ABSTRACT
Two mollicute species belonging to the Mesoplasma and Spiroplasma genera have been detected in several species of fungus-growing ants using molecular methods. However, their ecological roles remain largely inferred from metagenomic data. To better understand their diversity and specialization, we cultured both of these Mesoplasma and Spiroplasma symbionts from the fungus-growing ant Trachymyrmex septentrionalis, providing the first isolated mollicutes from any fungus-growing ant species. The genomes of our isolates and related metagenome-assembled genomes (MAGs) from T. septentrionalis fungus gardens comprise two unique phylogenetic lineages compared to previously described Mesoplasma and Spiroplasma species, and from related MAGs previously sequenced from the leaf-cutting ant Acromyrmex echinatior. This suggests that the T. septentrionalis symbionts comprise undescribed species that can exclude each other from a niche that is largely shared between them. Mesoplasma genomes and MAGs also demonstrate regional specificity with their T. septentrionalis ant hosts. Both Mesoplasma and Spiroplasma strains from T. septentrionalis can catabolize glucose and fructose; both sugars are common in the ant’s diet. Similarly, both these Mesoplasma and Spiroplasma can catabolize arginine, but only Mesoplasma can catabolize N-acetylglucosamine; both could produce ammonia for the ants or fungus garden. Based on our genomic and phenotypic analyses, we describe these T. septentrionalis symbionts as Mesoplasma whartonense sp. nov. and Spiroplasma attinicola sp. nov., providing insight into their genomic and phenotypic diversity and cultures to facilitate future studies of how these common but poorly understood members of the fungus-growing ant symbiosis separately colonize different ant colonies despite having highly overlapping niches.
IMPORTANCE
Fungus-growing ants partner with multiple microbial symbionts to obtain food and remain free from disease. Of these symbionts, those inhabiting the ant gut remain the least understood and are known only from environmental surveys. Such surveys can infer potential functions of gut symbionts, but cultures are required to experimentally validate these hypotheses. Here, we describe the first cultures of the ant gut symbionts of the fungus-growing ant Trachymyrmex septentrionalis, using comparative genomics and phenotypic experiments to describe them as two novel species: Mesoplasma whartonense sp. nov. and Spiroplasma attinicola sp. nov. This genomic analysis suggests that these species are highly specialized to T. septentrionalis and are distinct from related environmental data generated from the related ant species Acromyrmex echinatior, implying substantial host specificity. Our phenotypic experiments and genomic reconstructions highlight the highly overlapping niches and likely costs and benefits of these symbionts to their ant host, setting the stage for further experimentation.
KEYWORDS: fungus-growing ants, symbiosis, Spiroplasma, Mesoplasma, taxonomy, comparative genomics, host specialization
INTRODUCTION
Symbiosis is defined as two or more organisms living together in close association (1). Bacterial symbionts have many different types of relationships with such hosts (e.g., pathogenic, commensal, or mutualistic) (2). These relationships can result in tissue damage (3), manipulation of reproduction (4), nutrient provision (5), or protection (6) of the host. Symbionts can be transmitted by vertical transmission (from mother to offspring), horizontal transmission (from the environment or an individual of the same generation), or a mixture of both (7). Bacterial symbionts often undergo genome reduction, evolving to have smaller genomes by eliminating genes that are not needed by these symbionts (8, 9). Reduced genomes can prevent such symbionts from making amino acids or replicating outside of their host, thus ultimately tightening the bacteria-host symbiosis.
One well-known class of genome-reduced bacterial symbionts is the Mollicutes. Mollicutes are rapidly evolving via high mutation rates and frequent genome rearrangements and have reduced genome sizes (580–2,200 kbp) and low %GC contents compared to most other bacterial classes (10). They lack cell walls and stain Gram-negative despite evolving from a Gram-positive lineage. Mollicutes are strictly host-associated and include the well-studied genera Mycoplasma, Mesoplasma, Entomoplasma, Spiroplasma, and many others (11). Mesoplasma species grow in serum-free media, do not require cholesterol, and commonly ferment glucose. They have been isolated from many different plants and insects but have no known pathogenicity or benefit toward these hosts (11). Spiroplasma species are better studied and are associated with diverse plants, insects, crustaceans, and mammals (11, 12). They can ferment glucose and require serum but not sterols for growth in culture. Some Spiroplasma species are pathogens that infect citrus and corn plants, causing citrus stubborn disease and corn stunt disease, respectively (13, 14). Others manipulate sex ratios in Drosophila or cause tissue damage in mosquitos (15, 16). Spiroplasma can also be mutualistic, as in Drosophila neotestacea where they defend against nematode infections (17).
Recent work has described the common presence of Mollicute bacteria belonging to the order Entomoplasmatales (which contains both Mesoplasma and Spiroplasma) in fungus-growing ants (Formicidae: tribe Attini). These ants evolved 55 to 65 million years ago in South America (18–20) and today include 19 genera and approximately 250 known species that range from northern United States to southern Argentina (20–24). Many of these ants farm a coevolved fungus from the genus Leucoagaricus as their obligate food source (18, 22, 23). The ants forage materials for this fungus, including leaves, dried plant material, and insect frass (25), and the fungus produces hyphal swellings called gongylidia that the ants eat (26, 27). Except for the defensive actinobacterium Pseudonocardia and the fungal pathogen Escovopsis (28, 29), other members of the symbiosis remain poorly studied.
Recent environmental 16S rRNA gene sequencing studies of ant guts have revealed the frequent and abundant presence of Mesoplasma and Spiroplasma in several attine ant genera (30–34). An amplicon sequencing variant (ASV; a unique sequence variant obtained by clustering environmental 16S rRNA gene sequencing data sets), named EntAcro1 and phylogenetically related to the genus Mesoplasma, was detected in worker ants, larvae, and pupae of Atta cephalotes and Acromyrmex echinatior (32), and in Atta colombica, Atta sexdens, Acromyrmex octospinosus, and Paratrachymyrmex (previously Trachymyrmex) cornetzi worker ants (33, 34). A second ASV, named EntAcro10 and phylogenetically related to the genus Spiroplasma, was detected in worker ants and pupae from At. cephalotes and in worker ants from At. colombica, At. sexdens, Ac. octospinosus, P. cornetzi, Sericomyrmex amabilis, Mycetomoellerius (previously Trachymyrmex) zeteki, Mycocepurus smithii, Cyphomyrmex longiscapus, and Apterostigma dentigerum. These ASVs are distinct from but related to separate lineages of Mollicute bacteria found on non-fungus-growing ants (35–37).
EntAcro1 was more abundant than EntAcro10 in At. cephalotes, At. colombica, At. sexdens. Ac. octospinosus, and P. cornetzi, and the opposite was true for S. amabilis and M. zeteki; these differences were hypothesized to be due to differences in foraging between ant species (33, 34). The abundances of EntAcro1 and EntAcro10 differed between ants, and not every ant in a colony was colonized by either species; many (34/84) colonies also lacked either species (34). When present, EntAcro1 and EntAcro10 almost never co-occurred within a colony; either EntAcro1 or EntAcro10 was present in 48 of 50 sampled colonies, but both were present in only 2 of 50 (34). 16S rRNA gene ASVs that were related to both of these Mesoplasma and Spiroplasma lineages were also detected in most Trachymyrmex septentrionalis ant colonies (30, 38). Once again, only one of Mesoplasma or Spiroplasma was present in individual T. septentrionalis colonies (38 colonies sampled), and their abundances varied between individual ants (38). The abundances of these ASVs also varied between ant castes and following adaptation of ant colonies to the laboratory environment (38). Although these studies demonstrate the symbiotic relationship between Mollicutes and different genera of fungus-growing ants and the strong co-exclusion of different Mollicute symbionts, these 16S rRNA gene data sets lack the phylogenetic resolution necessary to provide information concerning the specificity and evolutionary history of Mesoplasma and Spiroplasma in the fungus-growing ant symbiosis and are unable to reveal the ecological consequences of their relationships with their ant hosts.
It is unknown how mollicute bacteria are acquired by their ant hosts. Potentially, ants might acquire them from a natural vector (e.g., plants) or from another ant colony (39). Neither Mesoplasma nor Spiroplasma is likely to be transferred vertically between ant generations, because fluorescence in situ hybridization imaging showed that EntAcro1 and EntAcro10 colonized the fat bodies, midgut, Malpighian tubules, ileum, and rectum of At. cephalotes, At. colombica, Ac. echinatior, Ac. octospinosus, M. zeteki, and S. amabilis, the fat bodies and ileum of P. cornetzi, and the midgut, ileum, and rectum of C. longiscapus (32, 33). The common tissue distribution within ant guts suggests the potential for niche overlap between EntAcro1 and EntAcro10. Neither EntAcro1 nor EntAcro10 was found in the reproductive organs of any of these ants, or either in or on ant eggs. Instead, mollicute bacteria are most likely socially transmitted from worker to worker, as demonstrated by the colonization of Ac. echinatior callows (freshly emerged ants) only when they were allowed to interact with older, colonized worker ants (32).
Metagenomic sequencing can provide a semi-complete genome of microbes that are difficult to culture in isolation. Metagenome-assembled genomes (MAGs) can be computationally reconstructed from such data to generate hypotheses about a microbe’s potential function, but microbial isolates are needed to test such hypotheses. Although MAGs provide more functional insight than the 16S rRNA gene, a MAG may not comprise a complete bacterial genome (40, 41), and the expense of metagenomic sequencing can limit the number of unique MAGs available to study an individual bacterial species.
Metagenome-assembled genomes have been generated from a single Ac. echinatior metagenome for both Mesoplasma EntAcro1 and Spiroplasma EntAcro10 (42). The MAGs for both Mesoplasma EntAcro1 and Spiroplasma EntAcro10 included genes predicted to decompose excess arginine into ammonium, which were hypothesized to be provided to the fungus garden via ant fecal droplets (42). Mesoplasma EntAcro1 was also predicted to catabolize citrate, which might be acquired from fruit, leaves, or other plant material foraged by the ants, transforming it into acetate that might then be secreted by the bacterium and imported by the ants. Based on these data, Sapountzis et al. (42) hypothesized that EntAcro1 and EntAcro10 are nutritional mutualists that use these citrate and arginine catabolic pathways to provide nutrients for the ants and fungus garden. However, these hypotheses have yet to be experimentally tested, especially using cultured bacteria.
The negative correlation between EntAcro1-like (Mesoplasma) and EntAcro10-like (Spiroplasma) symbionts of fungus-growing ants, their shared tissue distributions, and their similar metabolic reconstructions suggest that their niches overlap significantly. Niche theory predicts that organisms whose niches overlap will exclude each other (43). Such exclusion can be due to competition via direct antagonism between taxa for a single niche (e.g., reference 44). Symbionts might also separately exist in the same niche if each provides a different context-dependent benefit to the host (i.e., niche overlap is partial and symbiosis is costly or has imperfect transmission [45, 46]). Other ecological dynamics within host and symbiont populations determine when species with overlapping niches will be present or absent (47). The mechanism(s) that cause niche exclusion remain cryptic for most host-microbe symbioses.
To better understand the mechanisms responsible for symbiont co-exclusion and overcome the limitations of the sequence-based techniques that have thus far been used to characterize mollicute symbionts of fungus-growing ants, we isolated several Mesoplasma and Spiroplasma symbionts from T. septentrionalis ant guts. Our genomic comparisons using these isolates and related MAGs assembled from multiple T. septentrionalis fungus garden metagenomes (48) show how T. septentrionalis symbiont genomes differ in size, gene content, and phylogenetic position from the EntAcro1 and EntAcro10 MAGs isolated from Ac. echinatior, suggesting that distinct phylogenetic lineages of Mollicutes are specific to different fungus-growing ant hosts. We also discovered that Mesoplasma symbionts from T. septentrionalis form phylogenetic clusters that are each unique to specific geographic regions. The results of our phenotypic tests partially validated the predictions that we made based on our gene annotations and confirmed the high niche overlap between both symbionts. Together, these results reveal a highly specific relationship between T. septentrionalis ants and their mollicute symbionts and set the stage for future experiments that move beyond exclusively culture-independent approaches to experimentally confirm the effects of these Mollicutes on the other members of the fungus-growing ant symbiosis and the mechanisms by which they co-exclude each other.
MATERIALS AND METHODS
Sample collection
Trachymyrmex septentrionalis colonies were collected from New Jersey, North Carolina, Georgia, and Florida between 2013 and 2020 (Table S1). Permits for collection include New Jersey Department of Environmental Protection Division of Parks and Forestry State Park Service unnumbered letters of authorization, North Carolina Division of Parks and Recreation scientific collection and research permit 2015_0030, Georgia Department of Natural Resources State Parks and Historic Sites scientific research and collection permit 032015, and Florida Department of Agriculture and Consumer Services unnumbered letters of authorization. Directly after colony collections, fungus garden samples were stored in 20% dimethyl sulfoxide, 250 mM disodium EDTA, and saturated sodium chloride on dry ice (49). The remainder of these ant colonies was transported to the University of Connecticut (US Department of Agriculture permit P526P-14-00684) and maintained in a temperature-controlled room. Similar to reference 50, colonies were housed in 6 3/4 in × 4 13/16 in × 2 3/8 in plastic boxes lined with plaster of Paris, watered biweekly to maintain near 100% humidity, and provided sterile cornmeal ad libitum as food for the fungus garden. Frozen fungus garden samples were stored at −80°C until DNA extraction.
Isolation and growth
Live ants were taken from the lab colonies, submerged in ethanol for 10 s, then submerged again in phosphate buffered saline (PBS) for 10 s; washes were repeated three times per ant. The gut (hindgut, midgut, and rectum) was dissected from each ant abdomen using Swiss Style Superfine Tip 112 mm forceps (BioQuip, Rancho Dominguez, CA), placed into a 2 mL screw top tube filled with 1 mL PBS, and ground using a small sterile pestle. Cultures were grown in SP-4 modified from reference 51 as follows: Difco PPLO Broth Base (without crystal violet) replaced the mycoplasma broth base, and both 20 mg of phenol red and 630 mL of water were added to the basal medium before autoclaving. The supplements used were 200 mL of fetal bovine serum, 25 mL of CMRL 1066, 5 mL of 1 N NaOH, and 5 mL of a 40% L-arginine solution (replacing α-ketoglutaric acid). No penicillin was added at this stage. These supplements were sterilized using a 0.45 µm filter before being added to the autoclaved basal medium. One hundred microliters of homogenized ant gut solution was added to 15 mL Falcon tubes containing either (i) 4 mL of modified SP-4 broth, (ii) 2 mL of modified SP-4, or (iii) 4 mL modified SP-4 containing 40 mg/mL penicillin (Fisher Scientific, Hampton, NH). Tubes 1 and 3 were incubated at 30°C. Tube 2 was incubated at 37°C for 1 hour, filtered using a 0.45 µM 30 mm polypropylene membrane syringe filter into 2 mL of fresh modified SP-4 resulting in 4 mL final volume, and incubated at 30°C. Cultures were removed from the incubator after they changed color from red to orange/yellow or became turbid, typically within 5 to 14 days. Cultures that remained red with no turbidity were removed after 4 weeks. We purchased Mesoplasma lactucae 831-C4 (ATCC 49193) and Spiroplasma platyhelix PALS-1 (ATCC 51748) as reference strains. The freeze-dried pellets received from the ATCC were added to SP-4 broth and incubated at 30°C until they changed color from red to orange/yellow. We described the sequencing of S. platyhelix ATCC 51748 previously (52).
DNA extraction and sequencing
To choose cultures for purification, we performed community 16S rRNA sequencing using 1 mL of broth from each culture. DNA was extracted using the Epicentre MasterPure Complete DNA and RNA Purification Kit (Lucigen, Middleton WI) following the DNA purification protocol for cell samples and quantified using the Qubit dsDNA high-sensitivity assay protocol and a Qubit 3.0 fluorimeter (Invitrogen, Carlsbad, CA). The presence of bacterial DNA was confirmed using PCR. Ten nanograms of template DNA was added to 5 µL GoTaq Green Reaction Mix Buffer (Promega, Madison, WI), 1.25 units of GoTaq DNA Polymerase (Promega, Madison, WI), 10 µmol of primers 515F and 806R (targeting the bacterial 16S rRNA gene V4 region) (53), and 300 ng bovine serum albumin (BSA; New England BioLabs Inc. Ipswitch, MA), to which nuclease-free H2O was added to a volume of 25 µL. Thermocycler conditions (BioRad, Hercules, CA) were 3 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 50°C, and 60 s at 72°C, followed by a 5 min cycle at 72°C and then an indefinite hold at 4°C. Gel electrophoresis confirmed the expected band size of 300 bp–350 bp.
Samples were prepared for 16S rRNA community amplicon sequencing at the University of Connecticut Microbial Analysis, Resources and Services (MARS) facility. Approximately 30 ng of DNA from each sample was added to a 96-well plate containing 10 µmol each of the forward and reverse Illumina-barcoded versions of primers 515F and 806R, 5 µL AccuPrime buffer (Invitrogen, Carlsbad, CA), 50 mM MgSO4 (Invitrogen, Carlsbad, CA), 300 ng/µL BSA (New England BioLabs Inc. Ipswitch, MA), a 1 µmol spike-in of non-barcoded primers 515F and 806R, and 1 unit AccuPrime polymerase (Invitrogen, Carlsbad, CA), to which nuclease-free H2O was added to a volume of 50 µL. Reaction mixes were separated into triplicate reactions (each with a volume of 16.7 µL) in a 384-well plate using an epMotion 5075 liquid handling robot (Eppendorf, Hamburg, Germany). This 384-well plate was transferred to a thermocycler (Eppendorf, Hamburg, Germany) that used the following conditions: 2 min at 95°C, 30 cycles of 15 s at 95°C, 60 s at 55°C, and 60 s at 68°C, followed by a final extension for 5 min at 68°C and then an indefinite hold at 4°C. After PCR, triplicate reactions were re-pooled using the epMotion, and DNA concentrations were quantified using a QIAxcel Advanced capillary electrophoresis system (QIAgen, Hilden, Germany). Samples with concentrations >0.5 ng/µL were pooled using equal weights of DNA to create the final sequencing libraries. Libraries were then bead cleaned using Mag-Bind RXNPure plus beads (OMEGA, Norcross, Georgia) in a 1:0.8 ratio of sequencing library to bead volume. Cleaned library pools were adjusted to a concentration of 1.1 ng/µL ± 0.1 ng/µL, which was confirmed using the Qubit dsDNA high-sensitivity assay on a Qubit 3.0 fluorimeter and sequenced as 2 × 250 bp libraries on an Illumina MiSeq (Illumina, San Diego, CA) at the UConn MARS facility. These data were deposited in the NCBI SRA database (SRR18059736–SRR18059804https://www.ncbi.nlm.nih.gov/sra/SRR18059804).
16S rRNA community amplicon sequencing reads were analyzed using R v.3.5.3 (54) and the DADA2 v.1.11.1 (55) pipeline for ASVs. Reads were classified using the SILVA database v.128 (56, 57).
Cultures that contained Spiroplasma or Mesoplasma based on the community 16S rRNA gene sequencing, and the culture containing our reference M. lactucae strain, were T-streaked onto modified SP-4 agar plates, wrapped in parafilm to prevent the agar from drying, placed in a plastic bag or a candle jar (without a candle), and incubated at 30°C. The identity and purity of each strain were confirmed by Sanger sequencing. Single colonies were taken from the agar plates and added to 5 µL GoTaq Green Reaction Mix Buffer (Promega, Madison, WI, USA), 1.25 units GoTaq DNA Polymerase (Promega, Madison, WI, USA), 10 µmol each of primers 27F and 1492R (58) (targeting the near-complete 16S rRNA gene), 300 ng/µL BSA (New England BioLabs Inc. Ipswitch, MA), and 50 mM MgCl2, to which nuclease-free H2O was added for a final volume of 25 µL. Thermocycler conditions were 2 min at 95°C, 34 cycles of 1 min at 95°C, 1 min at 54°C and 1:30 min at 72°C, a final extension for 5 min at 72°C, and then a final hold at 4°C. Gel electrophoresis confirmed the expected band size between 1,000 and 1,400 bp. These PCR products were bead cleaned using AMPure XP beads (Beckman Coulter, Brea, CA, USA). A total of 8 µL of cleaned PCR product was added separately to 4 µL of primers 27F and 1492R, and the reactions were sent to Eurofins Genomics (Louisville, KY) for Sanger sequencing. Sanger sequencing chromatograms were analyzed using Geneious v.2019.1.3, and their identities were confirmed using NCBI Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/, queried during February, March, May, and July of 2021). These data were deposited in the NCBI GenBank database (OM760043, OM812022–OM812029)
For strains identified as Mesoplasma or Spiroplasma, a single colony from each source agar plate was spread over another SP-4 agar plate to increase cell concentrations. Genomic DNA was extracted from the resulting colonies using the Epicentre MasterPure Complete DNA and RNA purification kit, quantified, and confirmed as bacterial as described above. Genome sequencing libraries were then prepared using the Illumina TruSeq DNA PCR Free kit by the UConn MARS facility and sequenced on an Illumina MiSeq at the UConn Center for Genome Innovation as 2 × 250 bp libraries (Illumina, San Diego, CA).
Metagenome-assembled and reference genomes
Fungus garden bacterial metagenomes from T. septentrionalis colonies (which differed from those used for the Mollicute isolations) were sequenced and assembled by the Joint Genome Institute (JGI), who then generated MAGs as described in reference 48. The MAGs from these data sets with a Bin Lineage annotation of “Mollicutes” were downloaded from the JGI Integrated Microbial Genomes and Microbiomes website (https://img.jgi.doe.gov/). Additional MAGs (EntAcro1 and EntAcro10) that had been previously generated from Ac. echinatior ants (42) and other reference Mesoplasma and Spiroplasma genomes were downloaded from the NCBI (Tables S2 to S4).
Genome assembly, quality checking, and analysis
Sequencing adapters were removed using Trimmomatic v.0.39 with default parameters (59), and the data were quality checked using FastQC v.0.11.8 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Trimmed reads were assembled using Unicycler v.0.4.8-beta (60), and all genome assemblies (including those obtained from NCBI) were annotated using Prokka v.1.13.3 (61). Contigs <2,000 bp were checked for contamination using NCBI’s Nucleotide BLAST (https://blast.ncbi.nlm.nih.gov/) and investigating if the taxonomic identity of their top BLAST hits matched to the publicly available Mesoplasma lactucae genome (NCBI accession GCA_002441935.1), MAG EntAcro1 (42), our published Spiroplasma platyhelix genome (52), or MAG EntAcro10 (42). Genome completeness was checked using BUSCO v.5.0.0 with the tenericutes_odb10 Tenericutes database (62). The assembled genomes were deposited in the NCBI WGS (Table S5), and the sequencing reads were deposited in the NCBI SRA database (SRR17695994–SRR17696005)
Prokka gene annotations for each genome were imported into anvi’o v.7 (63, 64) using the gff.parser script (https://merenlab.org/2017/05/18/working-with-prokka/), and an anvi’o database was created for each genome following the anvi’o metagenome tutorial (https://merenlab.org/2016/06/22/anvio-tutorial-v2/). We followed the anvi’o pangenome and phylogenomics tutorials (https://merenlab.org/2016/11/08/pangenomics-v2/ and https://merenlab.org/2017/06/07/phylogenomics/) to calculate the genome length, %GC, and number of single-copy core gene clusters (homologous genes present in all analyzed genomes), as well as dendrograms to show the presence/absence of gene clusters (i.e., homologous genes) in each genome. The anvi’o “--mcl-inflation” parameter used to identify homologous genes can be set between 2 (less strict, used for distantly related genomes) and 10 (more strict, used for closely related genomes, typically of the same “species” or “strain”). All analyses used an mcl inflation score of 7; results were the same using a score of 3 (Fig. S1).
Assembled genomes were also functionally annotated using the RAST (Rapid Annotations Using Subsystems Technology) webserver (65) (https://rast.nmpdr.org/), selecting the domain “Bacteria,” the appropriate genus (Mesoplasma or Spiroplasma), and “Genetic code 4” as options. We used the “Classic RAST” annotation scheme, RAST as the gene caller, and FIGfam version “Release70.” RAST annotations are collated in Table S6.
The anvi’o commands “anvi-get-sequences-for-gene-clusters” and “--concatenate-gene-clusters” were used to export a file that contained a concatenated alignment of all the amino acid sequences from the single-copy gene clusters, and the flag “--report-DNA-sequences” was used to export their corresponding nucleotide sequences. Amino acid phylogenies were generated using FastTree (v.2.1.10) (66) with the Whelan-And-Goldman 2001 and CAT substitution model (-wag flag) and local bootstrapping. Nucleotide single-copy core gene cluster phylogenies were generated similarly, but using the Jukes-Cantor model (-nt flag). Trees were visualized using iTOL (https://itol.embl.de/) (67). Average nucleotide identity (ANI) values were computed in anvi’o using the “anvi-compute-genome-similarity” command.
Five hundred and fifty “core” gene clusters, which include both single-copy core genes and other highly conserved core genes that were nearly always present (but not absolutely so, e.g., due to incomplete genome and MAG assembly), were selected from our Mesoplasma genomes and MAGs, the EntAcro1 MAG, and the M. lactucae reference genomes using the anvi’o “Search gene clusters using filters” tab in the anvi’o interactive interface. The “minimum number of genomes gene cluster occurs” was set to 18, and the “maximum functional homogeneity index” was set to 0.99. The six hundred and eighty-four “core” gene clusters were similarly selected from our Spiroplasma genomes, the EntAcro10 MAG, and the S. platyhelix reference genome, setting the “minimum number of genomes gene cluster occurs” to 3 and the “maximum functional homogeneity index” to 0.99. The command “anvi-get-sequences-for-gene-clusters” was used to export all the amino acid sequences from these core gene clusters, and the flag “--report-DNA-sequences” was used to export their corresponding nucleotide sequences. ORFcor (68) and a custom Perl script provided in the Supplemental Code were used to align and concatenate all these sequences into single .faa and .fna files for each genus. Phylogenies were created as described above.
Mollicute phylogeny
We used 56 taxa to create a phylogeny of Mollicute 16S rRNA genes that included 8 Mesoplasma strains, 3 Spiroplasma strains, and 2 Mesoplasma MAGs from T. septentrionalis, the EntAcro1 and EntAcro10 MAGs, the 15 taxa listed in Table S4, and 26 other reference taxa whose accession numbers are listed on Fig. S4. The sequence alignment and phylogeny construction were completed using the CIPRES Science Gateway (https://www.phylo.org/). The sequences were aligned using MUSCLE v.3.7 (69) with default parameters except specifying the “-seqtype DNA” parameter and trimmed to an equal length using MEGA-X v.10.2.4 (70). The phylogeny was constructed using RAxML-HPC BlackBox v.8.2.12 with default parameters (71) and visualized using iTOL v.7 (67).
Phenotypic tests
Metabolism of glucose, fructose, N-acetylglucosamine, urea, and arginine was tested following the methods in reference 72, except using 0.04% (wt/vol) for chitin (which was otherwise insoluble). Sterol tests contained modified SP-4 lacking serum, modified SP-4 with tween and palmitic acid, modified SP-4 with tween, palmitic acid, and ethanol, and modified SP-4 containing 0, 1, 5, 10, or 20 µg/mL of cholesterol (MP Biomedicals), following reference 73 but using modified SP-4 medium instead of mycoplasma broth. Growth at 20, 25, 30, or 37°C was tested in modified SP-4 medium without other supplements. Before inoculation, all strains were grown in 5 mL of modified SP-4 medium until an orange color became visible, indicating that the cells entered logarithmic growth phase, after which 100 µL was added to each phenotypic test. All cultures were incubated at 30°C for 4 weeks or until they changed color from red to orange/yellow (indicating acidification) or from red to magenta (indicating alkalinization), typically within 5 to 14 days. M. lactucae ATCC 49193 and S. platyhelix ATCC 51748 were used as controls.
Transmission electron microscopy imaging
Cultures of JKS002660 and JKS002670 were grown in 5 mL of modified SP-4 medium until an orange color became visible, indicating cells entering the logarithmic growth phase. These samples were then centrifuged at 5,000 rcf, and the supernatant was removed. The cell pellets were resuspended in 1 mL of 0.1 M phosphate buffer, centrifuged at 5,000 rcf, and the phosphate buffer supernatant removed. This was repeated three times. Cells were then fixed in 2% glutaraldehyde (Electron Microscopy Sciences, Hatfield, PA) for 1 hour and rinsed three times with 0.1 M phosphate buffer.
Imaging was conducted in the University of Connecticut’s Bioscience Electron Microscopy Laboratory using an FEI Tecnai 12 G2 Spirit BioTWIN transmission electron microscope. Three microliters of each cell suspension in 0.1 M phosphate buffer was added to a carbon copper mesh grid, to which 0.5% wt/vol uranium acetate (Electron Microscopy Sciences, Hatfield, PA) was added to the grid and allowed to air dry.
RESULTS
Isolation
We dissected the guts of 69 T. septentrionalis ants from 17 colonies. Individual guts were crushed in PBS and inoculated into each of three enrichment conditions: (i) modified SP-4 medium; (ii) modified SP-4 medium supplemented with penicillin; and (iii) modified SP-4 medium that was heated and filtered (Fig. S2). One hundred of the resulting 207 enrichment cultures became turbid or changed color (phenol red turns from red to orange/yellow due to acid production during fermentation; Fig. S2). We characterized a subset of these cultures using 16S rRNA community amplicon sequencing (Fig. S3) and inoculated samples containing exclusively Spiroplasma or Mesoplasma onto modified SP-4 agar plates. Of the 38 enrichment cultures that we characterized, 17 contained exclusively Spiroplasma or Mesoplasma, five contained both taxa, and six contained neither (Fig. S3). This is consistent with the natural pattern of symbiont co-exclusion that we observed previously (38) but suggests that minority symbionts are sometimes not entirely absent and might be enriched under certain conditions. A single colony from each plate with a “fried egg” morphology typical of Mollicutes was purified and taxonomically identified using 16S rRNA gene Sanger sequencing as being most closely related to known strains belonging to either the genus Mesoplasma or Spiroplasma (Fig. S4). Strains JKS002657 and JKS002669, JKS002658 and JKS002659, and JKS002663 and JKS002664 were isolated from different enrichment cultures inoculated using the same ant colonies (Table S1); all other strains are from different ant colonies. In total, we isolated and identified eight Mesoplasma and three Spiroplasma strains from nine unique ant colonies (strains JKS002657–JKS002664 and JKS002669–JKS002671, respectively; Tables S1 and S5).
Gene content
We sequenced the genomes of all eight Mesoplasma and three Spiroplasma strains that we isolated from dissected T. septentrionalis guts. The 16S rRNA genes in these genomes were 100% identical to the ASVs that we identified in the gut enrichment cultures (Table S7) and to ASVs that we previously identified in the T. septentrionalis microbiome (38). We also analyzed 11 Mesoplasma MAGs that our lab sequenced in collaboration with the Joint Genome Institute (Table S2) (48). There were no Spiroplasma MAGs assembled from these metagenomes. For comparison, we also downloaded the MAGs assembled by Sapountzis et al. (42) (Table S3). As controls, we also re-sequenced the genome Mesoplasma lactucae ATCC 49193 (which matched that sequenced previously; NCBI BioProject: PRJNA412357; Table S4) and included our previously sequenced Spiroplasma platyhelix ATCC 51748 genome (52).
All eight Mesoplasma strains that we isolated from T. septentrionalis guts had genome sizes of 762–785 kbp and GC contents of 32.68–32.79% (Table S5). The Mesoplasma MAGs generated from T. septentrionalis fungus gardens (48) had lengths of 385–760 kbp and 32.41–33.00% GC content. These MAGs were estimated to be 52.70–100% complete using BUSCO (Table S2), with the smallest genomes being the least complete. The Mesoplasma EntAcro1 MAG from the fungus-growing ant Ac. echinatior had a genome size of 866 kbp and a GC content of 33.74% (42), which was larger in length and slightly higher in GC content compared to the Mesoplasma genomes and MAGs from T. septentrionalis (Table S3). All of these genomes and MAGs were smaller than the M. lactucae ATCC 49193 genome (824 kbp), which also had a lower GC content (29.63%; Table S4).
The genomes from all three Spiroplasma strains that we isolated from T. septentrionalis guts had sizes of 835–884 kbp and 25.49–25.77% GC content (Table S5). The Spiroplasma EntAcro10 MAG from Ac. echinatior (42) had a similar genome size (838 kbp) and GC content (25.53%) to those of the Spiroplasma strains isolated from T. septentrionalis (Table S3). The S. platyhelix ATCC 51748 genome was shorter than all of these genomes and MAGs (739 kbp), and its GC content was higher (28.60%; Table S4).
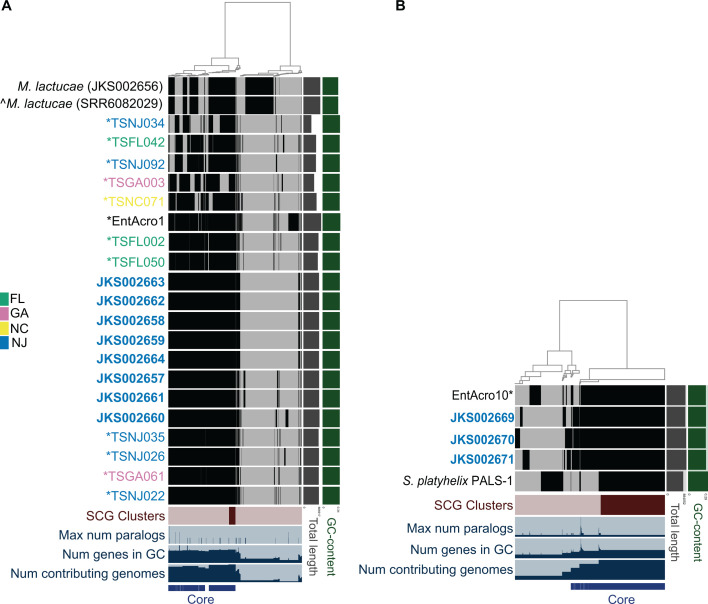
The Mesoplasma genomes from the strains that we isolated contained 660–689 coding sequences. Similarly, the T. septentrionalis MAGs with 100% complete BUSCO scores contained 654–663 coding sequences. MAG EntAcro1 contained substantially more coding sequences (757) than the genomes and MAGs from T. septentrionalis. The M. lactucae reference genome contained 691 coding sequences, similar to the isolate genomes. Our pangenome analysis separated gene clusters from these Mesoplasma genomes and MAGs into three bins: (i) “fully conserved,” containing anvi’o “single-copy core genes,” i.e., single-copy genes present in all analyzed genomes; (ii) “highly conserved,” genes found in most analyzed genomes; and (iii) “variable,” genes found in only a few genomes. There were 57, 583, and 555 gene clusters in the fully conserved, highly conserved, and variable bins (Fig. 1A). Consistent with the “variable” genes being part of a poorly characterized accessory genome, 87.57% of these genes had no Prokka annotation, compared to 39.96% and 35.08% of the “fully conserved” and “highly conserved” genes.
Fig 1.
Anvi’o pangenome analysis of (A) Mesoplasma whartonense genomes (bold) and MAGs (*), the EntoAcro1 MAG (*), and the M. lactucae genomes as a reference and (B) Spiroplasma attinicola genomes (bold), the EntAcro10 MAG (*), and the S. platyhelix genome as a reference. Text colors indicate strains or MAGs from the same state. Two M. lactucae genomes are shown, with the ^ indicating the genome downloaded from NCBI (SRR6082029) vs the other that we re-sequenced as a control (JKS002656). In the central heatmap, black and gray bars indicate homologous genes that are present or absent in each genome. The dendrogram clusters groups of homologous genes (anvi’o gene clusters) using Euclidean distances.
The Spiroplasma genomes from the strains that we isolated contained 768–820 coding sequences, similar to MAG EntAcro10, which had 774 (Table S6). The S. platyhelix reference genome contained significantly fewer coding sequences (670) than the Spiroplasma strains that we isolated. The Spiroplasma pangenome (Fig. 1B) had 1,111 gene clusters. There were 472, 156, and 483 gene clusters in the fully conserved, highly conserved, and variable bins with 26.81%, 80.77%, and 89.23% of these gene clusters having no gene annotation. The fewer Spiroplasma strains and lack of MAGs from T. septentrionalis compared to our Mesoplasma analysis likely caused the greater number of fully conserved genes and fewer highly conserved genes in our Spiroplasma analysis compared to that of Mesoplasma.
The Mesoplasma MAGs from T. septentrionalis fungus gardens closely resemble those of T. septentrionalis gut isolates, although those with low BUSCO scores lacked some “core” genes, consistent with BUSCO predicting that these genomes are incomplete. Some small gene content differences occurred between related strains in both the Mesoplasma and Spiroplasma pangenomes, with the strains having the most similar gene content also clustering together in our phylogenetic trees (Fig. 2A and B; Fig. S5 to S10). Compared to the T. septentrionalis genomes and MAGs, the EntAcro MAGs lacked very few “core” genes (i.e., those in the “fully conserved” and “highly conserved” gene bins). The reference genomes from M. lactucae and S. platyhelix had the most genes found only in their genome and lacked many of the “core” genes found in the ant symbiont genomes and MAGs. Both M. lactucae genomes had 265 gene clusters that were found only in their genomes and lacked 34 Mesoplasma “core” gene clusters. S. platyhelix had 158 gene clusters found only in its genome and lacked 22 Spiroplasma “core” genes. The MAGs EntAcro1 and EntAcro10 had 86 and 84 unique gene clusters, respectively, that were not found in the reference genomes or the T. septentrionalis genomes and MAGs. All genes unique to these reference genomes and MAGs were included in the “variable” bins of our pangenome analyses.
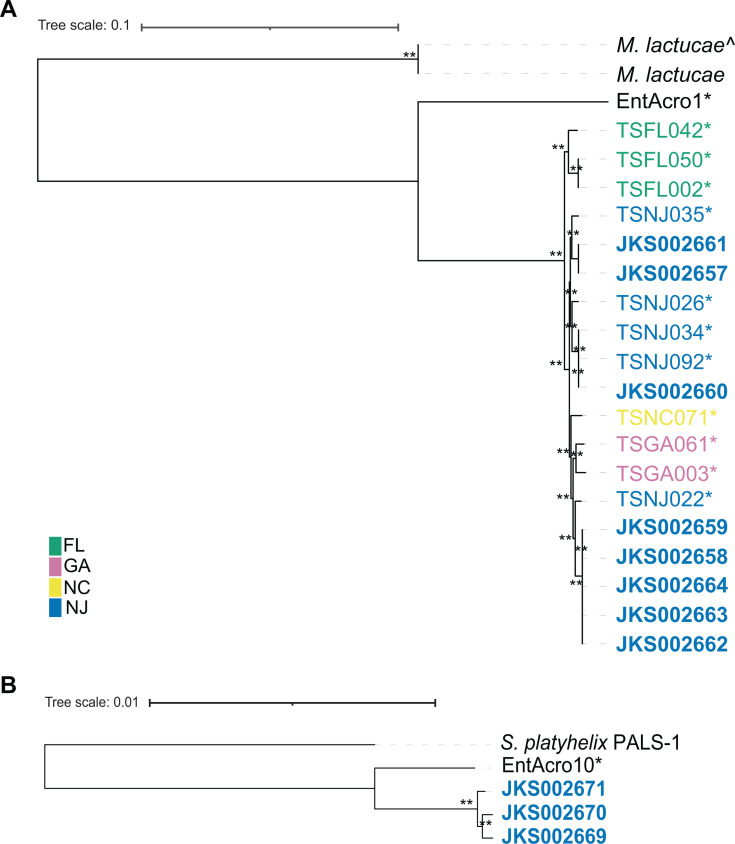
Fig 2.
(A) Mesoplasma nucleotide phylogeny created using 550 core gene clusters as input data and the FastTree JC + CAT substitution model. The tree was rooted using the midpoint of the branch leading to the M. lactucae genomes. The ^ indicates the NCBI (SRR6082029) genome. (B) Spiroplasma nucleotide phylogeny created using 684 core gene clusters as input data and the FastTree JC + CAT substitution model. The tree was rooted using the midpoint of the branch leading to the S. platyhelix genome. Asterisks indicate MAGs and bolded names indicate T. septentrionalis isolate genomes. Colors group strains by state. Local bootstrap values of 60–79 and 80–100 are indicated by * and **, respectively.
Phylogeny and average nucleotide identity
We created phylogenies using both amino acids and nucleotides from 21 single-copy gene clusters that were present in all ant-related Mesoplasma genomes and MAGs, as well as in several NCBI Mesoplasma reference genomes (Fig. S5 and S6). In both the amino acid and nucleotide phylogenies, the NCBI reference Mesoplasma genomes grouped with a similar topology to the tree in Gasparich (74), in which M. lactucae forms an outgroup to all other Mesoplasma strains. All Mesoplasma ant-related genomes and MAGs formed their own clade next to M. lactucae. Similarly, we created phylogenies using all 80 single-copy core gene clusters that were present in all Spiroplasma genomes and MAGs from both ants and NCBI, using both amino acid and nucleotide sequences (Fig. S7 and S8). Here, S. platyhelix formed an outgroup from the other NCBI Spiroplasma genomes, as in the Spiroplasma phylogenetic tree in Bergey’s Manual of Systematics of Archaea and Bacteria (11), and all Spiroplasma genomes from ants formed their own clade next to S. platyhelix. Thus, both our Mesoplasma and Spiroplasma isolate genomes formed monophyletic clades close to but distinct from the reference species M. lactucae and S. platyhelix, respectively. Because multiple conflicting proposals exist to reclassify these genera (74–76) that have not yet been formally adopted under the International Code of Nomenclature of Prokaryotes, we maintain the currently accepted genus names Mesoplasma and Spiroplasma while acknowledging the potential need for future reclassification.
To increase the phylogenetic resolution of our analyses, we created additional phylogenies using the core gene clusters (i.e., those in the “fully conserved” and “highly conserved” bins) in all our ant genomes, all ant MAGs, and their closest reference genomes. The pangenome including only the M. lactucae reference genome, MAG EntAcro1, and the Mesoplasma isolate genomes and MAGs from T. septentrionalis had 550 core gene clusters that were used for these phylogenies. The Spiroplasma pangenomes, including only the S. platyhelix reference genome, MAG EntAcro10, and the Spiroplasma isolate genomes from T. septentrionalis, had 684 core gene clusters that were used for these phylogenies.
In both the amino acid and DNA sequence-based trees, the EntAcro MAGs from A. echinatior grouped separately from the rest of the T. septentrionalis genomes and MAGs (Fig. 2A and B; Fig. S9 and S10), with the M. lactucae or S. platyhelix reference genomes forming outgroups. In all Mesoplasma phylogenies, the T. septentrionalis genomes and MAGs clustered by the geographic locations from which their corresponding ant colonies had been collected (Fig. 2A; Fig. S9). For example, all the Mesoplasma strains that we isolated were from ant colonies collected in New Jersey, and these genomes clustered together with New Jersey MAGs. North Carolina MAG TSNC071 formed its own branch in the DNA tree (Fig. 2A) but clustered with the New Jersey genomes and MAGs in the amino acid tree (Fig. S9). The Florida and Georgia MAGs formed their own clades in both the amino acid and DNA sequence trees. This suggests that T. septentrionalis Mesoplasma strains are specific to distinct geographic locations. Because we lack Spiroplasma genomes and MAGs sampled from across a similarly broad geographic range, whether their genomes show a similar biogeographic trend remains unknown.
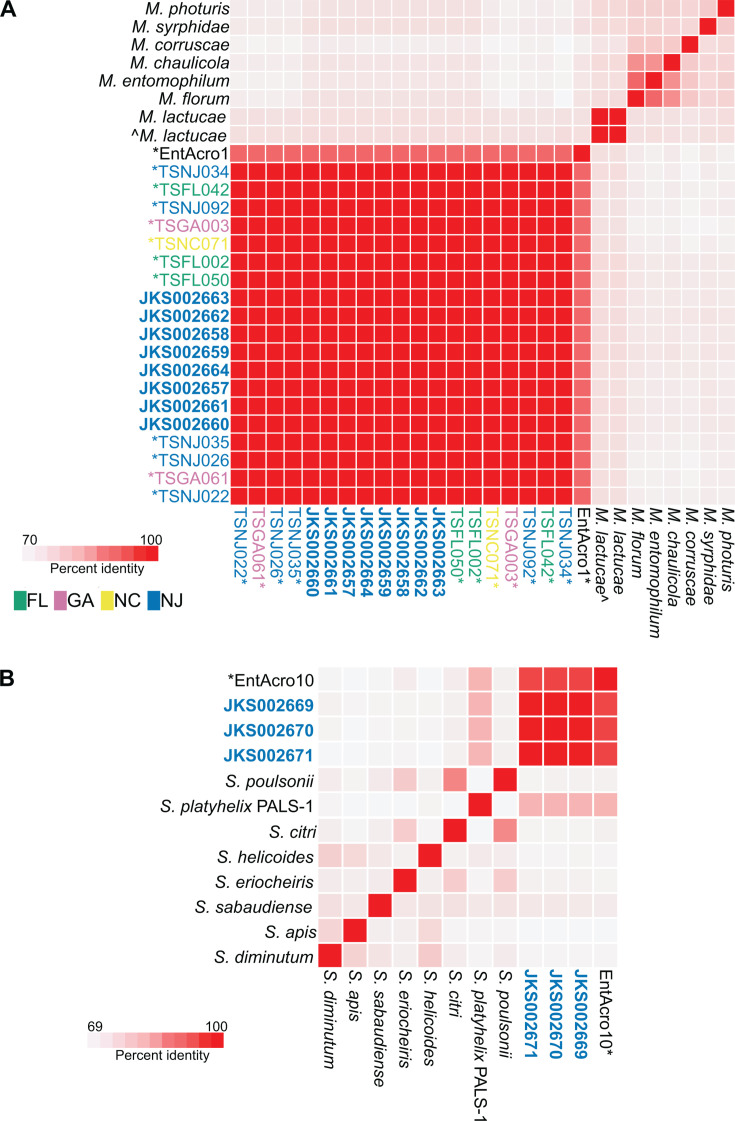
ANIs confirmed that all T. septentrionalis genomes and MAGs are distinct from the NCBI genomes and EntAcro MAGs (Fig. 3A and B). All Mesoplasma genomes and MAGs from T. septentrionalis had 72%–73% ANI to the M. lactucae reference genomes and 70%–73% ANI to the other NCBI reference genomes. Similarly, the Spiroplasma genomes from T. septentrionalis had 78% ANI to the S. platyhelix reference genome and 70%–78% ANI to the other NCBI reference genomes. These results confirm that the T. septentrionalis genomes and MAGs should not be classified as these species (77, 78). The Mesoplasma genomes and MAGs from T. septentrionalis had 87% ANI to the EntAcro1 MAG (Fig. 3A), and the Spiroplasma genomes from T. septentrionalis had 92% ANI to EntAcro10 (Fig. 3B). All Mesoplasma genomes and MAGs from T. septentrionalis had 99%–100% similarity to each other, and these ANIs were not differentiated by state as in the phylogenies (Fig. 2A; Fig. S9). Similarly, the Spiroplasma genomes from T. septentrionalis had ANIs of 98%–100% when compared to each other (Fig. 3B). These values are consistent with all T. septentrionalis Mesoplasma and Spiroplasma belonging to their own species that are distinct from the EntAcro MAGs (77, 78). We therefore named them Mesoplasma whartonense sp. nov. and Spiroplasma attinicola sp. nov. and characterized them further below.
Fig 3.
Average nucleotide identity heatmap of (A) M. whartonense genomes and MAGs, MAG EntAcro1, and Mesoplasma reference genomes, and (B) S. attinicola genomes, MAG EntAcro10, and Spiroplasma reference genomes, produced by anvi’o. Asterisks indicate MAGs, and bold names indicate our isolate genomes. The ^ indicates the NCBI (SRR6082029) genome, and colors indicate the state from which strains or MAGs were collected.
Gene functions
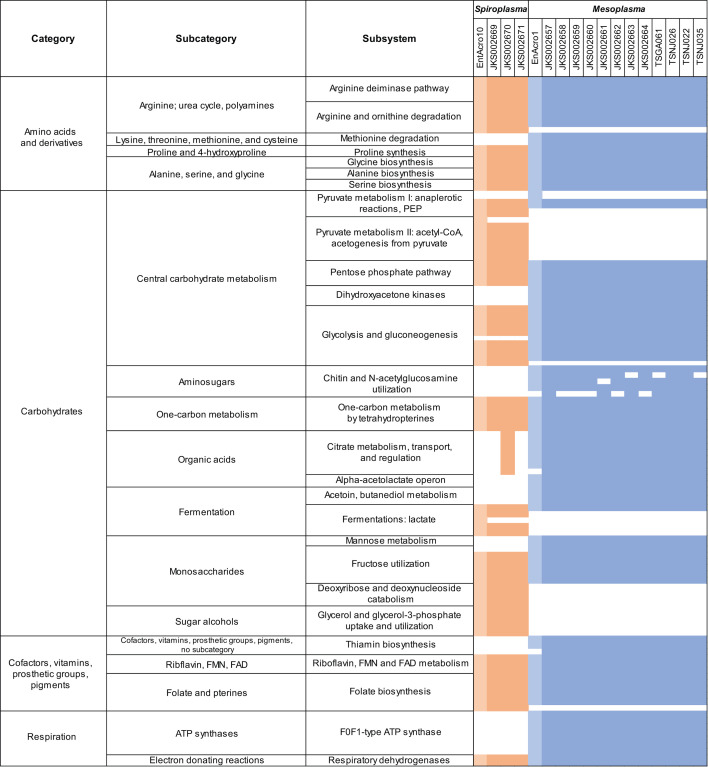
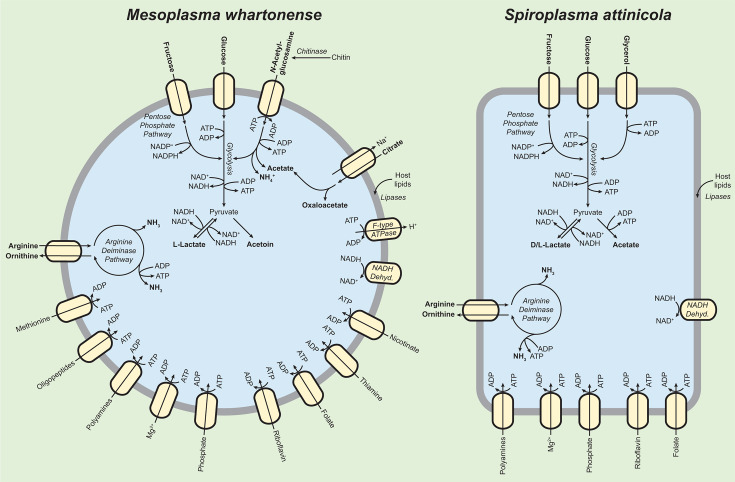
We used RAST to predict the functions of all genes in M. whartonense and S. attinicola (Fig. 4; Supplemental Code). As expected for Mollicutes, M. whartonense and S. attinicola lacked the genes necessary to produce a cell wall. Only M. whartonense had genes for the biosynthesis of glycerophospholipids, but both M. whartonense and S. attinicola had genes for lipases that likely facilitate the acquisition of host-derived fatty acids for cell membrane biogenesis. Both species lacked the genes needed to produce many amino acids but retained genes for nucleotide salvage, suggesting they also likely depend on their ant hosts to provide many amino acids. Both M. whartonense and S. attinicola had the genes necessary to import vitamin B, folate, and riboflavin, but only M. whartonense had thiamine utilization genes.
Fig 4.
Representative gene function predictions for ant-associated Spiroplasma and Mesoplasma, as predicted using RAST. Orange colors indicate genes present for Spiroplasma, and blue indicates genes present for Mesoplasma. The lighter of each color denotes genes present in MAGs EntAcro1 or EntAcro10. White indicates absence.
Both M. whartonense and S. attinicola had genes encoding for bacteriophage defense systems, including a type I restriction-modification system and clustered regularly interspaced short palindromic repeats (CRISPR) -Cas1, but neither had any obvious prophages. Only S. attinicola had genes for a type III restriction-modification system, and only M. whartonense had genes for CRISPR-Cas2 (Fig. 4; Supplemental Code). The conserved presence of such genes is consistent with both M. whartonense and S. attinicola being frequently exposed to bacteriophages, as also proposed for EntAcro1 and EntAcro10 (42).
Reconstructions of catabolism in M. whartonense and S. attinicola are shown in Fig. 5. We predict that both M. whartonense and S. attinicola can catabolize glucose and fructose using glycolysis and the pentose phosphate pathway to produce pyruvate, ATP, and NAD(P)H, and catabolize arginine to produce ornithine, ATP, and ammonium (NH4+); these catabolic pathways are present in many Mollicutes (Fig. 4; Supplemental Code) (79). Certain low-quality assemblies (especially the MAGs) lacked some genes present in their close relatives, but such differences are more likely technical than biological. Glucose and fructose likely originate from plant material that the ants forage or are produced during fungal digestion. The use of such digestion products is also suggested by M. whartonense having genes for chitin degradation and N-acetylglucosamine catabolism. Catabolism of sugars could produce pyruvate from the glycolysis or pentose phosphate pathways or provide precursors for the biosynthesis of purine and pyrimidines. We predict that both M. whartonense and S. attinicola possess fermentation pathways leading from pyruvate to L-lactate (both species), D-lactate (S. attinicola), acetoin (M. whartonense), and acetate (S. attinicola).
Fig 5.
Representative catabolic pathways in M. whartonense (left) and S. attinicola (right). Anabolic reactions are not shown, and reactions are not balanced. Major predicted catabolic substrates and products are bolded.
We predict that both M. whartonense and S. attinicola can catabolize dihydroxyacetone phosphate, but only M. whartonense can import glycerol. These are likely used to produce glyceraldehyde-3-phosphate that can enter the glycolysis pathway. Glycerol might also be produced by the lipases encoded by both M. whartonense and S. attinicola and may be used for glycerophospholipid biosynthesis in M. whartonense (but not S. attinicola, which lacks the corresponding genes). M. whartonense can also likely produce acetate, but from citrate (which could come from foraging materials) instead of from pyruvate as in S. attinicola (except for S. attinicola JKS002670, which we predict can produce acetate from both pyruvate and citrate). This acetate may be assimilated by the ant host (42). Both M. whartonense and S. attinicola can likely interconvert glycine and serine, convert aspartate into fumarate, import and metabolize polyamines, and encode an NADH oxidase. Only M. whartonense has genes for the import and metabolism of methionine, and F0F1 ATPase genes that are predicted to mainly hydrolyze ATP to maintain an electrochemical gradient in other Mollicutes (80, 81).
The EntAcro1 MAG and the M. whartonense genomes had very similar gene contents, except for a few differences. The EntAcro1 MAG lacked genes for methionine transport, Apo-citrate lyase phosphoribosyl-dephospho-CoA transferase, CRISPR-Cas2, and thiamine transport protein ThiT, which were all present in M. whartonense (Fig. 4; Supplemenetal Code). Whether these absences are due to incomplete MAG assembly is unknown. In contrast, the EntAcro1 MAG did contain an NADP-dependent malic enzyme gene and a TrkH potassium uptake gene that were lacking in M. whartonense. The EntAcro10 MAG and S. attinicola genomes were more similar in length and gene count compared to the Mesoplasma genomes (Fig. 4). The EntAcro10 MAG lacked genes for a DNA-cytosine methyltransferase and the TolA protein, which were both present in S. attinicola. However, the EntAcro10 MAG contained several genes lacking in S. attinicola, such as an acetaldehyde dehydrogenase gene, two coenzyme A biosynthesis genes, a methylglyoxal metabolism gene, and an internalin-like protein gene (Supplemental Code). Overall, however, the symbionts from Ac. echinatior and T. septentrionalis encode very similar sets of functional genes.
Phenotypic tests
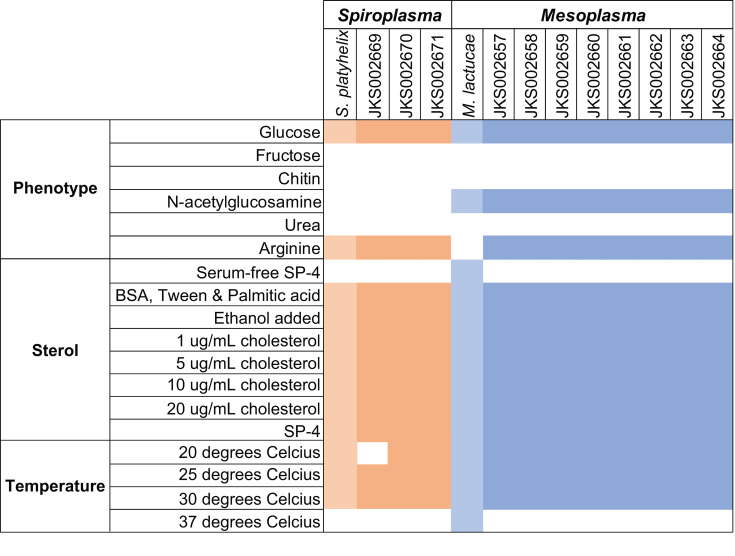
We used our M. whartonense and S. attinicola cultures, and M. lactucae and S. platyhelix as controls, for several phenotypic tests. Based on the predicted gene functions, we tested for acid production from glucose, fructose, N-acetylglucosamine, and chitin, and for alkalization from arginine and urea. All Mesoplasma and Spiroplasma isolates produced acid from glucose, and all, except M. lactucae, reduced the culture pH in the presence of arginine, confirming our predictions (Fig. 6). All Mesoplasma also produced acid from N-acetylglucosamine. However, no culture changed their pH in the presence of fructose, chitin, or urea.
Fig 6.
Results of phenotypic tests and growth at varying temperatures. Orange colors indicate phenotypes observed for Spiroplasma, and blue indicates phenotypes observed for Mesoplasma. The lighter of each color denotes phenotypes observed for the control strain, S. platyhelix or M. lactucae. White indicates no phenotype was observed.
We also tested the sterol and temperature requirements of M. whartonense and S. attinicola (Fig. 6). All Mesoplasma and Spiroplasma isolates grew in serum regardless of the presence of added cholesterol, but not without serum. No strains grew at 37°C. All M. whartonense isolates and S. attinicola strains JKS002670 and JKS002671 grew at 20, 25, and 30°C, while S. attinicola strain JKS002669 only grew at 25°C and 30°C.
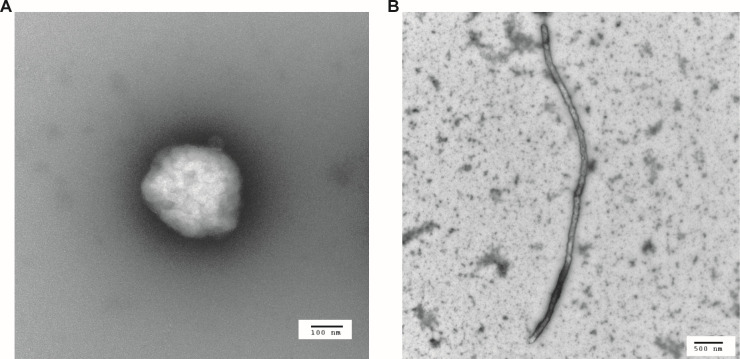
Using scanning transmission electron microscopy, M. whartonense cells were coccoid and 150 to 200 nm in diameter (Fig. 7A). S. attinicola cells were non-helical rods 4 to 5 µm long (Fig. 7B).
Fig 7.
Transmission electron microscope imaging of M. whartonense strain JKS002660 (A) and S. attinicola strain JKS002670 (B).
DISCUSSION
Here, we isolated novel Mesoplasma and Spiroplasma symbionts from the fungus-growing ant T. septentrionalis. They are phylogenetically related to, but distinct from, those of all previously described species and the EntAcro MAGs sequenced from Ac. echinatior, prompting us to describe them as Mesoplasma whartonense sp. nov. and Spiroplasma attinicola sp. nov., respectively. Although genomic data are lacking, M. whartonense and S. attinicola are also related to uncultured Mollicute bacteria detected in several species of non-fungus-growing ants, particularly army ants (35–37), based on homology between their partial 16S rRNA genes (38). The closest cultured relative to M. whartonense is M. lactucae, a strain originally isolated from lettuce leaves (82). Little is known about the ecology of this species, including whether it infects plants or is vectored to them by insects. The closest cultured relative to S. attinicola is S. platyhelix, another poorly studied species that was originally isolated from the dragonfly Pachydiplax longipennis (83).
Both M. whartonense and S. attinicola are predicted to encode for phenotypes that are similar to those encoded by the EntAcro MAGs, although with distinct genome sizes and genomic compositions (Fig. 4). The M. whartonense genomes and MAGs also phylogenetically cluster by the geographic region from which their host T. septentrionalis ants were collected (Fig. 2A). This contrasts with the lack of such clustering by the T. septentrionalis Pseudonocardia symbiont (84). Strain specificity in the gene content of these local symbiont populations may be conserved due to adaptation to local ecological conditions such as climate and forage availability (85).
It is possible that gene content and genome size differed between the M. whartonense genomes and the EntAcro1 MAG because the EntAcro1 MAG contained extra sequences due to computational errors that occurred during contig binning from the metagenome (41, 86). However, the M. whartonense MAGs assembled as part of a second study (48) had similar sizes as the M. whartonense isolate genomes. The difference in genome size between the EntAcro1 and M. whartonense MAGs may therefore be biological or due to the different pipelines used by each group to bin their metagenomic data. If we assume that the EntAcro1 MAG accurately characterizes the genome of the Mesoplasma symbiont of Ac. echinatior, the differences in gene content and phylogenetic position between it and M. whartonense suggest different evolutionary paths were followed by these symbionts that are unique to each fungus-growing ant species. Ac. echinatior ants are found in Central America, and as leaf-cutting ants represent the most evolutionary advanced form of ant agriculture with large colonies (hundreds of thousands of ants), caste differentiation, and specialization on fresh leaves and fruits. In contrast, T. septentrionalis ants are found throughout the Eastern USA but belong to the “higher attine” clade that branches basal to the true leaf-cutting ants. They have smaller colonies (thousands of ants), monomorphic worker castes, and different forage preferences including insect frass, oat catkins, and leaves including oak and ferns. The cultivar fungi grown by Ac. echinatior and T. septentrionalis also largely belong to different genetic lineages (20, 23, 24, 27, 87). Whether Mollicute symbionts are specific to the different modes of ant agriculture or different attine ant genera is an interesting hypothesis arising from our results that will require a wide sampling of ants from different genera and geographic regions to test. Regardless, the relatively close phylogenetic relationships (belonging to the same genus at least) between either M. whartonense and EntAcro1 or S. attinicola and EntAcro10 are inconsistent with either pair co-diverging with their T. septentrionalis and Ac. echinatior ant hosts, which belong to different genera that diverged ~19 million years ago (88).
The closest known species to M. whartonense and S. attinicola are M. lactucae and S. platyhelix, respectively, but these strains form separate groups in the phylogenetic trees (Fig. 2A and B; Fig. S5 to S10) and have different gene contents (Fig. 4). M. whartonense and EntAcro1 both have genes for the arginine deiminase pathway, pyruvate metabolism, and pyrimidine conversion, all of which M. lactucae lacks. The last common ancestor of M. lactucae and M. whartonense/EntAcro1 may have contained these genes, with M. lactucae losing them because they were not required for its symbiosis with plants. Alternatively, M. whartonense and EntAcro1 may have uniquely gained these genes that were not present in their last common ancestor with M. lactucae. Either of these explanations would imply that M. whartonense and EntAcro may have retained or gained these genes to survive within the ant gut.
The M. whartonense genomes are also ~100 kbp smaller than those of M. lactucae and MAG EntAcro1, which could mean that the many hypothetical genes in M. lactucae and MAG EntAcro1 were lost in M. whartonense. These different genome sizes could be due to M. whartonense losing almost all the hypothetical genes present in MAG EntAcro1 because they are not required for the T. septentrionalis symbiosis or, alternatively, that these hypothetical genes were uniquely gained by MAG EntAcro1. These gene content differences might be the result of the specialization of these Mesoplasma to each ant host, or due to technical artifacts introduced during MAG construction.
The S. attinicola and EntAcro10 ant symbionts differ in gene content from their nearest classified neighbor, S. platyhelix. Both S. attinicola and EntAcro10 have genes for the arginine deiminase pathway, but S. platyhelix does not. The S. platyhelix genome is smaller than those of S. attinicola and MAG EntAcro10 but contains chitin degradation and F0F1-type ATPase genes, unlike the Spiroplasma ant symbionts. The last common ancestor of S. platyhelix and S. attinicola/EntAcro10 may have contained these genes, which were then lost by S. attinicola and EntAcro10. Alternatively, these genes may have been gained uniquely by S. platyhelix following its divergence from S. attinicola and EntAcro10. The lack of chitin degradation genes in S. attinicola and EntAcro10 suggests that these strains cannot digest chitin derived from the cell wall of the ants’ cultivar food source. However, this lack may benefit the ant if this chitinase could instead degrade chitin in ant tissues (89). Differences in the distribution of hypothetical genes between the MAG EntAcro10 and S. attinicola could arise following the same mechanisms described above for M. whartonense and EntAcro1, with genes being uniquely gained or lost in either lineage. Such differences in the content of hypothetical genes between M. whartonense and EntAcro1 or S. attinicola and EntAcro10 could indicate different evolutionary adaptations to each ant host and type of fungus-growing agriculture.
M. whartonense and S. attinicola symbionts of fungus-growing ants have largely overlapping but sometimes distinct functions, most of which are also conserved in EntAcro1 and EntAcro10, respectively. Our gene annotations (Fig. 4 and 5) suggest that both M. whartonense and S. attinicola likely ferment glucose and fructose to produce L-lactate (both species), D-lactate and acetate (S. attinicola), or acetoin (M. whartonense). These fermentation products would be available for uptake in the ant gut or secretion onto the fungus garden. However, our phenotypic tests showed that both M. whartonense and S. attinicola strains catabolized glucose but not fructose under laboratory conditions. Only M. whartonense genomes possessed the genes needed to degrade chitin, which might also produce acetate and ammonia that could become available to the ants or fungus garden. Under our experimental conditions, M. whartonense degraded N-acetylglucosamine but not chitin.
All M. whartonense genomes and MAGs contained genes for citrate degradation (encoding citrate lyase; Fig. 4), as did one S. attinicola genome. Sapountzis et al. (42) reported citrate degradation genes in only the MAG EntAcro1 and hypothesized that citrate originated from ant foraging materials and that the acetate resulting from its degradation could be provided to the ants. Thus, both M. whartonense and S. attinicola can both likely produce acetate but from different precursors (citrate and pyruvate). However, how citrate degradation benefits M. whartonense remains vague, because genes are lacking that might encode further transformations of oxaloacetate that might provide an energetic benefit. Biochemical evidence for malate dehydrogenase activity (which could regenerate NAD+ by reducing oxaloacetate) exists for many mollicutes, despite their lacking homologs to known malate dehydrogenase genes (81). The benefit of citrate metabolism for M. whartonense (and the one S. attinicola strain) and the degree to which it provides acetate for the ant host therefore remains unclear.
S. attinicola, but not M. whartonense, encodes genes for glycerol degradation (Fig. 4), which is tied to pathogenicity in Mycoplasma, another Mollicute genus, via the production of hydrogen peroxide (90). However, M. whartonense and S. attinicola lack genes coding for GlpO, which is needed to produce hydrogen peroxide. Glycerol likely originates from the ant host and can be broken down into glyceraldehyde-3-phosphate that enters the glycolysis or pentose phosphate pathways. Glycerol may be produced by the lipases encoded by M. whartonense and S. attinicola (by which they presumably obtain host lipids for membrane biogenesis) and/or used for glycerophospholipid biosynthesis by M. whartonense (but not S. attinicola, which lacks the corresponding genes).
Both M. whartonense and S. attinicola strains all possess the genes needed to degrade arginine (via the arginine deiminase pathway; Fig. 4) and could do so in culture (Fig. 6). Although present in many Mollicutes, these genes are present in only one other Mesoplasma genome (M. photuris) but are sometimes found in Spiroplasma genomes (91). Both Ac. echinatior and T. septentrionalis ants lack genes for arginine metabolism and receive this amino acid from their fungal cultivar (92, 93). The degradation of arginine produces ammonium, and Sapountzis et al. (42) hypothesized that EntAcro1 and EntAcro10 recycled excess arginine into ammonium and provided it to the fungus garden for use in protein synthesis. This hypothesis was supported by these bacteria expressing arginine transporters in the ant hindgut and the presence of ammonium in Ac. echinatior ant fecal droplets (42). Our study also supports this hypothesis by showing that M. whartonense and S. attinicola catabolize arginine in culture. However, glycolytic Mollicutes (such as M. whartonense and S. attinicola) can generate ATP via glucose hydrolysis, and therefore may not require the high flux through the arginine deiminase pathway to generate ATP needed by non-glycolytic Mollicute species (81), concomitantly producing less ammonium in ant guts. The arginine deiminase pathway can also help Mollicutes overcome acid stress (94), consistent with the low pH values measured for ant guts (95). Determining the function of the arginine deiminase pathway in ant guts and if its products are, in fact, provided to the fungus garden will require future experiments.
In summary, our isolation and characterization of M. whartonense and S. attinicola set the stage for future experiments to quantify the relative benefits or costs that Mollicute symbionts have on fungus-growing ant symbioses. Such benefits might include acetate and ammonium production, and costs might involve the consumption of other metabolites derived from the ants or ant food, e.g., lipids, nucleobases, vitamins, arginine (primarily for catabolism), other amino acids (for anabolism), and other carbon sources. It is possible that the cost of consuming such nutrients exceeds the provision of benefits to the ant host, more consistent with parasitism than mutualism. Such dynamics may be context-dependent or involve non-nutritive benefits such as colonization resistance or immune priming.
Our isolation of the novel ant symbionts M. whartonense and S. attinicola also sets the stage for future research removing and reintroducing both Mollicutes to the ants individually and in competition to determine why these two bacterial species do not co-occur (38). Our genome analyses identified limited (some M. whartonense) or no (S. attinicola) genes that might encode for toxins (Supplemental Code), suggesting that direct antagonism is unlikely to cause co-exclusion of these species. Their limited and overlapping metabolic complexity (Fig. 4 to 6) also suggests that both species have a similar nutrient niche. Other niche axes will require more detailed study, especially those that involve differences in growth rate (vs growth ability) that are difficult to predict from genome sequences alone.
Ecological dynamics may also cause the co-exclusion of M. whartonense and S. attinicola in ant guts. M. whartonense and S. attinicola encode different defense gene repertoires, suggesting that bacteriophages may differentially shape the realized niches of these species (Supplemental Code). This may allow their co-existence at the population level but not within individual ant colonies. The potentially context-dependent fitness costs and benefits of hosting these symbionts may also promote their co-existence at the population level (45, 46) via transmission between local patches of infected vs uninfected host colonies and priority effects that facilitate rapid dissemination throughout newly infected colonies. Although further studies are needed to test these hypotheses, the unique tractability of the T. septentrionalis symbiosis, now including our isolation and characterization of M. whartonense and S. attinicola, offers strong potential to unravel such dynamics. We anticipate that the principles governing co-exclusion in this experimentally tractable system can be broadly applied to many other microbiomes.
Description of Mesoplasma whartonense
Etymology: whartonense, neutr. of Wharton State Park, referring to the geographic location of where the ant was collected from which the bacterium was isolated.
Isolated from the guts of T. septentrionalis ants collected in Wharton State Park, New Jersey, USA, with no known pathogenicity. Cells are coccoid in shape, 150 to 200 nm in size, lack a cell wall, and are filterable through 0.45 µm membranes. Colonies are a fried-egg shape, grow on SP-4 agar in 4 to 7 days at 30°C, and can grow between 20 and 30°C. Produces acid from glucose and N-acetylglucosamine and hydrolyzes arginine but not urea. Requires serum for growth but not cholesterol. The 16S rRNA gene of the proposed type strain (JKS002660) was 93.45% similar to that of Mesoplasma lactucae 831-C4T. The genome of Mesoplasma whartonense JKS002660 has 72.80% average nucleotide identity to that of M. lactucae 831-C4T. Its genome size is 786,541 bp and its %GC content is 32.68%. The type strain is JKS002660 (NCTC 14863; DMSZ 115716) and other strains include JKS002657, JKS002658, JKS002659, JKS002661, JKS002662, JKS002663, and JKS002664.
Description of Spiroplasma attinicola
Etymology: attini- Attine, -cola neutr. Inhabitant/dweller. An inhabitant of a fungus-growing ant from Tribe Attini
Isolated from the guts T. septentrionalis ants collected in Wharton State Park, New Jersey, USA, with no known pathogenicity. Cells are rod and non-helical in shape, 4 to 5 µm long, lack a cell wall, and are filterable through 0.45 µm membranes. Colonies are a fried-egg shape, grow on SP-4 agar in 7 days at 30°C, and can grow between 20 and 30°C. Produces acid from glucose and does not hydrolyze arginine or urea. Requires serum for growth but not cholesterol. The 16S rRNA gene of the proposed type strain (JKS002670) was 94.12% similar to Spiroplasma platyhelix. The genome of Spiroplasma attinicola has 77.63% average nucleotide identity to S. platyhelix. Its genome size is 872,740 bp and its %GC content is 25.49%. The type strain is JKS002670 (NCTC 14964; DMSZ 115717), and other strains include JKS002669 and JKS002671.
ACKNOWLEDGMENTS
We thank the New Jersey State Forest staff for their assistance with our fieldwork and the members of the Klassen lab for help collecting ant colonies and thoughtful comments on the paper. We also thank Dr. Kendra Maas and the University of Connecticut Microbiology Analysis, Resources, and Services facility for sequencing and Dr. Xuanhao Sun at University of Connecticut’s Bioscience Electron Microscopy Laboratory for imaging.
This research was funded by the National Science Foundation (IOS-1656475) and the University of Connecticut.
Contributor Information
Jonathan L. Klassen, Email: jonathan.klassen@uconn.edu.
Katherine P. Lemon, Baylor College of Medicine, Houston, Texas, USA
DATA AVAILABILITY
Cultures isolated during this study were deposited in the NCTC and DMSZ culture collections as M. whartonense JKS002660 (NCTC 14863; DMSZ 115716) and S. attinicola JKS002670 (NCTC 14964; DMSZ 115717). All assembled genomes were deposited in the NCBI WGS database (Table S5), and their corresponding sequencing reads were deposited in the NCBI SRA database (SRR17695994 to SRR17696005). All community amplicon sequencing data were also deposited in the NCBI SRA database (SRR18059736 to SRR18059804), and all 16S rRNA gene sequences generated from pure cultures were deposited in the NCBI GenBank database (OM760043, OM812022-OM812029).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/mbio.00893-25.
All code needed to recapitulate the analyses in the paper.
Supplemental figures and tables.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Margulis L. 1971. Symbiosis and evolution. Sci Am 225:48–57. doi: 10.1038/scientificamerican0871-48 [DOI] [PubMed] [Google Scholar]
- 2. McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA 110:3229–3236. doi: 10.1073/pnas.1218525110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vallet-Gely I, Lemaitre B, Boccard F. 2008. Bacterial strategies to overcome insect defences. Nat Rev Microbiol 6:302–313. doi: 10.1038/nrmicro1870 [DOI] [PubMed] [Google Scholar]
- 4. Elnagdy S, Messing S, Majerus MEN. 2013. Two strains of male-killing Wolbachia in a ladybird, Coccinella undecimpunctata, from a hot climate. PLoS One 8:e54218. doi: 10.1371/journal.pone.0054218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas AE, Prosser WA. 1992. Synthesis of the essential amino acid tryptophan in the pea aphid (Acyrthosiphon pisum) symbiosis. J Insect Physiol 38:565–568. doi: 10.1016/0022-1910(92)90107-O [DOI] [Google Scholar]
- 6. Haine ER. 2008. Symbiont-mediated protection. Proc R Soc B 275:353–361. doi: 10.1098/rspb.2007.1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ebert D. 2013. The epidemiology and evolution of symbionts with mixed-mode transmission. Annu Rev Ecol Evol Syst 44:623–643. doi: 10.1146/annurev-ecolsys-032513-100555 [DOI] [Google Scholar]
- 8. Dale C, Moran NA. 2006. Molecular interactions between bacterial symbionts and their hosts. Cell 126:453–465. doi: 10.1016/j.cell.2006.07.014 [DOI] [PubMed] [Google Scholar]
- 9. Hansen AK, Moran NA. 2011. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids. Proc Natl Acad Sci USA 108:2849–2854. doi: 10.1073/pnas.1013465108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sirand-Pugnet P, Citti C, Barré A, Blanchard A. 2007. Evolution of mollicutes: down a bumpy road with twists and turns. Res Microbiol 158:754–766. doi: 10.1016/j.resmic.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 11. Whitman WB. 2015. Bergey’s manual of systematics of archaea and bacteria. Bergey’s Manual Trust, Hoboken, NJ, USA. [Google Scholar]
- 12. Bolaños LM, Servín-Garcidueñas LE, Martínez-Romero E. 2015. Arthropod-Spiroplasma relationship in the genomic era. FEMS Microbiol Ecol 91:1–8. doi: 10.1093/femsec/fiu008 [DOI] [PubMed] [Google Scholar]
- 13. Fudl-Allah AE. 1972. Culture of a mycoplasmalike organism associated with stubborn disease of citrus. Phytopathology 62:729. doi: 10.1094/Phyto-62-729 [DOI] [Google Scholar]
- 14. Bai X, Hogenhout SA. 2002. A genome sequence survey of the mollicute corn stunt spiroplasma Spiroplasma kunkelii. FEMS Microbiol Lett 210:7–17. doi: 10.1111/j.1574-6968.2002.tb11153.x [DOI] [PubMed] [Google Scholar]
- 15. Ramond E, Maclachlan C, Clerc-Rosset S, Knott GW, Lemaitre B. 2016. Cell division by longitudinal scission in the insect endosymbiont Spiroplasma poulsonii. mBio 7:e00881-16. doi: 10.1128/mBio.00881-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphery-Smith I, Grulet O, Le Goff F, Chastel C. 1991. Spiroplasma (Mollicutes: Spiroplasmataceae) pathogenic for Aedes aegypti and Anopheles stephensi (Diptera: Culicidae). J Med Entomol 28:219–222. doi: 10.1093/jmedent/28.2.219 [DOI] [PubMed] [Google Scholar]
- 17. Hamilton PT, Peng F, Boulanger MJ, Perlman SJ. 2016. A ribosome-inactivating protein in a Drosophila defensive symbiont. Proc Natl Acad Sci USA 113:350–355. doi: 10.1073/pnas.1518648113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson EO. 1971. The insect societies. Harvard University Press, Cambridge, MA, USA. [Google Scholar]
- 19. Mueller UG, Schultz TR, Currie CR, Adams RM, Malloch D. 2001. The origin of the attine ant-fungus mutualism. Q Rev Biol 76:169–197. doi: 10.1086/393867 [DOI] [PubMed] [Google Scholar]
- 20. Schultz TR, Brady SG. 2008. Major evolutionary transitions in ant agriculture. Proc Natl Acad Sci USA 105:5435–5440. doi: 10.1073/pnas.0711024105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wheeler WM. 1907. The fungus-growing ants of North America. Bull Am Museum Nat Hist 23:669–807. [Google Scholar]
- 22. Weber NA. 1972. The fungus-culturing behavior of ants. Am Zool 12:577–587. doi: 10.1093/icb/12.3.577 [DOI] [Google Scholar]
- 23. Hölldobler B, Wilson EO. 1990. The ants. Belknap Press, Cambridge, MA, USA. [Google Scholar]
- 24. Solomon SE, Rabeling C, Sosa‐Calvo J, Lopes CT, Rodrigues A, Vasconcelos HL, Bacci M Jr, Mueller UG, Schultz TR. 2019. The molecular phylogenetics of Trachymyrmex Forel ants and their fungal cultivars provide insights into the origin and coevolutionary history of “higher‐attine” ant agriculture. Syst Entomol 44:939–956. doi: 10.1111/syen.12370 [DOI] [Google Scholar]
- 25. Wirth R, Beyschlag W, Ryel RJ, Hölldobler B. 1997. Annual foraging of the leaf-cutting ant Atta colombica in a semideciduous rain forest in Panama. J Trop Ecol 13:741–757. doi: 10.1017/S0266467400010907 [DOI] [Google Scholar]
- 26. Weber NA. 1966. Fungus-growing ants. Science 153:587–604. doi: 10.1126/science.153.3736.587 [DOI] [PubMed] [Google Scholar]
- 27. De Fine Licht HH, Boomsma JJ. 2010. Forage collection, substrate preparation, and diet composition in fungus-growing ants. Ecol Entomol 35:259–269. doi: 10.1111/j.1365-2311.2010.01193.x [DOI] [Google Scholar]
- 28. Currie CR, Mueller UG, Malloch D. 1999. The agricultural pathology of ant fungus gardens. Proc Natl Acad Sci USA 96:7998–8002. doi: 10.1073/pnas.96.14.7998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Goldstein SL, Klassen JL. 2020. Pseudonocardia symbionts of fungus-growing ants and the evolution of defensive secondary metabolism. Front Microbiol 11:621041. doi: 10.3389/fmicb.2020.621041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishak HD, Miller JL, Sen R, Dowd SE, Meyer E, Mueller UG. 2011. Microbiomes of ant castes implicate new microbial roles in the fungus-growing ant Trachymyrmex septentrionalis. Sci Rep 1:204. doi: 10.1038/srep00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liberti J, Sapountzis P, Hansen LH, Sørensen SJ, Adams RMM, Boomsma JJ. 2015. Bacterial symbiont sharing in Megalomyrmex social parasites and their fungus-growing ant hosts. Mol Ecol 24:3151–3169. doi: 10.1111/mec.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhukova M, Sapountzis P, Schiøtt M, Boomsma JJ. 2017. Diversity and transmission of gut bacteria in Atta and Acromyrmex leaf-cutting ants during development. Front Microbiol 8:1942. doi: 10.3389/fmicb.2017.01942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sapountzis P, Zhukova M, Hansen LH, Sørensen SJ, Schiøtt M, Boomsma JJ. 2015. Acromyrmex leaf-cutting ants have simple gut microbiota with nitrogen-fixing potential. Appl Environ Microbiol 81:5527–5537. doi: 10.1128/AEM.00961-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sapountzis P, Nash DR, Schiøtt M, Boomsma JJ. 2019. The evolution of abdominal microbiomes in fungus-growing ants. Mol Ecol 28:879–899. doi: 10.1111/mec.14931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Funaro CF, Kronauer DJC, Moreau CS, Goldman-Huertas B, Pierce NE, Russell JA. 2011. Army ants harbor a host-specific clade of Entomoplasmatales bacteria. Appl Environ Microbiol 77:346–350. doi: 10.1128/AEM.01896-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kautz S, Rubin BER, Moreau CS. 2013. Bacterial Infections across the ants: frequency and prevalence of Wolbachia, Spiroplasma, and Asaia. Psyche (Stuttg) 936341. doi: 10.1155/2013/936341 [DOI] [Google Scholar]
- 37. Łukasik P, Newton JA, Sanders JG, Hu Y, Moreau CS, Kronauer DJC, O’Donnell S, Koga R, Russell JA. 2017. The structured diversity of specialized gut symbionts of the New World army ants. Mol Ecol 26:3808–3825. doi: 10.1111/mec.14140 [DOI] [PubMed] [Google Scholar]
- 38. Green EA, Klassen JL. 2022. Trachymyrmex septentrionalis ant microbiome assembly is unique to individual colonies and castes. mSphere 7:e0098921. doi: 10.1128/msphere.00989-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howe J, Schiøtt M, Boomsma JJ. 2019. Horizontal partner exchange does not preclude stable mutualism in fungus-growing ants. Behav Ecol 30:372–382. doi: 10.1093/beheco/ary176 [DOI] [Google Scholar]
- 40. Meziti A, Rodriguez-R LM, Hatt JK, Peña-Gonzalez A, Levy K, Konstantinidis KT. 2021. The reliability of metagenome-assembled genomes (MAGs) in representing natural populations: insights from comparing mags against isolate genomes derived from the same fecal sample. Appl Environ Microbiol 87:e02593-20. doi: 10.1128/AEM.02593-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen L-X, Anantharaman K, Shaiber A, Eren AM, Banfield JF. 2020. Accurate and complete genomes from metagenomes. Genome Res 30:315–333. doi: 10.1101/gr.258640.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sapountzis P, Zhukova M, Shik JZ, Schiott M, Boomsma JJ. 2018. Reconstructing the functions of endosymbiotic Mollicutes in fungus-growing ants. eLife 7:e39209. doi: 10.7554/eLife.39209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hutchinson GE. 1957. Concluding remarks. Cold Spring Harb Symp Quant Biol 22:415–427. doi: 10.1101/SQB.1957.022.01.039 [DOI] [Google Scholar]
- 44. Wexler AG, Bao Y, Whitney JC, Bobay L-M, Xavier JB, Schofield WB, Barry NA, Russell AB, Tran BQ, Goo YA, Goodlett DR, Ochman H, Mougous JD, Goodman AL. 2016. Human symbionts inject and neutralize antibacterial toxins to persist in the gut. Proc Natl Acad Sci USA 113:3639–3644. doi: 10.1073/pnas.1525637113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rock DI, Smith AH, Joffe J, Albertus A, Wong N, O’Connor M, Oliver KM, Russell JA. 2018. Context-dependent vertical transmission shapes strong endosymbiont community structure in the pea aphid, Acyrthosiphon pisum. Mol Ecol 27:2039–2056. doi: 10.1111/mec.14449 [DOI] [PubMed] [Google Scholar]
- 46. Oliver KM, Smith AH, Russell JA. 2014. Defensive symbiosis in the real world – advancing ecological studies of heritable, protective bacteria in aphids and beyond. Funct Ecol 28:341–355. doi: 10.1111/1365-2435.12133 [DOI] [Google Scholar]
- 47. Leibold MA, McPeek MA. 2006. Coexistence of the niche and neutral perspectives in community ecology. Ecology 87:1399–1410. doi: 10.1890/0012-9658(2006)87[1399:cotnan]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 48. Nayfach S, Roux S, Seshadri R, Udwary D, Varghese N, Schulz F, Wu D, Paez-Espino D, Chen I-M, Huntemann M, et al. 2021. A genomic catalog of Earth’s microbiomes. Nat Biotechnol 39:499–509. doi: 10.1038/s41587-020-0718-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee KM, Adams M, Klassen JL. 2019. Evaluation of DESS as a storage medium for microbial community analysis. PeerJ 7:e6414. doi: 10.7717/peerj.6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sosa-Calvo J, Jesovnik A, Okonski E, Schultz TR. 2015. Locating, collecting, and maintaining colonies of fungus-farming ants (Hymenoptera: Myrmicinae: Attini). Sociobiology 62:300–320. doi: 10.13102/sociobiology.v62i2.300-320 [DOI] [Google Scholar]
- 51. Tully JG, Whitcomb RF, Clark HF, Williamson DL. 1977. Pathogenic mycoplasmas: cultivation and vertebrate pathogenicity of a new Spiroplasma. Science 195:892–894. doi: 10.1126/science.841314 [DOI] [PubMed] [Google Scholar]
- 52. Green EA, Klassen JL. 2020. Draft genome sequence of Spiroplasma platyhelix ATCC 51748, isolated from a dragonfly. Microbiol Resour Announc 9:e00422-20. doi: 10.1128/MRA.00422-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, Fierer N, Knight R. 2011. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108 Suppl 1:4516–4522. doi: 10.1073/pnas.1000080107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. R Core Team . 2018. R: a language and environment for statistical computing
- 55. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–D596. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner FO. 2014. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res 42:D643–D648. doi: 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lane DJ. 1991. 16S/23S rRNA sequencing, p 115–175. In Stackebrandt E, Goodfellow M (ed), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, New York. [Google Scholar]
- 59. Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 62. Seppey M, Manni M, Zdobnov EM. 2019. BUSCO: assessing genome assembly and annotation completeness, p 227–245. In Kollmar M (ed), Gene prediction: methods and protocols. Springer, New York, NY. [DOI] [PubMed] [Google Scholar]
- 63. Eren AM, Esen ÖC, Quince C, Vineis JH, Morrison HG, Sogin ML, Delmont TO. 2015. Anvi’o: an advanced analysis and visualization platform for ’omics data. PeerJ 3:e1319. doi: 10.7717/peerj.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eren A Murat, Kiefl E, Shaiber A, Veseli I, Miller SE, Schechter MS, Fink I, Pan JN, Yousef M, Fogarty EC, et al. 2021. Community-led, integrated, reproducible multi-omics with anvi’o. Nat Microbiol 6:3–6. doi: 10.1038/s41564-020-00834-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. 2008. The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Price MN, Dehal PS, Arkin AP. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol 26:1641–1650. doi: 10.1093/molbev/msp077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Letunic I, Bork P. 2021. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49:W293–W296. doi: 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Klassen JL, Currie CR. 2013. ORFcor: identifying and accommodating ORF prediction inconsistencies for phylogenetic analysis. PLoS One 8:e58387. doi: 10.1371/journal.pone.0058387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. doi: 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Aluotto BB, Wittler RG, Williams CO, Faber JE. 1970. Standardized bacteriologic techniques for the characterization of Mycoplasma species. Int J Syst Bacteriol 20:35–58. doi: 10.1099/00207713-20-1-35 [DOI] [Google Scholar]
- 73. Tully JG. 1983. Biochemical and enzymatic tests in Mycoplasma identification: tests for digitonin sensitivity and cholesterol requirement. Methods Mycoplasmol 1:355–362. doi: 10.1016/B978-0-12-583801-6.50059-7 [DOI] [Google Scholar]
- 74. Gasparich GE, Kuo C-H. 2019. Genome analysis-based union of the genus Mesoplasma with the genus Entomoplasma. Int J Syst Evol Microbiol 69:2735–2738. doi: 10.1099/ijsem.0.003548 [DOI] [PubMed] [Google Scholar]
- 75. Gupta RS, Sawnani S, Adeolu M, Alnajar S, Oren A. 2018. Phylogenetic framework for the phylum Tenericutes based on genome sequence data: proposal for the creation of a new order Mycoplasmoidales ord. nov., containing two new families Mycoplasmoidaceae fam. nov. and Metamycoplasmataceae fam. nov. harbouring Eperythrozoon, Ureaplasma and five novel genera. Antonie Van Leeuwenhoek 111:1583–1630. doi: 10.1007/s10482-018-1047-3 [DOI] [PubMed] [Google Scholar]
- 76. Yan X-H, Pei S-C, Yen H-C, Blanchard A, Sirand-Pugnet P, Baby V, Gasparich GE, Kuo C-H. 2024. Delineating bacterial genera based on gene content analysis: a case study of the Mycoplasmatales–Entomoplasmatales clade within the class Mollicutes. Microb Genom 10:001321. doi: 10.1099/mgen.0.001321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. 2007. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57:81–91. doi: 10.1099/ijs.0.64483-0 [DOI] [PubMed] [Google Scholar]
- 78. Richter M, Rosselló-Móra R. 2009. Shifting the genomic gold standard for the prokaryotic species definition. Proc Natl Acad Sci USA 106:19126–19131. doi: 10.1073/pnas.0906412106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Keçeli SA, Miles RJ. 2002. Differential inhibition of mollicute growth: an approach to development of selective media for specific mollicutes. Appl Environ Microbiol 68:5012–5016. doi: 10.1128/AEM.68.10.5012-5016.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Béven L, Charenton C, Dautant A, Bouyssou G, Labroussaa F, Sköllermo A, Persson A, Blanchard A, Sirand-Pugnet P. 2012. Specific evolution of F1-Like ATPases in Mycoplasmas. PLoS One 7:e38793. doi: 10.1371/journal.pone.0038793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Pollack JD, Williams MV, McElhaney RN. 1997. The comparative metabolism of the mollicutes (Mycoplasmas): the utility for taxonomic classification and the relationship of putative gene annotation and phylogeny to enzymatic function in the smallest free-living cells. Crit Rev Microbiol 23:269–354. doi: 10.3109/10408419709115140 [DOI] [PubMed] [Google Scholar]
- 82. Rose DL, Kocka JP, Somerson NL, Tully JG, Whitcomb RF, Carle P, Bove JM, Colflesh DE, Williamson DL. 1990. Mycoplasma lactucae sp. nov., a sterol-requiring Mollicute from a plant surface. Int J Syst Bacteriol 40:138–142. doi: 10.1099/00207713-40-2-138 [DOI] [PubMed] [Google Scholar]
- 83. Williamson DL, Adams JR, Whitcomb RF, Tully JG, Carle P, Konai M, Bove JM, Henegar RB. 1997. Spiroplasma platyhelix sp. nov., a new mollicute with unusual morphology and genome size from the dragonfly Pachydiplax longipennis. Int J Syst Bacteriol 47:763–766. doi: 10.1099/00207713-47-3-763 [DOI] [PubMed] [Google Scholar]
- 84. Goldstein S. 2020. Exploring the genomic diversity of the Trachymyrmex septentrionalis-associated Pseudonocardia symbiont. University of Connecticut. [Google Scholar]
- 85. Ginete DR, Goodrich-Blair H. 2021. From binary model systems to the human microbiome: factors that drive strain specificity in host-symbiont associations. Front Ecol Evol 9:614197. doi: 10.3389/fevo.2021.614197 [DOI] [Google Scholar]
- 86. Saheb Kashaf S, Almeida A, Segre JA, Finn RD. 2021. Recovering prokaryotic genomes from host-associated, short-read shotgun metagenomic sequencing data. Nat Protoc 16:2520–2541. doi: 10.1038/s41596-021-00508-2 [DOI] [PubMed] [Google Scholar]
- 87. Seal JN, Tschinkel WR. 2008. Food limitation in the fungus‐gardening ant, Trachymyrmex septentrionalis. Ecol Entomol 33:597–607. doi: 10.1111/j.1365-2311.2008.01010.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barrera CA, Sosa‐Calvo J, Schultz TR, Rabeling C, Bacci M Jr. 2022. Phylogenomic reconstruction reveals new insights into the evolution and biogeography of Atta leaf-cutting ants (Hymenoptera: Formicidae). Syst Entomol 47:13–35. doi: 10.1111/syen.12513 [DOI] [Google Scholar]
- 89. Merzendorfer H, Zimoch L. 2003. Chitin metabolism in insects: structure, function and regulation of chitin synthases and chitinases. J Exp Biol 206:4393–4412. doi: 10.1242/jeb.00709 [DOI] [PubMed] [Google Scholar]
- 90. Blötz C, Stülke J. 2017. Glycerol metabolism and its implication in virulence in Mycoplasma. FEMS Microbiol Rev 41:640–652. doi: 10.1093/femsre/fux033 [DOI] [PubMed] [Google Scholar]
- 91. Tully JG, Whitcomb RF, Hackett KJ, Rose DL, Henegar RB, Bové JM, Carle P, Williamson DL, Clark TB. 1994. Taxonomic descriptions of eight new non-sterol-requiring mollicutes assigned to the genus Mesoplasma. Int J Syst Bacteriol 44:685–693. doi: 10.1099/00207713-44-4-685 [DOI] [PubMed] [Google Scholar]
- 92. Nygaard S, Hu H, Li C, Schiøtt M, Chen Z, Yang Z, Xie Q, Ma C, Deng Y, Dikow RB, Rabeling C, Nash DR, Wcislo WT, Brady SG, Schultz TR, Zhang G, Boomsma JJ. 2016. Reciprocal genomic evolution in the ant-fungus agricultural symbiosis. Nat Commun 7:12233. doi: 10.1038/ncomms12233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nygaard S, Zhang G, Schiøtt M, Li C, Wurm Y, Hu H, Zhou J, Ji L, Qiu F, Rasmussen M, Pan H, Hauser F, Krogh A, Grimmelikhuijzen CJP, Wang J, Boomsma JJ. 2011. The genome of the leaf-cutting ant Acromyrmex echinatior suggests key adaptations to advanced social life and fungus farming. Genome Res 21:1339–1348. doi: 10.1101/gr.121392.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Pereyre S, Sirand-Pugnet P, Beven L, Charron A, Renaudin H, Barré A, Avenaud P, Jacob D, Couloux A, Barbe V, de Daruvar A, Blanchard A, Bébéar C. 2009. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet 5:e1000677. doi: 10.1371/journal.pgen.1000677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Erthal M Jr, Peres Silva C, Samuels RI. 2004. Digestive enzymes of leaf-cutting ants, Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini): distribution in the gut of adult workers and partial characterization. J Insect Physiol 50:881–891. doi: 10.1016/j.jinsphys.2004.06.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All code needed to recapitulate the analyses in the paper.
Supplemental figures and tables.
Data Availability Statement
Cultures isolated during this study were deposited in the NCTC and DMSZ culture collections as M. whartonense JKS002660 (NCTC 14863; DMSZ 115716) and S. attinicola JKS002670 (NCTC 14964; DMSZ 115717). All assembled genomes were deposited in the NCBI WGS database (Table S5), and their corresponding sequencing reads were deposited in the NCBI SRA database (SRR17695994 to SRR17696005). All community amplicon sequencing data were also deposited in the NCBI SRA database (SRR18059736 to SRR18059804), and all 16S rRNA gene sequences generated from pure cultures were deposited in the NCBI GenBank database (OM760043, OM812022-OM812029).