Abstract
Target gene activation by nuclear hormone receptors, including estrogen receptors (ERs), is thought to be mediated by a variety of interacting cofactors. Here we identify a number of nuclear extract-derived proteins that interact with immobilized ER ligand binding domains in a 17β-estradiol-dependent manner. The most prominent of these are components of the thyroid hormone receptor-associated protein (TRAP)/Mediator coactivator complex, which interacts with ERα and ERβ in both unfractionated nuclear extracts and purified form. Studies with extracts from TRAP220−/− fibroblasts reveal that these interactions depend on TRAP220, a TRAP/Mediator subunit previously shown to interact with ER and other nuclear receptors in a ligand-dependent manner. The physiological relevance of the in vitro interaction is documented further by the isolation of an ERα–TRAP/Mediator complex from cultured cells expressing an epitope-tagged ERα. Finally, the complete TRAP/Mediator complex is shown to enhance ER function directly in a highly purified cell-free transcription system. These studies firmly establish a direct role for TRAP/Mediator, through TRAP220, in ER function.
Nuclear hormone receptors comprise a superfamily of transcriptional activators that bind to and, in a ligand-dependent manner, activate target genes involved in diverse physiological processes (1). Conserved nuclear receptor domains include the central DNA binding domain and a C-terminal ligand binding domain (LBD) that contains the ligand-induced AF-2 activation domain. Many receptors also contain N-terminal AF-1 activation domains that are less conserved (2). The function of nuclear receptors on target genes involves a variety of commonly used coactivators that in many cases show ligand-dependent interactions (directly or indirectly) with the AF-2 domain (3–5). One prominent group includes the p160/SRC family and the interacting p300/CBP and PCAF proteins, which function at least in part through intrinsic histone acetyltransferase activities that modify chromatin structure to facilitate subsequent receptor/coactivator-mediated recruitment and/or function of the general transcription machinery (3–5).
Another coactivator of increasing importance for nuclear receptors is the thyroid hormone receptor-associated protein (TRAP)/Mediator complex. Although now known to mediate the activity of a number of distinct activators through specific subunit interactions (refs. 6 and 7; reviewed in refs. 8 and 9), TRAP/Mediator was identified first through a ligand-dependent interaction with thyroid hormone receptor (TR) and shown to be essential for TR function on DNA templates in a reconstituted cell-free system (10). The TRAP220 subunit was identified as the main anchor for TR on the basis of a selective ligand-dependent interaction of isolated TRAP220 with TR (6), and analyses with TRAP220−/− fibroblasts confirmed a receptor-selective function for TRAP220 (11, 12). The early demonstration of ligand-dependent interactions of TRAP220 with a number of other nuclear receptors further suggested a broader role for TRAP220 through TRAP/Mediator in nuclear receptor function (6, 13), as was shown subsequently for vitamin D receptor (VDR; ref. 14).
The possibility that TRAP/Mediator might function with class I (steroid hormone) nuclear receptors in addition to class II nuclear receptors such as TR and VDR was suggested first by the observation of a ligand-dependent interaction of intact TRAP220 with estrogen receptor (ER)α (6). In support of this notion, subsequent studies confirmed physical interactions of TRAP220 with ERα (15–17), demonstrated inhibitory effects of an ER-interacting fragment of TRAP220 (16) and an anti-TRAP220 antibody (18) on ERα function in transfected cells, and established the presence of TRAP220 on the promoters of endogenous estrogen-responsive genes (19). However, interpretation of these studies is complicated variously by (i) the stable association of TRAP220 with other TRAP/Mediator components that may mediate (via different activators) TRAP/Mediator recruitment and function, (ii) the failure to analyze suitable control genes and other cofactors for broader effects of agents designed to block TRAP220 functions, and (iii) an inability to demonstrate interactions of ER with the intact TRAP/Mediator complex, rather than isolated TRAP220 or TRAP220 fragments. In fact, it was suggested on the basis of the latter results that ER might function through TRAP220 alone or through a different TRAP220 (sub)complex (16). Furthermore, there is some discrepancy regarding the ability of TRAP220 to interact with ERα vs. ERβ (16, 17).
As part of a broader effort to identify novel ER-interacting factors (presumptive cofactors) that might show specificity for ER α and β subtypes (20) and/or mediate tissue-selective functions of selective ER modulators (reviewed in ref. 21), we have demonstrated ligand-dependent interactions of the complete TRAP/Mediator complex with both ERα and ERβ. We further show that these interactions are direct and dependent on both TRAP220 and the ER LBD (but apparently modulated by the AF-1 domain) and that TRAP/Mediator directly facilitates ER function.
Materials and Methods
Construction of Plasmids.
Details of subcloning (not shown here) are available on request. Briefly, plasmids encoding glutathione S-transferase (GST)–ERαAB(1–180), GST–ERαLBD(302–595), GST–ERβAB(1–153), and GST–ERβLBD(243–530) were created by inserting the corresponding PCR-generated human ER (hER) derivatives into the pGEX vector (Amersham Pharmacia). A plasmid expressing FLAG-tagged hERαΔAB (f:hERαΔAB) was created by inserting the FLAG-tagged C-terminal part (residues 160–595) of hERα into pIRES-neo vector (CLONTECH).
Cell Line Establishment.
A HeLa-derived cell line (C8) that stably expresses f:hERαΔAB was established by transfection using Lipofectamine (GIBCO/BRL). At 72 h posttransfection, cells were passaged 1:10 in selective medium (DMEM containing 10% FBS and 0.5 mg/ml G418). After 2 weeks of selection, G418-resistant colonies were picked and expanded. Mouse embryo fibroblasts (MEFs) from wild-type and TRAP220−/− mice (11) were immortalized by transformation with simian virus 40 large T antigen.
Cell Culture, Extract Preparation, and Immunoaffinity Purification.
Cell lines expressing FLAG (f)-tagged proteins f:TR (10), f:Nut2 (22), f:CDK8/SRB10 (23), f:TRAP220AB (C.-X.Y. and R.G.R, unpublished data), and f:hERαΔAB were grown in DMEM-PO4 medium containing 10% calf serum. Corresponding complexes containing these FLAG-tagged proteins were purified from derived nuclear extracts by binding to M2 agarose and elution with FLAG peptide as described (10, 22, 23). MEFs were grown in DMEM containing 10% FBS.
Recombinant Protein Purification.
GST and GST-fusion proteins were expressed and purified as described (16). Recombinant FLAG-tagged ERs were expressed via baculovirus vectors and purified as described (24).
GST Pull-Down Assays.
GST pull-down assays were performed as described (25). Before incubation with HeLa nuclear extract, all glutathione-Sepharose bead-immobilized GST or GST-fusion proteins were normalized to equimolarity after quantitation by SDS/PAGE. After binding [20 mM Hepes, pH 7.9/20% glycerol/0.2 mM EDTA/180 mM KCl/1 mM DTT/0.05% Nonidet P-40/0.5 mM PMSF/1 μM 17β-estradiol (E2) as indicated] and washing (20 mM Hepes, pH 7.9/20% glycerol/0.2 mM EDTA/180 mM KCl/1 mM DTT/0.1% Nonidet P-40/0.5 mM PMSF), samples were eluted with 0.2% Sarkosyl in wash buffer, subjected to 4–20% gradient SDS/PAGE, and then analyzed by either silver staining with the Rapid Ag stain kit (ICN) or Western blot.
Antibodies and Western Blot Analysis.
Antibodies against TRAP/Mediator subunits were described previously (6, 7, 22). Antibodies against SRC-1 and RPB1 were from Santa Cruz Biotechnology. Western blot analysis involved standard procedures with an enhanced chemiluminescence detection kit (Amersham Pharmacia).
In Vitro Transcription Assays.
Reactions contained transcription factor (TF)IID, TFIIB, TFIIE, TFIIF, TFIIH, RNA polymerase II, PC4, and other components (TRAP/Mediator and ERs) as indicated. All factors were either recombinant or natural affinity-purified components that were isolated and used under previously described conditions (26).
Results
Estrogen-Dependent Interactions of Nuclear Extract Proteins with ER LBDs.
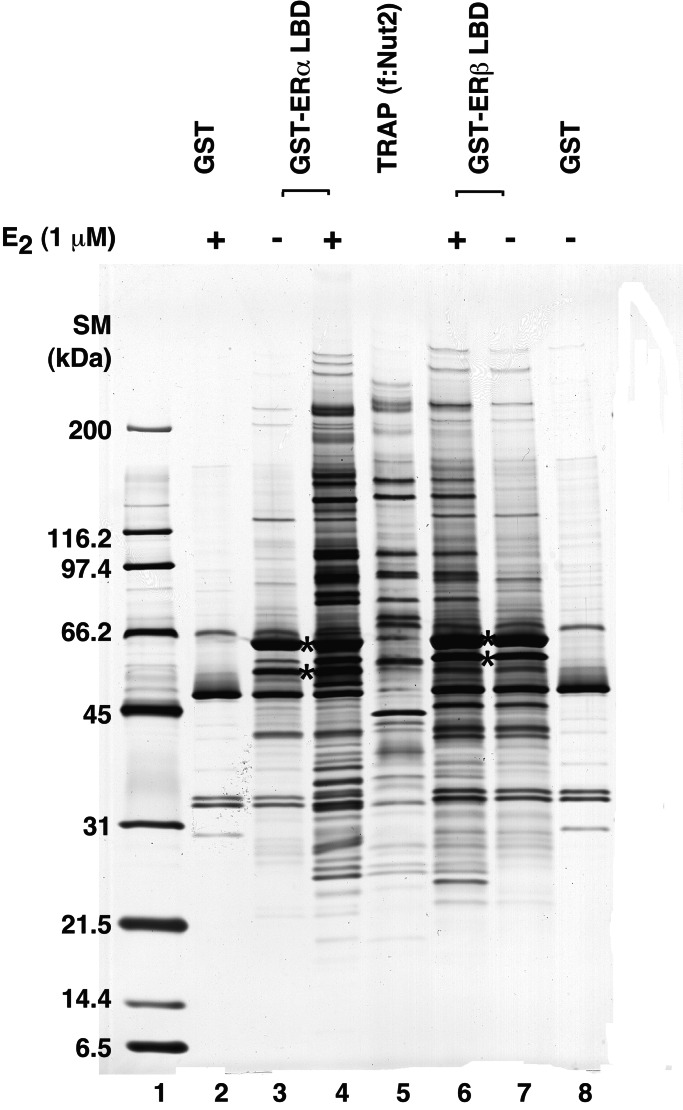
To identify nuclear proteins (presumptive cofactors) that interact independently or cooperatively with ERs, GST-fused ERα (residues 302–595) and ERβ (residues 243–530) LBDs were expressed, purified, and immobilized on glutathione-Sepharose beads. After incubation with HeLa nuclear extract in the presence or absence of E2, beads were washed extensively and bound proteins were eluted and analyzed by SDS/PAGE and silver staining. As shown in Fig. 1, ≈5–10 proteins bound specifically to GST-LBDs, relative to GST alone, in an E2-independent manner; and these proteins largely (but not completely) overlapped for ERα and ERβ. Much larger groups of proteins (≈30–40 in each case) showed E2-dependent interactions with the GST–LBDs, and these also largely (but not completely) overlapped for ERα and ERβ.
Figure 1.
E2-dependent interactions of HeLa nuclear extract proteins with ERα and ERβ LBDs. Immobilized GST (lanes 2 and 8), GST–ERαLBD (lanes 3 and 4) and GST–ERβLBD (lanes 6 and 7) proteins were incubated with HeLa nuclear extract in the absence (−) or presence (+) of 1 μM E2, and bound proteins were eluted and analyzed by SDS/PAGE and silver staining as described in Materials and Methods. Purified TRAP/Mediator complex from f:Nut2-expressing cells is analyzed in lane 5. Standard molecular mass markers (SM) with sizes in kDa indicated on the left were present in lane 1. Bands marked with an asterisk represent degradation products of GST–ER fusion proteins.
Many of these polypeptides appeared similar in size to components of the ≈25-subunit TRAP/Mediator complex, as indicated further (Fig. 1) by a direct comparison of the independently purified TRAP/Mediator complex (which contains some polypeptides that nonspecifically bind to M2 agarose; ref. 23 and Fig. 6B). Subsequent to confirmation of TRAP/Mediator interactions with the LBDs by Western blot (below), mass spectral analyses also confirmed the presence of specific TRAPs (e.g., TRAP220, TRAP170, hSur2, TIG1/PAQ, SOH1, p37, p36, and MED6) and further revealed several other known and previously uncharacterized proteins (Y.K.K., W. Zhang, B. Chait, and R.G.R., unpublished observations). Although these components will be detailed elsewhere, it is relevant to note that they included the RPB2 subunit of RNA polymerase II, the 450-kDa DNA-PK, and the 400-kDa TRRAP component of GCN5/PCAF complexes (27). The latter result is consistent with the documented E2-dependent recruitment of PCAF to ER-regulated target genes (19). Although the identities and functional relevance of the additional subtype-independent and subtype-specific proteins remain to be established, these results are consistent with both common and unique features of ERα and ERβ structure and function (20).
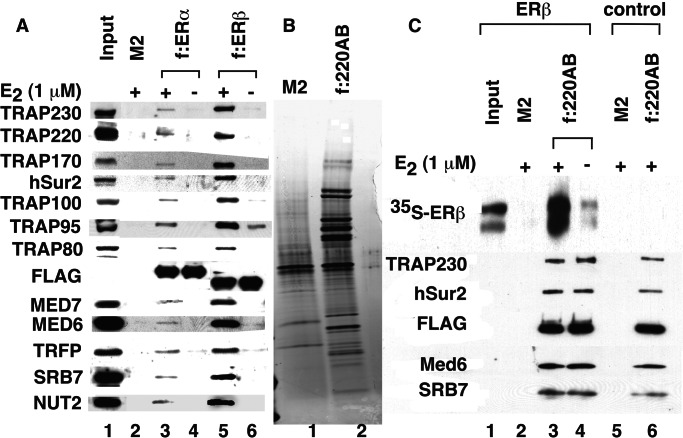
Figure 6.
TRAP/Mediator interactions with full-length ERα and ERβ. (A) Ligand-dependent interactions between TRAP/Mediator and ERs in HeLa nuclear extract. M2 agarose-immobilized FLAG-ERα and FLAG-ERβ were incubated with HeLa nuclear extracts in the absence (−) or presence (+) of 1 μM E2, and bound proteins were eluted with FLAG peptide and analyzed by SDS/PAGE and Western blot (with antibodies to proteins indicated in the left) as described in Materials and Methods. As a control, HeLa nuclear extract proteins bound to M2 agarose alone were analyzed in lane 2. A 1/10th equivalent of input nuclear extract was analyzed in lane 1. (B) Purified f:TRAP220AB TRAP/Mediator complex. The complex was affinity-purified from cells expressing a FLAG-tagged TRAP220 lacking the C-terminal domain (f:TRAP220AB) and analyzed by SDS/PAGE and silver staining (lane 2). Lane 1 shows HeLa nuclear extract proteins bound nonspecifically to M2 agarose. (C) Direct interactions between purified TRAP/Mediator complex and full-length ERβ. M2 agarose-immobilized TRAP/Mediator (f:TRAP220AB) complex (lanes 3, 4, and 6) and M2 agarose alone (lanes 2 and 5) were incubated with 35S-labeled (in vitro translated) full-length ERβ in the presence (lane 3) or absence (lane 4) of 1 μM E2 or with control lysate (lanes 5 and 6). After washing, bound proteins were eluted with FLAG peptide and analyzed by autoradiography (Upper) or Western blot with antibodies to the indicated components of TRAP/Mediator complex (Lower). Lane 1 shows 1/20th of the 35S-labeled full-length ERβ input.
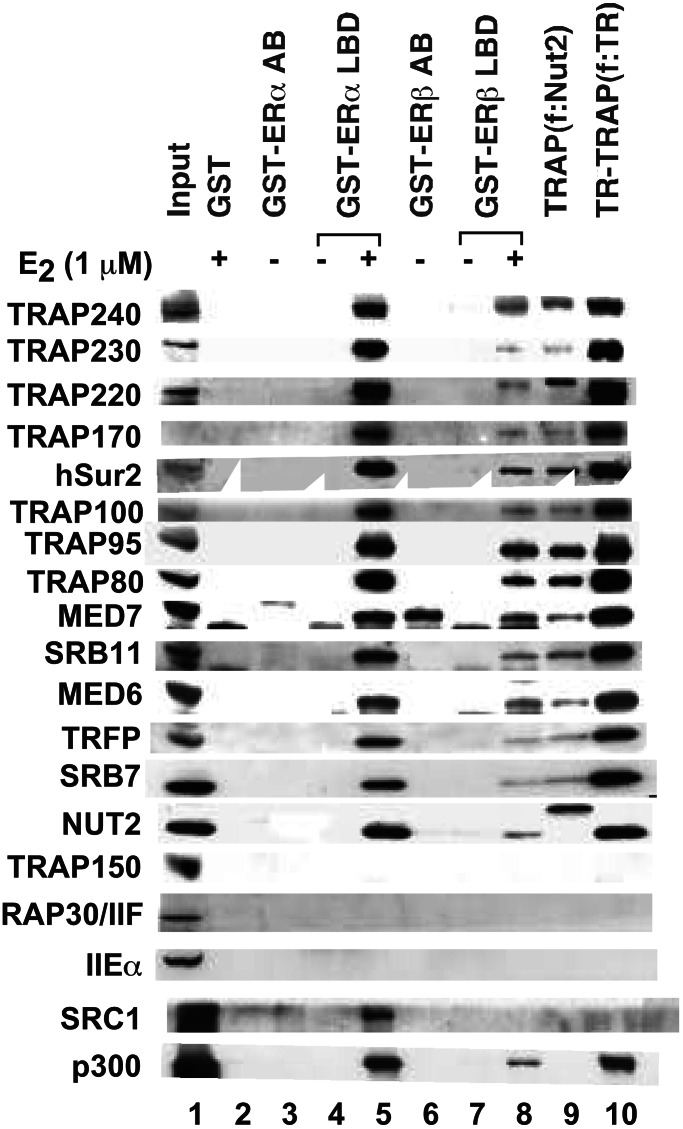
Estrogen-Dependent Interactions of TRAP/Mediator with ER LBDs in Nuclear Extracts.
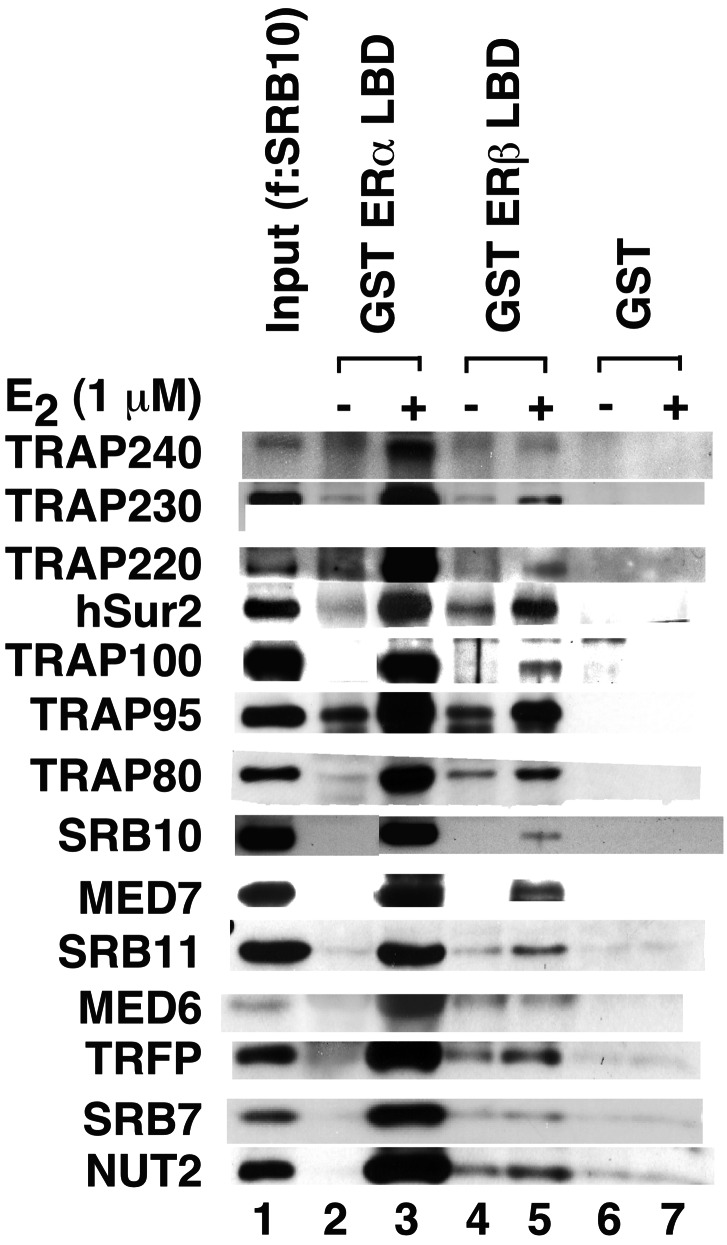
To extend the analyses of Fig. 1, proteins bound to ER LBDs in the presence and absence of E2 were analyzed by Western blot with antibodies to representative TRAP/Mediator components. Strikingly, as shown in Fig. 2 and by comparison with purified TRAP (f:Nut2) and TR–TRAP (f:TR) complexes, all TRAP/Mediator components examined (other than TRAP150, which may not be a bona fide integral subunit) were found to associate with ERα and ERβ LBDs in an E2-dependent manner. Because equimolar amounts of immobilized fusion proteins were used for the binding assays, the results also reveal a higher affinity of TRAP/Mediator complex for the isolated ERα LBD than for the isolated ERβ LBD. The assays in Fig. 2 further show ligand-dependent interactions of SRC-1 and p300 with ER LBDs. As observed for TRAP/Mediator, these interactions are stronger for ERα than for ERβ. Consistent with the results of our previous analyses of TRAP/Mediator complexes (22, 23) and indicative of specificity of factor binding to ER LBDs, no interactions of general transcription factors TFIIE (IIEα) and TFIIF (RAP30) were observed.
Figure 2.
E2-dependent interactions of TRAP/Mediator with ERα and ERβ LBDs in nuclear extract. Immobilized GST (lane 2), GST–ERαAB (lane 3), GST–ERαLBD (lanes 4 and 5), GST–ERβAB (lane 6), and GST–ERβLBD (lanes 7 and 8) proteins were incubated with HeLa nuclear extract in the absence (−) or presence (+) of 1 μM E2, and bound proteins were eluted and analyzed by SDS/PAGE and Western blot (with antibodies to proteins indicated on the left) as described in Materials and Methods. A 1/10th equivalent of the input nuclear extract is shown in lane 1. TRAP/Mediator and TR–TRAP complexes immunopurified from cells expressing f:Nut2 and f:TR, respectively, are shown in lanes 9 and 10. The band observed in lane 6 with MED7 antibody is a nonspecific band that crossreacts with this antibody.
Also of note is the lack of any detectable binding of TRAP/Mediator components to GST-fused ERα and ERβ AB regions that contain the AF-1 domains (Fig. 2). This result contrasts with reports that other nuclear receptor coactivators, including p160/SRC members, can interact not only with AF-2 domains in a ligand-dependent manner but also with AF-1 domains in a ligand-independent manner (reviewed in ref. 5). It is notable, however, that in our assays interactions of these components (at normal nuclear levels) with ERα and ERβ AB domains were either undetectable (p300) or barely detectable (SRC-1). The inability to see TRAP/Mediator interactions with AB domains also contrasts with the demonstration of a ligand-independent interaction of independently expressed TRAP170 with the AF-1 domain of the glucocorticoid receptor (28). However, our results do not exclude possible modulatory effects of AB and associated AF-1 domains on interactions of TRAP/Mediator with LBD and associated AF-2 domains.
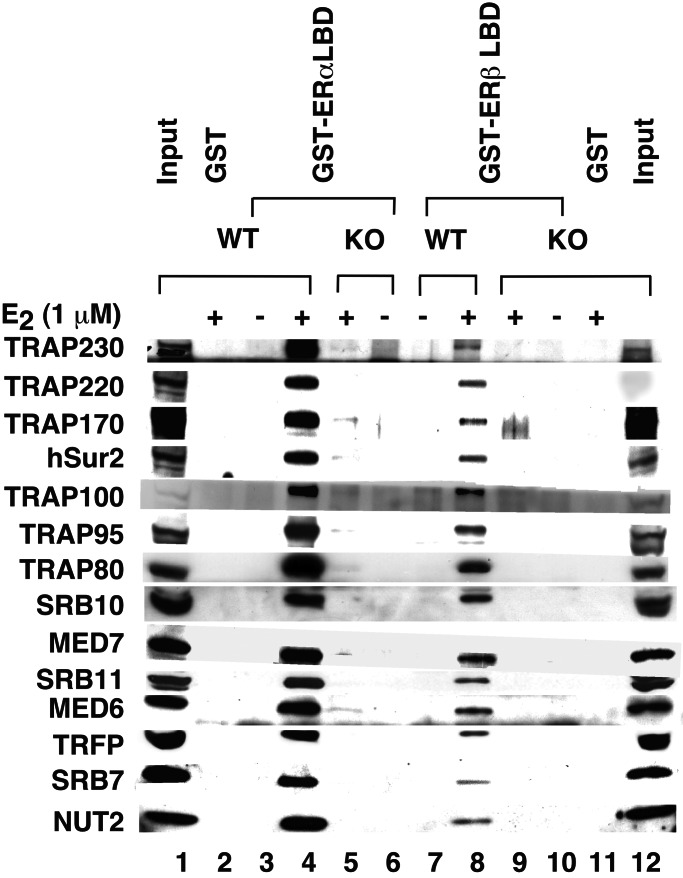
TRAP220-Dependent Interactions of TRAP/Mediator with ER LBDs in Nuclear Extracts.
The demonstration of ligand-dependent interactions of ERα and ERβ with isolated TRAP220 (6, 15–17) has led to speculation that TRAP220 may anchor ERs to the TRAP/Mediator complex, as observed for TR and VDR, even though previous studies (16) had failed to detect ER–TRAP/Mediator interactions. In view of our current demonstration of ER–TRAP/Mediator interactions, we used extracts from TRAP220−/− MEFs (11) to test the relevance of TRAP220 for the observed interactions. As shown in Fig. 3, TRAP/Mediator components from control MEFs bound in an E2-dependent manner to GST-fused ER LBDs, whereas TRAP/Mediator components from TRAP220−/− MEFs did not. Other studies have shown that TRAP220−/− cells contain a residual TRAP complex lacking only TRAP220 and that this complex interacts normally with other activators such as VP16 (C.-X.Y., S. Malik, and R.G.R., unpublished observation). These results firmly establish a role for TRAP220 in anchoring TRAP/Mediator to ERα and ERβ and again show that TRAP/Mediator has a stronger affinity for the isolated ERα LBD than for the isolated ERβ LBD in the context of other nuclear proteins.
Figure 3.
TRAP220-dependent interactions of TRAP/Mediator with ER LBDs in nuclear extract. Immobilized GST (lanes 2 and 11), GST–ERα LBD (lanes 3–6), and GST–ERβ LBD (lanes 7–10) were incubated with nuclear extracts from wild-type (WT, lanes 2, 3, 4, 7, and 8) and TRAP220−/− (KO, lanes 5, 6, 9, 10, and 11) MEFs in the absence (−) or presence (+) of 1 μM E2, and bound proteins were eluted and analyzed by SDS/PAGE and Western blot (with antibodies to proteins indicated on the left) as detailed in Materials and Methods. One-tenth equivalents of input nuclear extracts were analyzed in lanes 1 (WT) and 12 (KO).
Direct Interactions of Purified TRAP/Mediator Complex with ER LBDs.
The studies described above have clearly shown ligand-dependent interactions of TRAP/Mediator with ER LBDs but, because they used nuclear extracts as a source of TRAP/Mediator, did not establish whether other factors were essential for these interactions. To investigate this question further, we used a highly purified TRAP/Mediator complex immunopurified from cells expressing a FLAG-tagged SRB10/CDK8 subunit (23). As shown in Fig. 4, this complex showed an E2-dependent interaction with the ERα LBD and an E2-enhanced interaction with the ERβ LBD. Overall interactions in the presence of E2 again were stronger for ERα than for ERβ, as in the analyses with nuclear extracts (above). Although we do not know the basis for the decreased ligand dependency of the ERβ LBD interaction with purified TRAP/Mediator relative to TRAP/Mediator in nuclear extracts (above), this result may reflect the absence of negative constraints (interacting factors) that increase the ligand dependency of interactions in nuclear extracts. This observation is consistent also with the observation of ligand-dependent interactions of ERβ with a TRAP220 fragment in gel-shift assays (17).
Figure 4.
Direct interactions of purified TRAP/Mediator with LBDs of ERα and ERβ. Immobilized GST (lanes 6 and 7), GST–ERα LBD (lanes 2 and 3), and GST–ERβ LBD (lanes 4 and 5) were incubated with immunopurified TRAP/Mediator complex from cells expressing f:CDK8/SRB10 in the absence (−) or presence (+) of 1 μM E2. After extensive washing, bound proteins were eluted and analyzed by SDS/PAGE and Western blot (with antibodies to proteins indicated on the left) as described in Materials and Methods. A 1/10th equivalent of the input TRAP/Mediator preparation was analyzed in lane 1.
Intracellular Association of TRAP/Mediator with ERαΔAB.
To provide evidence for a physiological association of ER with TRAP/Mediator, as demonstrated previously for TR (10), we established a HeLa cell line (C8) that stably expresses a FLAG-tagged ERα lacking the AB domain (f:hERαΔAB). The tagged ERαΔAB and associated proteins were affinity-purified from C8-derived nuclear extracts on M2 agarose beads. A Western blot of the bound and eluted proteins revealed the presence of FLAG-tagged ERαΔAB (scored by both ERα and FLAG antibodies) as well as all TRAP/Mediator components that were analyzed (Fig. 5). An intracellular association of ERαΔAB with p300 was detected also. None of these proteins were bound to M2 agarose when a control extract from HeLa cells was applied. These results thus indicate an intracellular association of the entire TRAP/Mediator complex with ER, as described originally for TR.
Figure 5.
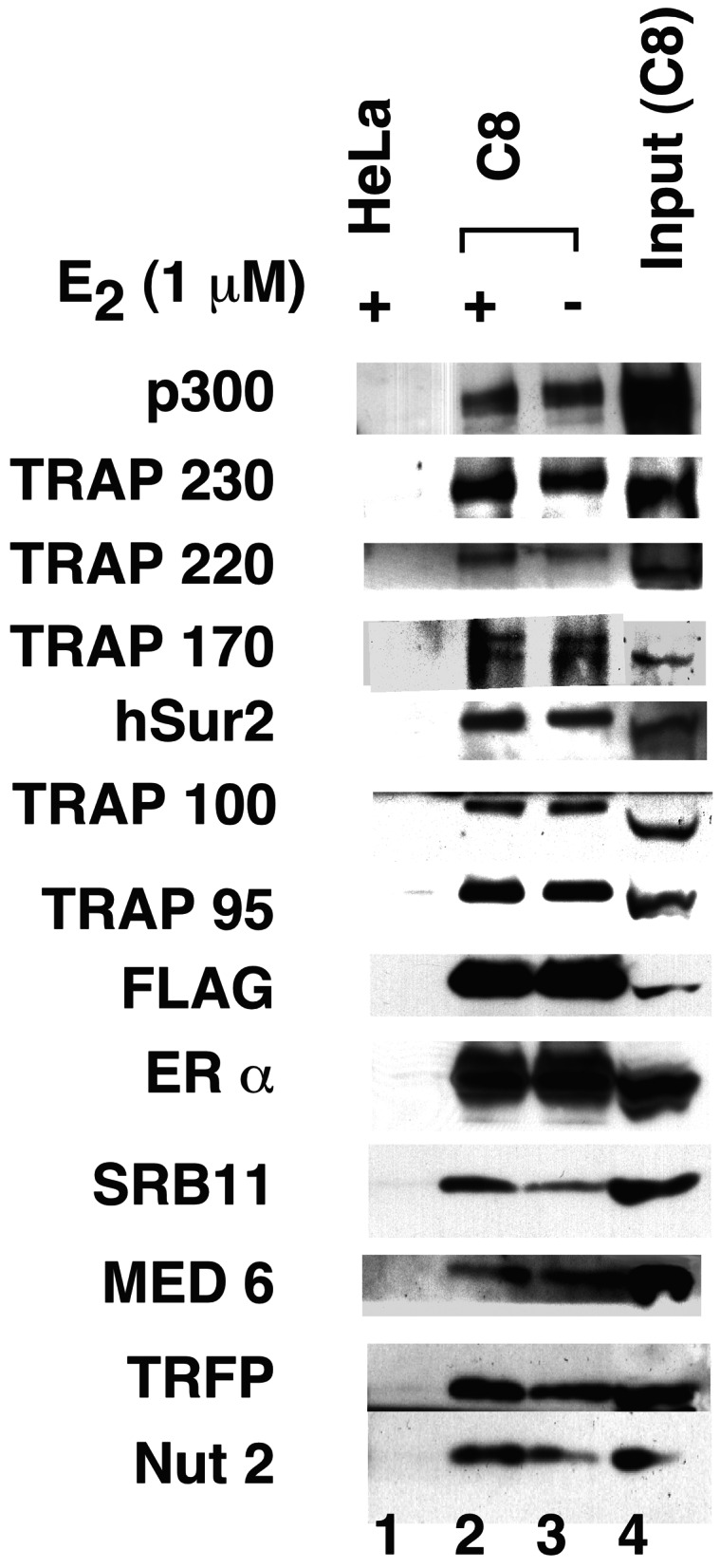
Intracellular association of TRAP/Mediator with ERαΔAB. C8 cells that express FLAG-tagged ERα lacking the AB domain (f:hERαΔAB) were maintained in DMEM-PO4 medium supplemented with 10% calf serum. f:ERαΔAB and associated proteins were affinity-purified from C8-derived nuclear extract on M2 agarose. Proteins bound in the presence (+) or absence (−) of 1 μM E2 during purification were eluted with FLAG peptide and analyzed by SDS/PAGE and Western blot with antibodies to proteins indicated on the left. M2 agarose-bound proteins from HeLa extract were analyzed in lane 1, and a 1/10th equivalent of C8 nuclear extract input was analyzed in lane 4.
In the current analysis, the probable E2 dependence of this association could not be assessed, because the C8 cells lost receptors and exhibited poor viability in steroid-depleted medium; and whereas purification of the ERαΔAB-containing complex in the presence and absence of E2 yielded comparable results (Fig. 5, lane 2 vs. lane 3), this result likely reflects prior occupancy and stability of the ER ligand binding pocket by estrogen or estrogen-like compounds in the culture medium.
Interactions of TRAP/Mediator with Intact ERs.
The above results have established E2-dependent interactions of TRAP/Mediator with isolated ER LBDs, but these interactions can be considered physiologically relevant only if they are demonstrable in the context of natural full-length receptors. In an analysis of this question, full-length FLAG-tagged ERs were expressed via baculovirus vectors, affinity-purified, and immobilized on M2 agarose beads. Nuclear extracts were applied to beads containing immobilized receptors and, after washing, bound proteins were eluted and analyzed by SDS/PAGE and Western blotting with antibodies to representative TRAP/Mediator components. As indicated in Fig. 6A, TRAP/Mediator bound to both ERα and ERβ in an E2-dependent manner. However, in contrast to the results with ER LBDs, TRAP/Mediator showed stronger interactions with ERβ than with ERα. Although not comparable directly, these results are nonetheless consistent with a prior report that isolated TRAP220 interacts more strongly with intact ERβ than with ERα (17). Given that the assays for TRAP/Mediator interactions with intact ERs (Fig. 6) vs. ER LBDs (Fig. 2) were performed under comparable conditions, these results in toto suggest that the ERα and ERβ AB domains differentially modulate interactions of TRAP/Mediator with corresponding LBDs.
To investigate the possibility that additional nuclear proteins might have facilitated intact ER-TRAP/Mediator interactions in these assays, the binding of 35S-labeled full-length ERβ to purified and immobilized TRAP/Mediator (Fig. 6B) was analyzed. (For technical reasons relating to the presence of FLAG tags on both recombinant ERs and the purified TRAP complex, an analysis of TRAP/Mediator binding to M2 agarose-immobilized ERs was not possible.) As shown in Fig. 6C, 35S-labeled full-length ERβ bound to purified TRAP/Mediator in an E2-dependent manner, thus indicating direct ER-TRAP/Mediator interactions. Consistent with the results of Fig. 6A, ERβ interacted more strongly with purified TRAP/Mediator in this assay than did ERα (data not shown).
TRAP/Mediator Directly Enhances ER Function in a Purified in Vitro Transcription Assay.
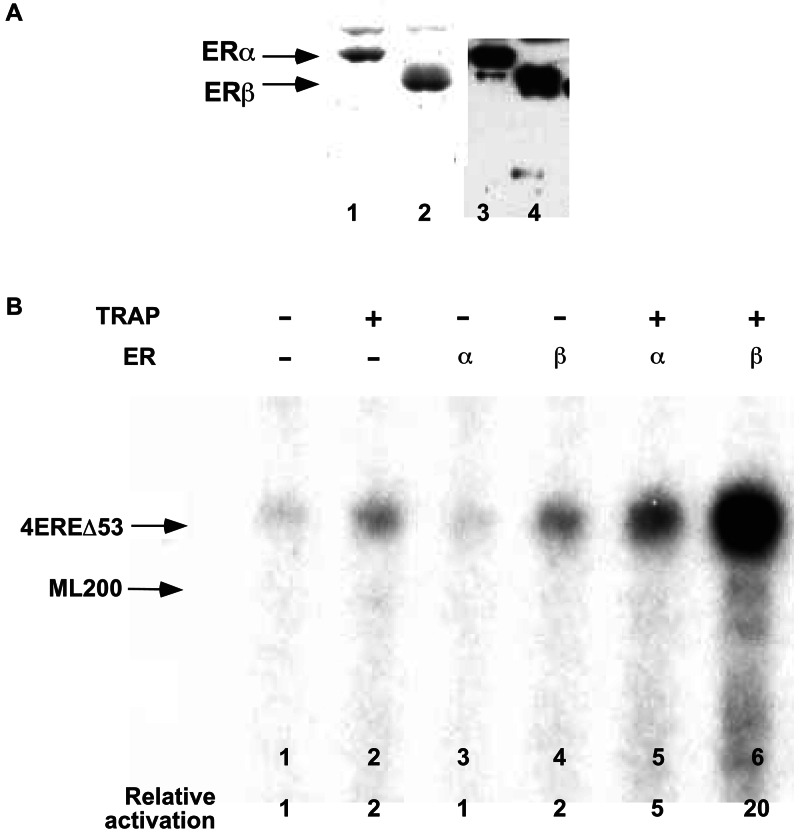
TRAP/Mediator has been shown to enhance the transcription activity of several activators, including nuclear receptors, from DNA templates in purified cell-free systems (7, 10, 22, 23). To assess the role of the complete TRAP/Mediator complex in ER-mediated transcription, we used a cell-free system reconstituted with highly purified general initiation factors and cofactors (TFIIB, TFIID, TFIIE, TFIIF, TFIIH, RNA polymerase II, and PC4), combinations of baculovirus-expressed and purified receptors (Fig. 7A), affinity-purified TRAP/Mediator (Fig. 6B), and a DNA template containing four copies of an estrogen response element (ERE) upstream of the adenovirus major late core promoter (24). In this assay (Fig. 7B), basal activity (lane 1) was unaffected by ERα alone (lane 3) and weakly (≈2-fold) enhanced by ERβ alone (lane 4) or by TRAP/Mediator alone (lane 2). In the presence of TRAP/Mediator, activity with ERα was ≈5-fold above basal (lane 5) and activity with ERβ was ≈20-fold above basal (lane 6). These results clearly show a direct role for TRAP/Mediator in ER function as well as a greater activity for ERβ than for ERα. Although somewhat surprising, the latter result is consistent with the higher affinity of TRAP/Mediator for intact ERβ relative to intact ERα.
Figure 7.
TRAP/Mediator complex directly mediates ER function in vitro. (A) Recombinant FLAG-tagged ER proteins. Affinity-purified ERs were analyzed by SDS/PAGE and either Coomassie brilliant blue R-250 staining (lanes 1 and 2) or Western blot with corresponding antibodies (lanes 3 and 4). (B) ER-dependent in vitro transcription. Reactions contained purified general TFs and cofactors, purified TRAP/Mediator complex, and ER proteins (20 nM) as indicated and both 4EREΔ53 G-less reporter and ML200 G-less control templates. TRAP/Mediator was purified from the cell line expressing f:TRAP220AB (Fig. 6B).
Discussion
The diversity of cofactors implicated in nuclear receptor function is somewhat surprising in view of the DNA binding specificity intrinsic to the receptors. However, this diversity provides (i) the means to overcome (or reinforce) barriers to transcription imposed by chromatin structure, as well as a variety of interfaces for the structurally complex general transcription machinery, (ii) mechanisms, through cofactor modulation, to integrate secondary signaling pathways with ligand signaling pathways, and (iii) the possibility of cofactor redundancy and alternate activation pathways for different cell types, promoters, and ligands. Understanding the cofactors that regulate ER function is particularly interesting and important because of the existence of receptor subtypes (α and β) with different tissue distributions, functions, and ligand responses; cell and promoter context-dependent functions of resident (AF-1 and AF-2) activation domains; and the existence of selective ER modulators (reviewed in refs. 5, 20, and 21). After earlier reports of ligand-dependent interactions of ERs with the isolated TRAP220 subunit of the multicomponent (≈25 subunit) TRAP/Mediator complex, we provide evidence for direct TRAP220 and ligand-dependent interactions of ERs with the intact TRAP/Mediator complex and a direct role for the complex in ER function through the general transcription machinery.
TRAP/Mediator Is a Bona Fide ER Coactivator.
Although a role for the complete TRAP/Mediator in ER function has been inferred from TRAP220 binding studies (6, 15–17) and from precedent established with TR and VDR (6, 14), the current results provide direct evidence for this hypothesis. Consistent with the earlier results, and with structural conservation of the AF-2 domain implicated in ligand-dependent interactions of many cofactors, we show ligand-dependent interactions of TRAP/Mediator in nuclear extracts and in purified form with both ERα and ERβ LBDs. The latter result establishes that these interactions are direct, whereas analyses with extracts from TRAP220−/− fibroblasts establish an essential role for TRAP220 in these interactions. Consistent with their role in TR and VDR interactions, the TRAP220 LXXLL motifs (most notably motif 1) have been implicated in ligand-dependent ERα and ERβ interactions (16, 17). Thus, although reported ligand-independent interactions of ERs with other TRAPs (16) could be relevant, they are insufficient for demonstrable interactions of the entire TRAP complex with ERs. Related, our analyses failed to show any direct interactions of TRAP/Mediator with ER N-terminal AB domains.
Importantly, the physiological relevance of the in vitro interactions is substantiated by our demonstration of an in vivo association of the entire TRAP/Mediator complex with an ERα derivative lacking the AB domain. Along with the in vitro data, this observation makes improbable the suggestion (16) that TRAP220 may mediate ER function independently of many or all the other TRAP/Mediator subunits. In a further analysis of the significance of TRAP/Mediator interactions, and perhaps most importantly, we demonstrate that the purified TRAP/Mediator complex markedly enhances ER function in a fully defined in vitro transcription assay with DNA templates. This latter result firmly establishes a direct function for TRAP/Mediator in mediating the action of ERs on target promoters in conjunction with the general transcription machinery.
Subtype Specificity of ERs.
Although previous studies have reported activation by ERα in crude cell-free systems (24, 29), this report compares the intrinsic activities of ERα and ERβ in a fully defined system reconstituted with essentially homogeneous factors. Our observation that ERβ is more active than ERα in the TRAP/Mediator-dependent assay system is somewhat surprising in view of reports that ERα generally is more active than ERβ in transfection assays with ectopic reporters (reviewed in refs. 5, 20, and 30). However, the in vitro transcription data correlate directly with our observation that intact ERβ has a higher affinity for TRAP/Mediator than does intact ERα. Furthermore, promoter and cell context-dependent effects of ER AF-1 and AF-2 domain are well established (reviewed in refs. 5, 20, and 30). Especially relevant to the present findings, an ERβ lacking the AB domain is much more active than an ERα lacking the AB domain in certain (e.g., HeLa) cell types (30), which reflects the well established synergy of ERα AF-1 and AF-2 domains (20) and an apparent inhibitory effect of ERβ AF-1 on ERβ AF-2 function (30). Because ERα AF-1 function has been shown to involve site-specific phosphorylation and recruitment of cofactors not present in our in vitro assay (ref. 31 and references therein), our results likely reflect an intrinsic AF-2 function mediated through TRAP/Mediator interactions. Also of importance is our observation that the differential affinities of TRAP/Mediator for intact ERs (β > α) is reversed when the AB domains are absent (α > β), which clearly indicates an ability of the AB domains to modulate interactions of the LBD (AF-2) domains with TRAP/Mediator (and probably other cofactors), although it is not clear whether this reflects an inhibitory effect of the ERα AB domain on ERα AF-2 interactions and/or a stimulatory effect of the ERβ AB domain on ERβ AF-2 interactions with TRAP/Mediator. However, the results reported here likely reflect intrinsic (intramolecular) effects of the AB domain(s) rather than effects of additional AB domain-interacting factors, because similar effects were observed with unpurified (nuclear extract) and highly purified TRAP/Mediator.
Although we (Y.K.K. and R.G.R., unpublished data) and Freedman and coworkers (16) failed to see differential interactions of ERα and ERβ with isolated TRAP220, Treuter and coworkers (17) have reported stronger interactions of isolated TRAP220 (and TRAP220 fragments) with ERβ than with ERα. Although the basis for these discrepancies is not clear, the latter results are consistent with our observation of stronger ERβ interactions with TRAP220 in the more physiological context of the functional TRAP/Mediator complex. Given that other AF-2-interacting coactivators such as p160/SRC members show little subtype selectivity in ER binding (16, 17), and as discussed by Warnmark et al. (17), the differential binding of TRAP/Mediator to ERα and ERβ has significant implications for differential functions of these receptors in diverse tissues that could show modulations of TRAP/Mediator components (15). The differential recruitment of these factors also may contribute to the tissue-selective action of selective ER modulators. Finally, apart from a number of other interacting factors (reviewed in ref. 5) implicated in ER functions, it will be important to characterize further the additional ER-interacting polypeptides identified here (Fig. 1) for possible subtype-specific functions and interactions with TRAP/Mediator, other cofactors, and the general transcription machinery.
Acknowledgments
We thank Dr. M. Muramatsu for the ERβ cDNA, Dr. C. M. Chiang for the 4EREΔ53 template, Dr. M. Ito for the GST-ERαLBD construct, Dr. Z. Wang for preliminary transcription assays, Dr. S. Malik for technical advice and support, and Mr. H. J. Baek for the anti-hSur2 antibody. This work was supported by grants from the National Institutes of Health (to R.G.R.).
Abbreviations
- LBD
ligand binding domain
- TRAP
thyroid hormone receptor-associated protein
- TR
thyroid hormone receptor
- VDR
vitamin D receptor
- ER
estrogen receptor
- GST
glutathione S-transferase
- hER
human ER
- MEF
mouse embryo fibroblast
- E2
17β-estradiol
- TF
transcription factor
References
- 1.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, et al. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owen G I, Zelent A. Cell Mol Life Sci. 2000;57:809–827. doi: 10.1007/s000180050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKenna N J, Lanz R B, O'Malley B W. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 4.Glass C K, Rosenfeld M G. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 5.Nilsson S, Makela S, Treuter E, Tujague M, Thomsen J, Andersson G, Enmark E, Pettersson K, Warner M, Gustafsson J A. Physiol Rev. 2001;81:1535–1565. doi: 10.1152/physrev.2001.81.4.1535. [DOI] [PubMed] [Google Scholar]
- 6.Yuan C X, Ito M, Fondell J D, Fu Z Y, Roeder R G. Proc Natl Acad Sci USA. 1998;95:7939–7944. doi: 10.1073/pnas.95.14.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito M, Yuan C X, Malik S, Gu W, Fondell J D, Yamamura S, Fu Z Y, Zhang X, Qin J, Roeder R G. Mol Cell. 1999;3:361–370. doi: 10.1016/s1097-2765(00)80463-3. [DOI] [PubMed] [Google Scholar]
- 8.Ito M, Roeder R G. Trends Endocrinol Metab. 2001;12:127–134. doi: 10.1016/s1043-2760(00)00355-6. [DOI] [PubMed] [Google Scholar]
- 9.Malik S, Roeder R G. Trends Biochem Sci. 2000;25:277–283. doi: 10.1016/s0968-0004(00)01596-6. [DOI] [PubMed] [Google Scholar]
- 10.Fondell J D, Ge H, Roeder R G. Proc Natl Acad Sci USA. 1996;93:8329–8333. doi: 10.1073/pnas.93.16.8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito M, Yuan C X, Okano H J, Darnell R B, Roeder R G. Mol Cell. 2000;5:683–693. doi: 10.1016/s1097-2765(00)80247-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Qi C, Jia Y, Nye J S, Rao M S, Reddy J K. J Biol Chem. 2000;275:14779–14782. doi: 10.1074/jbc.C000121200. [DOI] [PubMed] [Google Scholar]
- 13.Zhu Y, Qi C, Jain S, Rao M S, Reddy J K. J Biol Chem. 1997;272:25500–25506. doi: 10.1074/jbc.272.41.25500. [DOI] [PubMed] [Google Scholar]
- 14.Rachez C, Lemon B D, Suldan Z, Bromleigh V, Gamble M, Naar A M, Erdjument-Bromage H, Tempst P, Freedman L P. Nature (London) 1999;398:824–828. doi: 10.1038/19783. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, Qi C, Jain S, Le Beau M M, Espinosa R, 3rd, Atkins G B, Lazar M A, Yeldandi A V, Rao M S, Reddy J K. Proc Natl Acad Sci USA. 1999;96:10848–10853. doi: 10.1073/pnas.96.19.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burakov D, Wong C W, Rachez C, Cheskis B J, Freedman L P. J Biol Chem. 2000;275:20928–20934. doi: 10.1074/jbc.M002013200. [DOI] [PubMed] [Google Scholar]
- 17.Warnmark A, Almlof T, Leers J, Gustafsson J A, Treuter E. J Biol Chem. 2001;276:23397–23404. doi: 10.1074/jbc.M011651200. [DOI] [PubMed] [Google Scholar]
- 18.Llopis J, Westin S, Ricote M, Wang Z, Cho C Y, Kurokawa R, Mullen T M, Rose D W, Rosenfeld M G, Tsien R Y, Glass C K, Wang J. Proc Natl Acad Sci USA. 2000;97:4363–4368. doi: 10.1073/pnas.97.8.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shang Y, Hu X, DiRenzo J, Lazar M A, Brown M. Cell. 2000;103:843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 20.Pettersson K, Gustafsson J A. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 21.McDonnell D P. Trends Endocrinol Metab. 1999;10:301–311. doi: 10.1016/s1043-2760(99)00177-0. [DOI] [PubMed] [Google Scholar]
- 22.Malik S, Gu W, Wu W, Qin J, Roeder R G. Mol Cell. 2000;5:753–760. doi: 10.1016/s1097-2765(00)80254-3. [DOI] [PubMed] [Google Scholar]
- 23.Gu W, Malik S, Ito M, Yuan C X, Fondell J D, Zhang X, Martinez E, Qin J, Roeder R G. Mol Cell. 1999;3:97–108. doi: 10.1016/s1097-2765(00)80178-1. [DOI] [PubMed] [Google Scholar]
- 24.Wu S Y, Thomas M C, Hou S Y, Likhite V, Chiang C M. J Biol Chem. 1999;274:23480–23490. doi: 10.1074/jbc.274.33.23480. [DOI] [PubMed] [Google Scholar]
- 25.Rachez C, Suldan Z, Ward J, Chang C P, Burakov D, Erdjument-Bromage H, Tempst P, Freedman L P. Genes Dev. 1998;12:1787–1800. doi: 10.1101/gad.12.12.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guermah M, Tao Y, Roeder R G. Mol Cell Biol. 2001;21:6882–6894. doi: 10.1128/MCB.21.20.6882-6894.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassilev A, Yamauchi J, Kotani T, Prives C, Avantaggiati M L, Qin J, Nakatani Y. Mol Cell. 1998;2:869–875. doi: 10.1016/s1097-2765(00)80301-9. [DOI] [PubMed] [Google Scholar]
- 28.Hittelman A B, Burakov D, Iniguez-Lluhi J A, Freedman L P, Garabedian M J. EMBO J. 1999;18:5380–5388. doi: 10.1093/emboj/18.19.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraus W L, Kadonaga J T. Genes Dev. 1998;12:331–342. doi: 10.1101/gad.12.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall J M, McDonnell D P. Endocrinology. 1999;140:5566–5578. doi: 10.1210/endo.140.12.7179. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe M, Yanagisawa J, Kitagawa H, Takeyama K, Ogawa S, Arao Y, Suzawa M, Kobayashi Y, Yano T, Yoshikawa H, Masuhiro Y, Kato S. EMBO J. 2001;20:1341–1352. doi: 10.1093/emboj/20.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]