contributes to genomic instability. Both pathways converge to promote HCC proliferation. The red arrows indicate downregulation, while green arrows indicate upregulation. Created with BioRender (BioRender.com)
-
5.
HNF4α and immune response modulation in HCC progression
The immune system plays a vital role in both protecting the liver and contributing to its pathogenesis, particularly in HCC. Recent studies have highlighted the immunomodulatory role of stromal cells such as mesenchymal stem cells in regulating immune responses, especially in regulating immune cells like CD4+ T cells, which are crucial for effective immune function in HCC [63, 64]. Under normal conditions, the immune response is tightly regulated to maintain liver function and prevent excessive inflammation. However, when this regulation is disrupted, it can lead to chronic inflammation, increased oxidative stress, and the accumulation of toxic metabolites, creating a microenvironment that promotes tumor development. This is further supported by studies highlighting the complex interplay between innate immunity and cellular senescence, processes that can contribute to chronic inflammation and tumorigenesis [65].
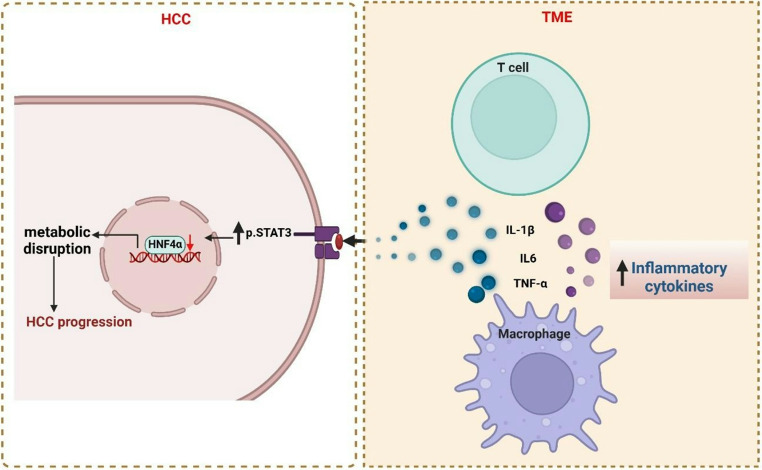
The liver microenvironment is characterized by a complex interplay between HNF4α, macrophage activation, and inflammation. Infiltrating immune cells, including macrophages with both anti-tumor and pro-tumorigenic capabilities, and T cells, release pro-inflammatory cytokines (IL-1β, IL-6, TNF-α) [66, 67]. These cytokines activate the p-STAT3 pathway in hepatocytes, resulting in HNF4α downregulation [66, 67]. This disruption of HNF4α, a critical regulator of hepatic metabolism and homeostasis, is implicated in inflammation-driven carcinogenesis, suggesting its potential as a therapeutic target. While a controlled inflammatory response is typical in healthy liver tissue, chronic inflammation disrupts homeostasis, fostering tumorigenesis [68, 69].
Macrophage-mediated phagocytosis is essential for maintaining liver homeostasis. Although a direct link between HNF4α and phagocytic activity remains unestablished, HNF4α’s pivotal role in hepatocyte development and function suggests an indirect influence on phagocytosis, potentially through regulation of genes governing Kupffer cells differentiation, activation, and phagocytic capacity [68, 69]. In summary, a feedback loop exists between inflammatory cell activation (particularly macrophages), cytokine release, and HNF4α regulation. This intricate relationship highlights the need for further research to elucidate the precise mechanisms connecting HNF4α, macrophage function (including phagocytosis), and the inflammatory response in liver cancer progression. The impact of macrophage activation on HNF4α levels and its subsequent consequences for liver homeostasis and tumorigenesis require further investigation (Fig. 6).
Fig. 6.
HNF4α Depletion Mediated by p-STAT3 Signaling in Hepatocytes Drives Metabolic Disruption and HCC Progression: Pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, secreted by macrophages and infiltrating T cells, activate the p-STAT3 signaling pathway in hepatocytes. This activation downregulates HNF4α, a key regulator of liver metabolism and tumor suppression. ultimately contributing to HCC progression. Created with BioRender (BioRender.com)
Research indicates that HNF4α is critical for regulating liver inflammation by maintaining active chromatin states. Under normal conditions, HNF4α represses acute-phase response genes; however, during inflammation, it can allow the upregulation of proteins such as serum amyloid A and haptoglobin, which can exacerbate the inflammatory process [70]. Cancer-induced inflammation is driven by the secretion of cytokines and chemokines, which facilitate the infiltration of immune cells into the liver. In healthy individuals, exposure to foreign substances triggers a regulated inflammatory response. However, in pathological conditions, persistent inflammation disrupts liver homeostasis and creates a microenvironment that supports tumor development [71]. T-cell infiltration is influenced by chemokine gradients and interactions with the tumor vasculature. Dysfunctional endothelial cells within tumors can hinder T cell trafficking, which impairs immune surveillance and reduces the effectiveness of cancer immunotherapies. To enhance T cell-mediated immune responses in HCC, it is essential to overcome these barriers. Additionally, excessive downregulation of HNF4α exacerbates liver inflammation and accelerates tumorigenesis [71, 72].
In the context of viral hepatitis, particularly hepatitis C virus (HCV) infection, the suppression of HNF4α is critically involved in liver cancer progression. Persistent HCV infection activates unfolded protein response (UPR) signaling through the PERK pathway, resulting in NRF2-mediated STAT3 activation that suppresses HNF4α expression [73]. This suppression disrupts the regulatory feedback loop involving miR-122, a liver-specific microRNA essential for managing inflammation and immune responses in HCC. Studies indicate that interferon (IFN)-based therapies may be more effective than direct-acting antivirals (DAAs) in restoring miR-122 levels, suggesting potential therapeutic strategies to modulate the immune response and mitigate HCC progression. Additionally, the downregulation of HNF4α can be reversed by inhibiting the ERK signaling pathway, indicating its significant role during HBV infection [74]. Preclinical models have highlighted the protective role of HNF4α in maintaining liver health. For instance, overexpression of HNF4α in liver organoid models has been shown to preserve liver function despite inflammation-induced damage [75]. In conclusion, HNF4α serves as a crucial regulator of inflammation and gene expression within the liver. Its suppression, particularly during viral infections such as HCV, leads to increased inflammation and facilitates the development of HCC.
The impact of post-translational modification (PTMs) on HNF4α activity and its influence on immune response modulation are increasingly recognized. While the specific PTMs involved and their precise effects require further investigation, HNF4α’s susceptibility to various PTMs [40] and its regulatory role in PTM-related gene expression suggest a complex interplay between PTMs, HNF4α function, and the immune response within the HCC tumor microenvironment (TME) [76, 77]. For instance, alterations in HNF4α phosphorylation could affect its interaction with coactivators or corepressors, thereby influencing the expression of genes involved in immune regulation [78]. Additionally, long non-coding RNAs, such as HNF4α-AS1, modulate HNF4α’s regulatory functions, fine-tuning its activity during liver development and disease [79]. This complex interplay underscores the intricate nature of the immune response and its impact on HCC progression.
-
6.
The transcriptional control of HNF4α in hepatic oncogenesis
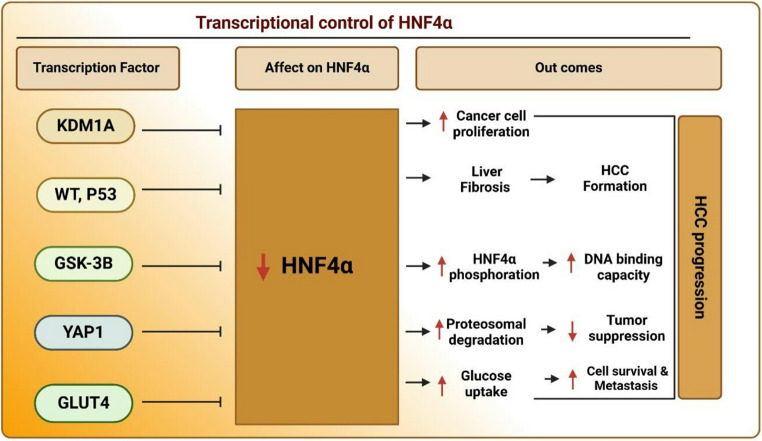
In the complex landscape of HCC, HNF4α acts as a pivotal transcription factor, maintaining a delicate balance between cellular homeostasis and cancer progression. However, its protective effects are often disrupted by various regulatory mechanisms. A key factor in this process is the activation of transposable lements—mobile genetic elements that, when triggered, can enhance the expression of the KDM1A gene. This epigenetic modifier silences HNF4α, thereby promoting unchecked cell proliferation. The downregulation of HNF4α facilitates tumor growth, highlighting it as a potential therapeutic target in the fight against HCC [27]. The downregulation of HNF4α is not the result of a single factor but rather a complex network of influences. Chronic liver injuries, characterized by inflammation and fibrosis, introduce factors such as Wilms tumor 1 (WT1) and p53. These factors which are elevated in fibrotic and cirrhotic livers further suppress HNF4α expression, accelerating its decline [82, 83]. Additionally, the activation of mitogen-activated protein kinase (MAPK) signaling interferes with the binding of other transcription factors like HNF1α and One Cut Homeobox 1 (ONECUT1) to enhancers that typically promote HNF4α expression [84]. This multifaceted repression creates a detrimental environment for liver health, as the cumulative effects of these factors, significantly accelerate the decline of HNF4α, exacerbating liver dysfunction and tumor progression.
The challenges facing HNF4α extend beyond transcriptional regulation to include PTMs that modulate its activity. GSK-3β, an oncogenic kinase, can phosphorylate HNF4α, reduce its DNA-binding capacity, and mark it for proteasomal degradation. Additionally, oncogenic proteins such as YAP1 further exacerbate this degradation and impair HNF4α’s tumor-suppressive functions [81]. This complex interplay of phosphorylation and ubiquitination is essential for controlling HNF4α’s stability and activity within the cancer microenvironment, rendering it vulnerable to aggressive cancer progression. Moreover, HNF4α is intricately involved in metabolic pathways, particularly in response to nutrient deprivation.
Under these stress conditions, it serves as a key regulator within the AMPKα2BORISGLUT4 pathway, facilitating glucose uptake crucial for the survival and metastasis of cancer cells [85]. HNF4α is regulated by factors like WT1, p53, and MAPK signaling, which reduce its expression and promote tumor growth in HCC. Post-translational modifications further impair its tumor-suppressive role. These regulatory disruptions make HNF4α a potential target for therapeutic strategies aimed at restoring its protective function in liver cancer (Fig. 7).
Fig. 7.
Transcriptional control of HNF4α and its impact on HCC progression: HNF4α expression and activity are modulated by various transcription factors (KDM1A, WT1/P53, GSK-3β, YAP1, and GLUT4). Downregulation of HNF4α promotes HCC progression by increasing cell proliferation, liver fibrosis, and metastasis, while impairing DNA binding, tumor suppression, and cell survival, ultimately leading to worse outcomes. Created with BioRender (BioRender.com)
-
7.
Translational applications of HNF4α in HCC therapy
The pathogenesis of HCC is significantly influenced by HNF4α, a crucial regulator of liver function. Dysregulation of HNF4α in HCC presents a promising avenues for improved diagnostics and targeted therapies. Its altered expression is a potential diagnostic biomarker, potentially improving early detection, patient prognosis, and overall outcomes [24, 80, 81]. While HNF4α’s role in tumorigenesis is complex [80], its consistent dysregulation in HCC [24] and influence on endothelial cell function [14] suggest its potential utility in monitoring angiogenesis and metastasis. Further research is needed to validate its diagnostic utility, particularly in early detection and risk stratification, potentially enabling earlier and more effective interventions [4, 49]. Moreover, HNF4α expression levels may predict patient response to treatments and overall survival, which could pave the way for personalized therapies tailored to individual patient characteristics [28, 49, 82, 83].
Given HNF4α’s crucial role in HCC development, it is a highly attractive therapeutic target. Therapeutic strategies under investigation include gene therapy to restore HNF4α expression, such as delivering HNF4α mRNA [80] or utilizing gene editing techniques [46], to reverse metabolic and cellular dysregulation in HCC. Another approach involves developing small molecule activators to specifically activate HNF4α [84]. While N-trans-caffeoyltyramine (NCT), a known HNF4α agonist, shows promise in preventing weight gain and hepatic steatosis [84], further research is crucial to develop more potent and specific activators with minimal off-target effects to enhance efficacy and reduce side effects [85]. Targeting metabolic reprogramming associated with HNF4α downregulation (e.g., using mannose and glucose oxidase [86]and the glycolytic pathway [25] could complement HNF4α-based therapies. Additionally, inducing ferroptosis, a process suppressed by HNF4α, could be explored in combination with HNF4α-targeted therapies (e.g., silencing BAP31) [87].
Challenges remain in translating these findings into effective clinical treatments. These include developing specific and effective HNF4α modulators with minimal off-target effects [88], and gaining a more comprehensive understanding of HNF4α’s intricate regulation by epigenetic modifications, transcription factors, and post-translational modifications [40, 78]. Developing such modulators requires a deep understanding of the complex interplay of proteins and their modifications, as demonstrated by studies using integrated proteomic and phosphoproteomic approaches to predict drug responses in cancer [89, 90]. Preclinical studies show promise, but rigorous clinical trials are essential to evaluate the safety, efficacy, and optimal dosing regimens of HNF4α-based therapies in HCC patients [80, 84]. Continued research is vital to fully realize the potential of HNF4α in HCC diagnostics and therapeutics.
Despite the significant promise of HNF4α for translational applications, several challenges remain. A comprehensive understanding of HNF4α’s intricate regulation, encompassing epigenetic modifications, transcription factors, and post-translational modifications, is crucial for developing targeted therapies. Furthermore, creating safe and effective drugs that specifically target HNF4α without impacting other essential transcription factors presents a major hurdle. Finally, the pleiotropic effects of HNF4α necessitate careful preclinical studies to identify and mitigate potential off-target effects and minimize associated risks.
Conclusion
HNF4α stands as a critical regulator in HCC progression, orchestrating EMT, metabolic reprogramming, and immune responses. Its downregulation accelerates tumor growth and invasion by disrupting metabolic balance, solidifying its potential as a key significant therapeutic target. Future research should prioritize the development of HNF4α-targeted therapies, such as mRNA-based approaches or small-molecule activators, to restore its expression and tumor-suppressive functions. Exploring the role of HNF4α in ferroptosis and interactions with immune cells may further unlock new avenues for cancer cell death. and synergistic combination therapies. Ultimately, a deeper understanding of HNF4α’s molecular mechanisms promises more effective HCC treatments and improved patient outcomes.
Acknowledgements
We thank Dr. Jing Li for their helpful suggestion and comments on the manuscript.
Author contributions
Hayam Hamdy, Jiashun Xu, and Chang Shen conceptualized and drafted the review manuscript. Die Fanand Yiwen Zhang critically reviewed and refined the content. Hui Li, Yonglong Wei, and Jianwei Sun provided supervision, contributed critical insights, and approved the final version ofthe manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (NSFC) fund (82273460, 82273089), the Yunnan Fundamental Research Projects (202401AS070133, 202501AS070120 and XDYC-YLWS-2023-0056), grant (KLTIPT-2023-03) from Key Laboratory of Tumor Immunological Prevention and Treatment in Yunnan Province, Yan’an Hospital Affiliated to Kunming Medical University, and grants (KC-23236563 and 2024Y014) from Yunnan University.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hayam Hamdy, Chang Shen and Jiashun Xu contributed equally to this work
Contributor Information
Hui Li, Email: lihuidoc@126.com.
Yonglong Wei, Email: weiyl@ynu.edu.cn.
Jianwei Sun, Email: jwsun@ynu.edu.cn.
References
- 1.P. Konyn, A. Ahmed, D. Kim, Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 15, 1295–1307 (2021). 10.1080/17474124.2021.1991792 [DOI] [PubMed]
- 2.Z. Li, N. Zhang, Z. Dong, X. Wang, J. Zhou, J. Gao, Y. Yang, J. Li, F. Guan, Y. Zhou, Z. Tan, Integrating transcriptomics, glycomics and glycoproteomics to characterize hepatitis B virus-associated hepatocellular carcinoma. Cell. Commun. Signal. 22, 200–215 (2024). 10.1186/s12964-024-01569-y [DOI] [PMC free article] [PubMed]
- 3.L. Sang, X. Wang, W. Bai, J. Shen, Y. Zeng, J. Sun, The role of hepatocyte nuclear factor 4α (HNF4α) in tumorigenesis. Front. Oncol. 12, 1011230–1011237 (2022). 10.3389/fonc.2022.1011230 [DOI] [PMC free article] [PubMed]
- 4.D. Suresh, A.N. Srinivas, D.P. Kumar, Etiology of hepatocellular carcinoma: special focus on fatty liver disease. Front. Oncol. 10, 601710–601719 (2020). 10.3389/fonc.2020.601710 [DOI] [PMC free article] [PubMed]
- 5.E. Haque, A.S. Teeli, D. Winiarczyk, M. Taguchi, S. Sakuraba, H. Kono, P. Leszczyński, M. Pierzchała, H. Taniguchi, HNF1A POU domain mutations found in Japanese liver Cancer patients cause downregulation of HNF4A promoter activity with possible disruption in transcription networks. Genes 13, 413–434 (2022). 10.3390/genes13030413 [DOI] [PMC free article] [PubMed]
- 6.B.J. Lambert, E. Levesque, D. Boisvert, F. Boudreau, A244 role of the nuclear receptor hnf4α and its many isoforms in colorectal cancer. J. Can. Association Gastroenterol. 1, 356–357 (2018). 10.1093/jcag/gwy009.244
- 7.P. Fang, W.A. Orellana, B. Abstract, Characterizing the role of HNF4α P1 and P2 isoforms in the classical subtype of pancreatic ductal adenocarcinoma. Cancer Research. 82, B034-B034 (2022). 10.1158/1538-7445.Panca22-b034
- 8.T. Tanaka, S. Jiang, H. Hotta, K. Takano, H. Iwanari, K. Sumi, K. Daigo, R. Ohashi, M. Sugai, C. Ikegame, H. Umezu, Y. Hirayama, Y. Midorikawa, Y. Hippo, A. Watanabe, Y. Uchiyama, G. Hasegawa, P.C. Reid, H. Aburatani, T. Hamakubo, J. Sakai, M. Naito, T. Kodama, Dysregulated expression of P1 and P2 promoter-driven hepatocyte nuclear factor-4alpha in the pathogenesis of human cancer. J Pathol. 5, 662–672 (2006). 10.1002/path.1928 [DOI] [PubMed]
- 9.S. Wilson, J. Babeu, F. Boisvert, F. Boudreau, A242 the p2 isoform class of the transcription factor hnf4a plays Dna repair role in colorectal cancer. J. Can. Assoc. Gastroenterol. 1, 423–424 (2018). 10.1093/jcag/gwy008.243
- 10.A.L. Hunter, T.M. Poolman, D. Kim, F.J. Gonzalez, D.A. Bechtold, A.S.I. Loudon, M. Iqbal, D.W. Ray, HNF4A modulates glucocorticoid action in the liver. Cell. Rep. 39, 110697 [DOI] [PMC free article] [PubMed]
- 11.L. Zheng, M. Ji, H. Zhang, L. Chang, Regulatory mechanisms of mechanotransduction in genome instability. Genome Instability Disease. 3, 311–316 (2022). 10.1007/s42764-022-00086-x
- 12.S. Matsuo, M. Ogawa, M.U. Muckenthaler, Y. Mizui, S. Sasaki, T. Fujimura, M. Takizawa, N. Ariga, H. Ozaki, M. Sakaguchi, F.J. Gonzalez, Y. Inoue, Hepatocyte nuclear factor 4α controls Iron metabolism and regulates transferrin receptor 2 in mouse liver. 290, 30855–30865 (2015). 10.1074/jbc.M115.694414 [DOI] [PMC free article] [PubMed]
- 13.E. Thymiakou, M. Tzardi, D. Kardassis, Impaired hepatic glucose metabolism and liver-α-cell axis in mice with liver-specific ablation of the Hepatocyte Nuclear Factor 4α (Hnf4a) gene. Metabolism: clinical and experimental. 139, 155371 (2023). 10.1016/j.metabol.2022.155371 [DOI] [PubMed]
- 14.M. Osanai, H. Chiba, T. Kojima, M. Fujibe, K. Kuwahara, H. Kimura, M. Satoh, N. Sawada, Hepatocyte nuclear factor (HNF)-4alpha induces expression of endothelial Fas ligand (FasL) to prevent cancer cell transmigration: a novel defense mechanism of endothelium against cancer metastasis. Japanese J. cancer Research: Gann. 93, 532–541 (2002). 10.1111/j.1349-7006.2002.tb01288.x [DOI] [PMC free article] [PubMed]
- 15.C. Cicchini, D. Filippini, S. Coen, A. Marchetti, C. Cavallari, I. Laudadio, F.M. Spagnoli, T. Alonzi, M. Tripodi, Snail controls differentiation of hepatocytes by repressing HNF4alpha expression. J. Cell. Physiol. 209, 230–238 (2006). 10.1002/jcp.20730 [DOI] [PubMed]
- 16.M. Kotulkar, D.R. Robarts, U. Apte, HNF4α in hepatocyte health and disease. Semin Liver Dis. 43, 234–244 (2023). 10.1055/a-2097-0660 [DOI] [PMC free article] [PubMed]
- 17.C. Walesky, W. Goessling, A. Balancing Act, Role of HNF4α and Β -catenin in hepatobiliary development and cholangiocarcinoma pathogenesis. The FASEB journal. 30, 56.54–56.54 (2016). 10.1096/fasebj.30.1_supplement.56.4
- 18.S. Wattanavanitchakorn, P. Rojvirat, T. Chavalit, M.J. Macdonald, S. Jitrapakdee, CCAAT-enhancer binding protein-α (C/EBPα) and hepatocyte nuclear factor 4α (HNF4α) regulate expression of the human fructose-1,6-bisphosphatase 1 (FBP1) gene in human hepatocellular carcinoma HepG2 cells. PLoS One 13, e0194252–e0194272 (2018). 10.1371/journal.pone.0194252 [DOI] [PMC free article] [PubMed]
- 19.L. Santangelo, A. Marchetti, C. Cicchini, A. Conigliaro, B. Conti, C. Mancone, J.A. Bonzo, F.J. Gonzalez, T. Alonzi, L. Amicone, M. Tripodi, The stable repression of mesenchymal program is required for hepatocyte identity: a novel role for hepatocyte nuclear factor 4α. Hepatol. (Baltimore Md) 53 2063–2074 (2011). 10.1002/hep.24280 [DOI] [PMC free article] [PubMed]
- 20.Q. Huang, M. Pu, G. Zhao, B. Dai, Z. Bian, H. Tang, C. Chen, W. Liu, X. Qu, L. Shen, K. Tao, Tg737 regulates epithelial-mesenchymal transition and cancer stem cell properties via a negative feedback circuit between snail and HNF4α during liver stem cell malignant transformation. Cancer Lett. 402, 52–60 (2017). 10.1016/j.canlet.2017.05.005 [DOI] [PubMed]
- 21.C. Battistelli, G. Sabarese, L. Santangelo, C. Montaldo, F.J. Gonzalez, M. Tripodi, C. Cicchini, The LncRNA HOTAIR transcription is controlled by HNF4α-induced chromatin topology modulation. Cell. Death Differ. 26, 890–901 (2019). 10.1038/s41418-018-0170-z [DOI] [PMC free article] [PubMed]
- 22.J. Kuang, T. Duan, C. Gao, C. Liu, S. Chen, L.Y. Zhu, L. Min, C. Lu, W. Wang, L. Zhu, RNF8 depletion attenuates hepatocellular carcinoma progression by inhibiting epithelial-mesenchymal transition and enhancing drug sensitivity. Acta Biochim. Et Biophys. Sinica. 55, 661–671 (2023). 10.3724/abbs.2023076 [DOI] [PMC free article] [PubMed]
- 23.B. Shokouhian, B. Negahdari, Z. Heydari, M. Totonchi, H. Aboulkheyr Es, A. Piryaei, E. Mostafavi, M. Vosough, HNF4α is possibly the missing link between epithelial-mesenchymal transition and Warburg effect during hepatocarcinogenesis. Cancer Sci. 114, 1337–1352 (2023). 10.1111/cas.15686 [DOI] [PMC free article] [PubMed]
- 24.A.S. Teeli, K. Łuczyńska, E. Haque, M.A. Gayas, D. Winiarczyk, H. Taniguchi, Disruption of tumor suppressors HNF4α/HNF1α causes tumorigenesis in liver. Cancers. 13, 5357–3578 (2021). 10.3390/cancers13215357 [DOI] [PMC free article] [PubMed]
- 25.F. Lin, W. Zhou, X. Yuan, S. Liu, Z. He, Mechanistic study of Quercetin in the treatment of hepatocellular carcinoma with diabetes via MEK/ERK pathway. Int. Immunopharmacol. 142, 113194 (2024). 10.1016/j.intimp.2024.113194 [DOI] [PubMed]
- 26.H. Chen, Q. Wu, L. Peng, T. Cao, M.-L. Deng, Y.-W. Liu, J. Huang, Y. Hu, N. Fu, K.-B. Zhou, M.-L. Yang, X.-F. Yang, S. Tan, Mechanism, Clinical Significance, and Treatment Strategy of Warburg Effect in Hepatocellular Carcinoma. Journal of Nanomaterials. 2021, 1–10 (2021). 10.1155/2021/5164100
- 27.Q. Xu, Y. Li, X. Gao, K. Kang, J.G. Williams, L. Tong, J. Liu, M. Ji, L.J. Deterding, X. Tong, J.W. Locasale, L. Li, I. Shats, X. Li, HNF4α regulates sulfur amino acid metabolism and confers sensitivity to methionine restriction in liver cancer. Nat. Commun. 1, 3978–3995 (2020). 10.1038/s41467-020-17818-w [DOI] [PMC free article] [PubMed]
- 28.X. Wang, J. Shi, M. Huang, J. Chen, J. Dan, Y. Tang, Z. Guo, X. He, Q. Zhao, TUBB2B facilitates progression of hepatocellular carcinoma by regulating cholesterol metabolism through targeting HNF4A/CYP27A1. Cell death & disease. 14, 179–188 (2023). 10.1038/s41419-023-05687-2 [DOI] [PMC free article] [PubMed]
- 29.G. Fu, S.T. Li, Z. Jiang, Q. Mao, N. Xiong, X. Li, Y. Hao, H. Zhang, PGAM5 deacetylation mediated by SIRT2 facilitates lipid metabolism and liver cancer proliferation. Acta Biochim. Et Biophys. Sinica. 55, 1370–1379 (2023). 10.3724/abbs.2023155 [DOI] [PMC free article] [PubMed]
- 30.C. Liu, J. Shi, Z. Jiang, S. Jiang, Y. Wu, D. Peng, J. Tang, L. Guo, RP11-495P10.1 promotes HCC cell proliferation by regulating reprogramming of glucose metabolism and acetylation of the NR4A3 promoter via the PDK1/PDH axis. Acta Biochimica et Biophysica Sinica. 56, 44–53. (2024). 10.3724/abbs.2023242 [DOI] [PMC free article] [PubMed]
- 31.Y. Ni, M. Huang, S. Chen, S. Wang, J. Chen, Integrins and NAFLD-associated liver diseases: clinical associations, pathophysiological mechanisms and Pharmacological implications: integrins and NAFLD-associated liver diseases. Acta Biochim. Biophys. Sin (Shanghai) 56, 1573–1583 (2024). 10.3724/abbs.2024149 [DOI] [PMC free article] [PubMed]
- 32.X. Yang, H. Feng, J. Kim, G. Ti, L. Wang, K. Wang, D. Song, PRR34-AS1 promotes mitochondrial division and glycolytic reprogramming in hepatocellular carcinoma cells through upregulation of MIEF2. Acta Biochimica et Biophysica Sinica. 56, 1604–1617 (2024). 10.3724/abbs.2024083 [DOI] [PMC free article] [PubMed]
- 33.B.F. Ning, J. Ding, C. Yin, W. Zhong, K. Wu, X. Zeng, W. Yang, Y.X. Chen, J.P. Zhang, X. Zhang, H.Y. Wang, W.F. Xie, Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer research. 70, 7640–7651. (2010). 10.1158/0008-5472.CAN-10-0824 [DOI] [PubMed]
- 34.D.D. Lv, L.Y. Zhou, H. Tang, Hepatocyte nuclear factor 4α and cancer-related cell signaling pathways: a promising insight into cancer treatment. Experimental Mol. Med. 53, 8–18 (2021). 10.1038/s12276-020-00551-1 [DOI] [PMC free article] [PubMed]
- 35.D.V.F. Tauriello, E. Batlle, Targeting the microenvironment in advanced colorectal cancer. Trends in Cancer. 2, 495–504 (2016). 10.1016/j.trecan.2016.08.001 [DOI] [PubMed]
- 36.L. Yin, H. Ma, X. Ge, P.A. Edwards, Y. Zhang, Hepatic hepatocyte nuclear factor 4α is essential for maintaining triglyceride and cholesterol homeostasis. Arteriosclerosis, thrombosis, and vascular biology. 11, 328–336 (2011). 10.1161/ATVBAHA.110.217828 [DOI] [PMC free article] [PubMed]
- 37.W.W. Hwang-Verslues, F.M. Sladek, HNF4α--role in drug metabolism and potential drug target? Current opinion in pharmacology. 10, 698–705 (2010). 10.1016/j.coph.2010.08.010 [DOI] [PMC free article] [PubMed]
- 38.É. Lambert, J.P. Babeu, J. Simoneau, J. Raisch, L. Lavergne, D. Lévesque, É. Jolibois, M. Avino, M.S. Scott, F. Boudreau, F.M. Boisvert, Human hepatocyte nuclear factor 4-α encodes isoforms with distinct transcriptional functions. Molecular & Cellular Proteomics. 19, 808–829 (2022). 10.1074/mcp.RA119.001909 [DOI] [PMC free article] [PubMed]
- 39.J.Y. Chiang, Hepatocyte nuclear factor 4alpha regulation of bile acid and drug metabolism. Expert Opin. Drug Metab. Toxicol. 2, 137–147 (2009). 10.1517/17425250802707342 [DOI] [PMC free article] [PubMed]
- 40.C. Walesky, U. Apte, Role of hepatocyte nuclear factor 4α (HNF4α) in cell proliferation and cancer. Gene Expression 16, 101–108 (2015). 10.3727/105221615X14181438356292 [DOI] [PMC free article] [PubMed]
- 41.J.H. Kim, H.J. Eom, G. Lim, S. Park, J. Lee, S. Nam, Y.H. Kim, J.H. Jeong, Differential effects, on oncogenic pathway signalling, by derivatives of the HNF4 α inhibitor BI6015. Br. J. cancer. 120, 488–498 (2019). 10.1038/s41416-018-0374-5 [DOI] [PMC free article] [PubMed]
- 42.M. Sun, X. Liu, W. Wei, N. Ge, S. Luo, S. Shen, R. Ge, BAP31 promotes proliferation, invasion, and metastasis of liver Cancer cells via activating PI3K/AKT pathway. J. Healthc. Eng. 11, 7686728 (2022). 10.1155/2022/7686728 [DOI] [PMC free article] [PubMed] [Retracted]
- 43.E. Dang, S. Yang, C. Song, D. Jiang, Z. Li, W. Fan, Y. Sun, L. Tao, J. Wang, T. Liu, C. Zhang, B. Jin, J. Wang, K. Yang, BAP31, a newly defined cancer/testis antigen, regulates proliferation, migration, and invasion to promote cervical cancer progression. Cell. Death Disease 9, 791–112 (2018). 10.1038/s41419-018-0824-2 [DOI] [PMC free article] [PubMed]
- 44.V. Marchesin, A. Pérez-Martí, G. Le Meur, R. Pichler, K. Grand, E.D. Klootwijk, A. Kesselheim, R. Kleta, S. Lienkamp, M. Simons, Molecular basis for Autosomal-Dominant renal Fanconi syndrome caused by HNF4A. Cell reports. 29, 4407–4421.e4405. (2019). 10.1016/j.celrep.2019.11.066 [DOI] [PMC free article] [PubMed]
- 45.A. Thakur, K. Park, R. Cullum, B.M. Fuglerud, M. Khoshnoodi, S. Drissler, T.L. Stephan, J. Lotto, D. Kim, F.J. Gonzalez, P.A. Hoodless, HNF4A guides the MLL4 complex to Establish and maintain H3K4me1 at gene regulatory elements. Commun. Biology. 7, 144–158 (2024). 10.1038/s42003-024-05835-0 [DOI] [PMC free article] [PubMed]
- 46.P.H. Tsai, M.L. Wang, J.H. Chang, A.A. Yarmishyn, P.N. Nhi Nguyen, W. Chen, Y. Chien, T.I. Huo, C.Y. Mou, S.H. Chiou, dual delivery of HNF4α and cisplatin by mesoporous silica nanoparticles inhibits Cancer pluripotency and tumorigenicity in Hepatoma-Derived CD133-Expressing stem cells. ACS Appl. Mater. Interfaces 11, 19808–19818 (2019). 10.1021/acsami.9b04474 [DOI] [PubMed]
- 47.Z. Wang, Y. Zhang, J. Zhang, Q. Deng, H. Liang, Controversial roles of hepatocyte nuclear receptor 4 α on tumorigenesis. Oncol. Lett. 21, 356–366 (2021). 10.3892/ol.2021.12617 [DOI] [PMC free article] [PubMed]
- 48.W. Lei, F. Liu, S.A. Ness, Positive and negative regulation of c-Myb by Cyclin D1, Cyclin-dependent kinases, and p27 Kip1. Blood 105, 3855–3861 (2005). 10.1182/blood-2004-08-3342 [DOI] [PMC free article] [PubMed]
- 49.J. Li, Y. Zhu, Recent advances in liver Cancer stem cells: Non-coding RNAs, oncogenes and oncoproteins. Front. Cell. Dev. Biology 8, 548335–548351 (2020). 10.3389/fcell.2020.548335 [DOI] [PMC free article] [PubMed]
- 50.H. Wang, P. Wang, M. Xu, X. Song, H. Wu, M. Evert, D.F. Calvisi, Y. Zeng, X. Chen, Distinct functions of transforming growth factor-β signaling in c-MYC driven hepatocellular carcinoma initiation and progression. Cell. Death Dis. 12, 200–216 (2021). 10.1038/s41419-021-03488-z [DOI] [PMC free article] [PubMed]
- 51.K. Qu, Z. Wang, H. Fan, J. Li, J. Liu, P. Li, Z. Liang, H. An, Y. Jiang, Q. Lin, X. Dong, P. Liu, C. Liu, MCM7 promotes cancer progression through cyclin D1-dependent signaling and serves as a prognostic marker for patients with hepatocellular carcinoma. Cell. Death Dis. 8, e2603–e2616 (2017). 10.1038/cddis.2016.352 [DOI] [PMC free article] [PubMed]
- 52.H. Wu, T. Reizel, Y.J. Wang, J.L. Lapiro, B.T. Kren, J. Schug, S. Rao, A. Morgan, A. Herman, L.L. Shekels, M.S. Rassette, A.N. Lane, T. Cassel, T.W.M. Fan, J.C. Manivel, S. Gunewardena, U. Apte, P. Sicinski, K.H. Kaestner, J.H. Albrecht, A negative reciprocal regulatory axis between Cyclin D1 and HNF4α modulates cell cycle progression and metabolism in the liver. Proc. Natl. Acad. Sci. United States Am. 117, 17177–17186 (2020). 10.1073/pnas.2002898117 [DOI] [PMC free article] [PubMed]
- 53.H. Taniguchi, A. Fujimoto, H. Kono, M. Furuta, M. Fujita, H. Nakagawa, Loss-of-function mutations in Zn-finger DNA-binding domain of HNF4A cause aberrant transcriptional regulation in liver cancer. Oncotarget 9, 26144–26156 (2018). 10.18632/oncotarget.25456 [DOI] [PMC free article] [PubMed]
- 54.E. Tashiro, H. Maruki, Y. Minato, Y. Doki, I.B. Weinstein, M. Imoto, Overexpression of Cyclin D1 Contributes to Malignancy by Up-Regulation of Fibroblast Growth Factor Receptor 1 via the pRB/E2F Pathway1. Cancer Research. 63, 424–431 (2003). [PubMed]
- 55.R.R. John, C. Ravindran, N. Malathi, R.M. Aruna, Evaluation of the role played by Cyclin D1 as a diagnostic and prognostic marker in the progression of oral carcinogenesis. J. Maxillofac. Oral Surg. 17, 389–395 (2018). 10.1007/s12663-018-1087-2 [DOI] [PMC free article] [PubMed]
- 56.F.I. Montalto, F. De Amicis, Cyclin D1 in cancer: A molecular connection for cell cycle control, adhesion and invasion in tumor and stroma. Cells. 9, 2648–2661 (2020). 10.3390/cells9122648 [DOI] [PMC free article] [PubMed]
- 57.S. Qie, J.A. Diehl, Cyclin D1, cancer progression, and opportunities in cancer treatment. J. Mol. Med. (Berl) 49, 1313–1326 (2016). 10.1007/s00109-016-1475-3 [DOI] [PMC free article] [PubMed]
- 58.V. D’Angiolella, V. Donato, F.M. Forrester, Y.T. Jeong, C. Pellacani, Y. Kudo, A. Saraf, L. Florens, M.P. Washburn, M. Pagano, Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell. 149, 1023–1034 (2012). 10.1016/j.cell.2012.03.043 [DOI] [PMC free article] [PubMed]
- 59.Q.O. Zhou, T. Liu, W. Qian, J. Ji, Q. Cai, Y. Jin, J. Jiang, J.O. Zhang, HNF4A-BAP31-VDAC1 axis synchronously regulates cell proliferation and ferroptosis in gastric cancer. Cell. Death Dis. 14, 356–370 (2023). 10.1038/s41419-023-05868-z [DOI] [PMC free article] [PubMed]
- 60.V. D’Angiolella, M. Esencay, M. Pagano, A Cyclin without Cyclin-dependent kinases: Cyclin F controls genome stability through ubiquitin-mediated proteolysis. Trends Cell. Biol. 23, 135–140 (2013). 10.1016/j.tcb.2012.10.011 [DOI] [PMC free article] [PubMed]
- 61.M. Kotulkar, D. Paine-Cabrera, K. Venneman, U. Apte, Role of HNF4alpha-cMyc interaction in liver regeneration after partial hepatectomy. Front. Endocrinol. (Lausanne) 31, 1404318–1404327 (2024). 10.3389/fendo.2024.1404318 [DOI] [PMC free article] [PubMed]
- 62.S. Qiu, Y. Pan, Y. Cui, M. Li, T. Yue, S. Pu, Q. Zhang, M. Wang, HNF4α improves hepatocyte regeneration by upregulating PXR. FASEB J. 38, e23830 (2024). 10.1096/fj.202400459RR [DOI] [PubMed]
- 63.Z. Lin, W. Cai, Y. Sun, B. Han, Y. Hu, Z. He, X. Chen, Mechanism and application of mesenchymal stem cells and their secreting extracellular vesicles in regulating CD4(+)T cells in immune diseases. Biophys. Rep. 10, 403–415 (2024). 10.52601/bpr.2024.240005 [DOI] [PMC free article] [PubMed]
- 64.H. Wang, F. Chu, X.F. Zhang, P. Zhang, L.X. Li, Y.L. Zhuang, X.F. Niu, X. He, Z.J. Li, Y. Bai, D. Mao, Z.W. Liu, D.L. Zhang, B. Li, TPX2 enhances the transcription factor activation of PXR and enhances the resistance of hepatocellular carcinoma cells to antitumor drugs. Cell. Death Dis. 27, 64–81 (2023). 10.1038/s41419-022-05537-7 [DOI] [PMC free article] [PubMed]
- 65.J. Hou, Y. Zheng, C. Gao, Regulation of cellular senescence by innate immunity. Biophys Rep. 9, 338–351 (2023). 10.52601/bpr.2023.230032 [DOI] [PMC free article] [PubMed]
- 66.P.X. Li, Q. Ou, S. Shi, C. Shao, Immunomodulatory properties of mesenchymal stem cells/dental stem cells and their therapeutic applications. Cell. Mol. Immunol. 20, 558–569 (2023). 10.1038/s41423-023-00998-y [DOI] [PMC free article] [PubMed]
- 67.O. Goldman, L.N. Adler, E. Hajaj, T. Croese, N. Darzi, S. Galai, H. Tishler, Y. Ariav, D. Lavie, L. Fellus-Alyagor, R. Oren, Y. Kuznetsov, E. David, R. Jaschek, C. Stossel, O.Singer, S. Malitsky, R. Barak, R. Seger, N. Erez, I. Amit, A. Tanay, A. Saada, T. Golan, T. Rubinek, J. Sang Lee, S. Ben-Shachar, I. Wolf, A. Erez, Early Infiltration of Innate Immune Cells to the Liver Depletes HNF4α and Promotes Extrahepatic Carcinogenesis. Cancer Discov. 13, 1616–1635 (2023). 10.1158/2159-8290.CD-22-1062 [DOI] [PMC free article] [PubMed]
- 68.A. Delaforest, K. Nagaoka, K. Si-Tayeb, F.K. Noto, G. Konopka, M.A. Battle, S.A. Duncan, HNF4A is essential for specification of hepatic progenitors from human pluripotent stem cells. Development. 138, 4143–4153 (2011). 10.1242/dev.062547 [DOI] [PMC free article] [PubMed]
- 69.A. Thakur, J.C.H. Wong, E.Y. Wang, J. Lotto, D. Kim, J.C. Cheng, M. Mingay, R. Cullum, V. Moudgil, N. Ahmed, S.H. Tsai, W. Wei, C.P. Walsh, T. Stephan, M. Bilenky, B.M. Fuglerud, M.M. Karimi, F.J. Gonzalez, M. Hirst, P.A. Hoodless, hepatocyte nuclear factor 4-Alpha is essential for the active epigenetic state at enhancers in mouse liver. Hepatol. 70, 1360–1376 (2019). 10.1002/hep.30631 [DOI] [PMC free article] [PubMed]
- 70.D. Li, T. Zhang, Y. Guo, C. Bi, M. Liu, G. Wang, Biological impact and therapeutic implication of tumor-associated macrophages in hepatocellular carcinoma. Cell. Death Dis. 15, 498–4103 (2024). 10.1038/s41419-024-06888-z [DOI] [PMC free article] [PubMed]
- 71.M.W. Robinson, C. Harmon, C. O’farrelly, Liver immunology and its role in inflammation and homeostasis. Cell. Mol. Immunol. 13, 267–276 (2016). 10.1038/cmi.2016.3 [DOI] [PMC free article] [PubMed]
- 72.C. Ehle, A. Iyer-Bierhoff, Y. Wu, S. Xing, M. Kiehntopf, A.S. Mosig, M. Godmann, T. Heinzel, Downregulation of HNF4A enables transcriptomic reprogramming during the hepatic acute-phase response. Commun. Biol. 7, 589–5103 (2024). 10.1038/s42003-024-06288-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.S. Guo, H. Lu, Novel mechanisms of regulation of the expression and transcriptional activity of hepatocyte nuclear factor 4α. J. Cell. Biochem. 120, 519–532 (2019). 10.1002/jcb.27407 [DOI] [PMC free article] [PubMed]
- 74.Y. Aydin, R. Kurt, K. Song, D. Lin, H. Osman, B. Youngquist, J.W. Scott, N.J. Shores, P. Thevenot, A. Cohen, S. Dash, Hepatic stress response in HCV infection promotes STAT3-Mediated Inhibition of HNF4A-miR-122 feedback loop in liver fibrosis and Cancer progression. Cancers (Basel). 11, 1407–1432 (2019). 10.3390/cancers11101407 [DOI] [PMC free article] [PubMed]
- 75.S. Park, Y.N. Ha, M. Dezhbord, A.R. Lee, E.S. Park, Y.K. Park, J. Won, N.Y. Kim, S.Y. Choo, J.J. Shin, C.H. Ahn, K.K.H. Kim, Suppression of Hepatocyte Nuclear Factor 4 α by Long-term Infection of Hepatitis B Virus Contributes to Tumor Cell Proliferation. Int J Mol Sci. 21, 948–967 (2020). 10.3390/ijms21030948 [DOI] [PMC free article] [PubMed]
- 76.T.M. Filtz, W.K. Vogel, M. Leid, Regulation of transcription factor activity by interconnected post-translational modifications. Trends Pharmacol. Sci. 35, 76–85 (2014). 10.1016/j.tips.2013.11.005 [DOI] [PMC free article] [PubMed]
- 77.W. Zhou, Z. Hannoun, E. Jaffray, C.N. Medine, J.R. Black, S. Greenhough, L. Zhu, J.A. Ross, S. Forbes, I. Wilmut, J.P. Iredale, R.T. Hay, D.C. Hay, Sumoylation of HNF4α regulates protein stability and hepatocyte function. J. Cell. Sci. 125, 3630–3635 (2012). 10.1242/jcs.102889 [DOI] [PMC free article] [PubMed]
- 78.B. Vető, D. Bojcsuk, C. Bacquet, J. Kiss, S. Sipeki, L. Martin, L. Buday, B.L. Bálint, T. Arányi, The transcriptional activity of hepatocyte nuclear factor 4 alpha is inhibited via phosphorylation by ERK1/2. PLoS One. e0172020–e0172039 (2017). 10.1371/journal.pone.0172020 [DOI] [PMC free article] [PubMed]
- 79.R. Zhang, L. Liu, Assaying antigen-specific T cell trans-endothelial migration in vitro with the transwell system: application in tumor immunology. Biophys. Rep. 10, 1–8 (2024). 10.52601/bpr.2024.240032 [DOI] [PMC free article] [PubMed]
- 80.T. Yang, M. Poenisch, R. Khanal, Q. Hu, Z. Dai, R. Li, G. Song, Q. Yuan, Q. Yao, X. Shen, R. Taubert, B. Engel, E. Jaeckel, A. Vogel, C.S. Falk, A. Schambach, D. Gerovska, M.J. Araúzo-Bravo, F.W.R. Vondran, T. Cantz, N. Horscroft, A. Balakrishnan, F. Chevessier, M. Ott, A.D. Sharma, Therapeutic HNF4A mRNA attenuates liver fibrosis in a preclinical model. J. Hepatol. 75, 1420–1433 (2021). 10.1016/j.jhep.2021.08.011 [DOI] [PubMed]
- 81.M. Osanai, H. Chiba, T. Kojima, M. Fujibe, K. Kuwahara, H. Kimura, M. Satoh, N. Sawada, Hepatocyte nuclear factor (HNF)-4alpha induces expression of endothelial Fas ligand (FasL) to prevent cancer cell transmigration: a novel defense mechanism of endothelium against cancer metastasis. Japanese J. Cancer Research. 93, 532–541 (2002). 10.1111/j.1349-7006.2002.tb01288.x [DOI] [PMC free article] [PubMed]
- 82.P. Konyn, A. Ahmed, D. Kim, Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 11, 1295–1307 (2021). 10.1080/17474124.2021.1991792 [DOI] [PubMed]
- 83.C. Jiang, Y. Wu, J. Zhou, J. Zhao, Novel targets and small molecular interventions for liver cancer. Biomed. Res. Int. 2014, 148783–148785 (2014). 10.1155/2014/148783 [DOI] [PMC free article] [PubMed]
- 84.J. Inoue, S. Ikeda, T. Kanayama, R. Sato, The flavonoid derivative 4’-nitro-6-hydroxyflavone suppresses the activity of HNF4α and stimulates the degradation of HNF4α protein through the activation of AMPK. Biosci. Biotechnol. Biochem. 8, 1548–1552 (2017). 10.1080/09168451.2017.1325316 [DOI] [PubMed]
- 85.A. Caddeo, S. Romeo, Precision medicine and nucleotide-based therapeutics to treat MASH. Clin. Mol. Hepatol. 31, (2025). 10.3350/cmh.2024.0438. S76-S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.X. Zeng, Y. Ruan, L. Wang, J. Deng, S. Yan, Synergistic Glycolysis disturbance for cancer therapy by a MOF-based nanospoiler. Biophys. Rep. 3, 134–145 (2023). 10.52601/bpr.2023.230003 [DOI] [PMC free article] [PubMed]
- 87.M. Sun, X. Liu, W. Wei, N. Ge, S. Luo, S. Shen, R. Ge, Retracted: BAP31 Promotes Proliferation, Invasion, and Metastasis of Liver Cancer Cells via Activating PI3K/AKT Pathway. Journal of healthcare engineering. 11, 7686728 (2022). 10.1155/2022/7686728 [DOI] [PMC free article] [PubMed] [Retracted]
- 88.H.R. Chang, S. Nam, M.C. Kook, K.T. Kim, X. Liu, H. Yao, H.R. Jung, R. Jr. Lemos, H.H. Seo, H.S. Park, Y. Gim, D. Hong, I. Huh, Y.W. Kim, D. Tan, C.G. Liu, G. Powis, T. Park, H. Liang, Y.H. Kim, HNF4α is a therapeutic target that links AMPK to WNT signalling in early-stage gastric cancer. Gut. 2, 19–32 (2016). 10.1136/gutjnl-2014-307918 [DOI] [PMC free article] [PubMed]
- 89.X. Li, Y. Huang, K. Zheng, G. Yu, Q. Wang, L. Gu, J. Li, H. Wang, W. Zhang, Y. Sun, C. Li, Integrated proteomic and phosphoproteomic data-independent acquisition data evaluate the personalized drug responses of primary and metastatic tumors in colorectal cancer. Biophys. Rep. 2, 67–81 (2023). 10.52601/bpr.2022.210048 [DOI] [PMC free article] [PubMed]
- 90.Y. Di, W. Li, B. Salovska, Q. Ba, Z. Hu, S. Wang, Y. Liu, A basic phosphoproteomic-DIA workflow integrating precise quantification of phosphosites in systems biology. Biophys. Rep. 2, 82–98 (2023). 10.52601/bpr.2023.230007 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.