Abstract
Recently, several chromatin remodeling complexes in yeast, Drosophila, and mammals have been shown to contain actin and actin-related proteins (arps). However, the function of actin in these complexes is unclear. Here, we show that the mammalian SWI/SNF-like BAF complex binds to phosphatidylinositol 4,5-bisphosphate (PIP2) micelles and PIP2-containing mixed lipid vesicles, and that PIP2 binding allows the complex to associate with actin pointed ends and branch points. Actin binds to at least two distinct domains in the C terminus of the Brg1 protein, and interaction with only one of these domains is sensitive to PIP2. Based on these findings, we propose a model for PIP2 activation of actin binding by relief of intramolecular capping of actin by Brg1.
Much of the DNA in eukaryotic cells is packaged into chromatin structures that are significant barriers to transcription (see ref. 1 for review). Evidence indicates that at least two types of activities counteract these repressive chromatin structures. Histone acetyl transferases are frequently recruited to promoters by DNA-binding transcription factors and control acetylation of lysines in histone N termini. A second class of chromatin-remodeling activities consists of ATP-dependent multiprotein complexes related to the yeast Swi/Snf complex. These complexes have a Swi2-like ATPase as a core subunit, which is associated with up to 11 other subunits (see ref. 2 for review). The canonical yeast Swi/Snf complex contains 11 subunits that cofractionate in a 2-megadalton complex. Recent inroads have been made in the understanding of these complexes, but significant questions remain concerning how these complexes are targeted to specific promoters in vivo, what role they play in transcriptional activation, and how they function mechanistically.
Several proteins have been discovered in the Swi2 helicase superfamily in mammals, including Brg1 and hBrm, which all share homology with Swi2 in the putative helicase region but not through the rest of the protein. Brg1 and hBrm are mutually exclusive subunits of large (2 megadalton) complexes with 10 other proteins, collectively referred to as the BAF complex (Brg- or Brm-associated factors). The BAF complex, like the related SWI/SNF (yeast), RSC (yeast), and BAP (Drosophila) complexes, can remodel mononucleosomes in vitro, and at least the RSC complex can also transfer a histone octamer from one template to another (3–5). However, it is not known to what degree these in vitro assays accurately reflect the in vivo activities of the complexes. Interestingly, it has been observed that the Swi2-like Brg1 protein alone has in vitro nucleosome disruption activity (6), which is augmented by the Swi3-like BAF155 and BAF170 subunits and the human Snf5 homolog. However, mutation of any of the swi/snf genes in yeast leads to the swi/snf phenotype. These results demonstrate that there are roles for the non-core subunits of these complexes in vivo beyond those required for simple mononucleosome disruption. Interestingly, the core subunits are all homologs of yeast swi/snf genes (Swi2, Swi3, Snf5). However, many of the non-core subunits in the BAF complex are unique, in that their orthologs are not found in the yeast complex. These subunits include BAF400, BAF250, BAF110, BAF57, and β actin (unpublished results and W. Wang, personal communication). The role of the unique subunits, whether it be targeting, signaling, resolution of higher order chromatin structure, or some other function, remains unclear.
Surprisingly, subunit purification and sequencing revealed actin to be an integral component of the mammalian BAF and PBAF complexes (7, 8). Actin is tightly bound to the BAF complex at a 1:1 stoichiometry and can only be removed by denaturing conditions. Within the complex, actin directly interacts with the Brg1 protein. Recently, peptides from the 47-kDa component of the Drosophila BAP complex were sequenced and found to be either gamma or beta actin (9). In addition, all of the large swi/snf-like complexes examined thus far (Swi/Snf, PBAF, RSC, BAP, and BAF) contain at least one actin-related protein (arp). In the mammalian BAF complex, BAF53 is 38% identical to actin and is most closely related to Arp3 (7). The yeast Swi/Snf complex contains Arp7 and Arp9, mutants in either of which exhibit the swi/snf phenotype (10), demonstrating the requirement for these subunits for the in vivo function of Swi/Snf. Recently, the chromatin remodeling Ino80 complex (whose ATPase is the Snf2-like Ino80), as well as the histone acetylase complexes NuA4 (which contains the histone acetyltransferase Esa1) and TIP60, have been shown to contain actin-related proteins as well as actin itself (11–13). The ubiquity of actin and arps in chromatin remodeling complexes led us to investigate what the roles of these subunits might be in the process of chromatin remodeling.
Materials and Methods
Purification of SW13 and HeLa Complexes.
Nuclear extract (20 mg) from HeLa or SW13 cells was immunoprecipitated as previously described (14) by using 50 μl of polyclonal antisera to BAF57 and 300 μl of protein A beads. Beads were washed as described, then bound proteins were eluted with 450 μl of ice-cold 0.1 M glycine, pH 2.5/0.5 M NaCl/0.1 mg/ml BSA. The eluate was immediately neutralized with 45 μl of 0.1 M NaHCO3, then dialyzed against 50 mM Hepes, pH 7.8/50 mM KCl/10% glycerol/1 mM MgCl2/0.1 mM CaCl2/1 mM DTT/1 mM ATP. BAF complex obtained this way behaved identically in all assays tested to BAF complex obtained by using nondenaturing purification from a Jurkat cell line stably expressing hemagglutinin-tagged BAF57 (15). This complex was preferable to the hemagglutinin-tagged complex given that it could be purified in larger quantities and the SW13 complex could be made in parallel as a control. For radioactive phosphatidylinositol 4,5-bisphosphate (PIP2) immunoprecipitations, 600 μl of buffer, SW13 nuclear extract (6 mg), or HeLa nuclear extract (6 mg) were immunoprecipitated with 15 μl of antibody onto 90 μl of protein A beads. Beads were washed as above, then washed once with 25°C binding buffer (20 mM Hepes, pH 7.8/150 mM KCl/1 mM MgCl2/0.1 mM CaCl2/1 mM DTT/1 mM ATP). Beads were incubated for 15 min at 25°C in 200 μl of binding buffer containing 10 μM sonicated vesicles of unlabeled PIP2 doped with ≈30,000 dpm total 3H-labeled PIP2 (NEN). Beads were spun down for 30 s in a tabletop microcentrifuge and washed with ice-cold binding buffer; all bound proteins were eluted with two washes of 200 μl of acid glycine. Glycine eluates were counted in a scintillation counter.
Lipids.
Lipids were purchased either from Sigma or Avanti Polar Lipids. Phosphatidylinositol 4-monophosphate (PIP) and PIP2 micelles were made by suspending the lipid in distilled water at a concentration of 1 mM, followed by several minutes of sonication at maximum power. Lipid vesicles were formed by mixing chloroform solutions of phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, PIP or PIP2, and drying under nitrogen followed by rehydration in 5 mM Hepes, pH 7.4/0.1 mM EDTA/211 mM sucrose for 4 h at 37°C. Vesicles for light scattering were extruded through a 100-nm nylon filter by using a Liposofast extruder, whereas those to be used for actin polymerization studies were sonicated, and those to be used for electron microscopy were neither sonicated nor extruded.
Light Scattering.
Two hundred microliters of HeLa or SW13 complex (both ≈8–10 nM as assessed by silver-stained gels) was centrifuged at 14,000 × g for 2 min at room temperature before being placed in a laser-scattering tube. Scattering of 633-nm light emitted from a 5-mW Siemens HeNe laser was measured at 90 degrees by using a Brookhaven BI-2030AT digital correlator with a BI-12RC goniometer. Light scattering was measured over 300 s with 10-ms delay times. Static light scattering was taken to be the total intensity of light scattered, whereas the effective diameter of particles was calculated from the autocorrelation function of fluctuations in light intensity.
Pyrenyl Actin Polymerization.
Five percent pyrene–iodoacetamide-labeled human actin was prepared by standard methods (16). G actin obtained after 3 days of dialysis against G buffer (2 mM Tris, pH 8.0/0.2 mM CaCl2/0.2 mM ATP/0.2 mM β-mercaptoethanol) at 4°C was centrifuged at 100,000 × g for 2 h. This actin was added at a final concentration of 3 μM to a 125-μl reaction containing G buffer, 50 μl of HeLa or SW13 complex, any indicated lipids, and 12.5 μl of 10× polymerization buffer consisting of 1 M KCl/100 mM Tris, pH 8.0/20 mM MgCl2/10 mM ATP. Polymerization was followed at 30°C on an Aminco-Bowman Series 2 luminescence spectrophotometer by using an excitation wavelength of 365 nm and an emission wavelength of 407 nm, with data points taken every 4 s.
Electron Microscopy.
Six-microliter reactions containing 3 μl of BAF complex, 100 μM lipid (either vesicles or sonicated micelles), and 3 μM actin were incubated at room temperature for 2 h. The reaction mixture was spotted onto 200 mesh carbon electron microscope grids and negatively stained with 2% uranyl acetate. Grids were examined by using a transmission electron microscope.
Peptide Synthesis.
Brg peptide 1402–1418 (EEVRQKKSSRKRKRDS) was synthesized on an Applied Biosystems 431A peptide synthesizer and N-terminally acetylated before deprotection and purification. The peptide was >95% pure as determined by HPLC and mass spectrometry.
Actin Binding.
Glutathione S-transferase fusion proteins were expressed in bacteria and purified on glutathione beads. Beads were incubated with 10 μM purified G actin in G buffer for 60 min at 4°C, then washed with binding buffer (20 mM Tris, pH 7.4/100 mM KCl/0.2 mM CaCl2). Actin-bound beads were split into two pools, incubated in binding buffer with or without 100 μM PIP2 micelles. Beads were incubated for 30 min at 4°C, then spun down and incubated for a further 30 min at 25°C in their respective buffers. Beads were then washed four times for 10 min on ice with 20 mM Tris, pH 7.4/300 mM NaCl/0.1% Triton X-100 at 4°C before addition of SDS/PAGE loading buffer.
Results
The BAF Complex Binds PIP2 Vesicles and Micelles.
The experiments that follow make use of two forms of the BAF complex, a complete complex from the HeLa cell line and an incomplete one from the SW13 cell line, a human pheochromocytoma that fails to express the Brg1 and hBrm ATPase subunits (17). The SW13 cell line was of particular use for determining the role of actin and the actin-related BAF53 protein in the BAF complex because Brg1 is the subunit of the BAF complex that directly interacts with actin and BAF53. Thus, although SW13 cells express these subunits as well as the other eight subunits of the BAF complex, they assemble a subcomplex lacking actin and BAF53 as well as Brg1 but containing the other BAF complex subunits (7, 18). As both the HeLa complex and the SW13 complex contain the HMG-like BAF57 subunit (15), each complex could be isolated by immunoprecipitation with antibody to BAF57.
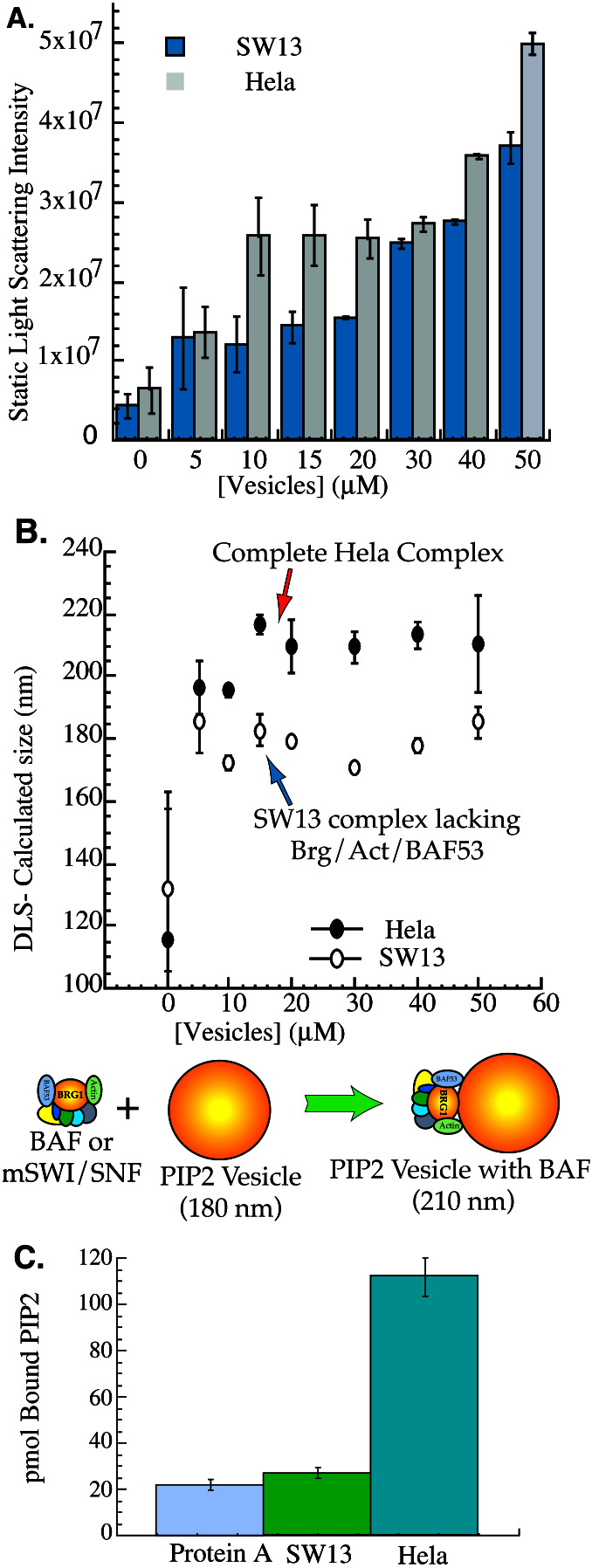
We have previously shown that the lipid PIP2 can cause increased association of the BAF complex with the nuclear matrix in vitro (7). These results raised the question of whether PIP2 acts by binding to a protein in the nuclear matrix or to the BAF complex itself. Given the large size of the BAF complex (≈50-nm diameter, see Fig. 3), it was possible to test whether BAF binds PIP2 by examining laser scattering (19) by 175-nm PIP2-containing vesicles in dilute solution with and without BAF complex. The extent of laser scattering was found to be greater by vesicles added to BAF complex isolated from HeLa cells than by those added to SW13-derived complex (Fig. 1 A and B). To estimate the size increase of the vesicles upon complex binding, dynamic light scattering was measured, and the effective diameter of an average spherical scattering particle was calculated. The effective diameter of PIP2-containing vesicles increased from ≈180 nm in the presence of SW13 complex to ≈210 nm when bound to the HeLa complex. The calculated 30-nm size increase of the vesicles is consistent with formation of an oblong composite particle by binding of a 180-nm vesicle to a 50-nm sphere, rather than nonspecific aggregation of multiple vesicles.
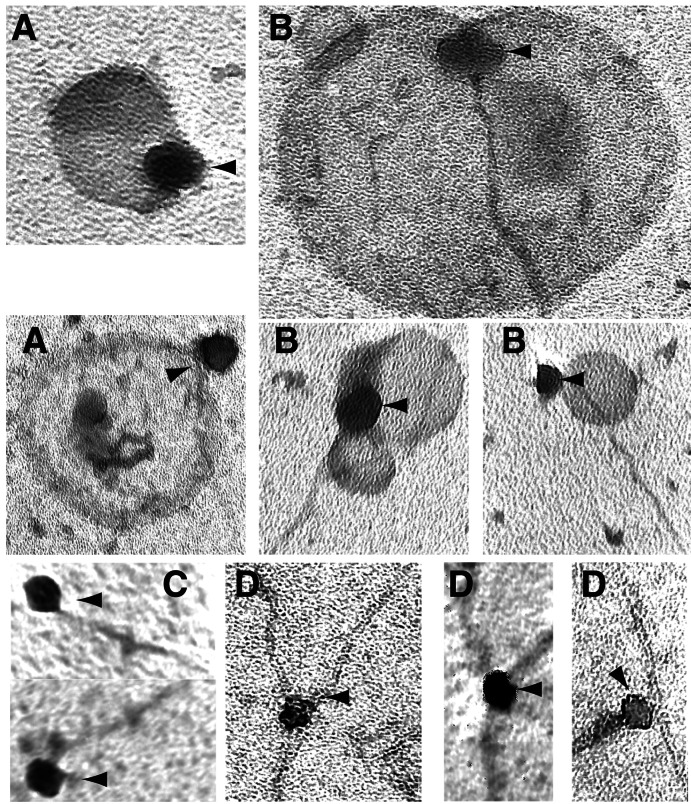
Figure 3.
Electron microscopy of the BAF complex bound to actin and lipid vesicles. (A) BAF complex binds PIP2-containing vesicles. BAF complex was added to 100 μM vesicles similar to those used in Fig. 1 but that had not been extruded to any particular size. This mixture was incubated at room temperature for 1 h, then stained with 2% uranyl acetate and examined by electron microscopy. Arrowheads indicate BAF complex. Scale bar is 100 nm. (B) BAF complex bound to PIP2-containing vesicles also binds actin filament ends. BAF complex was incubated in polymerization buffer with PIP2-containing vesicles and 3 μM actin for 2 h, then stained and examined as above. (C) BAF complex bound to PIP2 micelles binds filament ends. As in B, except sonicated PIP2 micelles were used in place of vesicles. (D) BAF complex bound to PIP2 micelles also binds branch points.
Figure 1.
The SWI/SNF-like BAF complex directly binds PIP2. (A) Static light scattering by the HeLa and SW13 complexes. HeLa or SW13 complexes were incubated alone or with the indicated concentration of 175-nm vesicles com- posed of 10% PIP2/30% phosphatidylcholine/40% phosphatidylethanolamine/20% phosphatidylserine, and light scattering was measured. (B) Dynamic light scattering by the HeLa and SW13 complexes. As in A, except effective diameter was calculated for an ideal sphere from the time autocorrelation function of fluctuations in light-scattering intensity. (C) PIP2 binding by the BAF complex requires Brg, actin, and BAF53. SW13 (complex lacking Brg, actin, and BAF53) and HeLa (complete complex) complexes were purified and incubated with radioactive PIP2 micelles. Approximately 1 pmol of SW13 or HeLa complex was bound in total to the protein A beads as assessed by silver staining of a small aliquot of beads. Tritium counts bound to the beads after washing were quantitated, and the total number of picomoles of PIP2 bound was calculated.
A second approach to study PIP2 binding by the BAF complex involving immunoprecipitation was also used. Both HeLa and SW13 complex were bound to protein A beads using antibody to BAF57 and then incubated with radiolabeled PIP2 micelles. Beads were then pelleted and washed, and bound counts were measured. There was little difference in counts bound by the SW13 complex or by protein A beads (Fig. 1C). In contrast, the HeLa complex specifically bound approximately 90 mol of PIP2 per mole of complex, consistent with the binding of one micelle containing 88–90 molecules (20) of PIP2 to one complex. Thus, we conclude that the complete BAF complex with Brg1, BAF53, and β-actin is able to bind to PIP2 in mixed vesicular bilayers similar to physiologic membranes as well as to pure PIP2 micelles.
PIP2 Stimulates Binding of BAF to Actin Filaments.
The ability of the BAF complex to bind PIP2 suggests that the increased nuclear retention observed in the presence of PIP2 (7) is likely a direct effect of PIP2 on the complex rather than an effect of PIP2 on a nuclear actin-binding protein. The presence of β-actin and a novel actin homolog in the complex plus the extensive evidence demonstrating PIP2 regulation of actin-binding proteins (21) together suggest that PIP2 binding may regulate BAF complex interactions with actin. Actin is an abundant, extensively studied cytoskeletal protein involved in diverse processes in the cell ranging from cell motility to signal transduction (see ref. 22 for review). Whereas the role of actin in the nucleus remains unclear and controversial, there are numerous reports of actin association with the nuclear matrix (23–25). Thus, PIP2 regulation of actin binding by the BAF complex could provide a means of regulating the subnuclear location of the complex. The presence of monomeric β-actin and an actin-related monomer (BAF53) in the BAF complex, coupled with the observations by multiple laboratories that PIP2 typically dissociates actin-binding proteins from actin (26–28), pointed toward an actin monomer sequestering mechanism. However, we found that the subunit composition of the complex did not change upon incubation with or without PIP2 (data not shown). In addition, there was no measurable exchange of rhodamine-labeled actin with the actin in the complex when the immunoprecipitated complex was incubated overnight with a 1,000-fold molar excess of rhodamine-actin in the presence of either PIP2 or no lipid (data not shown). Thus, we considered the possibility that PIP2 might instead regulate BAF complex binding to actin filaments rather than to monomers.
To examine this possibility, the polymerization of pyrene-labeled actin was used as a means of biochemically detecting whether the BAF complex changes either the extent or kinetics of actin polymerization. Presumably, any binding of BAF complex to actin filaments would be due either to the Brg1 subunit itself, which directly contacts both β-actin and BAF53 (7), or to BAF53 and/or actin. Thus, polymerization of actin in the presence of HeLa complex was compared with polymerization in the presence of SW13 complex.
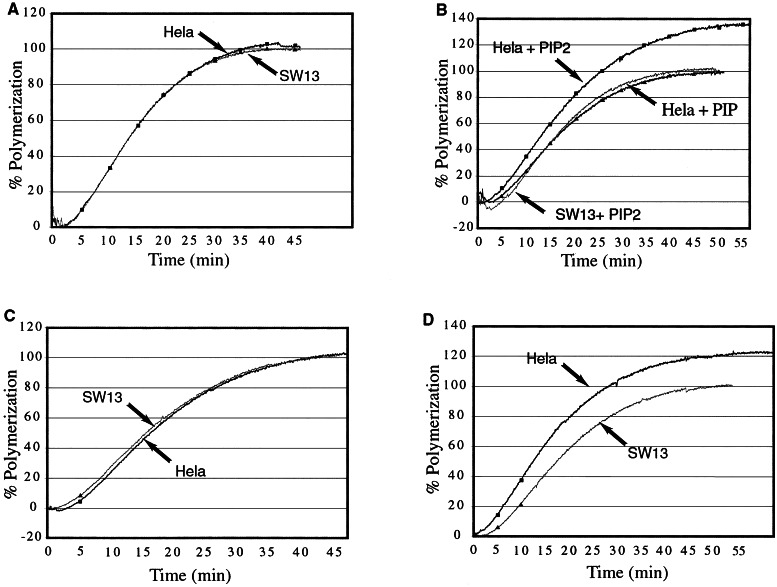
Pyrenyl actin polymerized in the presence of the HeLa complex with the same time course and to the same extent as actin polymerized in the presence of the SW13 complex or with buffer alone (Fig. 2A and data not shown). This suggests that either the complex fails to bind actin filaments or that the complex binds but does not perturb actin polymerization (as might be seen with a filament side-binder). However, when PIP2 micelles were added concurrently with the complexes, the total extent of actin polymerization increased in the presence of the HeLa-derived BAF complex (Fig. 2B). Addition of the same concentration of PIP to the BAF complex did not produce any increase in polymerization (Fig. 2B). This is consistent with the observation that most actin-binding proteins bind PIP2 with higher affinity than PIP, and that PIP2 is 10-fold more effective than PIP in inducing stable binding of the BAF complex to nuclei (7).
Figure 2.
The BAF complex stabilizes actin filaments in the presence of PIP2. (A) Polymerization of actin with no lipids added. HeLa or SW13 complexes were purified using BAF57 antibody; 5% pyrene-labeled actin was polymerized in the presence of either HeLa or SW13 complex at a concentration of ≈5 nM (as assessed by silver-stained gels) at 30°C. Polymerization is expressed by subtracting intrinsic fluorescence of pyrene-labeled G actin (as measured using 3 μM G actin), then calculating fluorescence as a percentage of final fluorescence of the SW13 sample. (B) As in A, except HeLa or SW13 complex was added to actin and buffer along with sonicated micelles of either PIP2 or PIP at a concentration of 50 μM. (C) As in A, except HeLa or SW13 complex was added to actin in the presence of 50 μM sonicated vesicles of phosphatidylcholine. (D) As in A, except HeLa or SW13 complex was added to actin in the presence of 50 μM sonicated vesicles of phosphatidylcholine containing 10% PIP2.
To further confirm that the effect seen with PIP2 micelles was indeed specific for PIP2, 10% PIP2 was incorporated into phosphatidylcholine vesicles, and these were added to the polymerization reaction. In the presence of control vesicles, HeLa and SW13 complexes behaved identically (Fig. 2C), whereas the total extent of actin polymerization increased when HeLa complex was added in the presence of PIP2 (Fig. 2D). It has proven difficult thus far to make pure complex more concentrated than 8–10 nM (as estimated from silver-stained gels); thus, no more than 5–6 nM complex has been added to the pyrenyl actin assay. Lower concentrations of the complex produce lesser effects on actin polymerization (data not shown), implying that the most concentrated complex we can obtain is unable to saturate filament binding.
BAF Complex Binds Actin Filament Ends and Branch Points.
To further investigate the PIP2-regulated, BAF-dependent stabilization of actin filaments, actin polymerized with BAF complex and large PIP2-containing vesicles was studied by electron microscopy. The binding of BAF complex to PIP2-containing vesicles was clearly visible under these conditions (Fig. 3A). In addition, BAF complexes bound to both a vesicle and an actin filament end were frequently visualized (Fig. 3B). Myosin S1 decoration revealed the bound end to be the pointed (“minus”) end of the filament (data not shown). In a typical experiment, virtually all BAF complexes observed (>95%) were bound to PIP2-containing vesicles, whereas in a parallel experiment 1% of complexes observed (2 of 200) were associated with control vesicles lacking PIP2. In the same experiment, 2 of the 200 complexes observed (not the 2 bound to vesicles) were bound to actin filaments, one to an end and one to a side. In contrast, 19 of 120 complexes observed in the presence of PIP2 were associated with actin filament ends, whereas 2 were associated with the side of a filament. No SW13 complexes were seen associated with filaments (data not shown).
The electron microscopy experiments were also repeated using PIP2 micelles rather than vesicles. Under these conditions, most complexes bound to filaments were bound to filament ends, as seen with vesicles (Fig. 3C). Surprisingly, 5–10% of BAF complexes bound to filaments were also side-bound, forming branch points (Fig. 3D) reminiscent of those seen associated with the Arp2/3 complex (29). This percentage is to be expected if the Kd for end-binding is significantly lower than the Kd for side-binding (as seen with the Arp2/3 complex). Higher concentrations of complex should allow more frequent observation of branch point binding (Mullins et al. use 80 nM Arp complex in their electron microscopy studies; ref. 29). Of significant interest is the fact that branch points are observed only when micelles of PIP2 are used, providing evidence that lipid curvature or charge density might potentially play some role in regulating how the BAF complex binds to actin filaments.
Intracomplex Actin Capping by Brg1 and Relief of Capping by PIP2.
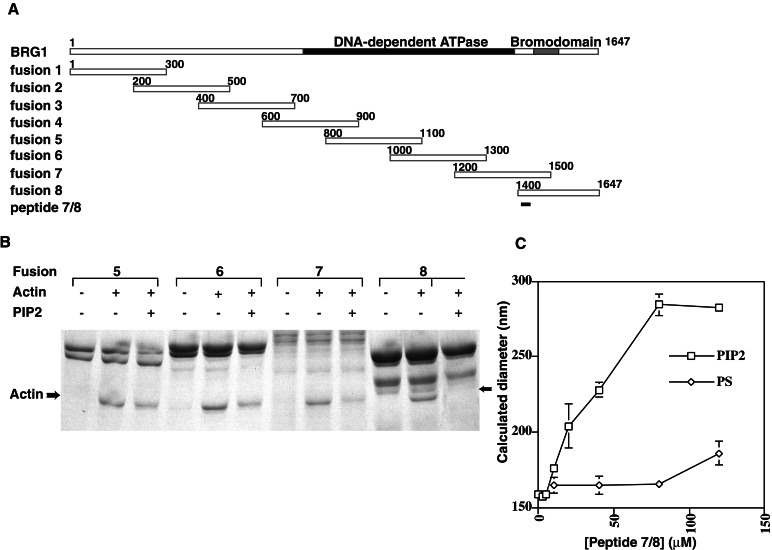
The observation that PIP2 activates actin filament binding by BAF suggests a mechanism in which PIP2 binds to a subunit of the complex and thereby relieves capping of the associated actin and/or BAF53, allowing the actins in the complex to interact with filaments. The most likely candidate for the autocapping protein is Brg1, because Brg1 directly interacts with β actin as well as the Arp3-like BAF53 and given that the SW13 complex, lacking only the actins and Brg1, cannot bind PIP2 (Fig. 1). To test this model, glutathione S-transferase fusion proteins were made to overlapping 300-aa stretches of the Brg1 protein (Fig. 4A). Fusion proteins were incubated with actin, washed several times, then incubated with PIP2 and washed again. Fusions 1 through 4 were unable to bind actin (data not shown), whereas 5, 6, 7, and 8 all bound actin, suggesting that actin contacts at least two distinct regions of the Brg1 protein. Interestingly, PIP2 inhibited actin binding by fragment 8 and, to a lesser extent, fragment 7 but caused only a very slight decrease in actin binding by fragments 5 and 6 (Fig. 4B), suggesting that in the presence of PIP2, actin is partially uncapped while remaining associated with Brg1. Several candidate PIP2-binding peptides were identified in Brg1 and synthesized, including positively charged peptides from the region 3/4 overlap, the region 4/5 overlap, region 5, and the region7/8 overlap. However, only the peptide identified in the region common to fragments 7 and 8 (Brg peptide 1402–1418, EEVRQKKSSRKRKRDS) was able to specifically bind PIP2-containing vesicles as assayed by light scattering (Fig. 4C and data not shown).
Figure 4.
PIP2 regulation of actin binding by Brg1 fragments. (A) Fusion constructs used in this study. Glutathione S-transferase fusions to the N terminus of the indicated peptide fragments of Brg1 were expressed in bacteria, whereas peptide 7/8 was synthesized by machine and purified by HPLC. (B) Binding of actin to C-terminal fusions 7 and 8 is inhibited by PIP2. Fusion constructs 5 through 8 were expressed and purified on glutathione beads, then incubated with 10 μM actin. Bead pools were split and incubated in binding buffer with or without 100 μM PIP2 and then washed. Bound proteins were separated by SDS/PAGE. Actin band is indicated by an arrow. (C) Binding of PIP2-containing vesicles by a peptide from Brg1. Peptide 7/8 (N-acetyl EEVRQKKSSRKRKRDS) was added at the indicated concentrations to 10 μM vesicles containing either 10% PIP2 (PIP2) or 30% phosphatidylserine (PS). DLS was measured, and the diameter of complexes was calculated. Note that in this experiment, calculated changes in vesicle size were likely because of peptide-mediated changes in vesicle shape, fusion, or aggregation.
Discussion
The discovery that actin is a subunit of chromatin remodeling complexes in yeast, Drosphila, and mammals has raised the question of the role of actin in the function of these complexes and revives the decades-old questions concerning actin's function in the nucleus. Although the very presence of actin in the nucleus has been hotly contested, present evidence indicates that β-actin spends at least some time in the nucleus. Blockade of nuclear export with the cell-permeant small molecule leptomycin B causes accumulation of actin in the nucleus (30), demonstrating conclusively that wild-type actin shuttles to and from the nucleus. In addition, β-actin can be crosslinked to the nuclear BAF complex in vivo (7), demonstrating that some actin is indeed localized to the nucleus in vivo. However, despite much study, there has been little understanding of the role that actin plays within the nucleus.
Actin polymerization is regulated primarily by Ca2+ and PIP2 (and perhaps other phosphoinositides) (21) that bind to and regulate actin-binding proteins whose roles range from capping to crosslinking to sequestering. We have shown previously that PIP2 induces stable association of the BAF complex with permeabilized nuclei, raising the question of the mechanism underlying this regulation. Here, we have shown that PIP2 enhances actin binding by the BAF complex. These results identify PIP2 as an attractive candidate for a matrix localization signal for the BAF complex. It must be noted, however, that it is not clear whether the targeting molecule in vivo is PIP2 itself, another phosphoinositide, or perhaps a protein that can mimic PIP2.
PIP2 binding to the purified BAF complex allows the complex to bind and stabilize actin filaments. This function requires a full BAF complex containing actin, BAF53, and Brg1 as shown by studies using incomplete, functionally inactive complexes. In addition, we have found that Brg1 can interact with actin using at least two separate domains, and PIP2 can selectively displace actin from one of these two sites. We expect that in the intact BAF complex, the actin monomer is bound to Brg1 at both of these sites. We suggest that in the presence of PIP2, Brg1 subregions 5 and 6 will remain bound to actin, whereas subregions 7 and 8 will dissociate from actin and bind to PIP2 and thereby reveal a previously occluded site on the actin monomer. This model suggests that in mammalian cells, PIP2 or a related molecule directly activates actin filament binding by the BAF complex by binding to the Brg1 subunit, relieving capping of BAF53 and actin by the Brg1 C terminus. The uncapped complex is then able to bind actin filaments through the newly exposed surfaces of the still-bound actin and BAF53. This model is highly speculative, but it explains how PIP2 might activate actin binding and is consistent with the discovery of a PIP2-sensitive and a PIP2-insensitive actin-binding domain on the same protein.
In several respects, the BAF complex is similar to the Arp2/3 complex, which is involved in a variety of processes requiring dynamic regulation of actin polymerization (29, 31, 32). Both are large multisubunit complexes, both contain two actin-like subunits, and Arp3 is the closest identified homolog of BAF53 (7). Our studies point out further commonalities between the two complexes: both bind actin filament pointed ends and filament branch points. The latter characteristic is thought to contribute to the generation of force at the leading edge of moving cells. Given that BAF complex binding to actin is more complicated than simple filament side binding, we suggest that there might be roles for actin binding in the function of the BAF complex beyond simple subnuclear localization. For example, by analogy with the Arp2/3 complex, BAF complex stabilization of actin branch points could result in force generation. Force generation could play a role in the resolution of higher order chromatin structure.
Acknowledgments
We thank Stuart L. Schreiber for support and discussions; X. Chen, S. Harrison, and J. Hartwig for help with electron microscopy; J. Chen for help with peptide synthesis and purification; M. Footer and C. Murphy for technical help; and A. Gasch, C. Hassig, J. Theriot, J. Tong, and members of the Crabtree lab for discussions and critical reading of the manuscript.
Abbreviations
- PIP2
phosphatidylinositol 4,5-bisphosphate
- PIP
phosphatidylinositol 4-monophosphate
References
- 1.Wolffe A P. Curr Opin Genet Dev. 1994;4:245–254. doi: 10.1016/s0959-437x(05)80051-6. [DOI] [PubMed] [Google Scholar]
- 2.Carlson M, Laurent B C. Curr Opin Cell Biol. 1994;6:396–402. doi: 10.1016/0955-0674(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 3.Lorch Y, Cairns B R, Zhang M, Kornberg R D. Cell. 1998;94:29–34. doi: 10.1016/s0092-8674(00)81218-0. [DOI] [PubMed] [Google Scholar]
- 4.Lorch Y, Zhang M, Kornberg R D. Cell. 1999;96:389–392. doi: 10.1016/s0092-8674(00)80551-6. [DOI] [PubMed] [Google Scholar]
- 5.Schnitzler G, Sif S, Kingston R E. Cell. 1998;94:17–27. doi: 10.1016/s0092-8674(00)81217-9. [DOI] [PubMed] [Google Scholar]
- 6.Phelan M L, Sif S, Narlikar G J, Kingston R E. Mol Cell. 1999;3:247–253. doi: 10.1016/s1097-2765(00)80315-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhao K, Wang W, Rando O J, Xue Y, Swiderek K, Kuo A, Crabtree G R. Cell. 1998;95:625–636. doi: 10.1016/s0092-8674(00)81633-5. [DOI] [PubMed] [Google Scholar]
- 8.Xue Y, Canman J C, Lee C S, Nie Z, Yang D, Moreno G T, Young M K, Salmon E D, Wang W. Proc Natl Acad Sci USA. 2000;97:13015–13020. doi: 10.1073/pnas.240208597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papoulas O, Beek S J, Moseley S L, McCallum C M, Sarte M, Shearn A, Tamkun J W. Development (Cambridge, UK) 1998;125:3955–3966. doi: 10.1242/dev.125.20.3955. [DOI] [PubMed] [Google Scholar]
- 10.Cairns B R, Erdjument-Bromage H, Tempst P, Winston F, Kornberg R D. Mol Cell. 1998;2:639–651. doi: 10.1016/s1097-2765(00)80162-8. [DOI] [PubMed] [Google Scholar]
- 11.Ikura T, Ogryzko V V, Grigoriev M, Groisman R, Wang J, Horikoshi M, Scully R, Qin J, Nakatani Y. Cell. 2000;102:463–473. doi: 10.1016/s0092-8674(00)00051-9. [DOI] [PubMed] [Google Scholar]
- 12.Galarneau L, Nourani A, Boudreault A A, Zhang Y, Heliot L, Allard S, Savard J, Lane W S, Stillman D J, Cote J. Mol Cell. 2000;5:927–937. doi: 10.1016/s1097-2765(00)80258-0. [DOI] [PubMed] [Google Scholar]
- 13.Shen X, Mizuguchi G, Hamiche A, Wu C. Nature (London) 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 14.Wang W, Xue Y, Zhou S, Kuo A, Cairns B R, Crabtree G R. Genes Dev. 1996;10:2117–2130. doi: 10.1101/gad.10.17.2117. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Chi T, Xue Y, Zhou S, Kuo A, Crabtree G R. Proc Natl Acad Sci USA. 1998;95:492–498. doi: 10.1073/pnas.95.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kouyama T, Mihashi K. Eur J Biochem. 1981;114:33–38. [PubMed] [Google Scholar]
- 17.Dunaief J L, Strober B E, Guha S, Khavari P A, Alin K, Luban J, Begemann M, Crabtree G R, Goff S P. Cell. 1994;79:119–130. doi: 10.1016/0092-8674(94)90405-7. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Cote J, Xue Y, Zhou S, Khavari P A, Biggar S R, Muchardt C, Kalpana G V, Goff S P, Yaniv M, et al. EMBO J. 1996;15:5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 19.Lamb J A, Allen P G, Tuan B Y, Janmey P A. J Biol Chem. 1993;268:8999–9004. [PubMed] [Google Scholar]
- 20.Sugiura Y. Biochim Biophys Acta. 1981;641:148–159. doi: 10.1016/0005-2736(81)90578-2. [DOI] [PubMed] [Google Scholar]
- 21.Janmey P A. Annu Rev Physiol. 1994;56:169–191. doi: 10.1146/annurev.ph.56.030194.001125. [DOI] [PubMed] [Google Scholar]
- 22.Carlier M F, Valentin-Ranc C, Combeau C, Fievez S, Pantoloni D. Adv Exp Med Biol. 1994;358:71–81. doi: 10.1007/978-1-4615-2578-3_7. [DOI] [PubMed] [Google Scholar]
- 23.Nakayasu H, Ueda K. Cell Struct Funct. 1985;10:305–309. doi: 10.1247/csf.10.305. [DOI] [PubMed] [Google Scholar]
- 24.Clark T G, Rosenbaum J L. Cell. 1979;18:1101–1108. doi: 10.1016/0092-8674(79)90223-x. [DOI] [PubMed] [Google Scholar]
- 25.Valkov N I, Ivanova M I, Uscheva A A, Krachmarov C P. Mol Cell Biochem. 1989;87:47–56. doi: 10.1007/BF00421082. [DOI] [PubMed] [Google Scholar]
- 26.Lassing I, Lindberg U. Nature (London) 1985;314:472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- 27.Janmey P A, Stossel T P. Nature (London) 1987;325:362–364. doi: 10.1038/325362a0. [DOI] [PubMed] [Google Scholar]
- 28.Steimle P A, Hoffert J D, Adey N B, Craig S W. J Biol Chem. 1999;274:18414–18420. doi: 10.1074/jbc.274.26.18414. [DOI] [PubMed] [Google Scholar]
- 29.Mullins R D, Heuser J A, Pollard T D. Proc Natl Acad Sci USA. 1998;95:6181–6186. doi: 10.1073/pnas.95.11.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wada A, Fukuda M, Mishima M, Nishida E. EMBO J. 1998;17:1635–1641. doi: 10.1093/emboj/17.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winter D, Podtelejnikov A V, Mann M, Li R. Curr Biol. 1997;7:519–529. doi: 10.1016/s0960-9822(06)00223-5. [DOI] [PubMed] [Google Scholar]
- 32.Welch M D, Iwamatsu A, Mitchison T J. Nature (London) 1997;385:265–269. doi: 10.1038/385265a0. [DOI] [PubMed] [Google Scholar]