Abstract
This paper presents a new wide-ranging reference correlation for the viscosity of argon, incorporating recent ab initio dilute-gas calculations and critically evaluated experimental data. The correlation is designed to be used with a high-accuracy Helmholtz equation of state that extends from the triple point (83.8058 K) to 700 K, and at pressures up to 1000 MPa. The estimated uncertainty of the correlation based on comparisons with the best experimental data indicate that the uncertainty for the gas at pressures from zero to 0.1 MPa for temperatures from 202 K to 394 K is 0.076% (at k = 2), the uncertainty of the best experimental data, offering a significant improvement over the current reference equation that has an uncertainty in this region of 0.5%. A zero-density correlation based on ab-initio values is incorporated that is valid over a temperature range between 84 K and 10 000 K and has an uncertainty of 0.12% (at the 95% confidence level). The estimated uncertainty for moderate pressures from 1 MPa to 100 MPa is 1% for temperatures from roughly 195 K to 300 K, rising to 2% at 175 K. For the high-pressure region, the estimated uncertainty of the correlation is about 2% for temperatures between 175 K and 308 K at pressures from 100 MPa to 606 MPa. For temperatures from 308 K to 700 K at pressures to 5.2 GPa, the equation has an estimated uncertainty of 10%. The estimated uncertainty in the liquid phase at pressures up to 34 MPa is 3%. The correlation behaves in a physically reasonable manner over the full range of applicability of the EOS, although uncertainties may be higher in regions where data were not available for full validation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10765-025-03603-8.
Keyword: Argon, Transport properties, Viscosity
Introduction
Argon is a common fluid that has a wide range of industrial uses. As a result of the passage of the CHIPS Act [1] (Creating Helpful Incentives to Produce Semiconductors) of 2022, there is increased interest in improving the calibration of the flow of gases used in semiconductor processing. In the production of semiconductors, flow meters are often calibrated with one gas, and then calibration coefficients for other gases are determined using gas-property data [2]. Viscosity is one of the properties used in the development of some flow-meter models. Recent advances in quantum-chemical ab initio computations [3–6] allow improvement in the representation of the dilute-gas viscosity of argon. It is our goal to incorporate ab initio results to develop an improved correlation for the viscosity of argon that can not only be used for gas-calibration purposes, but that is valid over the entire fluid range incorporating gas, liquid, and supercritical regions.
The viscosity correlation published in 2004 by Lemmon and Jacobsen [7] is widely used, and is considered a standard, valid over liquid, vapor, and supercritical states. According to Lemmon and Jacobsen [7], the uncertainty.
For the dilute-gas viscosity (defined in their manuscript as p < 1 MPa) is generally within about 0.5%, increasing near the triple point.
In the range 270 to 300 K at pressures less than 100 MPa, for the non-dilute gas and in the liquid, the uncertainty is as low as 1%.
In the range 180 to 270 K, the uncertainty is about 2%.
Below 180 K, and away from the critical region, the uncertainty steadily increases to about 5% at the triple point of the fluid.
The uncertainty in the critical region is higher.
The correlation of Lemmon and Jacobsen [7] was based on the best available experimental data at the time, and hence was limited by the uncertainty of those measurements. The goal of this work is to develop a new viscosity correlation that will incorporate the new dilute-gas limit ab initio calculations for viscosity, to both extend the temperature range, and to lower the uncertainty in the dilute-gas region. In addition, we will include new critically-assessed literature data that became available after 2004.
In a series of papers published over the last ten years, we reported new reference correlations over extended temperature and pressure ranges for the viscosity of some simple fluids (xenon [8], hydrocarbons (n-hexane [9], n-heptane,[10], n-undecane [11], n-hexadecane [12], benzene [13], toluene [14], cyclopentane [15]), alcohols (methanol [16], ethanol [17]), glycols (ethane-1,2-diol [18], propane-1,2-diol [19]) and some refrigerants (R-1234yf and R-1234ze(E) [20], R-134a [21], R-161 [22], R-245fa [23], and R-32 [24]). In this paper, the same methodology adopted in any of the aforementioned papers is extended to developing a new reference correlation for the viscosity of argon.
The analysis we use is based on the best available experimental data. A prerequisite to the analysis is a critical assessment of the experimental data. Here we define two categories of experimental data: primary data, employed in the development of the correlation, and secondary data, used simply for comparison purposes. According to the recommendation adopted by the Subcommittee on Transport Properties (now known as The International Association for Transport Properties) of the International Union of Pure and Applied Chemistry, the primary data are identified by a well-established set of criteria [25]. These criteria have been successfully employed to establish standard reference values for the viscosity and thermal conductivity of fluids over wide ranges of conditions, with uncertainties in the range of 1%. However, in many cases, such a narrow definition unacceptably limits the range of the data representation. Consequently, within the primary data set, it is also necessary to include results that extend over a wide range of conditions, albeit with a poorer accuracy, provided they are consistent with other more accurate data or with theory. In all cases, the accuracy claimed for the final recommended data must reflect the estimated uncertainty in the primary information.
The form of correlation we use expresses the viscosity as a function of temperature and density. Experimental data are generally reported in terms of pressure and temperature and an equation of state (EOS) is needed to obtain corresponding densities. If necessary, we first convert temperatures to ITS-90 [26, 27], then use the Helmholtz EOS published by Tegeler et al. [28] to obtain the density for a given temperature–pressure state point. We also use the critical and triple point associated with this EOS; the critical point and other constants for this EOS are given in Table 1. The uncertainty in density of the EOS is less than 0.02% for pressures up to 12 MPa and temperatures up to 340 K except for the critical region, and less than 0.03% for pressures up to 30 MPa and temperatures between 235 K and 520 K. Elsewhere, the uncertainty in density is generally within 0.2%. The EOS is recommended for use from the melting line to 700 K at pressures up to 1000 MPa, but gives physically reasonable extrapolation behavior up to very high pressures and temperatures [28].
Table 1.
Critical point and fixed constants for the EOS of Tegeler et al. [28]
| Property | Symbol | Units | Value | |
|---|---|---|---|---|
| Critical temperature | Tc | K | 150.687 | |
| Critical pressure | Pc | MPa | 4.863 | |
| Critical density | ρc | kg·m−3 | 535.6 | |
| Triple-point temperature | Ttp | K | 83.8058 | |
| Molar mass | M | g·mol−1 | 39.948 | |
| Molar gas constant | R | J·mol−1·K−1 | 8.31451 | |
The Viscosity Correlation
The viscosity η can be expressed [8–24, 29–31] as the sum of four independent contributions,
| 1 |
where ρ is the molar density, T is the absolute temperature, and the first term, η0(Τ) = η(0,Τ), is the contribution to the viscosity in the dilute-gas limit, where only two-body molecular interactions occur. The linear-in-density term, η1(Τ) ρ, known as the initial density dependence term, can be separately established using Rainwater-Friend theory [32–34] for the transport properties of moderately dense gases. The critical enhancement term, Δηc(ρ,Τ), arises from the long-range density fluctuations that occur in a fluid near its critical point, which contribute to divergence of the viscosity at the critical point. This term for viscosity is significant only in the region near the critical point, as shown in Vesovic et al. [35] and Hendl et al. [36]. For CO2, Vesovic et al. [35] showed that the enhancement contributes greater than 1% to the viscosity only in the small region bounded by 0.986 < Tr < 1.019 and 0.642 < ρr < 1.283 (where Tr and ρr denote the reduced temperature Tr = T/Tc and reduced density ρr = ρ/ρc). Since data close to the critical point are unavailable, Δηc(ρ,Τ) will be set to zero in Eq. 1 and not discussed further. The reader should note that theory indicates that the viscosity diverges at the critical point [37], and our model does not have the correct theoretical behavior. Finally, the term Δη(ρ,T), the residual term, represents the contribution of all other effects to the viscosity of the fluid at elevated densities including many-body collisions, molecular-velocity correlations, and collisional transfer.
The identification of these four separate contributions to the viscosity and to transport properties in general is useful because it is possible to some extent to treat η0(Τ), and η1(Τ) theoretically. In addition, it is possible to derive information about both η0(Τ) and η1(Τ) from experiment. In contrast, there is little theoretical guidance concerning the residual contribution, Δη(ρ,Τ), and therefore its evaluation is based entirely on an empirical equation obtained by fitting experimental data.
In addition to performing literature searches and using content in previous correlations, we made extensive use of the NIST ThermoData Engine [38] to identify data sources. Table 2 summarizes, to the best of our knowledge, the experimental measurements of the viscosity of argon reported in the literature. Data sources in italics indicate that the particular set was also employed in the development of the correlation of Lemmon and Jacobsen [7]. With few exceptions, we only included in the primary dataset measurements where the technique employed, and the uncertainty of the measurement are specified. Very few data sets specifically call out if the uncertainty is on a k = 1 or k = 2 basis; we assume k = 2 when no information is given. Furthermore, with the exceptions discussed below, we preferred measurements with uncertainty less than 1%. In the remainder of this manuscript all uncertainties are at the k = 2 level unless specified otherwise.
Table 2.
Viscosity measurements of argon
| Investigators/referencea | Publ Year | Techniqueb | Purity (%) | Uncertainty (%) | No. data | Temperatur range (K) | Pressure range (MPa) |
|---|---|---|---|---|---|---|---|
| Primary data | |||||||
| Zhou et al. [39] | 2024 | VBW | 99.999 | 4.212σ | 19 | 89–200 | 0.3–5.0 |
| Xiao et al. [5, 40] | 2020 | 2CAP | 99.999 | 0.0762σ | 42 | 202–395 | 0, 0.1 |
| Humberg and Richter [41] | 2019 | RCyl | 99.999 | 0.22σ | 46 | 253–473 | 0.1–1 |
| Lin et al. [42] | 2014 | 2CAP | 99.9997 | 0.1242σ | 14 | 298–653 | 0 |
| Berg and Burton [43] | 2013 | CAP | na | 0.062σ | 1 | 298 | 0 |
| Zhang et al. [44] | 2013 | 2CAP | 99.999 | 0.1642σ | 17 | 243–393 | 0.1 |
| Abramson [45] | 2011 | RBall | 99.995 | na | 31 | 293–689 | 490–5170 |
| Vogel [46–48] | 2010 | OscDQ | 99.999 | 0.15–0.20 | 81 | 291–682 | 0.05–0.16 |
| Wang et al. [49] | 2010 | OscD | 99.99 | 22σ | 17 | 300 | 0.1–4.5 |
| Hurly et al. [50] | 2003 | GV | 99.99 | 0.3 | 124 | 293–373 | 0.1–3.8 |
| Evers et al. [51] | 2002 | RCyl | 99.9996 | 0.15–0.4 | 81 | 293–523 | 0.09–28 |
| Wilhelm and Vogel [52] | 2000 | VBW | 99.998 | 0.20 | 160 | 298–423 | 0.1–20 |
| Diller and Frederick [53] | 1989 | PCV | 99.99 | 2 | 91 | 292–501 | 1–57 |
| Hobley et al. [54] | 1989 | CAP | na | 0.7 | 5 | 301–521 | 0.1 |
| Mostert [55, 56] | 1989 | VBW | na | na | 25 | 174.7 | 16–471 |
| Kestin and Ro [57] | 1982 | CAP | na | 0.3 | 5 | 298–473 | 0.1 |
| Matthews et al. [58] | 1982 | CAP | 99.9 | 1 | 11 | 118–1598 | 0.1 |
| Barr et al. [59] | 1981 | CAP | 99.99 | 1 | 19 | 173–1598 | 0.1 |
| Kestin et al. [60] | 1978 | OscD | 99.995 | 0.1–0.3 | 9 | 298–773 | 0.1 |
| Hongo [61] | 1978 | OscD | 99.994 | 0.3 | 52 | 298–373 | 0.1–13 |
| Clifford et al. [62] | 1975 | CAP | 99.9995 | 0.1 | 9 | 321–1300 | 0.1 |
| Haynes [63] | 1973 | TorC | 99.76 | 2 | 167 | 85–298 | 0.07–35 |
| Vermesse and Vidal [64] | 1973 | CAP | na | 0.5 | 25 | 308 | 12–606 |
| Rabinovich et al. [65] | 1971 | CAP | na | 1.2 | 63 | 298–523 | 2–59 |
| Timrot et al. [66] | 1969 | OscD | 99.95 | 0.1 | 7 | 300–600 | 0.1 |
| Guevara et al. [67] | 1969 | CAP | na | 0.4 | 21 | 1100–2100 | 0.1 |
| Gracki et al. [68] | 1969 | CAP | 99.998 | 0.2–0.3 | 44 | 173–298 | 0.6–17 |
| Boon et al. [69, 70] | 1967 | CAP | 99.98 | na | 6 | 83–89 | 0.07–0.1 |
| Flynn et al. [71] | 1963 | CAP | 99.995 | 0.1 | 27 | 194–373 | 2–18 |
| De Rocco and Halford [72] | 1958 | CAP | na | 0.5 | 20 | 210–471 | 0.1 |
| Secondary data | |||||||
| Borjan et al. [73] | 2022 | VVHPO | 99.998 | na | 34 | 313,353 | 0.1–51 |
| Goodwin et al. [74] | 2006 | MEMS | na | 32σ | 43 | 323–423 | 7–69 |
| May et al. [75] | 2006 | 2CAP | 99.9995 | 0.1682σ | 21 | 202–394 | 0 |
| Lukin et al. [76] | 1983 | CAP | na | 0.3 | 23 | 76–293 | 0.1 |
| Malbrunot et al. [77] | 1983 | AcA | na | na | 9 | 84–97 | 0.07–0.25 |
| Abachi et al. [78] | 1980 | VBW | na | 2 | 12 | 83–90 | 0.06–0.14 |
| Trappeniers et al. [79] | 1980 | VBW | na | 2 | 44 | 223–323 | 99–897 |
| Vidal et al. [80] | 1980 | CAP | na | 0.5 | 7 | 298 | 0.1–600 |
| Kestin and Wakeham [81] | 1979 | OscD | 99.9 | 0.2 | 5 | 300–473 | 0.1 |
| Kestin et al. [82] | 1977 | OscD | 99.9995 | 0.1–0.2 | 8 | 298–673 | 0.1 |
| Kestin and Ro [83] | 1976 | OscD | na | 0.6 | 9 | 298–1273 | 0.1 |
| Lyusternik and Lavushev [84] | 1976 | FPor | na | na | 49 | 403–1950 | 0.02–0.1 |
| Gough et al. [85] | 1976 | CAP | 99.8 | 0.5–1.0 | 11 | 120–320 | 0.1 |
| Baharudin et al. [86] | 1975 | BRIL | na | 5 | 6 | 85–110 | 0.1 |
| Timrot et al. [87] | 1975 | OscD | na | 1.5 | 39 | 292–573 | 0.1–14.5 |
| Carey et al. [88] | 1974 | AcA | na | 0.1 | 15 | 294–298 | 0.1–14 |
| Casparian and Cole [89] | 1974 | CAP | 99.98 | na | 4 | 293–422 | 0.1 |
| Hellemans et al. [90] | 1974 | OscD | na | 0.3 | 8 | 298–973 | 0.1 |
| Kurin and Golubev [91] | 1974 | CAP | 99.981 | 2 | 99 | 273–423 | 9–380 |
| Maitland and Smith [92] | 1974 | CAP | 99.995 | 1 | 11 | 295–1533 | 0.1 |
| Hellemans et al. [93] | 1973 | OscD | 99.99 | 0.1–0.3 | 6 | 298–770 | 0.1 |
| Rakshit et al. [94] | 1973 | OscD | na | 1 | 4 | 238–308 | 0.1 |
| Slyusar et al. [95] | 1973 | FCyl | na | 4 | 215 | 83–300 | 0.08–343 |
| Kestin et al. [96] | 1972 | OscD | 99.9995 | 0.1 | 8 | 298–973 | 0.1 |
| Kestin et al. [97] | 1972 | OscD | 99.9995 | 0.1 | 7 | 298.973 | 0.1 |
| Kestin et al. [98] | 1971 | OscD | na | 0.2 | 40 | 298 | 0.1–10 |
| Dawe and Smith [99] | 1970 | CAP | 99.995 | 0.5 | 15 | 293–1600 | 0.1 |
| Golubev [100, 101] | 1970 | CAP | na | na | 49 | 273–473 | 0.1–48 |
| Hellemans and Zink [102] | 1970 | OscD | na | 2 | 44 | 104–147 | 0.5–9.6 |
| Kalelkar and Kestin [103] | 1970 | OscD | na | 0.5 | 9 | 298–1124 | 0.1 |
| Kestin et al. [104] | 1970 | OscD | na | 0.1 | 8 | 298–973 | 0.1 |
| Clarke and Smith [105] | 1968 | CAP | 99.9 | 0.5 | 12 | 114–375 | 0.1 |
| De Bock et al. [106] | 1967 | TorQ | na | 3 | 19 | 90 | 0.1–14 |
| De Bock et al. [107] | 1967 | TorQ | na | 3 | 86 | 88–140 | 0.1–20 |
| DiPippo and Kestin [108] | 1967 | OscD | na | 0.1 | 23 | 297–575 | 0.03–0.18 |
| DiPippo et al. [109] | 1967 | OscD | na | 0.1 | 10 | 293, 303 | 0.1–2.3 |
| Andreev et al. [110] | 1966 | CAP | 99.9 | 1–3 | 40 | 294–923 | 5–51 |
| van Itterbeek et al. [111] | 1966 | OscD | na | na | 10 | 84, 89 | 0.1–9.8 |
| Naugle [112] | 1966 | UAtt | 99.99 | na | 4 | 84–112 | 0.8 |
| Naugle et al. [113] | 1966 | UAtt | 99.99 | na | 59 | 86–142 | 2–15 |
| Rigby and Smith [114] | 1966 | CAP | 99.95 | 0.3 | 15 | 293–972 | 0.1 |
| Chakraborti and Gray [115] | 1965 | CAP | na | 1 | 1 | 298 | 0.1 |
| Saji and Okuda [116] | 1965 | CAP | 99.9 | na | 5 | 84–87 | 0.1 |
| Iwasaki et al. [117] | 1964 | OscD | 99.997 | 0.1 | 14 | 293–303 | 0.1–5.3 |
| Kestin and Nagashima [118] | 1964 | OscD | 99.997 | 0.4 | 20 | 293–303 | 0.1–5.2 |
| Lowry et al. [119] | 1964 | TorQ | na | 2 | 20 | 102, 128 | 0.1–51 |
| Reynes and Thodos [120] | 1964 | CAP | 99.998 | na | 35 | 373–423 | 7–82 |
| Saji and Kobayashi [121] | 1964 | CAP | 99.9 | na | 5 | 84–86 | 0.1 |
| Forster [122] | 1963 | OscD | 99.8 | na | 8 | 85–114 | 0.1–0.9 |
| Iwasaki and Kestin [123] | 1963 | OscD | 99.997 | 0.1 | 14 | 293–303 | 0.1–5.3 |
| Kestin and Whitelaw [124] | 1963 | OscD | 99.997 | 0.5 | 48 | 296–537 | 0.1–14 |
| Filippova and Ishkin [125] | 1961 | 2CAP | 99.8 | 1.5 | 52 | 90–273 | 3.5–15 |
| Thornton [126] | 1960 | CAP | 99.8 | 1 | 1 | 291 | 0.1 |
| Filippova and Ishkin [127] | 1959 | 2CAP | 99.8 | 1.5 | 31 | 90–273 | 0.1–15 |
| Kestin and Leidenfrost [128] | 1959 | OscD | 99.979 | 0.05 | 15 | 293–298 | 0.03–3.2 |
| Makita [129] | 1957 | RBall | 99.9 | na | 45 | 298–423 | 0.1–78 |
| Zhdanova [130] | 1957 | na | na | na | 10 | 90–149 | 0.1–4.6 |
| Bonilla et al. [131] | 1956 | CAP | 99.9 | na | 22 | 273–2073 | 0.1 |
| Jackson [132] | 1956 | CAP | 99.93 | na | 1 | 298 | 0.1 |
| Makita [133] | 1955 | RBall | 97.8 | na | 30 | 323–573 | 0.1–10 |
| Michels et al. [134] | 1954 | CAP | na | na | 100 | 273–348 | 0.1–200 |
| Rietveld et al. [135] | 1953 | OscD | na | 1 | 9 | 72–291 | 0.01–0.1 |
| Kiyama and Makita [136] | 1952 | RBall | 97.8 | na | 40 | 323–573 | 0.1–10 |
| Johnston and Grilly [137] | 1942 | VBW | na | 0.5 | 17 | 77–296 | 0.1 |
| Wobser and Mueller [138] | 1941 | FBall | na | 1.5 | 5 | 293–371 | 0.1 |
| van Itterbeek and van Paemel [139] | 1938 | OscD | na | na | 6 | 55–294 | 0–0.13 |
| Rudenko and Schubnikow [140] | 1934 | CAP | na | 1.4 | 4 | 84–87 | 0.1 |
| Trautz and Binikele [141] | 1930 | CAP | na | na | 4 | 293–473 | 0.1 |
| Trautz and Zink [142] | 1930 | CAP | 99 | na | 21 | 567–1100 | 0.1 |
| Trautz and Ludewigs [143] | 1929 | CAP | 99.8 | na | 4 | 288–523 | 0.1 |
| Ishida [144] | 1923 | OilD | na | 3 | 1 | 296 | 0.1 |
| Rankine [145] | 1910 | CAP | na | na | 1 | 285 | 0.1 |
| Tanzler [146] | 1906 | CAP | na | na | 4 | 273–456 | 0.1 |
| Schultze [147] | 1901 | CAP | na | na | 6 | 291–456 | 0.1 |
aData sources in italics indicate that the particular set was also employed in the development of the correlation of Lemmon and Jacobsen [7]
b2CAP, 2-Capillary; AcA, Acoustic Attenuator; BRIL, Brillouin lines; CAP, Capillary; FBall, Falling Ball; FPor, Flow through Porous media; GV, Greenspan Viscometer; MEMS, Method of Microelectromechanical Systems; OilD, Oil Drop; OscD, Oscillating Disk; OscDQ, Oscillating Disk Quartz viscometer; PCV, Piezoelectric Crystal Viscometer; RBall, Rolling Ball; TorQ, Torsional Quartz, UAtt, Ultrasonic Attenuator; VBW, Vibrating Wire; VVHPO, Variable-Volume High-Pressure Optical view cell; na not available
2σuncertainty either explicitly stated or converted to the 95% confidence level
Hence, in the primary sets we included.
All measurements with stated uncertainty equal, or less than, 1% (or 2–2.1% at k = 2).
We included some measurements with larger uncertainties than 1%, or unspecified uncertainties, in order to extend the primary data set to higher pressures (Diller and Frederick [53], Abramson [45]), or low temperatures (Zhou et al. [39], and Haynes [63]). These were included with weights adjusted so that the fit was not overly influenced by them. Abramson [45] did not specify an uncertainty; we assigned the data an estimated uncertainty of 10% and used the data mainly to guide extrapolations to very high (5 GPa) pressures. In order to improve coverage of the liquid region, we added the measurements of Boon et al. [69] to the primary set.
The following points about data usage should also be made:
The specific measurements of the group of Kestin [96, 98, 103, 108, 109, 124], performed in 1972 and earlier, with the instrument originally constructed by DiPippo [108, 109], as was pointed out both by Vogel [46] and Maitland et al. [148], are subject to a temperature error, and hence were not included in the primary dataset.
In cases where measurements are superseded by more recent ones at the same or wider conditions, we report only the latest, e.g. the group of Kestin has previous measurements [81, 82, 93, 97, 104, 117, 118, 123, 128, 149, 150], also the group of Smith [85, 92, 99, 105, 114], and Vogel [47, 48, 151].
The measurements of Trappeniers et al. [79] were not included as Mostert et al. [55] found at cryogenic temperatures inaccuracies in the measurements, due to the interference between the cooling system and the electrical resistance of the leads.
In some cases, the authors re-evaluated their earlier work and we retained the most recent publications. The 2010 work of Vogel [46] includes both new measurements and re-analyzed values from his earlier works [47, 48]. Also, Mostert et al. [55] recalculated the values of Van der Gulik and Trappeniers [56] with an additional correction factor and thus is included in the primary set instead of Van der Gulik and Trappeniers [56].
All remaining measurements were considered as secondary as they did not satisfy the aforementioned criteria.
Figure 1 shows the temperature–pressure and temperature-density ranges of the primary measurements outlined in Table 2, and the phase boundary. The temperature axis is restricted to 750 K, as measurements in the region above that up to 2100 K are only at atmospheric pressure.
Fig. 1.
Temperature–pressure and temperature-density ranges of the primary experimental viscosity data for argon, (––) saturation curve. Zhou et al. [39] ( ), Xiao et al. [5, 40] (×), Humberg and Richter [41] (■), Lin et al. [42] (
), Xiao et al. [5, 40] (×), Humberg and Richter [41] (■), Lin et al. [42] ( ), Berg and Burton [43] (
), Berg and Burton [43] ( ), Zhang [44] (▲), Abramson [45] (△), Vogel [46] (♦), Wang et al. [49] (□), Hurly et al. [50] (+), Evers et al. [51] (
), Zhang [44] (▲), Abramson [45] (△), Vogel [46] (♦), Wang et al. [49] (□), Hurly et al. [50] (+), Evers et al. [51] ( ), Wilhelm and Vogel [52] (
), Wilhelm and Vogel [52] ( ), Diller and Frederick [53] (
), Diller and Frederick [53] ( ), Hobley et al. [54] (
), Hobley et al. [54] (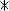 ), Mostert et al. [55] (
), Mostert et al. [55] ( ), Kestin and Ro [57] (
), Kestin and Ro [57] ( ), Matthews et al. [58] (
), Matthews et al. [58] (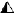 ), Barr et al. [59] (
), Barr et al. [59] ( ), Kestin et al. [60] (
), Kestin et al. [60] (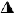 ), Hongo [61] (
), Hongo [61] ( ), Clifford et al. [62] (
), Clifford et al. [62] (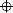 ), Haynes [63] (●), Vermesse and Vidal [64] (
), Haynes [63] (●), Vermesse and Vidal [64] ( ), Rabinovich et al. [65] (
), Rabinovich et al. [65] ( ), Timrot et al. [66] (○), Gracki et al. [68] (
), Timrot et al. [66] (○), Gracki et al. [68] (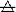 ), Boon et al. [69] (∗), Flynn et al. [71] (
), Boon et al. [69] (∗), Flynn et al. [71] (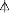 ), de Rocco and Halford [72] (
), de Rocco and Halford [72] (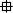 )
)
The Viscosity in the Dilute-Gas Limit
The dilute-gas limit viscosity, η0(Τ) is a function only of temperature and can be analyzed independently of all other contributions in Eq. 1. In 2010, Vogel et al. [4] employed an argon–argon interatomic potential energy curve determined from quantum–mechanical ab initio calculations [3] to calculate the thermophysical properties of argon governed only by two-body interactions. The dilute-gas viscosity was computed from 83.8 K to 10 000 K. The calculated values for the different thermophysical properties were compared with available experimental data and values computed with other argon–argon potentials. An extensive analysis showed that the proposed potential was superior to all previous ones and that the calculated viscosity values were accurate enough to be applied as standard values for the complete temperature range of the calculations.
In 2020, Xiao et al. [5] presented a reference correlation for the dilute-gas viscosity of argon with an uncertainty of about 0.06% (at the k = 1 confidence level). Vogel et al. [4] used ab initio computations to compute a reference value of 22.552 μPa·s at 298.15 K for the zero-density gas viscosity of argon. The correlation proposed by Xiao et al. [5] is based on the ab initio computations of Vogel et al. [4], but scaled to match an updated reference value of 22.5666 μPa·s at 298.15 K that is based on the viscosity ratio measurements of May et al. [40].
Very recently, in 2024, Lang et al. [6] determined a new ab initio quantum potential by the inclusion of the two-electron relativistic and leading-order quantum electrodynamics effects. Moreover, the long-range retardation effects were considered to properly describe the dissociation limit.
In this work, it was decided to employ the dilute-gas viscosity correlation proposed by Xiao et al. [5], based on the work of Vogel et al. [4], because the results have been scaled to agree with the most accurate viscosity ratio measurements. This correlation is
| 2 |
where η0(298.15 K) = 22.5666 μPa·s, and the coefficients ai are shown in Table 3. The computed viscosity values in the dilute-gas limit of Eq. 2 cover a temperature range between 84 K and 10 000 K with an uncertainty of 0.12% (at the 95% confidence level) [5].
Table 3.
| i | ai | i | ai | i | ai |
|---|---|---|---|---|---|
| 1 | 8.395115 × 10–1 | 5 | − 8.881774 × 10–3 | 9 | − 2.544782 × 10–5 |
| 2 | − 1.062564 × 10–1 | 6 | − 9.613779 × 10–5 | 10 | 4.398471 × 10–5 |
| 3 | 1.065796 × 10–2 | 7 | 1.404406 × 10–3 | 11 | − 9.997908 × 10–6 |
| 4 | 1.879809 × 10–2 | 8 | − 4.321739 × 10–4 | 12 | 7.753453 × 10–7 |
Figure 2 plotted only up to 2000 K, shows the deviations of the viscosity values calculated from the potentials of Vogel et al. [4], Lang et al. [6], and the correlation of Lemmon and Jacobsen [7] from Eq. 2. The values from Vogel et al. [4] have a small constant offset of about 0.06%, due to the use of a different reference value at 298.15 K, as mentioned earlier. Values from Lang et al. [6] are essentially identical to those from Eq. 2 for temperatures above about 700 K, and have the largest deviation of about 0.09% near 175 K. The correlation of Lemmon and Jacobsen has much larger deviations, with the maximum deviation of 1.3% at about 100 K. As previously mentioned, this correlation was developed only using available experimental data, and incorporating the ab initio results offers significant improvement.
Fig. 2.

Relative deviations of the dilute-gas viscosity values η0 from ab-initio calculations and the correlation of Lemmon and Jacobsen [7] from the values calculated by Eq. 2
The Initial-Density Dependence Viscosity Term
We will use theoretical results to guide the development of the initial-density dependence of the viscosity, rather than rely solely on experimental data as was recently done for nitrogen [152]. The temperature dependence of the linear-in-density coefficient of the viscosity η1(T) in Eq. 1 is very large at subcritical temperatures and must be considered to obtain an accurate representation of the behavior of the viscosity in the vapor phase. It changes sign from positive to negative as the temperature decreases. Therefore, the viscosity along an isotherm should first decrease in the vapor phase and subsequently increase with increasing density [153]. Vogel et al. [154] have shown that fluids exhibit the same general behavior of the initial density dependence of viscosity, which can also be expressed by means of the second viscosity virial coefficient Bη(T), as
| 3 |
The second viscosity virial coefficient can be obtained according to the theory of Rainwater and Friend [32, 33] as a function of a reduced second viscosity virial coefficient, , as
| 4 |
where NA is the Avogadro constant, σ is an intermolecular potential distance parameter, and T* is a scaled temperature that will depend on the intermolecular potential.
depends on what potential is used. Vogel et al. [153] presented an expression for based on a Lennard–Jones potential that has been widely used for a variety of fluids including for example, aromatics [13, 14] and alkanes [9–11]. Najafi et al. [155] used potentials that are more accurate than the Lennard–Jones, specifically that of that of Aziz [156] and Boyes [157] and gave correlations for . We will adopt the Najafi et al. [155] correlation based up the Aziz potential [156] as it gives slightly better performance at 300 K [155] than the correlation based on the Boyes potential, and is in better agreement with experimental values than the correlation of Vogel et al. [153]. The Najafi et al. [155] correlation is recommended for use for densities up to 2 mol/L. It is expressed as
| 5 |
with coefficients ci given in Table 4, and T* = T/(ε/kB) is a scaled temperature, with parameters ε/kB = 143.235 K and σ = 0.33501 nm [155].
Table 4.
| i | ci |
|---|---|
| 0 | − 0.2571 |
| 1 | 3.033 |
| 2 | 1.144 |
| 3 | − 5.586 |
| 4 | 3.089 |
| 5 | − 0.8824 |
| 6 | − 0.03856 |
Figure 3 shows experimentally-derived values of Bη along with values computed from the correlation of Vogel et al. [153] (incorporating Lennard–Jones parameters recommended by Bich [34]), values computed from the correlation given by Najafi et al. [155] based on the Aziz potential [156], and values derived from the correlation of Lemmon and Jacobsen [7]. The correlation of Lemmon and Jacobsen [7] did not incorporate any theory and is based solely on experimental data; since there were no data at high temperatures the correlation deviates from theory as the temperature increases. It also deviates from theory at very low temperatures, again in a region where experimental data were unavailable. The correlation of Vogel appears to have a lower peak than the experimental data and it is shifted slightly.
Fig. 3.

The second viscosity virial coefficient of argon, B, as a function of temperature. Correlation of Lemmon and Jacobsen [7] dotted line, Correlation of Vogel et al. [153] dashed line, Correlation of Najafi et al. [155] solid line, Humberg and Richter [41] (×), Hurly et al. [50] (□), Evers et al. [51] (◊), Wilhelm and Vogel [52] (●), Hongo [61] (▲), Haynes [63] (◆), Gracki et al. [68] (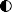 ), Flynn et al. [71] (+)
), Flynn et al. [71] (+)
The Viscosity Residual Term
The residual viscosity term Δη(ρ,T), represents the contribution of all other effects to the viscosity of the fluid at elevated densities including many-body collisions, molecular-velocity correlations, and collisional transfer. Because there is little theoretical guidance concerning this term, its evaluation is based entirely on experimentally obtained data.
The procedure adopted during this analysis used symbolic regression software [158] to fit the primary data to obtain the residual viscosity correlation Δη(ρ,T). The functional form is not known at the start of the regression process; symbolic regression is used to determine not only the coefficients but also the functional form of the correlation. Symbolic regression is a type of genetic programming that allows the exploration of arbitrary functional forms to regress data. The functional form is obtained by use of a set of operators, parameters, and variables as building blocks. In the present work we restricted the operators to the set (+ , − ,*,/) and the operands (constant, Tr, ρr), with Tr = T/Tc and ρr = ρ/ρc. In addition, we adopted a form suggested from the hard-sphere model employed by Assael et al. [159] Δη(ρr,Tr) = (ρr2/3Tr1/2)F(ρr,Tr), where the symbolic regression method was used to determine the functional form for F(ρr,Tr). For this task, the dilute-gas limit and the initial density dependence terms were calculated for each experimental point (employing Eqs. 2–5) and subtracted from the experimental viscosity to obtain the residual term. We increased the weights on the data as necessary to ensure the residual contribution was near zero for densities less than 2 mol⋅L−1 to retain the theoretical values. The final equation obtained was
| 6 |
The coefficients are given in Table 5, and Δη is in μPa·s. A parameter file suitable for use with the NIST REFPROP [160] program is included in the supplemental information that gives the full correlation Eqs. 1–6. When using symbolic regression programs, we have noticed that the resulting correlation often has mathematical poles. This is true for the correlation here as well, as there are discontinuities when the denominator of Eq. 6 is zero. For integration into software that may encounter evaluation beyond physically meaningful conditions, we recommend users check that the region Tr = ρr—f4 is avoided to ensure discontinuities will not be encountered and cause numerical instabilities. For argon, we know the location of the melting line, and the line of poles in Eq. 6 is well into the solid region as shown in Fig. 4. The melting line is that given by Tegeler et al. [28]. An explicit range of applicability is not given, but it is said to behave reasonably up to ~ 750 K and 6000 MPa.
Table 5.
Coefficients fi for Eq. 6
| i | fi |
|---|---|
| 1 | 3.62648753859904 |
| 2 | 6.655428299399591 |
| 3 | 0.397511608257391 |
| 4 | 2.6697983930209 |
| 5 | 0.0472018570860789 |
Fig. 4.

The melting line for argon and location of poles of Eq. 6. Melting line from Tegeler et al. [28] solid line, line of poles from Eq. 6 dashed line
Comparison with Data
The final correlation model consists of Eqs. 1–6 with the critical enhancement term set to zero. Table 6 summarizes comparisons of the primary data with the present correlation, while Table 7 gives comparisons of the secondary data. Comparisons with the correlation of Lemmon and Jacobsen [7] are also given. We use the following expressions for the percent deviation (PCT), average absolute relative deviation (AARD) and BIAS
| 7 |
| 8 |
| 9 |
where n is the number of data points, ηexp is the experimental value of the viscosity and ηcalc is the value calculated from the correlation. The maximum deviation (positive or negative) is also given.
Table 6.
Evaluation of the argon viscosity correlation for the primary data
| Investigators/references | Year Publ | AARD (%) | BIAS (%) | MAX (%) | AARD (%) | BIAS (%) | MAX (%) |
|---|---|---|---|---|---|---|---|
| Present work | Lemmon and Jacobsen [7] | ||||||
| Zhou et al. [39] | 2024 | 1.79 | 0.56 | 3.86 | 1.15 | − 0.05 | 2.55 |
| Xiao et al. [5, 40] | 2010 | 0.01 | 0.00 | − 0.03 | 0.28 | − 0.28 | − 0.62 |
| Humberg and Richter [41] | 2019 | 0.05 | − 0.04 | − 0.13 | 0.21 | − 0.21 | − 0.40 |
| Lin et al. [42] | 2014 | 0.07 | − 0.07 | − 0.13 | 0.41 | − 0.41 | − 0.54 |
| Berg and Burton [43] | 2013 | 0.06 | − 0.06 | − 0.06 | 0.23 | − 0.23 | − 0.23 |
| Zhang et al. [44] | 2013 | 0.07 | − 0.07 | − 0.09 | 0.27 | − 0.27 | − 0.40 |
| Abramson [45] | 2011 | 3.13 | − 1.21 | 9.73 | 21.26 | − 20.05 | − 55.61 |
| Vogel [46] | 2010 | 0.05 | − 0.05 | − 0.18 | 0.38 | − 0.38 | − 0.67 |
| Wang et al. [49] | 2010 | 0.31 | − 0.02 | 0.73 | 0.36 | 0.08 | 0.87 |
| Hurly et al. [50] | 2003 | 0.26 | 0.26 | 0.34 | 0.33 | 0.33 | 0.56 |
| Evers et al. [51] | 2002 | 0.30 | − 0.17 | − 2.34 | 0.23 | − 0.09 | − 1.92 |
| Wilhelm and Vogel [52] | 2000 | 0.22 | − 0.18 | − 0.68 | 0.12 | − 0.01 | − 0.43 |
| Mostert et al. [55, 56] | 1989 | 0.77 | 0.45 | 2.48 | 1.16 | − 0.70 | − 2.92 |
| Diller and Frederick [53] | 1989 | 1.30 | 1.04 | 4.27 | 1.43 | 1.28 | 3.92 |
| Hobley et al. [54] | 1989 | 0.07 | 0.06 | 0.19 | 0.19 | − 0.19 | − 0.22 |
| Kestin and Ro [57] | 1982 | 0.81 | 0.81 | 1.20 | 0.60 | 0.59 | 0.95 |
| Matthews et al. [58] | 1982 | 0.53 | 0.11 | − 1.59 | 0.48 | − 0.36 | − 1.06 |
| Barr et al. [59] | 1981 | 0.49 | 0.04 | − 1.98 | 0.44 | − 0.33 | − 2.96 |
| Kestin et al. [60] | 1978 | 0..60 | 0.60 | 1.13 | 0.31 | 0.20 | 0.95 |
| Hongo [61] | 1978 | 0.31 | 0.18 | 0.86 | 0.40 | 0.34 | 1.39 |
| Clifford et al. [62] | 1975 | 0.37 | − 0.05 | − 0.78 | 0.50 | − 0.34 | − 1.09 |
| Haynes [63] | 1973 | 0.76 | 0.41 | 4.83 | 0.93 | 0.21 | 5.72 |
| Vermesse and Vidal [64] | 1973 | 1.09 | 0.68 | 2.98 | 2.44 | 2.27 | 7.44 |
| Rabinovich et al. [65] | 1971 | 0.69 | − 0.65 | − 1.55 | 0.60 | − 0.37 | − 1.54 |
| Timrot et al. [66] | 1969 | 0.88 | 0.88 | 2.08 | 0.60 | 0.57 | 1.58 |
| Guevara et al. [67] | 1969 | 0.55 | 0.07 | − 0.97 | 0.59 | 0.59 | 0.97 |
| Gracki et al. [68] | 1969 | 0.44 | − 0.09 | − 1.61 | 1.10 | − 1.09 | − 3.22 |
| Boon et al. [69] | 1967 | 0.48 | − 0.05 | − 1.00 | 0.48 | 0.0 | − 0.90 |
| Flynn et al. [71] | 1963 | 0.49 | 0.15 | 1.94 | 0.24 | 0.12 | 1.12 |
| De Rocco and Halford [72] | 1958 | 0.49 | − 0.40 | − 0.90 | 0.65 | − 0.63 | − 1.22 |
| Total | 0.55 | 0.10 | 1.11 | − 0.42 | |||
Table 7.
Evaluation of the argon viscosity correlation for the secondary data
| Investigators/references | Year Publ | AARD (%) | BIAS (%) | MAX (%) | AARD (%) | BIAS (%) | MAX (%) |
|---|---|---|---|---|---|---|---|
| Present work | Lemmon and Jacobsen [7] | ||||||
| Borjan et al. [73] | 2022 | 0.51 | − 0.51 | − 0.85 | 0.22 | − 0.07 | − 0.70 |
| Goodwin et al. [74] | 2006 | 2.66 | 0.33 | 8.07 | 2.60 | 0.77 | 8.49 |
| May et al. [75] | 2006 | 0.05 | 0.05 | 0.07 | 0.23 | − 0.23 | − 0.58 |
| Lukin et al. [76] | 1983 | 1.39 | 1.20 | 4.28 | 0.75 | 0.33 | 4.31 |
| Malbrunot et al. [77] | 1983 | 2.51 | 2.51 | 4.55 | 2.75 | 2.75 | 5.29 |
| Abachi et al. [78] | 1980 | 2.79 | 2.79 | 6.75 | 2.87 | 2.87 | 6.82 |
| Trappeniers et al. [79] | 1980 | 5.05 | 4.15 | 13.38 | 5.27 | 3.24 | − 14.11 |
| Vidal et al. [80] | 1980 | 2.58 | − 1.68 | − 4.34 | 2.56 | − 0.04 | − 4.49 |
| Kestin and Wakeham [81] | 1979 | 0.80 | 0.80 | 1.09 | 0.59 | 0.59 | 0.84 |
| Kestin et al. [82] | 1977 | 0.73 | 0.73 | 1.06 | 0.42 | 0.37 | 0.81 |
| Kestin and Ro [83] | 1976 | 0.47 | 0.47 | 0.64 | 0.25 | 0.22 | 0.46 |
| Lyusternik and Lavushev [84] | 1976 | 0.91 | − 0.78 | − 1.69 | 0.85 | − 0.76 | − 1.95 |
| Gough et al. [85] | 1976 | 0.36 | 0.36 | 0.73 | 0.38 | − 0.33 | − 1.00 |
| Baharudin et al. [86] | 1975 | 20.59 | − 2.57 | 59.88 | 20.45 | − 1.70 | 62.20 |
| Timrot et al. [87] | 1975 | 0.35 | 0.24 | 1.28 | 0.39 | 0.34 | 1.13 |
| Carey et al. [88] | 1974 | 0.49 | 0.39 | 1.42 | 0.69 | 0.65 | 1.70 |
| Casparian and Cole [89] | 1974 | 0.68 | 0.68 | 1.45 | 0.58 | 0.50 | 1.26 |
| Hellemans et al. [90] | 1974 | 0.52 | 0.44 | 1.24 | 0.44 | 0.05 | 0.90 |
| Kurin and Golubev [91] | 1974 | 1.50 | − 0.43 | − 8.28 | 1.25 | 0.63 | 6.77 |
| Maitland and Smith [92] | 1974 | 0.53 | − 0.09 | − 1.13 | 0.46 | − 0.30 | − 0.99 |
| Hellemans et al. [93] | 1973 | 0.66 | 0.66 | 1.10 | 0.33 | 0.29 | 0.77 |
| Rakshit et al. [94] | 1973 | 1.51 | 1.51 | 4.04 | 1.32 | 1.25 | 3.65 |
| Slyusar et al. [95] | 1973 | 1.63 | 0.42 | 17.47 | 1.31 | 0.93 | 15.82 |
| Kestin et al. [96] | 1972 | 0.59 | 0.56 | 1.07 | 0.41 | 0.17 | 0.73 |
| Kestin et al. [97] | 1972 | 0.54 | 0.54 | 1.01 | 0.30 | 0.17 | 0.67 |
| Kestin et al. [98] | 1971 | 0.14 | 0.12 | 0.39 | 0.28 | 0.28 | 0.83 |
| Dawe and Smith [99] | 1970 | 0.65 | − 0.32 | − 1.21 | 0.65 | − 0.48 | − 0.98 |
| Golubev [100, 101] | 1970 | 4.09 | 3.93 | 19.64 | 4.43 | 4.21 | 19.65 |
| Hellemans and Zink [102] | 1970 | 7.19 | − 6.85 | − 33.58 | 6.96 | − 6.23 | − 33.49 |
| Kalelkar and Kestin [103] | 1970 | 0.38 | 0.20 | 0.73 | 0.43 | − 0.16 | − 0.67 |
| Kestin et al. [104] | 1970 | 0.43 | 0.40 | 0.80 | 0.31 | 0.01 | 0.59 |
| Clarke and Smith [105] | 1968 | 0.25 | 0.25 | 0.51 | 0.52 | − 0.41 | − 1.80 |
| De Bock et al. [106] | 1967 | 3.13 | 3.13 | 5.69 | 2.18 | 2.18 | 5.13 |
| De Bock et al. [107] | 1967 | 4.73 | 3.91 | 20.19 | 4.72 | 3.54 | 20.92 |
| DiPippo and Kestin [108] | 1967 | 0.67 | 0.67 | 1.14 | 0.44 | 0.42 | 0.72 |
| DiPippo et al. [109] | 1967 | 0.10 | 0.10 | 0.21 | 0.13 | 0.11 | 0.27 |
| Andreev et al. [110] | 1966 | 1.42 | − 1.40 | − 3.21 | 1.77 | − 1.57 | − 4.08 |
| van Itterbeek et al. [111] | 1966 | 1.04 | 0.95 | 3.98 | 1.03 | 0.08 | 2.88 |
| Naugle [112] | 1966 | 1.37 | − 1.37 | − 2.78 | 1.05 | − 1.05 | − 1.42 |
| Naugle et al. [113] | 1966 | 9.39 | − 9.39 | − 17.28 | 9.81 | − 9.81 | − 17.39 |
| Rigby and Smith [114] | 1966 | 1.67 | − 1.67 | − 3.25 | 2.10 | − 2.10 | − 3.65 |
| Chakraborti and Gray [115] | 1965 | 0.20 | − 0.20 | − 0.20 | 0.37 | − 0.37 | − 0.37 |
| Saji and Okuda [116] | 1965 | 0.43 | 0.31 | 0.79 | 1.30 | 1.19 | 2.99 |
| Iwasaki et al. [117] | 1964 | 0.12 | − 0.05 | − 0.26 | 0.15 | 0.09 | 0.42 |
| Kestin and Nagashima [118] | 1964 | 0.10 | 0.08 | 0.34 | 0.23 | 0.23 | 0.64 |
| Lowry et al. [119] | 1964 | 6.90 | − 6.84 | − 15.79 | 8.25 | − 8.25 | − 15.30 |
| Reynes and Thodos [120] | 1964 | 3.43 | 3.39 | 6.07 | 3.59 | 3.57 | 6.22 |
| Saji and Kobayashi [121] | 1964 | 0.44 | 0.33 | 0.80 | 0.45 | 0.36 | 0.84 |
| Forster [122] | 1963 | 2.80 | 2.80 | 5.63 | 3.61 | 3.61 | 7.01 |
| Iwasaki and Kestin [123] | 1963 | 0.12 | − 0.05 | − 0.26 | 0.15 | 0.09 | 0.42 |
| Kestin and Whitelaw [124] | 1963 | 1.51 | 1.45 | 3.06 | 1.65 | 1.62 | 3.48 |
| Filippova and Ishkin [125] | 1961 | 10.64 | 1.89 | 52.90 | 9.80 | 0.91 | 45.04 |
| Thornton [126] | 1960 | 0.17 | − 0.17 | − 0.17 | 0.35 | − 0.35 | − 0.35 |
| Filippova and Ishkin [127] | 1959 | 7.14 | 3.99 | − 16.41 | 6.40 | 3.22 | − 16.36 |
| Kestin and Leidenfrost [128] | 1959 | 0.09 | 0.01 | 0.23 | 0.07 | 0.01 | 0.21 |
| Makita [129] | 1957 | 0.99 | − 0.59 | − 3.72 | 0.83 | − 0.13 | − 3.51 |
| Zhdanova [130] | 1957 | 8.22 | 4.27 | 21.25 | 8.69 | 5.41 | 22.08 |
| Bonilla et al. [131] | 1956 | 4.74 | − 4.72 | − 9.74 | 4.67 | − 4.67 | − 8.67 |
| Jackson [132] | 1956 | 0.29 | − 0.29 | − 0.29 | 0.46 | − 0.46 | − 0.46 |
| Makita [133] | 1955 | 1.81 | 0.54 | 5.68 | 2.07 | 0.62 | 6.26 |
| Michels et al. [134] | 1954 | 0.61 | − 0.56 | − 1.46 | 0.26 | − 0.11 | − 1.54 |
| Rietveld et al. [135] | 1953 | 3.14 | 1.19 | 7.38 | 3.17 | 0.86 | 9.11 |
| Kiyama and Makita [136] | 1952 | 1.66 | 0.33 | 6.14 | 1.90 | 0.39 | 6.73 |
| Johnston and Grilly [137] | 1942 | 1.32 | 1.32 | 6.51 | 0.72 | 0.50 | 6.55 |
| Wobser and Mueller [138] | 1941 | 0.10 | 0.01 | 0.18 | 0.17 | − 0.16 | − 0.29 |
| van Itterbeek and van Paemel [139] | 1938 | 4.51 | 4.08 | 5.42 | 6.80 | 6.32 | 13.23 |
| Rudenko and Schubnikow [140] | 1934 | 2.73 | − 2.73 | − 3.22 | 2.70 | − 2.70 | − 3.18 |
| Trautz and Binikele [141] | 1930 | 1.26 | − 1.26 | − 1.80 | 1.53 | − 1.53 | − 2.20 |
| Trautz and Zink [142] | 1930 | 3.24 | − 3.24 | − 4.98 | 3.68 | − 3.68 | − 5.20 |
| Trautz and Ludewigs [143] | 1929 | 1.12 | − 1.12 | − 1.91 | 1.40 | − 1.40 | − 2.31 |
| Ishida [144] | 1923 | 1.59 | − 1.59 | − 1.59 | 1.76 | − 1.76 | − 1.76 |
| Rankine [145] | 1910 | 0.42 | − 0.42 | − 0.42 | 0.61 | − 0.61 | − 0.61 |
| Tanzler [146] | 1906 | 1.42 | 1.42 | 1.84 | 1.20 | 1.20 | 1.53 |
| Schultze [147] | 1901 | 0.53 | 0.53 | 1.09 | 0.31 | 0.31 | 0.78 |
One of the primary drivers for this work is to improve upon the correlation of Lemmon and Jacobsen [7] by incorporating developments in theory. This was done by incorporating Eqs. 2–5 for densities below about 2 mol⋅liter−1 (80 kg⋅m−3). Although we have identified a primary data set, the data were not fit to determine coefficients for this region since the behavior is fixed by Eqs.2–5. Figure 5 shows comparisons with the most reliable low-uncertainty measurements at pressures up to 1 MPa for the present correlation, Eqs. 1–6, and for the Lemmon and Jacobsen [7] correlation. The Lemmon and Jacobsen results are generally within about 0.5%, as claimed. The present correlation gives improved results in this region. Comparisons with the re-analyzed data of May [40] presented in Xiao et al. [5], that cover temperatures from 202 K to 394 K at pressures of zero and 0.1 MPa, show the correlation represents the data to within their stated uncertainty, 0.076% at k = 2. For temperatures above 394 K, comparisons with the data of Vogel et al. [46] show agreement to within the uncertainty of the experimental data, which Vogel conservatively reported as 0.15–0.2% with the highest uncertainty at the highest temperature. The Vogel et al. [46] data were calibrated with a reference value of 22.552 μPa s, that is slightly lower than the reference value used in this work, 22.5666 μPa s, and as indicated in Fig. 5 exhibit a small systematic deviation. As noted by Xiao et al. [5], the data of Lin et al. [42] show very good agreement up to about 393 K but have larger deviations as the temperature increases. The zero-density correlation, Eq. 2, [5] incorporated into this work has an estimated uncertainty of 0.12 (at k = 2) for the entire range from 83.8 K to 10,000 K.
Fig. 5.

Percentage deviations of selected data at low pressures (p < 1 MPa) calculated by the present model and the model of Lemmon and Jacobsen [7]. Xiao et al. [5, 40] ( ×), Humberg and Richter [41] (■), Lin et al. [42] ( ), Berg and Burton [43] (
), Berg and Burton [43] ( ), Zhang [44] (▲), Vogel [46] (♦), Wilhelm and Vogel [52] (
), Zhang [44] (▲), Vogel [46] (♦), Wilhelm and Vogel [52] ( )
)
For the mid-range pressure region (1 MPa < p < 100 MPa), Fig. 6 indicates that the results of the Lemmon and Jacobsen [7] model and the present model are very similar. Lemmon and Jacobsen [7] state an uncertainty of 1% for the temperature range from 270 to 300 K for pressures between 1 and 100 MPa, and that is the same found for the present correlation. For the temperature range of 180 K to 270 K and pressures between 1 and 100 MPa, the Lemmon and Jacobsen correlation [7] claims a 2% uncertainty, the present correlation gives 1% for this region. However, the Lemmon and Jacobsen [7] manuscript did not identify a primary data set and used all available data for comparisons, including some with larger deviations, such as Trappeniers et al. [79], that were excluded from our primary data set. When the Lemmon and Jacobsen [7] correlation is compared with our primary data set, it also has a 1% uncertainty for the temperature range of 180 K to 270 K and pressures between 1 and 100 MPa. Below 180 K, the most significant data sets useful for comparisons are Gracki et al. [68], Haynes [63], and Mostert et al. [55, 56], all of which have measurements that do not go below 173 K. For this region the uncertainties rise to about 3%. Below 173 K, there are not enough data at pressures between 1 and 100 MPa to do significant comparisons; only Zhou et al. [39] has one point at 150 K and 3.5 MPa that indicate that the uncertainty is at least 4% in this region. Additional high-quality measurements at temperatures below 173 K and at pressures up to 100 MPa could help improve the correlation in this region.
Fig. 6.

Percentage deviations of the primary data pressures between 1 and 100 MPa calculated by the present model and the model of Lemmon and Jacobsen [7]. Zhou et al. [39] ( ), Wang et al. [49] (□), Hurly et al. [50] (+), Evers et al. [51] (
), Wang et al. [49] (□), Hurly et al. [50] (+), Evers et al. [51] ( ), Wilhelm and Vogel [52] (
), Wilhelm and Vogel [52] ( ), Diller and Frederick [53] (
), Diller and Frederick [53] ( ), Mostert et al. [55] (
), Mostert et al. [55] ( ), Hongo [61] (
), Hongo [61] ( ), Haynes [63] (●), Vermesse and Vidal [64] (
), Haynes [63] (●), Vermesse and Vidal [64] ( ), Rabinovich et al. [65] (
), Rabinovich et al. [65] ( ), Gracki et al. [68] (
), Gracki et al. [68] ( ), (Flynn et al. [71] (
), (Flynn et al. [71] ( )
)
Figure 7 shows the deviations for both models as a function of temperature for the high-pressure region above 100 MPa. In this region the present correlation has improved performance over the Lemmon and Jacobsen correlation [7] due to the inclusion of the data of Abramson et al. [45] that were unavailable to Lemmon and Jacobsen [7]; these measurements extend to very high pressures (5.17 GPa). Unfortunately Abramson et al. [45] did not give uncertainties for the measurements, and we have assigned an uncertainty of 10% based primarily on the observed scatter in the data and performance of the instrument on other fluids [152, 161]. Based on the measurements of Mostert et al. [55] and Vermesse and Vidal [64], the estimated uncertainty of the correlation is about 2% for temperatures between 175 K and 308 K at pressures from 100 MPa to 606 MPa. Until additional data are available, we can only claim 10% uncertainty for temperatures above 308 K and high pressures.
Fig. 7.

Percentage deviations of the primary data pressures between 100 and 6000 MPa calculated by the present model and the model of Lemmon and Jacobsen [7]. Abramson [45] (△), Mostert et al. [55] ( ),Vermesse and Vidal [64] (
),Vermesse and Vidal [64] ( )
)
Figure 8 shows deviations for both correlations for liquid-phase measurements. These extend up to 34 MPa. The performance of both correlations is similar; the estimated uncertainty in this region based on comparisons with the experimental data is about 3%.
Fig. 8.

Percentage deviations of the primary data in the liquid phase at pressures up to 34 MPa calculated by the present model and the model of Lemmon and Jacobsen [7]. Zhou et al. [39] ( ), Haynes [63] (●), Boon et al. [69] (∗)
), Haynes [63] (●), Boon et al. [69] (∗)
Finally, Fig. 9 shows a plot of the viscosity of argon as a function of the temperature for different pressures. The plot demonstrates the reasonable extrapolation behavior at pressures up to 5 GPa and temperatures to 2000 K, that exceed the limits of the current EOS of Tegeler et al. [28] (700 K and 1 GPa). It is difficult to assign an uncertainty at conditions where there are no experimental data, so we can only state that the behavior is physically reasonable (no discontinuities, and the isobars on the temperature-viscosity plot do not cross). The extrapolated melting line of Tegeler et al. [28] is indicated by the dotted line, and the correlation does not have unphysical behavior such as mathematical poles within the fluid region. As noted earlier, the correlation does exhibit unphysical behavior outside of this region and users should be aware of this possibility.
Fig. 9.

Viscosity of argon as a function of the temperature for different pressures. The extrapolated melting line of Tegeler et al. [28] is indicated by the dotted line
Recommended Values and Computer-Program Verification
Recommended Values
In Table 8, viscosity values are given along the saturation boundary, calculated from the present proposed correlation between 90 K and 150 K, while in Table 9, viscosity values are given for temperatures between 80 K and 150 K and at selected pressures. Saturation density values for selected temperatures, as well as the density values for the selected temperature and pressure are obtained from the equation of state of Tegeler et al. [28]. The values in the tables are calculated from the given temperatures and densities according to Eqs. 1–6.
Table 8.
Viscosity values of argon along the saturation boundary, calculated by the present scheme
| Τ (Κ) | ρliq (kg·m−3) | ρvap (kg·m−3) | ηliq (μPa·s) | ηvap (μPa·s) |
|---|---|---|---|---|
| 90 | 1378.6 | 7.4362 | 240.45 | 7.2246 |
| 100 | 1313.7 | 16.859 | 183.07 | 8.0043 |
| 110 | 1242.8 | 33.287 | 142.48 | 8.8721 |
| 120 | 1162.8 | 60.144 | 111.66 | 9.9017 |
| 130 | 1068.1 | 103.56 | 86.679 | 11.248 |
| 140 | 943.71 | 178.86 | 64.471 | 13.370 |
| 150 | 680.43 | 394.50 | 36.712 | 20.533 |
Table 9.
Viscosity values of argon at selected temperatures and pressures, calculated by the present scheme
| p (MPa) | T (K) | ρ (kg·m−3) | (μPa·s) |
|---|---|---|---|
| 0.1 | 100 | 4.9152 | 8.0810 |
| 150 | 3.2255 | 12.095 | |
| 200 | 2.4093 | 15.889 | |
| 400 | 1.2012 | 28.642 | |
| 600 | 0.80058 | 38.804 | |
| 800 | 0.60042 | 47.571 | |
| 1000 | 0.48034 | 55.485 | |
| 1500 | 0.32025 | 73.039 | |
| 2000 | 0.24019 | 88.656 | |
| 10 | 100 | 1349.4 | 204.28 |
| 150 | 964.88 | 67.573 | |
| 200 | 337.74 | 23.007 | |
| 400 | 119.43 | 30.618 | |
| 600 | 78.026 | 39.869 | |
| 800 | 58.472 | 48.214 | |
| 1000 | 46.880 | 55.893 | |
| 1500 | 31.435 | 73.167 | |
| 2000 | 23.672 | 88.666 | |
| 50 | 100 | 1448.5 | 290.45 |
| 150 | 1234.3 | 129.45 | |
| 200 | 1023.7 | 79.043 | |
| 400 | 511.19 | 43.770 | |
| 600 | 342.22 | 46.526 | |
| 800 | 261.22 | 52.428 | |
| 1000 | 212.57 | 58.820 | |
| 1500 | 146.25 | 74.560 | |
| 2000 | 111.88 | 89.378 | |
| 100 | 100 | 1528.5 | solid |
| 150 | 1363.4 | 187.48 | |
| 200 | 1213.1 | 121.19 | |
| 400 | 787.37 | 61.681 | |
| 600 | 574.15 | 56.530 | |
| 800 | 454.97 | 59.113 | |
| 1000 | 378.65 | 63.713 | |
| 1500 | 269.06 | 77.260 | |
| 2000 | 209.61 | 91.054 | |
| 200 | 100 | 1635.8 | solid |
| 150 | 1510.1 | 309.68 | |
| 200 | 1398.8 | 198.36 | |
| 400 | 1065.5 | 93.995 | |
| 600 | 856.29 | 76.295 | |
| 800 | 717.53 | 73.178 | |
| 1000 | 619.24 | 74.551 | |
| 1500 | 464.37 | 83.938 | |
| 2000 | 373.04 | 95.656 | |
| 500 | 100 | 1824.2 | solid |
| 150 | 1739.3 | solid | |
| 200 | 1663.4 | 486.65 | |
| 400 | 1425.0 | 191.05 | |
| 600 | 1253.5 | 134.40 | |
| 800 | 1123.0 | 114.78 | |
| 1000 | 1019.9 | 107.27 | |
| 1500 | 834.82 | 105.83 | |
| 2000 | 709.96 | 112.26 |
Computer-Program Verification
For checking computer implementations of the correlation, the following points may be used for the given T, ρ conditions: T = 300 K, ρ = 0 kg·m−3, η = 22.6840 μPa·s, T = 300 K, ρ = 4.0 kg⋅m−3, η = 22.7334 μPa·s, and T = 300 K, ρ = 700 kg⋅m−3, η = 49.3360 μPa·s.
Conclusions
A new, wide-ranging correlation for the viscosity of argon based on critically evaluated experimental data was presented. This correlation is designed to be used over the range of applicability of the equation of state of Tegeler et al. [28] that extends from the triple-point temperature (83.81 K) to 700 K, at pressures up to 1000 MPa. The estimated uncertainty of the correlation based on comparisons with the best experimental data indicate that the uncertainty for the gas at pressures from zero to 0.1 MPa for temperatures from 202 K to 394 K is 0.076%, within the uncertainty of the experimental data [5]. For temperatures above 394 K, comparisons with the data of Vogel et al. [46] show agreement to within the uncertainty of the experimental data, which Vogel conservatively reported as 0.15- 0.2% with the highest uncertainty at the highest temperature. This represents a significant improvement over the current reference correlation of Lemmon and Jacobsen [7] that has an estimated uncertainty of 0.5% in this region. The estimated uncertainty for moderate pressures from 1 MPa to 100 MPa is 1% for temperatures from roughly 195 K to 300 K, rising to 2% at 175 K. For the high-pressure region, the estimated uncertainty of the correlation is about 2% for temperatures between 175 K and 308 K at pressures from 100 MPa to 606 MPa. For temperatures above 308 K to 700 K at pressures to 5.2 GPa, the equation has an estimated uncertainty of 10%. The estimated uncertainty in the liquid phase at pressures up to 34 MPa is 3%.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank our NIST colleagues Ala Bazyleva and Vladimir Diky for assistance in collecting and evaluating literature data.
Author Contributions
All authors contributed equally to preparing and reviewing this manuscript.
Funding
Marcia L. Huber received funding from the CHIPS Metrology Program, part of CHIPS for America, National Institute of Standards and Technology, U.S. Department of Commerce.
Data Availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
Marc J. Assael is the Editor-in-Chief and Konstantinos D. Antoniadis and Marcia L. Huber are Associate Editors of the International Journal of Thermophysics.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CHIPS Act of 2022, H.R. 4346, 117th Congress of the United States of America (2022). https://www.congress.gov/bill/117th-congress/house-bill/4346
- 2.J.D. Wright, T. Cobu, R.F. Berg, M.R. Moldover, Flow Meas. Instrum. 25, 8 (2012). 10.1016/j.flowmeasinst.2011.08.007 [Google Scholar]
- 3.B. Jäger, R. Hellmann, E. Bich, E. Vogel, Mol. Phys. 107, 2181 (2009). 10.1080/00268970903213305 [Google Scholar]
- 4.E. Vogel, B. Jäger, R. Hellmann, E. Bich, Mol. Phys. 108, 3335 (2010). 10.1080/00268976.2010.507557 [Google Scholar]
- 5.X. Xiao, D. Rowland, S.Z.S. Al Ghafri, E.F. May, J. Chem. Ref. Data 49, 013101 (2020). 10.1063/1.5125100 [Google Scholar]
- 6.J. Lang, M. Przybytek, M. Lesiuk, Phys. Rev. 109, 052803 (2024). 10.1103/PhysRevA.109.052803 [Google Scholar]
- 7.E.W. Lemmon, R.T. Jacobsen, Int. J. Thermophys. 25, 21 (2004). 10.1023/B:IJOT.0000022327.04529.f3 [Google Scholar]
- 8.D. Velliadou, K. Tasidou, K.D. Antoniadis, M.J. Assael, R.A. Perkins, M.L. Huber, Int. J. Thermophys. 42, 74 (2021). 10.1007/s10765-021-02818-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.E.K. Michailidou, M.J. Assael, M.L. Huber, R.A. Perkins, J. Phys. Chem. Ref. Data 42, 033104 (2013). 10.1063/1.4818980 [Google Scholar]
- 10.E.K. Michailidou, M.J. Assael, M. Huber, I. Abdulagatov, R.A. Perkins, J. Phys. Chem. Ref. Data 43, 023103 (2014). 10.1063/1.4875930 [Google Scholar]
- 11.M.J. Assael, T.B. Papalas, M.L. Huber, J. Phys. Chem. Ref. Data 46, 033103 (2017). 10.1063/1.4996885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.S.A. Monogenidou, M.J. Assael, M.L. Huber, J. Phys. Chem. Ref. Data 47, 013103 (2018). 10.1063/1.5021459 [Google Scholar]
- 13.S. Avgeri, M.J. Assael, M.L. Huber, R.A. Perkins, J. Phys. Chem. Ref. Data 43, 033103 (2014). 10.1063/1.4892935 [Google Scholar]
- 14.S. Avgeri, M.J. Assael, M.L. Huber, R.A. Perkins, J. Phys. Chem. Ref. Data 44, 033101 (2015). 10.1063/1.4926955 [Google Scholar]
- 15.K. Tasidou, M.L. Huber, M.J. Assael, J. Phys. Chem. Ref. Data 48, 043101 (2019). 10.1063/1.5128321 [Google Scholar]
- 16.E.A. Sykioti, M.J. Assael, M.L. Huber, R.A. Perkins, J. Phys. Chem. Ref. Data 42, 043101 (2013). 10.1063/1.4829449 [Google Scholar]
- 17.S. Sotiriadou, E. Ntonti, D. Velliadou, K.D. Antoniadis, M.J. Assael, M.L. Huber, Int. J. Thermophys. 40, 44 (2023). 10.1007/s10765-022-03149-z [Google Scholar]
- 18.M. Mebellis, D. Velliadou, M.J. Assael, M.L. Huber, Int. J. Thermophys. 42, 116 (2021). 10.1007/s10765-021-02867-0 [Google Scholar]
- 19.D. Velliadou, K.D. Antoniadis, M.J. Assael, M.L. Huber, Int. J. Thermophys. 43, 42 (2022). 10.1007/s10765-021-02970-2 [Google Scholar]
- 20.M.L. Huber, M.J. Assael, Int. J. Refrig. 71, 39 (2016). 10.1016/j.ijrefrig.2016.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D. Velliadou, M.J. Assael, M.L. Huber, Int. J. Thermophys. 43, 105 (2022). 10.1007/s10765-022-03029-6 [Google Scholar]
- 22.C.M. Tsolakidou, M.J. Assael, M.L. Huber, R.A. Perkins, J. Phys. Chem. Ref. Data 46, 023103 (2017). 10.1063/1.4983027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R.A. Perkins, M.L. Huber, M.J. Assael, J. Chem. Eng. Data 61, 3286 (2016). 10.1021/acs.jced.6b00350 [Google Scholar]
- 24.D. Velliadou, K.D. Antoniadis, M.J. Assael, M.L. Huber, Int. J. Thermophys. 43, 129 (2022). 10.1007/s10765-022-03050-9 [Google Scholar]
- 25.M.J. Assael, A.E. Kalyva, S.A. Monogenidou, M.L. Huber, R.A. Perkins, D.G. Friend, E.F. May, J. Phys. Chem. Ref. Data 47, 021501 (2018). 10.1063/1.5036625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.H. Preston-Thomas, Metrologia 27, 3 (1990). 10.1088/0026-1394/27/1/002 [Google Scholar]
- 27.H. Preston-Thomas, Metrologia 27, 107 (1990). 10.1088/0026-1394/27/2/010 [Google Scholar]
- 28.C. Tegeler, R. Span, W. Wagner, J. Phys. Chem. Ref. Data 28, 779 (1999). 10.1063/1.556037 [Google Scholar]
- 29.M.L. Huber, R.A. Perkins, A. Laesecke, D.G. Friend, J.V. Sengers, M.J. Assael, I.N. Metaxa, E. Vogel, R. Mares, K. Miyagawa, J. Phys. Chem. Ref. Data 38, 101 (2009). 10.1063/1.3088050 [Google Scholar]
- 30.M.J. Assael, S.A. Monogenidou, M.L. Huber, R.A. Perkins, J.V. Sengers, J. Phys. Chem. Ref. Data 50, 033102 (2021). 10.1063/5.0048711 [Google Scholar]
- 31.S.A. Monogenidou, M.J. Assael, M.L. Huber, J. Phys. Chem. Ref. Data 47, 02310 (2018). 10.1063/1.5036724 [Google Scholar]
- 32.D.G. Friend, J.C. Rainwater, Chem. Phys. Lett. 107, 590 (1984). 10.1016/S0009-2614(84)85163-5 [Google Scholar]
- 33.J.C. Rainwater, D.G. Friend, Phys. Rev. A 36, 4062 (1987). 10.1103/physreva.36.4062 [DOI] [PubMed] [Google Scholar]
- 34.E. Bich, E. Vogel, Chap. 5.2, in Transport properties of fluids. Their correlation, prediction and estimation (Cambridge University Press, Cambridge, 1996)
- 35.V. Vesovic, W.A. Wakeham, G.A. Olchowy, J.V. Sengers, J.T.R. Watson, J. Millat, J. Phys. Chem. Ref. Data 19, 763 (1990). 10.1063/1.555875 [Google Scholar]
- 36.S. Hendl, J. Millat, E. Vogel, V. Vesovic, W.A. Wakeham, J. Luettmer-Strathmann, J.V. Sengers, M.J. Assael, Int. J. Thermophys. 15, 1 (1994). 10.1007/BF01439245 [Google Scholar]
- 37.J.V. Sengers, Int. J. Thermophys. 6(3), 203 (1985). 10.1007/BF00522145 [Google Scholar]
- 38.V. Diky, D.R. Chirico, M. Frenkel, A. Bazyleva, J.W. Magee, E. Paulechka, A. Kazakov, E.W. Lemmon, C.D. Muzny, A.Y. Smolyanitsky, S. Townsend, K. Kroenlein, ThermoData Engine (TDE) Version 10.4, NIST Standard Database #103b, National Institute of Standards and Technology, Gaithersburg, MD (2019). https://www.nist.gov/mml/acmd/trc/thermodata-engine/srd-nist-tde-103b
- 39.X. Zhou, W. Qin, Z. Li, J. Wu, X. Meng, Int. J. Thermophys. 45, 30 (2024). 10.1007/s10765-023-03325-9 [Google Scholar]
- 40.E.F. May, R.F. Berg, M.R. Moldover, Int. J. Thermophys. 28, 1085 (2007). 10.1007/s10765-007-0198-7 [Google Scholar]
- 41.K. Humberg, M. Richter, Ind. Eng. Chem. Res. 58, 8878 (2019). 10.1021/acs.iecr.9b01533 [Google Scholar]
- 42.H. Lin, X.J. Feng, J.T. Zhang, C. Liu, J. Chem. Phys. 141, 234311 (2014). 10.1063/1.4903960 [DOI] [PubMed] [Google Scholar]
- 43.R.F. Berg, W.C. Burton, Mol. Phys. 111, 195 (2013). 10.1080/00268976.2012.713132 [Google Scholar]
- 44.J.T. Zhang, H. Lin, J. Che, Metrologia 50, 377 (2013). 10.1088/0026-1394/50/4/377 [Google Scholar]
- 45.E.H. Abramson, High Press. Res. 31(4), 544 (2011). 10.1080/08957959.2011.625554 [Google Scholar]
- 46.E. Vogel, Int. J. Thermophys. 31, 447 (2010). 10.1007/s10765-010-0760-6 [Google Scholar]
- 47.E. Vogel, Ber. Bunsenges. Phys. Chem. 88, 997 (1984). 10.1002/bbpc.19840881014 [Google Scholar]
- 48.E. Vogel, Wiss, Z. Univ. Rostock. Math. Naturwiss. Reihe 21(2), 169 (1972) [Google Scholar]
- 49.X. Wang, J. Wu, Z. Liu, J. Chem. Eng. Data 55, 496 (2010). 10.1021/je900279z [Google Scholar]
- 50.J.J. Hurly, K.A. Gillis, J.B. Mehl, M.R. Moldover, Int. J. Thermophys. 24, 1441 (2003). 10.1023/B:IJOT.0000004088.04964.4c [Google Scholar]
- 51.C. Evers, H.W. Loesch, W. Wagner, Int. J. Thermophys. 23, 1411 (2002). 10.1023/A:1020784330515 [Google Scholar]
- 52.J. Wilhelm, E. Vogel, Int. J. Thermophys. 21, 301 (2000). 10.1023/A:1006667125801 [Google Scholar]
- 53.D.E. Diller, N.V. Frederick, Int. J. Thermophys. 10, 145 (1989). 10.1007/BF00500715 [Google Scholar]
- 54.A. Hobley, G.P. Matthews, A. Townsend, Int. J. Thermophys. 10, 1165 (1989). 10.1007/BF00500569 [Google Scholar]
- 55.R. Mostert, P.S. van der Gulik, H.R. van den Berg, Physica A 156, 921 (1989). 10.1016/0378-4371(89)90028-9 [Google Scholar]
- 56.P.S. van der Gulik, N.J. Trappeniers, Physica A (Amsterdam) 135, 1 (1986). 10.1016/0378-4371(86)90103-2 [Google Scholar]
- 57.J. Kestin, S.T. Ro, Ber. Bunsenges. Phys. Chem. 86, 948 (1982). 10.1002/bbpc.19820861016 [Google Scholar]
- 58.G.P. Matthews, H. Schofield, E.B. Smith, A.R. Tindell, J. Chem. Soc. Faraday Trans. 1(78), 2529 (1982). 10.1039/F19827802529 [Google Scholar]
- 59.I.A. Barr, G.P. Matthews, E.B. Smith, A.R. Tindell, J. Phys. Chem. 85(22), 3342 (1981). 10.1021/j150622a027 [Google Scholar]
- 60.J. Kestin, H.E. Khalifa, W.A. Wakeham, Physica A 90, 215 (1978). 10.1016/0378-4371(78)90110-3 [Google Scholar]
- 61.M. Hongo, Rev. Phys. Chem. Japan 48(2), 63 (1978) [Google Scholar]
- 62.A.A. Clifford, P. Gray, A.C. Scott, J. Chem. Soc. Faraday Trans. 71(4), 875 (1975). 10.1039/f19757100875 [Google Scholar]
- 63.W.M. Haynes, Physica (Amsterdam) 67, 440 (1973). 10.1016/0031-8914(73)90162-6 [Google Scholar]
- 64.J. Vermesse, D. Vidal, C.R. Hebd, Seances Acad. Sci. Ser. B 277(9), 191 (1973) [Google Scholar]
- 65.V.A. Rabinovich, V.A. Kryuchkov, E.A. Zikeev, Teplofiz. Svoistva Veshchestv Mater., 56 (1971).
- 66.D.L. Timrot, M.A. Serednitskaya, S.A. Traktueva, Teploenergetika 16(1), 83 (1969) [Google Scholar]
- 67.F.A. Guevara, B.B. McInteer, W.E. Wageman, Phys. Fluids 12, 2493 (1969). 10.1063/1.1692386 [Google Scholar]
- 68.J.A. Gracki, G.P. Flynn, J. Ross, J. Chem. Phys. 51, 3856 (1969). 10.1063/1.1672602 [Google Scholar]
- 69.J.P. Boon, J.C. Legros, G. Thomaes, Physica (Amsterdam) 33, 547 (1967). 10.1016/0031-8914(67)90203-0 [Google Scholar]
- 70.J.P. Boon, G. Thomaes, Physica 29, 208 (1963). 10.1016/S0031-8914(63)80278-5 [Google Scholar]
- 71.G.P. Flynn, R.V. Hanks, N.A. Lemaire, J. Ross, J. Chem. Phys. 38, 154 (1963). 10.1063/1.1733455 [Google Scholar]
- 72.A.G. De Rocco, J.O. Halford, J. Chem. Phys. 28(6), 1152 (1958). 10.1063/1.1744359 [Google Scholar]
- 73.D. Borjan, M. Gracnar, Z. Knez, M.K. Marevci, Processes 10, 2275 (2022). 10.3390/pr10112275 [Google Scholar]
- 74.A.R.H. Goodwin, A.D. Fitt, K.A. Ronaldson, W.A. Wakeham, Int. J. Thermophys. 27, 1650 (2006). 10.1007/s10765-006-0114-6 [Google Scholar]
- 75.E.F. May, M.R. Moldover, R.F. Berg, J.H. Hurly, Metrologia 43, 247 (2006). 10.1088/0026-1394/43/3/007 [Google Scholar]
- 76.V.I. Lukin, B.A. Ivakin, P.E. Suetin, Sov. Phys. Tech. Phys. 28(5), 597 (1983) [Google Scholar]
- 77.P. Malbrunot, A. Boyer, E. Charles, H. Abachi, Phys. Rev. A 27, 1523 (1983). 10.1103/PhysRevA.27.1523 [Google Scholar]
- 78.H. Abachi, J. Molenat, P. Malbrunot, Phys. Lett. A 80(2/3), 171 (1980). 10.1016/0375-9601/2880/2990214-5 [Google Scholar]
- 79.N.J. Trappeniers, P.S. vanderGulik, H. van den Hooff, Chem. Phys. Lett. 70, 438 (1980). 10.1016/0009-2614(80)80100-X
- 80.D. Vidal, R. Tufeu, Y. Garrabos, B. Le Neindre, In Molecular and Chemical Physics, Chemistry, Biological Effects, Geo and Planetary Sciences, New Resources, Dynamic Pressures, High Pressure Safety, ed. by B. Vodar, P. Marteau (Pergamon, 1980), p. 692–698
- 81.J. Kestin, W.A. Wakeham, Ber. Bunsenges. Phys. Chem. 83, 573 (1979). 10.1002/bbpc.19790830607 [Google Scholar]
- 82.J. Kestin, H.E. Khalifa, S.T. Ro, W.A. Wakeham, Physica A 88, 242 (1977). 10.1016/0378-4371(77)90003-6 [Google Scholar]
- 83.J. Kestin, S.T. Ro, Ber. Bunsenges. Phys. Chem. 80, 619 (1976). 10.1002/bbpc.19760800709 [Google Scholar]
- 84.V.E. Lyusternik, A.V. Lavushev, Teplofiz. Vys. Temp. 14, 970 (1976) [Google Scholar]
- 85.D.W. Gough, G.P. Matthews, E.B. Smith, J. Chem. Soc. Faraday Trans. 1 72 (3), 645 (1976). 10.1039/F19767200645
- 86.B.Y. Baharudin, D.A. Jackson, P.E. Schoen, Phys. Lett. A 51, 409 (1975). 10.1016/0375-9601(75)90750-1 [Google Scholar]
- 87.D.L. Timrot, M.A. Serednitskaya, S.A. Traktueva, Teploenergetika 22, 84 (1975) [Google Scholar]
- 88.C. Carey, J. Bradshaw, E. Lin, E.H. Carnevale, Experimental determination of gas properties at high temperatures and/or pressures, Panametrics Inc., No. AEDC-TR-74-33:Waltham, MA (1974).
- 89.A.S. Casparian, R.H. Cole, J. Chem. Phys. 60, 1106 (1974). 10.1063/1.1681120 [Google Scholar]
- 90.J.M. Hellemans, J. Kestin, S.T. Ro, Physica (1974). 10.1016/0031-8914(74)90043-3 [Google Scholar]
- 91.V.I. Kurin, I.F. Golubev, Teploenergetika 21, 125 (1974) [Google Scholar]
- 92.G.C. Maitland, E.B. Smith, J. Chem. Soc. Faraday Trans. 70, 1191 (1974). 10.1039/F19747001191 [Google Scholar]
- 93.J.M. Hellemans, J. Kestin, S.T. Ro, Physica 65, 362 (1973). 10.1016/0031-8914(73)90351-0 [Google Scholar]
- 94.A.B. Rakshit, C.S. Roy, A.K. Barua, J. Chem. Phys. 59, 3633 (1973). 10.1063/1.1680530 [Google Scholar]
- 95.V.P. Slyusar, N.S. Rudenko, V.M. Tret’yakov, Teplofiz. Svoistva Veshchestv Mater. 7, 50 (1973) [Google Scholar]
- 96.J. Kestin, S.T. Ro, W.A. Wakeham, J. Chem. Phys. 56, 4119 (1972). 10.1063/1.1677824 [Google Scholar]
- 97.J. Kestin, S.T. Ro, W.A. Wakeham, J. Chem. Phys. 56, 4086 (1972). 10.1063/1.1677819 [Google Scholar]
- 98.J. Kestin, E. Paykoc, J.V. Sengers, Physica (Amsterdam) 54, 1 (1971). 10.1016/0031-8914(71)90059-0 [Google Scholar]
- 99.R.A. Dawe, E.B. Smith, J. Chem. Phys. 52, 693 (1970). 10.1063/1.1673042 [Google Scholar]
- 100.I. F. Golubev, in Viscosity of Gases and Gas Mixtures. A Handbook (Israel Program Sci. Transl., 1970), pp. 245
- 101.I.F. Golubev, V.A. Petrov, Trudy GIAP 1, 7 (1953) [Google Scholar]
- 102.J. Hellemans, H. Zink, O. Van Paemel, Physica (Amsterdam) 46, 395 (1970). 10.1016/0031-8914(70)90013-3 [Google Scholar]
- 103.A.S. Kalelkar, J. Kestin, J. Chem. Phys. 52, 4248 (1970). 10.1063/1.1673636 [Google Scholar]
- 104.J. Kestin, W.A. Wakeham, K. Watanabe, J. Chem. Phys. 53(10), 3773 (1970). 10.1063/1.1673840 [Google Scholar]
- 105.A.G. Clarke, E.B. Smith, J. Chem. Phys. 48, 3988 (1968). 10.1063/1.1669725 [Google Scholar]
- 106.A. DeBock, W. Grevendonk, H. Awouters, Physica 34, 49 (1967). 10.1016/0031-8914(67)90053-5 [Google Scholar]
- 107.A. DeBock, W. Grevendonk, W. Herreman, Physica (Amsterdam) 37, 227 (1967). 10.1016/0031-8914(67)90153-X [Google Scholar]
- 108.R. DiPippo, J. Kestin, The Viscosity of Seven Gases up to 500deg C and Its Statistical Interpretation, Natl. Sci. Found. Res. Grant, No. GK 1305 (1967)
- 109.R. DiPippo, J. Kestin, K. Oguchi, J. Chem. Phys. 46, 4758 (1967). 10.1063/1.1840632 [Google Scholar]
- 110.I.I. Andreev, N.V. Tsderberg, V.N. Popov, Teploenergetika, 78 (1966)
- 111.A. Van Itterbeek, J. Hellemans, H. Zink, M. Van Cauteren, Physica 32, 2171 (1966). 10.1016/0031-8914(66)90178-9 [Google Scholar]
- 112.D.G. Naugle, J. Chem. Phys. 44(2), 741 (1966). 10.1063/1.1726755 [Google Scholar]
- 113.D.G. Naugle, J.H. Lunsford, J.R. Singer, J. Chem. Phys. 45, 4669 (1966). 10.1063/1.1727553 [Google Scholar]
- 114.M. Rigby, E.B. Smith, Trans. Faraday Soc. 62, 54 (1966). 10.1039/TF9666200054 [Google Scholar]
- 115.P.K. Chakraborti, P. Gray, Trans. Faraday Soc. 61(515), 2422 (1965). 10.1039/TF9666201769 [Google Scholar]
- 116.Y. Saji, T. Okuda, Adv. Cryo. Eng. 10, 209 (1965) [Google Scholar]
- 117.H. Iwasaki, J. Kestin, A. Nagashima, J. Chem. Phys. 40(10), 2988 (1964). 10.1063/1.1724938 [Google Scholar]
- 118.J. Kestin, A. Nagashima, J. Chem. Phys. 40, 3648 (1964). 10.1063/1.1725066 [Google Scholar]
- 119.B.A. Lowry, S.A. Rice, P. Gray, J. Chem. Phys. 40(12), 3673 (1964). 10.1063/1.1725072 [Google Scholar]
- 120.E.G. Reynes, G. Thodos, Physica (Amsterdam) 30, 1529 (1964). 10.1016/0031-8914(64)90177-6 [Google Scholar]
- 121.Y. Saji, S. Kobayashi, Cryogenics 4, 136 (1964). 10.1016/S0011-2275(64)80002-3 [Google Scholar]
- 122.S. Forster, Cryogenics 3, 176 (1963). 10.1016/0011-2275(63)90012-2 [Google Scholar]
- 123.H. Iwasaki, J. Kestin, Physica (Amsterdam) 29, 1345 (1963). 10.1016/S0031-8914(63)80243-8 [Google Scholar]
- 124.J. Kestin, J.H. Whitelaw, Physica (Amsterdam) 29, 335 (1963). 10.1016/S0031-8914(63)80294-3 [Google Scholar]
- 125.G.P. Filippova, I.P. Ishkin, Inzh.-Fis. Zh. 4(3), 105 (1961) [Google Scholar]
- 126.E. Thornton, Proc. Phys. Soc. (London) 76, 104 (1960). 10.1088/0370-1328/76/1/313 [Google Scholar]
- 127.G.P. Filippova, I.P. Ishkin, Kislorod 2, 38 (1959) [Google Scholar]
- 128.J. Kestin, W. Leidenfrost, Physica (Amsterdam) 25, 1033 (1959). 10.1016/0031-8914(59)90024-2 [Google Scholar]
- 129.T. Makita, Rev. Phys. Chem. Jpn. 27, 16 (1957) [Google Scholar]
- 130.N.F. Zhdanova, Sov. Phys. - JETP 4, 749 (1957) [Google Scholar]
- 131.C.F. Bonilla, S.J. Wang, H. Weiner, Trans. ASME 78, 1285 (1956). 10.1115/1.4014012 [Google Scholar]
- 132.W.M. Jackson, J. Phys. Chem. 60, 789 (1956). 10.1021/j150540a021 [Google Scholar]
- 133.T. Makita, The viscosity of gases under high pressure. Mem. Fac. Ind. Arts Kyoto Tech. Univ. Sci. Technol. 4, 19 (1955) [Google Scholar]
- 134.A. Michels, A. Botzen, W. Schuurman, Physica (Amsterdam) 20, 1141 (1954). 10.1016/S0031-8914(54)80257-6 [Google Scholar]
- 135.A.O. Rietveld, A. Van Itterbeek, G.J.K. Van Den Berg, Physica (The Hague) 19, 517 (1953). 10.1016/S0031-8914(53)80055-8 [Google Scholar]
- 136.R. Kiyama, T. Makita, Rev. Phys. Chem. Jpn. 12, 49 (1952) [Google Scholar]
- 137.H.I. Johnston, E.R. Grilly, J. Phys. Chem. 46, 948 (1942). 10.1021/j150422a019 [Google Scholar]
- 138.R. Wobser, F. Mueller, Kolloid-Beih. 52, 165 (1941) [Google Scholar]
- 139.A. van Itterbeek, O. van Paemel, Physica V 5, 1009 (1938). 10.1016/S0031-8914(38)80046-X [Google Scholar]
- 140.N.S. Rudenko, L.V. Shubnikov, Phys. Z. Sowjetunion 6, 470 (1934) [Google Scholar]
- 141.M. Trautz, H.E. Binkele, Ann. Phys. 397(5), 561 (1930). 10.1002/andp.19303970503 [Google Scholar]
- 142.M. Trautz, R. Zink, Ann. Phys. (Leipzig) 7, 427 (1930). 10.1002/andp.19303990405 [Google Scholar]
- 143.M. Trautz, W. Ludewigs, Ann. Phys. (Leipzig) 3(5), 409 (1929). 10.1002/andp.19293950308 [Google Scholar]
- 144.Y. Ishida, Phys. Rev. 21, 550 (1923). 10.1103/PhysRev.21.550 [Google Scholar]
- 145.A.O. Rankine, Proc. R. Soc. London Ser. A 83, 516 (1910). 10.1098/rspa.1910.0040 [Google Scholar]
- 146.P. Tanzler, Ber. Dtsch. Chem. Ges. 4, 222 (1906) [Google Scholar]
- 147.H. Schultze, Ann. Phys. (Leipzig) 310, 140 (1901). 10.1002/andp.19013100508 [Google Scholar]
- 148.G.C. Maitland, E.B. Smith, J. Chem. Eng. Data 17(2), 150 (1972). 10.1021/je60053a015 [Google Scholar]
- 149.R. DiPippo, J. Kestin, Proc. 4th Symp. Thermophys. Prop., Maryland: April 1–4, p. 304 (1968)
- 150.J. Kestin, H.E. Wang, Trans. ASME 80, 11 (1958). 10.1115/1.4012237 [Google Scholar]
- 151.T. Strehlow, Ph.D. Thesis, Wilhelm-Pieck-Universitat Rostock, Rostock, Germany (1987)
- 152.M.L. Huber, R.A. Perkins, E.W. Lemmon, Int. J. Thermophys. 45(10), 146 (2024). 10.1007/s10765-024-03440-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ε Vogel, C. Küchenmeister, Ε Bich, A. Laesecke, J. Phys. Chem. Ref. Data 27, 947 (1998). 10.1063/1.556025 [Google Scholar]
- 154.E. Vogel, E. Bich, R. Nimz, Phys. A 139, 188 (1986). 10.1016/0378-4371(86)90012-9 [Google Scholar]
- 155.B. Najafi, Y. Ghayeb, J.C. Rainwater, S. Alavi, R.F. Snider, Physica A 260(1), 31 (1998). 10.1016/S0378-4371(98)00287-8 [Google Scholar]
- 156.R.A. Aziz, J. Chem. Phys. 99(6), 4518 (1993). 10.1063/1.466051 [Google Scholar]
- 157.S.J. Boyes, Chem. Phys. Lett. 221(5), 467 (1994). 10.1016/0009-2614(94)00303-3 [Google Scholar]
- 158.EUREQA Formulize v.098.1, Nutonian Inc., Cambridge MA, USA (Nutonian Inc., Cambridge MA, USA). Commercial equipment, instruments, or materials are identified only in order to adequately specify certain procedures. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and technology, nor does it imply that the products identified are necessarily the best available for the purpose.
- 159.M.J. Assael, J.H. Dymond, M. Papadaki, P.M. Patterson, Int. J. Thermophys. 13, 269 (1992). 10.1007/BF00504436 [Google Scholar]
- 160.E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, REFPROP, NIST Standard Reference Database 23,Version 10.0, NIST, Standard Reference Data Program, Gaithersburg, MD (2018). 10.18434/T4/1502528
- 161.A. Laesecke, C.D. Muzny, J. Phys. Chem. Ref. Data 46, 013107 (2017). 10.1063/1.4977429 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.



