Abstract
Background
Central retinal vein occlusion (CRVO) is a rare but severe complication following cataract surgery. This report highlights a unique case of CRVO secondary to phacoemulsification in a patient with multiple comorbidities.
Case presentation
A 60-year-old female presented with left-eye blurred vision, photophobia, and elevated intraocular pressure. Preoperative findings included neovascular glaucoma, nuclear cataract, and suspected retinitis pigmentosa. Postoperatively, she developed CRVO with flame-shaped retinal hemorrhages, confirmed via fundoscopy and optical coherence tomography (OCT). Laboratory tests revealed hypertriglyceridemia (3× normal).
Conclusion
This case underscores the synergistic roles of vascular pathology (neovascular glaucoma), surgical stress (IOP fluctuations), and metabolic dysfunction (hypertriglyceridemia) in CRVO pathogenesis. Perioperative monitoring of blood rheology and targeted interventions (e.g., anti-VEGF therapy) may mitigate risks in high-risk patients.
Keywords: Central retinal vein occlusion, Neovascular glaucoma, Cataract surgery, Hypertriglyceridemia, Retinitis pigmentosa, Phacoemulsification, Blood hypercoagulability
Background
CRVO is a vision-threatening condition characterized by thrombosis of the central retinal vein, leading to retinal hemorrhage, edema, and potential neovascular complications. It is typically associated with systemic risk factors such as hypertension, diabetes, and hyperlipidemia, with an estimated annual incidence of 0.4–0.8% in individuals over 50 years of age [1]. While cataract surgery is one of the most frequently performed and generally safe ophthalmic procedures, rare postoperative complications—including endophthalmitis, cystoid macular edema, and retinal detachment—are well-documented [2]. However, CRVO following cataract surgery remains exceedingly uncommon, with only sporadic case reports in the literature [3]. The pathogenesis of CRVO in this context may involve multifactorial mechanisms. Elevated intraocular pressure (IOP) during phacoemulsification, even transiently, can compress retinal vessels and impair venous return, particularly in patients with preexisting glaucoma [4]. Additionally, surgical trauma and postoperative inflammation may induce a hypercoagulable state through platelet activation and fibrinogen elevation. Metabolic disorders such as hypertriglyceridemia further exacerbate thrombosis risk by altering blood rheology. Despite these theoretical associations, comprehensive studies on the interplay of these factors in postoperative CRVO are lacking, underscoring the need for detailed case analyses to guide clinical management.
Case presentation
A 60-year-old female patient presented with left - eye blurred vision for over 10 days, accompanied by symptoms such as ocular obstruction, distension, photophobia, lacrimation, and occasional left-sided headache. The patient was diagnosed with left eye glaucoma and bilateral cataracts at a local county hospital. Due to limited local medical conditions, the etiology of the left eye glaucoma was not clarified. However, the patient was temporarily administered intravenous mannitol infusion combined with brinzolamide eye drops in the left eye to reduce intraocular pressure, and then transferred to our hospital for further treatment. Ophthalmological examination revealed corrected visual acuity of 0.1 in the left eye, left-eye conjunctival hyperemia mixta, mild corneal edema, anterior chamber depth of approximately 2 CT, negative Tyndall phenomenon, indistinct iris texture, peripheral iris neovascularization, dilated pupil with a diameter of about 6 mm and loss of light reflex, peripheral anterior chamber angle narrowing, and suspected angle closure at 3 and 9 o’clock positions. Bilateral nuclear cataract, vitreous opacification, clear and pale optic discs, scattered gray - black pigmentary disorders in the retina, and no macular reflex were observed. The left-eye intraocular pressure after treatment was 15 mmHg.
The preliminary diagnosis was as follows: (1) Left-eye neovascular glaucoma complicated by cataract; (2) Suspected bilateral retinitis pigmentosa. After excluding surgical contraindications, left-eye phacoemulsification cataract extraction, intraocular lens implantation, and goniosynechialysis were performed. The surgery was uneventful. Pre-operative fundus photography is presented in Fig. 1A, while the fundus photography 24 h post - operation is shown in Fig. 1B and D, and the postoperative macular optical coherence tomography is depicted in Fig. 1C. Color Doppler ultrasonography of blood vessels demonstrated no significant abnormalities in the bilateral common carotid arteries, the origins of the internal and external carotid arteries, and the extracranial segments of the vertebral arteries. Additionally, the patient’s triglyceride level was three - fold higher than normal.
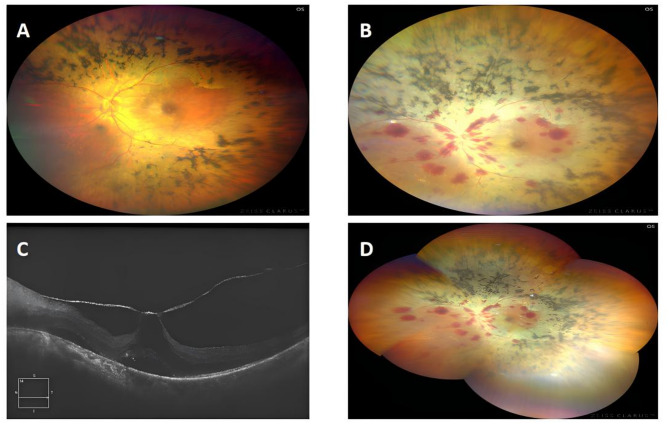
Fig. 1.
A Fundus before cataract surgery: scattered foci of osteoclast-like pigmentation in the retina, and dark macular area, B and D Fundus taken 24 h after surgery: flame-like haemorrhage along the vascular line was seen in the left eye, massive osteoclast-like pigmentation was seen in the posterior pole and periphery of the retina, C Macular OCT: macular morphology was absent and low-reflective dark cavities were seen in the interlaminar layers, linear high-reflective pulling was seen in the anterior retina
Discussion
CRVO is extremely rare as a postoperative complication of cataract surgery and is rarely reported in the available literature. Possible causes of CRVO secondary to cataract surgery in this case include:
Vascular pathologic factors
1. Relationship between glaucoma and CRVO: Chronic elevation of IOP in patients with glaucoma exerts pressure on the central retinal artery and vein at the optic disc sieve plate, impeding venous return. It has been shown that for every 1 mmHg increase in IOP, the risk of glaucoma increases by 12% [5], and then the risk of CRVO increases with it. 2. Neovascular glaucoma and CRVO: Regarding the causal relationship between neovascular glaucoma and CRVO, current research suggests that retinal ischemia and hypoxia after CRVO stimulate the secretion of cytokines, such as vascular endothelial growth factor (VEGF), which can contribute to the formation of neovessels, which can lead to the development of neovascular glaucoma [6]. In this process, VEGF not only induces neovascularization, but also disrupts the normal function of vascular endothelial cells, leading to an increase in vascular permeability and a series of pathologic changes in the eye. 3. Retinitis pigmentosa and CRVO: In patients with retinitis pigmentosa, the retinal blood vessels become progressively thinner and are subjected to prolonged ischemia and hypoxia, which activates the coagulation system and alters blood rheology. A similar case report [7] was previously reported, noting that patients with ischemic central retinal vein occlusion combined with retinitis pigmentosa have a lower risk of concurrent neovascularization of the fundus. In this case, however, the patient had neovascularization of the iris, which induced neovascular glaucoma.
Surgical factors
1. Surgical stress and hypercoagulability: The intraoperative IOP fluctuations during phacoemulsification, even in uneventful cases. Postoperatively, the body develops a stress response with increased secretion of hormones such as catecholamines and cortisol. It is closely associated with increased platelet counts, enhanced platelet aggregation, and elevated levels of fibrinogen in the blood, which together lead to a hypercoagulable state of the blood. At the same time, postoperative repair of ocular tissues and inflammatory responses exacerbate microcirculatory disturbances and impede blood return to the central retinal vein, ultimately leading to the development of CRVO. 2. Metabolic factors: Hypertriglyceridemia is associated with CRVO. High triglycerides increase the risk of CRVO by affecting blood mobility and platelet function, making the blood more prone to thrombus formation [8].
This case exemplifies the intricate interplay of multiple risk factors leading to CRVO following cataract surgery—a rare but vision-threatening complication. The patient’s unique profile, including preexisting neovascular glaucoma, untreated hypertriglyceridemia, and suspected retinitis pigmentosa, highlights three critical insights: Vascular-Mechanical Synergy: Chronic IOP elevation in glaucoma likely compressed retinal vasculature, while phacoemulsification-induced intraoperative IOP fluctuations exacerbated venous stasis. This aligns with prior evidence suggesting that even transient IOP spikes may trigger CRVO in susceptible individuals.Metabolic-Surgical Crosstalk: Hypertriglyceridemia, a known prothrombotic factor, synergized with postoperative hypercoagulability (platelet activation, fibrinogen rise) to accelerate thrombosis. This dual mechanism—rarely emphasized in existing literature—suggests that metabolic screening and preoperative lipid control could mitigate CRVO risk. Retinitis Pigmentosa Paradox: While retinitis pigmentosa typically reduces retinal neovascularization risk due to attenuated VEGF expression, this patient developed iris neovascularization, underscoring disease heterogeneity. This divergence implies that localized ischemia (e.g., anterior segment hypoxia) may override systemic VEGF dynamics, warranting tailored antiangiogenic strategies.
Indeed, this case requires differentiation from hemorrhagic occlusive retinal vasculitis (HORV) syndrome following cataract surgery. A 2015 study [9] published in Ophthalmology reported 6 cases of HORV syndrome occurring after cataract surgery, where pathogenesis may be associated with intraoperative use of intraocular vancomycin (especially at high concentrations). This agent can directly damage retinal vascular endothelium, leading to vascular occlusion, hemorrhage, and inflammation—features similar to the fundus retinal hemorrhage signs observed in this patient. However, no vancomycin was used intraoperatively in this case; only a small amount of low-concentration cefuroxime was instilled after intraocular lens implantation for postoperative infection prophylaxis. Therefore, the diagnosis of postoperative HORV syndrome remains unclear, and future analysis and summarization of more similar clinical cases are needed. A 2022 study [10] reported a case of HORV occurrence following PPV (Pars Plana Vitrectomy). This research proposed that the use of HBBG dye might be the cause of HORV, mediated through Müller cell damage; additionally, dye contamination triggering an inflammatory response could not be excluded. However, the patient in this case did not undergo PPV, and no staining agents were used during anterior capsulorhexis. Therefore, HORV caused by similar mechanisms is not currently considered.
Conclusion
Clinical Implications: Proactive Monitoring: High-risk patients (glaucoma, metabolic disorders) should undergo perioperative blood rheology tests and lipid profiling. Targeted Interventions: Early anti-VEGF therapy and short-term anticoagulation may disrupt thrombotic cascades without delaying surgical recovery. Multidisciplinary Collaboration: Coordinated care among ophthalmologists, endocrinologists, and hematologists is essential for optimizing outcomes. Innovative Perspective: To our knowledge, this is the first report linking retinitis pigmentosa-associated vascular changes to postoperative CRVO pathogenesis, proposing a novel “triple-hit” model. Future studies should validate this model through multicenter registries and explore personalized anticoagulant protocols in similar cohorts.
Authors’ contributions
LXin and LX designed the study and drafted the manuscript as the co-first author. LXin and QX carried out the literature search.CM and XX contributed to data extraction and quality assessment. LX supervised the study and LX as the corresponding author. All authors contributed to the article and approved the submitted version.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient/parents/legal guardian/next of kin of the participant for publication of the case report and the images.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sophie R, McIntosh RL, Ning C, et al. The prevalence of retinal vein occlusion: pooled data from population studies from the United States, Europe, Asia, and Australia. Ophthalmology. 2009;117(2):313-9.e1. [DOI] [PMC free article] [PubMed]
- 2.Yu-Chi L, Mark W, Terry K, et al. Cataracts. Lancet. 2017;390(10094):600–12. [DOI] [PubMed] [Google Scholar]
- 3.Sangwan VS, Gupta S, Das S. Cataract surgery in ocular surface diseases: clinical challenges and outcomes. Curr Opin Ophthalmol. 2017;29(1):81–7. [DOI] [PubMed]
- 4.Francesco S, Rossella M, Sandra F, et al. High lipoprotein (a) levels are associated with an increased risk of retinal vein occlusion. Atherosclerosis. 2009;210(1):278–81. [DOI] [PubMed] [Google Scholar]
- 5.NemesureB HR. Incident open-angle glaucoma and intraocular pressure. Ophthalmology. 2007;114(10):1810–5. [DOI] [PubMed] [Google Scholar]
- 6.Călugăru D, Călugăru M. Predictors of neovascular glaucoma in central retinal vein Occlusion. Am J Ophthalmol. 2019;205:200–1. [DOI] [PubMed]
- 7.Paxhia MJ, Ting TD, Fekrat S. Ischemic central retinal vein occlusion and retinitis pigmentosa: lower risk of neovascularization? Retina. 2001;2:179–80. [DOI] [PubMed]
- 8.O’Mahoney PR, Wong DT, Ray JG. Retinal vein occlusion and traditional risk factors for atherosclerosis. Arch Ophthalmol. 2008;5:692-9. [DOI] [PubMed]
- 9.Witkin AJ, Shah AR, Engstrom RE, et al. Postoperative hemorrhagic occlusive retinal vasculitis: expanding the clinical spectrum and possible association with vancomycin. Ophthalmology. 2015;122(7):1438-51. [DOI] [PubMed]
- 10.Markan A, Singla P, Singh R. Development of retinal vasculitis following an uneventful pars plana vitrectomy. BMJ Case Rep. 2022;15(8):251–7 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.