Abstract
Background
Animal pigmentation serves as an excellent model for studying genetics, development, and evolution. Among yak breeds, the all-white yak breed (Bos grunniens) is the only indigenous variety with pristine white fur, in stark contrast to the black coat color of the wild yak and most domesticated yaks.
Results
Using whole-genome sequencing data from 387 yaks, we analyzed the population genetic structure of all-white yaks and discovered that they clustered into two distinct genetic groups. Further, by conducting a genome-wide association study (GWAS) based on whole-genome variants (SNPs and indels) between all-white and wild-type yaks, we identified a 14-bp deletion in the promoter of KIT, which decreased its expression in all-white yaks. The following knock-in experiments in mouse confirmed that the absence of the yak 14-bp motif decreases the expression of KIT. Deletion of a human orthologue of the yak 14-bp motif by using the CRISPR/Cas9 system reduces the melanin accumulation in human melanoma cells.
Conclusions
Overall, our study revealed the genetic basis of all-white yaks and underscored the importance of studying livestock phenotypes to uncover conserved genetic regulators in mammals.
Supplementary information
The online version contains supplementary material available at 10.1186/s12915-025-02311-x.
Keywords: All-white yaks, KIT gene, 14-bp motif, Gene editing, Melanin
Background
Animal pigmentation plays a key role in mate choice, signaling, predator distraction, and prey predation [1]. The primary colors of animal skin, hair, and eyes are determined by the relative ratio of pheomelanin to eumelanin [2]. Genetic variations in melanin biosynthesis or transfer led to differences in hair, eye, and skin color and can be subject to positive selection [3]. In farm animals, pigmentation characteristics are typically selected by human preference of specific colors and patterns. As a result, animal pigmentation is an obvious and highly variable trait in domestic animals, which is targeted by artificial selection and has attracted considerable attention [4–12].
Abnormalities in melanin synthesis also play a role in various health conditions and diseases, including albinism and melanoma cancer in humans [13, 14]. The KIT gene has been implicated in melanin accumulation and the development of diverse pigmentation phenotypes across mammalian species [6–8, 15–17]. However, the specific key regulators involved in this process remain largely unknown.
Domestic and wild yaks (Bos grunniens and B. mutus, respectively) are the largest mammals inhabiting the Qinghai-Tibet Plateau (QTP) [18, 19], and both of them have predominantly a black coat color. However, varying degrees of pigmentation result in white spots on different body parts or in a completely white animals, referred to as “partially white” and all-white phenotypes [20]. Both phenotypes result from a deficiency of melanocytes in the skin and hair follicles, also known as leucoderma or philodermia, which is attributed to defects in melanocyte development or migration [2, 21–23]. The all-white yak breed has been highly valued for around 2000 years in China due to ecological, cultural, and economic importance.
Our previous research primarily focused on investigating the origin of all-white yaks [17]. Through analysis of the pan-genome and graph-genome structural variations (SVs) for all bovine species, we discovered that partially white yaks were the result of an introgression of translocated and rearranged SV alleles (Cs6) from color-sided cattle. Furthermore, we identified that a subsequent rearrangement generated a tandem repeat of three copies of about 600 kb, which led to the formation of a yak-specific allele (SCs6) and the suppression the expression of KIT in all-white yaks [17]. Yet, it is not clear how the SCs6 allele causes the white color in yaks. In this study, by generating and analyzing 387 whole-genome sequencing data, we identified a 14-bp deletion in the promoter of KIT region in the SCs6 allele causing all-white yaks via decreasing the KIT expression. The same yak promoter variants introduced in transgenetic mice regulated the coat color in a similar way, and the deletion of human homologous 14-bp motif decreases the melanin expression in human melanoma cells.
Results
Genome re-sequencing and population genetic structure
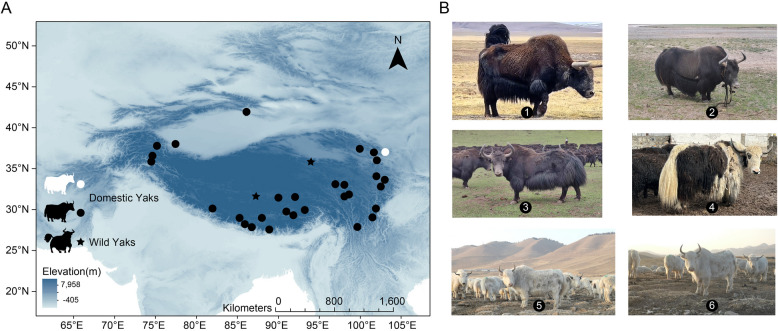
We carried out whole-genome sequencing (WGS) of 140 domestic yaks from the QTP (Fig. 1A, B and Additional file 2: Tables S1, S2). We obtained an average sequencing coverage of 13.23 × (ranging from 8.56 × to 20.75 × , Additional file 2: Table S2) across these animals. These newly generated data were further combined with previously published WGS data of 247 yaks [17, 19, 24–28]. In total, the dataset comprised 18 wild yaks, 89 all-white yaks, and 280 domestic yaks with other coat colors, representing nearly the entire distribution area of yaks (Fig. 1A, B and Additional file 2: Tables S1–S3). A total of 13,099,643 high-quality genomic variants were detected, which included 12,229,673 single nucleotide polymorphisms (SNPs) and 869,970 insertions/deletions (indels). Using wild yaks as the outgroup, the neighbor-joining (NJ) tree based on SNPs revealed the presence of four major clusters within the domestic yaks (Additional file 1: Fig. S1A). All-white yaks clustered into two groups (W1 and W2) by NJ tree, principal component analysis (PCA), and admixture (Additional file 1: Fig. S1B–D), suggesting that all-white yaks may have originated from hybridization between different ancestral populations of domestic yaks and cattle [17]. Additionally, extensive genetic admixture was observed among domestic yaks, with the exception of Pakistani and Xinjiang yaks, providing further supports for the phylogenetic and PCA analysis (Additional file 1: Fig. S1A–D).
Fig. 1.
Distribution of domestic and wild yaks. A Main geographical distribution and B phenotypic characteristics of domestic and wild yaks included in this study. 1: wild yak, 2: F1 generation male yak hybrid between wild yak and domestic yak, 3–4: domestic yaks with all-black and white spotting phenotype, 5–6: domestic yaks with all-white phenotype
Causal genetic mutations for all-white yaks
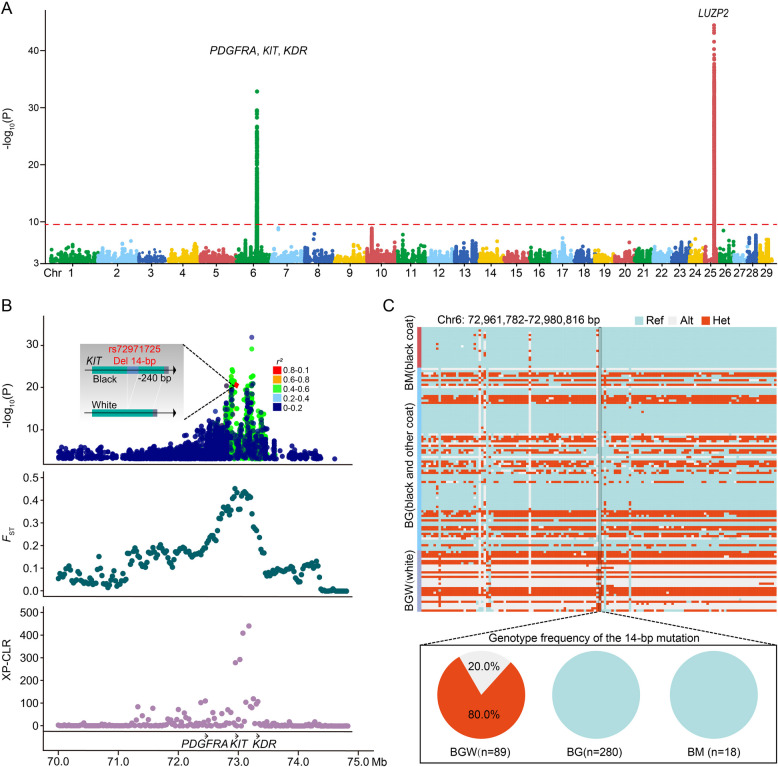
Using our comprehensive panel of genomes, we compared all-white yaks with other yaks by GWAS, fixation index (FST), and the cross-population composite likelihood ratio test (XP-CLR; Fig. 2A and Additional file 1: Fig. S2). Consistent with our previous report [17], two regions, from 72.13 to 73.47 Mb on chromosome 6 and from 31.26 to 31.69 Mb on chromosome 25, displayed strong GWAS signals (Fig. 2A), which was further confirmed by selective sweep analyses (Fig. 2B). The first selection signal on chromosome 6 includes three genes, PDGFRA, KIT, and KDR (Fig. 2A and B), all of which encode receptor tyrosine kinases and share similar functions through duplication from a common ancestral gene [29]. These three genes have been linked to regulation of pigmentation in many mammalian species, including humans, mice, goats, horses, cattle, and non-human primates [6, 7, 29–35]. The second notable selection signal encompassed the only LUZP2 gene and corresponds to the rearrangement that copied the last four exons of LUZP2 into the KIT locus (the β-γ fragment without implying any other involvement of LUZP2) [6]. Remarkedly, apart from the SCs6 structural variant [17], among these significant regions, we identified a novel 14-bp deletion in the promoter region of the KIT gene in all-white yaks, whereas other loci are located in intronic and intergenic regions (Chr6: 72,971,725; Fig. 2B). Notably, this indel exhibits only weak linkage disequilibrium (LD, r2 < 0.8) with both nearby and more distant SNPs and indels (Fig. 2B). It is homozygous (20.0%) or heterozygous (80.0%) absent in all-white yaks and present in other domestic or wild yaks (Fig. 2C and Additional file 2: Table S4). The PCR genotyping also confirmed that the 14-bp deletion existed distinctly in all of 40 re-sampled all-white yaks (Additional file 1: Fig. S3A–C and Additional file 2: Table S5). Combining these results with previous findings indicates that the 14-bp deletion evolved together with the two additional copies of the (B-C-β-γ
 segment in color-sided cattle and color-sided yaks, which resulted in the all-white phenotype in yaks [6, 17].
segment in color-sided cattle and color-sided yaks, which resulted in the all-white phenotype in yaks [6, 17].
Fig. 2.
Genomic regions with strong selection signals and GWAS in all-white yaks. A GWAS was performed by whole-genome SNPs and indels of all-white and other yaks. The red dashed line indicates the threshold line is equal to 9. B GWAS, FST, and XP-CLR values within the about 70.0–74.9 Mb regions on chromosome 6 and LD values between the 14-bp indel and other loci. The opacity of the GWAS dots is 70%. C Genotype frequency plots of variations in Chr6:72,961,782–72,980,816 in all-white and other yaks. Ref: homozygous reference genotype, Alt: homozygous mutant genotype, Het: heterozygous mutant genotype. BM: wild yaks, BG: domestic yaks, BGW: domestic white coat yaks
The 14-bp deletion in the active promoter region of KIT inhibits the binding activity of E2F3/6 and decreases in its expression
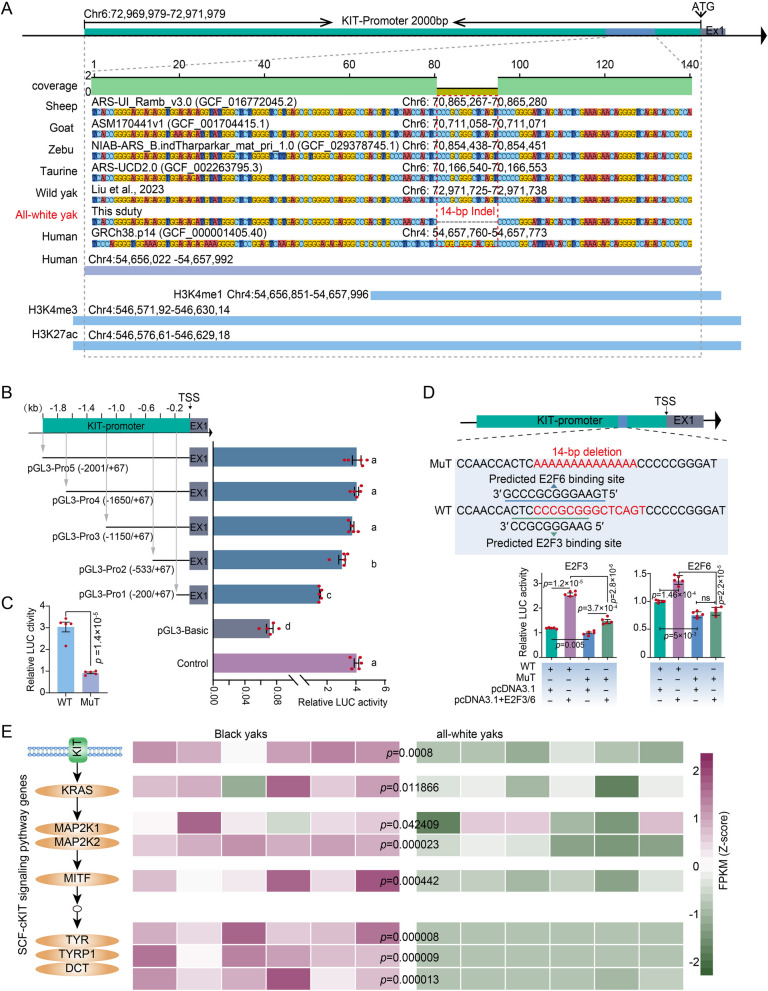
The 2 kb DNA sequence upstream of the transcription start site (TSS) of KIT gene were strongly constrained across 12 mammal species (Additional file 2: Table S6). The 14-bp orthologous sequence that is absent in all-white yaks overlaps with histone modification signals of H3K4me1, H3K4me3, and H3K27ac in human foreskin melanocyte cells (Fig. 3A) based on human ENCOED project [36]. To confirm the deletion within the active promoter of KIT, we inserted different promoter fragments in the luciferase reporter vector pGL3-Basic for co-transfection of five plasmids with pRL-TK as internal reference into HEK293T cells. The fluorescence of the five target vectors was stronger than that of the pGL3-Basic vector (p < 0.01; Fig. 3B and Additional file 3: Table S1). The fluorescence activity increased as the promoter fragment was extended until reaching a plateau at pGL3-Pro3 (p < 0.01; Fig. 3B and Additional file 3: Table S1), indicating an active KIT promoter region between pGL3-Pro3 (− 1150 bp) and TSS. We then constructed the pGL3-Pro2 plasmid (named WT) and without the conserved 14-bp fragment (named Mut), respectively. WT induced a significantly higher luciferase activity than Mut (Fig. 3C and Additional file 3: Table S1), indicating that the deletion of 14-bp inhibited KIT expression.
Fig. 3.
Promoter activity and 14-bp function analysis of KIT gene. A The 14-bp mutation within the promoter region of the KIT gene in bovine species and humans, along with its flanking sequences was visualized by Geneious. Red boxes highlight the specific genomic locations of this 14-bp mutation in main Bovidae species and humans, allowing for the assessment of their homology and characteristics. Based on the ENCODE project, the human histone modification signals corresponding to this 14-bp mutation and its flanking regions were examined. B Validation of promoter active of KIT gene (see Methods). Lowercase letters indicate significant differences (p < 0.01). C 14-bp mutation function verification by dual-luciferase assay. WT stands for the pGL3-Pro2, while MuT represents the pGL3-Pro2 with a 14-bp deletion. D The 14-bp mutation was verified to bind to the transcription factor motif by dual-luciferase assay. WT stands for the pGL3-Pro2, while MuT represents the pGL3-Pro2 with a 14-bp deletion. Values are shown as means ± SD from five biological replicates; exact p values are shown; two-sided Student’s t-test. See also Additional file 3: Tables S1 and S2. E PFKM of SCF-cKIT signaling pathway genes was calculated from RNA-seq data of 6 black coat and 6 white coat yak ear tissues. Exact p values are shown; two-sided Student’s t-test
According to AnimalTFDB3.0 database, there are in the region of the deletion potential binding motif of the E2F and AP-2 transcription factors in this region (Additional file 2: Table S7). Both families of E2F and AP-2 transcription factors are known to play crucial roles in cell proliferation and cycle regulation and they are involved in the reprogramming, transformation, and progression of melanocytes and melanoma cells [37–39]. Dual-luciferase reporter experiments demonstrated that only E2F3 (motif 3′-CCGCGGGAAG-5′) and E2F6 (3′-GCCCGCGGGAAGT5′), respectively inserted in the pcDNA3.1 vector, stimulated the expression more if contransfected with WT plasmid than if cotransfected with Mut (Fig. 3D and Additional file 3: Table S2), suggesting that this fragment may be the binding site for the two transcription factors E2F3 and E2F6. In the melanin synthesis pathway SCF-cKIT, the KIT gene belongs to the transmembrane gene category. It traverses the cell membrane and interacts with the intracellular and extracellular environment. By transcriptome analysis and western blot (WB) experiment, we found that KIT gene has significantly low expression in all-white yak ear tissues compared with black yaks (Fig. 3E and Additional file 1: Fig. S4). The other core genes in the SCF-cKIT signaling pathway also showed similar expression trends (Fig. 3E).
Effect of the 14-bp deletion on the expression of KIT gene in transgenic mice.
To further investigate the function of a 14-bp deletion within the yak KIT gene promoter region with respect to coat color phenotypes, we used CRISPR/Cas9 technology to replace the mouse KIT promoter with the active promoters from all-white and black yaks, respectively (Fig. 4A and Additional file 1: Fig. S5). This resulted in two gene-edited mouse models, the yak-mouse wild allele (ymKIT+) and yak-mouse allele promoter deletion (ymKITPro14Del). By genotyping, we screened 3 positive F0 ymKIT+ and 2 positive F0 ymKITPro14Del transgenic individuals (Additional file 1: Figs. S6 and S7). Subsequently, by generating F2 mice, we observed that homozygous F2 ymKIT+/+ mice exhibited overall black coat color, while homozygous F2 ymKITPro14Del/Pro14Del mice displayed white spots and white hairs on their abdomens (Fig. 4B, C and Additional file 1: Fig. S8). We further observed a significant decrease in the expression of KIT mRNA in the white-spot tissues of ymKITPro14Del/Pro14Del mice compared to ymKIT+/+ mice skin tissues (Fig. 4D and Additional file 3: Table S3). Altogether the gene-edited mice confirm that deletion of the 14-bp fragment inhibits the expression of KIT, decreases melanin synthesis, and finally changes the coat color.
Fig. 4.
Functional validation of the 14-bp absence in the KIT promoter in mice. A A new KIT knockin (KI) model between C57BL/6 J mice and yaks by CRISPR/Cas-mediated genome engineering. B Homozygous knockin ymKIT+/+ mice. C Homozygous point mutation knockin ymKITPro14Del/Pro14Del mice. D Relative mRNA expression level of KIT in ymKIT+/+ and ymKIT.Pro14Del/Pro14Del mice. Bars of different colors correspond to the locations of the tissue in Fig. 4B and C. Values are shown as means ± SD from three biological replicates. Exact p values from the two-sided Student’s t-test are shown. See also Additional file 3: Table S3
Functional impacts of the 14-bp promoter fragment of KIT in human melanoma cells.
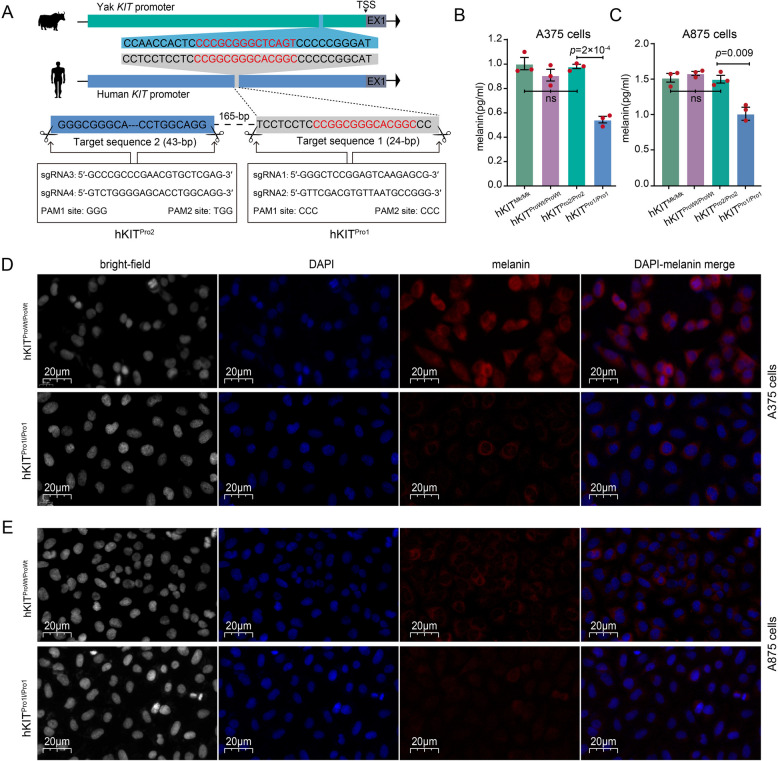
To explore the functional impacts of the 14-bp fragment in melanin deposition in humans, we constructed two CRISPR/Cas9 system models in the human melanoma A375 and A875 cell lines to knock out a 24-bp segment containing the 14-bp mutation (hKITPro1) in the KIT promoter region (Fig. 5A). As negative control, we deleted a 43-bp sequence upstream (hKITPro2; Fig. 5A). For a second negative control, we used the empty lentivirus vector without sgRNA (mock, hKITMk/Mk). Interestingly, we found that hKITPro1/Pro1 significantly reduced the melanin content relative to the control (hKITProWt/ProWt, hKITPro2/Pro2, and hKITMk/Mk) in both A375 and A875 cells (Fig. 5B, C and Additional file 3: Table S4).
Fig. 5.
Effect of deletion in KIT promoter in human A375 and A875 cells. A The strategy for editing KIT promoter in human A375 and A875 cells. B and C The melanin levels were assessed across distinct cellular populations, including untreated wild-type cells (the complete promoter region, hKITProWt/ProWt), cells harboring the 24-bp deletion spanning the homologue of the 14-bp yak indel sequence (hKITPro1/Pro1), as control cells with the 43-bp deletion at 165-bp upstream of target sequence 1 (hKITPro2/Pro2) and as second control cells infected with an empty lentivirus vector that does not contain sgRNA (mock, hKITMk/Mk). Values are shown as means ± SD from three biological replicates. Exact p values from the two-sided Student’s t-test are shown. See also Additional file 3: Table S4. D and E Immunofluorescence (IF) analysis of hKITPro1/Pro1 and hKITProWt/ProWt A375 or A875 cells. The DAPI column represents the cell nucleus stained in blue using 4′,6-diamidino-2-phenylindole (DAPI), while the melanin column illustrates the presence of melanin within the cells, depicted in red through melanin antibody labeling
We visualized pigment granules by immunofluorescence analysis of the hKITPro1/Pro1 A375 and A875 cells. The majority of hKITWt/Wt A375 and A875 cells exhibited dense pigmentation. In contrast, most hKITPro1/Pro1 cells were either non-pigmented or lightly pigmented (Fig. 5D and E). Notably, the hKITWt/Wt cells showed predominant detection in “rings” around pigment granules, indicating localization to the limiting membrane of mature melanosomes (Fig. 5D and E). Transcriptome profiling of hKITPro1/Pro1 and hKITWt/Wt A375 cells revealed 1019 upregulated and 1189 downregulated differentially expressed genes (DEGs; Additional file 1: Fig. S9A–C). Functional annotation analysis of these DEGs highlighted their important roles in melanoma, melanosome, melanogenesis, HIF-1 signaling pathway, MAPK signaling pathway, mTOR signaling pathway, p53 signaling pathway, and DNA replication (Additional file 1: Fig. S9D).
Discussion
In the present study, we revealed a distinct 14-bp deletion in the KIT promoter that is present in all-white yaks. Although this indel variant does not exhibit the strongest association signal, its low LD with other variants suggests it is a potential independent genetic locus not fully accounted for by neighboring variants. We hypothesize that this unique indel may regulate KIT gene expression by altering transcription factor binding sites, thereby influencing the phenotype of white yak. Combined with our previous findings [17], this indel, together with two large-sized copies (SCs6), may contribute to the transition from “partially white” to “all-white” phenotype in domestic yaks (Fig. 6).
Fig. 6.

A genetic model for evolution from all-black to all-white yak. In white coat yaks, the KITPro14Del_Hap1−SCs6 together inhibit the expression of KIT gene, resulting in expression decrease of genes in the SCF-cKIT signaling pathway, ultimately reducing the production of melanocytes. The KIT promoter region is defined as 2 kb upstream of the transcription start site
This 14-bp fragment in the KIT active promoter is conserved across different mammals and overlapped with multiple key histone modification signals, suggesting that this mutation is a potential target responsible for skin diseases in humans and coat color variations associated with KIT expression in both humans and animals. This hypothesis was further supported by three additional experiments. First, sequence analysis and luciferase reporter experiments showed that this 14-bp may be a binding site for the E2F transcription factor family, and its deletion significantly reduced the expression of KIT. Second, this could be reproduced in transgenic mice in which a part of the murine KIT promoter was replaced by segments of the yak promoter with and without the 14-bp fragment. This resulted in white spots on their abdomens in mice without the fragment. Third, the 14-bp deletion in human melanocytes also resulted in reduced melanin productions.
KIT is related to development, and its functional role in various phenotypes is highly complex. It regulates the development of melanocytes, blood cells, primordial germ cells, auditory hair cells, mast cells, and small intestinal Cajal cells [15]. In mice, mutations in the regulatory or coding regions of the KIT can cause skin pigmentation patterns and reproductive system disorders, resulting in the inability to produce offspring [16, 40, 41]. However, mutation in the KIT active promoter region of all-white yak did not lead to reproductive system disorders and produced a stable inherited white-spotted phenotype. Yaks, mice, and humans likely acquire their color patterns through different genetic mechanisms [13, 16, 40, 41], as observed in other studies that reported species-specific differences in pigmentation phenotypes [42–44].
Conclusions
Overall, our results show that the all-white phenotype in yaks is associated with the 14-bp deletion and SCs6 structure variants [17] (Fig. 6). However, the specific mechanisms of these two mutations governing KIT expression in all-white yaks remain unknown and should be addressed in future research. Despite these limitations, our findings suggest that the 14-bp fragment that was deleted in all-white yaks is conserved in mammals and plays a critical role in melanin accumulation across different species. Furthermore, our study highlights the importance of investigating diverse animal phenotypes to identify novel and conserved genetic regulators for key genes across different species.
Methods
Collection and whole-genome sequencing of study samples
In this study, the high-quality DNA was extracted from blood or tissue of 140 domestic yaks. All of these 140 DNA samples were sequenced to generate WGS short reads by BGISEQ-500platform. Besides, we also downloaded genomic data of 247 yaks from the NCBI database [17, 19, 24–28]. In total, WGS data were collected for 387 yaks, covering the geographical distribution and breeds of yaks.
Alignment of clean reads and identification of polymorphisms
After quality and adapter trimming of the raw reads with Cutadapt v. 2.8 [45], clean reads were aligned to our assembled wild yak reference genome [17] using the BWA-MEM algorithm [46] with the default parameters. PCR duplicates of BAM files were removed with the MarkDuplicates module in the Picard tool package v2.9.0 (http://broadinstitute.github.io/picard/). The BAM files were converted to the GVCF format files with the method HaplotypeCaller from GATK v3.8 (https://software.broadinstitute.org/gatk/), after which the genotype was called using the GenotypeGVCFs method. SNPs and indels were extracted by Selectvariants, and then the HardFilter was performed with the following parameters “QD < 2.0, FS > 60.0 || MQ < 40.0, MQRankSum < − 12.5, and ReadPosRankSum < − 8.0” for SNPs and “QD < 2.0, FS > 200.0, and ReadPosRankSum < − 20.0” for indels. The subsequent filtering steps were performed with VCFtools v0.1.16 [47] using the following thresholds: Only biallelic variants that met the conditions of minimum allele frequency (MAF) > 5%, call rate > 80%, and Hardy–Weinberg equilibrium (HWE) < 10−6 were retained.
Population genetics analysis
An individual-based neighbor-join (NJ) tree was constructed from the population scale SNPs using phylip software (https://evolution.genetics.washington.edu/phylip.html) and visualized using Figtree v1.4.4 (http://tree.bio.ed.ac.uk/software/figtree/). The population structure was analyzed by ADMIXTURE v1.23 [48] and the appropriate software for PCA is GCTA v.1.92.4 [49], respectively. A LD pruning step was performed with PopLDdecay (https://github.com/BGI-shenzhen/PopLDdecay).
Detection of selection signatures in white yaks
The GWAS was performed using PLINK v1.9b4.6 [50] with logistic regression model, PC1–PC3 as covariates, white phenotype was represented by “1,” and other coat color phenotypes were represented by “0.” The detailed parameters are as follows: “-pheno, –logistic, –covar.” The threshold line was calculated by dividing 0.01 by the total number of variants. FST and XP-CLR between black and white yak populations were calculated with a 50 kb sliding window and 25 kb step size by VCFtools v0.1.16 [47] and XP-CLR (https://github.com/hardingnj/xpclr), respectively. The GWAS, FST, and XP-CLR results were visualized using the qqman R package v0.1.4 (https://CRAN.R-project.org/package=qqman). The GWAS, FST, and XP-CLR overlap regions of each method were considered candidate signatures of selection.
Validation of the 14-bp deletion
For validation of the GWAS and selection sweep signals, we re-sampled blood or ear tissues for DNA extraction from 100 all-black yaks, 40 all-white yaks, and 20 cattle. Three pairs of primers were designed using the primer software to identify this indel, and only one pair of primers (F: CGGTGGCGGACCTTTATTGT; R: TCTTTCGAGGTGCTGATCCC) could be used for genotype detection in 160 individuals. The detailed PCR system and procedures are shown below. A touch-down PCR reaction was done in a total volume of 25 μL, containing 50 ng genomic DNA [51]. PCR thermocycling was done as follows: initial denaturation at 95 °C for 5 min; 18 cycles at 94 °C for 30 s, 68 °C for 30 s with a decrease of 1 °C per cycle, and 72 °C for 12 s; another 25 cycles at 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 12 s. PCR products were subjected to electrophoresis using a 3.5% agarose gel stained with ethidium bromide to identify the indel locus. Association of different indel genotypes with coat color of yaks were analyzed using least-squares mean (LSM) test model in SPSS software version 24.0 (IBM Corp., NY, USA).
KIT promoter plasmid construction.
The promoter sequence of the yak KIT was defined as 2 kb upstream of the transcription start site (TSS). The homology of the yak KIT promoter was evaluated based on the BLAT module in the UCSC database (http://genome.ucsc.edu/), and 12 representative species with high homology were finally selected in Additional file 2: Table S6. The transcription factor binding sites that may interact with the 14-bp variant were predicted based on the AnimalTFDB3.0 database [52], and prediction results are shown in Additional file 2: Table S7. According to the prediction results of transcription factor binding sites, five fragments of the KIT promoter region were amplified by PCR using different primer pairs. The large 2068-bp fragment spanning position − 2001 to + 83 was cloned into a PMD19-T vector (Takara, Tokyo, Japan). For luciferase reporter construction, the insert was released by NheI and HindIII digestion, and then subcloned into the luciferase reporter vector pGL3-Basic (Promega, Madison, WI, USA), which designated as pGL3-Pro5. The serial deletion vectors pGL3-Pro4 (− 1650 to + 67), pGL3-Pro3 (− 1150 to + 67), pGL3-Pro2 (− 533 to + 67), and pGL3-Pro1 (− 200 to + 67) were constructed using primers for NheI and Hind III-recognized sites, respectively. To test the function of the KIT indel, we also constructed a mutant vector (pGL3-Pro2-Mut, named MuT). The vector pRL-TK (Promega, Madison, WI, USA) was used as an internal reference in the luciferase reporter assay.
HEK293T cells culture
The HEK293T (Human Embryonic Kidney 293 T) cells were seeded at a density of 5 cells per well in 96-well plates containing 100 mL of growth medium and grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen) and double antibiotic (1% penicillin and streptomycin) at 37 °C in a humidified 5% CO2 atmosphere.
Transient transfection and luciferase reporter assay
Luciferase reporter constructs containing the KIT promoter plasmid (200 ng) and pGL3-(Pro1-5 and MuT) were temporarily transfected into human HEK293T cells using Lipofectamine 3000 (Invitrogen, Carlsbad, CA) based on the manufacturer’s protocol. To normalize the transfection efficiency, 40 ng of the pRL-TK Renilla transfection control plasmid was co-transfected into the cells. After 24 h of serum starvation, the cells were lysed, and the luciferase activity was measured using the Dual Luciferase Assay System (Promega, Madison, WI, USA) and the SpectraMax M5 reader (Shanpu, Shanghai, China). The luciferase signal from the all reporter constructs was calculated and normalized to the Renilla luciferase activity. The data were obtained from at least five independent experiments and presented as the means ± standard error (SE). Individual comparisons were assessed using Student’s t-test. The p < 0.05 was considered to indicate significant differences.
Validation of transcription factor binding sites
In order to further verify the reliability of the prediction results, we conducted the dual-luciferase assay experiment. The WT and MuT types of KIT promoter, respectively, were cloned into the pGL3-basic vector. The transcription factors’ CDS region from Pl-Pika was cloned into the pcDNA3.1 vector. HEK 293 T cells were cultured in DMEM medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, and were seeded into a 96-well plate at a density of 1.5 × 104 cells/well, and then transfected using Hieff TransTM Liposomal Transfection Reagent (Yeasen, Shanghai, China) as per the manufacturer’s instructions, along with PRL-TK used as an internal control. After 48 h, luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) on a SuPerMax 3100 Labsystems Multiskan (Shanpu, Shanghai, China).
Protein extraction, western blot experiments, and transcriptome analysis
Ear tissues of 5 black yaks and 5 all-white yaks aged between 1 and 2 years old were sampled for protein extraction. Approximately 20 mg of ear tissue was homogenized in RIPA lysis buffer (ZHHC Bio, Shaanxi, China) supplemented with PMSF and phosphatase inhibitors (DeeYee Bio, Shanghai, China). The homogenate was incubated on ice for 1 h, centrifuged at 12,000 g for 10 min at 4 °C, and the supernatant was collected. Protein concentration was quantified using a BCA assay kit (Proandy, Shaanxi, China), and samples were denatured with 5 × SDS-PAGE loading buffer (Solarbio, Beijing, China) at 95 °C for 10 min. Equal amounts of protein (20 μg) were separated by SDS-PAGE (4% stacking gel and 12.5% resolving gel) and transferred to a 0.45-μm PVDF membrane (Millipore, USA) via wet transfer (250 mA, 2 h). The membrane was blocked with rapid blocking buffer (Epizyme, Shanghai, China) for 30 min at room temperature, washed with TBST, and incubated overnight at 4 °C with primary antibodies: anti-c-Kit (Bioss, bs-10005R, 1:800) or anti-β-actin (Proteintech, 66,009–1-Ig, 1:20,000). After washing, membranes were probed with HRP-conjugated secondary antibodies: Goat Anti-Rabbit IgG (Aladdin, ab170144, 1:10,000) or Goat Anti-Mouse IgG (Aladdin, ab138040, 1:10,000) for 2 h at room temperature. Signals were detected using ECL Plus chemiluminescent substrate (Epizyme, Shanghai, China) and visualized with an Amersham ImageQuant 800 system (GE Healthcare, USA).
The FPKM (fragments per kilobase of exon model per million mapped fragments) values of SCF-cKIT signaling pathway genes was calculated from RNA-seq data of black (n = 6) and white yaks (n = 6) ear tissues [17]. Briefly, the clean reads from 12 ear tissues of yaks mapped to the yak reference genomes WY18 [17] (http://bovpan.lzu.edu.cn) using Hisat2 (v2.1.0) [53] and gene expression levels were calculated in terms of FPKM by StringTie (v2.0.6) [54] with default parameters. The DEGs were calculated using Cufflinks [55].
Functional validation of KIT promoter mutation in the mice.
All C57BL/6 mice used for experiments were bred and raised under the standard conditions in the investigator’s colony at the animal core facility Lanzhou University (EAF2022030). All experimental procedures were approved by Lanzhou University (SYXK- “Gan”−2021–0001) and performed according to the guidelines of the National Institutes of Health for laboratory animals (http://www.nc3rs.org.uk/arrive-guidelines).
To more accurately simulate the yak coat color variation, we created a new KIT knockin model (KI) in C57BL/6 J mice by CRISPR/Cas-mediated genome engineering. For KI model, the mouse promoter region and 5′UTR (943-bp upstream of the ATG start codon) were replaced by the KIT active promoter of wild-type (yKIT+, 1200 bp region upstream of the ATG start codon of KIT gene in all-black yaks) and mutant (yKITPro14Del, 14-bp deletion in all-white yaks) of yak, respectively. This editing approach was designed to ensure that the KIT gene promoter sequences in the two edited mouse models differ only by a 14-bp indel. To engineer the donor vector, homology arms were generated by PCR using BAC clone RP23-232H18 as a template. An equal mixture of two sgRNA was used to create ymKIT+ and ymKITPro14Del Cas9 RNP: targets sites gRNA1-5′ AGAAACAGATACACGAGCAA 3′ and gRNA2-5′ AGATACACGAGCAATGGCAA 3′. Before performing oocyte injections, we tested the ability of the Cas9 RNP to digest a PCR product that spans the target site. Then, Cas9 and gRNA will be co-injected into fertilized eggs with the donor vector for mice production. All of these mice were kept the same C57BL/6 J genetic background. All F0 mice were screened for mutations in KIT promoter by different PCR and sanger sequence. The 3 positive ymKIT+ F0 generation and 2 positive ymKITPro14Del F0 generation mice were identified. The positiveF F0 generation mice were mated with healthy WT C57BL/6 J mice to obtain a positive F1 generation mouse model that can be stably inherited. The resulting F1 offspring were intercrossed to generate the F2, and the resulting progeny were then genotyped by PCR (homozygous point mutation mice: ymKITPro14Del/Pro14Del, homozygous without mutation mice: ymKIT+/+).
We isolated total RNA from abdominal and back tissues of three ymKITPro14Del/Pro14Del mice and three ymKIT+/+ mice using the TRIzol method. The quantified RNA was then reversely transcribed into cDNA using Hifair®III 1st Strand cDNA Synthesis Kit (Yeasen Biotech Co., Ltd, Shanghai, China). Quantitative real-time PCR was performed using an ABI QuantStudio 5 Real-Time PCR Detection System (ABI, USA) with SYBR qPCR Master Mix (Vazyme, China) and primer sets. Primer sequences used for qRT-PCR analysis of KIT expression were as follows: F-5′-GAGTGTAAGGCCTCCAACGA-3′ and reverse primer, R-5′-GGCCTGGATTTGCTCTTTGTTG-3′. Each experiment was independently performed three times and the 2−ΔΔCT analysis was normalized relative to GAPDH.
Functional validation of KIT promoter mutation in the A375 and A875 cells.
Lentiviral cloning and production
To establish whether melanin production can be influenced by 14-bp mutation of KIT promoter in white yaks, we used two human melanoma cell lines: A375 and A875 cells, which both cells come from human skin tissue. The A375 and A875 cell lines demonstrate distinct advantages for investigating melanogenesis mechanisms, including well-established culture systems, clearly defined genetic backgrounds, and highly reproducible experimental results [56]. Briefly, based on the homology between the yak KIT promoter region (from TSS to − 1200 bp) and the human KIT promoter region, we anchored the position of the human KIT promoter corresponding to the 14-bp variation. The single-guide RNAs (sgRNAs) were designed in the promoter region of the human KIT gene using an online tool (https://zlab.bio/guide-design-resources). We employed lentiCRISPR v2 EGFP and lentiCRISPR v2 mCherry plasmids as vector constructs. Specifically, sgRNA1 (5′-GGGCTCCGGAGTCAAGAGCG-3′) and sgRNA2 (5′-GTTCGACGTGTTAATGCCGGG-3′), targeting the variation region of KIT promoter, were individually integrated into lentiCRISPR v2 EGFP and lentiCRISPR v2 mCherry. Additionally, sgRNA3 (5′-GCCCGCCCGAACGTGCTCGAG-3′) and sgRNA4 (5′-GTCTGGGGAGCACCTGGCAGG-3′), directed towards the upstream 165 bp of the KIT promoter variation, were also separately inserted into lentiCRISPR v2 EGFP and lentiCRISPR v2 mCherry. The standard procedures for viral production, as previously documented, were followed. Specifically, 5 million HEK293T cells were seeded per 10 cm dish, and when cellular confluence reached 80%, transfection was conducted using Lipofectamine 2000. The transfection mixture comprised 10 μg of the transfer vector, 2.5 μg of pMD2G, and 7.5 μg of psPAX2. Viral supernatant was harvested at 48 h and 72 h post-media change, filtered through a 0.45-μm membrane, and concentrated utilizing Lentivirus Concentration Solution (YEASEN #41101ES50).
Generation of knockout cell lines
A375 and A875 cells were passaged at an appropriate density in a 6-well tissue culture plate with 2 mL of DMEM media containing 10% FBS. After 24 h, harvested virus was introduced to the cells with polybrene and gently mixed to ensure uniform coverage. Subsequently, 48 h later, the virus-containing media was removed and replaced with normal DMEM media supplemented with 10% FBS. Detach cells by adding 0.5 mL of trypsin to each well. Transfer all of cells to 5 mL FACS tube. For the generation of homogeneous cell lines, fluorescence-activated cell sorting (FACS) was employed to isolate EGFP and mCherry-positive cells by flow cell sorter (BD FACSAria TM III). Co-infection cells with both types of viruses were carried out, and these cells were most likely to have undergone genetic editing. Finally, the cells infected with sgRNA1 and sgRNA2 were designated as hKITPro1/Pro1, infected with sgRNA3 and sgRNA4 were denoted as hKITPro2/Pro2, infected with empty lentivirus vector that does not contain sgRNA designated as the mock, hKITMk/Mk, and untreated wild-type cells denoted as hKITProWt/ProWt.
Measurement of melanin
hKITPro1/Pro1, hKITPro2/Pro2, hKITProWt/ProWt, and hKITMk/Mk A375 and A875 cells were seeded in a 6-well tissue culture plate. Subsequently, these cells were detached using trypsin and counted using the CKK8 method. The cell lysates were prepared by RIPA buffer (Solarbio) supplemented with protease inhibitors. To achieve a final concentration of 1 × 107 cells/mL, the amount of RIPA lysis buffer was adjusted based on the cell count results. The lysates were centrifuged at 15,000 rpm for 20 min at 4 °C. The precipitate was discarded, and the melanin content in the supernatant from different cell types was quantified using a melanin ELISA assay kit (Ruixin Biotech) on microplate reader (TECAN PLEX).
Immunofluorescence analysis
hKITPro1/Pro1 and wild-type hKITProWt/ProWt cells were fixed with 4% paraformaldehyde for 10 min. Fixed cells were incubated with rabbit polyclonal melanin antibodies (Absolute Biotech, Ab02814-230) and 2% bovine serum albumin (BSA) at 37 °C for 1 h. After washing in PBS, the cells were incubated with Cy3 labeled antirabbit IgG secondary antibodies and 2% BSA at 37 °C for 1 h. One microgram per milliliter DAPI was used to dye the nuclear DNA. Cy3 was detected with a main beam splitter at 550 nm and a 570 nm long pass emission filter. DAPI was detected with a main beam splitter at 359 nm and a 570 nm long pass emission filter. Stained cells were viewed under a confocal laser scanning microscope (Olympus, FV3000).
RNA sequencing and analysis A375 and A875 cells
Total RNA was extracted using TRIzol reagent (Invitrogen, USA) from hKITPro1/Pro1 and wild-type hKITProWt/ProWt A375 cells following the manufacturer’s protocols. For the raw RNA-seq data, the sequencing adapter reads with unknown nucleotides and the bases with low quality were removed with Picard tool package v2.9.0 (http://broadinstitute.github.io/picard/). The clean data were mapped to the human reference genome (GRCh38) using Hisat2 v2.1.0 [53] and transcripts and genes were quantified using StringTie v2.1.6 [54]. The DEGs were calculated using Cufflinks [55] and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis and GO terms were performed using Metascape [57].
Supplementary information
Additional file 1: Figs. S1–S9. Fig. S1 Population genetics structure of domestic and wild yaks. Fig. S2 The qqplot of GWAS of all-white and other coat color yaks. Fig. S3 Validation of 14-bp mutation by PCR and Sanger sequencing. Fig. S4 The expression of KIT protein in the skin tissue of black (n = 5) and all-white (n = 5) yaks was analyzed by western blot experiment. M: marker, W: all-white yak, B: black yak. Fig. S5 New KIT knockin (KI) model sequences were showed between C57BL/6 J mouse and yak by CRISPR/Cas-mediated genome engineering. Fig. S6 Genotyping validation strategy for positive all-black yak KIT active promoter knock-in edited F0 mice by different PCR and sanger sequence. Samples 24, 26, and 27 were identified positive and without random insertion by vector backbone PCR screening. Fig S7 Genotyping validation strategy for positive all-white yak KIT active promoter knock-in edited F0 mice by different PCR and sanger sequence. Samples 1 and 16 were identified positive and without random insertion by vector backbone PCR screening. Fig S8 Genotyping validation strategy for positive all-white yak KIT active promoter knock-in edited F2 mice by different PCR. Fig S9 The RNA-seq of hKITWt/Wt and hKITPro14Del/Pro14Del A375 cells.
Additional file 2: Tables S1–S7. Table S1 List of samples used for novel whole-genome sequencing. Table S2 Overview of novel whole-genome sequencing data. Table S3 Information of public whole-genome sequencing data. Table S4 Genotypes with a 14-bp mutation in all yaks. Table S5 Verification of the 14-bp mutation. Table S6 Homology alignment of the yak KIT promoter sequence with other species. Table S7 Predicting potential transcription factor binding sites for 14-bp indel based on AnimalTFDB3.0 database.
Additional file 3: Tables S1–S4. Supporting data related to the experiments (Figs. 3B, C, D, 4D, 5B and C).
Acknowledgements
We thank Big Data Computing Platform for Western Ecological Environment and Regional Development, and the Supercomputing Center of Lanzhou University. We thank Dr. X. Luo from the Kunming Institute of Zoology, Chinese Academy of Sciences for his valuable suggestions and insightful comments throughout this study.
Abbreviations
- GWAS
Genome-wide association study
- SNPs
Single nucleotide polymorphisms
- SVs
Structural variations
- WGS
Whole-genome sequencing
- QTP
Qinghai-Tibetan Plateau
- NJ
Neighbor-joining
- PCA
Principal component analysis
- LD
Linkage disequilibrium
- FST
Fixation index
- MAF
Minimum allele frequency
- HWE
Hardy-Weinberg equilibrium
- XP-EHH
The cross-population composite likelihood ratio test
- FPKM
Fragments per kilobase of exon model per million mapped fragments
- TSS
Transcription start site
- LSM
Least-squares mean
- BSA
Bovine serum albumin
- DEGs
Differentially expressed genes
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- GO
Gene Ontology
Author contribution
J.Q.L., L.Z.F., B.W.L., and M.H.K. conceived the project and designed the research. X.F.L., T.C.W., and Z.Y.Z. performed the majority of the data analysis. X.F.L., C.C.D., W.Y.L., Y.B.Y., X.Y.W., and Q.Q. collected materials. B.W.L., X.F.L., and Z.M.N. designed experiments for the 14-bp mutation of KIT gene. X.F.L., and T.C.W. raised mice. X.F.L., and B.W.L. wrote original the manuscript. J.Q.L., B.W.L., L.Z.F., and M.H.K., Y.Z.Y., K.X.L., JA. L., and P.Y. revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grants U23A20227, U22A20447 and 32471557), Second Tibetan Plateau Scientific Expedition and Research Program (Grant no. 2019QZKK0502), Agricultural Science and Technology Innovation Program (Grant no. 25-LZIHPS-01), and National Key Research and Development Programs (Grant no. 2021YFD1200901).
Data availability
The new raw reads for all WGS data from the yaks have been deposited at the Sequence Read Archive under project number PRJNA1069901, the SRR number can be found in the Additional file 2: Table S2. The publicly available WGS data were downloaded according to SRR number in the Additional file 2: Table S3 [17, 19, 24–28]. The raw RNA-seq data from A375 cells have been deposited at the Sequence Read Archive under project number PRJNA1281012. Supporting data related to the experiments can be found in Supplementary file. All data generated or analysed during this study are included in this published article and its supplementary information files and publicly available repositories.
Declarations
Ethics approval
Blood samples and ear tissues of yaks were collected during routine veterinary treatments with the logistical support and agreement of relevant agricultural institutions.
Consent to participate
Animal collection and utility protocols were approved by the Animal Ethics Committee of College of Ecology, Lanzhou University (ethical approval form no. EAF2022030).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xinfeng Liu, Tongcheng Wang, and Zeyu Zheng contributed equally to this work.
Contributor Information
Minghui Kang, Email: kangminghui0106@gmail.com.
Bowen Li, Email: libowen@lzu.edu.cn.
Lingzhao Fang, Email: lingzhao.fang@qgg.au.dk.
Jianquan Liu, Email: liujq@lzu.edu.cn.
References
- 1.Cuthill IC, Allen WL, Arbuckle K, Caspers B, Chaplin G, Hauber ME, et al. The biology of color. Science. 2017; 357(6350):eaan0221357. [DOI] [PubMed]
- 2.Protas ME, Patel NH. Evolution of coloration patterns. Annu Rev Cell Dev Biol. 2008;24:425–46. [DOI] [PubMed] [Google Scholar]
- 3.Mallick CB, Iliescu FM, Chaubey G, Goto R, Ho SYW, Gallego I, et al. The light skin allele of SLC24A5 in South Asians and Europeans shares identity by descent. PLoS Genet. 2013;9(11): e1003912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruders R, van Hollebeke H, Osborne EJ, Kronenberg Z, Maclary E, Yandell M, et al. A copy number variant is associated with a spectrum of pigmentation patterns in the rock pigeon (Columba livia). PLoS Genet. 2020;16(5): e1008274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cieslak M, Reissmann M, Hofreiter M, Ludwig A. Colours of domestication. Biol Rev. 2011;86(4):885–99. [DOI] [PubMed] [Google Scholar]
- 6.Durkin K, Coppieters W, Drögüller C, Ahariz N, Cambisano N, Druet T, et al. Serial translocation by means of circular intermediates underlies colour sidedness in cattle. Nature. 2012;482(7383):81–4. [DOI] [PubMed] [Google Scholar]
- 7.Haase B, Brooks SA, Schlumbaum A, Azor PJ, Bailey E, Alaeddine F, et al. Allelic heterogeneity at the equine KIT locus in dominant white (W) horses. PLoS Genet. 2007;3(11):e195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henkel J, Saif R, Jagannathan V, Schmocker C, Zeindler F, Bangerter E, et al. Selection signatures in goats reveal copy number variants underlying breed-defining coat color phenotypes. PLoS Genet. 2019;5(12):e1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang D, Zhao P, Si J, Fang L, Pairo-Castineira E, Hu X, et al. Genomic analysis revealed a convergent evolution of LINE-1 in coat color: a case study in water buffaloes (Bubalus bubalis). Mol Biol Evol. 2021;38(3):1122–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Norris BJ, Whan VA. A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome Res. 2008;18(8):1282–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trigo BB, Utsunomiya ATH, Fortunato AAAD, Milanesi M, Torrecilha RBP, Lamb H, et al. Variants at the ASIP locus contribute to coat color darkening in Nellore cattle. Genet Sel Evol. 2021;53(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Li H, Guo Y, Huang J, Sun Y, Min J, et al. Donkey genomes provide new insights into domestication and selection for coat color. Nat Commun. 2020;11(1):6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajpai VK, Swigut T, Mohammed J, Naqvi S, Arreola M, Tycko J, et al. A genome-wide genetic screen uncovers determinants of human pigmentation. Science. 2023;381(6658):eade6289. [DOI] [PMC free article] [PubMed]
- 14.Wang Q, Wang Z, Wang Y, Qi Z, Bai D, Wang C, et al. A gain-of-function TPC2 variant R210C increases affinity to PI(3,5)P2 and causes lysosome acidi fi cation and hypopigmentation. Nat Commun. 2023;14(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cable J, Jaenisch DHR, Steel KE. Effects of mutations at the W locus (c-kit) on inner ear pigmentation and function in the mouse. Pigment Cell Res. 1994;7(1):17–32. [DOI] [PubMed] [Google Scholar]
- 16.Chabot B, Stephenson DA, Chapman VM, Besmer P, Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988;335(6185):88–9. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Liu W, Lenstra JA, Zheng Z, Wu X, Yang J, et al. Evolutionary origin of genomic structural variations in domestic yaks. Nat Commun. 2023;14(1):5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang K, Hu Q, Ma H, Wang L, Yang Y, Luo W, et al. Genome-wide variation within and between wild and domestic yak. Mol Ecol Resour. 2014;14(4):794–801. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Q, Wang L, Wang K, Yang Y, Ma T, Wang Z, et al. Yak whole-genome resequencing reveals domestication signatures and prehistoric population expansions. Nat Commun. 2015;6:10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang MQ, Xu X, Luo SJ. The genetics of brown coat color and white spotting in domestic yaks (Bos grunniens). Anim Genet. 2014;45(5):652–9. [DOI] [PubMed] [Google Scholar]
- 21.Barsh GS. The genetics of pigmentation: from fancy genes to complex traits. Trends Genet. 1996;12(8):299–305. [DOI] [PubMed] [Google Scholar]
- 22.Greenhill ER, Rocco A, Vibert L, Nikaido M, Kelsh RN. An iterative genetic and dynamical modelling approach identifies novel features of the gene regulatory network underlying melanocyte development. PLoS Genet. 2011;7(9): e1002265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackson IJ. Homologous pigmentation mutations in human, mouse and other model organisms. Hum Mol Genet. 1997;6(10):1613–24. [DOI] [PubMed] [Google Scholar]
- 24.Chai Z xin, Xin J wei, Zhang C fu, Dawayangla, Luosang, Zhang Q, et al. Whole-genome resequencing provides insights into the evolution and divergence of the native domestic yaks of the Qinghai–Tibet Plateau. BMC Evol Biol. 2020;20(1):137. [DOI] [PMC free article] [PubMed]
- 25.Liang C, Wang L, Wu X, Wang K, Ding X, Wang M, et al. Genome-wide association study identifies loci for the polled phenotype in yak. PLoS ONE. 2016;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medugorac I, Graf A, Grohs C, Rothammer S, Zagdsuren Y, Gladyr E, et al. Whole-genome analysis of introgressive hybridization and characterization of the bovine legacy of Mongolian yaks. Nat Genet. 2017;49:470–5. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Liu W, Liu X, Du X, Zhang K, Zhang Y, et al. Structural variants selected during yak domestication inferred from Long-read whole-genome sequencing. Mol Biol Evol. 2021;38(9):3676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Wang C, Xu H, Xia X, Luo X, Li K, et al. Genomic analysis reveals a KIT-related chromosomal translocation associated with the white coat phenotype in yak. J Anim Breed Genet. 2023;140(3):330–42. [DOI] [PubMed] [Google Scholar]
- 29.Grassot J, Gouy M, Perrière G, Mouchiroud G. Origin and molecular evolution of receptor tyrosine kinases with immunoglobulin-like domains. Mol Biol Evol. 2006;23(6):1232–41. [DOI] [PubMed] [Google Scholar]
- 30.Id JH, Saif R, Jagannathan V, Schmocker C, Zeindler F, Bangerter E, et al. Selection signatures in goats reveal copy number variants underlying breed-defining coat color phenotypes. PLoS Genet. 2019;15(12): e1008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu Q, Luo Y, Chao Z, Zhang J, Liu X, Tu D, et al. Discovery of potential candidate genes for coat colour in Wuzhishan pigs by integrating SNPs and mRNA expression analysis. Animals. 2024;14(23):3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunisada T. Review: melanocyte migration and survival controlled by SCF/c-kit expression. J Investig Dermatology Symp Proc. 2001;6(1):1–5. [DOI] [PubMed] [Google Scholar]
- 33.Illa SK, Mukherjee S, Nath S, Mukherjee A. Genome-wide scanning for signatures of selection revealed the putative genomic regions and candidate genes controlling milk composition and coat color traits in Sahiwal cattle. Front Genet. 2021;12: 699422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabirova E, Ryzhkova A, Lukyanchikova V, Khabarova A, Korablev A, Shnaider T, et al. TAD border deletion at the Kit locus causes tissue-specific ectopic activation of a neighboring gene. Nat Commun. 2024;15(1):4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang B, Chen W, Wang Z, Pang W, Luo M, Wang S, et al. Comparative genomics reveals the hybrid origin of a macaque group. Sci Adv. 2023;9(22):eadd3580. [DOI] [PMC free article] [PubMed]
- 36.Rosenbloom KR, Sloan CA, Malladi VS, Dreszer TR, Learned K, Kirkup VM, et al. ENCODE data in the UCSC Genome Browser: year 5 update (Database issue). Nucleic Acids Res. 2013; 41:D56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maurus K, Hufnagel A, Geiger F, Graf S, Berking C, Heinemann A, et al. The AP-1 transcription factor FOSL1 causes melanocyte reprogramming and transformation. Oncogene. 2017;36(36):5110–21. [DOI] [PubMed] [Google Scholar]
- 38.Zehavi L, Schayek H, Jacob-hirsch J, Sidi Y, Leibowitz-amit R. MiR-377 targets E2F3 and alters the NF-kB signaling pathway through MAP3K7 in malignant melanoma. Mol Cancer. 2015;14:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Halaban BR, Cheng E, Smicun Y, Germino J. Deregulated E2F transcriptional activity in autonomously growing melanoma cells. J Exp Med. 2000;191(6):1005–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loveland KL, Schlatt S. Stem cell factor and c-kit in the mammalian testis: lessons originating from mother nature’s gene knockouts. J Endocrinol. 1997;153(3):337–44. [DOI] [PubMed] [Google Scholar]
- 41.Geissler EN, Ryan MA, Housman DE. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988;55(1):185–92. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-elfring A, Sabin CE, Iouchmanov AL, Hendry AP, Menke DB, Barrett RDH, et al. Report piebaldism and chromatophore development in reptiles are linked to the tfec gene. Curr Biol. 2023;33(4):755–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan D, Sun S, Song L, Zhao P, Nie Y, Huang X. Taking a color photo: a homozygous 25-bp deletion in Bace2 may cause brown-and-white coat. Proc Natl Acad Sci. 2024;121(11): e2317430121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang G, Zhang Y, Zhang W, Wei F. Genetic mechanisms of animal camouflage: an interdisciplinary perspective. Trends Genet. 2024;40(7):613–20. [DOI] [PubMed] [Google Scholar]
- 45.Saeidipour B, Bakhshi S. The relationship between organizational culture and knowledge management, & their simultaneous effects on customer relation management. Adv Environ Biol. 2013;7(10):2803–9. [Google Scholar]
- 46.Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, et al. The variant call format and VCFtools. Bioinformatics. 2011;27(15):2156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang Z, Zhang S, He L, Zhu H, Wang Z, Yan H, et al. A 14-bp functional deletion within the CMTM2 gene is significantly associated with litter size in goat. Theriogenology. 2019;139:49–57. [DOI] [PubMed] [Google Scholar]
- 52.Hu H, Miao YR, Jia LH, Yu QY, Zhang Q, Guo AY. AnimalTFDB 3.0: a comprehensive resource for annotation and prediction of animal transcription factors. Nucleic Acids Res. 2019; 47(D1):D33-D38. [DOI] [PMC free article] [PubMed]
- 53.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pertea M, Pertea GM, Antonescu CM, Chang TC, Mendell JT, Salzberg SL. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33(3):290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu M, Ma X, Wang Y, Yu Z, Zheng X, Dai H, et al. Developing a prognostic model for skin melanoma based on the persistent tumor mutation burden and determining IL17REL as a therapeutic target. J Cancer Res Clin Oncol. 2024;150(6):313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Zhou B, Pache L, Chang M, Khodabakhshi AH, Tanaseichuk O, et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat Commun. 2019;10(1):1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figs. S1–S9. Fig. S1 Population genetics structure of domestic and wild yaks. Fig. S2 The qqplot of GWAS of all-white and other coat color yaks. Fig. S3 Validation of 14-bp mutation by PCR and Sanger sequencing. Fig. S4 The expression of KIT protein in the skin tissue of black (n = 5) and all-white (n = 5) yaks was analyzed by western blot experiment. M: marker, W: all-white yak, B: black yak. Fig. S5 New KIT knockin (KI) model sequences were showed between C57BL/6 J mouse and yak by CRISPR/Cas-mediated genome engineering. Fig. S6 Genotyping validation strategy for positive all-black yak KIT active promoter knock-in edited F0 mice by different PCR and sanger sequence. Samples 24, 26, and 27 were identified positive and without random insertion by vector backbone PCR screening. Fig S7 Genotyping validation strategy for positive all-white yak KIT active promoter knock-in edited F0 mice by different PCR and sanger sequence. Samples 1 and 16 were identified positive and without random insertion by vector backbone PCR screening. Fig S8 Genotyping validation strategy for positive all-white yak KIT active promoter knock-in edited F2 mice by different PCR. Fig S9 The RNA-seq of hKITWt/Wt and hKITPro14Del/Pro14Del A375 cells.
Additional file 2: Tables S1–S7. Table S1 List of samples used for novel whole-genome sequencing. Table S2 Overview of novel whole-genome sequencing data. Table S3 Information of public whole-genome sequencing data. Table S4 Genotypes with a 14-bp mutation in all yaks. Table S5 Verification of the 14-bp mutation. Table S6 Homology alignment of the yak KIT promoter sequence with other species. Table S7 Predicting potential transcription factor binding sites for 14-bp indel based on AnimalTFDB3.0 database.
Additional file 3: Tables S1–S4. Supporting data related to the experiments (Figs. 3B, C, D, 4D, 5B and C).
Data Availability Statement
The new raw reads for all WGS data from the yaks have been deposited at the Sequence Read Archive under project number PRJNA1069901, the SRR number can be found in the Additional file 2: Table S2. The publicly available WGS data were downloaded according to SRR number in the Additional file 2: Table S3 [17, 19, 24–28]. The raw RNA-seq data from A375 cells have been deposited at the Sequence Read Archive under project number PRJNA1281012. Supporting data related to the experiments can be found in Supplementary file. All data generated or analysed during this study are included in this published article and its supplementary information files and publicly available repositories.