Abstract
Individuals differ in the extent to which they experience negative mood states over time. To explore the relationship between individual differences in negative affect (NA) and brain activity, we asked healthy subjects participating in positron-emission tomography scans to rate the extent to which they had experienced NA terms during the month before scanning. In two independent samples of subjects, resting regional cerebral blood flow within the ventromedial prefrontal cortex (VMPFC) correlated with ratings of NA. The finding converges with recent evidence implicating the VMPFC in emotional and autonomic processing. Moreover, it demonstrates that variability in basal VMPFC activity across subjects is related to individual differences in subjective emotional experience.
Negative affect (NA) emerges as a higher order factor in almost every study of self-rated mood (1). The factor reflects general subjective distress and consists of a range of unpleasant mood states, such as irritability, anxiety, and anger (2). Individuals show marked differences in the extent to which they experience NA over time. These individual differences in affective experience show stability over time (3) and influence the risk of developing psychiatric conditions. Specifically, subjects with high trait levels of NA demonstrate a significantly elevated risk of developing anxiety disorders and clinical depression (4).
Differences in the basal activity within certain cortical and subcortical brain regions involved in emotional processing may underlie individual differences in personality and affective disposition (5). Neuroimaging techniques, such as positron-emission tomography (PET), provide a means of assessing these relationships (6, 7). However, the large number of subjects necessary to provide adequate statistical power and confidence limits has generally limited assessment of these issues.
To examine the relationship between regional cerebral blood flow (rCBF, an index of regional neuronal activity) and NA, we routinely collected data during one or more baseline scans as a part of H215O PET studies ongoing in our laboratory. The baseline scans consisted of subjects resting with their eyes closed in a quiet darkened room. Brain activity in this condition is characterized by an organized and consistent pattern of rCBF levels and uniform oxygen extraction factors across all nonvisual cortical regions (8). Measurement of rCBF in this state shows reasonable within and across session reliability, and comparable variability across studies.¶,‖,** Thus, although rCBF is dynamic and changes with task demands, measurement of resting rCBF provides a relatively stable estimate of brain activity in the absence of stimulation. Here we report on two studies involving large samples of subjects. In both studies a correlation emerged between self-ratings of NA for the month before scanning and resting rCBF within the ventral frontal lobe.
Materials and Methods
Human Subjects.
Fifty-one right-handed subjects participated in Study 1 (28 males and 23 females; age 18–50 years, median age = 23). Thirty-eight right-handed subjects participated in Study 2 (24 males and 14 females; age 19–55 years, median age = 26). All subjects completed a brief screening questionnaire. Exclusion criteria for both studies included: history of neurologic or medical disorders; history of major head trauma; and current use of psychotropic medications. All potential subjects were screened for psychiatric disorders with a computerized, self-administered questionnaire (12) and excluded on meeting criteria for any major psychiatric diagnoses (two subjects were included when subsequent detailed clinical interviews revealed false-positive diagnoses). Subjects were also excluded if they had received exposure to any emotionally evocative stimuli (e.g., aversive or highly rewarding stimuli) during the scanning session. All subjects completed written informed consent approved by the Minneapolis Veterans Affairs Medical Center's Institutional Review Board.
Affect Ratings.
All subjects retrospectively rated the extent to which they had experienced NA-related mood states over the past month by using the Positive Affect Negative Affect Schedule (PANAS) (13). NA measured over such a time span provides an estimate of the subjects' general level of affective experience, but couches the question in the context of a definable period. Subjects rated their experience of each positive affect (PA) and NA mood term on the PANAS, using a 5-point, anchored scale. NA scores represent the aggregate of the 10-NA terms with a minimum possible score of 10 and a maximum possible score of 50. To assess state levels of negative mood during scanning, subjects rated their average mood during the scanning session with the Profile of Mood States (POMS) (14) immediately following the scanning session. The POMS provides a more extensive assessment of different mood terms than the PANAS, and includes terms that do not selectively correlate with NA or PA. We therefore limited analysis of the POMS to the Tension–Anxiety and Anger–Hostility subscales, which both reflect lower-order factors of NA. Although each subscale represents only one aspect of NA, they provide a reasonable proxy because of the heavy loadings of the anxiety and hostility subfactors on the higher-order NA factor (15). All subjects in the first study completed the POMS. Only 29 subjects in the second study completed the POMS (because some studies did not include a POMS rating as part of their standard protocol). POMS scores were converted to T-scores before analysis by using the scale conversions provided by the POMS manual.
PET Imaging and Analysis.
rCBF was assayed using an ECAT 953B camera (Siemens, Knoxville, TN) with septa retracted; a slow-bolus injection of H215O [0.25 mCi/kg; e.g., 17.5 mCi (648 MBq) for a 70-kg person; 1 Ci = 37 GBq] infused at a constant rate over 30 s; and a 90-s scan acquisition. Images were reconstructed with a three-dimensional reconstruction algorithm using a 0.5 cycle-per-pixel Hanning filter (16). We applied no additional smoothing to maintain the highest possible spatial resolution. Post hoc analyses performed after additional spatial smoothing confirmed the primary study results. AUTOMATED software (17) performed normalization for global activity (scaled to a mean of 1,000 counts), detection of the intercommisural plane, intrasubject scan alignment, resampling to a pixel size of 2.25 mm3, and nonlinear warping to Talairach space (18). Forty-two of the subjects in Study 1, and 17 of the subjects in Study 2 received two or more resting scans that were averaged together before analysis. All images were inspected with in-house visualization software to ensure that the ventral frontal and temporal lobes were within the field of view of the PET camera and were not subject to interpolation errors associated with the three-dimensional image reconstruction. Correlational analyses were performed with in-house software that applied Pearson product moment calculations on a pixel-by-pixel basis, with subsequent confirmation of peak correlations using SPSS-10 (SPSS, Chicago). Cytoarchitectural labeling of areas demonstrating a correlation with NA follows the nomenclature of Öngur and Price (19).
Results
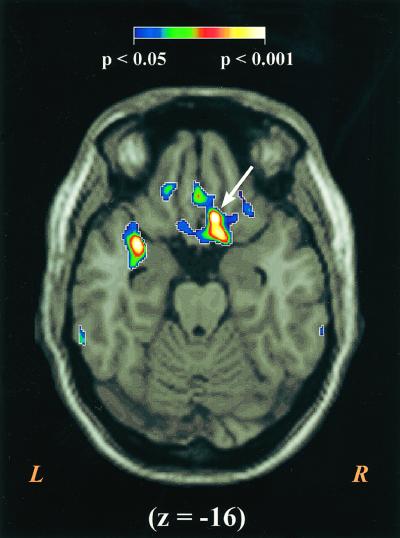
In the first study, the largest volume demonstrating a significant correlation between rCBF and NA (P < 0.001, uncorrected for multiple comparisons) localized to a posterior portion of the ventromedial prefrontal cortex (VMPFC) (see Table 1 and Figs. 1 and 2a). The peak correlation in VMPFC fell within the right hemisphere near the boundary between areas 10m, 25, and 32m. A statistical difficulty arises in this type of analysis as a consequence of the large number of pixels in the brain. Multiple comparisons across all pixels increase the risk of a spurious correlation emerging somewhere in the brain by chance, but leaves the Bonferroni correction (i.e., adjusting the threshold for statistical significance by the total number of voxels examined in the brain) too restrictive. Although the large volume (i.e., 261 mm3) of correlated cortex argues against chance, we decided to collect a second, independent sample of subjects to test for replication.
Table 1.
Location of areas correlating with NA over the past month in both studies
| Study | Brain area | Peak coordinate (x, y, z)* | Peak correlation (r, P value) | Volume (mm3) |
|---|---|---|---|---|
| 1 | Posterior VMPFC (10m/25/32m) | 8, 18, −16 | 0.44, <0.001 | 261 |
| 2 | Posterior VMPFC (10m/25/32m) | 3, 18, −14 | 0.46, <0.005 | 43 |
| 1 | Anterior VMPFC (10m/10r) | −7, 46, −11 | 0.36, <0.05 | 65 |
| 2 | Anterior VMPFC (10m/10r) | 3, 55, −9 | 0.46, <0.005 | 92 |
| −3, 44, −9 | 0.36, <0.05 |
Peak coordinates in Talairach space (18) in mm: x = medial–lateral to the midline (+ = right), y = anterior–posterior to the anterior commissure (+ = anterior), z = inferior–superior relative to the intercommissural plane (+ = superior).
Figure 1.
The figure displays the VMPFC area where rCBF correlates with NA in Study 1. For display purposes the correlation maps were resampled to 1 mm3 pixels, thresholded to only show areas with significant correlations, and overlayed on a high-resolution, Talairach-warped MRI template. The correlations are color coded by their level of statistical significance with areas in purple equivalent to P < 0.05, and areas in white equivalent to P < 0.001. The figure displays a transverse slice through z = −16 with the peak area of correlation marked with an arrow. A highly significant correlation can also be seen in the left parainsular region, but this focus failed to replicate. R, right; L, left.
Figure 2.
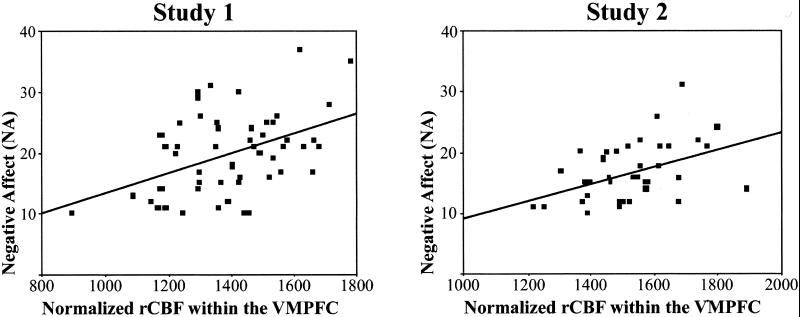
Scatter plots of NA and rCBF at the peak correlated pixel within the posterior VMPFC. Examination of both scatter plots reveals the presence of an outlier with particularly low VMPFC rCBF in each study. However, in both cases the correlation remained significant even after post hoc reanalysis with the outlier removed (r = 0.39, P = 0.005 for Study 1; r = 0.44, P = 0.005 for Study 2). Because no a priori reasons warranted removal of these subjects' data from the analyses, the data were included in all other reported analyses.
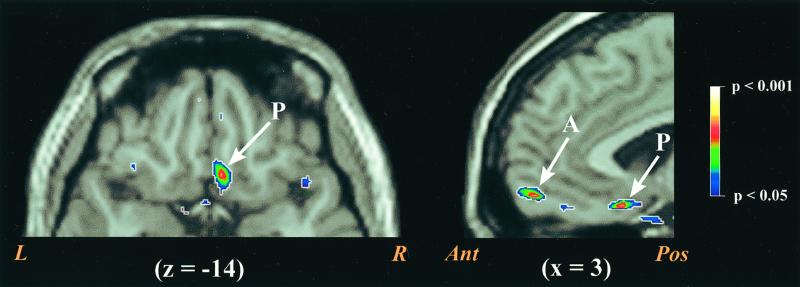
In the second study, rCBF within the VMPFC again demonstrated a significant correlation with NA ratings. Although the volume of correlated cortex was smaller in this replication sample, the magnitude and location of the correlated region were similar (the peak varied by no more than 4 mm in any direction—see Figs. 2b and 3). To confirm that the correlated areas in the two studies occupied similar areas of cortex, we performed a logistic convergence analysis of areas exceeding a P < 0.05 threshold. A volume of 29 mm3 of VMPFC showed statistically significant correlations in both studies. Although several other brain areas exceeded the P < 0.05 threshold in the first or second study, only one other area exceeded this threshold in both studies (see Table 1). This second area of overlap falls in a more anterior portion of VMPFC (area 10m/10r; 12 mm3 converging across studies). The area of overlap between the two studies is limited to the left anterior VMPFC, although in the second study the correlated region consists of two subpeaks that straddle the midline.
Figure 3.
VMPFC areas demonstrating correlations with NA in Study 2. The left side of the figure displays a transverse slice through z = −14. The right side of the figure displays a sagittal slice of the frontal lobe through x = 3. In both slices the posterior VMPFC focus is marked with a P. The right hemisphere component of the anterior VMPFC focus also appears on the sagittal slice (marked with an A). However, the anterior VMPFC area demonstrating convergence across the two studies falls strictly in the left hemisphere. R, right; L, left; Ant, anterior; Pos, posterior.
The causal direction of the observed association between NA and VMPFC activity cannot be addressed by a correlation analysis. For instance, the association might arise as a consequence of state levels of NA experienced during the scanning session. This possibility takes on added importance given recent findings that statewise increases in NA during anxiety provocations are associated with rCBF changes in the VMPFC (20, 21). However, in the present study, subjects were not exposed to any anxiety provocations and instead were resting during the period of scanning. To assess NA-related mood states during the period of scanning, we analyzed the Tension–Anxiety and Anger–Hostility subscales from the POMS, which were retrospectively rated immediately following the conclusion of scanning. Scores on the Anxiety–Hostility ratings were not normally distributed, with over half of the subjects receiving the lowest possible score (T = 37) on this scale (pooled mean = 39.5, SD = 4.5). These low scores reflect the lack of exposure to anything annoying or irritating during the scanning period. Not surprisingly, no correlations emerged between Anxiety–Hostility ratings and activity in the VMPFC. Although subject ratings of Tension–Anxiety were relatively low (pooled mean = 40.5, SD = 7.1, range = 30–60), they showed a more normal distribution and range. Across the two studies, correlations between resting rCBF and Tension–Anxiety ratings emerged in four areas of the brain: the right frontal pole, the retrosplenial cortex bilaterally, the right middle and superior temporal gyri along the posterior superior temporal sulcus, and the border of the left inferior and middle frontal gyri (see Table 2). No significant correlations arose in the VMPFC regions identified in Table 1. Thus, there was no evidence that the correlation between VMPFC and long-term levels of NA was a consequence of NA experienced during the scan session itself.
Table 2.
Location of areas correlating with Tension–Anxiety during the scan session in both studies
| Study | Brain area | Peak coordinate (x, y, z)* | Peak correlation (r, P value) | Volume (mm3) |
|---|---|---|---|---|
| 1 | Medial frontal pole (10) | 12, 64, 16 | 0.44, <0.005 | 113 |
| 2 | Medial frontal pole/pregenual cingulate (10/32) | 10, 50, 14 | 0.47, <0.01 | 104 |
| 1 | Superior temporal sulcus (39) | 37, −64, 16 | 0.41, <0.005 | 115 |
| 2 | Superior temporal sulcus (22/39/40) | 34, −39, 18 | 0.72, <0.00005 | 504 |
| 1 | Retrosplenial cortex (29/30) | −3, −46, 16 | 0.37, <0.01 | 45 |
| 2 | Retrosplenial cortex (30) | −3, −50, 16 | 0.47, <0.01 | 43 |
| 1 | Inferior and middle frontal gyri (45/46) | −48, 37, 14 | 0.32, <0.05 | 79 |
| 2 | Inferior and middle frontal gyri (45/46) | −48, 44, 11 | 0.60, <0.001 | 252 |
We also examined whether VMPFC activity was uniquely related to self-reported NA, or was related to an overall level of emotional experience that also involved variation in self-reported PA. One would not predict such a relationship given the independence of PA and NA when measured over long time periods. As expected, there were no significant correlations between PA and rCBF in the VMPFC. Thus the correlations between NA and VMPFC activity appears specific to the NA dimension of mood.
Discussion
The present studies indicate that resting rCBF in the VMPFC correlates with self-reported ratings of NA during the month preceding the scan. Increasing evidence indicates that the VMPFC plays a role in linking emotional appraisals with autonomic responses (22). For instance, patients with brain lesions in this area fail to show normal autonomic responses to socially and emotionally meaningful stimuli (23). Moreover, these patients demonstrate a general pattern of decreased NA states. They display a markedly impaired ability to appreciate future risks and fail to produce anticipatory autonomic responses to potential rewards or punishments (24, 25). The posterior aspects of the VMPFC (i.e., infralimbic cortex) represent a primary cortical region for the control of somatovisceral functions in many mammalian species (26). In primates, the region sends direct projections to a variety of subcortical areas that in turn control autonomic effector sites (27). Electrical stimulation of the posterior VMPFC produces marked modulations of autonomic functions (28). This association between long-term levels of NA and processing within a region that modulates autonomic pathways dovetails with the robust correlations between measures of NA and self-reported autonomic arousal (29). Less data directly addresses the functions of the anterior VMPFC (area 10m/10r), although given its pattern of efferent and afferent connections, its functions appear closely related to the visceral processing of the posterior VMPFC (19).
It is important to note that the NA ratings that correlated with VMPFC activity involved a retrospective rating of mood during the month before scanning. When measured over time periods such as a month, NA scores reflect an implicit aggregation of situations in which NA was elevated. Given the stability of such long-term ratings over time, they at least in part reflect trait-wise differences in the disposition to experience NA states. The present data thus raises the possibility that resting VMPFC activity is associated with a trait-wise disposition to experiencing NA. However, it must be noted that reporting biases and more temporally specific influences on mood states (i.e., unique events or situation occurring in the last month) also influence retrospective ratings of NA. Studies using longitudinal measures of both NA and VMPFC activity may in the future prove useful in determining the stability of the relationship between NA and VMPFC activity and help clarify the specific factors contributing to the relationship.
Although activity in the VMPFC showed no correlation with statewise levels of negative mood in the absence of overtly NA-provoking stressors, previous studies indicate that the extent to which VMPFC activity changes during overtly stressful situations is closely related to anxiety. The VMPFC shows reliable rCBF decreases when subjects move from a resting state to a more active, attention demanding cognitive state (30). The extent of this decrease appears inversely correlated with the amount of anxiety experienced by subjects in the more active state (20). For example, when anticipating an electric shock, subjects experiencing high anxiety levels show little or no change in VMPFC rCBF, whereas those experiencing little anxiety show substantial decreases (21). Thus, those subjects who fail to lower activity in the VMPFC experience the greatest level of transient anxiety when anticipating a potentially aversive stimulus. Two areas of the VMPFC show this relationship with transient anxiety. One falls in the posterior VMPFC (at y = 18) and the other in the anterior VMPFC (at y = 41). Thus, the same two regions whose resting activity predicts long-term NA ratings show an association with transient anticipatory anxiety during exposure to an overtly anxiety provoking situation. This convergence suggests a potential mechanism through which higher VMPFC activity at baseline might directly relate to a greater disposition or vulnerability to experiencing NA over time. Specifically, because of their higher starting point, these individuals may experience greater difficulty suppressing VMPFC to the extent necessary to minimize anxiety or other NA states.
In psychiatrically healthy subjects, moment to moment, state levels of NA are generally low (31) in the absence of stressors, and are reflected in the low scores observed on POMS Tension–Anxiety and Anger–Hostility scales collected during scanning (although receiving a PET scan might be considered a mild stressor in itself, most subjects are typically quite relaxed by the time scanning actually starts). Nevertheless, individual differences emerged in ratings of Tension–Anxiety for the scanning period, which correlated with resting rCBF in the right medial frontal pole, a posterior portion of the right superior temporal sulcus region, left inferior/middle frontal gyri, and the retrosplenial cortex. All of these areas, along with the VMPFC, have been previously identified as part of a “default” or “baseline” network of structures, which are more active during rest than during active tasks or sensory stimulation (30, 32, 33). The present data suggest that activity within several components of this default network is associated with the current emotional tone of the subject during the resting state. The association with the medial frontal pole is intriguing given previous data suggesting its importance in self-referential processes, such as self-monitoring of ones current emotional state (34). The correlation with activity in the retropsplenial cortex is even more intriguing in that the retrosplenial region frequently activates during exposure to emotion-inducing stimuli (with particularly frequent activations arising during exposure to unpleasant or fear inducing stimuli) (9). Thus, activity in the retrosplenial area appears related to negative mood factors both in the presence and absence of overtly anxiety-provoking stimuli. This pattern stands in contrast to the VMPFC, whose activity shows no association with moment to moment anxiety levels at rest.
Psychologists have long speculated that aspects of personality and affective experience arise from individual differences in brain functioning. Previous electrophysiological (5) and neuropharmacological studies (10, 11) have supported these hypotheses, but have lacked the spatial resolution to localize the specific brain areas contributing to dispositional features in healthy normals. By capitalizing on PET's ability to quantitate baseline physiological activity, we were able to demonstrate and replicate an association between long-term NA ratings and activity within specific portions of the VMPFC. The finding converges with previous evidence implicating this area of the brain in emotional disturbances arising in neurologic conditions. However, the major implications of the present results fall within the normal boundaries of human affective experience.
Acknowledgments
We thank our volunteer subjects and the personnel of the Minneapolis Veterans Affairs Medical Center Cognitive Neuroimaging Unit and PET Imaging Service and the staff of the Vanderbilt University Affective Neuroimaging Lab. This work was supported by the Department of Veterans Affairs, the Mark A. Nugent Medical Research Foundation, the National Association for Research in Schizophrenia and Affective Disorders (J.V.P.), Vanderbilt University, National Institutes of Mental Health Grant F32 MH11641-01A1, and National Institutes on Drug Abuse Grant T32 DA07097 (to D.H.Z.).
Abbreviations
- NA
negative affect
- PA
positive affect
- VMPFC
ventromedial prefrontal cortex
- PET
positron-emission tomography
- rCBF
regional cerebral blood flow
- POMS
Profile of Mood States
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Ball, S., Fox, P., Herscovitch, P. & Raichle, M. (1988) Neurology 38, Suppl. 1, 363 (abstr.).
Ball, S., Fox, P., Pardo, J. V. & Raichle, M. (1988) Neurology 38, Suppl. 1, 362 (abstr.).
Dickhaut, J. W., Nagode, J. C. & Pardo, J. V. (2000) Neuroimage 11, 591 (abstr.).
References
- 1.Watson D, Tellegen A. Psychol Bull. 1985;92:426–457. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- 2.Watson D, Clark L A. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- 3.Watson D, Walker L M. J Pers Soc Psychol. 1996;70:567–577. doi: 10.1037//0022-3514.70.3.567. [DOI] [PubMed] [Google Scholar]
- 4.Clark L A, Watson D, Mineka S. J Abnorm Psychol. 1994;103:103–116. [PubMed] [Google Scholar]
- 5.Davidson R J, Irwin W. Trends Cognit Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 6.Fredrikson M, Wik G, Fischer H. Person Indiv Diff. 1999;26:265–270. [Google Scholar]
- 7.Johnson D L, Wiebe J S, Gold S M, Andreasen N C, Hichwa R D, Watkins G L, Boles Ponto L L. Am J Psychiatry. 1999;156:252–257. doi: 10.1176/ajp.156.2.252. [DOI] [PubMed] [Google Scholar]
- 8.Raichle M E, MacLeod A M, Snyder A Z, Powers W J, Gusnard D A, Shulman G L. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maddock R J. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 10.Depue R, Zald D H. In: Basic Issues in Psychopathology. Costello C, editor. New York: Guilford Press; 1993. pp. 127–237. [Google Scholar]
- 11.Zald D H, Depue R A. Person Indiv Diff. 2001;30:71–86. [Google Scholar]
- 12.Robins L N, Marcus S C. COMPUTER-ADMINISTERED DIAGNOSTIC INTERVIEW SCHEDULE. St. Louis: Washington University; 1988. , Version I. [Google Scholar]
- 13.Watson D, Clark L, Tellegen A. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 14.McNair D M, Lorr M, Droppleman L F. Manual Profile of Mood States. San Diego: Educational and Industrial Testing Service; 1981. [Google Scholar]
- 15.Watson D, Clark L. J Pers Soc Psychol. 1992;62:489–505. doi: 10.1037//0022-3514.63.6.1011. [DOI] [PubMed] [Google Scholar]
- 16.Kinahan P E, Rogers J G. IEEE Trans Nucl Sci. 1989;36:964–986. [Google Scholar]
- 17.Minoshima S, Koeppe R A, Frey K A, Kuhl D E. J Nucl Med. 1994;35:1528–1537. [PubMed] [Google Scholar]
- 18.Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 19.Ongur D, Price J L. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 20.Simpson J R, Snyder A Z, Gusnard D A, Raichle M E. Proc Natl Acad Sci USA. 2001;98:683–687. doi: 10.1073/pnas.98.2.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson J R, Drevets W C, Snyder A Z, Gusnard D A, Raichle M E. Proc Natl Acad Sci USA. 2001;98:688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Damasio A R. Philos Trans R Soc London B. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 23.Damasio A R, Tranel D, Damasio H. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 24.Bechara A, Damasio A R, Damasio H, Anderson S W. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 25.Bechara A, Tranel D, Damasio H, Damasio A R. Cereb Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- 26.Neafsey E, Terreberry R, Hurley K, Ruit K, Frysztak R. In: Neurobiology of Cingulate Cortex and Limbic Thalamus. Vogt B A, Gabriel M, editors. Boston: Birchäuser; 1993. pp. 206–223. [Google Scholar]
- 27.Freedman L, Insel T R, Smith Y. J Comp Neurol. 2000;421:172–188. [PubMed] [Google Scholar]
- 28.Kaada B R. In: Handbook of Physiology. Magoun H W, editor. Washington, DC: Am. Physiological Soc.; 1960. pp. 1345–1372. [Google Scholar]
- 29.Brown T A, Chorpita B F, Barlow D H. J Abnorm Psychol. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- 30.Shulman G I, Fiez J, Corbetta M, Buckner R L, Miezin F M, Raichle M, Petersen S E. J Cognit Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- 31.Zevon M, Tellegen A. J Pers Soc Psychol. 1992;43:11–122. [Google Scholar]
- 32.Binder J R, Frost J A, Hammeke T A, Bellgowan P S, Rao S M, Cox R W. J Cognit Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 33.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, Crivello F, Joliot M, Petit L, Tzourio-Mazoyer N. Brain Res Bull. 2001;54:287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 34.Lane R D, Fink G R, Chau P M, Dolan R J. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]