Abstract
Background
Gastrointestinal helminths of the Strongylus genus can be very pathogenic in equids mainly because of migratory larval and subadult stages invading arteries of the mesenterium. However, the extraintestinal, aberrant presence of these stages has been observed.
Case presentation
A 19-year-old male donkey presented with severe lameness in the right front limb. After unsuccessful hoof wraps, diagnostic imaging was performed. Removal of the sole horn led to the discovery of a sizeable abscess containing a single nematode. The helminth was removed and parasitologically examined. The abscess was treated with surgical drainage, wound care, and medicinal fly larvae. Anthelmintic treatment with oral ivermectin (200 µg/kg body weight) was initiated. Parasitological follow-up examinations were conducted two weeks afterwards and at seven and ten months after initial diagnosis. The nematode was identified as adult female specimen of Strongylus vulgaris. Coproscopic examination of the animal and four herd mates confirmed the presence of S. vulgaris in the group. The hoof abscess healed within two months and lameness completely resolved. All parasitological follow-up examination demonstrated anthelmintic efficacy and absence of S. vulgaris, indicating sustained parasite control.
Conclusions
This case represents the first report of ectopic occurrence of S. vulgaris causing a hoof abscess inducing lameness in a donkey. The healing of the abscess was without complications as soon as the source of the condition was removed.
Keywords: Strongyle parasite, Migrating intestinal nematode, Ectopic location, Lameness, Equid
Background
Gastrointestinal helminths of the Strongylus genus rank amongst the most pathogenic internal parasites in equid species. The high pathogenic potential even at low infection levels is mainly attributed to the extraintestinal, intra-arterial migration of larval and subadult stages [1]. Strongylus vulgaris in particular has been regarded as the most pathogenic gastrointestinal parasite in equids with migratory fourth-stage larvae invading mesenteric arteries and causing fibrosis, arteritis, and infarction of ileum, caecum, and colon as well as fibrin tracts on the intima of arteries reaching as far as into the abdominal aorta [2, 3]. Even though lesions have most commonly been observed in the cranial mesenteric artery and in the arteries of the ileum, caecum, and colon, there have also been reports of lesions in unusual sites such as the coronary artery [4], kidney [5], external iliac artery [6], or the internal carotid artery [7]. Here, we report a case of ectopic occurrence of S. vulgaris causing a hoof abscess in a donkey.
Case presentation
In early spring 2023, a 19-year-old, male donkey presented with lameness in the right front limb. At a sanctuary for donkeys, the animal was housed in a group with four herd mates on a paddock with limited access to pasture. Anthelmintic treatment was implemented twice a year using an oral formulation of ivermectin or moxidectin together with praziquantel in winter, i.e. between November and January (Equimax®: 200 µg ivermectin and 1.5 mg praziquantel per kg body weight (BW); Equest® Pramox: 400 µg moxidectin/kg BW and 2.5 mg praziquantel/kg BW; both off-label use) and oral fenbendazole as a one-time treatment in summer, i.e. between June and August (Panacur®: 7.5 mg/kg BW). One animal of the group had had a history of weight loss of unclarified origin and had received an additional anthelmintic treatment based on a pyrantel formulation for oral administration (Strongid®: 6.5 mg/kg BW, off-label use).
As the owners had suspected a hoof abscess as the cause of lameness, they had made a wet hoof wrap soaked with iodine solution (Betadine®, Mundipharma, Cambridge, United Kingdom). On the day of the presentation of the animal to the primary veterinarian, a heavy thrush in the collateral grooves had become apparent. The donkey was lame grade 5/5 based on the American Association of Equine Practitioners (AAEP) lameness scale, showed an evident pulsation of the Aa. digitales lateralis and medialis, a warm hoof, and the forceps test was strongly positive lateral of the frog and slightly positive side-to-side [8]. The abaxial sesamoid nerve block was never completely positive. Differential diagnoses included a hoof abscess, a hoof haematoma, and a fracture of the distal phalanx. X-ray images were obtained, which included a lateromedial and a dorsopalmar 60° image. No abnormal findings could be detected, a fracture of the coffin bone could be ruled out. Hoof haematoma could be excluded as well. One week of conservative treatment was carried out which included wet and dry hoof wraps and non-steroidal anti-inflammatory drugs. The donkey was treated for three days with 3.1 mg/kg BW oral suxibuzon (Danilon®, Dr. E. Graeub AG) and for another six days with 1.1 mg/kg BW flunixin (Fluniximin®, Dr. E. Graeub AG). As conservative therapy was not successful, the hoof was opened to exclude a hoof abscess. After firmly trimming the sole and removing one centimeter of the sole horn, about 3 ml of pus could be drained. The abscess extended from the collateral groove to the lateral hoof wall and stretched over the whole quarters in length. Furthermore, the coffin bone was palpable with a probe. In the middle of the abscess cavity, a worm specimen was found (Fig. 1). The nematode was recovered, stored in a plastic tube, and immediately submitted to microscopic, parasitological examination. Microscopic examination confirmed the presence of a female specimen of Strongylus vulgaris due to characteristic structures, including a globulous buccal capsule with two lobed, rounded teeth at its base, a dorsal gutter reaching the anterior end, and two sets of leaf crowns at its opening and a vulva located approximately 8–9 mm from the posterior end (Fig. 2) [9].
Fig. 1.

Opened abcess cavity with clear view on the worm (arrowhead)
Fig. 2.

Morphological characteristics of the retrieved Strongylus vulgaris specimen. Left: Note the typical mouthparts including a globulous buccal capsule with two lobed, rounded teeth (white arrowheads) at its base, a dorsal gutter (yellow arrowhead) reaching the anterior end, and two sets of leaf crowns at its its opening (black arrowheads). Right: Posterior end with vulva. Scale bar 200 μm
The donkey spent two weeks at the clinic where the abscess was regularly flushed with sterile 0.9% sodium chloride solution (Fresenius Kabi AG, Bad Homburg, Germany), after which a gauze soaked with chlorhexidine (Hibidil 0.5 mg/ml, CPS Cito Pharma Services GmbH, Uster, Switzerland) and a hoof bandage were applied. A therapy of medicinal fly larvae was applied for three days in order to create the least amount of further damage possible and to remove necrotic tissue [10]. A dorsopalmar 60° image was captured again a week after the first x-ray. The abscess healed completely within two months (Fig. 3).
Fig. 3.
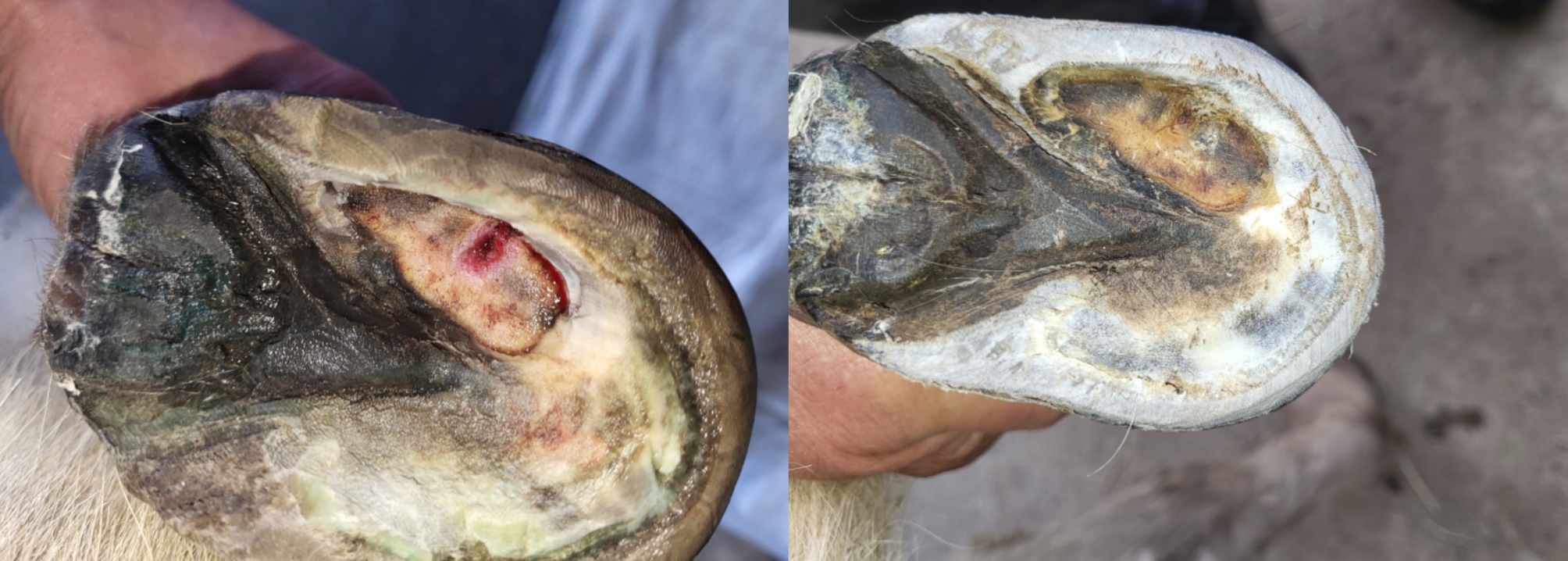
Healing progress of the hoof abscess. Left: The hoof abscess one week after the initial opening. Right: Abscess site one month after initial opening; an even, thin layer of horn is present covering the lesion
After identification of S. vulgaris, fresh faecal samples were collected over a period of three consecutive days from the five donkeys including the patient, pooled for each donkey, and subjected to coproscopic examination using the McMaster method [9] to obtain a faecal egg count for horse strongyles (FEC; small and large strongyles). FEC for strongyle eggs were 300, 350 (patient donkey), 700, 1,260, and 1,600 eggs per gram faeces (EPG) for the five individual donkeys, respectively. No other parasitic stages such as e.g. eggs of Parascaris spp. or Anoplocephala spp., were detected. Subsequently, coproculture [9] was initiated to obtain third-stage (L3) strongyle larvae and to differentiate those of the Strongylus genus. After twelve days, coprocultures were harvested into 50 ml tubes and centrifuged at 500 x g for 3 min. Supernatant was removed and the pellet containing the L3 was resuspended in water. Up to 100 larvae per sample were microscopically examined. The analysis of the material revealed several small horse strongyle larvae and two L3 of S. vulgaris in the faecal sample of one of the five donkeys. These L3, characterised by a filamentous sheath, measured 643 μm and 597 μm in total body length, with tail sheath lengths of 256 μm, and 261 μm, respectively. They also exhibited a filariform oesophagus and 32 triangular intestinal cells (Fig. 4) [11]. Subsequently, DNA was extracted from the resuspended larvae using the QIAmp® DNA Mini Kit (Quiagen, Hilden, Germany) according to the manufacturer’s instructions. PCR was conducted to verify the presence of S. vulgaris, i.e. to amplify a 170 bp (bp) sequence of the mitochondrial ITS2 gene DNA [12]. Strongylus vulgaris DNA was detected in all five coproculture samples.
Fig. 4.

Third-stage larva from coproculture. Characteristic sheathed third-stage larva of Strongylus vulgaris. Total body length: 643.6 μm, total sheath length: 256.4 μm, number of triangular intestinal cells: 32
Due to the high FEC as well as due to the identification of S. vulgaris, immediate anthelmintic treatment using oral ivermectin (Eraquell®: 200 µg/kg BW) was administered. Two weeks after treatment, EPG of all animals was < 50 and no L3 or DNA of S. vulgaris could be detected in coproculture of these samples (PCR and microscopical examination). The presence of S. vulgaris L3 in coprocultures and the adult worm may indicate post-treatment reinfection by ingesting L3 larvae after the winter treatment. Alternatively, the survival of an incomplete or suboptimal kill of migrating larvae at the time of treatment may have allowed the adult worm to persist and cause pathology.
Coproscopic examinations as well as PCR were repeated seven and ten months after treatment. After seven months, FECs of 200–700 EPG were determined in the five donkeys. This entailed anthelmintic treatment with oral fenbendazole (Panacur®: 7.5 mg/kg) which resulted in a reduction to a FEC of < 50 EPG in all animals after 2 weeks. Coproculture was negative for S. vulgaris after seven months both before and after the treatment with fenbendazole. Ten months after the first treatment following the extraction of the adult specimen of S. vulgaris from the hoof abscess, FECs of all animals were < 50 EPG and coproculture did not indicate the presence of S. vulgaris. PCR for S. vulgaris was negative throughout the follow-up period, i.e. both after seven and ten months.
Discussion and conclusions
Gastrointestinal parasites in equids have been associated with considerable impacts on animal health, welfare, and longevity. Among these, strongylid nematodes of the Strongylus genus stand out as notorious, potentially lethal pathogens that cause extensive damage in affected hosts [13–15]. Historically, there has been controversy about the development of S. vulgaris in equines particularly in regard to extraintestinal migrations [15]. Whereas Olt [16] suggested an extraintestinal migration similar to Ascaris spp., Enigk [17, 18] was able to confirm the migration of pre-adult stages of S. vulgaris to the cranial mesenteric artery after penetration of intestinal arterioles. However, lesions in the coronary arteries [4], kidney [5], external iliac artery [6], and internal carotid artery [19] have been associated with aberrant migrations of S. vulgaris larvae as well. To our knowledge, this represents the first report of ectopic occurrence of S. vulgaris in the hoof of an equid. All donkeys were clinically unsuspicious apart from the hoof abscess in one animal. Despite its high pathogenic potential even at low infection rates, S. vulgaris can remain subclinical in some cases [1, 9]. Due to this and the fact that FEC of strongyle eggs in general were relatively high, anthelmintic treatment of all animals was initiated without further delay using previously applied anthelmintic compounds [20]. Subsequent FEC confirmed the efficacy of ivermectin, and S. vulgaris was not detectable anymore. However, the detection of S. vulgaris L3 in all animals via ITS-2 PCR, despite a regular, calendar-based deworming schedule, raises important considerations. Due to the prepatent period of S. vulgaris, which can be as long as 6–7 months [9, 20], one might expect that twice-yearly treatments should have prevented the establishment of new infections. However, a few factors could explain this finding. First, it is possible that the donkeys were exposed to new sources of infective larvae shortly before or after their most recent deworming. If larvae were ingested just before or after treatment, they could have migrated and matured into adults, potentially producing eggs and larvae that were detectable in subsequent faecal samples. Second, there is the possibility of anthelmintic resistance developing in the parasite population. The history of an intense, calendar-based deworming regime raises concerns about the potential for resistance to macrocyclic lactones like ivermectin, which could result in incomplete elimination of migrating larvae or even adults, leading to persistent infections. Hence, the outcome of the present case was very fortunate. This is even more important given the situation that although infections with S. vulgaris have become relatively rare after the widespread use of anthelmintic drugs, many resistances of different types of equine intestinal parasites to virtually all available compounds have developed throughout the world [21–23]. Therefore, selected deworming strategy based on the presence of strongyle eggs in faecal samples has been proposed in order to tackle this problem and to reduce the frequency of deworming [24–26]. Accordingly, equids should be treated only if FEC were above 200 EPG . Importantly, the recommendations include coprocultures to identify the presence of large strongyles and FEC reduction tests after treatments to slow down the development of anthelmintic resistances [20]. The occurrence of these parasites was strongly reduced but their potential re-emergence needs to be monitored. In Switzerland, the selected deworming strategy is widely applied in a large proportion of the horse population and includes the anthelmintic treatment of all horses positive for large strongyles irrespective of the FEC [27]. In the present case, the owners were advised to switch towards a risk-oriented deworming strategy based on pasture hygiene, quarantines, and most importantly, coproscopic examinations four times a year rather than calendar-based anthelmintic treatments. Additionally, it is important to note that S. vulgaris larvae may have survived post-treatment and completed their life cycle despite the deworming depending on the time of exposure of L3 to the drug, potential incomplete efficacy of treatment, compliance in regard to treatment administration, or residual larvae escaping the drugs’ effect [28–30].
Due to the extensive extraintestinal migrations of S. vulgaris larval stages and a potential prepatent period of 6–7 months, it remains challenging to assess the direct success of anthelmintic treatment, since extraintestinal developmental stages may endure despite of elimination of adult stages. As a consequence, repeated control examinations and a surveillance of the conditions are necessary in cases where S. vulgaris has been detected in order to reduce infection pressure and risk and to prevent severe clinical cases [20]. However, since S. vulgaris cannot be ruled out by the standard FEC procedure and given the pathological potential of the parasite necessitating treatment, further analyses such as coproculture need to be conducted . In the present case, S. vulgaris was not detected at either seven or ten months after the initial treatment, indicating that all donkeys remained free from infection. However, reinfections could emerge from pasture grounds which requires prolonged surveillance and additional diagnostic measures, i.e., continuous re-examinations specifically for ruling out the presence of S. vulgaris [25, 31]. Importantly, potentially false-negative S. vulgaris results due to methodological reasons need to be considered. For example, L3 of S. vulgaris could be detected in only one of the five animals examined whereas subsequent PCR from coprocultures yielded positive results in all animals.
The specific healing of the hoof abscess was without complications. We speculate that this was due to the sterile nature of the abscess, which was managed to stay clean without secondary bacterial infection. Therefore, quick healing was possible as soon as the source of the problem was removed.
Abbreviations
- BW
Body Weight
- EPG
Eggs per gram faeces
- FEC(s)
Faecal egg count(s)
- L3
Third-stage larva
Author contributions
LS, NB, and AOE collected the data. LS, NB, AOE, FG, and MS reviewed and interpreted the data. LS and AOE wrote the manuscript with input from all co-authors. All authors read and approved the final manuscript.
Funding
Not applicable.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Studzińska MB, Tomczuk K, Demkowska-Kutrzepa M, Szczepaniak K. The Strongylidae belonging to Strongylus Genus in horses from southeastern Poland. Parasitol Res. 2012;111:1417–21. 10.1007/s00436-012-3087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan J, Pirie H. The pathogenesis of single experimental infections with Strongylus Vulgaris in foals. Res Vet Sci. 1975;18:82–93. [PubMed] [Google Scholar]
- 3.Drudge J, Lyons E, Szanto J. Pathogenesis of migrating stages of helminths, with special reference to Strongylus Vulgaris. In: Soulsby EJL, editor. Biology of parasites: emphasis on Veterinary parasites. New York: Academic; 1966. pp. 199–214. [Google Scholar]
- 4.Cronin M, Leader G. Coronary occlusion in a thoroughbred colt. Vet Rec. 1952;64.
- 5.Mahaffey L, Adam N. Strongylus vulgaris in the urinary tract of a foal and some observations upon the habits of the parasite. Vet Rec. 1963;75:561–6. [Google Scholar]
- 6.Wright A. Verminous arteritis as a cause of colic in the horse. Equine Vet J. 1972;4:169–74. [Google Scholar]
- 7.Little P. Cerebrospinal nematodiasis of Equidae. JAVMA. 1972;60:1407–13. [PubMed] [Google Scholar]
- 8.AAEP (American Association of Equine Practitioners). Lameness exams: evaluating the lame horse. 2024. https://aaep.org/horsehealth/lameness-exams-evaluating-lame-horse. Accessed 12 March 2024.
- 9.Deplazes P, Eckert J, Mathis A, von Samson-Himmelstjerna G, Zahner H. Parasitology in Veterinary Medicine. 1st ed. The Netherlands: Wageningen Academic; 2016. [Google Scholar]
- 10.Sherman RA, Morrison S, Ng D. Maggot debridement therapy for serious horse wounds-a survey of practitioners. Vet J. 2007;174:86–91. 10.1016/j.tvjl.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Amer MM, Desouky AY, Helmy NM, Abdou AM, Sorour SS. Identifying 3rd larval stages of common strongylid and non-strongylid nematodes (class: Nematoda) infecting Egyptian equines based on morphometric analysis. BMC Vet Res. 2022;18:432. 10.1186/s12917-022-03526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bracken MK, Wøhlk CB, Petersen SL, Nielsen MK. Evaluation of conventional PCR for detection of Strongylus vulgaris on horse farms. Vet Parasitol. 2012;184:387–91. 10.1016/j.vetpar.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Pihl TH, Nielsen MK, Olsen SN, Leifsson PS, Jacobsen S. Nonstrangulating intestinal infarctions associated with Strongylus vulgaris: clinical presentation and treatment outcomes of 30 horses (2008–2016). Equine Vet J. 2018;50:474–80. 10.1111/evj.12779. [DOI] [PubMed] [Google Scholar]
- 14.Andersen UV, Reinemeyer CR, Toft N, Olsen SN, Jacobsen S, Nielsen MK. Physiologic and systemic acute phase inflammatory responses in young horses repeatedly infected with cyathostomins and Strongylus Vulgaris. Vet Parasitol. 2014;201:67–74. 10.1016/j.vetpar.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 15.McCraw B, Slocombe J. Strongylus vulgaris in the horse: a review. Can Vet J. 1976;17:150. [PMC free article] [PubMed] [Google Scholar]
- 16.Olt A. Das Aneurysma Verminosum Des Pferdes und seine unbekannten Beziehungen Zur Kolik. Dtsch Tierarztl Wschr. 1932;40:326–32. [Google Scholar]
- 17.Enigk K. Weitere Untersuchungen Zur Biologie Von Strongylus Vulgaris (Nematodes) Im Wirtstiere. Z Tropenmed Parasitol. 1951;2:523–35. [PubMed] [Google Scholar]
- 18.Enigk K. Zur Entwicklung Von Strongylus Vulgaris (Nematodes) Im Wirtstier. Z Tropenmed Parasitol. 1950;2:287–306. [PubMed] [Google Scholar]
- 19.Little P, Lwin U, Fretz P. Verminous encephalitis of horses: experimental induction with Strongylus Vulgaris larvae. Am J Vet Res. 1974;35:1501–10. [PubMed] [Google Scholar]
- 20.European Scientific Counsel Companion Animal Parasites (ESCCAP). Behandlung und Kontrolle gastrointestinaler Parasiten bei Pferden und anderen Equiden. Adaption der ESCCAP-Empfehlung Nr. 8 für die Schweiz. August 2019. https://www.esccap.ch/demo/wp-content/uploads/2020/01/ESCCAP-CH_GL8_Pferdeparasiten_d_def.pdf. Accessed 8th October 2024.
- 21.Peregrine AS, Molento MB, Kaplan RM, Nielsen MK. Anthelmintic resistance in important parasites of horses: does it really matter? Vet Parasitol. 2014;201:1–8. 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Meier A, Hertzberg H. Equine strongyles II. Occurrence of anthelmintic resistance in Switzerland. Schweiz Arch Tierheilkd. 2005;147:389–96. 10.1024/0036-7281.147.9.389. [DOI] [PubMed] [Google Scholar]
- 23.Meier A, Hertzberg H. Equine strongyles. I. Development of anthelmintic resistance. Schweiz Arch Tierheilkd. 2005;147:381–8. 10.1024/0036-7281.147.9.381. [DOI] [PubMed] [Google Scholar]
- 24.Osterman-Lind E, Holmberg M, Grandi G. Selective anthelmintic treatment in horses in Sweden based on coprological analyses: ten-year results. Animals. 2023;13. 10.3390/ani13172741. [DOI] [PMC free article] [PubMed]
- 25.von Samson-Himmelstjerna G. Anthelmintic resistance in equine parasites - detection, potential clinical relevance and implications for control. Vet Parasitol. 2012;185:2–8. 10.1016/j.vetpar.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Hertzberg H, Schwarzwald CC, Grimm F, Frey CF, Gottstein B, Gerber V. Helminth control in the adult horse: the need for a re-orientation. Schweiz Arch Tierheilkd. 2014;156:61–70. 10.1024/0036-7281/a000552. [DOI] [PubMed] [Google Scholar]
- 27.Lüthin S, Zollinger A, Basso W, Bisig M, Caspari N, Eng V, et al. Strongyle faecal egg counts in Swiss horses: a retrospective analysis after the introduction of a selective treatment strategy. Vet Parasitol. 2023;323:110027. 10.1016/j.vetpar.2023.110027. [DOI] [PubMed] [Google Scholar]
- 28.Harvey AM, Meggiolaro MN, Hall E, Watts ET, Ramp D, Šlapeta J. Wild horse populations in south-east Australia have a high prevalence of Strongylus Vulgaris and may act as a reservoir of infection for domestic horses. Int J Parasitol Parasites Wildl. 2019;8:156–63. 10.1016/j.ijppaw.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Osterman-Lind E, Hedberg Alm Y, Hassler H, Wilderoth H, Thorolfson H, Tydén E. Evaluation of strategies to reduce equine Strongyle Infective Larvae on pasture and study of Larval Migration and Overwintering in a nordic climate. Animals. 2022;12:3093. 10.3390/ani12223093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wallace J. Equine endoparasite resistance and its management - a vet practice perspective. Vet Rec. 2022;191:e2512. 10.1002/vetr.2512. [DOI] [PubMed] [Google Scholar]
- 31.Tydén E, Enemark HL, Franko MA, Höglund J, Osterman-Lind E. Prevalence of Strongylus Vulgaris in horses after ten years of prescription usage of anthelmintics in Sweden. Vet Parasitol X. 2019;276S:100013. 10.1016/j.vpoa.2019.100013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.


