Abstract
The cytotoxic effect of microcin E492, a low-molecular-mass channel-forming bacteriocin (7,887 Da) produced by a strain of Klebsiella pneumoniae, was characterized in HeLa cells. At low (5 μg/ml) and intermediate (10 μg/ml) concentrations, microcin E492 induced biochemical and morphological changes typical of apoptosis, such as cell shrinkage, DNA fragmentation, extracellular exposure of phosphatidylserine, caspase activation, and loss of mitochondrial membrane potential. Treatment with zVAD-fmk, a general caspase inhibitor, completely blocked the cytotoxic effect of this bacteriocin. At higher microcin concentrations (>20 μg/ml) a necrotic phenotype was observed. Induction of apoptosis by microcin E492 was associated with the release of calcium from intracellular stores, probably after microcin-triggered ion channel formation. Microcin E492 also presented a cytotoxic effect on Jurkat and RJ2.25 cells, but had no effect on KG-1 cells nor on a primary culture of human tonsil endothelial cells, suggesting that there is a specific interaction of the bacteriocin with components of the target cell surface. This report describes a bacteriocin that has the capacity to induce apoptosis in human cell lines.
Bacteriocins are antimicrobial proteins produced by bacteria that are active against closely related species. They are produced by almost all major lineages of Eubacteria and in Archaebacteria (1) and secreted to the extracellular medium where they recognize specific receptors on the surface of sensitive bacterial cells (2). It has been proposed that the primary role of bacteriocins is to mediate population dynamics within closely related species (1). Bacteriocins can induce toxicity by a variety of mechanisms, most notably the formation of membrane pores. These compounds have received increasing attention for their potential use as preservatives in the food industry and as antibiotics for clinical usage (3–6).
Bacteria also produce other toxic proteins, released by pathogens into the host's body, that are important virulence factors causing disease symptoms (tetanus, diphtheria, cholera, etc.). These toxic agents are commonly called bacterial toxins and may attack a variety of different cell types (cytotoxins) or specific cell types (neurotoxins, hemolysins, etc). Among these bacterial toxins some can also form membrane pores and kill eukaryotic cells by disrupting the membrane permeability barrier. Lately, increasing interest has developed in the study of some toxins for their potential therapeutic use as antitumoral drugs. Thus, cytotoxic studies of toxins such as verotoxin 1 (7) and a derivative of a Pseudomonas exotoxin (8) have demonstrated that these toxins display antitumor activity in vitro and in vivo in ovarian and breast cancer, respectively. Another aspect that has aroused considerable interest is the role of toxin-producing pathogens in promoting apoptosis (9). In contrast to necrosis, apoptosis eliminates cells without inducing an inflammatory response, i.e., in an immunologically silent manner subverting the normal host defense responses. Among the pore-forming toxins that induce apoptosis is α-toxin, the major cytolysin from Staphylococcus aureus (10), aerolysin, produced by Aeromonas hydrophyla (11), and the porin PorB from Neisseria gonorrhoeae (12).
Apoptosis is a genetically determined form of cell death that plays a central role during development and homeostasis of multicellular organisms (13, 14). Necrotic cell death is usually the consequence of physical injury and does not involve the active participation of the cell. Apoptosis can be distinguished from necrosis on the basis of several morphological as well as biochemical parameters, such as nuclear condensation, loss of cell volume (shrinkage), DNA fragmentation (14), and phosphatidylserine exposure to the outer face of plasma membrane (15). This kind of cell death avoids spillage of intracellular contents in contrast to necrotic cell death, typified by cell and organelle swelling and membrane disruption, resulting in an inflammatory response (15). The central executioners of apoptosis are a set of cysteine proteases that are part of a large protein family known as caspases (16).
Some bacterial toxins share common features with bacteriocins, such as insertion into or transport across the plasma membrane, recognition of the target cell's membrane, pore formation, etc. The known action of bacterial toxins and bacteriocins has been restricted to eukaryotic cells and bacteria, respectively, probably because of specific interactions between the receptor and the toxin. The only bacteriocin reported to be lytic for eukaryotic as well as prokaryotic cells is a cytolysin from Enterococcus faecalis (17). Although it is known that this toxin induces cell lysis, no evidence has been presented as to whether cytotoxicity is caused by apoptosis or necrosis.
The aim of this work was to study the ability of microcin E492 to induce apoptosis. Microcin E492 is a low-molecular-mass (7,887 Da) channel-forming bacteriocin (18, 19) produced by Klebsiella pneumoniae RYC492, which has a rather broad target range, being active on strains of the family Enterobacteriaceae (20). The genetic determinants involved in the production of active microcin and its immunity protein have been cloned and expressed in Escherichia coli (21) and comprise a 13-kb DNA fragment encoding for at least 10 genes, necessary for the synthesis and export of active microcin (22). Analysis of the amino acid sequence of microcin E492 indicates that this bacteriocin does not share structural motif with any other known protein, and thus, it seems that this microcin belongs to a novel class of pore-forming bacteriocins not related to colicin-like channel-forming bacteriocin from Gram-negative bacteria or to channel-forming bacteriocins from Gram-positive bacteria (19). Results presented in this article show that in certain human cell lines microcin E492 promotes morphological and biochemical changes typical of apoptosis.
Materials and Methods
Isolation, Purification, and Fluorescent Labeling of Microcin E492.
Microcin E492 was extracted and purified (20) from the supernatant cultures of E. coli VCS257pJEM15. Microcin activity was estimated and expressed in arbitrary antibiotic units (AU) (23). Normally, 10 μg of microcin E492 corresponded to 500 AU. Labeling of microcin with fluorescamine and PAGE were carried out as described (21).
Cell Lines.
The following human cell lines (American Type Culture Collection) were used: HeLa, an epithelial cell line derived from human cervix carcinoma; KG-1, a monocyte-macrophage cell line; RJ2.25, a variant of the Raji B-LCL (24); Jurkat, a T cell derived from acute T cell leukemia, and Ramos, a B cell line originated from Burkitt's lymphoma. The human endothelial cells from human tonsils (AMG-3) were isolated as described (25). Ramos, Jurkat, RJ2.2.5, KG-1, and AMG-3 cells were maintained in RPMI 1640 medium supplemented with 10% FCS and antibiotics. HeLa cell line were maintained in DMEM with 10% FCS and antibiotics.
Cytotoxicity and Apoptosis Assays.
Cells were seeded at 2 × 104 cells/well in 96-well plates 24 h before microcin E492 addition, further incubated for the indicated time with microcin E492, trypsinized and washed, suspended in PBS, stained with 1 μg/ml propidium iodide (PI), and analyzed by using a FACScan (Becton Dickinson) with cell quest software. The loss of mitochondrial membrane potential was assessed by using 3,3′-dihexyloxacarbocyanine [DiOC6 (3)] (Molecular Probes) as described (26). Exposure of phosphatidylserine to the outer leaflet of the plasma membrane was determined by flow cytometry with annexin V coupled to FITC (Annexin-V-FLUOS staining kit, Roche Molecular Biochemicals) as described (27). Lactate dehydrogenase (LDH) activity was assessed by using the LDH kit from Sigma following the manufacturer's instructions. In situ detection of fragmented DNA was carried out by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay using the Apoptosis Detection System, fluorescein (Promega) following the manufacturer's instructions, and visualized with a Zeiss confocal microscope.
Determination of Intracellular Calcium Levels and Inmunofluorescence Assay.
Intracellular Ca2+ levels were determined as described (27, 28), using Fluo-3AM (Molecular Probes) and measuring the fluorescence by confocal microscopy (excitation 488/emission 505 nm). Human lymphocyte function-associated antigen 1 (LFA-1) (CD11a) was detected at the cell surface by using the mAb TS1/22.1.1 (29). Subconfluent cultures were analyzed by indirect immunofluorescence assays as described (30).
Caspase-3/Caspase-1 and Cytochrome c Release Assays.
HeLa cells incubated with microcin were harvested with a strip, washed in PBS, disrupted in 10 mM Tris⋅HCl (pH 7.4), 5 mM 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, 2 mM MgCl2, 150 mM NaCl, 5 mM EDTA, and 2 mM DTT, and centrifuged for 15 min at 11,500 g. Fifty micromolars of the colorimetric caspase-3 substrate Ac-DEVD-pNA or caspase-1 substrate Ac-YVAD-pNA was added (Calbiochem) and incubated for 1 h at 37°C, and absorbance was measured in an ELISA reader at 405 nm. For detection of cytochrome c release, HeLa cells treated with microcin E492 were resuspended in 10 mM Hepes (pH 7.5), 1 mM EGTA, 70 mM sucrose, 20 mM manitol, and protease inhibitors (PMSF, antipain, benzamidine), homogenized, and centrifuged at 1,000 rpm for 1 min, and the supernatant was centrifuged at 10,000 g for 1 h. The pellet was resuspended in Laemmli buffer, and soluble proteins were precipitated with methanol-chloroform and resuspended in Laemmli buffer, and the presence of cytochrome c was probed by blotting and Western blot with a mAb (Zyned, 33–8500). Bound antibody was detected by using SuperSignal West Pico chemiluminescent substrate (Pierce).
Results and Discussion
The Cytotoxic Effect of Microcin E492 Is Specific for Particular Human Cell Lines.
Cytotoxicity of microcin E492 was assessed on several human cell lines with different morphologies and physiology: HeLa, Jurkat, RJ2.2.5, KG-1, Ramos, and the primary culture AMG-3. Table 1 shows cell viability after incubation for 24 h in a medium containing 680 AU/ml (≈14 μg/ml) microcin E492. KG-1 and AMG-3 were insensitive to microcin E492. Ramos presented a slight sensitivity to this bacteriocin, whereas HeLa, RJ2.2.5, and Jurkat cell lines were sensitive, at different degrees, to the microcin E492 toxic effect.
Table 1.
Effect of microcin E492 on different human cells types
| Cell type | Cell survival, % |
|---|---|
| Jurkat | 4 ± 3 |
| HeLa | 56 ± 6 |
| RJ2.2.5 | 57 ± 11 |
| Ramos | 79 ± 19 |
| KG-1 | 91 ± 1 |
| AMG-3 | 99 ± 1 |
Cells were incubated for 24 h in the absence (untreated control) or presence of 680 AU/ml of microcin E492. The percentages of cell survival were determined in respect to the untreated control and are the averages of three independent measurements. Cells were stained with PI, and cell death was determined by flow cytometry analysis.
Microcin E492 has no hemolytic activity on blood agar and did not present any evident toxic effect after i.p. injection into mice. Hence, the cytotoxic effect observed seems to be restricted to particular cell lines.
Pore-forming bacterial toxins, such as hemolysins and leukotoxins, exert their lethal effect by binding to a cell-surface receptor identified as LFA-1, a member of the integrin family (31). To determine whether the cytotoxic specificity of microcin E492 correlates with the expression of LFA-1, the expression of this antigen at the cell surface of the six cell types used in this work was studied by indirect immunofluorescence. The primary culture AMG-3, Ramos, and HeLa cell lines did not present LFA-1 at the cell surface, whereas KG-1, RJ2.2.5, and Jurkat cell lines expressed high cell surface levels of LFA-1. The lack of correlation between toxicity and expression of LFA-1 discards this protein as the microcin E492 receptor. Although the cytotoxicity of microcin is specific on particular cell types, suggesting that the restricted host cell specificity can be caused by the binding to an unknown cell-surface receptor, the receptor does not necessarily have to be a protein. For instance, aerolysin interacts with a specific posttranslational modification, a glycosylphosphatidylinositol anchor (32), and perfringolysin O, a pore-forming cytolysin, recognizes the lipid composition of the cell membrane (33). Microcin protein sequence was analyzed by using advanced searches for domains or similarities with eukaryotic proteins that may correlate this bacteriocin with a possible receptor. However, no recognizable domain or structural homologies that may connect this bacteriocin as a ligand for an eukaryotic receptor was found. Alternatively, the resistance of some cell lines to microcin could be caused by a differential expression of apoptosis regulatory proteins when exposed to this bacteriocin.
Microcin E492 Induces Apoptosis in HeLa Cells.
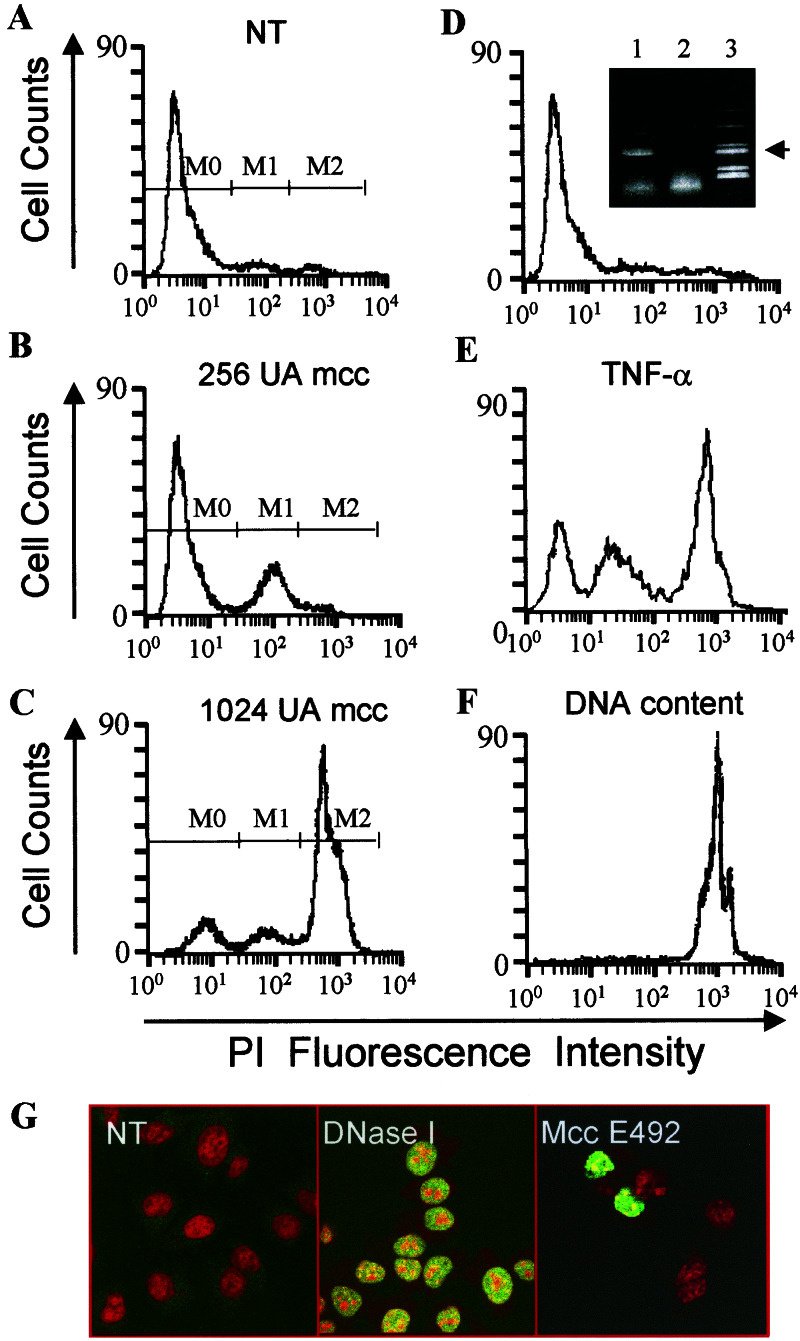
HeLa cells were chosen to characterize the cytotoxic effect of microcin E492. The PI exclusion method detects dead cells by the loss of membrane integrity. Once PI is inside the cell, it binds to DNA and its fluorescence is proportional to the DNA content. Fig. 1A shows the basal fluorescence of untreated viable cells. After treatment with 256 AU/ml (≈5 μg/ml) microcin E492 most dead cells showed decreased PI fluorescence (Fig. 1B) when compared with the total DNA content of HeLa cells (Fig. 1F). This so-called hypodiploid part of the population (M1) would correspond to apoptotic cells with degraded DNA that bind less PI than dead cells with intact DNA. The microcin concentration required for apoptosis induction was similar to that used to induce apoptosis on HeLa cells with the porin PorB (12). HeLa cells killed with a high dose of microcin E492 (1,024 AU/ml, ≈20 μg/ml) presented mainly an intact DNA content (M2 in Fig. 1C), indicating a death by necrosis. Treatment of HeLa cells with an intermediate dose of microcin E492 (512 AU/ml, ≈10 μg/ml) produced a mixture of the two populations with different DNA content (data not shown), i.e., death by necrosis and apoptosis. Apoptosis and necrosis were also induced after treatment of HeLa cells with tumor necrosis factor α (TNF-α) (Fig. 1E). To discard the possibility that cytotoxicity was induced by a minor contaminant that usually copurifies with microcin E492 and that is detected only with fluorescamine (Fig. 1D Inset, lane 1), a cytotoxic assay was carried out by using a preparation starting from a supernatant of E. coli VCS257np108. This mutant does not export microcin E492 to the extracellular space (Fig. 1D Inset, lane 2) (22), but produces the other component that copurifies with microcin E492. This impurity was not detected by other staining methods, because when Coomassie blue or silver staining were used only the protein band corresponding to microcin was observed. Fig. 1D shows that this preparation had no effect on HeLa cell viability, demonstrating that the cytotoxic effect observed is associated with the presence of microcin E492. DNA fragmentation was also determined in situ by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling analysis (Fig. 1G). Chromatin condensation and loss of epithelial morphology was also observed (not shown).
Figure 1.
Microcin E492 induces cell death with DNA fragmentation in a dose-dependent manner. HeLa cells (0.4 × 106 cells in 1 ml) were incubated during 24 h under different conditions and analyzed by the PI exclusion method. (A) Untreated control. NT, nontreated. (B) Cells incubated with 256 AU microcin E492. (C) Cells incubated with 1,024 AU microcin E492. Dead cells permeable to PI are indicated in A–C as M1 and M2 populations. M0 population represents viable cells, not permeable to PI. mcc, microcin E492. (D) Cells incubated with a preparation from E. coli VCS257np108 with an OD280 equivalent to 10 μg. (Inset) A PAGE of microcin E492 (labeled with fluorescamine) purified from the microcin-producing strain E. coli VCS257pJEM15 (lane 1) and a purified fraction from the nonproducing strain E. coli VCS257 np108 (lane 2). The arrow indicates the microcin protein band, and lane 3 corresponds to the molecular mass markers (myoglobin fragments of 16,950, 14,440, 8,160, 6,210, 3,460, and 2,510). (E) Cells treated with 1 ng/ml TNF-α and 5 μg/ml cycloheximide. (F) Total DNA content was measured by permeabilization of nontreated cells with 95% methanol. A total of 10,000 cells per sample was analyzed by flow cytometry. (G) Fragmented DNA of HeLa cells incubated with 250 AU microcin E492 for 18 h detected by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay. (Left) Nontreated (NT) cells. (Center) Cells incubated with DNase I. (Right) Cells treated with microcin E492. Apoptotic nuclei are shown in green (dUTP-FITC incorporation) and total nuclei are shown in red (PI staining). (Magnification: ×400.)
Microcin E492 Induces Cell Shrinkage, a Decrease on Mitochondrial Membrane Potential, Cytochrome c Release, and Caspase Activation.
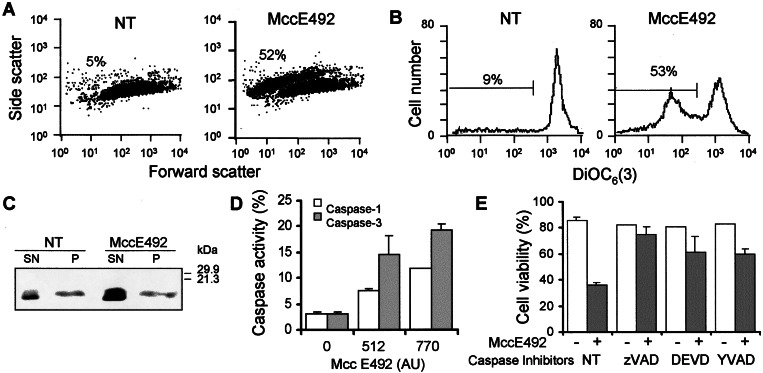
A relationship between cell shrinkage, loss of mitochondrial membrane potential, and release of cytochrome c after treatment with microcin E492 was investigated. Cell volume was assessed by light scattering on a forward-scatter versus side-scatter dot plot, and DiOC6 (3), a carbocyanine dye that accumulates in active mitochondria, was used to detect changes in the mitochondrial membrane potential. Fig. 2A shows that after treatment with 500 AU/ml (≈10 μg/ml) of microcin E492 HeLa cells presented a decrease in forward-scattered light and an increase in side-scattered light, indicative of shrunken cells, with a bimodal size distribution, in which 52% of the cells had a decrease in cell volume whereas the nontreated control displayed only 5% of cells with this characteristic. The loss of cell volume correlated very well with a reduction of mitochondrial membrane potential (53% of the cells), detected by a shift to lower fluorescence emission of DiOC6 (3) when compared with the untreated control (Fig. 2B). As expected, the cell population that presented a reduction in fluorescence emission corresponded to the shrunken cell population (not shown). The loss of mitochondrial membrane potential was accompanied by cytochrome c release to the cytoplasm, assessed by Western blot analysis (Fig. 2C).
Figure 2.
Microcin E492 induces caspase activation and mitochondrial dysfunction. (A) HeLa cells (0.2 × 106 cells in 1 ml) were treated with 500 AU/ml microcin E492 for 10 h and examined by flow cytometry, and the light-scattering properties (A) were assessed on a forward-scatter versus side-scatter dot plot, whereas the mitochondrial membrane potential (B) of these samples was evaluated by staining the cells with DiOC6 (3). The percentages of cells with decreased cell volume or mitochondrial membrane potential are indicated. NT corresponds to the untreated control. (C) HeLa cells (15 × 106 cells in 1 ml) were incubated with 700 AU microcin E492 for 7 h, and the cell extracts were separated into cytoplasmic (SN) and nuclear-mitochondrial (P) components and analyzed for the presence of cytochrome c by immunoblotting. (D) HeLa cells treated with microcin E492 for 12 h were disrupted and incubated with 50 μM of caspase-1 substrate Ac-YVAD-pma or caspase-3 substrate Ac-DEVD-pma. The results presented are the average of two independent experiments. (E) HeLa cells were incubated with or without 100 μM of Ac-DEVD-cho (DEVD), Ac-YVAD-cho (YVAD), or z-VAD-fmk (zVAD) for 2.5 h before addition of 700 AU microcin E492. After 24 h, cells were collected and stained with PI for cell viability analysis by FACS. The results correspond to the mean and standard deviations of three independent assays.
Association of cytochrome c to procaspase-9 and Apaf-1 is necessary for the apoptosome formation, which in turn activates caspase-3 (16). Thus, caspases activation such as caspase-1 and caspase-3 after microcin E492 treatment was measured. Fig. 2D shows that activation of these caspases depends on the microcin dose used. Conversely, caspase inhibitors interfered with cell death induced by microcin. Treatment of HeLa cells with 100 μM of zVAD-fmk, a general caspase inhibitor, abolished apoptosis induced by 700 AU/ml (≈14 μg/ml) microcin E492 (Fig. 2E). Treatment with 100 μM Ac-DEVD-cho, a caspase-3 inhibitor, or Ac-YVAD-cho, an inhibitor of ICE proteases like caspase-1, partially counteracted the cytotoxic effect of microcin E492 on HeLa cells (Fig. 2E). These findings allow us to conclude that apoptosis induction by microcin E492 is associated with activation of caspases.
A possible mechanism for apoptosis induction by microcin E492 could be through pore formation in mitochondria, such as the case described for the porin PorB (34). Here, it is postulated that this porin, like the mitochondrial voltage-dependent anion channel, functions at the mitochondrial checkpoint to mediate apoptosis. The decrease in mitochondrial membrane potential and cytochrome c release induced by microcin E492 is compatible with an opening of a permeability transition pore. However, this hypothesis should be tested by studying the subcellular localization of this bacteriocin and determining whether, as in the case of PorB, microcin E492 is able to produce the same effect in purified mitochondria.
High Doses of Microcin E492 Induce Necrosis and Apoptosis.
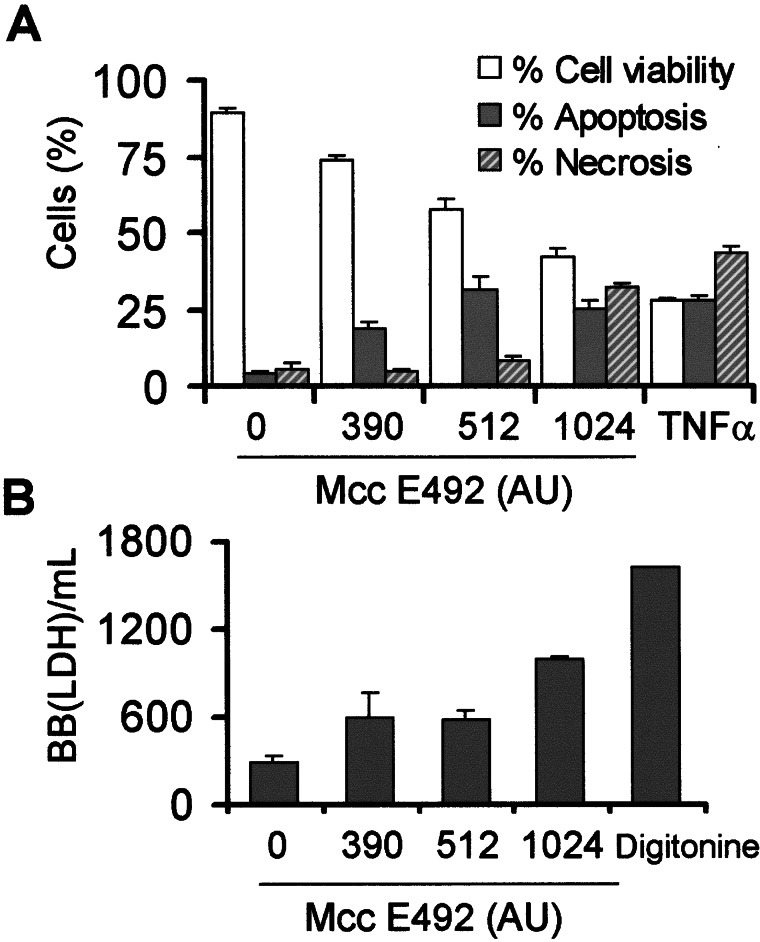
Many cytotoxic agents that induce apoptosis at low concentrations when used at higher doses produce necrosis (27, 35, 36). Fig. 1C shows mainly the induction of cell death without DNA fragmentation after treatment with 1,024 AU/ml microcin E492, suggesting that high doses of microcin E492 could induce cell death by necrosis. To assess the necrosis/apoptosis ratio depending on the microcin dose used, phosphatidylserine exposure at the cell surface was measured. This change occurs in early stages of apoptosis and can be recognized by binding of annexin V labeled with FITC. In early stages of apoptosis cells are not permeable to PI, and counterstaining with this fluorescent probe distinguishes the necrotic cells. HeLa cells treated with 390 and 512 AU/ml microcin E492 for 12 h showed 19% and 32% of apoptosis, respectively (Fig. 3A), with 5% and 9% of necrotic cells, respectively. Incubation with higher doses of microcin E492 (1,024 AU/ml; ≈20 μg/ml) increased the necrotic population to 32%, in contrast with the 25% of apoptosis (Fig. 3A). The apparent discrepancy in cell viability observed by using the PI cytotoxic assay (Fig. 1) and annexin V may be caused by the fact that the measurements of the latter were performed only after 12 h of incubation, a stage in apoptosis in which the cells are not permeable to PI, whereas the PI cytotoxic assay was performed after 24 h of incubation with microcin E492. These results were further confirmed by measuring a cell necrosis marker, the release of LDH to the culture medium. As expected, 390 and 512 AU/ml microcin E492 induced a lower leakage of LDH when compared with HeLa cells treated with a high dose of microcin E492 (Fig. 3B).
Figure 3.
Microcin E492 dose effect on the induction of necrosis and apoptosis in HeLa cells. Cells were incubated with microcin E492 for 12 h. (A) Phosphatidylserine exposure on the external plasma membrane leaflet was assessed by annexin V-FITC binding and loss of membrane integrity by PI uptake. A total of 20,000 cells per sample was analyzed by flow cytometry. As positive control, HeLa cells were incubated with 1 ng/ml TNF-α and 5 μg/ml cycloheximide. The bars are the average of three determinations. One hundred percent of cells is the addition of viable, apoptotic, and necrotic cells. (B) LDH activity from the supernatant cultures. One B-B(LDH) unit is defined as the amount of LDH that reduces 4.8 × 10−4 μmol of piruvate per min at 37°C.
Dose dependence associated with the induction of apoptosis or necrosis by bacterial toxins has been also described for α-toxin (35). T lymphocytes exposed to this toxin presented DNA degradation, typical of apoptosis, after incubation with low concentrations of this toxin (<200 nM). At higher doses, cells underwent necrosis, characterized by lysis without DNA degradation.
Microcin E492 Induces Calcium Fluxes in HeLa Cell Line.
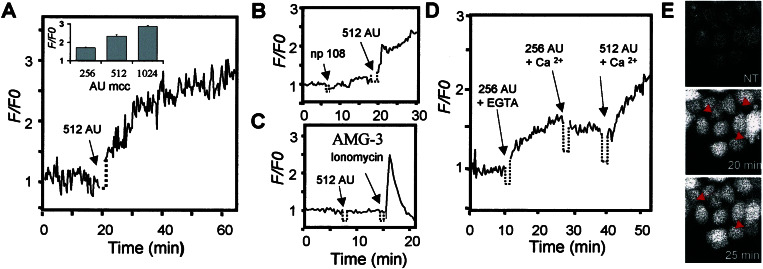
Changes in intracellular Ca2+ have been reported to occur at the beginning of apoptosis induced by pore-forming bacterial toxins, like α-toxin (35), PorB (12), and aerolysin (11). The fact that microcin E492 forms ion channels in planar phospholipid bilayers (18) prompted us to investigate the effect of microcin E492 treatment on intracellular Ca2+ levels. HeLa cells were loaded with the Ca2+-responsive fluorophore, Fluo-3, and the basal Ca2+ concentration in these cells was estimated to be approximately 55 nM. Cytosolic Ca2+ levels increased 2.5-fold after the addition of 512 AU/ml of microcin E492 (Fig. 4A), and this effect was dose-dependent (see Fig. 4A Inset). A microcin preparation obtained from the supernatant of the nonproducing mutant strain E. coli VCS257np108 had no significant effect on the intracellular Ca2+ concentration (Fig. 4B), confirming that the increase on intracellular Ca2+ was caused by the presence of microcin E492. Treatment of AMG-3 cells (insensitive to the cytotoxic effect of microcin E492) with microcin did not have any effect on cytosolic Ca2+ levels (Fig. 4C), which supports the idea that the cytotoxic effect is related to changes in intracellular Ca2+ concentrations.
Figure 4.
Microcin E492 induces an increase of intracellular Ca2+ levels as the result of Ca2+ release from intracellular stores. HeLa cells (A, B, D, and E) were loaded with the Ca2+ dye Fluo-3 (10 μg/ml). Changes in fluorescence intensity were measured by confocal microscopy. Relative fluorescence intensity (variations of fluorescence intensity normalized by the basal fluorescence) was plotted against time and represents the average of 20 individual cells. (A) Addition of 512 AU/ml microcin E492 (arrow). (Inset) The variation of intracellular Ca2+ levels after 30 min of treatment with different doses of microcin E492. (B) Treatment of HeLa cells with a preparation from the supernatant of a nonproducing strain (negative control, left arrow), followed by the addition of 512 AU/ml microcin E492 (positive control, right arrow). (C) A total of 512 AU/ml microcin E492 was added to AMG-3 cells (left arrow). As control of Ca2+ loading, cells were treated with 2 μg/ml ionomycin (right arrow). (D) HeLa cells were treated with 256 AU/ml microcin in the presence of 5 mM EGTA (left arrow). The solution was replaced with 20 mM Hepes, 136 mM NaCl, 4.7 mM KCl, 1.3 mM MgSO4, 1.3 mM CaCl2, 25 mM glucose, pH 7.4 plus 256 AU/ml microcin E492 (center arrow). After 10 min, this solution was replaced by a fresh one containing 512 AU/ml microcin E492 (right arrow). (E) High doses of microcin E492 induce morphological features of necrotic cell death. HeLa cells loaded with Fluo-3 were incubated with 2,048 AU/ml microcin E492. (Top) Untreated cells, (Center) after 20 min, and (Bottom) after 25 min of incubation with microcin E492. Arrows show the appearance of blebs at the cell surface. (Magnification: ×400.)
A rise in cytosolic Ca2+ could be originated by the release of Ca2+ from intracellular stores, such as the endoplasmic reticulum, or by uptake from the extracellular space. When extracellular Ca2+ was chelated by the addition of 5 mM EGTA, the increase of the intracellular Ca2+ level was to the same extent as that observed without the chelating agent (Fig. 4D). This observation suggests that microcin E492 triggers the release of Ca2+ from intracellular stores and discards the possibility that Ca2+ may enter through a Ca2+ channel, such as the case described for PorB (12). On the other hand, aerolysin promotes both Ca2+ influx across the plasma membrane and release of Ca2+ from intracellular stores (37). Increase of intracellular Ca2+ level caused only by the release from intracellular stores has been reported for cells treated with the specific K+ ionophores, valinomycin, and beauvericin (38) and by treatment with α-toxin (35). In all cases described before, the rise of Ca2+ levels occurs during the onset of apoptosis. Ca2+ fluxes have been implicated in the activation of apoptosis, because depletion of Ca2+ stored within the endoplasmic reticulum would produce Ca2+-overloaded mitochondria, resulting in abnormal mitochondrial metabolism, which activates apoptosis (39).
HeLa cells treated with very high doses of microcin E492 (2,048 AU/ml; ≈40 μg/ml) showed an increase in cell volume and membrane blebbing with a significant increase of Ca2+ levels (up to 4.5-fold), which preceded cell lysis by necrosis (Fig. 4E).
Infection of HeLa Cells with a Microcin E492-Producing Strain Induces Cell Death.
The potential cytotoxic effect of microcin E492-producing strains on in vitro infections of HeLa cells was investigated. Infection with E. coli VCS257pJEM15 for 48 h at a multiplicity of infection of 1 or 10 resulted in an induction of apoptosis (31%) or necrosis (52%), respectively. To analyze the possible participation of the bacterial strain per se in the cytotoxic process, the nonproducing microcin E492 bacterial strain E. coli VCS257 transformed with pHC79, the vector in which the genetic determinants needed for microcin E492 production are cloned (21), was used to infect HeLa cells at both multiplicities of infection. No effect on cell viability with respect to the untreated cells was observed (≥90% of survival in all cases). These results strongly suggest that the cause of the cytotoxic effect of these bacterial strains is microcin E492 production. The amount of microcin produced by the bacterial cells during the infections is at least 100-fold less than the concentration required to induce apoptosis by using the purified protein. Probably, the purification process alters the efficacy of microcin E492 to induce apoptosis.
Conclusions
Microcin E492 is a bacteriocin that has the capacity to induce apoptosis in human cell lines. Microcin E492 is currently described as a bacteriocin for its well-characterized bactericidal activity over different strains of Enterobacteriaceae (20). The proposed mechanism of action is through the formation of ion channels in the bacterial membrane (18). Although microcin E492 has a cytotoxic effect on several human cell lines, for the time being it may be not appropriate to define this bacteriocin as a classical bacterial toxin. Essentially, a toxin is defined by the toxic effect that it causes on an animal host. This raises the question of specificity of recognition of some cellular types that somehow may be related to the wide host range observed in bacteria. The low molecular weight of microcin E492 makes this bacteriocin very attractive for structural studies to understanding the toxin-receptor interactions and dynamics of insertion into the membrane. Probably, in both mechanisms, oligomerization and conformational changes play a pivotal role. In addition, the study of the mechanism by which microcin E492 induces apoptosis may provide insights to understanding this complex phenomena.
Acknowledgments
We thank Catherine Connelly and Octavio Monasterio for critical reading of the manuscript. This work was supported by Grants 1991017 and 8000011 from Fondo Nacional de Investigación Científica y Tecnológica. C.H. is a recipient of a predoctoral fellowship from Fundación Andes.
Abbreviations
- AU
antibiotic units
- PI
propidium iodide
- DiOC6
dihexyloxacarbocyanine
- LDH
lactate dehydrogenase
- LFA-1
lymphocyte function-associated antigen 1
- TNF-α
tumor necrosis factor α
References
- 1.Riley M A, Gordon D M. Trends Microbiol. 1999;7:129–133. doi: 10.1016/s0966-842x(99)01459-6. [DOI] [PubMed] [Google Scholar]
- 2.Kolter R, Moreno F. Annu Rev Microbiol. 1992;46:141–163. doi: 10.1146/annurev.mi.46.100192.001041. [DOI] [PubMed] [Google Scholar]
- 3.Jack R W, Tagg J R, Ray B. Microbiol Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riley M A. Annu Rev Genet. 1998;32:255–278. doi: 10.1146/annurev.genet.32.1.255. [DOI] [PubMed] [Google Scholar]
- 5.Baba T, Schneewind O. Trends Microbiol. 1998;6:66–71. doi: 10.1016/S0966-842X(97)01196-7. [DOI] [PubMed] [Google Scholar]
- 6.Ennahar S, Sashihara T, Sonomoto K, Ishizaki A. FEMS Microbiol Rev. 2000;24:85–106. doi: 10.1111/j.1574-6976.2000.tb00534.x. [DOI] [PubMed] [Google Scholar]
- 7.Farkas-Himsley H, Hill R, Rosen B, Lingwood C A. Proc Natl Acad Sci USA. 1995;92:6996–7000. doi: 10.1073/pnas.92.15.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leland P, Taguchi J, Husain S R, Kreitman R J, Pastan I, Puri R K. Mol Med. 2000;6:165–178. [PMC free article] [PubMed] [Google Scholar]
- 9.Weinrauch Y, Zychlinsky A. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 10.Dinges M M, Orwin P M, Schlievert P M. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nelson K L, Brodsky L, Buckley J T. Cell Microbiol. 1999;1:69–74. doi: 10.1046/j.1462-5822.1999.00009.x. [DOI] [PubMed] [Google Scholar]
- 12.Müller A, Günther D, Düx F, Naumann M, Meyer T F, Rudel T. EMBO J. 1999;18:339–352. doi: 10.1093/emboj/18.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaux D L, Korsmeyer S J. Cell. 1999;96:245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson M D, Weil M, Raff M. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- 15.Savill J, Fadok V. Nature (London) 2000;407:784–788. doi: 10.1038/35037722. [DOI] [PubMed] [Google Scholar]
- 16.Hengartner M O. Nature (London) 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 17.Booth M C, Bgie C P, Sahl H-G, Siezen R J, Hatter K L, Gilmore M S. Mol Microbiol. 1996;21:1175–1184. doi: 10.1046/j.1365-2958.1996.831449.x. [DOI] [PubMed] [Google Scholar]
- 18.Lagos R, Wilkens M, Vergara C, Cecchi X, Monasterio O. FEBS Lett. 1993;321:145–148. doi: 10.1016/0014-5793(93)80096-d. [DOI] [PubMed] [Google Scholar]
- 19.Lagos R, Villanueva J, Monasterio O. J Bacteriol. 1999;181:212–217. doi: 10.1128/jb.181.1.212-217.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Lorenzo V. Arch Microbiol. 1984;139:72–75. doi: 10.1007/BF00692715. [DOI] [PubMed] [Google Scholar]
- 21.Wilkens M, Villanueva J E, Cofré J, Chnaiderman J, Lagos R. J Bacteriol. 1997;179:4789–4794. doi: 10.1128/jb.179.15.4789-4794.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagos R, Baeza M, Corsini G, Hetz C, Strahsburger E, Castillo J A, Vergara C, Monasterio O. Mol Microbiol. 2001;42:229–243. doi: 10.1046/j.1365-2958.2001.02630.x. [DOI] [PubMed] [Google Scholar]
- 23.Mayr-Harting A, Hedges A J, Berkeley C W. Methods Microbiol. 1972;7A:315–422. [Google Scholar]
- 24.Accolla R S. J Exp Med. 1983;157:1053–1058. doi: 10.1084/jem.157.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castro A, Bono M R, Simon V, Rosenblatt M. Eur J Cell Biol. 1996;70:61–68. [PubMed] [Google Scholar]
- 26.Zamzami N, Susin N A, Marchetti P, Hirsch T, Gómez-Monterrey I, Castedo M, Kroemer G. J Exp Med. 1996;183:1533–1544. doi: 10.1084/jem.183.4.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barros L F, Stutzin A, Calixto A, Catalán M, Castro J, Hetz C, Hermosilla T. Hepatology. 2001;33:114–122. doi: 10.1053/jhep.2001.20530. [DOI] [PubMed] [Google Scholar]
- 28.Kao J P, Harootunian A T, Tsien R Y. J Biol Chem. 1989;264:8179–8184. [PubMed] [Google Scholar]
- 29.Sánchez-Madrid F, Krensky A M, Ware C F, Robbins E, Strominger J L, Burakoff S J, Springer T A. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bono M R, Benech P, Couillin P, Alcaide-Loridan C, Grisard M C, Jouin H, Fisher D G, Fellous M. Somatic Cell Mol Genet. 1989;15:513–523. doi: 10.1007/BF01534912. [DOI] [PubMed] [Google Scholar]
- 31.Lally E T, Hill R B, Kieba I R, Korostoff J. Trends Microbiol. 1999;7:356–361. doi: 10.1016/s0966-842x(99)01530-9. [DOI] [PubMed] [Google Scholar]
- 32.Abrami L, Fivaz M, van der Goot F G. Trends Microbiol. 2000;8:168–172. doi: 10.1016/s0966-842x(00)01722-4. [DOI] [PubMed] [Google Scholar]
- 33.Shatursky O, Heuck A P, Shepard L A, Rossjohn J, Parker M W, Johnson A E, Tweten R K. Cell. 1999;99:293–299. doi: 10.1016/s0092-8674(00)81660-8. [DOI] [PubMed] [Google Scholar]
- 34.Müller A, Günther D, Brinkmann V, Hurwitz R, Meyer T F, Rudel T. EMBO J. 2000;19:5332–5343. doi: 10.1093/emboj/19.20.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dypbukt J M, Ankarcrona M, Burkitt M, Sjoholm A, Strom K, Orrenius S, Nicotera P. J Biol Chem. 1994;269:30553–30560. [PubMed] [Google Scholar]
- 37.Krause K-H, Fivaz M, Monod A, van der Goot F G. J Biol Chem. 1998;273:18122–18129. doi: 10.1074/jbc.273.29.18122. [DOI] [PubMed] [Google Scholar]
- 38.Ojcius D M, Zychlinsky A, Zheng L M, Young J D-E. Exp Cell Res. 1991;197:43–49. doi: 10.1016/0014-4827(91)90477-c. [DOI] [PubMed] [Google Scholar]
- 39.Berridge M J, Bootman M D, Lipp P. Nature (London) 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]