ABSTRACT
Background
Fibrostenosis is a serious complication of eosinophilic oesophagitis, but there is a lack of consensus regarding its definition and assessment. This poses a barrier in clinical care and research.
Aim
To perform a systematic review to examine existing definitions and diagnostic methods of detection regarding fibrostenosis in eosinophilic oesophagitis.
Methods
We searched MEDLINE, Cochrane Library, EMBASE, Scopus, and Web of Science and included studies of paediatric and adult eosinophilic oesophagitis patients with fibrostenosis based on endoscopy, imaging, histopathology, functional studies, and biomarkers. We excluded studies with <10 patients. We chose fibrostenosis as the umbrella term, encompassing all definitions.
Results
We identified 230 studies. The four categories of fibrostenosis definitions were: (1) structural findings (stricture, rings, and/or narrowings) (n=204, 88.7%), (2) histology (n=85, 37.0%), (3) functional (functional lumen imaging probe) (n=15, 6.5%), and (4) biomarkers (n=7, 3.0%). Multiple definitions were used in 78 studies. Methods used to detect structural fibrostenosis included Eosinophilic Oesophagitis Endoscopic Reference Score fibrostenotic components, luminal diameter (endoscopy or imaging), need for dilation, and endoscopist or radiologist global impression. Methods used to detect histologic fibrostenosis included Eosinophilic Oesophagitis Histologic Scoring System lamina propria fibrosis, pathologist global impression, and basal zone hyperplasia. Methods used to detect functional fibrostenosis included distensibility and compliance.
Conclusions
Significant variability exists in definitions and diagnostic methods of detection regarding fibrostenosis in eosinophilic oesophagitis. Lack of agreement hampers progress in further investigating this complication. Development of consensus criteria is necessary to provide clarity for clinical care and research.
Keywords: fibrosis, fibrostenosis, remodelling eosinophilic oesophagitis
Significant variability exists in definitions and diagnostic methods of detection regarding fibrostenosis in eosinophilic oesophagitis. Lack of consensus poses a barrier in clinical care and research.

Abbreviations
- EGJ
oesophagogastric junction
- EoE
eosinophilic oesophagitis
- EoE‐HSS
EoE Histologic Scoring System
- EREFS
EoE Endoscopic Reference Score
- FLIP
functional lumen imaging probe
- LPF
lamina propria fibrosis
- MeSH
medical subject heading
- mL
millilitre
- mm
millimetre
- PRISMA
preferred reporting items for systematic review and meta‐analyses
1. Introduction
Eosinophilic oesophagitis (EoE) is a chronic inflammatory condition characterised by oesophageal eosinophilia, the disease burden of which is increasing in both incidence and prevalence [1, 2, 3, 4, 5]. EoE is a progressive disease with three distinct clinical phenotypes based on the presence of inflammation and excessive accumulation of extracellular matrix: inflammatory, fibrostenotic, or mixed inflammatory and fibrostenotic. The natural history of untreated EoE involves oesophageal remodelling leading to fibrostenosis [4, 6, 7], frequently resulting in dysphagia, food impactions and repeated endoscopic dilations.
Currently, there is a lack of consensus regarding the definition of fibrostenosing EoE [8, 9, 10]. In this systematic review, fibrostenosis was chosen as the umbrella term, encompassing all definitions related to luminal narrowing and remodelling. Definitions include structural (e.g., stricture), histology, functional (functional lumen imaging probe), and biomarkers. There is also a wide range of diagnostic methods of detection used in the scientific literature and in clinical practice for each definition. For example, methods of detection may include radiographic oesophageal lumen diameter or EoE Endoscopic Reference Score (EREFS) [11]. The lack of consensus poses a challenge for investigating fibrostenosis as a clinical problem and discovering treatments directed against fibrostenosis in EoE. The aim of this systematic review was to comprehensively summarise existing definitions and diagnostic methods of detection in the literature regarding fibrostenosis in adult and paediatric EoE patients.
2. Methods
2.1. Screening Strategy
We conducted a systematic review in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) guidelines [12]. Studies were identified by searching Medline (via Ovid SP), Embase (via Ovid SP), Web of Science (via Clarivate Analytics), Scopus (via Elsevier), Cinahl (via EBSCOhost), and Cochrane (via Wiley). The databases were searched from the start of databases through 06/2024, limited to English literature. Each database was searched using medical subject headings (MeSH) and keywords and synonyms derived from them, including eosinophilic oesophagitis, fibrosis, fibrostenosis, obstruction, stenosis, rings, and stricture. Food impaction was omitted, as a food impaction can occur due to inflammation without fibrostenosis. A detailed description of the search strategies for each of the databases is listed in Tables S1–S4. Identified studies were first screened in abstract form, with the removal of duplicates and the exclusion of abstracts outside of eligibility criteria. Full‐text articles were then reviewed to identify those meeting inclusion criteria. All references of full‐text articles were also reviewed manually to identify any additional relevant publications. The final identified studies were then used for data extraction (Table S5).
2.2. Eligibility Criteria
Eligibility criteria were set a priori by the study authors. The study protocol was added to PROSPERO under ID CRD42024558352. Inclusion criteria were original studies with the following study designs: prospective cohort studies, retrospective cohort studies, cross‐sectional studies, case–control studies, case series involving at least 10 patients, consensus statements, randomised or non‐randomised observational cohort studies, survey‐based studies, pilot studies, validation studies, open‐label trials, and randomised controlled trials; patients with EoE and fibrostenosis (defined as fibrosis, stenosis, obstruction, rings, stricture, narrowing, fibrostenosis). Exclusion criteria were narrative reviews or editorials, commentary articles, letters to the editor, languages other than English, studies with fewer than 10 patients, murine models, conference abstracts, pre‐clinical studies (in vitro, human oesophageal biopsies or fibroblasts), non‐fibrotic causes of stenosis (e.g., muscle spasm), or studies about EoE not evaluating fibrostenosis.
2.3. Outcomes of Interest
The outcomes of interest were definitions, diagnostic modalities, and methods of detection used for fibrostenotic EoE.
2.4. Study Selection and Data Extraction
Two reviewers (NF, CMaruggi) independently screened all abstracts identified and selected studies for inclusion based on eligibility criteria. They also extracted data in duplicate. Any disagreements regarding eligibility for inclusion or data extraction were resolved through consensus with a third reviewer (CAB). The following variables were extracted from each of the studies: first author name, journal, year of publication, study design, number of patients, patient cohort (adult, paediatric), definition of fibrostenosis, diagnostic modality, and methods used to describe fibrostenosis for each modality.
2.5. Risk of Bias
Two reviewers (NF, CMaruggi) independently assessed all studies for risk of bias. The following were used: Cochrane ROB2.0 Tool for randomised controlled trials, ROBINS‐I V2 for non‐randomised studies of interventions, Joanna Briggs Institute critical appraisal checklist for cross‐sectional studies and case series, and the Ottawa‐Newcastle scale for cohort and case–control studies (Table S6) [13, 14, 15, 16, 17, 18, 19]. Any discrepancies in scoring were resolved via discussion among the two reviewers, and if no resolution was achieved, the final decision was made by CAB.
2.6. Terminology
In this review article, fibrostenosis was chosen as the umbrella term, encompassing all definitions related to fibrosis, luminal narrowing, stenosis, and remodelling (e.g., fibrosis, stenosis, obstruction, rings, stricture, narrowing, narrow calibre oesophagus, fibrostenosis, remodelling).
3. Results
Of 7324 studies, 4098 were duplicates and excluded. A total of 3226 studies underwent abstract review. Of these, 2363 were excluded due to not meeting eligibility criteria. 863 studies were assessed in full‐text format, with 633 excluded due to only being available in abstract form, review articles, editorials, commentaries, letters to the editor, pre‐clinical studies, fewer than 10 patients, or articles not pertaining to fibrostenotic EoE. A total of 230 studies were included in the final qualitative analysis (Figure 1): cohort (n=151), cross‐sectional (n=24), case series (n=18), randomised control trials (n=15), case–control (n=8), survey‐based (n=5), consensus (n=3), validation (n=2), open‐label trials (n=2), and pilot studies (n=2). 110 studies were exclusively adult patients, and 56 studies were exclusively paediatric patients (Table S5).
FIGURE 1.

PRISMA diagram of included studies. Flow diagram of the number of records identified, included and excluded, and the reasons for exclusions.
3.1. Definitions
In the 230 studies, the following definitions were used to diagnose fibrostenosis (non‐exclusive): (1) structural (e.g., strictures) (n=204, 88.7%); (2) histology (n=85, 37.0%); (3) functional (functional lumen imaging probe) (n=15, 6.5%); 4) biomarkers (n=7, 3.0%) (Tables 1 and 2). Multiple definitions in the same publication were used in 78 studies [7, 24, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 225, 226, 249]. In these studies, more than one definition was accepted and did not require all definitions to be present. Multiple methods of detection in the same publication were used in 148 studies [7, 11, 20, 21, 22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 225, 226, 249].
TABLE 1.
Definitions and diagnostic methods of detection for fibrostenosis in eosinophilic esophagitis.
| Method of detection | Gold standard | Accuracy | Other | |
|---|---|---|---|---|
| Definition: stuctural (stricture, rings, and/or narrowings) (N=204) | ||||
| Endoscopy (N=202) | EREFS (N=111) | None | N/A |
Interobserver agreement [11]
Predictive ability to identify EoE from control (AUC) [20]
|
| Dilation (N=89) | None | N/A | N/A | |
| Endoscopist discretion (N=73) | Oesophagram (radiologist discretion) | 20% [21] | N/A | |
| Luminal diameter (N=25) | Oesophagram (narrowing or tablet delay [22]; <10th percentile healthy controls [23]) | 45% [22] |
Sensitivity: 14.7% Specificity: 79.2% [23] |
|
| Luminal narrowing (N=16) | None | N/A | N/A | |
| Imaging (N=12) |
Luminal narrowing by oesophageal length (N=5) |
Endoscopy (based on resistance or inability to pass endoscope) [24] | 70.6% [24] | N/A |
| Outside of 90%–95% percentile range for healthy controls (N=4) | Healthy control [23, 25, 26, 27] | N/A | N/A | |
| Luminal narrowing (N=4) | Endoscopy (EREFS) [28] | 74% | Detection using oesophagram versus endoscopy [28]
|
|
| Barium tablet delay or retention (N=2) | None | N/A | N/A | |
| Radiologist discretion (N=1) | None | N/A | N/A | |
| Luminal diameter (N=1) | None | N/A | N/A | |
| Oesophageal luminal diameter<15mm (N=1) | None | N/A | N/A | |
| Definition: histology (N=85) | ||||
| Lamina propria fibrosis (N=84) | EoE‐HSS: LPF stage & grade (N=33) | None | N/A |
Intra‐rater reliability
Intra‐observer agreement [30]
|
| Pathologist discretion (N=31) | None | N/A | N/A | |
| Other severity scoring systems (N=11) | None | N/A | N/A | |
| Presence LPF (N=9) | None | N/A | N/A | |
| Extracellular matrix remodelling or deposition (N=1) | None | N/A | N/A | |
| Presence of LPF or BZH. Presence of DEC if no lamina propria (EoE‐HSS) (N=1) | None | N/A | N/A | |
| Basal zone hyperplasia (N=3) | Pathologist discretion (N=1) | None | N/A | N/A |
| Remodelling score (N=1) | None | N/A | N/A | |
| Architectural features of EoE‐HSS (N=1) | None | N/A | N/A | |
| Definition: functional (functional lumen imaging probe) (N=15) | ||||
| Distensibility (N=14) | Distensibility Plateau (N=7) |
|
N/A |
|
| Distensibility (N=5) |
|
N/A | ||
| Distensibility slope (N=2) | EREFS rings 3 | N/A | Spearman's r=−0.33, p=0.004 [36] | |
| Distensibility index (N=2) | EREFS: rings or stricture | N/A | Presence versus absence: mean 3.7±1.8 versus 6.6±1.8mm2/mmHg, p=0.0003 [35] | |
| Compliance (N=5) | Compliance |
|
N/A |
|
| EGJ diameter (N=1) | Maximum EGJ diameter | Healthy control | N/A |
≤16mm (abnormal) [38] <12mm (severely abnormal) [38] |
| Definition: biomarkers (N=7) | ||||
| Human oesophageal biopsies | Vimentin, E‐cadherin expression (N=2) | None | N/A | N/A |
| TGFβ1 and pSMAD2/3 expression (N=1) | None | N/A | N/A | |
| TGFβ1 expression (N=1) | None | N/A | N/A | |
| Plasminogen Activator Inhibitor −1 expression (N=1) | None | N/A | N/A | |
| Profibrogenic gene expression (N=1) | None | N/A | N/A | |
| PRO‐C3; CTX‐III; PRO‐C6 (N=1) | None | N/A | N/A | |
Note: When available, additional information is provided such as accuracy, interobserver agreement, sensitivity, specificity and more. There are two articles that are not part of the systematic review but included in this table, as they are the reference for healthy control thresholds for distensibility plateau and oesophagogastric junction diameter [34, 38].
Abbreviations: AUC, area‐under‐the‐curve; BZH, basal zone hyperplasia; CCL18, CC chemokine ligand 18; CTX‐III, cross‐linked type III collagen; DEC, dyskeratotic epithelial cells; DP, distensibility plateau; EGJ, oesophagogastric junction; EoE‐HSS, Eosinophilic Oesophagitis Histologic Scoring System; EREFS, EoE Endoscopic References Score; FGF9, fibroblast growth factor 9; hpf, high power field; ICD, international classification of diseases; IL‐5, interleukin‐5; LPF, lamina propria fibrosis; ng, nanogram; PRO‐C3 and C6, type III and type VI collagen; pSMAD2/3, phospho‐SMAD2/3; TGFβ1, transforming growth factor beta.
TABLE 2.
Details on detection for fibrostenosis in eosinophilic oesophagitis.
| Method of detection | Literature | |
|---|---|---|
| Definition: structural (N = 204) [7, 11, 20, 21, 22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224] | ||
| Endoscopy (N = 202) [7, 11, 20, 21, 22, 23, 24, 25, 26, 28, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224] | EREFS a | N = 111 [7, 11, 20, 28, 32, 33, 35, 36, 37, 39, 40, 45, 46, 49, 50, 54, 55, 56, 57, 59, 60, 61, 62, 65, 67, 68, 70, 71, 72, 73, 74, 76, 78, 79, 80, 81, 83, 84, 85, 86, 87, 88, 89, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 110, 115, 122, 124, 126, 127, 128, 130, 131, 133, 136, 137, 138, 147, 148, 150, 151, 152, 153, 156, 157, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 214, 216, 219, 220, 221, 224] |
|
||
|
||
|
||
| Dilation | N = 89 [20, 23, 26, 32, 33, 35, 40, 44, 48, 49, 50, 51, 52, 53, 54, 55, 56, 60, 61, 67, 76, 77, 81, 82, 93, 94, 95, 96, 97, 106, 107, 108, 109, 111, 112, 113, 115, 116, 117, 118, 119, 120, 121, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 150, 151, 152, 153, 154, 155, 156, 159, 160, 161, 162, 164, 186, 187, 188, 189, 190, 191, 192, 193] | |
|
|
|
|
||
|
||
|
|
|
|
|
|
| Endoscopist discretion | N = 73 [20, 21, 23, 25, 26, 30, 31, 41, 42, 43, 44, 48, 50, 51, 52, 53, 58, 63, 64, 66, 69, 75, 77, 82, 105, 106, 107, 108, 111, 112, 113, 117, 118, 119, 121, 123, 132, 134, 135, 140, 141, 142, 143, 144, 145, 146, 147, 148, 154, 155, 157, 160, 162, 164, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 215, 217] | |
| Lumen diameter b | N = 25 [7, 22, 24, 41, 44, 46, 49, 56, 60, 83, 105, 106, 108, 109, 110, 113, 114, 120, 122, 129, 139, 149, 211, 212, 218] | |
|
||
|
||
|
||
|
||
|
|
|
|
|
|
|
|
|
|
|
|
| Luminal narrowing c | N = 16 [11, 22, 44, 47, 77, 79, 90, 109, 114, 116, 125, 129, 151, 213, 222, 223] | |
|
||
|
|
|
|
||
|
||
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
| Imaging (N = 12) [21, 22, 23, 24, 25, 26, 27, 28, 44, 89, 108, 158] | Luminal narrowing by oesophageal length d | N = 5 [22, 23, 24, 26, 44] |
|
||
|
||
|
||
|
||
|
|
|
| Outside of 90%–95% percentile range for healthy controls | N = 4 [23, 25, 26, 27] | |
| Luminal narrowing | N = 4 [22, 28, 108, 158] | |
| Barium tablet delay or retention | N = 2 [22, 28] | |
| Radiologist discretion | N = 1 [21] | |
| Luminal diameter | N = 1 [158] | |
| Oesophageal luminal diameter < 15 mm | N = 1 [89] | |
| Definition: histology (N = 85) [7, 24, 29, 30, 31, 35, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 92, 102, 103, 104, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248] | ||
| LPF (N = 84) [7, 24, 29, 30, 31, 35, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 102, 103, 104, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248] | EoE‐HSS: LPF stage & grade | N = 33 [7, 29, 30, 31, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 102, 104, 237, 238, 239, 240, 241, 242, 243, 245, 246] |
| Pathologist discretion | N = 31 [35, 39, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 234, 235, 236, 244, 247, 248] | |
| Other severity scoring systems e | N = 11 [40, 68, 69, 70, 103, 225, 226, 227, 231, 232, 233] | |
|
||
|
||
|
||
| Presence LPF f | N = 9 [24, 37, 41, 42, 43, 44, 228, 229, 230] | |
| Extracellular matrix remodelling or deposition | N = 1 [40] | |
| Presence of LPF or BZH. Presence of DEC if no lamina propria (EoE‐HSS) | N = 1 [7] | |
| Basal zone hyperplasia (N = 3) [39, 74, 92] | Pathologist discretion | N = 1 [39] |
| Remodelling score g | N = 1 [92] | |
| Architectural features of EoE‐HSS h | N = 1 [74] | |
| Definition: functional (N = 15) [32, 33, 35, 36, 37, 74, 77, 90, 92, 94, 95, 97, 98, 99, 249] | ||
| Distensibility (N = 14) [32, 33, 35, 36, 37, 74, 77, 90, 92, 94, 95, 97, 98, 99] | Distensibility plateau | N = 7 [32, 33, 77, 95, 97, 98, 99] |
|
||
|
||
|
|
|
|
|
|
| Distensibility | N = 5 [37, 74, 90, 92, 94] | |
| Distensibility slope | N = 2 [36, 95] | |
| Distensibility index | N = 2 [35, 90] | |
| Compliance (N = 5) [33, 92, 97, 99, 249] | Compliance | |
|
||
|
||
|
|
|
| Diameter (N = 1) [99] |
Maximum EGJ diameter
|
|
| Definition: biomarkers (N = 7) [71, 74, 101, 102, 225, 226, 249] | ||
| Human oesophageal biopsies |
Vimentin, E‐cadherin expression
|
N = 2 [71, 74] |
TGFβ
1
and pSMAD2/3 expression
|
N = 1 [225] | |
| TGFβ1 expression (no further detail) | N = 1 [102] | |
Plasminogen Activator Inhibitor −1 expression
|
N = 1 [249] | |
Profibrogenic gene expression
|
N = 1 [226] | |
PRO‐C3; CTX‐III; PRO‐C6
|
N = 1 [101] | |
| Multiple definitions: N = 78 [7, 24, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 225, 226, 249] | ||
| Multiple methods of detection: N = 148 [7, 11, 20, 21, 22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 225, 226, 249] | ||
Abbreviations: BZH, basal zone hyperplasia; cm, centimetre; CCL18, CC chemokine ligand 18; CTX‐III, cross‐linked type III collagen; DEC, dyskeratotic epithelial cells; EGJ, oesophagogastric junction; EoE‐HSS, Eosinophilic Oesophagitis Histologic Scoring System; EREFS, EoE Endoscopic References Score; FGF9, fibroblast growth factor 9; hpf, high power field; ICD, international classification of diseases; IL5, interleukin‐5; LPF, lamina propria fibrosis; mm, millimetre; mL, millilitre; ng, nanogram; PRO‐C3 and C6, type III and type VI collagen; pSMAD2/3, phospho‐SMAD2/3; TGFβ1, transforming growth factor beta.
Presence of EREFS means that there were rings and/or stricture identified on EREFS but no scoring performed. Severity of EREFS means that the scoring (grade of rings and stricture) was provided. Of note, only 1 article used rings 2–3 [172], but all other articles used rings 1–3. Fibrostenotic subscore of EREFS means that the fibrostenotic subscore was provided.
If not otherwise mentioned, lumen diameter was measured based on resistance or inability to pass an endoscope with a corresponding outer diameter (e.g., neonatal, paediatric, adult). The severity score entailed: 1 point for present but scope passes easily; 2 points for present but requires dilation or snug fit with standard endoscope; 15 points: unable to pass standard upper endoscope or repeated dilations in adult or any dilation in child
Ring severity was graded as 0 (normal), 1 (mild), 2 (moderate), and 3 (severe) with no further details. Ring presence was defined as circumferential mucosal plications during maximal insufflation, oriented in a perpendicular plane. Schatzki's ring severity was defined as either narrow (difficult to pass standard endoscope) or wide (easy to pass endoscope). Short versus long narrowing: short refers to 1 cm to one‐third oesophageal length, and long refers to more than one‐third oesophageal length.
Narrowing severity defined as short (1 cm to one‐third of oesophageal length) or long (more than one‐third oesophageal length)
(1) Score of 1–3 was based on collagen bundle thickness, accumulation, and number of fibroblasts using H&E or Masson's trichrome staining. (2) LPF defined as Grade 2 was based on dense and compact collagen deposition on Masson's trichrome staining. (3) Mild, moderate, or severe LPF was based on deposition of extracellular matrix on H&E stain with minimal detail.
Presence of fibrosis was based on dense collagen deposition with or without compression or elongation of intact fibroblasts. This was assessed on Masson's trichrome staining, light microscopy, or digital morphometry.
Remodelling Score: H&E staining. Composite of: severity of basal zone hyperplasia (scored 0–3), presence/absence of dilated intercellular spaces (scored 0/1), and the presence/absence of epithelial desquamation (scored 0/1). Maximum score of 5
Architectural features EoE‐HSS. Combination of any of the following features using grade and severity: basal zone hyperplasia, dilated intercellular spaces, dyskeratotic epithelial cells, and lamina propria fibrosis
Table 1 provides the broad definitions and methods of detection as well as associated literature, such as accuracy and interobserver agreement. Table 2 provides more details on the definitions and methods of detection along with citations for the 230 studies. Tables S7 and S8 provide the details on the definitions and methods of detection along with citations for adult and paediatric studies, respectively. Table S9 provides details listed per study.
3.2. Definition: Structural
The structural definition of fibrostenosis included stricture, rings, and/or narrowings [7, 11, 20, 21, 22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224]. Out of the 204 studies, structural findings were found on endoscopy (n=202, 99.0%) [7, 11, 20, 21, 22, 23, 24, 25, 26, 28, 30, 31, 32, 33, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 149, 150, 151, 152, 153, 154, 155, 156, 157, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 211, 212, 213, 214, 215, 216, 217, 218, 219, 220, 221, 222, 223, 224] or imaging (n=12, 5.9%) [21, 22, 23, 24, 25, 26, 27, 28, 44, 89, 108, 158].
3.2.1. Endoscopic Methods of Detection
Of 202 studies using endoscopy to detect stricture, rings and/or narrowings, the majority relied on the EoE Endoscopic Reference Score (EREFS) (n=111, 55.0%) [7, 11, 20, 28, 32, 33, 35, 36, 37, 39, 40, 45, 46, 49, 50, 54, 55, 56, 57, 59, 60, 61, 62, 65, 67, 68, 70, 71, 72, 73, 74, 76, 78, 79, 80, 81, 83, 84, 85, 86, 87, 88, 89, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 110, 115, 122, 124, 126, 127, 128, 130, 131, 133, 136, 137, 138, 147, 148, 150, 151, 152, 153, 156, 157, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176, 177, 178, 179, 180, 181, 182, 183, 184, 185, 214, 216, 219, 220, 221, 224]. This validated endoscopic scoring system assesses fibrostenotic features (rings and/or strictures) [11]. 28 studies reported the presence or absence of fibrostenotic features [37, 46, 49, 56, 57, 59, 67, 73, 87, 92, 102, 115, 124, 130, 131, 152, 153, 161, 166, 167, 168, 185, 214, 216, 219, 220, 221, 224], 63 studies reported the scoring (rings: 0–3, stricture 0–1) [11, 28, 35, 36, 39, 40, 50, 54, 55, 59, 60, 61, 62, 65, 68, 70, 72, 74, 76, 78, 79, 80, 83, 84, 85, 86, 88, 89, 91, 93, 94, 95, 96, 97, 98, 99, 100, 101, 103, 104, 110, 136, 147, 148, 150, 151, 156, 157, 159, 160, 162, 163, 164, 165, 169, 170, 171, 172, 180, 181, 182, 183, 184], and 30 calculated a fibrostenotic subscore [7, 20, 32, 33, 45, 71, 81, 84, 89, 97, 99, 101, 104, 122, 126, 127, 128, 133, 137, 138, 163, 165, 173, 174, 175, 176, 177, 178, 179, 180]. EREFS scoring provided in Table S10.
The next most commonly used method of detection was oesophageal dilation (n=89, 44.1%) [20, 23, 26, 32, 33, 35, 40, 44, 48, 49, 50, 51, 52, 53, 54, 55, 56, 60, 61, 67, 76, 77, 81, 82, 93, 94, 95, 96, 97, 106, 107, 108, 109, 111, 112, 113, 115, 116, 117, 118, 119, 120, 121, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 150, 151, 152, 153, 154, 155, 156, 159, 160, 161, 162, 164, 186, 187, 188, 189, 190, 191, 192, 193]. This was often retrospective and based on the endoscopy report with limited (if any) description (n=79) [20, 23, 26, 32, 33, 35, 40, 48, 49, 50, 51, 52, 53, 54, 55, 56, 60, 61, 67, 76, 77, 81, 93, 94, 95, 96, 97, 106, 107, 109, 111, 112, 113, 115, 117, 118, 119, 120, 121, 123, 124, 126, 127, 128, 129, 130, 131, 132, 133, 135, 136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147, 148, 150, 151, 152, 153, 155, 156, 159, 160, 161, 162, 164, 186, 187, 188, 189, 193]. Other studies provided more details: stricture requiring dilation (n=5) [82, 108, 134, 154, 190], ICD code (n=3) [116, 125, 191], patient reported history (n=1) [192], and a decreased lumen diameter requiring dilation (n=1) [44].
73 studies (36.1%) extracted data from the endoscopy report alone regarding strictures, rings, and/or narrowings [20, 21, 23, 25, 26, 30, 31, 41, 42, 43, 44, 48, 50, 51, 52, 53, 58, 63, 64, 66, 69, 75, 77, 82, 105, 106, 107, 108, 111, 112, 113, 117, 118, 119, 121, 123, 132, 134, 135, 140, 141, 142, 143, 144, 145, 146, 147, 148, 154, 155, 157, 160, 162, 164, 194, 195, 196, 197, 198, 199, 200, 201, 202, 203, 204, 205, 206, 207, 208, 209, 210, 227, 250]. There were no further details or criteria given.
25 studies (12.4%) specified criteria for the luminal diameter [7, 22, 24, 41, 44, 46, 49, 56, 60, 83, 105, 106, 108, 109, 110, 113, 114, 120, 122, 129, 139, 149, 211, 212, 218]. This was mostly based on resistance or inability to pass upper endoscopes (n=14) [22, 24, 44, 46, 49, 83, 105, 108, 120, 122, 129, 139, 149, 211]. This included adult, paediatric, and neonatal endoscopes. The next most commonly used criteria included measuring the luminal diameter by the dilator size that resulted in mucosal disruption (n=7) [41, 60, 106, 109, 113, 114, 211]. Other studies specified narrowings by the length of a segment with luminal constriction (Table 2).
3.2.2. Radiographic Methods of Detection
The only imaging used to detect stricture, ring and/or narrowing was a barium oesophagram. Of the 12 studies, 9 (75.0%) used oesophageal diameter or length of affected segment with inconsistent terminology [22, 23, 24, 26, 28, 44, 89, 108, 158]. For example, two studies defined a luminal narrowing of ≤1cm as a stricture [24, 44], whereas two other studies defined a luminal narrowing of ≤1cm as a ring and a luminal narrowing between 1 and 8cm in length as a stricture [23, 26]. Four studies (33.3%) defined an abnormal narrowing as a measurement outside of the 90%–95% percentile range for healthy controls [23, 25, 26, 27].
3.3. Definition: Histology
The definition of histology included 85 studies [7, 24, 29, 30, 31, 35, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 92, 102, 103, 104, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248]. For the most part, the histologic definition of fibrostenosis encompassed lamina propria fibrosis (LPF) (n=84; 98.8%) [7, 24, 29, 30, 31, 35, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 102, 103, 104, 225, 226, 227, 228, 229, 230, 231, 232, 233, 234, 235, 236, 237, 238, 239, 240, 241, 242, 243, 244, 245, 246, 247, 248] with only three studies (3.5%) assessing basal cell hyperplasia [39, 74, 92]. Criteria for LPF included increased, thickened, and altered collagen deposition as well as alterations in fibroblasts and epithelial thickness.
3.3.1. Histologic Methods of Detection
Of the 85 studies, 44 (51.8%) studies used a severity scoring system for LPF [7, 29, 30, 31, 40, 68, 69, 70, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 102, 103, 104, 225, 226, 227, 231, 232, 233, 237, 238, 239, 240, 241, 242, 243, 245, 246]. The grade and extent of LPF in the validated EoE histologic scoring system (EoE‐HSS) [29] are the most commonly used histologic scores (n=33) [7, 29, 30, 31, 71, 72, 73, 74, 75, 76, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 102, 104, 237, 238, 239, 240, 241, 242, 243, 245, 246]. Details on EoE‐HSS scoring are given in Table S11. Additional items of detection for LPF are listed in Tables 1 and 2. Presence of dense collagen deposition using Masson's trichrome staining was used in 9 studies with no detail on severity or quantification [24, 37, 41, 42, 43, 44, 228, 229, 230]. Pathologist discretion was used in 31 studies [35, 39, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 234, 235, 236, 244, 247, 248].
3.4. Definition: Functional
This definition encompasses functional lumen imaging probe (FLIP) parameters (N=15) [32, 33, 35, 36, 37, 74, 77, 90, 92, 94, 95, 97, 98, 99, 249].
3.4.1. FLIP Methods of Detection
Methods of detection include distensibility (n=14, 93.3%) [32, 33, 35, 36, 37, 74, 77, 90, 92, 94, 95, 97, 98, 99], compliance (n=5, 33.3%) [33, 92, 97, 99, 249], and maximum oesophagogastric junction diameter (n=1, 6.7%) [99]. More details are given below.
3.4.1.1. Distensibility Plateau
The most commonly utilised method of detection for distensibility was the distensibility plateau (n=7) [32, 33, 77, 95, 97, 98, 99]. Specific thresholds varied by study. An abnormal distensibility plateau were defined as ≤17mm (n=6) [32, 33, 95, 97, 98, 99] and a severely reduced distensibility plateau was defined as <13mm [32, 95] or <14mm [99]. Thresholds were initially determined using a history of food impaction as a surrogate marker [95], but were confirmed when assessing healthy controls [34]. Another study using thresholds defined an abnormal distensibility plateau as <15mm [77]. However, there was no clear rationale for this definition.
3.4.1.2. Distensibility, Distensibility Slope, Distensibility Index
The three other methods of distensibility detection include distensibility (n=5) [37, 74, 90, 92, 94], distensibility slope (n=2) [36, 95], and distensibility index (n=2) [35, 90]. Description regarding measurements can be found in Table S12.
3.4.1.3. Compliance
Five studies used compliance as a readout for fibrostenosis (Table S12) [33, 92, 97, 99, 249]. In a prospective cohort study of 171 adult EoE patients, a compliance of >0.2mL/mmHg was defined as normal [33]. There were limited details on how this cutoff was determined. In subsequent studies, abnormal compliance was defined as <450mm3/mmHg and severely abnormal compliance was defined as <300mm3/mmHg [97, 99]. Thresholds were determined using that same prospective cohort study, but analysing the data from healthy controls [33].
3.4.1.4. Oesophagogastric Junction (EGJ) Diameter
Lastly, one study used the maximum EGJ diameter to determine fibrostenosis [99]. Abnormal was defined as ≤16mm and severely abnormal was defined as <12mm based on data from healthy controls [38].
3.5. Definition: Biomarkers
7 studies used biomarkers as the definition of fibrostenosis with varying methods of detection listed in Tables 1 and 2 [71, 74, 101, 102, 225, 226, 249]. Examples include expression of vimentin, E‐cadherin, TGFβ1, pSMAD2/3, SOX2, and plasminogen activator inhibitor‐1.
3.6. Oesophageal Luminal Diameter
Specific oesophageal luminal diameter thresholds were used in structural (endoscopic and imaging) and functional (FLIP) definitions (Figure 2). Endoscopic thresholds include <7mm, ≤10mm, and ≤13mm, which were determined by the resistance or inability to pass an endoscope [22, 24, 44, 46, 49, 83, 105, 108, 110, 120, 122, 129, 139, 149, 211, 218] When using oesophagram, the <10th percentile of healthy controls was frequently used, which corresponded to an oesophageal luminal diameter of ≤15mm [23, 25, 26, 27]. Tablet delay was less frequently used but corresponded to an oesophageal luminal diameter of ≤13mm [22]. Lastly, FLIP used thresholds of ≤16mm (abnormal) and <12mm (severely abnormal) [38].
FIGURE 2.
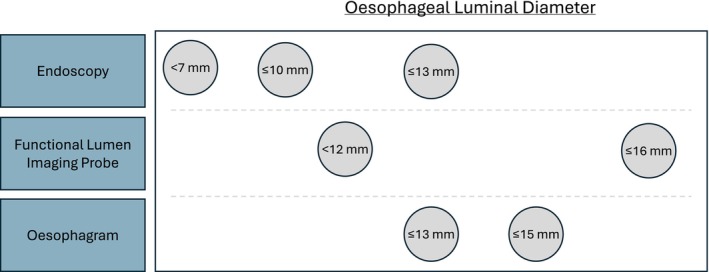
Fibrostenosis and oesophageal luminal diameters. Oesophageal luminal diameters that are highlighted by different methods of detection, such as endoscopy, imaging, and functional lumen imaging probe.
3.7. Comparing Adult and Paediatric Studies
Studies exclusively assessing adult versus paediatric patients were compared. Studies were excluded if both adult and paediatric patients were included or excluded if they were consensus, survey, or validation studies. Using Fisher's Exact Test and assuming a significance level of 0.05, structural definitions were more commonly used in adult studies compared with paediatric studies (98.2% vs. 69.6%, p<0.001). Alternatively, histologic definitions were more commonly used in paediatric studies than in adult studies (69.6% vs. 20.9%, p<0.001). There were no significant differences between frequencies of functional definitions, biomarker definitions, use of multiple definitions, and use of multiple methods of detection in adult and paediatric studies (9.1 vs. 8.9%; 2.7 vs. 5.4%; 32.7 vs. 46.5%; 66.4 vs. 57.1%). There were also no significant differences when comparing the frequencies of the specific methods of detection.
4. Discussion
Untreated eosinophilic oesophagitis can lead to remodelling and fibrostenosis with significant clinical implications [4, 6, 7]. However, there is no standardised definition of fibrostenosis in clinical practice or in clinical research studies. In this systematic review, we detected significant variability in the definitions of fibrostenosis as well as methods of detection. The two most common definitions included (1) structural presence of endoscopic or radiographic stricture, rings, and/or narrowings and (2) histologic presence of lamina propria fibrosis. However, many studies used more than one definition. The methods of detection for each modality were highly variable, and few validated scoring systems were used.
Regarding the structural definition of fibrostenosis, both endoscopy and radiology were used. A validated endoscopic scoring system (EREFS) for fibrostenotic EoE features exists. It is important to note that the reliability for determining the presence and severity of rings or strictures is limited. The interobserver agreement was only moderate for both (k=0.40 with 56% pairwise agreement; k=0.52 with 79% pairwise agreement, respectively) [11]. Novel techniques may help improve detection. For example, white light imaging combined with linked colour imaging had a superior interobserver agreement compared to white light imaging alone (stricture: ƙ=0.51 vs. 0.39, respectively, p=0.0072). However, the clinical applicability remains to be determined.
There were conflicting data comparing endoscopy to imaging regarding fibrostenosis identification. Three studies suggested that an oesophagram was superior to endoscopy for a diagnosis of a stricture (20%–45% strictures identified on endoscopy vs. 95.5%–100% on oesophagram) [21, 22, 23]. Given that an oesophagram was utilised as the gold standard, there is the possibility of a false positive by oesophagram. Using endoscopy as the gold standard, a different study found that barium oesophagram failed to identify small‐calibre oesophagus, ringed oesophagus, or stricture 29.4% of the time [24]. In a large retrospective cohort of 70 adult EoE patients, there was no significant difference between detection of fibrostenosis using an oesophagram and endoscopy (fibrotic subscore on EREFS): 74% and 84%, p=0.07 [28]. Overall, endoscopy is likely the more practical of the two methods. An oesophagram is solely diagnostic, but during an endoscopy, histology can be obtained and a therapeutic dilation performed.
Given that remodelling begins at the microscopic level, it is likely that early stages of fibrostenosis are missed in the current paradigm that uses endoscopy or imaging. Endoscopic biopsies can be very useful, but to date no standardised approach for histologic assessment has been developed. There is a standardised approach to obtaining biopsies (number and location), but it remains unclear if taking biopsies at the location of a ring or stricture would improve yield for detection of LPF. Only one validated scoring system (EoE‐HSS [29]) exists, but the majority of studies in this review did not use this score. For the EoE‐HSS, a strong interobserver agreement on stage and grade of fibrosis was noted (Kendall's coefficient: 0.8 and 0.82, respectively [29]), but the experience of the pathologist does play a role [30]. Artificial intelligence is a promising modality to aid pathologists with less experience (accuracy of 97% and 96% for assessing lamina propria and collagen deposition, respectively) [241]. Of note, the EoE‐HSS is a detailed and time‐consuming scoring system, which may limit the clinical applicability. Abbreviated scoring systems may be better adapted for clinical practice, but this will require future research and validation. For example, the histologic fibrostenotic component of the Index of Severity for Eosinophilic Esophagitis (I‐SEE) is clinically useful and is a simplified derivation of the EoE‐HSS [7]. However, the I‐SEE still requires validation. Lastly, a major limitation when relying on LPF is that the yield of obtaining adequate lamina propria without crush artefact can be low. Percentage of adequate lamina propria in routine oesophageal biopsies ranged from 15%–75.0% per individual biopsy (Table S13) [42, 68, 72, 73, 75, 78, 80, 236, 237]. However, taking multiple biopsies per patient, targeting distal oesophagus, and using a web‐based model to predict LPF are all methods to improve LPF detection. When multiple biopsies per patient are obtained, the yield can be as high as 96.2% [35, 42, 43, 49, 51, 73, 75, 78, 237] Incidence of evaluable lamina propria is higher in the distal oesophagus [42, 239]. At least 7 biopsies should be taken from the mid‐distal oesophagus as the probability of detecting LPF reached >95% [42]. Lastly, there is a promising web‐based model to predict LPF when there is inadequate lamina propria (grade and stage EoE‐HSS LPF: area under the curve with 95% confidence interval: 0.77 [0.69–0.84] and 0.75 [0.67–0.82], respectively; accuracy of 78% and 72%, respectively) [240].
Functional definitions (FLIP parameters) correlate significantly with endoscopic fibrostenotic findings [32, 33, 35, 36], but it remains unclear if FLIP will be useful as a primary or adjunctive modality for assessing fibrostenosis. Based on healthy controls and the risk of food impaction, thresholds for distensibility plateau (abnormal ≤17mm, severely abnormal <14mm), compliance (abnormal ≤450mm3/mmHg, severely abnormal <300mm3/mmHg), and EGJ diameter (abnormal ≤16mm, severely abnormal <12mm) have been defined. However, thresholds are based on a limited number of studies, which may require a larger validation cohort. Operational challenges may need to be overcome. FLIP is not readily available at all institutions or practices and incurs additional costs, and there have been no validation studies comparing reliability between community and academic endoscopists. However, there is research demonstrating that measurement technique may matter [96] Thus, standardisation of measurement is needed.
The inability to assess the transmural wall changes of the oesophagus remains a major limitation in the endoscopic and histopathologic assessment. Fibrostenosis is a transmural pathologic process, but our current methods provide minimal information regarding disease progression in deeper mural layers. As opposed to other disease states, such as inflammatory bowel diseases or diverticulitis, full‐thickness oesophageal resections are rarely performed in EoE, which greatly limits our knowledge and research capabilities. FLIP parameters, biomarkers, or other histologic assessments may hold promise for assessing oesophageal transmural properties and changes. However, future studies are needed.
There are several limitations to this systematic review. Many definitions and diagnostic methods of detection are not validated scoring systems. The majority of studies are retrospective, with few prospective or randomised controlled trials. There are very few studies that directly compare definitions or modalities with each other in the same patient. Non‐fibrotic causes of stenosis (e.g., muscle spasms) were not included. Given the heterogeneity of this systematic review, a meta‐analysis could not be performed. There are also multiple strengths of this article. This is the largest known systematic review to evaluate fibrostenosis in EoE. There have been systematic reviews of prevalence, treatment, perforation, natural history, and dilation among other topics, but none to our knowledge dedicated solely to fibrostenosis. A commonly accepted gold standard for fibrostenosis is missing, and our review provides the basis for future work in this area.
Based on this systematic review, we propose the following at this time: (1) In clinical practice, fibrostenosis should be defined as endoscopic findings (fibrostenotic parameters of the EREFS); and (2) in clinical research, there should be a combined assessment of endoscopic findings (fibrostenotic EREFS) and histopathologic findings (LPF on EoE‐HSS). Both EREFS and EoE‐HSS are validated scoring systems. FLIP can be used as an adjunctive measure in either scenario, but there is not enough data or widespread availability to support its primary use. Biomarkers, which may have future potential, are not a practical definition for fibrostenosis. Biomarkers are primarily a research‐only tool and are not readily available to clinicians and scientists.
In clinical practice, endoscopic evaluation is preferred for a few reasons. Endoscopy is more practical, as it is needed to diagnose and monitor response to therapy in EoE, while barium oesophagram is less frequently utilised. There is substantially more data behind the use of EREFS compared with imaging. For clinical research, it is reasonable to also include LPF on EoE‐HSS, as it may capture early findings of fibrostenosis that may not be endoscopically visible. For research, there is typically more time and resources available, which would allow for the collection of a sufficient number of biopsies and collaboration with an experienced GI pathologist. Further assessment is needed to determine clinically relevant thresholds of oesophageal lumen diameter, ring severity, and LPF severity.
Fibrostenosis is a challenging clinical problem for patients with EoE, resulting in dysphagia, food impactions, and repeated endoscopic dilations. In this systematic review, we show significant heterogeneity in definitions, diagnostic modalities, and methods of detection for fibrostenosis in EoE. Lack of agreement hampers progress in preventing, diagnosing, and managing this significant complication. Standardised definitions generated by EoE experts according to a UCLA/RAND process are a reasonable next step, which is already underway [251]. Only then will we have a robust basis for future investigation of fibrostenosing EoE.
Author Contributions
Claire A. Beveridge: conceptualization, investigation, writing – original draft, methodology, data curation, formal analysis, supervision, visualization, project administration, writing – review and editing. Natalie Farha: data curation, investigation. Christina Hermanns: data curation, investigation. Chiara Maruggi: data curation, investigation. Katherine Falloon: methodology. Shivani Thanawala: writing – review and editing. Mary Pat Harnegie: methodology, resources. J. Mark Brown: writing – review and editing. Andrei I. Ivanov: writing – review and editing. Evan S. Dellon: writing – review and editing. Gary W. Falk: writing – review and editing. Christopher Ma: writing – review and editing. Nirmala Gonsalves: writing – review and editing. Anthony Lembo: writing – review and editing. Amanda B. Muir: writing – review and editing. Scott Gabbard: writing – review and editing. Glenn T. Furuta: writing – review and editing. Florian Rieder: conceptualization, supervision, visualization, formal analysis, writing – original draft, writing – review and editing.
Conflicts of Interest
C.A.B.: consultant: Guidepoint, Takeda; speaking: GastroGirl. K.F.: grant funding: Crohn's and Colitis Foundation (916943), Pfizer (90146787); advisory panel: Janssen. E.S.D.: research funding: Adare/Ellodi, Allakos, Arena/Pfizer, AstraZeneca, Celldex, Eupraxia, Ferring, GSK, Meritage, Miraca, Nutricia, Celgene/Receptos/BMS, Regeneron, Revolo, Sanofi, Shire/Takeda; consultant: Abbvie, Adare/Ellodi, Akesobio, Alfasigma, ALK, Allakos, Amgen, Apollo, Aqilion, Arena/Pfizer, Aslan, AstraZeneca, Avir, Biocryst, Bryn, Calypso, Celgene/Receptos/BMS, Celldex, EsoCap, Eupraxia, Dr. Falk Pharma, Ferring, GI Reviewers, GSK, Holoclara, Invea, Knightpoint, LucidDx, Morphic, Nexstone Immunology/Uniquity, Nutricia, Parexel/Calyx, Phathom, Regeneron, Revolo, Robarts/Alimentiv, Sanofi, Shire/Takeda, Target RWE, Upstream Bio; educational grant: Allakos, Aqilion, Holoclara, Invea. G.W.F.: research funding: Celldex, Ellodi Pharmaceuticals, Nexteos, Arena/Pfizer, Bristol, Myers/Squibb, Regeneron/Sanofi, Lucid, Takeda; consulting: Ellodi Pharmaceuticals, Ubiquity Bio, Bristol Myers Squibb, Regeneron/Sanofi, Phathom Pharmaceuticals. C.M.: consultant: AbbVie, Alimentiv, Amgen, AVIR Pharma Inc., Bristol Myers Squibb, Celltrion, Eli Lilly, Ferring, Forte Biosciences, Fresenius Kabi, Gilead, Janssen, McKesson, Mirador Therapeutics, Mylan, Pendopharm, Pfizer, Prometheus Biosciences Inc., Roche, Sanofi, Takeda, Tillotts Pharma; speaker: AbbVie, Amgen, AVIR Pharma Inc., Alimentiv, Bristol Myers Squibb, Eli Lilly, Ferring, Fresenius Kabi, Janssen, Merck, Organon, Pendopharm, Pfizer, Sanofi, Takeda, Tillotts Pharma; royalties: Springer Publishing; research support: AbbVie, Eli Lilly, Ferring, Pfizer. N.G.: consultant: AstraZeneca, BMS, Sanofi, Regeneron, Takeda, Exact Sciences, Eupraxia, Uniquity; speaking: Takeda, Sanofi, Regeneron, BMS; royalties: Up‐to‐date. A.L.: consultant: Takeda, Ardelyx, Ironwood, Gemelli, Vibrant, Evoke, BioAmerica, Arena, Aeon, Amylyx, Pfizer, BMS, J&J. A.B.M.: medical advisory board: Regeneron/Sanofi, Bristol Meyers Squib, Uniquity, Takeda. S.G.: speaking: GastroGirl. G.T.F.: EnteroTrack (CMO). F.R.: consultant: Adiso, Adnovate, Agomab, Allergan, AbbVie, Arena, Astra Zeneca, Bausch & Lomb, Boehringer‐Ingelheim, Celgene/BMS, Celltrion, CDISC, Celsius, Cowen, Eugit, Ferring, Galapagos, Galmed, Genentech, Gilead, Gossamer, Granite, Guidepoint, Helmsley, Horizon Therapeutics, Image Analysis Limited, Index Pharma, Landos, Jannsen, Koutif, Mestag, Metacrine, Mirum, Mopac, Morphic, Myka Labs, Organovo, Origo, Palisade, Pfizer, Pliant, Prometheus Biosciences, Receptos, RedX, Roche, Samsung, Sanofi, Surmodics, Surrozen, Takeda, Techlab, Teva, Theravance, Thetis, Tr1x Bio, UCB, Ysios, 89Bio. N.F., C.H., C.M., S.T., M.P.H., J.M.B., A.I.I.: none.
Supporting information
Data S1.
Handling Editor: Colin Howden
Funding: The authors received no specific funding for this work.
Data Availability Statement
Data available upon request. Added to PROSPERO under ID CRD42024558352.
References
- 1. Hirano I., Chan E. S., Rank M. A., et al., “AGA Institute and the Joint Task Force on Allergy‐Immunology Practice Parameters Clinical Guidelines for the Management of Eosinophilic Esophagitis,” Gastroenterology 158 (2020): 1776–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lucendo A. J., Molina‐Infante J., Arias A., et al., “Guidelines on Eosinophilic Esophagitis: Evidence‐Based Statements and Recommendations for Diagnosis and Management in Children and Adults,” United European Gastroenterology Journal 5 (2017): 335–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beveridge C. A., Gabbard S., and Rieder F., “Not Hard to Swallow‐Understanding Endothelial‐Fibroblast Crosstalk in Eosinophilic Esophagitis,” Gastroenterology 162 (2022): 390–392. [DOI] [PubMed] [Google Scholar]
- 4. Muir A. and Falk G. W., “Eosinophilic Esophagitis: A Review,” JAMA 326 (2021): 1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roberts S. E., Morrison‐Rees S., Thapar N., and Williams J. G., “Incidence and Prevalence of Eosinophilic Oesophagitis Across Europe: A Systematic Review and Meta‐Analysis,” United European Gastroenterology Journal 12 (2024): 89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shaheen N. J., Mukkada V., Eichinger C. S., Schofield H., Todorova L., and Falk G. W., “Natural History of Eosinophilic Esophagitis: A Systematic Review of Epidemiology and Disease Course,” Diseases of the Esophagus 31 (2018): 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dellon E. S., Khoury P., Muir A. B., et al., “A Clinical Severity Index for Eosinophilic Esophagitis: Development, Consensus, and Future Directions,” Gastroenterology 163 (2022): 59–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aceves S. S., “Eosinophilic Esophagitis,” Immunology and Allergy Clinics of North America 35 (2015): 145–159. [DOI] [PubMed] [Google Scholar]
- 9. Dellon E. S. and Hirano I., “Epidemiology and Natural History of Eosinophilic Esophagitis,” Gastroenterology 154 (2018): 319–332.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menard‐Katcher C. and Aceves S., “Pathophysiology and Clinical Impact of Esophageal Remodeling and Fibrosis in Eosinophilic Esophagitis,” Immunology and Allergy Clinics of North America 44 (2024): 129–143. [DOI] [PubMed] [Google Scholar]
- 11. Hirano I., Moy N., Heckman M. G., Thomas C. S., Gonsalves N., and Achem S. R., “Endoscopic Assessment of the Oesophageal Features of Eosinophilic Oesophagitis: Validation of a Novel Classification and Grading System,” Gut 62 (2013): 489–495. [DOI] [PubMed] [Google Scholar]
- 12. Page M. J., McKenzie J. E., Bossuyt P. M., et al., “The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews,” BMJ (Clinical Research Ed.) 372 (2021): n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterne J. A., Savović J., Page M. J., et al., “RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials,” BMJ (Clinical Research Ed.) 366 (2019): l4898. [DOI] [PubMed] [Google Scholar]
- 14. Wells G. A., Wells G., Shea B., et al., “The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta‐Analyses,” (2014), https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15. Ma L. L., Wang Y. Y., Yang Z. H., Huang D., Weng H., and Zeng X. T., “Methodological Quality (Risk of Bias) Assessment Tools for Primary and Secondary Medical Studies: What Are They and Which Is Better?,” Military Medical Research 7 (2020): 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. ELC A., Porritt K., Pilla B., and Jordan Z., eds., JBI Manual for Evidence Synthesis (JBI, 2024). [Google Scholar]
- 17. Sterne J. A., Hernán M. A., Reeves B. C., et al., “ROBINS‐I: A Tool for Assessing Risk of Bias in Non‐Randomised Studies of Interventions,” BMJ (Clinical Research Ed.) 355 (2016): i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells G., Shea B., O'Connell D., et al., “The Newcastle‐Ottawa Scale (NOS) for Assessing the Quality of Case‐Control Studies in Meta‐Analyses,” European Journal of Epidemiology 25 (2011): 603–605. [DOI] [PubMed] [Google Scholar]
- 19. Moola S. M. Z., Tufanaru C., Aromataris E., et al., “Chapter 7: Systematic Reviews of Etiology and Risk,” in JBI Manual for Evidence Synthesis, ed. Aromataris E. M. Z. (JBI (Joanna Briggs Institute), 2020). [Google Scholar]
- 20. Dellon E. S., Cotton C. C., Gebhart J. H., et al., “Accuracy of the Eosinophilic Esophagitis Endoscopic Reference Score in Diagnosis and Determining Response to Treatment,” Clinical Gastroenterology and Hepatology 14 (2016): 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Binkovitz L. A., Lorenz E. A., Di Lorenzo C., et al., “Pediatric Eosinophilic Esophagitis: Radiologic Findings With Pathologic Correlation,” Pediatric Radiology 40 (2010): 714–719. [DOI] [PubMed] [Google Scholar]
- 22. Menard‐Katcher C., Swerdlow M. P., Mehta P., Furuta G. T., and Fenton L. Z., “Contribution of Esophagram to the Evaluation of Complicated Pediatric Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 61 (2015): 541–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gentile N., Katzka D., Ravi K., et al., “Oesophageal Narrowing Is Common and Frequently Under‐Appreciated at Endoscopy in Patients With Oesophageal Eosinophilia,” Alimentary Pharmacology & Therapeutics 40 (2014): 1333–1340. [DOI] [PubMed] [Google Scholar]
- 24. Al‐Hussaini A., AboZeid A., and Hai A., “How Does Esophagus Look on Barium Esophagram in Pediatric Eosinophilic Esophagitis?,” Abdominal Radiology (NY) 41 (2016): 1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee J., Huprich J., Kujath C., et al., “Esophageal Diameter Is Decreased in Some Patients With Eosinophilic Esophagitis and Might Increase With Topical Corticosteroid Therapy,” Clinical Gastroenterology and Hepatology 10 (2012): 481–486. [DOI] [PubMed] [Google Scholar]
- 26. Podboy A., Katzka D. A., Enders F., et al., “Oesophageal Narrowing on Barium Oesophagram Is More Common in Adult Patients With Eosinophilic Oesophagitis Than PPI‐Responsive Oesophageal Eosinophilia,” Alimentary Pharmacology & Therapeutics 43 (2016): 1168–1177. [DOI] [PubMed] [Google Scholar]
- 27. Muinuddin A., O'Brien P. G., Hurlbut D. J., and Paterson W. G., “Diffuse Esophageal Narrowing in Eosinophilic Esophagitis: A Barium Contrast Study,” Journal of the Canadian Association of Gastroenterology 2 (2019): 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nelson M. J., Miller F. H., Moy N., et al., “Comparison of Endoscopy and Radiographic Imaging for Detection of Esophageal Inflammation and Remodeling in Adults With Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 87 (2018): 962–968. [DOI] [PubMed] [Google Scholar]
- 29. Collins M. H., Martin L. J., Alexander E. S., et al., “Newly Developed and Validated Eosinophilic Esophagitis Histology Scoring System and Evidence That It Outperforms Peak Eosinophil Count for Disease Diagnosis and Monitoring,” Diseases of the Esophagus 30 (2017): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vieira M. C., Gugelmin E. S., Percicote A. P., et al., “Intra‐ and Interobserver Agreement of Histopathological Findings in Pediatric Patients With Eosinophilic Esophagitis,” Jornal de Pediatria 98 (2022): 26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Warners M. J., Ambarus C. A., Bredenoord A. J., et al., “Reliability of Histologic Assessment in Patients With Eosinophilic Oesophagitis,” Alimentary Pharmacology & Therapeutics 47 (2018): 940–950. [DOI] [PubMed] [Google Scholar]
- 32. Carlson D. A., Shehata C., Gonsalves N., et al., “Esophageal Dysmotility Is Associated With Disease Severity in Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 20 (2022): 1719–1728.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moosavi S., Shehata C., Kou W., et al., “Measuring Esophageal Compliance Using Functional Lumen Imaging Probe to Assess Remodeling in Eosinophilic Esophagitis,” Neurogastroenterology and Motility 35 (2023): e14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Carlson D. A., Kou W., Lin Z., et al., “Normal Values of Esophageal Distensibility and Distension‐Induced Contractility Measured by Functional Luminal Imaging Probe Panometry,” Clinical Gastroenterology and Hepatology 17 (2019): 674–681.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hoffmann N. V., Keeley K., and Wechsler J. B., “Esophageal Distensibility Defines Fibrostenotic Severity in Pediatric Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 21 (2023): 1188–1197.e4. [DOI] [PubMed] [Google Scholar]
- 36. Chen J. W., Pandolfino J. E., Lin Z., et al., “Severity of Endoscopically Identified Esophageal Rings Correlates With Reduced Esophageal Distensibility in Eosinophilic Esophagitis,” Endoscopy 48 (2016): 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menard‐Katcher C., Benitez A. J., Pan Z., et al., “Influence of Age and Eosinophilic Esophagitis on Esophageal Distensibility in a Pediatric Cohort,” American Journal of Gastroenterology 112 (2017): 1466–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Carlson D. A., Prescott J. E., Baumann A. J., et al., “Validation of Clinically Relevant Thresholds of Esophagogastric Junction Obstruction Using FLIP Panometry,” Clinical Gastroenterology and Hepatology 20 (2022): e1250–e1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sato H., Dellon E. S., Aceves S. S., et al., “Clinical and Molecular Correlates of the Index of Severity for Eosinophilic Esophagitis,” Journal of Allergy and Clinical Immunology 154 (2024): 375–386.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schreiner P., Safroneeva E., Rossel J. B., et al., “Sex Impacts Disease Activity but not Symptoms or Quality of Life in Adults With Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 20 (2022): 1729–1738.e1. [DOI] [PubMed] [Google Scholar]
- 41. Runge T. M., Eluri S., Cotton C. C., et al., “Causes and Outcomes of Esophageal Perforation in Eosinophilic Esophagitis,” Journal of Clinical Gastroenterology 51 (2017): 805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang J., Park J. Y., Huang R., et al., “Obtaining Adequate Lamina Propria for Subepithelial Fibrosis Evaluation in Pediatric Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 87 (2018): 1207–1214.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thaker A. I., Melo D. M., Samandi L. Z., Huang R., Park J. Y., and Cheng E., “Esophageal Fibrosis in Eosinophilic Gastrointestinal Diseases,” Journal of Pediatric Gastroenterology and Nutrition 72 (2021): 392–397. [DOI] [PubMed] [Google Scholar]
- 44. Al‐Hussaini A., “Savary Dilation Is Safe and Effective Treatment for Esophageal Narrowing Related to Pediatric Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 63 (2016): 474–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bon L., Safroneeva E., Bussmann C., et al., “Close Follow‐Up Is Associated With Fewer Stricture Formation and Results in Earlier Detection of Histological Relapse in the Long‐Term Management of Eosinophilic Esophagitis,” United European Gastroenterology Journal 10 (2022): 308–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kuchen T., Straumann A., Safroneeva E., et al., “Swallowed Topical Corticosteroids Reduce the Risk for Long‐Lasting Bolus Impactions in Eosinophilic Esophagitis,” Allergy 69 (2014): 1248–1254. [DOI] [PubMed] [Google Scholar]
- 47. Muller M., Eckardt A. J., Fisseler‐Eckhoff A., et al., “Endoscopic Findings in Patients With Schatzki Rings: Evidence for an Association With Eosinophilic Esophagitis,” World Journal of Gastroenterology 18 (2012): 6960–6966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Müller S., Pühl S., Vieth M., and Stolte M., “Analysis of Symptoms and Endoscopic Findings in 117 Patients With Histological Diagnoses of Eosinophilic Esophagitis,” Endoscopy 39 (2007): 339–344. [DOI] [PubMed] [Google Scholar]
- 49. Schoepfer A. M., Safroneeva E., Bussmann C., et al., “Delay in Diagnosis of Eosinophilic Esophagitis Increases Risk for Stricture Formation in a Time‐Dependent Manner,” Gastroenterology 145 (2013): 1230–1236.e1‐2. [DOI] [PubMed] [Google Scholar]
- 50. Singla M. B., Chehade M., Brizuela D., et al., “Early Comparison of Inflammatory vs. Fibrostenotic Phenotype in Eosinophilic Esophagitis in a Multicenter Longitudinal Study,” Clinical and Translational Gastroenterology 6 (2015): e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sperry S. L., Woosley J. T., Shaheen N. J., and Dellon E. S., “Influence of Race and Gender on the Presentation of Eosinophilic Esophagitis,” American Journal of Gastroenterology 107 (2012): 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Koutlas N. T., Eluri S., Rusin S., et al., “Impact of Smoking, Alcohol Consumption, and NSAID Use on Risk for and Phenotypes of Eosinophilic Esophagitis,” Diseases of the Esophagus 31 (2018): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eluri S., Selitsky S. R., Perjar I., et al., “Clinical and Molecular Factors Associated With Histologic Response to Topical Steroid Treatment in Patients With Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 17 (2019): 1081–1088.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Safroneeva E., Straumann A., Coslovsky M., et al., “Symptoms Have Modest Accuracy in Detecting Endoscopic and Histologic Remission in Adults With Eosinophilic Esophagitis,” Gastroenterology 150 (2016): 581–590.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Safroneeva E., Coslovsky M., Kuehni C. E., et al., “Eosinophilic Oesophagitis: Relationship of Quality of Life With Clinical, Endoscopic and Histological Activity,” Alimentary Pharmacology & Therapeutics 42 (2015): 1000–1010. [DOI] [PubMed] [Google Scholar]
- 56. Dellon E. S., Peterson K. A., Mitlyng B. L., et al., “Mepolizumab for Treatment of Adolescents and Adults With Eosinophilic Oesophagitis: A Multicentre, Randomised, Double‐Blind, Placebo‐Controlled Clinical Trial,” Gut 72 (2023): 1828–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scherer R., Schreiner P., Rossel J. B., et al., “Barrett's Esophagus in Eosinophilic Esophagitis in Swiss Eosinophilic Esophagitis Cohort Study (SEECS),” Digestive Diseases 41 (2023): 695–707. [DOI] [PubMed] [Google Scholar]
- 58. von Graffenried T., Safroneeva E., Braegger C., et al., “Pediatric Patients With Eosinophilic Esophagitis and Their Parents Identify Symptoms as the Most Important Treatment Outcome,” International Archives of Allergy and Immunology 185 (2024): 527–535. [DOI] [PubMed] [Google Scholar]
- 59. Nakata A., Tanaka F., Nadatani Y., et al., “Classification of Patients With Esophageal Eosinophilia by Patterns of Sensitization Revealed by a Diagnostic Assay for Multiple Allergen‐Specific IgEs,” Journal of Gastroenterology 56 (2021): 422–433. [DOI] [PubMed] [Google Scholar]
- 60. Cameron B. A., Anderson C. W., Jensen E. T., and Dellon E. S., “Vitamin D Levels as a Potential Modifier of Eosinophilic Esophagitis Severity in Adults,” Digestive Diseases and Sciences 69 (2024): 1287–1292. [DOI] [PubMed] [Google Scholar]
- 61. Thakkar K. P., Philpott H., Lafata S., et al., “Effect of Proton Pump Inhibitor Treatment in “PPI Non‐Responsive” Patients With Eosinophilic Esophagitis,” Journal of Gastrointestinal and Liver Diseases 32 (2023): 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cardullo A. T. and Robson J. O., “Side‐Opening Cutting Forceps and Esophageal Lamina Propria Yield in Pediatric Patients With Eosinophilic Esophagitis,” Clinical Endoscopy 57 (2024): 128–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nagarajan K. V., Krishnamurthy A. N., Yelsangikar A., et al., “Does Eosinophilic Esophagitis Exist in India?,” Indian Journal of Gastroenterology 42 (2023): 286–291. [DOI] [PubMed] [Google Scholar]
- 64. Morales‐Cabeza C., Infante S., Cabrera‐Freitag P., Fuentes‐Aparicio V., Zubeldia J. M., and Álvarez‐Perea A., “Oral Immunotherapy and Risk of Eosinophilic Esophagitis in Children: 15Years' Experience,” Journal of Pediatric Gastroenterology and Nutrition 76 (2023): 53–58. [DOI] [PubMed] [Google Scholar]
- 65. Wong S., Safaeian R., Zobel J., Holloway R. H., Ruszkiewicz A., and Nguyen N. Q., “Increase in Distal Esophageal Wall Thickness With Time in Adult Patients With Eosinophilic Esophagitis,” JGH Open 7 (2023): 178–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Altamimi E., Ahmad B., Abu‐Aqoulah A., and Rawabdeh N., “Clinico‐Pathological Characteristics of Eosinophilic Esophagitis in Jordanian Children,” Przegląd Gastroenterologiczny 17 (2022): 207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Dickerson A., Kolemen A., Kime K., et al., “The Index of Severity for Eosinophilic Esophagitis (I‐SEE) Reflects Longitudinal Clinicopathologic Changes in Children,” Clinical Gastroenterology and Hepatology 22 (2024): 732–740.e1. [DOI] [PubMed] [Google Scholar]
- 68. Andreae D. A., Hanna M. G., Magid M. S., et al., “Swallowed Fluticasone Propionate Is an Effective Long‐Term Maintenance Therapy for Children With Eosinophilic Esophagitis,” American Journal of Gastroenterology 111 (2016): 1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chehade M., Sampson H. A., Morotti R. A., and Magid M. S., “Esophageal Subepithelial Fibrosis in Children With Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 45 (2007): 319–328. [DOI] [PubMed] [Google Scholar]
- 70. Schoepfer A. M., Simko A., Bussmann C., et al., “Eosinophilic Esophagitis: Relationship of Subepithelial Eosinophilic Inflammation With Epithelial Histology, Endoscopy, Blood Eosinophils, and Symptoms,” American Journal of Gastroenterology 113 (2018): 348–357. [DOI] [PubMed] [Google Scholar]
- 71. Gann P. H., Deaton R. J., McMahon N., et al., “An Anti‐IL‐13 Antibody Reverses Epithelial‐Mesenchymal Transition Biomarkers in Eosinophilic Esophagitis: Phase 2 Trial Results,” Journal of Allergy and Clinical Immunology 146 (2020): 367–376.e3. [DOI] [PubMed] [Google Scholar]
- 72. Hiremath G., Choksi Y. A., Acra S., et al., “Factors Associated With Adequate Lamina Propria Sampling and Presence of Lamina Propria Fibrosis in Children With Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 19 (2021): 1814–1823.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lyles J. L., Martin L. J., Shoda T., et al., “Very Early Onset Eosinophilic Esophagitis Is Common, Responds to Standard Therapy, and Demonstrates Enrichment for CAPN14 Genetic Variants,” Journal of Allergy and Clinical Immunology 147 (2021): 244–254.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Muir A. B., Ackerman S. J., Pan Z., et al., “Esophageal Remodeling in Eosinophilic Esophagitis: Relationships to Luminal Captured Biomarkers of Inflammation and Periostin,” Journal of Allergy and Clinical Immunology 150 (2022): 649–656.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rajan J., Newbury R. O., Anilkumar A., et al., “Long‐Term Assessment of Esophageal Remodeling in Patients With Pediatric Eosinophilic Esophagitis Treated With Topical Corticosteroids,” Journal of Allergy and Clinical Immunology 137 (2016): 147–156.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Shoda T., Wen T., Caldwell J. M., et al., “Loss of Endothelial TSPAN12 Promotes Fibrostenotic Eosinophilic Esophagitis via Endothelial Cell‐Fibroblast Crosstalk,” Gastroenterology 162 (2022): 439–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lynch K. L., Benitez A. J., Godwin B., et al., “The Slender Esophagus: Unrecognized Esophageal Narrowing in Eosinophilic Esophagitis,” Clinical and Translational Gastroenterology 14 (2023): e00564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Collins M. H., Dellon E. S., Katzka D. A., Hirano I., Williams J., and Lan L., “Budesonide Oral Suspension Significantly Improves Eosinophilic Esophagitis Histology Scoring System Results: Analyses From a 12‐Week, Phase 2, Randomized, Placebo‐Controlled Trial,” American Journal of Surgical Pathology 43 (2019): 1501–1509. [DOI] [PubMed] [Google Scholar]
- 79. Shoda T., Wen T., Aceves S. S., et al., “Eosinophilic Oesophagitis Endotype Classification by Molecular, Clinical, and Histopathological Analyses: A Cross‐Sectional Study,” Lancet Gastroenterology & Hepatology 3 (2018): 477–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Hiremath G., Correa H., Acra S., et al., “Correlation of Endoscopic Signs and Mucosal Alterations in Children With Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 91 (2020): 785–794.e1. [DOI] [PubMed] [Google Scholar]
- 81. Safroneeva E., Pan Z., King E., et al., “Long‐Lasting Dissociation of Esophageal Eosinophilia and Symptoms After Dilation in Adults With Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 20 (2022): 766–775.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Becker R., Rigsby M., Suchi M., Lerner D. G., and Chugh A., “Dupilumab in Adolescent Eosinophilic Esophagitis: Experience With Fibrostenosis and Eosinophilic Gastrointestinal Disease With Esophageal Involvement,” Journal of Pediatric Gastroenterology and Nutrition 78 (2024): 1337–1341. [DOI] [PubMed] [Google Scholar]
- 83. Aceves S. S., Alexander J. A., Baron T. H., et al., “Endoscopic Approach to Eosinophilic Esophagitis: American Society for Gastrointestinal Endoscopy Consensus Conference,” Gastrointestinal Endoscopy 96 (2022): 576–592.e1. [DOI] [PubMed] [Google Scholar]
- 84. Rothenberg M. E., Dellon E. S., Collins M. H., et al., “Efficacy and Safety of Dupilumab up to 52Weeks in Adults and Adolescents With Eosinophilic Oesophagitis (LIBERTY EoE TREET Study): A Multicentre, Double‐Blind, Randomised, Placebo‐Controlled, Phase 3 Trial,” Lancet Gastroenterology & Hepatology 8 (2023): 990–1004. [DOI] [PubMed] [Google Scholar]
- 85. Jevtic J., Ristic N., Pavlovic V., et al., “The Usefulness of the Eosinophilic Esophagitis Histology Scoring System in Predicting Response to Proton Pump Inhibitor Monotherapy in Children With Eosinophilic Esophagitis,” Diagnostics (Basel) 13 (2023): 3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ruffner M. A., Shoda T., Lal M., et al., “Persistent Esophageal Changes After Histologic Remission in Eosinophilic Esophagitis,” Journal of Allergy and Clinical Immunology 153 (2024): 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shaul E., Kennedy K. V., Spergel Z. C., et al., “Endoscopic and Histologic Utility of Transnasal Endoscopy in Pediatric Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 78 (2024): 1155–1160. [DOI] [PubMed] [Google Scholar]
- 88. Bredenoord A. J., Dellon E. S., Hirano I., et al., “Dupilumab Demonstrated Efficacy and Was Well Tolerated Regardless of Prior Use of Swallowed Topical Corticosteroids in Adolescent and Adult Patients With Eosinophilic Oesophagitis: A Subgroup Analysis of the Phase 3 LIBERTY EoE TREET Study,” Gut 73 (2024): 398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Alexander R. G., Ravi K., Collins M. H., et al., “Eosinophilic Esophagitis Histologic Scoring System: Correlation With Histologic, Endoscopic, and Symptomatic Disease and Clinical Use,” Digestive Diseases and Sciences 68 (2023): 3573–3583. [DOI] [PubMed] [Google Scholar]
- 90. Kwiatek M. A., Hirano I., Kahrilas P. J., Rothe J., Luger D., and Pandolfino J. E., “Mechanical Properties of the Esophagus in Eosinophilic Esophagitis,” Gastroenterology 140 (2011): 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Carlson D. A., Hirano I., Zalewski A., Gonsalves N., Lin Z., and Pandolfino J. E., “Improvement in Esophageal Distensibility in Response to Medical and Diet Therapy in Eosinophilic Esophagitis,” Clinical and Translational Gastroenterology 8 (2017): e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hassan M., Aceves S., Dohil R., et al., “Esophageal Compliance Quantifies Epithelial Remodeling in Pediatric Patients With Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 68 (2019): 559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hirano I., Dellon E. S., Hamilton J. D., et al., “Efficacy of Dupilumab in a Phase 2 Randomized Trial of Adults With Active Eosinophilic Esophagitis,” Gastroenterology 158 (2020): 111–122.e10. [DOI] [PubMed] [Google Scholar]
- 94. Navarro P., Laserna‐Mendieta E. J., Guagnozzi D., et al., “Proton Pump Inhibitor Therapy Reverses Endoscopic Features of Fibrosis in Eosinophilic Esophagitis,” Digestive and Liver Disease 53 (2021): 1479–1485. [DOI] [PubMed] [Google Scholar]
- 95. Nicodeme F., Hirano I., Chen J., et al., “Esophageal Distensibility as a Measure of Disease Severity in Patients With Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 11 (2013): 1101–1107.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carlson D. A., Lin Z., Hirano I., Gonsalves N., Zalewski A., and Pandolfino J. E., “Evaluation of Esophageal Distensibility in Eosinophilic Esophagitis: An Update and Comparison of Functional Lumen Imaging Probe Analytic Methods,” Neurogastroenterology and Motility 28 (2016): 1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Carlson D. A., Hirano I., Gonsalves N., et al., “A PhysioMechanical Model of Esophageal Function in Eosinophilic Esophagitis,” Gastroenterology 165 (2023): 552–563.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Araujo I. K., Shehata C., Hirano I., et al., “The Severity of Reduced Esophageal Distensibility Parallels Eosinophilic Esophagitis Disease Duration,” Clinical Gastroenterology and Hepatology 22 (2024): 513–522.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Carlson D. A., Hirano I., Gonsalves N., et al., “Composite Score of Physiomechanical Esophageal Function Using Functional Lumen Imaging Probe Panometry in Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 99 (2024): 499–510.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kennedy K. V., Umeweni C. N., Alston M., et al., “Esophageal Remodeling Correlates With Eating Behaviors in Pediatric Eosinophilic Esophagitis,” American Journal of Gastroenterology 119 (2024): 1167–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pehrsson M., de Rooij W. E., Bay‐Jensen A. C., Karsdal M. A., Mortensen J. H., and Bredenoord A. J., “Extracellular Matrix Remodeling Proteins as Biomarkers for Clinical Assessment and Treatment Outcomes in Eosinophilic Esophagitis,” BMC Gastroenterology 23 (2023): 357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Abonia J. P., Rudman Spergel A. K., Hirano I., et al., “Losartan Treatment Reduces Esophageal Eosinophilic Inflammation in a Subset of Eosinophilic Esophagitis,” Journal of Allergy and Clinical Immunology: In Practice 12 (2024): 2427–2438.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Gueguen E., Morsy Y., Mamie C., et al., “Novel Transcriptomic Panel Identifies Histologically Active Eosinophilic Oesophagitis,” Gut 73 (2024): 1076–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pyne A. L., Uchida A. M., Hazel M. W., et al., “Effect of Benralizumab on Histopathology and Inflammatory Signatures in a Clinical Cohort of Eosinophilic Esophagitis,” Diseases of the Esophagus 38 (2025): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Eluri S., Runge T. M., Cotton C. C., et al., “The Extremely Narrow‐Caliber Esophagus Is a Treatment‐Resistant Subphenotype of Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 83 (2016): 1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Greenberg S., Chang N. C., Corder S. R., Reed C. C., Eluri S., and Dellon E. S., “Dilation‐Predominant Approach Versus Routine Care in Patients With Difficult‐To‐Treat Eosinophilic Esophagitis: A Retrospective Comparison,” Endoscopy 54 (2022): 243–250. [DOI] [PubMed] [Google Scholar]
- 107. Jung K. W., Gundersen N., Kopacova J., et al., “Occurrence of and Risk Factors for Complications After Endoscopic Dilation in Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 73 (2011): 15–21. [DOI] [PubMed] [Google Scholar]
- 108. Menard‐Katcher C., Furuta G. T., and Kramer R. E., “Dilation of Pediatric Eosinophilic Esophagitis: Adverse Events and Short‐Term Outcomes,” Journal of Pediatric Gastroenterology and Nutrition 64 (2017): 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Saligram S. and McGrath K., “The Safety of a Strict Wire‐Guided Dilation Protocol for Eosinophilic Esophagitis,” European Journal of Gastroenterology & Hepatology 26 (2014): 699–703. [DOI] [PubMed] [Google Scholar]
- 110. Schoepfer A. M., Henchoz S., Biedermann L., et al., “Technical Feasibility, Clinical Effectiveness, and Safety of Esophageal Stricture Dilation Using a Novel Endoscopic Attachment Cap in Adults With Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 94 (2021): 912–919.e2. [DOI] [PubMed] [Google Scholar]
- 111. Ally M. R., Dias J., Veerappan G. R., Maydonovitch C. L., Wong R. K., and Moawad F. J., “Safety of Dilation in Adults With Eosinophilic Esophagitis,” Diseases of the Esophagus 26 (2013): 241–245. [DOI] [PubMed] [Google Scholar]
- 112. Chehade M., Jones S. M., Pesek R. D., et al., “Phenotypic Characterization of Eosinophilic Esophagitis in a Large Multicenter Patient Population From the Consortium for Food Allergy Research,” Journal of Allergy and Clinical Immunology: In Practice 6 (2018): 1534–1544.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dellon E. S., Gibbs W. B., Rubinas T. C., et al., “Esophageal Dilation in Eosinophilic Esophagitis: Safety and Predictors of Clinical Response and Complications,” Gastrointestinal Endoscopy 71 (2010): 706–712. [DOI] [PubMed] [Google Scholar]
- 114. Eluri S., Tappata M., Huang K. Z., et al., “Distal Esophagus Is the Most Commonly Involved Site for Strictures in Patients With Eosinophilic Esophagitis,” Diseases of the Esophagus 33 (2020): 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Greuter T., Godat A., Ringel A., et al., “Effectiveness and Safety of High‐ Vs Low‐Dose Swallowed Topical Steroids for Maintenance Treatment of Eosinophilic Esophagitis: A Multicenter Observational Study,” Clinical Gastroenterology and Hepatology 19 (2021): 2514–2523.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miller D., Mago S., Birk J. W., Dellon E. S., Feustel P. J., and Tadros M., “Obesity Reduces the Requirement for Subsequent Esophageal Stricture Dilation in Adults With Eosinophilic Esophagitis,” Esophagus 18 (2021): 908–914. [DOI] [PubMed] [Google Scholar]
- 117. Moawad F. J., Dellon E. S., Achem S. R., et al., “Effects of Race and Sex on Features of Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 14 (2016): 23–30. [DOI] [PubMed] [Google Scholar]
- 118. Runge T. M., Eluri S., Cotton C. C., et al., “Outcomes of Esophageal Dilation in Eosinophilic Esophagitis: Safety, Efficacy, and Persistence of the Fibrostenotic Phenotype,” American Journal of Gastroenterology 111 (2016): 206–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Runge T. M., Eluri S., Woosley J. T., Shaheen N. J., and Dellon E. S., “Control of Inflammation Decreases the Need for Subsequent Esophageal Dilation in Patients With Eosinophilic Esophagitis,” Diseases of the Esophagus 30 (2017): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schoepfer A. M., Gschossmann J., Scheurer U., Seibold F., and Straumann A., “Esophageal Strictures in Adult Eosinophilic Esophagitis: Dilation Is an Effective and Safe Alternative After Failure of Topical Corticosteroids,” Endoscopy 40 (2008): 161–164. [DOI] [PubMed] [Google Scholar]
- 121. Ukleja A., Shiroky J., Agarwal A., and Allende D., “Esophageal Dilations in Eosinophilic Esophagitis: A Single Center Experience,” World Journal of Gastroenterology 20 (2014): 9549–9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Warners M. J., Oude Nijhuis R. A. B., de Wijkerslooth L. R. H., Smout A. J. P. M., and Bredenoord A. J., “The Natural Course of Eosinophilic Esophagitis and Long‐Term Consequences of Undiagnosed Disease in a Large Cohort,” American Journal of Gastroenterology 113 (2018): 836–844. [DOI] [PubMed] [Google Scholar]
- 123. Wolf W. A., Piazza N. A., Gebhart J. H., et al., “Association Between Body Mass Index and Clinical and Endoscopic Features of Eosinophilic Esophagitis,” Digestive Diseases and Sciences 62 (2017): 143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Laserna‐Mendieta E. J., Casabona S., Guagnozzi D., et al., “Efficacy of Proton Pump Inhibitor Therapy for Eosinophilic Oesophagitis in 630 Patients: Results From the EoE Connect Registry,” Alimentary Pharmacology & Therapeutics 52 (2020): 798–807. [DOI] [PubMed] [Google Scholar]
- 125. Trovato A., Tsang T., Manem N., et al., “The Impact of Obesity on the Fibrostenosis Progression of Eosinophilic Esophagitis in a U. S. Veterans Cohort,” Dysphagia 38 (2023): 866–873. [DOI] [PubMed] [Google Scholar]
- 126. Lucendo A. J., Miehlke S., Schlag C., et al., “Efficacy of Budesonide Orodispersible Tablets as Induction Therapy for Eosinophilic Esophagitis in a Randomized Placebo‐Controlled Trial,” Gastroenterology 157 (2019): 74–86.e15. [DOI] [PubMed] [Google Scholar]
- 127. Miehlke S., Schlag C., Lucendo A. J., et al., “Budesonide Orodispersible Tablets for Induction of Remission in Patients With Active Eosinophilic Oesophagitis: A 6‐Week Open‐Label Trial of the EOS‐2 Programme,” United European Gastroenterology Journal 10 (2022): 330–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Straumann A., Lucendo A. J., Miehlke S., et al., “Budesonide Orodispersible Tablets Maintain Remission in a Randomized, Placebo‐Controlled Trial of Patients With Eosinophilic Esophagitis,” Gastroenterology 159 (2020): 1672–1685.e5. [DOI] [PubMed] [Google Scholar]
- 129. Dellon E. S., Selitsky S. R., Genta R. M., Lash R. H., and Parker J. S., “Gene Expression‐Phenotype Associations in Adults With Eosinophilic Esophagitis,” Digestive and Liver Disease 50 (2018): 804–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Chang N. C., Thakkar K. P., Ketchem C. J., et al., “A Gap in Care Leads to Progression of Fibrosis in Eosinophilic Esophagitis Patients,” Clinical Gastroenterology and Hepatology 20 (2022): 1701–1708.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Laserna‐Mendieta E. J., Casabona S., Savarino E., et al., “Efficacy of Therapy for Eosinophilic Esophagitis in Real‐World Practice,” Clinical Gastroenterology and Hepatology 18 (2020): 2903–2911.e4. [DOI] [PubMed] [Google Scholar]
- 132. Wolf W. A., Cotton C. C., Green D. J., et al., “Predictors of Response to Steroid Therapy for Eosinophilic Esophagitis and Treatment of Steroid‐Refractory Patients,” Clinical Gastroenterology and Hepatology 13 (2015): 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Laserna‐Mendieta E. J., Navarro P., Casabona‐Frances S., et al., “Differences Between Childhood‐ and Adulthood‐Onset Eosinophilic Esophagitis: An Analysis From the EoE Connect Registry,” Digestive and Liver Disease 55 (2023): 350–359. [DOI] [PubMed] [Google Scholar]
- 134. Schupack D. A., Ravi K., Geno D. M., et al., “Effect of Maintenance Therapy for Eosinophilic Esophagitis on Need for Recurrent Dilation,” Digestive Diseases and Sciences 66 (2021): 503–510. [DOI] [PubMed] [Google Scholar]
- 135. Leigh L. Y. and Spergel J. M., “An In‐Depth Characterization of a Large Cohort of Adult Patients With Eosinophilic Esophagitis,” Annals of Allergy, Asthma & Immunology 122 (2019): 65–72.e1. [DOI] [PubMed] [Google Scholar]
- 136. Miehlke S., Hruz P., Vieth M., et al., “A Randomised, Double‐Blind Trial Comparing Budesonide Formulations and Dosages for Short‐Term Treatment of Eosinophilic Oesophagitis,” Gut 65 (2016): 390–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. de Rooij W. E., Diks M. A. P., Warners M. J., Ampting M. T. J. V., van Esch B. C. A. M., and Bredenoord A. J., “Gene Expression and Clinical Outcomes After Dietary Treatment for Eosinophilic Esophagitis: A Prospective Study,” Neurogastroenterology and Motility 34 (2022): e14367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Warners M. J., Vlieg‐Boerstra B. J., Verheij J., et al., “Elemental Diet Decreases Inflammation and Improves Symptoms in Adult Eosinophilic Oesophagitis Patients,” Alimentary Pharmacology & Therapeutics 45 (2017): 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Schoepfer A. M., Gonsalves N., Bussmann C., et al., “Esophageal Dilation in Eosinophilic Esophagitis: Effectiveness, Safety, and Impact on the Underlying Inflammation,” American Journal of Gastroenterology 105 (2010): 1062–1070. [DOI] [PubMed] [Google Scholar]
- 140. Cohen M. S., Kaufman A. B., Palazzo J. P., Nevin D., A. J. DiMarino, Jr. , and Cohen S., “An Audit of Endoscopic Complications in Adult Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 5 (2007): 1149–1153. [DOI] [PubMed] [Google Scholar]
- 141. Croese J., Fairley S. K., Masson J. W., et al., “Clinical and Endoscopic Features of Eosinophilic Esophagitis in Adults,” Gastrointestinal Endoscopy 58 (2003): 516–522. [DOI] [PubMed] [Google Scholar]
- 142. Enns R., Kazemi P., Chung W., et al., “Eosinophilic Esophagitis: Clinical Features, Endoscopic Findings and Response to Treatment,” Canadian Journal of Gastroenterology 24 (2010): 341–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Lucendo A. J., Arias Á., Molina‐Infante J., et al., “Diagnostic and Therapeutic Management of Eosinophilic Oesophagitis in Children and Adults: Results From a Spanish Registry of Clinical Practice,” Digestive and Liver Disease 45 (2013): 562–568. [DOI] [PubMed] [Google Scholar]
- 144. Pasha S. F., DiBaise J. K., Kim H. J., et al., “Patient Characteristics, Clinical, Endoscopic, and Histologic Findings in Adult Eosinophilic Esophagitis: A Case Series and Systematic Review of the Medical Literature,” Diseases of the Esophagus 20 (2007): 311–319. [DOI] [PubMed] [Google Scholar]
- 145. Robles‐Medranda C., Villard F., le Gall C., et al., “Severe Dysphagia in Children With Eosinophilic Esophagitis and Esophageal Stricture: An Indication for Balloon Dilation?,” Journal of Pediatric Gastroenterology and Nutrition 50 (2010): 516–520. [DOI] [PubMed] [Google Scholar]
- 146. Röjler L., Glimberg I., Walker M. M., Garber J. J., and Ludvigsson J. F., “Validation of the Diagnosis of Eosinophilic Esophagitis Based on Histopathology Reports in Sweden,” Upsala Journal of Medical Sciences 126 (2021): ujms7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Ocampo A. A. and Dellon E. S., “Worsened Fibrostenotic Outcomes in Eosinophilic Esophagitis Patients due to COVID‐19‐Related Endoscopy Cancellations,” Digestive Diseases and Sciences 68 (2023): 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Thakkar K. P., Fowler M., Keene S., Iuga A., and Dellon E. S., “Long‐Term Efficacy of Proton Pump Inhibitors as a Treatment Modality for Eosinophilic Esophagitis,” Digestive and Liver Disease 54 (2022): 1179–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Hoofien A., Rea F., Espinheira M. C., et al., “Systemic Steroids Have a Role in Treating Esophageal Strictures in Pediatric Eosinophilic Esophagitis,” Digestive and Liver Disease 53 (2021): 324–328. [DOI] [PubMed] [Google Scholar]
- 150. Safroneeva E., Cotton C. C., Schoepfer A. M., Zwahlen M., Woosley J. T., and Dellon E. S., “Dilation Modifies Association Between Symptoms and Esophageal Eosinophilia in Adult Patients With Eosinophilic Esophagitis,” American Journal of Gastroenterology 115 (2020): 2098–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kavitt R. T., Ates F., Slaughter J. C., et al., “Randomized Controlled Trial Comparing Esophageal Dilation to no Dilation Among Adults With Esophageal Eosinophilia and Dysphagia,” Diseases of the Esophagus 29 (2016): 983–991. [DOI] [PubMed] [Google Scholar]
- 152. Ketchem C. J., Ocampo A. A., Xue Z., et al., “Higher Body Mass Index Is Associated With Decreased Treatment Response to Topical Steroids in Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 21 (2023): 2252–2259.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ketchem C. J., Thakkar K. P., Xue A., et al., “Older Patients With Eosinophilic Esophagitis Have High Treatment Response to Topical Steroids,” Digestive and Liver Disease 54 (2022): 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Tang T. C., Leach S. T., and Krishnan U., “Proton Pump Inhibitors, Antibiotics, and Atopy Increase the Risk of Eosinophilic Esophagitis in Children With Esophageal Atresia,” Journal of Pediatric Gastroenterology and Nutrition 78 (2024): 1317–1328. [DOI] [PubMed] [Google Scholar]
- 155. Kolasinski N. T., Pasman E. A., Nylund C. M., et al., “Improved Outcomes in Eosinophilic Esophagitis With Higher Medication Possession Ratio,” Medicines (Basel) 11 (2024): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Greenberg S. B., Ocampo A. A., Xue Z., et al., “Increasing Rates of Esophageal Stricture and Dilation Over 2 Decades in Eosinophilic Esophagitis,” Gastro Hep Adv 2 (2023): 521–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Molina‐Jimenez F., Ugalde‐Trivino L., Arias‐Gonzalez L., et al., “Proton Pump Inhibitor Effect on Esophageal Protein Signature of Eosinophilic Esophagitis, Prediction, and Evaluation of Treatment Response,” Allergy 79 (2024): 3448–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Snyder D. L., Alexander J. A., Ravi K., Fidler J. L., and Katzka D. A., “Course of Esophageal Strictures in Eosinophilic Esophagitis Using Structured Esophagram Protocol,” Gastro Hep Advances 3 (2024): 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Kiran A., Cameron B. A., Xue Z., et al., “Increasing Age at the Time of Diagnosis and Evolving Phenotypes of Eosinophilic Esophagitis Over 20Years,” Digestive Diseases and Sciences 69 (2024): 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Redd W. D., Ocampo A. A., Xue Z., et al., “Eosinophilic Esophagitis Patients With Multiple Atopic Conditions: Clinical Characteristics and Treatment Response to Topical Steroids,” Annals of Allergy, Asthma & Immunology 131 (2023): 109–115.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Melgaard D., Hansen A. B., Pedersen C., et al., “An Improved Guideline Adherence and PPI Efficacy has Been Accompanied by a Decrease in Diagnostic Delay, and Strictures Before Diagnosis of Eosinophilic Esophagitis in the North Denmark Region—A Retrospective Registry Study of the DanEoE Cohorts,” Clinics and Research in Hepatology and Gastroenterology 47 (2023): 102159. [DOI] [PubMed] [Google Scholar]
- 162. Lee C. J. and Dellon E. S., “Real‐World Efficacy of Dupilumab in Severe, Treatment‐Refractory, and Fibrostenotic Patients With Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 22 (2024): 252–258. [DOI] [PubMed] [Google Scholar]
- 163. Fujiwara Y., Kanamori A., Sawada A., et al., “Prevalence of Elderly Eosinophilic Esophagitis and Their Clinical Characteristics,” Scandinavian Journal of Gastroenterology 58 (2023): 1222–1227. [DOI] [PubMed] [Google Scholar]
- 164. Ocampo A. A., Xue Z., Chang N. C., et al., “Clinical Features and Treatment Response to Topical Steroids in Ethnic and Racial Minority Patients With Eosinophilic Esophagitis,” American Journal of Gastroenterology 119 (2024): 262–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Gonzalez‐Cervera J., Arias A., Navarro P., et al., “Tolerance to Sterilised Cow's Milk in Patients With Eosinophilic Oesophagitis Triggered by Milk,” Alimentary Pharmacology & Therapeutics 56 (2022): 957–967. [DOI] [PubMed] [Google Scholar]
- 166. Dellon E. S., Kim H. P., Sperry S. L., et al., “A Phenotypic Analysis Shows That Eosinophilic Esophagitis Is a Progressive Fibrostenotic Disease,” Gastrointestinal Endoscopy 79 (2014): 577–585.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Colizzo J. M., Clayton S. B., and Richter J. E., “Intrabolus Pressure on High‐Resolution Manometry Distinguishes Fibrostenotic and Inflammatory Phenotypes of Eosinophilic Esophagitis,” Diseases of the Esophagus 29 (2016): 551–557. [DOI] [PubMed] [Google Scholar]
- 168. Dunn J. L. M., Shoda T., Caldwell J. M., et al., “Esophageal Type 2 Cytokine Expression Heterogeneity in Eosinophilic Esophagitis in a Multisite Cohort,” Journal of Allergy and Clinical Immunology 145 (2020): 1629–1640.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Dellon E. S., Katzka D. A., Collins M. H., et al., “Budesonide Oral Suspension Improves Symptomatic, Endoscopic, and Histologic Parameters Compared With Placebo in Patients With Eosinophilic Esophagitis,” Gastroenterology 152 (2017): 776–786.e5. [DOI] [PubMed] [Google Scholar]
- 170. Kon T., Abe Y., Sasaki Y., et al., “Clinical Features of Esophageal Eosinophilia According to Endoscopic Phenotypes,” Internal Medicine 59 (2020): 2971–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Hashimoto A., Sugawa T., Iwakura N., et al., “The Predictive Factors of Responsiveness to Proton Pump Inhibitor Therapy for Eosinophilic Esophagitis,” Gastrointestinal Disorders 1 (2019): 220–230. [Google Scholar]
- 172. Dellon E. S., Lucendo A. J., Schlag C., et al., “Fluticasone Propionate Orally Disintegrating Tablet (APT‐1011) for Eosinophilic Esophagitis: Randomized Controlled Trial,” Clinical Gastroenterology and Hepatology 20 (2022): 2485–2494.e15. [DOI] [PubMed] [Google Scholar]
- 173. Sarbinowska J., Wiatrak B., and Wasko‐Czopnik D., “Searching for Noninvasive Predictors of the Diagnosis and Monitoring of Eosinophilic Esophagitis‐The Importance of Biomarkers of the Inflammatory Reaction Involving Eosinophils,” Biomolecules 11 (2021): 890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. von Arnim U., Kandulski A., Weigt J., and Malfertheiner P., “Correlation of High‐Resolution Manometric Findings With Symptoms of Dysphagia and Endoscopic Features in Adults With Eosinophilic Esophagitis,” Digestive Diseases 35 (2017): 472–477. [DOI] [PubMed] [Google Scholar]
- 175. Yoon H. J., Youn Y. H., Park J. C., and Park H., “Reversibility of Endoscopic Features After Treatment for Eosinophilic Esophagitis,” Yonsei Medical Journal 62 (2021): 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Ma C., Bredenoord A. J., Dellon E. S., et al., “Reliability and Responsiveness of Endoscopic Disease Activity Assessment in Eosinophilic Esophagitis,” Gastrointestinal Endoscopy 95 (2022): 1126–1137.e2. [DOI] [PubMed] [Google Scholar]
- 177. Navarro P., Laserna‐Mendieta E. J., Casabona S., et al., “Accurate and Timely Diagnosis of Eosinophilic Esophagitis Improves Over Time in Europe. An Analysis of the EoE CONNECT Registry,” United European Gastroenterology Journal 10 (2022): 507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rodriguez‐Sanchez J., Barrio‐Andres J., Nantes Castillejo O., et al., “The Endoscopic Reference Score Shows Modest Accuracy to Predict Either Clinical or Histological Activity in Adult Patients With Eosinophilic Oesophagitis,” Alimentary Pharmacology & Therapeutics 45 (2017): 300–309. [DOI] [PubMed] [Google Scholar]
- 179. van Rhijn B. D., Verheij J., Smout A. J., et al., “The Endoscopic Reference Score Shows Modest Accuracy to Predict Histologic Remission in Adult Patients With Eosinophilic Esophagitis,” Neurogastroenterology and Motility 28 (2016): 1714–1722. [DOI] [PubMed] [Google Scholar]
- 180. Navarro P., Feo‐Ortega S., Casabona‐Frances S., et al., “Determinant Factors for First‐Line Treatment Choice and Effectiveness in Pediatric Eosinophilic Esophagitis: An Analysis of the EUREOS EoE CONNECT Registry,” European Journal of Pediatrics 183 (2024): 3567–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Abe Y., Sasaki Y., Yagi M., et al., “Linked Color Imaging Improves the Diagnostic Accuracy of Eosinophilic Esophagitis,” DEN Open 3 (2023): e146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Ayaki M., Manabe N., Nakamura J., Fujita M., Katsumata R., and Haruma K., “A Retrospective Study of the Differences in the Induction of Regulatory T Cells Between Adult Patients With Eosinophilic Esophagitis and Gastroesophageal Reflux Disease,” Digestive Diseases and Sciences 67 (2022): 4742–4748. [DOI] [PubMed] [Google Scholar]
- 183. Heil A., Kuehlewindt T., Godat A., et al., “Histological Phenotyping in Eosinophilic Esophagitis: Localized Proximal Disease Is Infrequent but Associated With Less Severe Disease and Better Disease Outcome,” International Archives of Allergy and Immunology 185 (2024): 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Lee S. Y., Nahm J. H., Kim M. J., et al., “Expression of Immunoglobulin G4 in Eosinophilic Esophagitis,” Journal of Clinical Medicine 13 (2024): 2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Sessions J., Purington N., McGhee S., et al., “Subtyping of Eosinophilic Esophagitis Based on Disease Presentation in a Pediatric Cohort,” Journal of Pediatric Gastroenterology and Nutrition 75 (2022): e67–e74. [DOI] [PubMed] [Google Scholar]
- 186. Bohm M., Richter J. E., Kelsen S., et al., “Esophageal Dilation: Simple and Effective Treatment for Adults With Eosinophilic Esophagitis and Esophageal Rings and Narrowing,” Diseases of the Esophagus 23 (2010): 377–385. [DOI] [PubMed] [Google Scholar]
- 187. Slack M. A., Ogbogu P. U., Phillips G., Platts‐Mills T. A. E., and Erwin E. A., “Serum Vitamin D Levels in a Cohort of Adult and Pediatric Patients With Eosinophilic Esophagitis,” Annals of Allergy, Asthma & Immunology 115 (2015): 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Safroneeva E., Hafner D., Kuehni C. E., et al., “Systematic Assessment of Adult Patients' Satisfaction With Various Eosinophilic Esophagitis Therapies,” International Archives of Allergy and Immunology 181 (2019): 211–220. [DOI] [PubMed] [Google Scholar]
- 189. Lipka S., Kumar A., and Richter J. E., “Successful Esophageal Dilation of Eosinophilic Esophagitis (EoE) Patients With a Previous Postdilation Complication: Start Low and Go Slow,” Journal of Clinical Gastroenterology 52 (2018): 773–777. [DOI] [PubMed] [Google Scholar]
- 190. Melgaard D., Westmark S., Laurberg P. T., and Krarup A. L., “A Diagnostic Delay of 10Years in the DanEoE Cohort Calls for Focus on Education ‐ a Population‐Based Cross‐Sectional Study of Incidence, Diagnostic Process and Complications of Eosinophilic Oesophagitis in the North Denmark Region,” United European Gastroenterology Journal 9 (2021): 688–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Maradey‐Romero C., Prakash R., Lewis S., Perzynski A., and Fass R., “The 2011–2014 Prevalence of Eosinophilic Oesophagitis in the Elderly Amongst 10 Million Patients in the United States,” Alimentary Pharmacology & Therapeutics 41 (2015): 1016–1022. [DOI] [PubMed] [Google Scholar]
- 192. Chang J. W., Rubenstein J. H., Mellinger J. L., et al., “Motivations, Barriers, and Outcomes of Patient‐Reported Shared Decision Making in Eosinophilic Esophagitis,” Digestive Diseases and Sciences 66 (2021): 1808–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Henriksen S. D., Hansen S. K., Heinesen M., et al., “The Phenotype of Adults With Complicated Eosinophilic Esophagitis Is Dominated by a 5‐Year Longer Diagnostic Delay: A Population‐Based Study of the DanEoE Cohort,” JGH Open 7 (2023): 553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Bohm M., Malik Z., Sebastiano C., et al., “Mucosal Eosinophilia: Prevalence and Racial/Ethnic Differences in Symptoms and Endoscopic Findings in Adults Over 10Years in an Urban Hospital,” Journal of Clinical Gastroenterology 46 (2012): 567–574. [DOI] [PubMed] [Google Scholar]
- 195. Lipka S., Keshishian J., Boyce H. W., Estores D., and Richter J. E., “The Natural History of Steroid‐Naive Eosinophilic Esophagitis in Adults Treated With Endoscopic Dilation and Proton Pump Inhibitor Therapy Over a Mean Duration of Nearly 14Years,” Gastrointestinal Endoscopy 80 (2014): 592–598. [DOI] [PubMed] [Google Scholar]
- 196. Madanick R. D., Shaheen N. J., and Dellon E. S., “A Novel Balloon Pull‐Through Technique for Esophageal Dilation in Eosinophilic Esophagitis (With Video),” Gastrointestinal Endoscopy 73 (2011): 138–142. [DOI] [PubMed] [Google Scholar]
- 197. Mangla S., Goldin A. H., Singal G., et al., “Endoscopic Features and Eosinophil Density Are Associated With Food Impaction in Adults With Esophageal Eosinophilia,” Digestive Diseases and Sciences 61 (2016): 2578–2584. [DOI] [PubMed] [Google Scholar]
- 198. Agullo‐Garcia A., Cubero J. L., Lezaun A., et al., “Clinical and Anatomopathological Features of Eosinophilic Oesophagitis in Children and Adults,” Allergologia et Immunopathologia 48 (2020): 560–567. [DOI] [PubMed] [Google Scholar]
- 199. Lucendo A. J., Pascual‐Turrión J. M., Navarro M., et al., “Endoscopic, Bioptic, and Manometric Findings in Eosinophilic Esophagitis Before and After Steroid Therapy: A Case Series,” Endoscopy 39 (2007): 765–771. [DOI] [PubMed] [Google Scholar]
- 200. Peterson K. A., Byrne K. R., Vinson L. A., et al., “Elemental Diet Induces Histologic Response in Adult Eosinophilic Esophagitis,” American Journal of Gastroenterology 108 (2013): 759–766. [DOI] [PubMed] [Google Scholar]
- 201. Reed C. C., Safta A. M., Qasem S., Angie Almond M., Dellon E. S., and Jensen E. T., “Combined and Alternating Topical Steroids and Food Elimination Diet for the Treatment of Eosinophilic Esophagitis,” Digestive Diseases and Sciences 63 (2018): 2381–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Strauss Starling A., Ren Y., Li H., et al., “Reducing Eosinophil Counts in Eosinophilic Esophagitis in Children Is Associated With Reduction in Later Stricture Development,” American Journal of Gastroenterology 119 (2024): 2002–2009. [DOI] [PubMed] [Google Scholar]
- 203. Edwards‐Salmon S., Moraczewski J., Offerle T., et al., “Comparing Eosinophilic Esophagitis in a Black and Non‐Black Pediatric Cohort,” Journal of Pediatric Gastroenterology and Nutrition 75 (2022): 485–490. [DOI] [PubMed] [Google Scholar]
- 204. Quitadamo P., Pascarella A., Gragnaniello P., et al., “Esophageal Food Bolus Impaction in Pediatric Age,” Journal of Pediatric Gastroenterology and Nutrition 78 (2024): 1398–1402. [DOI] [PubMed] [Google Scholar]
- 205. Kozon I. F. L., Izgi B., Trabjerg M. S., Hansen L. E., Melgaard D., and Krarup A. L., “No Gender Differences in Patients With Eosinophilic Oesophagitis,” Danish Medical Journal 70 (2023): A06220393. [PubMed] [Google Scholar]
- 206. Türkel Küçükmetin N., Tiftikçi A., Baba F., and Solakoglu T., “Clinical Presentation and Endoscopic Findings in Adult Patients With Eosinophilic Esophagitis,” Journal of Surgery and Medicine 6 (2022): 907–911. [Google Scholar]
- 207. Goldin A. H., Muftah M., Mangla S., et al., “Assessment of the Clinical and Allergy Profiles of PPI Responsive and Non‐Responsive Eosinophilic Esophagitis,” Diseases of the Esophagus 36 (2023): doac098. [DOI] [PubMed] [Google Scholar]
- 208. Alhmoud T., Ghazaleh S., Ghanim M., and Redfern R. E., “The Risk of Esophageal Food Impaction in Eosinophilic Esophagitis Patients: The Role of Clinical and Socioeconomic Factors,” Clinical and Experimental Gastroenterology 15 (2022): 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Gutierrez‐Junquera C., Fernandez‐Fernandez S., Dominguez‐Ortega G., et al., “Proton Pump Inhibitor Therapy in Pediatric Eosinophilic Esophagitis: Predictive Factors and Long‐Term Step‐Down Efficacy,” Journal of Pediatric Gastroenterology and Nutrition 76 (2023): 191–198. [DOI] [PubMed] [Google Scholar]
- 210. Plate J., Soderbergh T., Bergqvist J., et al., “Eosinophilic Esophagitis Prevalence, Incidence, and Presenting Features: A 22‐Year Population‐Based Observational Study From Southwest Sweden,” Diseases of the Esophagus 38 (2024): doae025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Kim J. P., Weingart G., Hiramoto B., Gregory D. L., Gonsalves N., and Hirano I., “Clinical Outcomes of Adults With Eosinophilic Esophagitis With Severe Stricture,” Gastrointestinal Endoscopy 92 (2020): 44–53. [DOI] [PubMed] [Google Scholar]
- 212. Lipka S., Kumar A., and Richter J. E., “Impact of Diagnostic Delay and Other Risk Factors on Eosinophilic Esophagitis Phenotype and Esophageal Diameter,” Journal of Clinical Gastroenterology 50 (2016): 134–140. [DOI] [PubMed] [Google Scholar]
- 213. Oliva S., Dias J. A., Rea F., et al., “Characterization of Eosinophilic Esophagitis From the European Pediatric Eosinophilic Esophagitis Registry (pEEr) of ESPGHAN,” Journal of Pediatric Gastroenterology and Nutrition 75 (2022): 325–333. [DOI] [PubMed] [Google Scholar]
- 214. Cotton C. C., Biswas M., and Dellon E. S., “Health State Utility Is Substantially Reduced With an Increasing Burden of Patient‐Reported Symptoms in Eosinophilic Esophagitis,” American Journal of Gastroenterology 120 (2024): 905–908. [DOI] [PubMed] [Google Scholar]
- 215. Delaney S., Harris Z., and Clayton S., “Potential Missed Opportunities for the Diagnosis, Treatment, and Follow‐Up of Eosinophilic Esophagitis: Analysis of Endoscopic Food Impaction Management,” Annals of Esophagus 7 (2024): 11. [Google Scholar]
- 216. Borinsky S. A., Weir A. A., LaFata S. S., et al., “Impact of Cannabis Use on Presentation and Treatment Response in Eosinophilic Esophagitis,” Diseases of the Esophagus 38 (2025): doae080. [DOI] [PubMed] [Google Scholar]
- 217. Eroglu Y., Lu H., Terry A., et al., “Pediatric Eosinophilic Esophagitis: Single‐Center Experience in Northwestern USA,” Pediatrics International 51 (2009): 612–616. [DOI] [PubMed] [Google Scholar]
- 218. Frandsen L. T., Melgaard D., Hansen S. K., Mørk K., and Krarup A. L., “Effectiveness of Treatment With Budesonide Orodispersible Tablets in 76 Patients With Eosinophilic Oesophagitis ‐ Real‐Life Experience From the Population‐Based DanEoE Cohort,” Scandinavian Journal of Gastroenterology 59 (2024): 1137–1143. [DOI] [PubMed] [Google Scholar]
- 219. Geow R., Arena G., Siah C., and Picardo S., “A Retrospective Real‐World Study on the Safety and Efficacy of Budesonide Orodispersible Tablets for the Induction Therapy of Eosinophilic Oesophagitis,” Therapeutic Advances in Gastroenterology 17 (2024): 17562848241290346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Njie C., Richman C., Rebhun J., et al., “Identification of Gaps in the Delivery of High‐Quality Care of Patients With Eosinophilic Esophagitis,” Diseases of the Esophagus 38 (2025): doae055. [DOI] [PubMed] [Google Scholar]
- 221. Oliva S., Rossetti D., Papoff P., et al., “A 12‐Week Maintenance Therapy With a New Prepared Viscous Budesonide in Pediatric Eosinophilic Esophagitis,” Digestive Diseases and Sciences 64 (2019): 1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sawada A., Ihara Y., Imai T., Tanaka F., and Fujiwara Y., “Real World Treatment Patterns in Patients With Eosinophilic Esophagitis in Japan,” Scientific Reports 14 (2024): 27490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Xu X., Chen S. Y., Maslova E., et al., “Clinical Symptoms, Comorbidities, Treatment Patterns and Time to Diagnosis in Patients With Eosinophilic Oesophagitis in England: A Retrospective Cohort Study,” Frontline Gastroenterology 15 (2024): 477–485. [Google Scholar]
- 224. Sorge A., Aldinio G., Marinoni B., et al., “Distribution of Esophageal Inflammation in Patients With Eosinophilic Esophagitis and Its Impact on Diagnosis and Outcome,” Digestive and Liver Disease 57 (2025): 260–265. [DOI] [PubMed] [Google Scholar]
- 225. Aceves S. S., Newbury R. O., Chen D., et al., “Resolution of Remodeling in Eosinophilic Esophagitis Correlates With Epithelial Response to Topical Corticosteroids,” Allergy 65 (2010): 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Lucendo A. J., Arias A., De Rezende L. C., et al., “Subepithelial Collagen Deposition, Profibrogenic Cytokine Gene Expression, and Changes After Prolonged Fluticasone Propionate Treatment in Adult Eosinophilic Esophagitis: A Prospective Study,” Journal of Allergy and Clinical Immunology 128 (2011): 1037–1046. [DOI] [PubMed] [Google Scholar]
- 227. Abu‐Sultaneh S. M., Durst P., Maynard V., and Elitsur Y., “Fluticasone and Food Allergen Elimination Reverse Sub‐Epithelial Fibrosis in Children With Eosinophilic Esophagitis,” Digestive Diseases and Sciences 56 (2011): 97–102. [DOI] [PubMed] [Google Scholar]
- 228. Lieberman J. A., Morotti R. A., Konstantinou G. N., Yershov O., and Chehade M., “Dietary Therapy Can Reverse Esophageal Subepithelial Fibrosis in Patients With Eosinophilic Esophagitis: A Historical Cohort,” Allergy 67 (2012): 1299–1307. [DOI] [PubMed] [Google Scholar]
- 229. Li‐Kim‐Moy J. P., Tobias V., Day A. S., Leach S., and Lemberg D. A., “Esophageal Subepithelial Fibrosis and Hyalinization Are Features of Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 52 (2011): 147–153. [DOI] [PubMed] [Google Scholar]
- 230. Thaker A. I., Smith J., Pathak M., and Park J. Y., “Challenges in Inter‐Rater Agreement on Lamina Propria Fibrosis in Esophageal Biopsies,” Pediatric and Developmental Pathology 26 (2023): 106–114. [DOI] [PubMed] [Google Scholar]
- 231. Beppu L., Yang T., Luk M., et al., “MMPs‐2 and ‐14 Are Elevated in Eosinophilic Esophagitis and Reduced Following Topical Corticosteroid Therapy,” Journal of Pediatric Gastroenterology and Nutrition 61 (2015): 194–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Dohil R., Newbury R., Fox L., Bastian J., and Aceves S., “Oral Viscous Budesonide Is Effective in Children With Eosinophilic Esophagitis in a Randomized, Placebo‐Controlled Trial,” Gastroenterology 139 (2010): 418–429. [DOI] [PubMed] [Google Scholar]
- 233. Pasman E. A., Rubin Z., Hooper A. R., et al., “Quantitative Analysis of Tug Sign: An Endoscopic Finding of Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 21 (2023): 1108–1110.e1. [DOI] [PubMed] [Google Scholar]
- 234. Wong E. C. L., Gleave A. L., Marshall J. K., and Narula N., “Predictors of Histologic Response to Mepolizumab in Pediatric Eosinophilic Esophagitis,” European Journal of Gastroenterology & Hepatology 35 (2023): 1131–1136. [DOI] [PubMed] [Google Scholar]
- 235. Castrodad‐Rodriguez C. A., Cheng J., Westerhoff M., et al., “Clinical and Pathological Correlation in Concomitant Celiac Disease and Eosinophilic Esophagitis Suggests Separate Etiologies,” International Journal of Surgical Pathology 32 (2024): 27–34. [DOI] [PubMed] [Google Scholar]
- 236. Dellon E. S., Speck O., Woodward K., et al., “Distribution and Variability of Esophageal Eosinophilia in Patients Undergoing Upper Endoscopy,” Modern Pathology 28 (2015): 383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Hiremath G., Sun L., Correa H., et al., “Development and Validation of Web‐Based Tool to Predict Lamina Propria Fibrosis in Eosinophilic Esophagitis,” American Journal of Gastroenterology 117 (2022): 272–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lopez‐Nunez O., Bernieh A., Kliewer K. L., et al., “Transnasal Endoscopy Acquires Esophageal Biopsies Adequate for Comprehensive Pathology Evaluation in Patients With Eosinophilic Esophagitis,” Pediatric and Developmental Pathology 27 (2024): 327–334. [DOI] [PubMed] [Google Scholar]
- 239. Hiremath G., Sun L., Collins M. H., et al., “Esophageal Epithelium and Lamina Propria Are Unevenly Involved in Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 21 (2023): 2807–2816.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Hiremath G., Arva N. C., and Wechsler J. B., “Reproducibility and Generalizability of the Web‐Based Tool to Predict Lamina Propria Fibrosis in Eosinophilic Esophagitis,” Journal of Pediatric Gastroenterology and Nutrition 77 (2023): 93–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Archila L. R., Smith L., Sihvo H. K., et al., “Development and Technical Validation of an Artificial Intelligence Model for Quantitative Analysis of Histopathologic Features of Eosinophilic Esophagitis,” Journal of Pathology Informatics 13 (2022): 100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Haugen E. J., Locke A. K., Correa H., et al., “Characterization of Lamina Propria Remodeling in Pediatric Eosinophilic Esophagitis Using Second Harmonic Generation Microscopy,” Translational Medicine Communications 9 (2024): 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Zhang S., Caldwell J. M., Rochman M., et al., “Machine Learning‐Based Identification and Characterization of Mast Cells in Eosinophilic Esophagitis,” Journal of Allergy and Clinical Immunology 153 (2024): 1381–1391.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Aceves S. S., Newbury R. O., Dohil R., Schwimmer J., and Bastian J. F., “Distinguishing Eosinophilic Esophagitis in Pediatric Patients: Clinical, Endoscopic, and Histologic Features of an Emerging Disorder,” Journal of Clinical Gastroenterology 41 (2007): 252–256. [DOI] [PubMed] [Google Scholar]
- 245. Lin B., Rabinowitz S., Haseeb M. A., and Gupta R., “Usefulness of the Eosinophilic Esophagitis Histologic Scoring System in Distinguishing Active Eosinophilic Esophagitis From Remission and Gastroesophageal Reflux Disease,” Gastroenterology Research 14 (2021): 220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Ma C., Jairath V., Feagan B. G., et al., “Responsiveness of a Histologic Scoring System Compared With Peak Eosinophil Count in Eosinophilic Esophagitis,” American Journal of Gastroenterology 117 (2022): 264–271. [DOI] [PubMed] [Google Scholar]
- 247. Nader L. S., Epifanio M., Coelho M. G., et al., “High Prevalence of Response to PPI Treatment in Children and Adolescents With Eosinophilic Esophagitis in Southern Brazil,” Frontiers in Allergy 5 (2024): 1346843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Dellon E. S., Gibbs W. B., Fritchie K. J., et al., “Clinical, Endoscopic, and Histologic Findings Distinguish Eosinophilic Esophagitis From Gastroesophageal Reflux Disease,” Clinical Gastroenterology and Hepatology 7 (2009): 1305–1313; quiz 1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Williamson P., Proudfoot J., Gharibans A., et al., “Plasminogen Activator Inhibitor‐1 as a Marker of Esophageal Functional Changes in Pediatric Eosinophilic Esophagitis,” Clinical Gastroenterology and Hepatology 20 (2022): 57–64.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kasagi Y., Dods K., Wang J. X., et al., “Fibrostenotic Eosinophilic Esophagitis Might Reflect Epithelial Lysyl Oxidase Induction by Fibroblast‐Derived TNF‐Alpha,” Journal of Allergy and Clinical Immunology 144 (2019): 171–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Fitch K., Bernstein S. J., Aguilar M. D., et al., The RAND/UCLA Appropriateness Method User's Manual (RAND, 2001). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
Data available upon request. Added to PROSPERO under ID CRD42024558352.


