Abstract
Cyclophilins A and B (CyPA and CyPB) are cyclosporin A-binding proteins that are involved in inflammatory events. We have reported that CyPB interacts with two types of cell-surface-binding sites. The first site corresponds to a functional receptor and requires interaction with the central core of CyPB. This region is highly conserved in cyclophilins, suggesting that CyPA and CyPB might share biological activities mediated by interaction with this receptor. The second site is identified with glycosaminoglycans (GAGs), the binding region located in the N terminus of CyPB. The difference in the N-terminal extensions of CyPA and CyPB suggests that a unique interaction with GAGs might account for selective activity of CyPB. To explore this hypothesis, we analyzed the lymphocyte responses triggered by CyPA, CyPB, and CyPBKKK−, a mutant unable to interact with GAGs. The three ligands seemed capable enough to elicit calcium signal and chemotaxis by binding to the same signaling receptor. In contrast, only CyPB enhanced firm adhesion of T cells to the extracellular matrix. This activity depended on the interactions with GAGs and signaling receptor. CyPB-mediated adhesion required CD147 presumably because it was a costimulatory molecule and was related to an activation of α4β1 and α4β7 integrins. Finally, we showed that CyPB was capable mainly to enhance T cell adhesion of the CD4+CD45RO+ subset. The present data indicate that CyPB rather than CyPA is a proinflammatory factor for T lymphocytes and highlight the crucial role of CyPB–GAG interaction in the chemokine-like activity of this protein.
Cyclophilins are the main binding proteins for the immunosuppressive drug cyclosporin A (CsA) (1, 2) and exhibit peptidyl-prolyl cis–trans isomerase activity (3, 4). Binding of CsA to cyclophilins leads to the formation of complexes that inhibit the phosphatase activity of calcineurin (5). The latter property is relevant to the inhibition of early T cell activation and constitutes the basis of the prevention of graft rejection (6). The first characterized isoform was cyclophilin A (CyPA), an abundant cytosolic isoform considered as the major target for CsA (1, 7, 8). Cyclophilin B (CyPB) was the second characterized member of the cyclophilin family (9, 10). It resides within the endoplasmic reticulum (11) and is secreted in human biological fluids, e.g., milk and plasma (10, 12).
Several data have suggested a role for CyPA and CyPB in inflammatory processes. High levels of both proteins were recovered in biological fluids as a response to inflammatory stimuli, e.g., in severe sepsis (13), HIV infection (14), or oxidative stress (15). Although the mechanism involved in the release of CyPA remained unclear (16, 17), the highest secretion of CyPB seemed to be related to an over-expression of the protein (18). CyPA was reported to trigger chemotactic activity for leukocytes (16, 17) and CyPB to enhance adhesion of platelets to collagen (19). The activities triggered by CyPA and CyPB suggest the existence of surface-binding sites on responsive cells. In this way, CyPA was reported to elicit Ca2+ responses through binding to membrane receptors on T lymphocytes (20). Most recently, CD147 was demonstrated to facilitate HIV-1 infection by interacting with virus-associated CyPA, making this glycoprotein a putative cell-surface receptor for extracellular CyPA (21). During past years, we described the presence of binding sites for CyPB on T lymphocytes (22–24), platelets (19), and endothelial cells (25). In our hands, however, CyPA was unable to compete with surface-bound radio-labeled CyPB, which may reflect either the existence of two distinct receptors or a large difference in binding affinities for the same receptor. Most recently, we demonstrated that surface binding of CyPB involved two classes of sites (26, 27). The first class, termed type I, was identified as a specific functional receptor, whereas the second class, termed type II, was identified as sulfated glycosaminoglycans (GAGs) of the heparan sulfate family (26). The location of binding regions in CyPB was delineated by engineering mutated proteins (27). Interaction with type I site involved the central conserved core of CyPB, which shares CsA-binding and catalytic domains, and can consequently be inhibited by CsA. By the way, it cannot be excluded that CyPA interacts with type I sites and initiates similar responses to CyPB. The central core is highly conserved between both cyclophilin isoforms, making CyPA a putative ligand for the CyPB type I site. Conversely, the binding region to GAGs was located in the N-terminal extension of CyPB and we clearly identified the sequences 3KKK5 and 15YFD17 as absolutely required for this interaction (27). CyPA does not possess these clusters, explaining why CyPB is a unique, highly specific ligand for type II site. The binding regions of CyPB are located on opposite sides of the molecule, suggesting that proteoglycan-bound ligand is presented to functional receptors (27). Such a mechanism might account for differences in the biological responses triggered by CyPA and CyPB.
We took advantage of these findings to explore whether lymphocyte responses triggered by CyPA and CyPB could be relevant to interaction with the same binding site. The mutant CyPBKKK− seemed to be a helpful tool for studying the involvement of proteoglycans in CyPB activity, because it is devoid of ability to interact with GAGs but binds to type I sites as well as wild-type ligands (27). We demonstrated that CyPA and CyPB elicit Ca2+ signals in T lymphocytes by binding to type I site. Interaction with type I receptor seemed capable enough for both agonists to trigger chemotaxis of T lymphocytes. In contrast, the participation of GAGs seemed absolutely required for CyPB to enhance integrin-mediated adhesion to the extracellular matrix (ECM). The ability to trigger chemotaxis and integrin-mediated adhesion of T cell subsets is currently attributed to proinflammatory factors of the chemokine family (28–30). We demonstrated that CyPB also shared this latter property and preferentially acted on memory helper/inducer CD4+/CD45RO+ lymphocyte subset. Altogether, our results suggest that CyPB might be added to the family of heparin-binding proteins that act in the context of T lymphocyte recruitment and function.
Materials and Methods
Materials.
Recombinant human CyPA and CyPB were produced and purified as described (7, 10). The mutant CyPBKKK− was engineered by site-directed mutagenesis (CyPBK3A,K4A,K5A) and purified as described (27). CsA was provided by Novartis (Basel, Switzerland). Human fibronectin was a gift from Ph. Delannoy (University of Lille, France). ECM was from Harbor Bio-Products (Norwood, MA). Monoclonal antibodies to integrin subunits (anti-α2, -α4, -α5, -β1, -β2, -β7) were from Calbiochem, to lymphocyte markers (anti-CD3, -CD4, -CD8, -CD45RA, -CD45RO) from Immunotech (Marseille, France), and to CD147 from PharMingen. Cell culture products were obtained from GIBCO/BRL. Pertussis toxin (PTX) and wortmannin were from Alexis Corporation (San Diego, CA), heparinase I (E.C. 4.2.2.7) from Sigma, Fluo-3/acetoxymethyl ester (Fluo-3/AM) from Molecular Probes, and peroxidase-labeled anti-IgG antibodies from BioSys (Compiègne, France), respectively. All other chemicals were purchased from Sigma.
Isolation of Human Peripheral Blood T Cells.
Human citrated venous blood samples from healthy donors were obtained from the local blood transfusion center (Etablissement de Transfusion Sanguine, Lille, France). Peripheral blood T lymphocytes were prepared as described (23). T cell subsets, CD4+, CD8+, CD45RA+, and CD45RO+, were prepared by exhaustive selection by using adsorbed monoclonal antibodies. The purity of the T cell populations was assessed by flow cytofluorimetry and found >95% (24). Heparinase-treated T cells were prepared as described (26).
Calcium Measurements.
T lymphocytes were loaded with 3 μM Fluo-3/AM for 30 min at 37°C. After washing off extracellular Fluo-3, the final cell concentration was adjusted to 1 × 105 cells per ml in Dulbecco's phosphate-buffered saline (DPBS), pH 7.4. Stimulation was induced by the addition of various concentrations of agonists at 37°C. Variations in cytosolic Ca2+ concentration were analyzed by flow cytofluorimetry by monitoring changes in fluorescence (19). Levels of cytosolic Ca2+ were calculated for each fluorescence mean value, with a Kd of 400 nM for Fluo-3 (31).
In Vitro Chemotaxis Assays.
T lymphocyte chemotaxis was assayed in vitro as described (32). In brief, freshly isolated T cells were adjusted at 10 × 106 cells per ml in RPMI-1640 culture medium supplemented with 0.5% BSA. Chemotactic activity was evaluated in a microchemotaxis chamber containing fibronectin-coated 5-μm pore polycarbonate membranes (Corning Costar). The chemotactic index was calculated as the number of cells migrating toward the test sample divided by the number of cells migrating toward control medium.
Adhesion Assays.
Immobilization of fibronectin was performed in 96-well microtiter plates (Nunc) overnight at 4°C in DPBS (1 μg/100 μl per well). ECM-coated plates were prepared as described by the manufacturer. Plates were washed extensively with DPBS, and nonspecific binding sites were blocked by the addition of 0.5% BSA in DPBS for 30 min at 37°C. For adhesion assays, freshly isolated T lymphocytes (10 × 106 cells per ml) were preincubated in DPBS/0.5% BSA with increasing concentrations of the proadhesive factors for 10 min at 37°C, and the mixture was then distributed into the wells (100 μl) for an additional 20-min incubation at 37°C. Thereafter, the plates were washed extensively to remove nonadherent cells, and the remaining firmly attached cells were fixed with 3% formaldehyde, pH 7.8, for 20 min at 4°C. Adherent cells were quantified by using two different methods. In the first method, cells were stained with 1% methylene blue in 100 mM borate buffer, pH 8.2, as described (33). In the second method, cells were rinsed with DPBS and incubated for 15 min in the presence of 0.1 M glycocoll to block remaining formaldehyde activity. The phenotype of adherent T cells was analyzed by incubation with antibodies to CD3, CD4, or CD8 in DPBS/0.5% BSA for 1 h at 37°C. After wash, cells were exposed to horseradish peroxidase-labeled anti-mouse IgG antibodies for another hour of incubation. Staining was performed with o-phenylene diamine kit (Sigma), and the absorbance was measured at 490 nm. In both staining methods, cell adhesion was estimated by using standard curves where absorbances were related to T cell numbers, and results were expressed as a percentage of the initially added cells remaining fixed to the substrates.
Statistics Analysis.
Results are expressed as mean values ± SD and representative for at least three independently performed experiments conducted with distinct blood donors (n). Statistical significance between the different values was analyzed by Student's t test for unpaired data with a threshold of P < 0.05.
Results
Cyclophilin-Mediated Ca2+ Rise in T Lymphocytes.
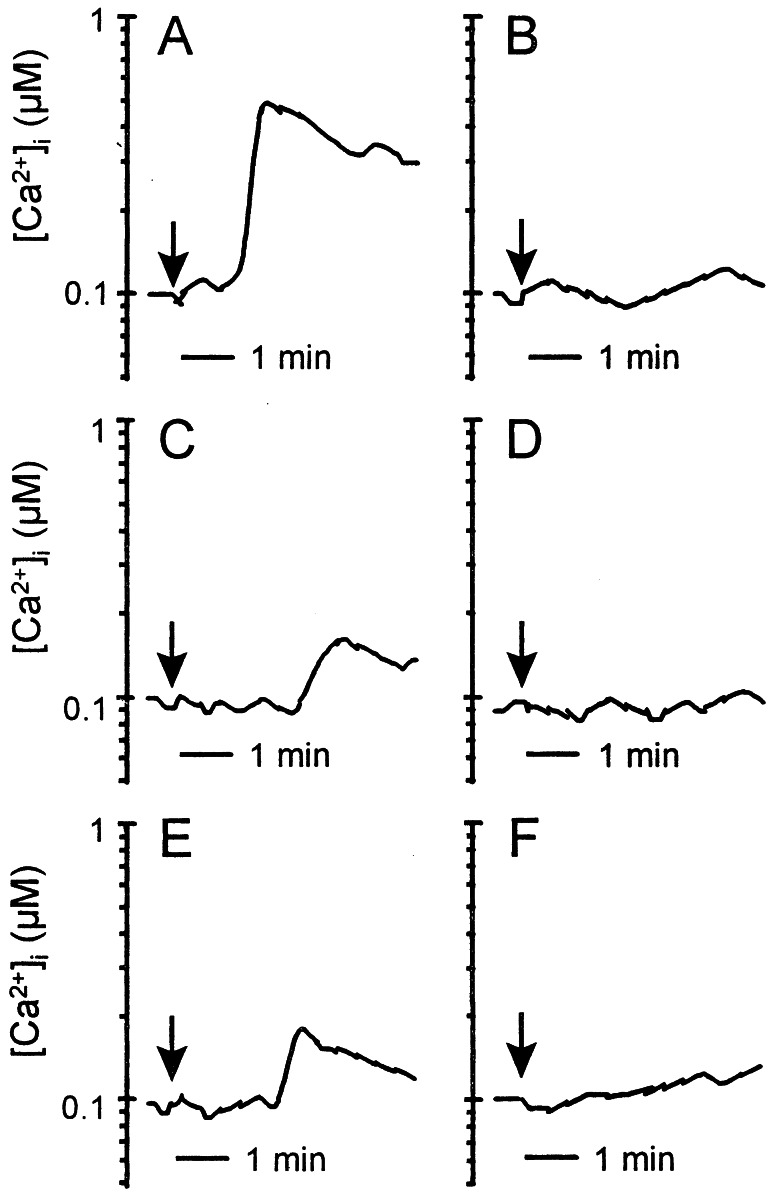
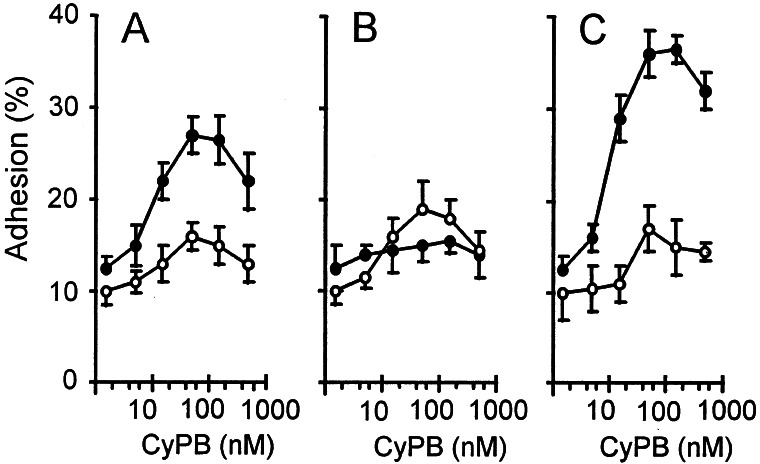
Stimulation of Fluo-3- loaded T cells with CyPB at concentrations varying from 5 to 500 nM induced Ca2+ mobilization in the first minute after the addition of ligand. The optimal response was observed at 50 nM CyPB (Fig. 1A). Stimulation resulted in a rise in cytosolic Ca2+ concentration from basal (95 ± 15 nM) to levels varying from 300 to 850 nM (550 ± 240 nM, n = 6). CyPA also generated intracellular Ca2+ rise but seemed less capable than CyPB (Fig. 1C). The optimal response was obtained with 250 nM the ligand and seemed delayed by comparison with CyPB. Cytosolic Ca2+ concentration was increased from basal to levels varying from 150 to 250 nM (220 ± 60 nM). As shown in Fig. 1B, preincubation with CyPA (250 nM) for 10 min at 37°C markedly affected the responsiveness to the subsequent stimulation with CyPB (50 nM). Similarly, the reverse experiment shows that prestimulation with CyPB renders the cells unresponsive to CyPA (Fig. 1D). These data indicate that CyPA and CyPB reciprocally desensitized the cells toward a subsequent stimulation with the other agonist, indicating that both agonists acted by means of interaction with the same binding site. CyPBKKK− also generated intracellular Ca2+ increase, with optimal response at about 50 nM (Fig. 1E). The elevation in cytosolic Ca2+ concentration seemed delayed and peaked at about 250 nM (235 ± 70 nM), indicating that failure in binding to GAGs rendered the mutant as capable as CyPA for eliciting Ca2+ signal. Stimulation of heparinase-treated cells with CyPB induced a delayed Ca2+ response by comparison with untreated cells, whereas the response triggered by CyPA remained unchanged (data not shown). These results confirmed that GAGs are not required for direct generation of Ca2+ signal but probably account for the higher efficiency of CyPB. Finally, when CyPB was preincubated in the presence of CsA, no Ca2+ response was observed (Fig. 1F), further supporting that the rise in cytosolic free Ca2+ level depended on the binding to type I site.
Figure 1.
Cyclophilin-mediated Ca2+ signaling in T lymphocytes. Ca2+ mobilization in Fluo-3-loaded T cells was measured in the presence of CyPB (50 nM) (A and B), CyPA (250 nM) (C and D), CyPBKKK− (50 nM) (E), or CyPB/CsA (50 nM) (F). The arrows indicate the addition of the agonist. Cells were either directly stimulated with the agonist for analysis (A, C, E, F) or prestimulated with CyPA (B) or CyPB (D) before a second stimulation. Changes in fluorescence, reflecting changes in cytosolic Ca2+ concentration, were monitored by flow cytofluorimetry. Tracings are from a single representative experiment.
Chemotactic Properties of CyPA and CyPB.
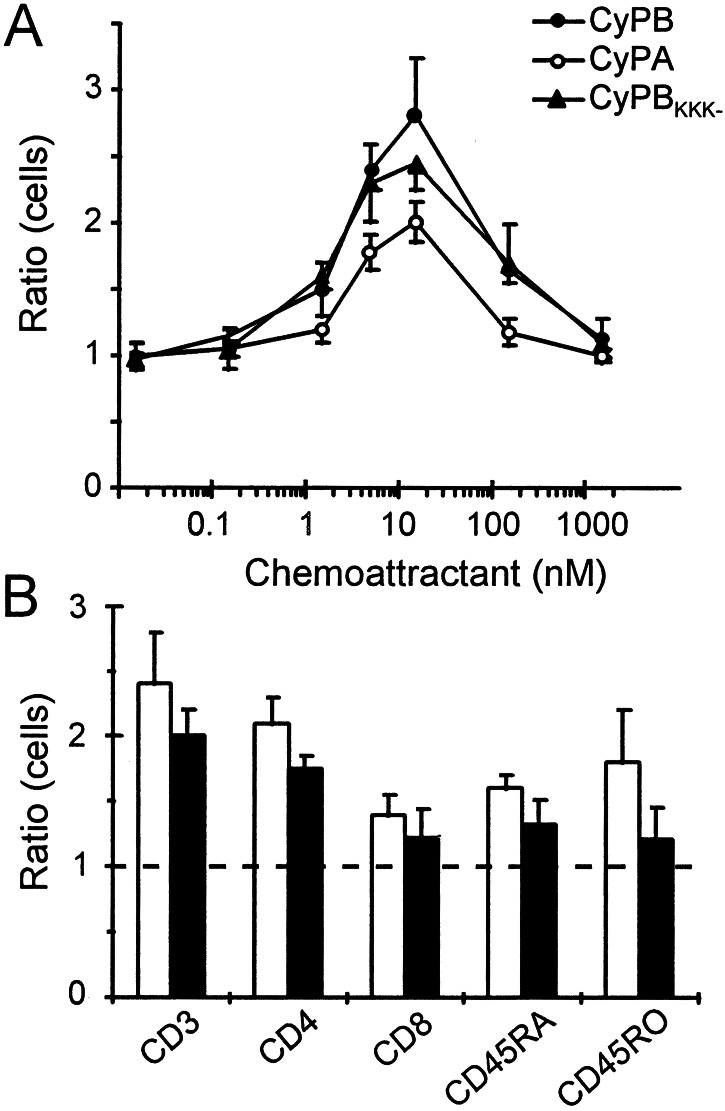
The chemotactic activities of CyPA and CyPB were evaluated in vitro and were compared with the efficacy of CyPBKKK− to attract peripheral blood T lymphocytes from the same donors. The shape of the dose-response curves was similar to the typical bell-shaped curve seen with a variety of chemotactic factors, indicating that human recombinant CyPA and CyPB were capable to attract T lymphocytes dose-dependently (Fig. 2A). When the concentration gradient across the membrane was destroyed by the addition of agonists to the upper and lower compartments, chemotactic activity was not seen, indicating that the effect of cyclophilins was caused by chemotaxis and not just by an increase in chemokinesis. The maximal activity of CyPA, CyPB, and CyPBKKK− was achieved at a concentration of 15 nM in the lower chamber. CyPA was approximately 1.5-fold less capable than CyPB to trigger T cell chemotaxis. At the concentration giving maximal activity, the index of migration was evaluated at 2.8 for CyPB versus 2.4 for CyPBKKK− and 2 for CyPA (n = 3), suggesting that interaction with GAGs was not required to induce chemotaxis. Removal of GAGs from T cell surface by heparinase treatment did not significantly affect the responses triggered by cyclophilins, supporting this hypothesis. Conversely, coincubation with 1 μM CsA completely abolished the chemotactic activity of CyPA and CyPB, indicating that chemotactic activity involved interaction between the central core of cyclophilins and the type I site (data not shown). To know whether cyclophilin-initiated chemotaxis is related to the phenotype of T lymphocytes, we reproduced our experiments with purified CD4+, CD8+, CD45RA+, and CD45RO+ lymphocyte subsets (Fig. 2B). At the concentration of CyPB giving optimal activity, indexes of migration were evaluated at 2.1 for CD4+ T cells versus 1.4 for CD8+ T cells, and at 1.8 for CD45RO+ T cells versus 1.6 for CD45RA+ T cells (n = 4). When the experiments were reproduced with CyPA, the same preferential subset migration was observed, excluding differential expression of cell-surface receptors for CyPA and CyPB among T cell subsets.
Figure 2.
Chemotactic activity of CyPA and CyPB. Results are expressed as the number of cells migrating toward the test sample divided by the number of cells migrating toward control medium. (A) Comparison of chemotactic activities of CyPB (●), CyPA (○), and CyPBKKK− (▴). Data represent means ± SD of triplicate from a representative experiment. (B) Chemotactic preferential responses of T cell subsets to CyPA (filled bars) or CyPB (open bars). Data are means ± SD from three separate experiments.
CyPB-Mediated Adhesion of T Lymphocytes to the ECM.
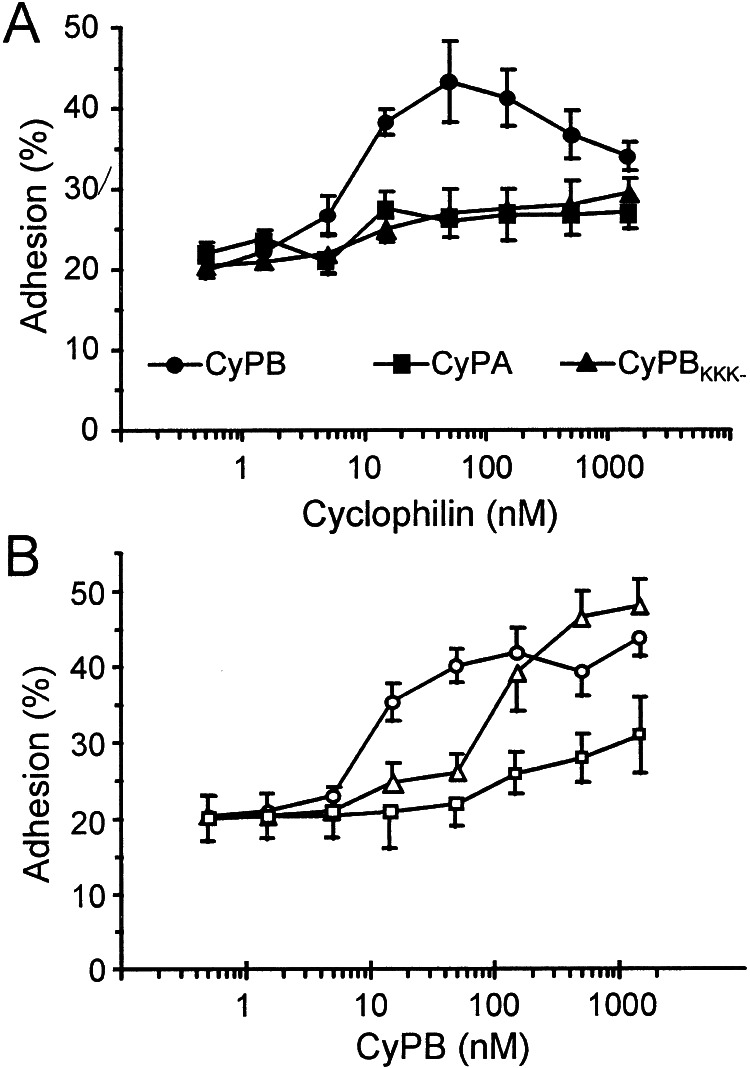
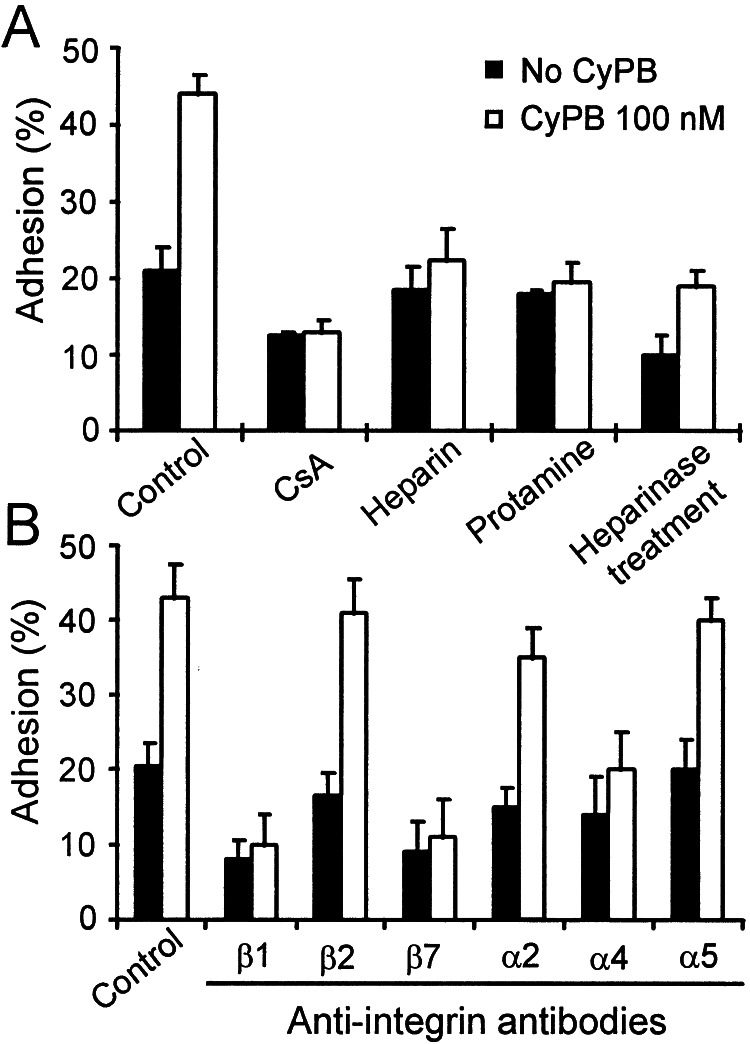
In the absence of exogeneous cyclophilins, the adhesion of T lymphocytes to ECM-coated plates was estimated at 20.5 to 31% of initially added cells (23.4 ± 4.2%). These values were obtained in separate experiments conducted with T lymphocytes from different donors (n = 6) and variations are likely to be caused by individual differences in the cell response. When the experiments were reproduced in the presence of recombinant cyclophilins, a significant increase in T cell adhesion was observed with CyPB. In contrast, no significant variation in cell adhesion was observed in the presence of CyPA or CyPBKKK− (Fig. 3A). CyPB was effective from 10 nM and optimal at approximately 50–100 nM. At this concentration, adherent T lymphocytes ranged from 39.5 to 52% (44.9 ± 4.1%), reflecting an almost 2-fold increase in cell adhesion compared with control. When ECM was pretreated with CyPB and extensively washed before the addition of T lymphocytes, increase in cell adhesion to ECM was observed, indicating that CyPB might act by forming a link between ECM and T cell membrane (Fig. 3B). In the reverse experiments, T lymphocytes were pretreated with CyPB for 20 min, washed to remove unbound ligand, and then added to ECM-coated wells. When the pretreatment was performed at 4°C, CyPB only triggered a poor increase in cell adhesion to ECM. In contrast, preincubation at 37°C restored the enhancing effect of diffusible CyPB, suggesting that the exposure at this temperature led to the generation of regulatory stimuli required for enhancing T cell adhesion (Fig. 3B). To define the involvement of CyPB-binding sites, the experiments were reproduced in the presence of CsA (1 μM), heparin (500 μg/ml), or protamine (12.5 μM) (Fig. 4A). CsA dramatically reduced T cell adhesion, both in the absence or presence of 100 nM CyPB, indicating that binding to the type I site is involved in CyPB-mediated cell adhesion. The addition of heparin or protamine strongly reduced the enhancing effect of CyPB, suggesting that interaction with proteoglycans present in the ECM are required for the enhancing effect of CyPB. To check this possibility, ECM-coated plates were pretreated with heparinase before the addition of T lymphocytes and CyPB. In this case, T cell adhesion was dramatically reduced by comparison with untreated ECM, both in the absence or presence of CyPB. The effect of CyPB was not completely abolished, however, indicating that proteoglycans present on T lymphocytes might also serve in the activity of CyPB.
Figure 3.
Enhancing effect of CyPB on T lymphocyte adhesion to the ECM. (A) Comparison of the effects of CyPB (●), CyPBKKK− (▴), and CyPA (■) on T cell adhesion. T lymphocytes were stimulated in the presence of increasing concentrations of recombinant cyclophilins and allowed to adhere into ECM-coated wells. (B) Effects of anchored or diffusible CyPB on T cell adhesion. In one procedure (Δ), ECM-coated plates were pretreated with CyPB and extensively washed before the addition of T lymphocytes. In the other procedures, T cells were pretreated with CyPB, either at 37°C (○) or at 4°C (□), and then allowed to adhere into ECM-coated wells. Results are expressed as percentages of initially added T lymphocytes (1 × 106 per well) remaining associated to the ECM-coated well. Points represent means ± SD of quadruplicates and are representative from at least three separate experiments.
Figure 4.
Inhibition of CyPB-mediated adhesion of peripheral blood T lymphocytes to the ECM. T lymphocytes were preincubated in the presence of inhibitors for 30 min at 37°C and thereafter used for adhesion assays in the absence (filled bars) or the presence of 100 nM CyPB (open bars). (A) Inhibitory properties of CsA (1 μM), heparin (500 μg/ml), protamine (12.5 μM), and treatment of the ECM by heparinase on CyPB-mediated adhesion. (B) Effect of blocking antibodies to integrin subunits on T lymphocyte adhesion to the ECM. Data are means ± SD of quadruplicates from at least three separate experiments.
The effect of blocking antibodies to integrins was then analyzed to know whether CyPB-mediated activity might be relevant to an alteration in the affinity of integrins for their extracellular ligands (Fig. 4B). In the presence of anti-β2 chain antibody, T cell adhesion to the ECM was not significantly modified, either in the absence or the presence of CyPB. In contrast, the addition of anti-β1 and anti-β7 chain antibodies markedly reduced cell adhesion in the absence of CyPB and completely abolished the increasing effect of CyPB. To approach the nature of the integrins involved in CyPB-mediated adhesion, experiments were reproduced with blocking antibodies to the α chains mainly associated with both β1 and β7 subunits. Antibodies to the α2 and α5 chains had no marked effect on the enhanced adhesion to the ECM, whereas only anti-α4 antibody strongly reduced CyPB-mediated adhesion of T lymphocytes. To confirm the effect of CyPB on an increase in integrin affinity rather than an overexpression of these molecules, cell-surface density of α4, β1, and β7 subunits was analyzed by flow cytofluorimetry. As expected, no significant modification in cell staining could be observed for any of the integrin subunits tested. Moreover, we check that blocking antibodies did not interfere with Ca2+ responses induced by CyPB, confirming that neutralization of CyPB-mediated adhesion is not related to inhibition of the interaction with binding sites and related signaling (data not shown). Altogether, these results indicate that CyPB supports T cell adhesion to the ECM through the up-regulation of α4β1 and α4β7 integrin activity.
Involvement of Cell-Surface Proteoglycans and Cosignaling Molecules in CyPB-Mediated Adhesion.
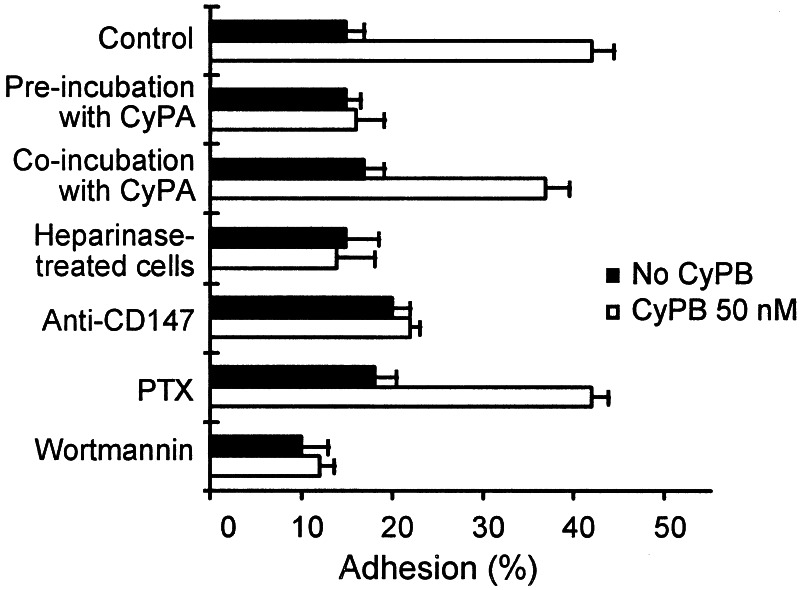
We attempted to identify some of the molecules involved in the selective proadhesive activity of CyPB. To avoid the appearance of additional events mediated by ECM components, adhesion assays were performed by incubating cells on immobilized fibronectin. At the optimal concentration of 50 nM, approximately 40% of initially added cells were retained on fibronectin in the presence of CyPB (42 ± 3, n = 6), reflecting an almost 3-fold increase by comparison with control. When T cells were pretreated with CyPA for 20 min at 37°C and thereafter allowed to adhere to fibronectin, the enhancing activity of CyPB was strongly reduced (Fig. 5). Because preincubation of T cells with CyPA led to the desensitization of signaling receptor, our data indicated that the same binding site is required for CyPB to initiate Ca2+ flux and enhanced T cell adhesion. In contrast, coincubation with CyPA did not significantly modify the effect of CyPB on cell adhesion (Fig. 5). This difference could be related to the inability of CyPA to compete with CyPB for the same binding sites, leading to an absence of desensitization. To test further the hypothesis whether binding to the cyclophilin receptor is not capable enough to mediate enhanced cell adhesion, T cells were treated with heparinase and thereafter stimulated by CyPB. Heparinase treatment of T lymphocytes led to a marked reduction in CyPB-mediated adhesion (Fig. 5), indicating that cell surface proteoglycans serve in the selective effect of CyPB. We then tested whether CD147 was related to CyPB activity. The addition of anti-CD147 antibody to lymphocyte mixture led to a strong inhibition of CyPB-mediated adhesion to fibronectin, indicating that CD147 is required for the enhancing effect of CyPB on T cell adhesion (Fig. 5). The same antibody did not inhibit the binding of radio-iodinated CyPB. These data did not mean that CD147 and CyPB receptor are distinct, because anti-CD147 and CyPB could interact with separate regions of the same molecule. We have demonstrated that CyPB binding initiated the endocytosis of type I binding site (26). We took advantage of these findings to test whether CyPB induced internalization of CD147. No modification in cell-surface binding of anti-CD147 could be observed in the presence of CyPB (data not shown), indicating that CD147 probably serves as a costimulatory molecule rather than a binding site for CyPB. To approach the signaling pathway by which CyPB triggers cell-substrate adhesion, T cells were preincubated with PTX (2 μg/ml), which causes ADP-ribosylation of Giα subunit and blocks dependent signal transduction, or wortmannin (50 nM) which selectively blocks phosphatidylinositol 3-kinase activity when used at low concentrations. PTX did not modify the activity of CyPB, whereas wortmannin strongly reduced CyPB-mediated adhesion, indicating that phosphatidylinositol kinases are required for CyPB to enhance lymphocyte adhesion.
Figure 5.
Involvement of cell-surface proteoglycans and cosignaling molecules in CyPB-mediated adhesion of T lymphocytes to fibronectin. Peripheral blood T cells from the same donors were either treated with heparinase or incubated with CyPA (500 nM), monoclonal antibody to CD147, PTX (2 μg/ml), or wortmannin (50 nM). T lymphocytes were then allowed to adhere into fibronectin-coated wells in the absence (filled bars) or presence of 50 nM CyPB (open bars). Data are means ± SD of quadruplicates from at least three separate experiments.
Selective Activity of CyPB on T Cell Subset Adhesion.
The phenotype of adherent T cells was analyzed by immunostaining with anti-CD4 and anti-CD8 antibodies (Fig. 6A). In the presence of CyPB, cell adhesion was increased in both CD4+ and CD8+ subsets, with an optimal effect observed at about 50 nM. At this concentration, 27% of the initially added T cells beared the CD4 marker, indicating an almost 2.2-fold increase in cell adhesion. In contrast, adhesion of CD8+ T cells was only increased by 1.6, indicating that CyPB was more active in promoting adhesion of CD4+ lymphocytes to fibronectin. To discriminate between native and memory T lymphocyte subsets, T cells were then separated within CD45RA+ and CD45RO+ subpopulations, and the phenotype of adherent cells was analyzed with anti-CD4 and anti-CD8 antibodies (Fig. 6 B and C). CyPB enhanced cell adhesion similarly in both naive and memory CD8+ subsets. Indeed, 19% of the initially added CD45RA+cells expressed the CD8 marker versus 17% for CD45RO+ cells. A significant increase was observed for CD4+CD45RO+ cells. When incubated with CyPB, 36.5% of adhered CD45RO+ T cells expressed the CD4 marker, reflecting a 3-fold increase in cell adhesion for this subpopulation. In contrast, no variation could be observed after staining of adherent CD45RA+ cells with anti-CD4, indicating that native CD4+ cells are not responsive to CyPB. Taken together, these results clearly demonstrated that the enhancing effect of CyPB on T cell adhesion is related to the phenotype of T cells.
Figure 6.
Phenotype of responsive T cells to CyPB. T lymphocytes were stimulated with increasing concentrations of CyPB and added to fibronectin-coated wells. Adherent cells were fixed and immunostained with either anti-CD4 (●) or anti-CD8 (○) monoclonal antibodies. Discrimination between CD4+ and CD8+ adherent T cells was performed in the whole T cell population (A) or after separation of T lymphocytes within CD45RA+ native (B) and CD45RO+ memory (C) T cell subsets. Data represent means ± SD of quadruplicates and are representative from at least three separate experiments.
Discussion
During inflammation, the recruitment of T cells is regulated by a cascade of molecular events resulting in adhesion to the endothelium followed by migration into tissue (36). Numerous promigratory factors, e.g., chemokines, have been identified as attractants of peripheral blood leukocytes (28–30). A common feature between chemokines and CyPB is their ability to interact with receptor and proteoglycans through two distinct regions of the proteins (26, 27, 34). This model has suggested that anchorage to proteoglycans might modulate the activity of chemokines (34–37). In this context, we investigated whether interactions between secreted cyclophilins and cell-surface-binding sites participate in the recruitment of peripheral blood T lymphocytes.
We confirmed that CyPA elicits Ca2+ signals in T cells and showed that CyPB shares the same activity with a higher efficiency. Cross-desensitization experiments allowed us to demonstrate that both CyPA and CyPB act by way of interaction with the same signaling receptor. The mutant CyPBKKK−, which was deprived of its ability to interact with GAGs, remained at least as capable as CyPA in inducing Ca2+ signal and chemotaxis. These data support our findings that lymphocyte responses triggered by CyPA and CyPB are actually related to interaction with the same signaling receptor, identified as type I site (26, 27). We then demonstrated that CyPB shares another crucial feature with chemokines—i.e., enhancement of T cell adhesion through activation of integrins. Neither CyPA nor CyPBKKK− possess this activity. We reported that CyPBKKK− exhibited binding affinity similar to CyPB for type I sites (27), ruling out the possibility that unique activity of CyPB was related to higher affinity for signaling receptor. CyPA remained able to desensitize signaling receptor and neutralize CyPB-mediated adhesion, indicating that signaling events mediated by type I receptor were required but not sufficient to promote cell adhesion. Conversely, proteoglycans present in the ECM or on T cells apparently were crucial for CyPB-mediated T cell adhesion, pointing out a complementary mechanism dependent on the interaction with GAGs. A recent study, however, reported a role for GAGs in HIV-1 attachment through binding to virus-associated CyPA (38), indicating that they may also act as binding sites for CyPA. A possible explanation for this discrepancy may be related to the marked differences in binding affinities for GAGs and/or to the positioning of the heparin-binding protein for presentation to signaling receptor (26, 27, 38). On the basis of our earlier studies showing that only CyPB was a high-affinity ligand for GAGs, interaction between CyPB and proteoglycans is most likely to take place in a physiological environment. Moreover, the heparin-binding domain of CyPB, which does not exist in CyPA and was removed in CyPBKKK−, might be determinant for GAGs to present efficiently the ligand to type I receptor. GAG–CyPB interaction might induce structural changes and/or stabilize the subsequent interaction with signaling receptor, leading to sustained intracellular signals. Such a mechanism has been proposed for explaining the participation of proteoglycans in the activity of growth factors such as hepatocyte growth factor (HGF) (39), which is also consistent with our findings that, even though interaction with GAGs did not seem to be required to induce Ca2+ signaling, they probably account for the highest response elicited by CyPB. Another possible explanation is that proteoglycans recognized by CyPB on T cells—i.e., type II sites, are coupled to signaling molecules that link with type I receptor. Therefore, CyPB–proteoglycan interaction might induce complementary signaling events that lead, in turn, to an enhancement in cell response. This notion is supported by the findings that mutations that reduced CyPB binding to GAGs or to the functional receptor strongly affected T cell adhesion to fibronectin (M.C., unpublished results). All these mechanisms are likely to function simultaneously and to account for higher stimulation responsible for the selective activity of CyPB on T cell adhesion. Recently, CD147 was described as a putative cell-surface receptor for CyPA (21). This glycoprotein is involved in various regulatory mechanisms, including T cell activation (40). The data presented in this report strongly suggest that CD147 is not directly involved in the binding of CyPB but presumably acts as a costimulatory molecule in cyclophilin-mediated signaling events. Together with the recent findings that antibodies to CD147 also neutralize cell aggregation mediated by CD98 (41), it may be speculated that CD147 plays a central role in cellular events associated with cytoskeleton rearrangement. CD147 and proteoglycans have been reported to facilitate HIV-1 cell entry, presumably through interactions with virus-associated CyPA (21, 38). The ability of HIV-1 to interact with chemokine receptors, such as CCR5 and CXCR4, is also well established (28, 29). Interactions of HIV-1 with cell-surface molecules that mediate the activity of chemoattractants could divert their original functions, e.g., cytoskeleton rearrangements, and promote facilitation of the virus entry. Further investigations on the cooperation between these molecules might provide new insights on the mechanisms controlling HIV-1 entry within target cells and lead to the development of therapeutic tools to block the virus infection. Even though CyPB exhibits similar activity as chemokine, early intracellular signals are distinct but probably lead downstream to a common pathway relevant to the up-regulation of integrin-mediated adhesion. Most of the promigratory factors are produced under pathological conditions, whereas others seem to fulfill housekeeping functions (28–30). The ability of promigratory factors to regulate the recruitment of some subsets of T cells preferentially seemed to be a crucial step that likely contributes to the distinct patterns of migration of the lymphocytes subsets in vivo (35, 37, 42). We recently characterized CyPB in the ECM where it was associated with proteoglycans. In addition, we showed that CyPB was more capable than RANTES to promote T cell adhesion to the ECM (F.A., unpublished results). However, the concentration of endogenous CyPB (less than 5 nM) did not seem sufficient to trigger lymphocyte responses, ruling out that it may be constitutively secreted to serve as a guidance for T cells infiltrating lymphoid organs. Conversely, inflammatory stimuli led to marked increase in the expression and secretion of CyPB (13, 18). The protein might diffuse from the inflamed site and thereby be presented to infiltrating T lymphocytes when immobilized to proteoglycans. The findings that CyPB enhances T cell adhesion mainly of the CD4+/CD45RO+ subpopulation further supports the hypothesis that it contributes to the regulation of specific T cell subset infiltration into inflammatory sites.
In conclusion, we demonstrated here that CyPB exhibits the main biological properties of the members of the chemokine family, e.g., chemotaxis and enhancement of integrin-mediated adhesion. Although it is structurally distinct from other proinflammatory factors, these data suggest that CyPB may participate in the recruitment of peripheral blood T lymphocytes into tissue. The findings presented here further broaden the concept that numerous GAG-binding factors, which may not be structurally related, regulate leukocyte recruitment and functions through synergistic interaction with sulfated GAGs and specific signaling receptors.
Acknowledgments
We are grateful to the Etablissement de Transfusion Sanguine (Lille, France) for providing us with human blood samples, to Dr. B. Haendler for helpful discussion, and to Dr. M. Kibe for critical reading of the manuscript. This investigation was supported in part by the Centre National de la Recherche Scientifique (UMR 8576) and by the Université des Sciences et Technologies de Lille, France.
Abbreviations
- CsA
cyclosporin A
- CyPA
cyclophilin A
- CyPB
cyclophilin B
- DPBS
Dulbecco's phosphate-buffered saline
- ECM
extracellular matrix
- GAGs
glycosaminoglycans
- HGF
hepatocyte growth factor
- PTX
pertussis toxin
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Handschumacher R E, Harding M W, Rice J, Drug R J, Speicher D W. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber S L. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 3.Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid F X. Nature (London) 1989;337:476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- 4.Takahashi N, Hayano T, Suzuki M. Nature (London) 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 5.Liu J, Farmer J D, Lane W S, Friedmann J, Weissman I, Schreiber S L. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber S L, Crabtree G R. Immunol Today. 1992;13:136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- 7.Haendler B, Hofer-Warbinek R, Hofer E. EMBO J. 1987;6:947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galat A. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- 9.Price E R, Zydowsky L D, Jin M, Baker C H, McKeon F D, Walsh C T. Proc Natl Acad SciUSA. 1991;88:1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spik G, Haendler B, Delmas O, Mariller C, Chamoux M, Maes P, Tartar A, Montreuil J, Stedman K, Kocher H, et al. J Biol Chem. 1991;266:10735–10738. [PubMed] [Google Scholar]
- 11.Arber S, Krause K H, Caroni P. J Cell Biol. 1992;116:113–125. doi: 10.1083/jcb.116.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allain F, Boutillon C, Mariller C, Spik G. J Immunol Methods. 1995;178:113–120. doi: 10.1016/0022-1759(94)00249-v. [DOI] [PubMed] [Google Scholar]
- 13.Tegeder I, Schumacher A, John S, Geiger H, Geisslinger G, Bang H, Brune K. J Clin Immunol. 1997;17:380–386. doi: 10.1023/a:1027364207544. [DOI] [PubMed] [Google Scholar]
- 14.Endrich M M, Gehring H. Eur J Biochem. 1998;252:441–446. doi: 10.1046/j.1432-1327.1998.2520441.x. [DOI] [PubMed] [Google Scholar]
- 15.Jin Z G, Melaragno M G, Liao D F, Yan C, Haendeler J, Suh Y A, Lambeth J D, Berk B C. Circ Res. 2000;87:789–796. doi: 10.1161/01.res.87.9.789. [DOI] [PubMed] [Google Scholar]
- 16.Sherry B, Yarlett N, Strupp A, Cerami A. Proc Natl Acad Sci USA. 1992;89:3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, Leiva M C, Fischkoff S A, Handschumacher R E, Lyttle C R. J Biol Chem. 1992;267:11968–11971. [PubMed] [Google Scholar]
- 18.Gonzalez-Cuadrado S, Bustos C, Ruiz-Ortega M, Ortiz A, Guijarro C, Plaza J J, Egido J. Clin Exp Immunol. 1996;106:518–522. doi: 10.1046/j.1365-2249.1996.d01-864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allain F, Durieux S, Denys A, Carpentier M, Spik G. Blood. 1999;94:976–983. [PubMed] [Google Scholar]
- 20.Sherry B, Zybarth G, Alfano M, Dubrovsky L, Mitchell R, Rich D, Ulrich P, Bucala R, Cerami A, Bukrinsky M. Proc Natl Acad Sci USA. 1998;95:1758–1763. doi: 10.1073/pnas.95.4.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pushkarsky T, Zybarth G, Dubrovsky L, Yurchenko V, Tang H, Guo H, Toole B, Sherry B, Bukrinsky M. Proc Natl Acad Sci USA. 2001;98:6360–6365. doi: 10.1073/pnas.111583198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allain F, Denys A, Spik G. J Biol Chem. 1994;269:16537–16540. [PubMed] [Google Scholar]
- 23.Allain F, Denys A, Spik G. Biochem J. 1996;317:565–570. doi: 10.1042/bj3170565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denys A, Allain F, Foxwell B, Spik G. Immunology. 1997;91:609–617. doi: 10.1046/j.1365-2567.1997.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carpentier M, Descamps L, Allain F, Denys A, Durieux S, Fenart L, Kieda C, Cecchelli R, Spik G. J Neurochem. 1999;73:260–270. doi: 10.1046/j.1471-4159.1999.0730260.x. [DOI] [PubMed] [Google Scholar]
- 26.Denys A, Allain F, Carpentier M, Spik G. Biochem J. 1998;336:689–697. doi: 10.1042/bj3360689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carpentier M, Allain F, Haendler B, Denys A, Mariller C, Benaïssa M, Spik G. J Biol Chem. 1999;274:10990–10998. doi: 10.1074/jbc.274.16.10990. [DOI] [PubMed] [Google Scholar]
- 28.Rollins B J. Blood. 1997;90:909–928. [PubMed] [Google Scholar]
- 29.Baggiolini M. Nature (London) 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 30.Moser B, Loetscher M, Piali L, Loetscher P. Int Rev Immunol. 1998;16:323–344. doi: 10.3109/08830189809043000. [DOI] [PubMed] [Google Scholar]
- 31.Minta A, Kao J P Y, Tsien R Y. J Biol Chem. 1989;264:8171–8178. [PubMed] [Google Scholar]
- 32.Taub D D, Key M L, Clark D, Turcovski-Corrales S M. J Immunol Methods. 1995;184:187–198. doi: 10.1016/0022-1759(95)00087-q. [DOI] [PubMed] [Google Scholar]
- 33.Bar-Shavit R, Sabbah V, Lampugnani M G, Marchisio P C, Fenton J W, Vlodavsky I, Dejana E. J Cell Biol. 1991;112:335–344. doi: 10.1083/jcb.112.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanaka Y, Adams D H, Shaw S. Immunol Today. 1993;14:111–115. doi: 10.1016/0167-5699(93)90209-4. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Adams D H, Hubscher S, Hirano H, Siebenlist U, Shaw S. Nature (London) 1993;361:79–82. doi: 10.1038/361079a0. [DOI] [PubMed] [Google Scholar]
- 36.Campbell J J, Hedrick J, Zlotnik A, Siani M A, Thompson D A, Butcher E C. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 37.Gilat D, Hershkoviz R, Mekori Y A, Vlodavsky I, Lider O. J Immunol. 1994;153:4899–4906. [PubMed] [Google Scholar]
- 38.Saphire A C, Bobardt M D, Gallay P A. EMBO J. 1999;18:6771–6785. doi: 10.1093/emboj/18.23.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sergeant N, Lyon M, Rudland P S, Fernig D G, Delehedde M. J Biol Chem. 2000;275:17094–17099. doi: 10.1074/jbc.M000237200. [DOI] [PubMed] [Google Scholar]
- 40.Koch C, Staffler G, Huttinger R, Hilgert I, Prager E, Cerny J, Steinlein P, Majdic O, Horejsi V, Stockinger H. Int Immunol. 1999;11:777–786. doi: 10.1093/intimm/11.5.777. [DOI] [PubMed] [Google Scholar]
- 41.Cho J Y, Fox D A, Horejsi V, Sagawa K, Skubitz K M, Katz D R, Chain B. Blood. 2001;98:374–382. doi: 10.1182/blood.v98.2.374. [DOI] [PubMed] [Google Scholar]
- 42.Adams D H, Harvath L, Bottaro D P, Interrante R, Catalano G, Tanaka Y, Strain A, Hubscher S G, Shaw S. Proc Natl Acad Sci USA. 1994;91:7144–7148. doi: 10.1073/pnas.91.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]