Abstract
Drought stress is one of the most significant climatic challenges, severely affecting the productivity of medicinal and aromatic plants. Kaolin, a mineral with highly effective reflective properties, has been identified as a useful tool for enhancing plant resilience to abiotic stresses. This study examined the effects of kaolin concentrations (0%, 2%, 4%, and 8%) on the growth, physiological, and phytochemical attributes of Thymus vulgaris L. under drought conditions (100%, 75%, and 50% of field capacity (FC)). The findings revealed that the best performance in growth parameters (plant diameter, height, leaf dimensions (length and width), fresh and dry weight, as well as chlorophyll concentration) was observed with the 4% kaolin foliar application under 100% field capacity conditions. The highest concentration of proline, malondialdehyhe, hydrogen peroxide and cell membrane damage was observed under drought stress (50% FC), However, foliar application of kaolin 8% led to reduced these levels compared to untreated plants grown under the same water deficit (50% FC). Foliar application of kaolin (8%) under water deficit (50% FC) also increased total phenolic concentration, total flavonoid concentration, antioxidant activity and essential oil percentage. Rosmarinic acid content in plants treated with kaolin (8%) under drought conditions (50% FC) increased 1.6 times compared to untreated plants. An investigation of changes in enzymatic and non-enzymatic systems showed that kaolin improves the antioxidant capacity of T. vulgaris under drought stress and increases its resistance. Notably, kaolin also facilitated faster restauration of physiological functions during stress recovery. In summary, kaolin foliar sprays alleviated the negative effects of water stress in T. vulgaris performance by modulating distinct physiological and biochemical responses. This suggests kaolin application can support the cultivation and production of T. vulgaris in low-yielding and arid lands.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-025-06847-6.
Keywords: Growth characteristics, Antioxidant, Essential oil, Rosmarinic acid, Drought stress
Introduction
Thymus vulgaris L., a perennial herb from the mint family, thrives across the Mediterranean, Southern Europe, Asia, and North Africa [1]. It is considered one of the most widely traded culinary and medicinal plants. Thyme herb is a valuable raw material listed in the European pharmacopeia (EP) and classified by European Medicine Agency (EMA) as a traditional herbal product. It contains about 2.5% essential oil (according to the EP, not less than 1.2%), which is reported to be in among top ten of essential oils used in the global industry [2]. Traditionally, it has been used as a traditional medication for bronchitis, cough, sore throat, rheumatism, and arthritis disorders [3, 4]. Due to its aromatic properties and valuable pharmaceutical compounds in the aerial parts, the plant has a wide range of applications, including use as herbal tea, food flavoring, and as source of active ingredients in various medicinal products [5]. The essential oil of T. vulgaris, rich in thymol and carvacrol, possesses antitussive, antispasmodic, antimicrobial, carminative, anticancer, and other medicinal properties [5, 6]. Additionally, flavonoids (such as apigenin and luteolin derivatives) and phenolic acids (including cinnamic, carnosic and rosmarinic acids) present in T. vulgaris extracts contribute to its antioxidant capacity and play a significant role in combating diseases such as cancer and chronic inflammation [7]. Its essential oil is also used as a natural preservative in the food industry and in food packaging systems to prevent spoilage [8].
The quantity and quality of phytochemical compounds in medicinal and aromatic plants, as well as the morphological and biochemical traits these plants, are significantly influenced by environmental factors, particularly abiotic stresses [9, 10]. Among these, water deficit is one of the most widespread abiotic stresses, affecting approximately 25% of global cropland [11]. Therefore, gaining a comprehensive understanding of plant responses and thoroughly investigating drought stress coping mechanisms are crucial for developing and cultivating stress-tolerant crops [12–14]. Drought stress is a major environmental challenge that not only impairs nutrient uptake but also disrupts growth, reproduction, gene expression, quality, and overall productivity [10, 15]. It interferes with cell division processes and the activity of essential enzymes involved in nutrient absorption, resulting in reduced plant height, leaf area, and both fresh and dry biomass [16]. Moreover, water deficiency negatively effects the photosynthetic system by damaging or disrupting enzymes critical for carbon fixation, the electron transport chain, and light stress responses, while also compromising membrane integrity and pigment stability [17]. In addition, drought stress can alter the behavior of bioactive compounds such as phenolic compounds, flavonoids, and essential oil content and composition in medicinal plants [18].
Kaolin (Al2Si2O5(OH)4) is an antitranspirant that has been widely used in organic farming in recent years as a safe material. Its most notable characteristic is its white color and ability to reflect light. Kaolin is an effective particle film with unique properties: the particles are chemically inert, have a diameter of less than 2 micrometers, exhibit high dispersibility, and can form a uniform coating [19]. The film created by kaolin is porous, does not interfere with leaf gas exchange, allows the passage of photosynthetically active radiation, and partially blocks infrared and ultraviolet radiation [20]. Kaolin increases the reflection of radiation reaching the leaf surface, thereby reducing the plant’s heat load and increasing its productivity [21]. A study by Semida et al. [22] on Phaseolus vulgaris demonstrated that foliar application of kaolin under drought stress improved phytochemical and biochemical contents, plant water status, membrane stability, and increased plant performance. Similarly, foliar spraying of grape leaves with kaolin under heat stress increased the content of phenolic compounds and enhanced antioxidant capacity [23]. Previous research has shown that foliar spraying with kaolin mitigates drought stress in Olea europaea [24], Gossypium hirsutum [20] and Zea mays [25]. Furthermore, kaolin application under drought stress conditions increased the essential oil content, phenolic compounds, flavonoids and antioxidant activity of Pelargonium graveolens [26].
To date, no research has investigated the effects of kaolin foliar application on the growth, performance, phytochemical properties, and enzymatic activity of T. vulgaris under drought conditions. This study was designed to fill that gap by examining the influence of kaolin foliar treatment on the agro-morphological, biochemical, and phytochemical characteristics of T. vulgaris in response to drought stress. The findings from this research have the potential to significantly improve the cultivation of T. vulgaris in arid regions, thereby supporting the supply of essential raw materials for the pharmaceutical and food industries.
Materials and methods
Plant materials and experimental setup
The seeds of T. vulgaris were obtained from the gene bank at the Research Institute of Forests and Rangelands in Tehran, Iran. For seed germination (in April 2024), trays were prepared using a growing medium composed of coco peat and perlite mixed in equal proportions by volume (1:1). Once the seedlings reached the four-leaf stage (21 days after sowing seeds), they were transplanted into pots (20 cm in diameter and 25 cm in depth) filled with sandy loam soil, consisting of 56.80% sand, 34.85% silt, and 8.35% clay (Table 1). The experiment followed a completely randomized factorial design with three replications, incorporating 12 treatment combinations. The setup included four kaolin levels (0% as control, 2%, 4%, and 8%) and three irrigation regimes corresponding to 100% (control), 75%, and 50% of field capacity (FC). Each replication consisted of four pots per treatment, resulting in a total of 144 pots for the study. Soil FC was determined following the method described by Pourmeidani et al. [27]. Five kilograms of soil were dried in an oven at 105 ºC for 72 h to obtain dry weight. The containers were then filled with the dried soil and weighed every two hours for 36 h after watering and saturating the soil. Once the container weights stabilized, a soil sample was taken from each for analysis. Equation (1) was utilized to calculate the percentage of soil water at field capacity.
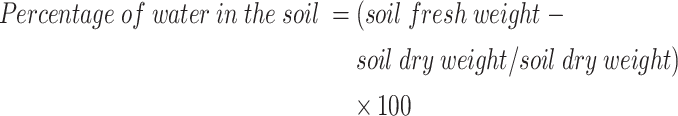 |
1 |
Table 1.
Soil physicochemical properties analyzed in this study
| Sand | Silt | Clay | Field capacity | Wilting Point | pH | EC (ds/cm) | OC (%) | N (%) | P (mg/kg) | K (mg/kg) | Ca (meq/l) | Mg (meq/l) | Cl (meq/l) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | |||||||||||||
| 56.80 | 34.85 | 8.35 | 16 | 7 | 7.8 | 2.34 | 1.74 | 0.08 | 25.12 | 241 | 14.1 | 12 | 14 |
EC electrical conductivity, OC organic carbon
After obtaining the weighted soil moisture values in FC, different treatments (100, 75, and 50% FC) were calculated. Irrigation cycles for each treatment were established based on the pots’ weight and water evaporation, with irrigation conducted every two days. Kaolin foliar spraying was first applied 25 days after transferring the seedlings, followed by a second application 20 days later. Throughout the experimental period, appropriate measures were taken to manage pests, diseases, and weeds. The pots were maintained in a greenhouse environment with night/day temperatures of 19 °C and 30 °C, respectively, and a relative humidity range of 60–75%.
Evaluation of growth and yield traits
During the full flowering stage (85 days after planting), measurements were taken using a ruler to determine plant height (cm), plant diameter (cm), leaf length (mm), and leaf width (mm). After harvest, the fresh weight of the shoots was recorded using a digital scale. Once the shoot had been dried at room temperature (25–27 °C) for five days, their dry weight was also measured.
Phytochemical traits
Essential oil extraction
According to the method described in the British Pharmacopoeia, the essential oil percentage was determined through hydro-distillation. The process involved using 50 g of dried aerial parts and a Clevenger apparatus for a duration of three hours. The essential oil yield was calculated based on 100 g of aerial parts following the 180-minute distillation period.
Preparation of phenolic extracts
Powdered aerial part samples (100 mg) were extracted using 80% methanol with ultrasound assistance for a duration of 44 min. After extraction, the mixtures were centrifuged at a speed of 4400×g for 15 min. The resulting supernatant was collected and used for phytochemical analysis.
Quantification of phenolic compounds by HPLC-DAD
Phenolic compounds were analyzed using a Knauer HPLC system from Germany, featuring dual Wellchrom-K1001 pumps and a K2800 PDA detector. The separation was carried out on an RP-C18 column with dimensions of 4.6 mm internal diameter and 250 mm length, manufactured by Eurosphr. The mobile phase consisted of methanol and HPLC-grade water. Peak detection was performed within a wavelength range of 200–600 nm. The sample injection volume was set at 20 µl, with the system maintained at a constant temperature of 25 °C.The pure phenol compound standards were sourced from Sigma Aldrich. Retention times and the analysis of the spiked oil extract, alongside a standard solution, were utilized to determine the presence of phenolic acids. Calibration curves were generated by injecting varying concentrations of standard compounds (5, 10, 20, 40, 60, 80, 100, 150, and 200 ppm) to quantify the phenolic acids. The results were expressed in milligrams per gram of dry weight (mg/g DW).
Estimation of total phenol concentration (TPC), total flavonoid concentration (TFC), and antioxidant activity
TPC was quantified the using the method described by Singleton et al. [28], which involves the Folin-Ciocalteu reagent. TFC was measured based at the method outlined by Dewanto et al. [29], utilizing aluminium chloride. Absorbance readings for TPC and TFC had been taken at wavelengths of 765 nm and 510 nm, respectively. The ferric reducing-antioxidant power (FRAP) assay of the methanolic extracts was determined using the method developed by Benzie and Strain [30] with some modification. The fresh FRAP reagent was prepared by mixing 300 mmol/l acetate buffer (pH 3.6), 10 mmol/l TPTZ in 40 mmol/l HCl, and 20 mmol/l FeCl3 at 10:1:1 (v/v/v). The reaction mixture was kept for 30 min at 37 °C. The absorption was then measured at 593 nm. A standard curve was generated using ferrous sulfate solution in concentrations ranging from 0.25 to 8 mmol dm − 3 (0.5–10 mg/ml). The result was expressed as µmol Fe+2 per g of DW.
Physiological traits
Relative moisture content (RWC)
To determine the relative water content (RWC) of the leaves, first, separated from the upper parts of the plant from each experimental unit, and their fresh weight was measured. To measure the turgor weight, these parts were placed in distilled water for 24 h under light intensity, and then, the turgor weight of the sample was read. The samples were placed in an oven at 75ºC for 48 h. The relative moisture content material become calculated using the following method [31]:
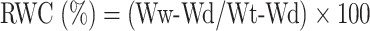 |
where Ww is the fresh weight, Wd is the dry weight and Wt is the turgor weight.
Total chlorophyll concentration measurement
To determine chlorophyll content, 0.5 g of freshly harvested leaves of T. vulgaris were finely ground with 80% acetone [32]. The extract was then transferred to a Falcon tube, and its volume reached 10 ml with 80% acetone. The extracted solution was centrifuged (for 10 min at 4400×g at 4 °C) and the absorbance of the extract was determined at 645 and 663 nm using a spectrophotometer. Eventually, the total chlorophyll content material was calculated in milligrams according to gram of clean weight based on the subsequent formula:
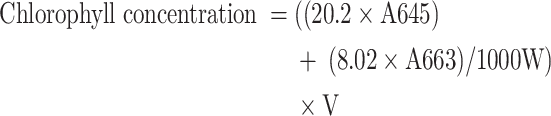 |
In which V, volume of solution; A, absorption of light at wavelengths of 663 and 645 nm; and W, wet weight of the sample (g).
Evaluation of biochemical traits and antioxidant enzyme activity
Enzyme activity was measured the using a spectrophotometer (Bio-Tek Instruments, Inc., USA). Fresh leaf samples (0.1 g) were homogenized with 1 ml of cold 50 mM phosphate buffer (pH = 7.8) and then centrifuged at 4400×g at 4 °C for 20 min. Catalase activity was estimated following the method of Aebi [33]. Peroxidase activity was determined according to the method of Zhang [34], and superoxide dismutase (SOD) activity was measured using the protocol described by Beachamp and Fridovich [35]. Proline extraction was carried out according to the method of Bates et al. [30]. Electrolyte leakage (EL) was measured using the method described by Lutts et al. [36]. Hydrogen peroxide (H2O2) was quantified utilizing a spectrophotometer at 390 nm according to Velikova and Loreto method [37]. To measure the concentration of malondialdehyde (MAD), 0.2 g of fresh leaf tissue was ground with 0.5 ml of 0.1% trichloroacetic acid (TCA), and the extract was centrifuged for 5 min. Then, 4.5 ml of 20% TCA solution containing 0.5% thiobarbituric acid (TBA) was mixed with 1 ml of the obtained supernatant. The mixture was centrifuged for 10 min at 4000 rpm. Absorbance was measured using a spectrophotometer at 532 nm, targeting the red MAD-TBA complex. Non-specific absorbance at 600 nm was also measured and subtracted from the 532 nm reading to correct for background interference [38].
Water use efficiency (WUE)
The WUE for each treatment was calculated in terms of grams of biomass produced per liter of water used. The following formula was applied:
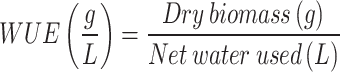 |
Where Dry biomass (g) represents the total weight of the plant’s dried above-ground components. Net water used (L) is the total water supplied minus the drained water (leachate), expressed in liters.
Statistical analyses
Two-way analysis of variance (ANOVA) was performed using SAS 9.1.3 software (SAS Institute Inc). The least significant difference (LSD) look at a chance degree of 0.05 (p-value < 0.05) changed into used to evaluate the suggest values of every variable. Origin 2021 software become used to examine the heatmap, and plotting graphs. The relationship between traits became performed the usage of R software program.
Results
Growth and performance traits
The variance analysis revealed that the interaction effects of drought stress and kaolin suspention application had a significant influence (p < 0.05) on the growth and performance characteristics of T. vulgaris. Drought caused a significant reduction in plant height and diameter, with both parameters decreasing as drought intensity increased. Foliar application of kaolin enhanced plant height and diameter, and the application of 4% kaolin had the greatest effect on plant height (25.36 ± 0.61 cm) and diameter (35.36 ± 0.26 cm) at different drought levels (Table 2). However, increasing kaolin concentration from 4 to 8% let to reduction in both plant height and diameter. Without kaolin application, leaf length and width reduced under drought condition. Kaolin foliar spraying increased leaf length and width in non-stress and drought stress conditions. The highest leaf length (9.19 ± 0.07 mm) and width (5.58 ± 0.08 mm) were obtained in the 100% FC and 4% kaolin treatments, which increased by 17.51 and 22.22%, respectively, compared to the control (Table 2). The fresh and dry weights of the plant decreased significantly with increasing drought stress in the absence of kaolin application, particularly at 50% FC. The fresh and dry weights of the shoot in the drought stress treatment (50% FC) decreased by 28.13 and 29.39%, respectively, compared to the control. The usage of 4% kaolin ended to a remarkable increase in the fresh (120.39 ± 1.01 g/plant) and dry (67.07 ± 0.88 g/plant) weights of the plant compared to the absence of kaolin application (Table 2). Foliar spraying with kaolin 4% at various drought stress levels effectively mitigated the adverse effect of drought. As a result, morphological and functional traits exhibited less decline compared to the control treatment.
Table 2.
Mean (± standard error) comparison of interaction effects of drought stress and Kaolin application on growth traits of Thymus vulgaris
| Treatment | Traits | ||||||
|---|---|---|---|---|---|---|---|
| Irrigation (% FC) | Kaolin (%) | Plant height (cm) | Plant diameter (cm) | Leaf length (mm) | Leaf width (mm) | Shoot fresh weight (g/plant) | Shoot dry weight (g/plant) |
| 100 | 0 | 19.79 ± 0.25d | 27.35 ± 43d | 7.58 ± 0.02d | 4.34 ± 0.11c | 84.05 ± 0.86d | 47.42 ± 0.52e |
| 100 | 2 | 22.24 ± 0.46c | 32.73 ± 0.47b | 8.12 ± 0.08c | 5.11 ± 0.03b | 101.78 ± 0.84c | 55.71 ± 0.57c |
| 100 | 4 | 25.36 ± 0.61a | 35.36 ± 0.26a | 9.19 ± 0.07a | 5.58 ± 0.08a | 120.39 ± 1.01a | 67.07 ± 0.88a |
| 100 | 8 | 23.50 ± 0.58b | 33.13 ± 0.42b | 8.58 ± 0.20b | 5.28 ± 0.0.2b | 115.70 ± 0.91b | 60.34 ± 0.55b |
| 75 | 0 | 15.64 ± 0.33f | 23.88 ± 0.31e | 6.43 ± 0.04f | 2.88 ± 0.05g | 72.33 ± 0.61f | 44.48 ± 0.55c |
| 75 | 2 | 17.95 ± 0.35e | 27.36 ± 0.45d | 6.71 ± 0.04e | 3.19 ± 0.09f | 78.06 ± 0.28e | 47.10 ± 0.28e |
| 75 | 4 | 20.69 ± 0.28d | 30.54 ± 0.47c | 7.43 ± 0.04d | 3.83 ± 0.04e | 84.26 ± 0.63d | 51.56 ± 0.41d |
| 75 | 8 | 18.53 ± 0.13e | 27.61 ± 0.46d | 6.91 ± 0.04e | 3.52 ± 0.06f | 77.96 ± 0.88e | 47.55 ± 0.48e |
| 50 | 0 | 11.23 ± 0.44h | 16.75 ± 0.46h | 4.87 ± 0.06i | 2.34 ± 0.05i | 60.04 ± 0.85h | 33.48 ± 0.48j |
| 50 | 2 | 13.03 ± 0.03g | 18.41 ± 0.31g | 5.33 ± 0.06h | 2.56 ± 0.03h | 64.45 ± 0.74g | 35.97 ± 0.37i |
| 50 | 4 | 15.80 ± 0.26f | 23.34 ± 0.50e | 5.91 ± 0.04g | 2.90 ± 0.05g | 70.19 ± 0.46f | 42.33 ± 0.61g |
| 50 | 8 | 14.13 ± 0.41g | 20.68 ± 0.61f | 5.43 ± 0.05fh | 2.73 ± 0.05gh | 66.42 ± 0.51g | 39.30 ± 0.61h |
Values in the same column followed by the same letter (s) are not significantly different at P < 0.05 according to the LSD test
Phytochemical traits
Essential oil percentage
The application of kaolin foliar spray and drought stress had a significant effect on essential oil percentage (p < 0.05). Both the individual and interactive effects of drought stress and kaolin foliar spray significantly increased essential oil content. Essential oil percentage expanded with increasing drought intensity with out using kaolin, which became 37.47% exceptional from the manage. The use of 8% kaolin resulted in a significant increase in the percentage of essential oil (1.37%) compared to the absence of kaolin (1.14%). The highest essential oil percentage (1.74%) was observed in the treatment of 50% FC and 8% kaolin, while the lowest was recorded in the control (Fig. 1a).
Fig. 1.
Effect of irrigation levels and kaolin on essential oil content (a), total phenol content (b), total flavonoid content (c), and antioxidant activity (d) of Thymus vulgaris. K: Kaolin; K0: without kaolin; K2: 2%; K4: 4%; K8: 8%. FC: Field capacity. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
TPC and TFC
The results of ANOVA showed that the total phenol and total flavonoid concentration were significantly affected by kaolin foliar application, drought stress, and their interaction (p < 0.05). In this study, various levels of kaolin and drought significantly increased both total phenol and flavonoid concentration. The application of 8% kaolin under 50% FC irrigation conditions had the highest total phenol concentration (16.11 mg GAE/g DW), which showed an increase of 86.67% compared to the control (8.63 mg GAE/g DW) (Fig. 1b). The total flavonoid concentration was also significantly influenced by drought stress and kaolin foliar application. The highest (9.32 mg QE/g DW) and lowest (4.20 mg QE/g DW) total flavonoid concentration was related to 8% kaolin foliar application under 50% FC irrigation and the control, respectively. The total flavonoid concentration in this treatment increased 2.1 times compared to the control (Fig. 1c).
Antioxidant activity
The analysis of variance revealed that both the individual and combined effects of kaolin and drought stress on antioxidant activity were statistically significant (P < 0.05). Antioxidant activity in the aerial parts of T. vulgaris increased by 1.23 times under drought stress combined with kaolin application compared to the control.
The maximum antioxidant activity recorded was 35.15 µmol Fe/g DW in thedrought treatment of 50% FC and the lowest (15.72 µmol Fe/g DW) was observed in the control (Fig. 1d).
Phenolic compounds
Drought stress and kaolin application had a significant impact (P < 0.01) on the phenolic compounds in the methanol extract of T. vulgaris. Among these compounds, rosmarinic acid was identified as the predominant component, as shown in Fig. 2. The maximum concentration of rosmarinic acid (11.05 mg/g DW) was observed under the treatment of 50% FC combined with 8% kaolin. Notably, under the highest level of drought stress, kaolin application led to a 1.6-fold increase in rosmarinic acid concentration compared to the control. Overall, increasing both drought intensity and kaolin concentrations resulted in higher levels of rosmarinic acid.
Fig. 2.
Effect of drought stress and kaolin on the phenolic compounds of Thymus vulgaris extract. K: Kaolin; K0: without kaolin; K2: 2%; K4: 4%; K8: 8%. DS: Drought stress; DS100: 100% FC, DS75: 75% FC, DS50: 50% FC
Biochemical traits
Relative water content (RWC)
The individual and combined effects of drought stress and kaolin application significantly influenced relative water content (p < 0.05). Analysis of their interaction revealed that, across all four kaolin levels, the 100% FC irrigation treatment resulted in the highest relative water content compared to the 75% and 50% FC drought treatments. The maximum relative water content was recorded under the 100% FC condition with an 8% kaolin application. In contrast, the lowest relative water content was observed under of 50% FC without kaolin application, showing a 41.15% decrease compared to the control (Fig. 3a).
Fig. 3.
Effect of irrigation levels and kaolin on relative water content (a), and cell membrane damage (b) of Thymus vulgaris. K: Kaolin; K0: without kaolin; K2: 2%; K4: 4%; K8: 8%. FC: Field capacity. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
Cell membrane damage
Stressed plants exhibited higher electrolyte leakage compared to control plants, indicating reduced cytoplasmic membrane stability. The analysis of variance showed that the individual effects of kaolin foliar application and drought stress, as well as their interaction, had a significant impact on cell membrane damage (p < 0.05). Cell membrane damage increased significantly with the intensification of drought stress compared to the control, while kaolin application reduced membrane damage in various drought conditions. The lowest cell membrane damage was observed in plants treated with 8% kaolin foliar application and 100% FC irrigation. In contrast, the highest level of membrane damage (74.26%) occurred under 50% FC without kaolin application in T. vulgaris (Fig. 3b).
Chlorophyll concentration
The application of a kaolin solution mitigated the adverse impacts of drought stress on chlorophyll concentration, resulting to a significant increase in chlorophyll levels across varying drought conditions (p < 0.05). As shown in Fig. 4a, chlorophyll concentration ranged between from 1.81 to 3.86 mg/g of fresh weight. Under drought conditions (50–75% FC), chlorophyll concentration in T. vulgaris plants decreased compared to plants under control conditions (100% FC). However, different levels of kaolin application significantly alleviated the negative effects of reduced irrigation water (from 100 to 50% FC) and increased chlorophyll concentration. Notably, a 4% kaolin foliar application resulted in a 45.11% increase in chlorophyll concentration compared to the control.
Fig. 4.
Effect of irrigation levels and kaolin on chlorophyll content (a), H2O2 content (b), malondialdehde (c), and proline (d) of Thymus vulgaris. K: Kaolin; K0: without kaolin; K2: 2%; K4: 4%; K8: 8%. FC: Field capacity. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
Hydrogen peroxide (H2O2), malondialdehyde (MDA), and proline concentration
The analysis of variance revealed that the application of kaolin, drought conditions, and their interaction had significant effects on H2O2, MDA, and proline levels (p < 0.05).As drought stress increased, H2O2 levels increased, with the highest level (6.29 nmol/g) observed in drought stress of 50% FC and without kaolin application. The application of 8% kaolin reduced H2O2 at all drought stress levels, with the lowest level (0.75 nmol/g) observed in the 8% and 100% FC kaolin treatments (Fig. 4b). The MDA level followed a similar trend to H2O2, T. vulgaris plants subjected to 50% FC without kaolin exhibited the highest MAD concentration (43.85 µmol/g). In contrast, the lowest MAD (13.16 µmol/g) was observed under 100% FC with 8% kaolin foliar application (Fig. 4c). Kaolin application also reduced proline content under drought conditions. The highest proline accumulation occurred under 50% FC irrigation without kaolin, while the lowest levels were observed with 8% kaolin under 80% and 100% FC conditions (Fig. 4d).
Antioxidant enzyme activity
According to the results of variance analysis, the effects of kaolin application, drought stress, and their interaction on SOD, POD and CAT enzymes activities were significant (p < 0.05). The results showed that there was no significant difference in SOD activity between foliar spray treatments of 0 and 2% kaolin foliar spray treatment under 100% FC. However, as the kaolin concentration increased, SOD activity decreased. The lowest SOD activity (26.70 U/g FW) was observed in the treatment of 8% kaolin and irrigation of 100 FC and the highest (144.33 U/g FW) was observed in drought of 50% FC and without the application of kaolin (Fig. 5a). POD and CAT activities of plants under water stress increased. However, foliar spraying with kaolin also increased their activities. The highest POD (1112.90 U/g FW) and CAT (52.68 U/g FW) activities were obtained in the treatment of 8% kaolin and 50% FC irrigation, and the lowest were observed in 100 FC irrigation and without kaolin application (Fig. 5b and c). Compared to the control, the activities of POD and CAT increased by 1.9 and 0.7 times, respectively.
Fig. 5.
Effect of irrigation levels and kaolin on superoxide dismutase (a), peroxidase (b), and catalase (c) of Thymus vulgaris. K: Kaolin; K0: without kaolin; K2: 2%; K4: 4%; K8: 8%. FC: Field capacity. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
Water use efficiency
WUE—defined as the ratio of plant biomass produced (g of dry weight) to the volume of water used (kg or L)—is a critical parameter for understanding plant responses to water availability. For T. vulgaris, which is characterized by its tolerance to arid Mediterranean climates, WUE varies significantly under different field capacity moisture levels (100%, 75%, and 50%). These variations are influenced by the interaction of water availability, plant physiological responses, and environmental factors. Kaolin application consistently improves water use efficiency in Thymus vulgaris across all moisture levels. The magnitude of improvement is most pronounced at 75% field capacity, where natural drought-tolerant mechanisms of the plant are complemented by kaolin’s reflective properties, resulting in optimal WUE. At 100% field capacity, kaolin reduces excessive transpiration, while at 50% field capacity, it mitigates severe drought stress effects by maintaining photosynthesis to a larger extent (Fig. 6). Overall, kaolin appears to be a promising tool for optimizing water use efficiency and sustaining plant productivity under varying moisture conditions, particularly in arid and semi-arid environments.
Fig. 6.
Effect of irrigation levels and kaolin on water use efficiency. K: Kaolin; K0: without kaolin; K2: 2%; K4: 4%; K8: 8%. FC: Field capacity. Bars with the same letter(s) are not significantly different at P < 0.05 according to the LSD test
Correlation analysis
The relationships between the studied traits of T. vulgaris under drought stress and kaolin application were illustrated in a network map based on the Pearson correlation (Fig. 7). Plant dry weight, an important economic trait, showed a positiv and significant correlattion with plant height, plant diameter, leaf length, leaf width, fresh weight, total chlorophyll, RWC. It was negatively and significantly correlated with TPC, TFC, MDA, H2O2, proline, SOD, POD, cell membrane damage. Positive and significant correlations were observed between RWC and plant height, plant diameter, leaf length, leaf width, fresh weight, total chlorophyll. It also had a negative and significant correlation with the essential oil percentage, TPC, TFC, antioxidant activity, MAD, H2O2, proline, and antioxidant enzymes. Growth traits and RWC were positively and significantly correlated with chlorophyll concentration. On the other hand, cell membrane damage, MAD, H2O2, proline and antioxidant enzymes were negatively a significantly correlated with chlorophyll.
Fig. 7.
Network correlation representation of agro-morphological, biochemical and phytochemical traits of Thymus vulgaris under kaolin and drought stress. TPC: Total phenol content; TFC: Total flavonoid content; FRAP: Antioxidant power assay; MDA: Malondialdehyde; H2O2: Hydrogen peroxide; CAT: catalase; SOD: Superoxide dismutase; POD: Peroxidase
Discussion
Drought, as an abiotic stress factor, can significantly impair plant performance and growth by disrupting key molecular, biochemical, and physiological processes such as protein metabolism, photosynthesis, and lipid biosynthesis [10, 38]. With the increasing prevalence of droughts worldwide, accurately assessing the water requirements for agricultural production has become essential to minimize water loss and enhance water-use efficiency [39]. This study investigates the effects of kaolin foliar application on the growth, phytochemical properties (including essential oil yields and phenolic compound content), and biochemical traits of T. vulgaris under drought conditions.
The findings revealed a decline in the growth and performance characteristics of plants subjected to drought stress compared to those under control conditions. This reduction may be attribute to increased evapotranspiration, reduced photosynthesis, stomatal closure, decreased CO2 absorption, lower cell turgor, and inhibited cell divisions under drought conditions [40]. Kaolin application improved growth traits, including plant height, plant diameter, leaf length and width, and both fresh and dry weight under drought stress. Under dry conditions, foliar spraying with kaolin enhances relative water content, water potential, and water use efficiency by increasing plant turgor. Additionally, by protecting the leaf surface, kaolin improves the rates of photosynthesis, transpiration as well as chlorophyll content, ultimately boosting yield and biomass [20]. These results are consistent with the findings of Segura-Monroy et al. [41] and Desoky et al. [42].
According to the results, kaolin increased the relative water content of T.vulgaris under drought conditions. Kaolin acts as a coating on the leaves and plant surface, reducing water evaporation from the leaf surface. This coating diminishes the intensity of direct sunlight on the plants and prevents the temperature increases on the leaf surface [20]. By lowering leaf surface temperature, kaolin enhances the plant’s relative water content, thereby improving its ability to withstand drought stress [43]. Additionally, Kaolin increases the plant’s resistance to biotic stresses by serving as a protective barrier against pests and diseases. As a result, the plant can use its resources more efficiently to conserve water and support physiological functions [44], thereby improving water use efficiency—particularly under drought stress [45].
Drought stress causes lipid peroxidation and harm to cell membranes through the production of reactive oxygen species (ROS) [13, 14, 46]. In this study, cell membrane damage increased with increasing drought stress intensity, and the application of kaolin significantly decreased the effects of stress, and the lowest membrane damage was observed in kaolin at 8%. Kaolin reduces the adverse effects of sunlight, temperature, and dryness by creating a protective layer on the leaf. Kaolin helps the proper functioning of the cell membrane by maintaining moisture [47]. Kaolin reduces the pressure on the cell membrane by reducing damage caused by pests and diseases, and protects the cell membrane by regulating cell metabolism and improving plant physiology and producing cell membrane-protective compounds, including antioxidants and secondary metabolites [25]. Kaolin reduces the production of free radicals and oxidative stress, and helps restore cell membranes and maintain their structural integrity [48].
Chlorophyll reduction is one of the key symptoms of environmental stress, particularly drought [49]. In this study, chlorophyll content decreased with increasing drought stress intensity, a trend has also been reported in other plants [50, 51]. Foliar application of kaolin moderated the effects of drought on chlorophyll concentration. Kaolin reduces the intensity of light reaching the chloroplast by creating a coating layer on the leaves; as a result, it prevents the photodegradation of chlorophyll and increases the chlorophyll content [52]. Kaolin prevents heat-induced chlorophyll degradation by reducing leaf surface temperature. Kaolin prevents the decrease in chlorophyll content caused by water deficiency by improving water conditions by reducing surface evaporation. Kaolin improves plant health and reduces stress, increasing nutrient uptake, which affects chlorophyll production [53, 54]. In one study, kaolin application at a concentration of 3% increased the content of chlorophyll a and b in Ocimum basilicum plants [55].
In drought conditions, H2O2 and MDA levels increased significantly. In addition, based on the results, kaolin application led to a decrease in these levels, which indicates the high potential of kaolin in reducing damage caused by ROS accumulation. H2O2 is a reactive oxygen species that is produced in response to environmental stresses and the cell signaling process. Kaolin indirectly affects antioxidant activity and H2O2 levels by providing less stress. Considering the role of kaolin in reducing temperature and increasing light reflectance, it reduces ROS, including H2O2 [56, 57]. MDA is a byproduct of lipid peroxidation, which indicates oxidative stress in plant cells. Increased MDA levels indicate cell membrane damage and impaired cell function. Kaolin directly prevents oxidative stress by covering the leaf surface from the intensity of light and heat and reduces MDA levels. Kaolin indirectly reduces MDA by reducing stress and better regulating the antioxidant system [25]. Kaolin can prevent drought damage and reduce MDA content by reducing evaporation from the leaf surface [25, 58].
Under stress conditions, proline accumulates as a protective osmolyte and contributes to osmotic regulation, protein stability and cell structure, and membrane protection [59]. In this study, drought stress increased proline concentration in T. vulgaris, while foliar application of kaolin reduced proline level by alleviating drought stress. Kaolin acts indirectly on proline accumulation by forming a protective coating on the leaves [60]. By mitigating drought stress, kaolin decreases the need for the accumulation of protective osmolytes such as proline [61]. In other words, it prevents excessive proline buildup. Kaolin achieves this by reducing oxidative stress and improving water use efficiency [25]. In this way, kaolin helps the plant allocate its energy for optimal growth by reducing the need to produce proline [62].
Kaolin helps improve the function of the antioxidant system and reduce free radicals [23]. Increasing resistance to drought stress causes physiological balance and reduces the need for proline accumulation [63]. In another study, kaolin at concentrations of 2.5% and 5% reduced proline accumulation in plants under drought stress conditions [64].
Polyphenols, as non-enzymatic antioxidants, inactivate lipid free radicals or prevent their formation, thereby reducing stress. They play a crucial role in minimizing oxidative damage by detoxifying ROS [65]. According to the results, the amount of phenolic compounds (especially rosmarinic acidas the main compound of the extract) and flavonoids increased under the influence of drought and foliar spraying of kaolin.
Kaolin reduces the intensity of light received by forming a white coating on the leaf surface, thereby mitigating light and heat stress. This reduction in environmental stress helps regulate the content of secondary metabolites, including phenolic compounds and flavonoids [24]. Although kaolin enhances the synthesis of phenolic compounds and flavonoids under light and heat stress, the extent of their production depends on the severity of the stress [57]. By reducing the adverse effect of stress, kaolin balances the production of phenolic compounds and prevents excess stress [65]. Kaolin helps increase the production of flavonoids under drought stress by maintaining photosynthetic capacity and reducing evaporation [64]. Kaolin also reduces oxidative damage by reducing leaf temperature and regulates the production of natural antioxidants, including flavonoids and phenols [56]. Kaolin had increasing effects on phenolic and flavonoid compounds in Mentha [66] and Ocimumbasilicum [67], with the extent of increase varying depending on the plant type, environmental conditions, and kaolin concentration. In the present study, the measurement of antioxidant activity in T. vulgaris samples revealed kaolin foliar application enhanced antioxidant activity under drought conditions. This increase may be attributed to the higher accumulation of phenolic and flavonoid compounds, as a strong and significant relationship between these compounds and antioxidant activity has been previously established [68].
The percentage of essential oils in plants is strongly influenced by both climatic and genetic factors [69]. In this study, the percentage of essential oils increased with the intensification of drought stress, a trend also observed in Coriandrum sativum [70] and Pelargonium graveolens [26] under similar conditions. It appears that kaolin indirectly influences the metabolic pathways involved essential oil production [71]. By forming a protective layer on the plant surface, kaolin helps prevent damage from pests and diseases, allowing the plant to allocate more resources toward essential oil synthesis [72]. Similar findings have been reported in Ocimum basilicum [67] and Pelargonium graveolens [26], where foliar application of kaolin led to increased essential oil content under drought stress.
The results showed an increase in antioxidant enzyme activity under drought stress. Foliar application of kaolin enhanced CAT and POD activities under drought conditions. By reducing oxidative stress and improving plant cell defense mechanisms, kaolin promotes the activity of antioxidant enzymes such as CAT and POD, thereby mitigating damage caused by lipid peroxidation [26]. In Capsicum annuum, kaolin significantly increased CAT and POD activities by alleviating drought stress. Kaolin reduces light intensity and leaf temperature by creating a protective layer on the leaf. This leads to reduced evaporation, water retention in the plant, and reduced reactive oxygen species. By strengthening the antioxidant system, it performs better in reducing oxidative stress [58].
Conclusion
The study demonstrated that foliar application kaolin improved the morphological, phytochemical, and biochemical traits of T. vulgaris, while also enhancing its resilience and adaptability to drought stress. The highest concentration of proline, MDA, H2O2 and cell membrane damage was observed under drought stress (50% FC), However, foliar application of kaolin 8% significantly reduced these levels compared to untreated plants grown under the same water deficit conditions (50% FC). Additionally, foliar application of kaolin 8% under water deficit (50% FC) increased total phenolic concentration, total flavonoid concentration, antioxidant activity, and essential oil percentage. The rosmarinic acid content in plants treated with kaolin 8% under drought conditions (50% FC) increased by 1.6 times compared to untreated plants. Kaolin enhances the antioxidant capacity of T. vulgaris under drought stress, thereby increasing its stress tolerance. It serves as a suitable and versatile tool in improving the morphological, biochemical and phytochemical traits of T. vulgaris. By mitigating stress, it keeps the plant productive and at the same time increases its medicinal properties by increasing secondary metabolites. The findings of this study support improved agricultural practices and greater productivity in the cultivation of T. vulgaris in arid and low-yielding environments. Kaolin is recommended as an effective strategy for promoting sustainable agriculture and resilience to climate change.
Supplementary Information
Acknowledgements
The authors gratefully acknowledge the Research Council of Shahid Beheshti University and for their support.
Ethical review
This study does not involve any human or animal testing.
Authors’ contributions
E.F: lab work, analysis data and writing-original draft; F.A: methodology, conceptualization, supervision, data curation, data analysis and editing; M.M: reviewing, and editing; G.E: methodology, conceptualization, data curation, reviewing, and editing.
Funding
Not applicable.
Data availability
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Declarations
Ethics approval and consent to participate
This manuscript is an original research and has not been published or submitted in other journals.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fateme Aghamir, Email: f.aghamir@sbu.ac.ir.
Ghasem Eghlima, Email: gh_eghlima@sbu.ac.ir.
References
- 1.Gupta N, Bhattacharya S, Dutta A, et al. Synthetic polyploidization induces enhanced phytochemical profile and biological activities in Thymus vulgaris L. essential oil. Sci Rep. 2024;14:5608. 10.1038/s41598-024-56378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kosakowska O, Węglarz Z, Bączek K. The effect of open field and foil tunnel on yield and quality of the common thyme (Thymus vulgaris L.), in organic farming. Agronomy. 2021;11(2):197. 10.3390/agronomy11020197. [Google Scholar]
- 3.Horváth G, Ács K. Essential oils in the treatment of respiratory tract diseases highlighting their role in bacterial infections and their anti-inflammatory action: A review. FlavourFragr J. 2015;30:331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I. Thymol and thyme essential oil—New insights into selected therapeutic applications. Molecules. 2020;25:4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tohidi B, Rahimmalek M, Arzani A, Sabzalian MR. Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem. 2020;307:125521. [DOI] [PubMed] [Google Scholar]
- 6.Salehi B, Abu-Darwish MS, Tarawneh AH, et al. Thymus spp. Plants—Food applications and phytopharmacy properties. Trends Food Sci Technol. 2019;85:287–306. 10.1016/j.tifs.2019.01.020. [Google Scholar]
- 7.Caprioli G, Maggi F, Bendif H, Miara MD, Cinque B, Lizzi AR, Brisdelli F, Celenza G. Thymus lanceolatusethanolic extract protects human cells from tBHP induced oxidative damage. Food Funct. 2018;9:3665–72. 10.3390/antiox13111287. [DOI] [PubMed] [Google Scholar]
- 8.Orhan-Yanıkan E, Gülseren G, Ayhan K. Antimicrobial characteristics of Thymus vulgaris and Rosa Damascena oils against some milk-borne bacteria. Microchem J. 2022;183:108069. [Google Scholar]
- 9.Zhu H, Liu C, Qian H. Pharmaceutical Potential of High-Altitude Plants for Fatigue-Related Disorders: A Review. Plants. 2022;11(5):2004. 10.3390/plants11152004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farouk S, AL-Huqail AA, El-Gamal SMA. Potential role of Biochar and silicon in improving physio-biochemical and yield characteristics of borage plants under different irrigation regimes. Plants. 2023;12(8):1605. 10.3390/plants12081605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan A, Pan X, Najeeb U, Tan DKY, Fahad S, Zahoor R, et al. Coping with drought: stress and adaptive mechanisms, and management through cultural and molecular alternatives in cotton as vital constituents for plant stress resilience and fitness. Biol Res. 2018;51(1):47. 10.1186/s40659-018-0198-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kordrostami M, Rabiei B, Kumleh H. Association analysis, genetic diversity and haplotyping of rice plants under salt stress using SSR markers linked to SalTol and morpho-physiological characteristics. Plant SystEvol. 2016;302(7):871–90. 10.1007/s00606-016-1304-8. [Google Scholar]
- 13.Metwaly ESE, Al-Yasi HM, Ali EF, Farouk HA, Farouk S. Deteriorating harmful effects of drought in cucumber by spraying Glycinebetaine. Agriculture. 2022;12:2166. 10.3390/agriculture12122166. [Google Scholar]
- 14.Seham MA, El-Gamal D, Wessam M, Serag E, Farouk S, Namait A, Mokhtar YO. Integrated effects of Biochar and potassium silicate on borage plant under different irrigation regimes in sandy soil. J Hort Sci Ornam Plants. 2021;13(1):60–76. 10.5829/idosi.jhsop.2021.60.76. [Google Scholar]
- 15.Guo R, Shi L, Jiao Y, Li M, Zhong X, Gu F, et al. Metabolic responses to drought stress in the tissues of drought-tolerant and drought-sensitive wheat genotype seedlings. AoB Plants. 2018;10:ply016. 10.1093/aobpla/ply016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YJ, Kwon DY, Koo SY, Truong TQ, Hong S-C, Choi J, Moon J, Kim SM. Identification of drought-responsive phenolic compounds and their biosynthetic regulation under drought stress in Ligulariafischeri. Front Plant Sci. 2023;14:1140509. 10.3389/fpls.2023.1140509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sourour A, Afef O, Mounir R, Mongi BY. A review: morphological, physiological, biochemical and molecular plant responses to water deficit stress. Int J Eng Sci. 2017;6:1–4. 10.9790/1813-0601010104. [Google Scholar]
- 18.Khalid MF, Zakir I, Khan RI, Irum S, Sabir S, Zafar N, Ahmad S, Abbas M, Ahmed T, Hussain S. Effect of water stress (drought and waterlogging) on medicinal plants. In: Husen A, Iqbal M, editors. Medicinal plants. Singapore: Springer; 2023. 10.1007/978-981-19-5611-9_6. [Google Scholar]
- 19.Glenn DM. Particle films: A new technology for agriculture. Hortic Reviews. 2005;31:1–44. [Google Scholar]
- 20.Nazim M, Ali M, Shahzad K, et al. Kaolin and jasmonic acid improved cotton productivity under water stress conditions. Saudi J Biol Sci. 2021;28:6606–14. 10.1016/j.sjbs.2021.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glenn DM, Cooley N, Walker R, Clingeleffer P, Shellie K. Impact of Kaolin particle film and water deficit on wine grape water use efficiency and plant water relations. Hort Sci. 2010;45:1178–87. [Google Scholar]
- 22.Semida W, Emara A, Ghoneim IM, Barakat MA. Kaolin foliar application enhanced physiological functions and pods quality of (Phaseolus vulgaris L.) under deficit irrigation regimes. Labyrinth: Fayoum J SciInterdisciplin Stud. 2023;1(1):84–94. 10.21608/ifjsis.2023.302858. [Google Scholar]
- 23.Dinis L-T, Bernardo S, Conde A, Pimentel D, Ferreira H, Félix L, Gerós H, Correia CM, Moutinho-Pereira J. Kaolin exogenous application boosts antioxidant capacity and phenolic content in berries and leaves of grapevine under summer stress. J Plant Physiol. 2016;191:45–53. 10.1016/j.jplph.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Brito C, Dinis L, Luzio A, et al. Kaolin and Salicylic acid alleviate summer stress in rainfed Olive orchards by modulation of distinct physiological and biochemical responses. Sci Hortic. 2019;246:201–11. 10.1016/j.scienta.2018.10.059. [Google Scholar]
- 25.Al-Mokadem AZ, Sheta MH, Mancy AG, Hussein HAA, Kenawy SKM, Sofy AR, Abu-Shahba MS, Mahdy HM, Sofy MR, Bakry A, AF., et al. Synergistic effects of Kaolin and silicon nanoparticles for ameliorating deficit irrigation stress in maize plants by upregulating antioxidant defense systems. Plants. 2023;12:2221. 10.3390/plants12112221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.AbuEl-Leil EF, AbdelRahman MAE, Desoukey SF. Effect of Kaolin on productivity, anatomical and biochemical responses to water deficit in Pelargonium graveolens grown in sandy soil. BMC Plant Biol. 2024;24:1111. 10.1186/s12870-024-05814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pourmeidani A, Jafari AA, Mirza M. Studying drought tolerance in Thymus Kotschyanus accessions for cultivation in dryland farming and low efficient grassland. J Rangel Sci. 2017;7(4):331–40. [Google Scholar]
- 28.Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. AJEV. 1965;16(3):144–58. 10.5344/ajev.1965.16.3.144. [Google Scholar]
- 29.Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010–4. 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- 30.Bates LS, Waldron RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–17. 10.1007/BF00018060. [Google Scholar]
- 31.Ghoulam C, Foursy A, Fares K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ Exp Bot. 2002;41(1):39–50. 10.1016/S0098-8472(01)00109-5. [Google Scholar]
- 32.Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949;24(1):1. 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 34.Zhang X. The measurement and mechanism of lipid peroxidation and SOD, POD and CAT activities in biological system. In: Zhang XZ, editor. Research methodology of crop physiology. Beijing: Agriculture; 1992. pp. 208–11. [Google Scholar]
- 35.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 36.Lutts S, Kinet JM, Bouharmont J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot. 1996;78:389–98. 10.1006/anbo.1996.0134. [Google Scholar]
- 37.Velikova V, Loreto F. On the relationship between isoprene emission and thermotolerance in Phragmitesaustralis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005;28(3):318–27. 10.1111/j.1365-3040.2004.01314.x. [Google Scholar]
- 38.Caser M, Chitarra W, D’Angiolillo F, Perrone I, Demasi S, Lovisolo C, Pistelli L, Pistelli L, Scariot V. Drought stress adaptation modulates plant secondary metabolite production in Salvia dolomitica Codd. Ind Crop Prod. 2019;129:85–96. 10.1016/j.indcrop.2018.11.068. [Google Scholar]
- 39.Berbel J, Exp´osito AA. Decision model for stochastic optimization of seasonal irrigation-water allocation. Agric Water Manag. 2022;262:107419. 10.1016/j.agwat.2021.107419. [Google Scholar]
- 40.Hossain MS, Li J, Sikdar A, Hasanuzzaman M, Uzizerimana F, Muhammad I, Yuan Y, Zhang C, Wang C, Feng B. Exogenous melatonin modulates the physiological and biochemical mechanisms of drought tolerance in Tartary buckwheat (Fagopyrum Tataricum (L.) Gaertn). Molecules. 2020;25(12):2828. 10.3390/molecules25122828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Segura-Monroy S, Uribe-Vallejo A, Ramirez-Godoy A, Restrepo-Diaz H. Effect of Kaolin application on growth, water use efficiency, and leaf epidermis characteristics of Physallisperuviana seedlings under two irrigation regimes. J Agric Sci Technol. 2015;17:1585–96. [Google Scholar]
- 42.Desoky ESM, Tohamy MRA, Eisa GSA, El-Sarkassy NM. Effect of some antitranspirant substances on growth, yield and flag leaf structure of wheat plant (Triticumaestivum L.) grown under water stress conditions. Zagazig J Agric Res. 2013;40:223–33. [Google Scholar]
- 43.Faghih S, Zamani Z, Fatahi R, Omidi M. Influence of Kaolin application on most important fruit Andleaf characteristics of two Apple cultivars under sustained deficit irrigation. Biol Res. 2021;54:1. 10.1186/s40659-020-00325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gharaghani A, Mohammadi A, Javarzari AR, Nejati R. Kaolin spray improves growth, physiological functions, yield, and nut quality of ‘tardy nonpareil’ almond under deficit irrigation regimens. App Fruit Sci. 2023;65:989–1001. 10.1007/s10341-022-00732-4. [Google Scholar]
- 45.Djurović N, Ćosić M, Stričević R, Savić S, Domazet M. Effect of irrigation regime and application of Kaolin on yield, quality and water use efficiency of tomato. Sci Hort. 2016;201:271–8. 10.1016/j.scienta.2016.02.017. [Google Scholar]
- 46.Shi Q, Ding F, Wang X, Wei M. Exogenous nitric oxides protect cucumber roots against oxidative stress induced by salt stress. Plant PhysiolBiochem. 2007;45:542–50. 10.1016/j.plaphy.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 47.de Abreu DP, de Roda N, Krohling M, Campostrini CA, Rakocevic E, M. Kaolin particle film mitigates supra-optimal temperature stress effects at leaf scale and increases bean size and productivity of Coffeacanephora. Expert Agri. 2023;59:e13. 10.1017/S001447972300011X. [Google Scholar]
- 48.Kato T, Toyooka T, Ibuki Y, et al. Effect of physicochemical character differences on the genotoxic potency of Kaolin. Genes Environ. 2017;39(12). 10.1186/s41021-017-0075-y. [DOI] [PMC free article] [PubMed]
- 49.Morshedloo MR, Salami A, Nazeri V, Craker LE. Prolonged water stress on growth and constituency of Iranian of oregano (Origanumvulgare L). J Med Act Plants. 2016;5:1–4. [Google Scholar]
- 50.Campos CN, Ávila RG, de Souza KRD, Azevedo LM, Alves JD. Melatonin reduces oxidative stress and promotes drought tolerance in young Coffeaarabica L. plants. Agric Water Manag. 2019;211:37–47. 10.1016/j.agwat.2018.09.025. [Google Scholar]
- 51.Sharma A, Wang J, Xu D, Tao S, Chong S, Yan D, Li Z, Yuan H, Zheng B. Melatonin regulates the functional components of photosynthesis, antioxidant system, gene expression, and metabolic pathways to induce drought resistance in grafted Caryacathayensis plants. Sci Total Env. 2020;713:136675. 10.1016/j.scitotenv.2020.136675. [DOI] [PubMed] [Google Scholar]
- 52.Garrido A, Serôdio J, De Vos R, Conde A, Cunha A. Influence of foliar Kaolin application and irrigation on photosynthetic activity of grape berries. Agronomy. 2019;9(11):685. 10.3390/agronomy9110685. [Google Scholar]
- 53.Xavier TS, Pereira VS, Nascimento YYG, Neto BP, Souza RR, Silva RR, Beckmann-Cavalcante MZ. Kaolin, boron, and zinc increase photosynthetic activity and mitigate the effects of light stress in heliconia grown under semi-arid conditions. Sci Hort. 2023;319:112134. 10.1016/j.scienta.2023.112134. [Google Scholar]
- 54.Sharma RR, Datta SC, Varghese E. Kaolin-based particle film sprays reduce the incidence of pests, diseases and storage disorders and improve postharvest quality of ‘delicious’ apples. Crop Prot. 2019;127104950. 10.1016/j.cropro.2019.104950.
- 55.Salari M, Sodaeizadeh H, Hakimzadeh MA, Yazdani-Biouki R. Investigating of kaolin in increasing of drought tolerance of Basil (Ocimumbasilicum var. purpurascens). Env. Stresses Crop Sci. 2020;13(1):171–183. 10.22077/escs.2019.1864.1442.
- 56.Bernardo S, Dinis LT, Luzio A, Pinto G, Meijón M, Valledor A, Conde H, Gerós CM, Correia J. Kaolin particle film application lowers oxidative damage and DNA methylation on grapevine (Vitisvinifera L.). Environ. Exp Bot. 2017;139:39–47. 10.1016/j.envexpbot.2017.04.002. [Google Scholar]
- 57.Khavari M, Fatahi R, Zamani Z. Salicylic acid and Kaolin effects on pomological, physiological, and phytochemical characters of hazelnut (Corylusavellana) at warm summer condition. Sci Rep. 2021;11:4568. 10.1038/s41598-021-83790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghanbari F, Cheraghi M, ErfaniMoghadam J. The effect of Kaolin on drought stress tolerance and some physiological responses of bell pepper (Capsicum annuumL). J Veg Sci. 2024;5(9):71–85. 10.22034/IUVS.2020.137652.1122. [Google Scholar]
- 59.Hayat S, Hayat Q, Alyemeni MN, Shafi AW, Pichtel J, Ahmad A. Role of proline under changing environmentsa review. Plant Signal Behav. 2012;7(11):1456–66. 10.4161/psb.21949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AbdAllah A. Impacts of Kaolin and pinoline foliar application on growth, yield and water use efficiency of tomato (Solanumlycopersicum L.) grown under water deficit: A comparative study. J Saudi Soc Agric Sci. 2019;18:256–68. 10.1016/j.jssas.2017.08.001. [Google Scholar]
- 61.Iqbal M, Bashir R, Hussain I, et al. Enhancing maize (Zea mays L.) tolerance to water stress using Kaolin and potassium silicate as protective agents. Cereal Res Commun. 2024. 10.1007/s42976-024-00532-4. [Google Scholar]
- 62.Furlan AL, Bianucci E, Giordano W, Castro S, Becker DF. Proline metabolic dynamics and implications in drought tolerance of peanut plants. Plant Physiol Biochem. 2020;151:566–78. 10.1016/j.plaphy.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 63.Mahmoudian M, Rahemi M, Karimi S, Yazdani N, Tajdini Z, Sarikhani S, Vahdati K. Role of Kaolin on drought tolerance and nut quality of Persian walnut. J Saudi Soc Agric Sci. 2021;20(6):409–16. 10.1016/j.jssas.2021.05.002. [Google Scholar]
- 64.Rahimi M, Kordrostami M, Mohamadhasani F, et al. Antioxidant gene expression analysis and evaluation of total phenol content and oxygen-scavenging system in tea accessions under normal and drought stress conditions. BMC Plant Biol. 2012;21:494. 10.1186/s12870-021-03275-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dinis L, Pereira S, Fraga I, Sílvia M, Costa R, Martins C, Vilela C, Arrobas A, Moutinho-Pereira M. Kaolin foliar spray induces positive modifications in volatile compounds and fruit quality of Touriga Nacional red wine. OENO One. 2024;58–2. 10.20870/oeno-one.2024.58.2.7945.
- 66.Wang Y, Xue T, Han X, Guan L, Zhang L. Kaolin particle film affects grapevine berry quality in Cv. Meili Humid Clim Conditions HORTSCIENCE. 2020;55(12). 10.21273/HORTSCI15364-20.
- 67.Rania MRK, Al-Azzony EAA. Kaolin and irrigation intervals affect growth and essential oil of sweet Basil. Middle East J Appl Sci. 2019;10(4):792–802. 10.36632/mejas/2020.10.4.70. [Google Scholar]
- 68.Moshari-Nasirkandi A, Iaccarino N, Romano F, Graziani G, Alirezalu A, Alipour H, Amato J. Chemometrics-based analysis of the phytochemical profile and antioxidant activity of Salvia species from Iran. Sci Rep. 2024;14:17317. 10.1038/s41598-02468421-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bajalan I, Rouzbahani R, Pirbalouti AG, Maggi F. Antioxidant and antibacterial activities of the essential oils obtained from seven Iranian populations of Rosmarinus officinalis. Ind Crops Prod. 2017;107:305–11. 10.1016/j.indcrop.2017.05.063. [Google Scholar]
- 70.Afshari M, Pazoki A, Sadeghipour O. Foliar-applied silicon and its nanoparticles stimulate physio-chemical changes to improve growth, yield and active constituents of coriander (CoriandrumSativum L.) essential oil under different irrigation regimes. Silicon. 2021;13:4177–88. 10.21203/rs.3.rs-176146/v1. [Google Scholar]
- 71.Conde A, Pimentel D, Neves A, Dinis L-T, Bernardo S, Correia CM, Moutinho-Pereira J. Kaolin foliar application has aStimulatory effect on phenylpropanoidand flavonoid pathways in grapeberries. Front Plant Sci. 2016;7:1150. 10.3389/fpls.2016.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reitz SR, Maiorino G, Olson S, Sprenkel R, Crescenzi A, Momol MT. Integrating plant essential oils and Kaolin for the sustainable management of thrips and tomato spotted wilt on tomato. Plant Dis. 2008;92:878–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.









