Abstract
Background
Gut microbiota has emerged as a promising therapeutic target for neurodegenerative disorders through regulation of neuroinflammatory responses, while its role in optic nerve degeneration remains incompletely characterized. This study elucidates the neuroprotective role of gut microbiota derived tryptophan metabolites in glaucoma through gut-eye communication and inhibition of microglia-mediated neuroinflammation.
Methods
Gut microbiota profiling (16 S rRNA sequencing) and serum indoleacetic acid (IAA) quantification were performed in glaucoma patients versus controls. Microbiota–metabolite relationships were further validated through fecal microbiota transplantation (FMT). The neuroprotective and anti-neuroinflammatory effect of Bacteroides fragilis (B. fragilis) and IAA was assessed in both microbead-induced ocular hypertension mice model and in vitro BV-2 microglial cell inflammation model via immunofluorescence, qPCR, Western blot and mice behavioral assays. To explore the underlying mechanisms, retinal transcriptomics and microglia-neuron co-cultures were also employed.
Result
Glaucoma patients exhibited gut dysbiosis characterized by depleted tryptophan-metabolizing bacteria (B. fragilis, Bacteroides thetaiotaomicron, Anaerostipes hadrus) and reduced serum IAA levels. Mice receiving FMT from glaucoma patients exhibited lower systemic IAA levels. In in vivo and in vitro models, B. fragilis or IAA restored AhR activation, suppressed inflammation by inhibiting microglial activation and the release of pro-inflammatory mediators throughout the retina, reduced retinal ganglion cells (RGCs) loss and preserved visual function. Mechanistically, IAA attenuated RAGE/NF-κB pathway activation via AhR-dependent signaling, conferring neuroprotection.
Conclusion
Our study proposes a novel AhR-mediated gut microbiota-eye axis in glaucoma pathogenesis and demonstrates that IAA serves as an effective neuroprotective strategy with clinical potential for managing RGCs neurodegeneration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12974-025-03505-4.
Keywords: Gut microbiota-eye axis, Glaucoma, Neuroinflammation, Tryptophan metabolites, AhR, Microglia
Background
Glaucoma, the leading cause of irreversible blindness, affects approximately 95 million people globally, including 60 million with primary open-angle glaucoma (POAG) [1]. Glaucomatous neurodegeneration is characterized by progressive retinal ganglion cells (RGCs) loss with axonal degeneration, while ocular hypertension remains the primary risk factor [2]. Besides ocular pathology, systemic factors influencing the progression of glaucoma and the underlying pathophysiological mechanisms require further investigation.
Notably, the gut microbiota-brain axis has been implicated in multiple neurodegenerative disorders [3–5]. Given the optic nerve’s anatomical continuity with the central nervous system, the gut-eye axis likely contributes to ophthalmic diseases [6]. Emerging evidence demonstrates gut microbiota’s inextricable linkage to glaucoma, showing distinct microbial alterations in glaucoma patients [7–10]. Our prior work revealed abnormal Prevotellaceae/Bacteroidaceae ratios in Posner-Schlossman syndrome (PSS, a glaucoma subtype) correlating with disease progression [11]. Yet, whether gut microbes exert protective or pathogenic roles in glaucoma remains unclear.
Microbial metabolites primarily mediate gut-nervous system crosstalk [12]. These metabolites (e.g., short-chain fatty acids, bile acids, and tryptophan derivatives) enter systemic circulation to modulate neuroimmune responses by regulating immune cell recruitment, activation, and inflammatory mediator release [13–15]. Tryptophan metabolism involves both host and microbial pathways [16]. Microbiota-derived metabolites like indoleacetic acid (IAA) and indole-3-propionic acid (IPA) exhibit neuroprotective effects via aryl hydrocarbon receptor (AhR) activation in Alzheimer’s disease and multiple sclerosis [17, 18]. In ocular diseases, reduced IAA levels correlate with high myopia progression, while exogenous supplementation has shown therapeutic promise [19]. Glaucoma patients exhibit distinct metabolic profiles in bile secretion [8], amino acids [10], and short-chain fatty acids [20], while microbiota-driven tryptophan metabolism is uncharacterized. Given tryptophan’s dual role in neuroinflammation and neuroprotection, defining its impact on retinal immune homeostasis is urgent.
This study investigates the gut microbiota-tryptophan metabolite-eye axis in glaucoma. Using POAG patient samples, MB-induced ocular hypertension models, and bacterial colonization, we identified depleted tryptophan-metabolizing bacteria in glaucoma patients. Supplementing Bacteroides fragilis (B. frigilis) alleviated glaucomatous damage in mice, and its metabolite IAA reduced RGC loss and suppressed microglial neuroinflammation in vivo and in vitro. Mechanistically, IAA activated AhR to inhibit the receptor for advanced glycation end products (RAGE) pathway, conferring neuroinflammation-modulatory and neuroprotective effects. These findings highlight therapeutic strategies targeting microbial tryptophan metabolites to enhance RGCs survival and impede glaucoma progression.
Results
Gut microbiota dysbiosis in POAG patients
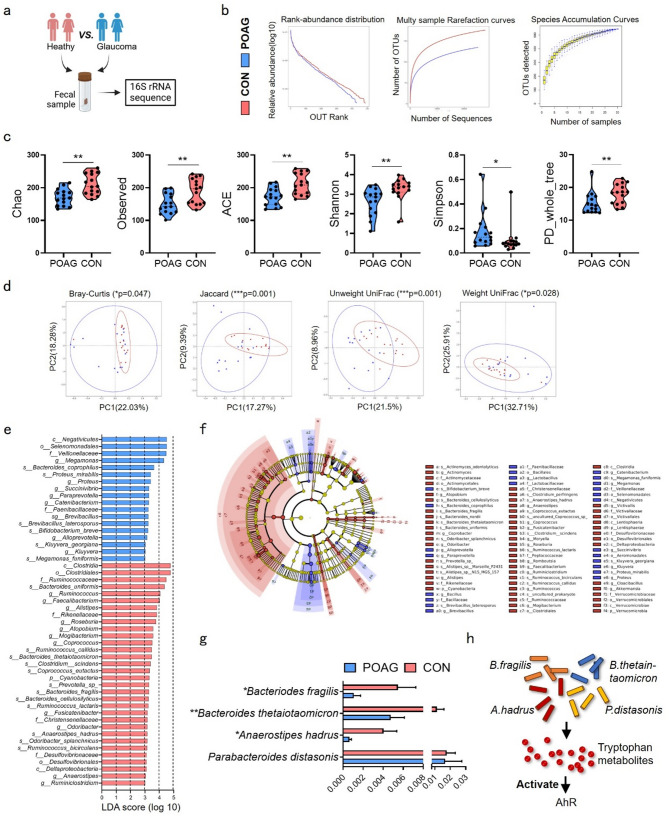
To investigate gut microbiota alterations in glaucoma, we recruited 15 POAG patients and 15 age-/sex-matched controls for fecal 16 S rRNA sequencing (Fig. 1a). Demographic parameters (gender, age, BMI) and ophthalmic metrics (intraocular pressure (IOP), cup-to-disc ratio (C/D), visual field defects, retinal nerve fiber layer (RNFL) thickness) were documented (Table S1).
Fig. 1.
Microbial atlas in POAG patients and healthy controls. (a) Fecal sample from heathy control and POAG patients were collected for 16 S rRNA sequence. (b) Rank abundance distribution, Multy sample rarefaction curves and Species accumulation curves. (c) Richness and evenness of gut microbiome were evaluated by Alpha-diversity indexes including Chao, Observed, ACE, Shannon, Simpson and PD whole tree. (d) Beta-diversity was showed by measuring Bray-Curtis, Jaccard, Unweight UniFrac and Weight UniFrac indexes. (e) LefSe was used to identify differences of bacterial taxa between the two groups. Taxa with LDA scores > 3 were showed under Kruskal-Wallis test. (f) The cladograms were constructed with LefSe analysis. (g) Comparations of four tryptophan-metabolizing bacteria between two groups were showed. (h) The diagram showed that four tryptophan-metabolizing bacteria activated AhR by producing tryptophan metabolites. POAG, POAG patients; CON, healthy control. Data represented the mean ± SEM, *P < 0.05, **P < 0.01. Alpha-diversity was showed under non-parametric Wilcox test, Beta-diversity were showed under MRPP
Sequencing generated 1,439,673 high-quality reads (703,448 POAG vs. 736,225 controls), averaging 47,989 ± 2,888 reads per sample with mean read length 418.1 ± 0.58 bp. Clustering at 97% identity yielded 587 operational taxonomic units (OTUs). Despite comparable microbial richness (rarefaction/rank abundance curves), POAG exhibited significant alpha-diversity reductions in Chao1 (p = 0.0014), observed species (p = 0.0042), ACE (p = 0.0027), Shannon (p = 0.0014), Simpson (p = 0.0113), and phylogenetic diversity (p = 0.0043). Beta-diversity analysis confirmed structural divergence via Bray-Curtis (p = 0.047), Jaccard (p = 0.001), unweighted UniFrac (p = 0.001), and weighted UniFrac (p = 0.028) metrics (Fig. 1b–d). LEfSe analysis identified 18 POAG-enriched taxa versus 32 control-enriched taxa (LDA > 3, Kruskal-Wallis test), visualized through phylogenetic cladogram (phylum to species level, Fig. 1e, f). Full taxonomic profiles are detailed in Tables S2-S7.
We analyzed previously reported tryptophan-metabolizing bacteria [21], identifying significant reductions in B. fragilis (p < 0.05, FDR-corrected), Bacteroides thetaiotaomicron (p < 0.01, FDR-corrected), and Anaerostipes hadrus (p < 0.01, FDR-corrected) among POAG patients, while Parabacteroides distasonis remained unchanged (Fig. 1g). Topical glaucoma medications showed no significant impact on these bacterial abundances (Fig. S1). Unlike host-endogenous tryptophan metabolism producing kynurenine or neurotransmitters, microbial-derived metabolites primarily act as AhR ligands to modulate immunity [22]. Reduced tryptophan-metabolizing bacteria may diminish AhR-activating metabolites, disrupting immunoregulation (Fig. 1h).
B. fragilis ameliorates RGCs degeneration and neuroinflammation
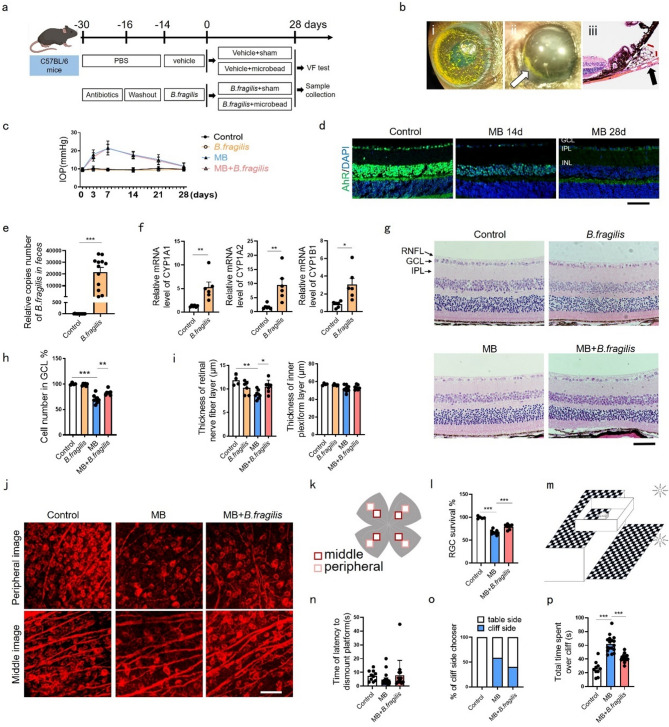
To determine if B. fragilis supplementation activates retinal AhR and protects against glaucomatous damage, we administered B. fragilis via oral gavage to C57BL/6J mice after antibiotic pretreatment (14 days) followed by MB-induced ocular hypertension (Fig. 2a, b). Retinal AhR expression progressively declined during high IOP (Fig. 2c, d), whereas B. fragilis colonization (confirmed by fecal qPCR, Fig. 2e) upregulated AhR target genes CYP1A1, CYP1A2, and CYP1B1 in retinal tissue (Fig. 2f), indicating gut-retina AhR activation. MB-injected mice exhibited reduced ganglion cell layer (GCL) density, RNFL thinning, and severe RGCs loss, all ameliorated by B. fragilis (Fig. 2g–l). Visual cliff testing revealed visual functional preservation with B. fragilis (Fig. 2m, n), with treated mice showing reduced cliff-side foot placements (Fig. 2o) and less time spent over the cliff (Fig. 2p). Histopathological assessment confirmed B. fragilis safety across major organs (Fig. S2a), and the non-toxic effects of B.fragilis on intestinal function in mice was assessed by testing the concentration of diamine oxidase (DAO) in serum [23] (Fig. S2b). These results demonstrated B. fragilis-mediated protection of retinal structure and function in glaucoma.
Fig. 2.
B. fragilis alleviated RGCs loss and retinal dysfunction in MB-induced ocular hypertension mice. (a) Schematic illustration of timeline and experimental design. (b) (i) mouse eye after injection of MB; (ii) MB deposition near the anterior chamber angle, photographed on the third day after injection; (iii) H&E-stained eye cross sections from day 28 after injection showing the accumulation of MB in the anterior chamber angle (arrows). (c) IOP changes with or without B. fragilis gavage administration after MB injection. Control or MB group, n = 17; B. fragilis or MB + B. fragilis group, n = 15. (d) Immunofluorescence staining showing gradually decreased expression of AhR (green) in mice retinal sections from control to 14 and 28 days after MB treatment (n = 4). Bar = 50 μm. (e) Quantitative PCR Analysis of B. fragilis 16 S gene copies in fecal DNA extracts after gavage administration of B. fragilis for 14 days. Control, n = 13; gavage group, n = 12. (f) Analysis of CYP1A1, CYP1A2, and CYP1B1 mRNA expression in the retina of mice with or without B. fragilis gavage administration for 14 days. (g) H&E-stained eye cross sections at day 28 after MB injection with or without B. fragilis gavage administration. Bar = 50 μm. (h, i) Quantitative analysis of cells number of GCL, thickness of RNFL and IPL in (g). Control, n = 4; B.fragilis, n = 6; MB, n = 8; MB + B. fragilis, n = 7. GCL, ganglion cell layer. (j) Representative confocal photomicrographs taken from the retinal flat mount of mice with βIII-tubulin (red) labeling RGCs at 28 days following MB injected. Bar = 30 μm. (k) The position of the field of view in (j). (l) Percentage of RGCs survival with or without B. fragilis gavage administration after MB injection. Control, n = 5; MB, n = 9; MB + B. fragilis, n = 7. (m) The diagram illustrating the setup of the visual cliff test. (n-p) Histograms showing time of latency to dismount platform of mice, number of mice with first foot on cliff side and total time spent over cliff with or without B. fragilis gavage administration after MB injection in visual cliff test. Control, n = 10; MB, n = 17; MB + B. fragilis, n = 15. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001
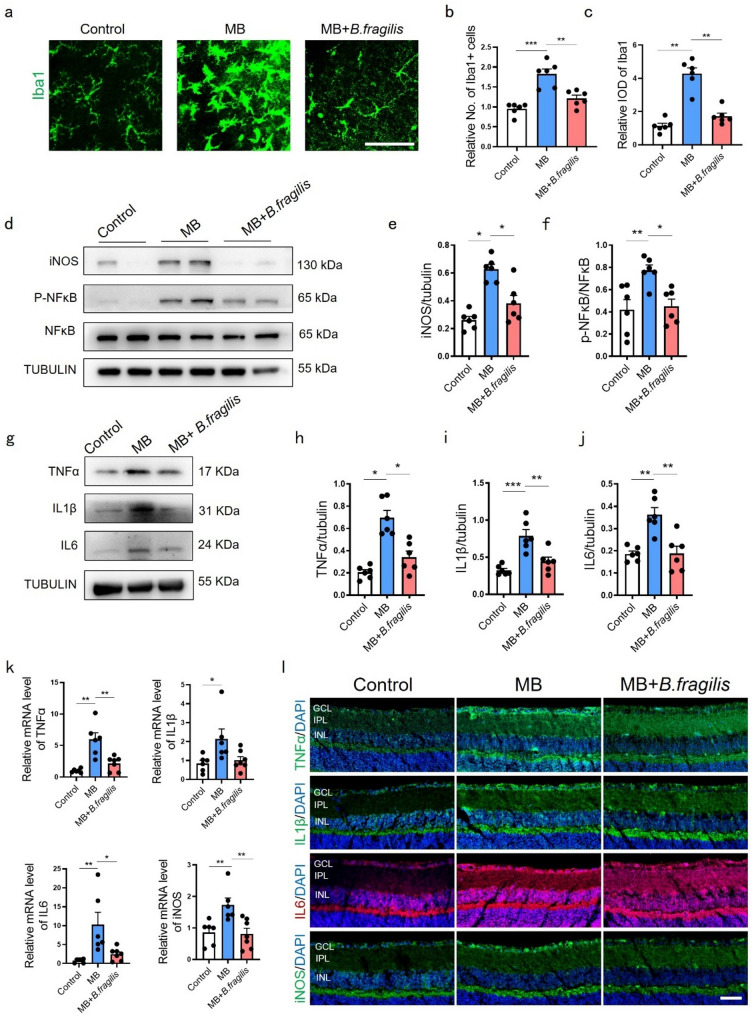
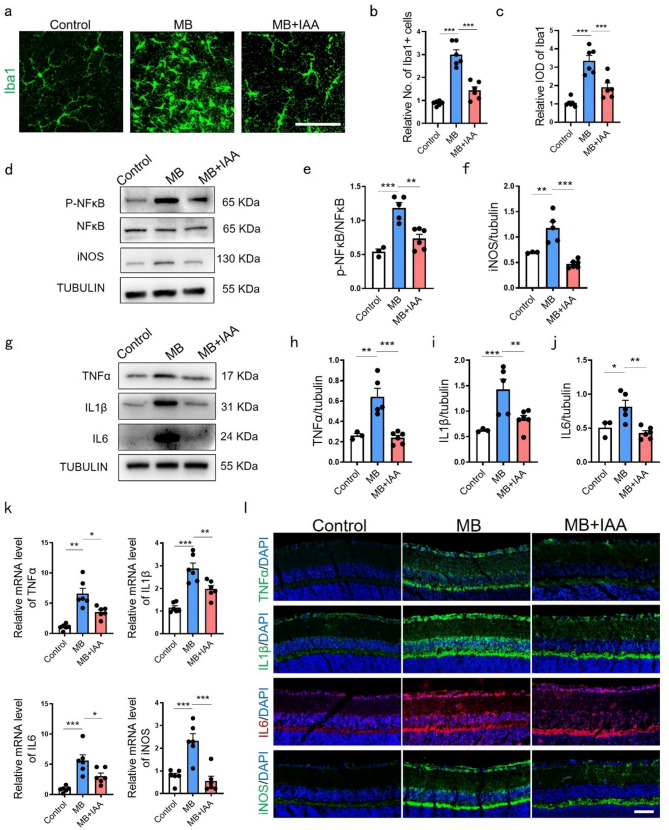
To assess retinal neuroinflammation in MB-induced ocular hypertension, we examined microglial activation and retinal inflammatory signaling. As principal initiators and amplifiers of neuroinflammation, microglial responses drive inflammatory changes that propagate throughout the retinal tissue [24, 25]. MB-treated mice showed increased Iba1 + microglial density and integrated optical density (IOD) compared to controls (Fig. 3a-c). Phospho-NFκB/NFκB ratio, iNOS, TNFα, IL1β, and IL6 were upregulated at protein (Fig. 3d–j) and mRNA levels (Fig. 3k), corroborated by immunofluorescence (Fig. 3l). B. fragilis supplementation suppressed microglial activation (Fig. 3a-c), NFκB phosphorylation (Fig. 3d, f), and overall retinal pro-inflammatory mediator expression (Fig. 3d-l). These findings demonstrated gut-derived AhR activation via B. fragilis modulates retinal inflammation through the gut-eye axis.
Fig. 3.
B. fragilis suppressed neuroinflammation caused by MB-induced ocular hypertension damage. (a) Immunofluorescence staining and relative expression of Iba1 (green) in retinal flat mounts of mice from control, MB and MB + B. fragilis groups. Bar = 50 μm. (b) Relative quantification of Iba1 + cells in retina at day 28 post MB injection with or without B. fragilis. n = 6. (c) Relative integral optical density (IOD) of Iba1 in retina at 28 days post MB injection with or without B. fragilis. n = 6. (d) Representative western blot showing the protein expression levels of iNOS, p-NFκB and NFκB in control, MB and MB + B. fragilis groups. (e-f) Quantification data of proteins in (d) were measured. iNOS was normalized to beta-tubulin, and p-NFκB was normalized to NFκB. n = 6. (g) Representative western blot showing the protein expression levels of TNFα, IL1β, IL6 in control, MB and MB + B. fragilis groups. (h-j) Quantification data of proteins in (g) were normalized to beta-tubulin. n = 6. (k) Relative mRNA expression of TNFα, IL1β, IL6 and iNOS in control, MB and MB + B. fragilis groups evaluated by quantitative real-time PCR. Control and MB groups, n = 6; MB + B. fragilis group, n = 7. (l) Representative immunofluorescence images showing localization and relative expression of TNFα, IL1β, IL6 and iNOS in the GCL, IPL and INL of mice retinal cross-sections. n = 4. Bar = 50 μm. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001
B. fragilis enhances IAA production
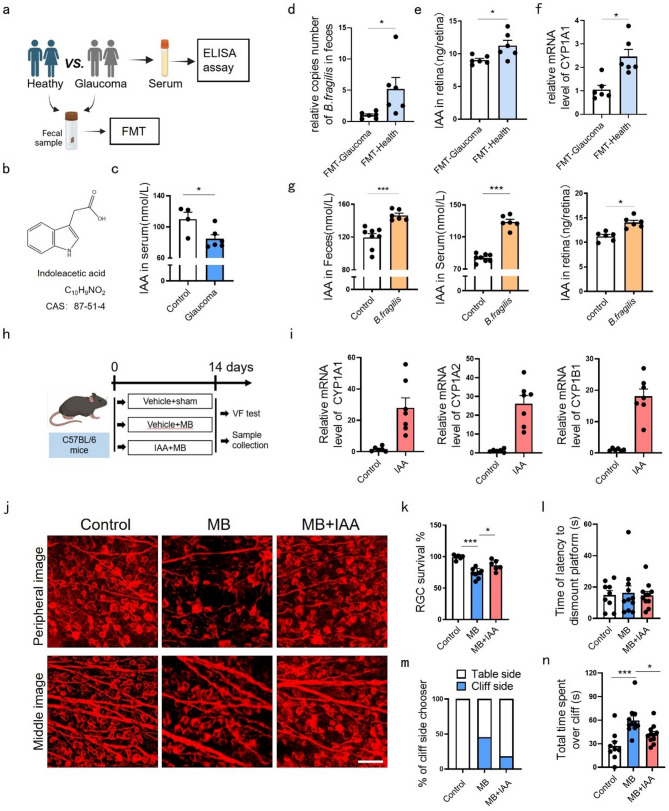
The gut microbiota-metabolite axis mediates gut-eye communication [7, 8]. IAA is B. fragilis’s main tryptophan metabolite [21, 26], and the chemical formula for IAA is shown in Fig. 4b. Clinical sample analysis revealed significantly lower serum IAA levels in glaucoma patients versus controls (Fig. 4a, c), correlating with reduced B. fragilis abundance. Fecal microbiota transplantation (FMT) from glaucoma patients to mice reduced gut B. fragilis colonization (Fig. 4d), retinal IAA level (Fig. 4e), and AhR activity (Fig. 4f) compared to healthy donor-FMT. Conversely, B. fragilis gavage elevated IAA levels in feces, serum, and retinas (Fig. 4g), establishing a causal relationship between B. fragilis and IAA.
Fig. 4.
IAA ameliorated RGCs loss and retinal function impairment in MB-induced ocular hypertension mice. (a) Serum samples from control and POAG patients were collected for ELISA and FMT experiments. (b) The chemical formula for indoleacetic acid. (c) Histograms showed levels of IAA in serum of POAG patients and controls. POAG group, n = 6; control group, n = 4. (d) Quantitative PCR Analysis of B. fragilis 16 S gene copies in fecal DNA extracts after FMT for 7days, n = 6. (e) Histograms showed levels of IAA in retinas of mice after FMT for 7days, n = 6. (f) Quantitative PCR Analysis of CYP1A1 mRNA expression, n = 6. (g) Levels of IAA in mice feces, serum or retinas 14 days post B. fragilis gavage administration tested by ELISA. Control group, n = 8 for feces and serum, n = 6 for retinas; B. fragilis group, n = 6. (h) Schematic illustration of timeline and experimental design. (i) Quantitative analysis of CYP1A1, CYP1A2, and CYP1B1 mRNA expression in the retina of mice with or without IAA administration. Control group, n = 6; IAA group, n = 7. (j) Representative confocal photomicrographs of mice retinal flat mounts with βIII-tubulin (red) labeling RGCs at 14 days following MB injected. Bar = 30 μm. (k) Percentage of RGCs survival with or without IAA administration post MB injection. Control group, n = 6; MB group, n = 8; MB + IAA group, n = 6. (l) Histograms showed time of latency to dismount platform of mice, (m) number of mice with first foot on cliff side and (n) total time spent over cliff with or without IAA administration post MB injection in visual cliff test. Control group, n = 9; MB group, n = 11; MB + IAA group, n = 11. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001
IAA supplementation mitigates RGCs loss and neuroinflammation
Intraperitoneal injection of IAA maintained elevated retinal IAA levels throughout the day (Fig. S3a), whereas topical ocular administration (5 g/L, 5 µL/dose) showed limited retinal bioavailability regardless of dosing frequency (twice or four times daily, Fig. S3b).
As a potent AhR agonist [27], IAA administration activated retinal AhR signaling (Fig. 4i). To evaluate its therapeutic potential, we delivered daily intraperitoneal IAA during MB-induced hypertension (Fig. 4h), which preserved RGCs survival (Fig. 4j, k) and restored visual function (Fig. 4l–n) without systemic toxicity (Fig. S4). Mechanistically, IAA suppressed microglial activation (Fig. 5a-c), reduced NFκB phosphorylation (Fig. 5d, e) and overall retinal inflammatory mediators (TNFα, IL1β, IL6, and iNOS) expression across mRNA (Fig. 5k), protein (Fig. 5d-j) and semi-quantified immunofluorescence signals (Fig. 5l).
Fig. 5.
IAA inhibited neuroinflammation caused by MB-induced ocular hypertension damage. (a) Immunofluorescence staining and relative expression of Iba1 (green) in retinal flat mounts of mice post MB with or without IAA. Bar = 50 μm. (b) Relative quantification of Iba1 + cells in retina at 14 days post MB injection with or without IAA. n = 6. (c) Relative IOD of Iba1 in retina at 14 days post MB injection with or without IAA. n = 6. (d) Representative western blot showing the protein expression levels of p-NFκB, NFκB and iNOS in control, MB and MB + IAA groups. (e-f) Quantitative protein levels in (d) were measured. iNOS was normalized to beta-tubulin, and p-NFκB was normalized to NFκB. Control group, n = 3; MB group, n = 5; MB + IAA group, n = 6. (g) Representative western blot showing the protein expression levels of TNFα, IL1β, IL6 in control, MB and MB + IAA groups. (h-j) Quantitative data of proteins in (g) were normalized to beta-tubulin. Control group, n = 3; MB group, n = 5; MB + IAA group, n = 6. (k) Relative mRNA expression of TNFα, IL1β, IL6 and iNOS in control, MB and MB + IAA groups evaluated by quantitative PCR. n = 6. (l) Representative immunofluorescence images showing localization and relative expression of TNFα, IL1β, IL6 and iNOS in the GCL, IPL and INL of mice retinal cross-sections. n = 5. Bar = 50 μm. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001
IAA suppresses microglial inflammation via AhR activation in vitro
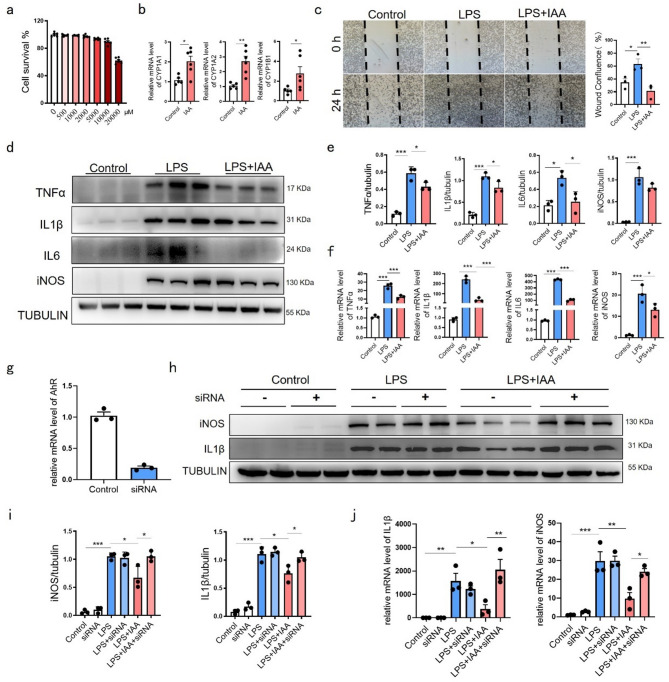
Using lipopolysaccharide (LPS)-stimulated BV-2 microglia, we evaluated IAA’s anti-inflammatory effects. We selected a safe IAA concentration based on published reports [27] and our concentration-gradient experiments (Fig. 6a). IAA activated AhR in BV-2 cells, evidenced by upregulated downstream targets (Fig. 6b). Scratch assays revealed IAA-mediated inhibition of microglial migration (Fig. 6c). Western blot and qPCR analyses confirmed IAA attenuated LPS-induced inflammatory markers (Fig. 6d–f). AhR knockdown (> 80% efficiency, Fig. 6g) abolished IAA’s suppression of IL-1β and iNOS at protein (Fig. 6h, i) and mRNA levels (Fig. 6j), establishing AhR-dependency for IAA’s anti-inflammatory action.
Fig. 6.
IAA relieved microglial inflammation by activating AhR. (a) The CCK8 assay was employed to assess the impact of varying concentrations of IAA on the viability of BV-2 cells. n = 6. (b) Analysis of CYP1A1, CYP1A2, and CYP1B1 mRNA expression of BV-2 cells with or without IAA administration. Control group, n = 5; IAA group, n = 6. (c) Histogram and photographs showing microglial wound confluence post scratch with or without IAA. n = 3. Bar = 200 μm. (d) Representative western blot showing the protein expression levels of TNFα, IL1β, IL6 and iNOS in control, LPS and LPS + IAA groups. (e) Quantitative data of proteins in (d) were normalized to beta-tubulin. n = 3. (f) Relative mRNA expression of TNFα, IL1β, IL6 and iNOS in control, LPS and LPS + IAA groups evaluated by quantitative PCR. n = 3. (g) AhR silencing efficiency > 80% reduction in AhR mRNA. n = 3. (h) Representative western blot showing the protein expression levels of iNOS and IL1β in control, siRNA AhR, LPS, LPS + siRNA AhR, LPS + IAA and LPS + IAA + siRNA AhR groups. (i) Quantitative data of proteins in (h) were normalized to beta-tubulin. n = 3. (j) Relative mRNA expression of iNOS and IL1β in control, siRNA AhR, LPS, LPS + siRNA AhR, LPS + IAA and LPS + IAA + siRNA AhR groups. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001
IAA-mediated retinal protection involves AhR and RAGE signaling
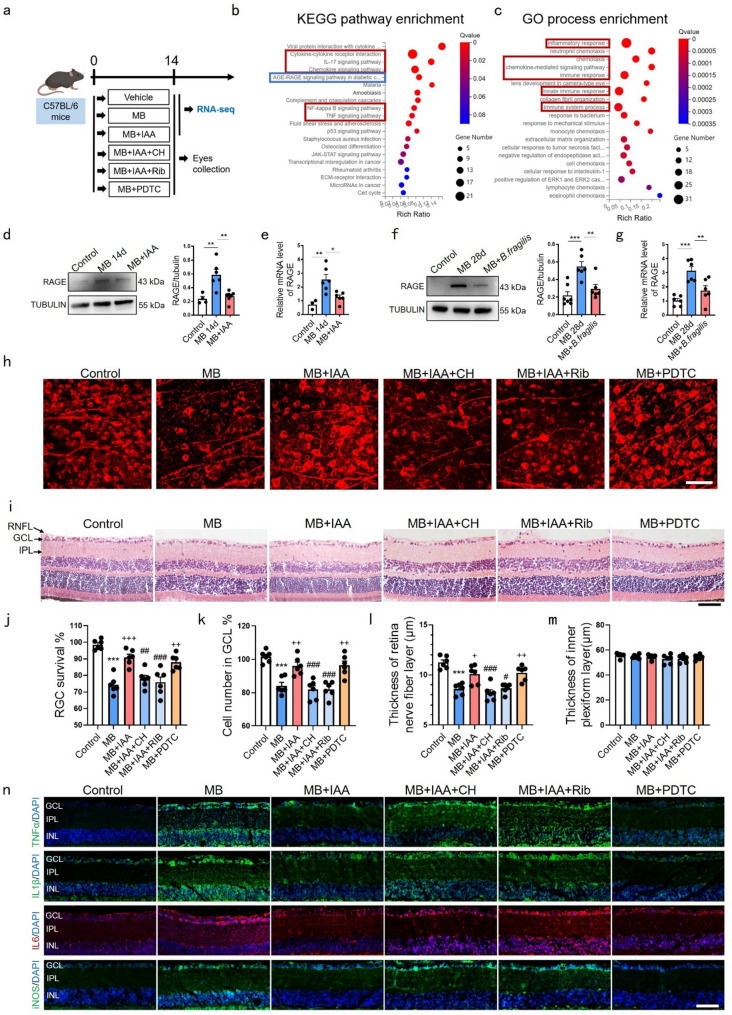
To delineate IAA’s molecular mechanisms, we performed RNA sequencing on mice retinas (NCBI accession: PRJNA1171984), analyzing differentially expressed genes between MB and MB + IAA groups (Fig. 7a). Pathway enrichment analysis revealed significant upregulation of immunoinflammatory pathways (red clusters) and RAGE signaling (blue cluster) in MB-treated retinas (Fig. 7b, c). Ocular hypertension induction elevated retinal RAGE mRNA/protein levels, which IAA administration suppressed at 14 days post-MB (Fig. 7d, e). Similarly, B. fragilis supplementation attenuated RAGE expression at 28 days post-MB (Fig. 7f, eg).
Fig. 7.
Effects of IAA on RGCs and inflammation depend on AhR activation and RAGE inhibition. (a) Schematic illustration of timeline and experimental design. (b) KEGG pathway enrichment and (c) GO process enrichment of the differences between MB and MB + IAA groups. (d) Representative western blot showing the protein expression levels of RAGE at 14 days post MB injection with or without IAA. Quantitative data of proteins were normalized to beta-tubulin. Control group, n = 4; MB group, n = 6; MB plus IAA group, n = 7. (e) Relative mRNA expression of RAGE in control, MB and MB + IAA groups. (f) Representative western blot showing the protein expression levels of RAGE at 28 days post MB injection with or without B. fragilis. Quantitative data of proteins were normalized to beta-tubulin. Control group, n = 7; MB group, n = 6; MB + IAA group, n = 6. (g) Relative mRNA expression of RAGE in control, MB and MB + B. fragilis groups. n = 6. (h) Representative confocal photomicrographs of mice retinal flat mounts with βIII-tubulin (red) labeling RGCs in control, MB, MB + IAA, MB + IAA + CH (CH223191, AhR inhibitor), MB + IAA + Rib (D-Ribose, RAGE agonist), MB + PDTC (NFκB antagonist) groups. Bar = 30 μm. (i) H&E-stained eye cross sections at day 14 post-MB in above mentioned 6 groups. Bar = 50 μm. (j) Percentage of RGCs survival in (h). n = 6. (k-m) Quantitative analysis of cells number of ganglion cell layer, thickness of RNFL and IPL in (i). n = 6. (n) Representative immunofluorescence images showing localization and relative expression of TNFα, IL1β, IL6 and iNOS in the GCL, IPL and INL of mice retinal cross-sections. n = 4. Bar = 50 μm. Data are presented as mean ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared to MB group. #p < 0.05, ##p < 0.01, ###p < 0.001 compared to MB + IAA group
We established six experimental groups: Control, MB, MB + IAA, MB + IAA + CH223191 (AhR inhibitor), MB + IAA + D-ribose (RAGE agonist [28]), and MB + PDTC (NFκB inhibitor, positive control) (Fig. 7a). Compared to the MB + IAA group, CH223191 or D-ribose co-treatment reduced RGCs survival (Fig. 7h, j), decreased GCL density, and attenuated RNFL (Fig. 7i, k-m). Immunofluorescence assays confirmed these interventions reversed IAA’s suppression of inflammatory mediators (Fig. 7n). These findings demonstrate IAA’s neuroprotective and anti-inflammatory effects depend on AhR activation and RAGE pathway inhibition.
IAA rescues microglial inflammation and microglia-mediated neurotoxicity through AhR/RAGE/NFκB pathway
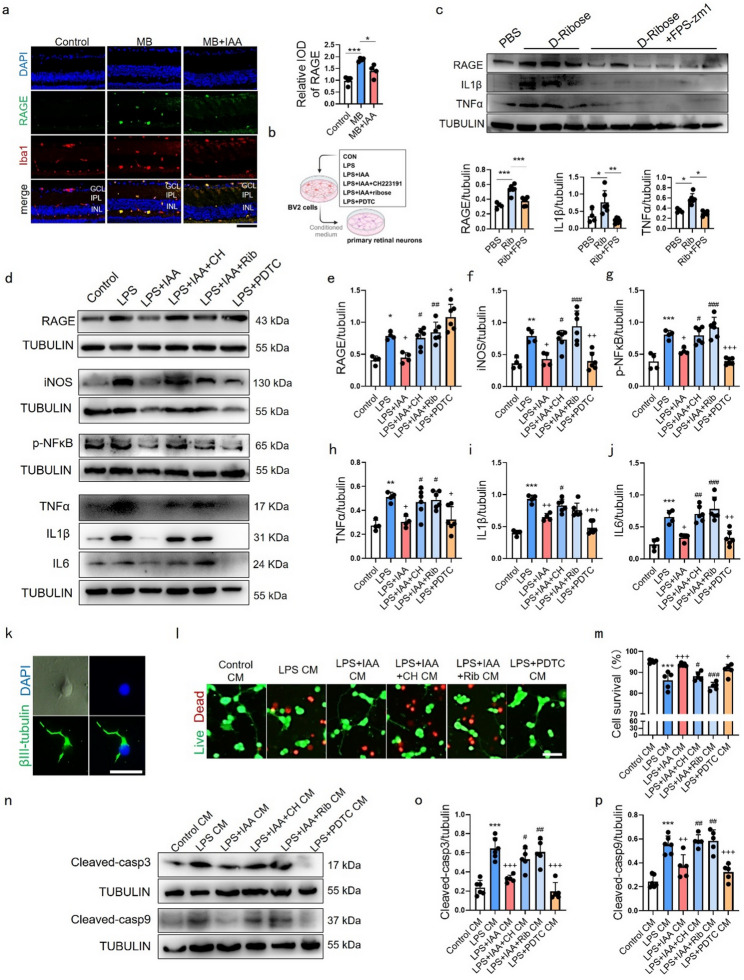
Immunofluorescence assay demonstrated that RAGE expression was co-localized with microglia on retinas and elevated after MB-induced ocular hypertension injury (Fig. 8a). We also observed minimal RAGE co-localization with astrocytes (GFAP+) in retinas (Fig. S5), suggesting astrocytic RAGE is not a major contributor in our model. To verify the effect of IAA on neuroinflammation and neurotoxicity in BV-2 cells, we set up control, LPS, LPS + IAA, LPS + IAA + CH223191, LPS + IAA + D-ribose and LPS + PDTC groups (Fig. 8b). RAGE, IL1β and TNFα expression in PBS-treated-, D-ribose-treated- or D-ribose + FPS-zm1 (RAGE inhibitor, to confirm the target specificity of D-ribose) treated BV-2 cells were detected by western blot, suggesting that D-ribose can lead an inflammatory cellular response in BV-2 microglia through activating RAGE (Fig. 8c). Our findings indicated that IAA reduced RAGE expression induced by LPS in BV-2 cells and the effect was eliminated by CH223191, D-ribose and PDTC, indicating that AhR was necessary for IAA to inhibit RAGE expression (Fig. 8d-e). PDTC (a positive control for anti-inflammation) effectively inhibited NFκB activity and reduced inflammatory cytokines but paradoxically increased RAGE levels, suggesting potential negative feedback or complex interplay within the AhR-RAGE-NFκB axis (Fig. 8d-j). IAA counteracted LPS-induced NFκB phosphorylation and inflammatory mediator expression (iNOS/TNFα/IL1β/IL6), with its efficacy diminished upon AhR inhibition or RAGE activation (Fig. 8d, f-j). Collectively, these results position IAA as acting through AhR to modulate RAGE expression and associated NFκB-mediated inflammation.
Fig. 8.
Effects of IAA on inflammatory response and cell neurotoxicity induced by LPS in BV-2 cells. (a) Representative immunofluorescence images showing relative expression of RAGE (green) and its co-localization with microglia marker Iba1 (red) in the GCL, IPL and INL of mice retinal cross-sections. The graph on the right demonstrates the IOD of RAGE in retina at 14 days post MB injection with or without IAA. n = 4. Bar = 50 μm. (b) Schematic illustration of the experimental design of BV-2 conditioned medium assay. (c) Representative western blot showing the protein expression levels of RAGE, IL1β and TNFα in PBS, D-Ribose and D-Ribose + FPS-zm1 groups. Quantitative data of proteins were measured and normalized to beta-tubulin. PBS group, n = 4; D-Ribose group, n = 6; D-Ribose + FPS-zm1 group, n = 6. *P < 0.05, **P < 0.001, ***P < 0.001. (d) Representative western blot showing the protein expression levels of RAGE, iNOS, p-NFκB, TNFα, IL1β, IL6 in control, LPS, LPS + IAA, LPS + IAA + CH, LPS + IAA + Rib, LPS + PDTC groups. (e-j) Quantitative data of proteins in (d) were normalized to beta-tubulin. control, LPS and LPS + IAA groups, n = 4; LPS + IAA + CH, LPS + IAA + Rib, LPS + PDTC groups, n = 6. *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared to LPS group. #p < 0.05, ##p < 0.01, ###p < 0.001 compared to LPS + IAA group. (k) Optical and immunofluorescent images of primary retinal neurons, RGCs were labeled by βIII-tubulin (green). Bar = 50 μm. (l) immunofluorescence of live (green)/dead (red) retinal neurons following CM treatment. Bar = 25 μm. (m) Percentage of neurons survival in (l). n = 5. (n) Representative western blot showing the protein expression levels of cleaved-caspase3 and cleaved-caspase9 in primary retinal neurons treated with conditioned medium from control, LPS, LPS + IAA, LPS + IAA + CH, LPS + IAA + Rib, LPS + PDTC BV-2 groups. (o, p) Quantitative data of proteins in (n) were normalized to beta-tubulin. Control CM group, LPS CM group and LPS + IAA CM group, n = 6; LPS + IAA + CH CM group, LPS + IAA + Rib CM group, LPS + PDTC CM group, n = 5. ***P < 0.001 compared to the control CM group; +p < 0.05, ++p < 0.01, +++p < 0.001 compared to LPS CM group. #p < 0.05, ##p < 0.01, ###p < 0.001 compared to LPS + IAA CM group. Data are presented as mean ± SEM
To assess IAA’s role in alleviating microglial neurotoxicity, we established a conditioned medium (CM) transfer system using BV-2 microglia and primary retinal neurons isolated from postnatal day 1 (P1) rats. Neurons were cultured for three days and validated by βIII-tubulin immunostaining (Fig. 8k). CM from six experimental BV-2 groups (Fig. 8b) was applied to neurons. Live-dead assays revealed that LPS-treated BV-2 CM induced neuronal death, while IAA- or PDTC-treated BV-2 CM attenuated neurotoxicity. This protection was abolished by AhR inhibition (CH223191) or RAGE activation (D-ribose) (Fig. 8l, m). Western blot analysis of cleaved caspase-3/9 confirmed IAA pretreatment in BV-2 cells suppressed LPS-induced neuronal apoptosis, an effect nullified by CH223191 or D-ribose (Fig. 8n–p).
Discussion
Host-associated microbiota serves as key modifiers in neuronal activity through metabolic pathway. Investigating microbial and metabolite dynamics in glaucoma pathogenesis unveils novel therapeutic targets. In this study, we elucidated the role of microbiota-derived tryptophan metabolism in glaucoma using clinical samples analysis, bacterial colonization, FMT and metabolite supplementation (Fig. 9). Our key findings demonstrate that: (1) glaucoma patients exhibit reduced abundance of tryptophan-metabolizing bacteria B. fragilis and decreased serum IAA; (2) B. fragilis colonization elevates retinal IAA levels, attenuating RGCs loss and neuroinflammatory responses in glaucomatous mice, replicating the effects of IAA administration; (3) IAA-mediated neuroprotection operates through AhR activation, which suppresses RAGE/NFκB signaling to mitigate microglia-driven neurotoxicity. These findings position the microbiota-IAA-AhR axis as a therapeutic strategy for glaucoma-associated neurodegeneration.
Fig. 9.
RGCs damage in glaucoma were ameliorated via Gut microbiota-IAA-eye signaling pathway. Dysbiosis of tryptophan metabolism-related microbiota B. fragili leads to low level of its metabolite IAA in glaucoma. By in vivo and in vitro experiment we demonstrated supplement of B.fragilis or IAA could activate AhR and inhibit RAGE/NFκB to suppress retinal neuroinflammation and neurotoxicity, and ultimately relieved the progression of RGCs loss
B. fragilis, recognized as a next-generation probiotic, demonstrates therapeutic potential in chronic kidney diseases [29], aging-related atrial fibrillation [30] and necrotizing enterocolitis [31] via immunomodulation. Its marked depletion in POAG patients, coupled with emerging mechanistic links to neuroprotective and immune homeostasis pathways [32–34], prompting its selection for therapeutic evaluation in glaucoma models. Administration of B. fragilis alleviated retinal neuroinflammation, improved RGCs survive and enhanced retinal function in glaucomatous mice. Gut microbiota-derived metabolite IAA—the predominant tryptophan derivative in humans [21]—were significantly reduced in POAG serum. B. fragilis colonization elevated IAA levels in mice serum, feces, and retinas, corroborating its role in IAA biosynthesis. Human studies further link IAA production to B. fragilis and B. thetaiotaomicron [35], aligning with our findings. IAA supplementation has shown therapeutic effects in a variety of diseases. Ji et al. found IAA relieved high fatty diet-induced fatty liver disease through reducing macrophage infiltration and inflammatory factors expression [36]. Li et al. demonstrated that IAA could decrease oxidative stress and neuroinflammation in mice brain through oral or intraperitoneal administration [37]. Our work extends IAA’s neuroprotective role to ocular pathologies, mediated through AhR-dependent immunomodulation.
A key question we addressed was how IAA exerted its neuroprotective effect. Microbiota-derived tryptophan metabolites, particularly IAA, serve as potent endogenous AhR ligands with distinct biological effects from environmental AhR agonists [21]. Our data demonstrate that IAA treatment attenuates microglial activation and reduces overall levels of NFκB-driven inflammatory mediators in the retina—key contributors to RGC death in glaucoma [25, 38–40]. Transcriptomic analyses identified IAA-mediated downregulation of RAGE signaling. RAGE is a multiligand pattern recognition receptor mediating damage-associated inflammatory responses [41–43], and was upregulated in the retinas and optic nerve heads of POAG patients [44]. Researchers found that inhibition of RAGE with lycium barbarum polysaccharides reduced retinal gliosis and improved neuronal survival in mice under acute ocular hypertension insult [45]. Chen et al. observed RAGE co-localization with activated microglia in glaucomatous retinas [46], consistent with our findings. Here, we mechanistically linked IAA’s effects to AhR-dependent RAGE suppression in our in vivo experiments and microglia: AhR inhibition significantly attenuated IAA’s ability to downregulate RAGE and its downstream inflammatory mediators. While astrocytes are also recognized drivers of retinal neuroinflammation [47], we did not observe significant RAGE expression specifically associated with astrocytes in this study. This suggests that microglial AhR-RAGE signaling plays a critical role in mediating the anti-neuroinflammatory effects of IAA. Future studies on the temporal interactions between glial cell subsets will be of great value, and parallel investigation into neuron-specific AhR functions could provide critical insights into compartmentalized neuroprotective pathways.
To isolate microglia-neuron crosstalk from systemic confounders, we employed a co-culture model where primary retinal neurons were exposed to CM from LPS-activated microglia—recapitulating glaucoma’s inflammatory milieu. This design exclusively attributes observed effects to microglial secretory factors, eliminating contributions from other retinal cells. Consistent with established mechanisms linking glial inflammation to Caspase-dependent apoptosis [47], LPS-microglial CM triggered profound neuronal death, which IAA preconditioning of microglia reversed. However, the effect was eliminated by AhR inhibitor or RAGE agonist. These results unambiguously demonstrate that microglial AhR/RAGE signaling—rather than neuronal intrinsic pathways—as the decisive mediator of IAA’s neuroprotection. This axis modulates retinal immune microenvironments to preserve RGCs viability.
Glaucoma is a refractory disease, and current treatment primarily relies on the use of eye drops and surgery to control IOP. In addition to treating symptoms, emerging strategies must address systemic contributors, including gut microbiota homeostasis and neuroinflammation modulation. While our study demonstrates B. fragilis-mediated preservation of RGCs and visual function, its dual role as an opportunistic pathogen necessitates caution. Certain strains have been implicated in clinical pathologies, including inflammatory bowel disease and neurological complications [34]. To mitigate these risks, stringent strain selection—prioritizing non-toxigenic variants lacking virulence factors—or alternative strategies such as engineered bacterial derivatives should be pursued to harness therapeutic benefits while minimizing potential adverse effects [48]. In addition, supplementation of microbial metabolites like IAA may offer superior safety and efficacy over live bacterial administration [49]. Although IAA remains investigational, its structural analog indomethacin—a clinically used analgesic nonsteroidal and anti-inflammatory drug with oral, rectal, and ocular formulations [48]—provides a translational roadmap [50]. In this study, we supplemented IAA by intraperitoneal injection, which allows the drug to quickly enter the circulatory system and mimic the effects of intravenous administration in clinical practice. Simpler and non-invasive ways of administration are worth exploring. Topical administration, though convenient, showed limited retinal penetration in our preliminary tests. Innovative delivery systems utilizing liposomes, nanomicelles, or chemical modifications may enhance posterior segment bioavailability [51–53]. Oral interventions also hold promise. Dietary strategies enriched in tryptophan modulate gut microbiota and mitigate neuroinflammation [54, 55]. In addition to the need for optimization of drug delivery, the limited human cohort sample in our study may also affect the generalizability and statistical power of the conclusions. Although a statistically significant difference was observed, it needs to be validated in larger multicenter studies. In summary, our findings establish the gut microbiota-eye axis—mediated by B. fragilis and IAA—as a viable therapeutic target for glaucoma. Translational success will require optimized delivery strategies, rigorous safety profiling, and clinical validation of metabolite-based interventions.
Conclusion
All our works have allowed us to infer a novel conceptual advancement in understanding the pathogenesis or risk factors of glaucoma. Glaucoma is not only an abnormality of ocular structure and function, but also involves complex crosstalk between the gut microbiota and the eye. The downregulation of tryptophan metabolism related microbiota during glaucoma progression leads to an inevitable decline in circulating IAA which can inhibit RAGE in an AhR-dependent manner to ameliorate the microglia-derived neuroinflammatory response and neurotoxicity and suppress the progression of glaucoma. More importantly, this study demonstrated the effect safety of tryptophan microbiota metabolite IAA on maintaining RGCs survival, which provided a new target and strategy for the treatment of glaucoma.
Methods
Human samples
Stool samples were collected from 30 individuals, including 15 POAG patients and 15 healthy controls. The two groups were matched in terms of age, gender, BMI, geographic location (all from the middle-lower Yangtze Plain in China), ethnicity and diet (traditional Chinese diet, primarily consuming refined grains). Serum samples were collected from POAG and age-/sex- matched cataract patients (offer ethical feasibility while exhibiting distinct pathological mechanisms from glaucoma). This project was approved by the Medical Ethnics Committee of Shanghai Ninth People’s hospital, Shanghai Jiao Tong University School of Medicine (No. SH9H-2021-T184-2). All participants provided written informed consent, and the protocol followed the principles of the Declaration of Helsinki. The diagnostic criteria for POAG are as follows: (1) IOP > 21mmHg in the affected eye at the initial diagnosis; (2) Excavation of optic disc: progressive increase of C/D ratio, C/D ratio > 0.5, or asymmetry of C/D ratio in eyes (≥ 0.2); (3) Visual field (MD value) changes: Characteristic glaucoma visual field defects consistent with ocular fundus examination; (4) open angles: Shaffer grade 3 and above. The following conditions will be excluded: (1) Use any of the following drugs: proton pump inhibitors, antidepressants, metformin, insulin, glucocorticoids, etc.; (2) Use of systemic (oral, intravenous, etc.) or ocular topical (eye drops, ointments, etc.) antibiotics within the past 4 weeks; (3) Suffering from digestive diseases such as inflammatory bowel disease, irritable bowel syndrome, chronic diarrhea, chronic constipation, etc.; (4) History of gastrointestinal surgery; (5) Suffering from any of the following diseases: high blood pressure, diabetes, heart disease, liver disease, kidney disease, malignant tumor, etc.; (6) Patients with other eye diseases (such as diabetic retinopathy, uveitis and other diseases); (7) Pregnant women and patients with major underlying diseases (liver and kidney failure, malignant tumors, etc.). BMI (height/weight2) and dietary habits of all participants were also collected.
Fecal DNA extraction
Each participant was provided with a fecal collection kit to collect about 2 g of fresh morning stool samples. These samples were immediately stored at -80 °C until further processing. Total genomic DNA was extracted by QIAamp DNA stool mini kit. The quality of DNA was checked on a 1.2% agarose gel. Blank extraction controls using sterile water instead of fecal samples were processed alongside experimental samples in every extraction batch.
16 S rRNA sequencing and analysis
Bidirectional sequencing was performed according to illumina’s high-throughput sequencing requirements, and the V3-V4 region and fusion primer with a “5 ‘connector -barcode- sequencing primer - specific primer − 3’” were designed. The specific primers were 357F 5’-ACTCCTACGGRAGGCAGCAG-3’; 806R 5’-GGACTACHVGGGTWTCTAAT-3’. A two-step PCR amplification method was used to construct the library. First, the target fragment was amplified by specific primers, the target fragment was recovered by AxyPrepDNA gel recovery kit (AXYGEN), and the recovered product was quantified by FTC-3000TM real-time PCR instrument. The recovered products were then used as templates for secondary PCR amplification.
The multi-sample parallel sequencing method was used and barcode sequence containing sample source information was introduced to distinguish samples. The original sequence is divided according to Barcode information, and the complete barcode label sequence is considered as a valid sequence. Trimmomatic(version:0.38) software was used for sequence quality control, and cutadapt(version:1.16) software was used for sequencing connectors and primers. FLASH (version:1.2.11) software was used to splice pairs of reads into a sequence according to the overlap between PEreads. The minimum overlap length was 10 bp, and the maximum mismatch ratio allowed in the overlap area of the splicing sequence was 0.2. Flash (Version 1.2.11) and mothur (version:1.39.5) were used for sequence optimization after merge. The CleanTags processed above were OTU clustered, and then OTU species were classified by OTU annotation. USEARCH software was used to cluster the merged sequences into OTU. All sequences were classified into OTU, and the OTU at 97% similarity level was analyzed for biological information, including OTU analysis based on sequence similarity clustering, species taxonomic analysis, α diversity analysis, β diversity analysis, and inter-group difference analysis. For microbiomes sequencing data, GraphPad Prizm and R (3.6.0) were performed for analysis. Kolmogorov-Smirnov test was used for normal distribution. Non-parametric tests were used to analyze the demographic data of POAG patients and healthy controls. For data without normal distribution, Mann-Whitney test was used. The differences in the abundance of various microbiomes between the two groups were elevated by non-parametric Wilcox. Benjamini-Hochberg false discovery rate (FDR) adjust < 0.25 was defined as statistically significant cutoff.
Mice and MB-induced ocular hypertension model
All mice experiments were approved by the Committee of Animal Care of the Shanghai 9th People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine (Approval no. SH9H-2022-A16-2) and performed in accordance with the institutional protocol guidelines and the Association for Research in Vision and Ophthalmology. 6–8 weeks old C57BL/6 mice were purchased from Jiesijie (Shanghai, China) and maintained under standard environmental temperature (21 ± 1 °C) with 14 h light and 10 h dark cycle. The experimental glaucomatous model was established by MB according to the previously reported method [56]. Briefly, the mice were anesthetized with zolitil/dexmedetomidine mixture, and 2 µl (about concentration of 5.0 × 106 MB/ml) MB (diameter of 15 μm, Invitrogen) were pushed into the anterior chamber of the mice with a 30G needle (Fig. 2b). The control group received sham surgery (without microbead injection). IOP was measured at Days 1, 3, 7, 14, 21, and 28 post-injection using Icare TonoLab (Icare Finland Oy, Finland) (Fig. 2c). Measurements were performed at 9:00 AM under isoflurane anesthesia, with five consecutive readings averaged per eye.
B. fragilis colonization
Mice were randomized into four groups: Control, MB, B. fragilis-colonized, and MB + B. fragilis. Before colonization, B. fragilis-treated groups received a 14-day antibiotic cocktail, including ampicillin (sigma) 1 g/L, metronidazole (Apexbio) 1 g/L, neomycin (Apexbio) 1 g/L and vancomycin (sigma) 0.5 g/L, in drinking water followed by a 2-day washout [57]. On Day 16, mice received daily oral gavage with 108 CFU/mL of B. fragilis ATCC25285 (non-toxigenic strain [58]) in 0.2 mL PBS or PBS vehicle. After 14 days, the MB-induced ocular hypertension modeling was performed, with thrice-weekly gavage maintenance until endpoint.
Drug application
For in vivo studies, IAA (20 mg/kg, sigma), CH223191 (10 mg/kg, Abmole, TX, USA), D-ribose (3.2 g/kg, sigma) or PDTC (100 mg/kg, Abmole, TX, USA) dissolved in same vehicle (5% DMSO in PBS) were injected intraperitoneally at the same time with MB induction and were administered daily subsequently. Mice in the control group received sham and vehicle. In vitro, BV-2 cells were pretreated with 1000 µM IAA, 10 µM CH223191, 50 mM D-ribose or 100 μm PDTC 4 h prior to LPS stimulation. For the FPS-zm1 treatment experiment, the BV-2 cells were pretreated with FPS-ZM1 (20 µM) for 2 h and then exposed to D-ribose (50 mM) for 24 h. The administration method and dosage of IAA [27, 59], CH223191 [59], D-ribose [28], FPS-zm1 [60] and PDTC [61, 62] were based on previous studies.
BV-2 culture and LPS stimulation model
The BV-2 murine microglia cell line was purchased from Cellcook Biotech Co.,Ltd (Guangzhou, China) and was cultured at 37 °C in a 5% CO2 humidifed incubator using Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Franklin Lakes, NJ, USA) supplemented 4.5 g/L D-Glucose, 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. For RNA and protein extraction, LPS (100 ng/ml, sigma) was administered after drug administration for 4 hours, and the cells were harvested after a further 4 hours; for the preparation of conditioned medium, the culture medium was replaced 4 hours after LPS administration and the cells were incubated for another 24 h. Cell viability was assessed using a CCK-8 assay kit (Yeasen, Shanghai, China). Briefly, BV-2 cells were seeded in 96-well culture plates at a density of 1 × 104 cells per well and incubated in DMEM supplemented with 10% FBS containing varying concentrations of IAA for 24 h. After incubation, the culture medium was removed, and 10% CCK-8 solution was added to each well. The cells were then incubated at 37 °C for 3 h, followed by measurement of the absorbance at 450 nm using a microplate reader.
SiRNA
The siRNA sequence was synthesized by Genomeditech (Shanghai). For siRNA-lipid complexes preparation, 50 nM AhR-targeting siRNA was diluted in Opti-MEM medium and mixed with Lipofectamine™ 3000 (also diluted with 50 µL Opti-MEM), followed by incubation at room temperature for 20 min. The siRNA complexes were then added dropwise to the wells and incubated for 24 h. After transfection, quantitative PCR was employed to assess the silencing efficiency, followed by subsequent experiments according to the designed groups.
Scratch wound healing assay
The scratch test references previous study [63]. BV-2 cells were seeded in 6-well plates to achieve 80% confluence and were then scratched with a sterile pipette with 200 µl tip followed by PBS washing to clean debris and dead cells. The cells were then divided into control, LPS, and LPS + IAA groups. Images were taken with a microscope at 0 h and 24 h, and cell migration was analyzed with imageJ.
Fecal microbiota transplantation experiment
The FMT procedure followed methods established in prior studies [64]. Stool samples from healthy controls and glaucoma patients (n = 5) were randomly selected and mixed in equal weights. The samples were then homogenized in pre-reduced phosphate-buffered saline. After vortexing for 5 min, the mixture was allowed to stand for another 5 min to allow larger particles to settle at the bottom of the tube. Mice pretreated with a broad-spectrum antibiotic cocktail were weight-matched and divided into two groups. Each group was administered 200 µl of the supernatant via oral gavage once daily for one week to establish colonization.
Histology and morphometric analysis
At 14 or 28 days after MB-induced ocular hypertension injury, mice were perfused with saline for 5 min to remove blood cells in the blood vessels, followed by fixation with 4% paraformaldehyde for 15 min. The eyeballs were immersed overnight with FAS eyeball fixture (Servicebio, Wuhan, China). The eyeballs were embedded in paraffin or optimal cutting temperature compound and cut into 10 μm slices, after which hematoxylin and eosin (H&E) staining was performed to analyze changes in retinal structure and the number of cells in GCL. The number of cells in 5 consecutive fields of retinal GCL was counted, averaged, and normalized with con group. The thickness of RNFL and inner plexiform layer (IPL) were measured at the same distance from the optic nerve head of each retina by ImageJ software. To explore the safety of B. fragilis or IAA, the heart, liver, spleen, lung and kidney tissues of mice were taken for paraffin embedding, section and H&E staining, and their structure changes were analyzed. Each group contained at least 3 samples.
Neurodegeneration evaluation
Neuronal degeneration was assessed by counting RGCs number at 14 days or 28 days after MB-induced ocular hypertension injury. Whole-mount retinas were obtained as previous reported [65]. Briefly, retinas were immersed in 4% PFA for 2 h and then dissected to flat-mount. Retinas were fixed with 4% PFA for 15 min followed by 0.3% Triton x-100 in PBS for permeabilization, and blocked with 5% goat serum (Boster, CA, USA) in 0.3% triton x-100 in PBS for 2 h at room temperature. Next, flat-mounts were incubated with primary antibody against βIII-tubulin (1:1000; Abcam, Cambridge, UK) overnight at 4℃ to label RGCs, followed by Alexa Fluor 594 anti-mouse secondary antibody (1:200; Abbkine, CA, USA) for 2 h at room temperature. Flat-mounts were photographed with confocal microscope (Nikon, Tokyo, Japan). A total of 8 visual fields in the middle and peripheral parts of the retina were obtained and counted using imageJ according to previous reported [66]. The average was calculated and standardized by control group.
Quantitative RT-PCR (qPCR)
Total RNA was extracted from retinal tissues using TRIzol (Invitrogen, CA, USA) and BV-2 cells using RNA extraction kit ((TaKaRa, Tokyo, Japan) as previous methods [59]. The concentration and purity of RNA was measured using Nanodrop (THERMO FISHER, USA) and reverse transcription was performed with PrimeScript RT reagent Kit (Takara, Kyoto, Japan). And fecal DNA of mice was extracted by MolPure Stool DNA Kit (Yeasen, Shanghai, China). LightCycler 480 II (Roche, Switzerland) with UNICON qPCR SYBR Green Master Mix (Yeasen, Shanghai, China) was used for gene expression quantitation. The primers used in this study refer to PrimerBank and were produced by Tsingke (Beijing, China). Primer sequences were list in Table S8. For gene expression analysis, beta-actin was used as endogenous control. For analysis of B.fragilis 16 S gene copies number in feces, 16 S rRNA was used as endogenous control. The fold changes were calculated by the 2−Δ(ΔCT) comparative method.
Western blot
Proteins were extracted from retinas or cells by RIPA (Sangon, Shanghai, China), and the concentration was measured using BCA Protein Assay Kit (Beyotime, Shanghai, China). Proteins were separated in the 10% SDS-PAGE and transferred to the polyvinylidene fluoride membranes. Then the membranes were blocked by 5% non-fat milk in Tris-buffered saline containing Tween-20. The membranes were incubated overnight at 4℃ with Primary antibodies as follows: TNFα (1:1000; Proteintech, Chicago, IL, USA), IL-1β (1:1000; Proteintech, Chicago, IL, USA), IL-6 (1:1000; Proteintech, Chicago, IL, USA), iNOS (1:1000; Abclonal, Wuhan, China), NFκB (1:1000; Proteintech, Chicago, IL, USA), p-NFκB (1:1000; Abcam, Cambrige, UK), RAGE (1:1000; Abmart, Shanghai, China), Tubulin (1:1000; Abmart, Shanghai, China), Cleaved Caspase-3 (1:1000; CST, USA), cleaved Caspase-9 (1:1000; Abclonal, Wuhan, China). Next, the secondary antibodies against rabbit (1:1000; Beyotime, Shanghai, China) or mouse (1:1000; Beyotime, Shanghai, China) were used to incubate the membranes at room temperature for 1 h. Blots were visualized by Tanon viewer system (Shanghai, China) with BeyoECL Moon (Beyotime, Shanghai, China) and quantified by ImageJ.
Immunofluorescence
For immunofluorescence, mice were perfused with saline for 5 min and followed by 4% PFA for 15 min to immobilize in situ. The eyeballs were removed and fixed in FAS eyeball fixture overnight before solidified in optimal cutting temperature compound. 10 μm slices were cut from the frozen eyeballs and permeabilized with 0.3% triton x-100 in PBS for 15 min at room temperature followed by 5% goat serum (Boster, CA, USA) in 0.3% triton x-100 in PBS for 1.5 h. The sections were incubated overnight at 4℃ with primary antibodies as follows: TNFα (1:200; Proteintech, Chicago, IL, USA), IL-1β (1:200; Proteintech, Chicago, IL, USA), IL-6 (1:200; Proteintech, Chicago, IL, USA), iNOS (1:200; Abclonal, Wuhan, China), RAGE (1:200; Abmart, Shanghai, China), Iba1; 1:100; Servicebio, Wuhan, China). Next, Alexa Fluor 488 anti-rabbit or Alexa Fluor 594 anti-mouse secondary antibodies (1:200; Abbkine, CA, USA) were used to incubate sections at room temperature for 1 h. The nuclear was stained by DAPI (Invitrogen, CA, USA). Then, the slices were photographed by confocal microscopy (Nikon, Tokyo, Japan). Each group included at least 3 samples. For the identification of microglial activation, Iba + cells number and integral optical density (IOD) were quantificated from five random fields of view of each retina and averaged, and each group contained 6 samples. For the identification of RAGE expression on retinas, IOD was quantificated from 5 consecutive fields of retinal sections and averaged. Results were analyzed by imageJ and normalized to controls.
ELISA
Mice blood, fecal and retinal samples as well as patient blood samples were collected for the detection of IAA levels using IAA research Elisa Kit (Abmart, Shanghai, China). Blood was collected using tubes free of heat and endotoxins, and serum was separated by centrifugation at 3000 rpm for 10 min. The fecal samples were added into normal saline, thoroughly crushed, and then centrifuged at 3000 rpm for 10 min to obtain the supernatant. Retinal samples were mashed in PBS and performed ultrasound and then centrifuged at 3000 rpm for 10 min to obtain the supernatant. The ELISA kit was allowed to equilibrate to for 60 min before use. Sampling and incubation were then carried out according to the ELISA kit’s instructions under room temperature. OD values at a wavelength of 450 nm were measured within 15 min after completion of the incubation steps, and standard curves were generated.
RNA sequencing
Mice retinas were collected 14 days after MB, with each group containing 3 samples and TRIzol was used to extract RNA from samples as previous manuscript. RNA was inspected using Fragment Analyze and Standard Sensitivity RNA Analysis Kit (15 nt) (DNF-471), and all samples met the requirements of database construction. Sequencing was performed by BGI (Beijing Genomic Institute, ShenZhen, China) [67]. In brief, 9 samples were sequenced with BGISEQ platform, averagely generating about 1.18G Gb bases per sample. The average mapping ratio with reference genome was 94.16%, the average mapping ratio with gene was 75.20%; 18,926 genes were identified. We used HISAT (v2.0.4) and Bowtie2 (v2.2.5) to align the clean reads to the reference genome followed by reads filtering. Mus_musculus from source of NCBI served as reference species, and reference genome version is GCF_000001635.26_GRCm38.p6. Clustering heat map, KEGG (Kyoto Encyclopedia of Genes and Genomes), and GO (Gene Ontology) were analyzed under identified by DEGseq. The results of RNA sequencing have been submitted to the NCBI database (accession number: PRJNA1171984).
Visual Cliff test
This experiment was based on the method of the previous report [68, 69]. An acrylic box (60 × 60 cm square×15 cm high) was placed on the edge of the bench with half extending over the edge to form a cliff side (90 cm above ground level) and half remained on the table to form a table side. A transparent platform (10 × 7 cm square×2 cm high) was placed in the center of the box. The table and the floor were covered with alternating black and white patterns of 2 cm in length to help the mice form depth vision. Light sources were placed 30 cm from the table and 30 cm from the ground to illuminate the experiment area. At the beginning of the experiment, the mouse was placed on the platform. The time it took for the mice to begin moving (recorded as the time of latency to dismount the platform) and their choice of direction (cliff side or table side, with the number of mice first placing a foot on the cliff side recorded) were noted. The total time of mice spent over the cliff in the first two minutes was also recorded. Each mouse was tested only once, and the platform and box were thoroughly cleaned after each test.
Isolation and treatment of primary retinal neurons
Primary retinal neurons culture was prepared based on previous studies [70]. Briefly, the retinas of postnatal day 1 (P1) rats were dissected and dissociated with papain (4 mg/mL) with DNase I (1 mg/mL). Then the tissues were filtered into single cell suspension using a 40-µm filter. The primary retinal neurons were then seeded (1.5 × 106/well for 6-well plate; 5 × 105/well for 24-well plate) in poly-L-lysine-coated (Gibco, Rockville, MD, USA) plate and cultured in a Neurobasal-A medium (Gibco, Rockville, MD, USA) supplemented with 2% B27 (Invitrogen, CA, USA), 2 mM L-glutamine (Beyotime, Shanghai, China) and 1% penicillin/streptomycin. RGCs were identified using βIII-tubulin. The neurons were cultured for 3 days and then incubated with BV2-conditioned medium for 24 h to conduct Western blot and live/dead assay. For the live/dead assay, the primary retinal neurons were incubated with calcein-AM/propidium iodide (Beyotime, China) for 30 min. Live and dead neurons were examined using a fluorescence microscope and counted in a total of eight fields at a standardized location at 10× magnification in a masked manner.
Statistical analysis
Data were expressed as mean ± SEM, and analyzed by Graphpad 8.0. The t-test was used to perform statistical analysis between two groups, and the one-way ANOVA followed by a Turkey’s test was used for multiple groups. P-value < 0.05 was regarded as statistically significant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Changjuan Zeng from Shanghai 9th People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine for her guidance and support in clinical sample collection. The schematic in this manuscript was created with BioRender.com.
Abbreviations
- RGCs
Retinal ganglion cells
- POAG
Primary open angle glaucoma
- AhR
Aryl hydrocarbon receptor
- IAA
Indoleacetic-3-acid
- MB
Microbead
- RAGE
Receptor for advanced glycation end products
- CM
Conditioned medium
- PDTC
Pyrrolidinedithiocarbamate ammonium
- GCL
Ganglion cell layer
- RNFL
Retinal nerve fiber layer
- IPL
Inner plexiform layer
- OTUs
Operational taxonomic units
Author contributions
NW, HS and WG conceived the study. NW, CS, YY, LH, CX, MW, MX and TY performed the experiments. NW, YW, LX, YJ and DZ analyzed the data. NW, CS, DZ, HS and WG drafted the manuscript. All authors read and approved the final version of manuscript.
Funding
This study was supported by National Natural Science Foundation of China (82171046, 81970796 and 82101114), Innovative research team of high-level local universities in Shanghai (SHSMU-ZDCX20210902), Post Graduate Medical Education Program 2022 (No.BYH20220403) and the Research Grant of the Shanghai Science and Technology Committee (No.20DZ2270800).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This study was approved by the Committee of Animal Care of the Shanghai 9th People’s Hospital Affiliated to Shanghai Jiaotong University School of Medicine (Approval no. SH9H-2022-A16-2). All animal experiments were performed in accordance with the Association for Research in Vision and Ophthalmology’s Statement for Use of Animals. The protocol for human serum collection were approved by the institutional review board of Ninth People’s hospital affiliated with the Shanghai Jiao Tong University School of Medicine (No. SH9H-2021-T184-2) and informed consent was obtained from all subjects.
Consent for publication
All consent included in this study are available upon request by contact with the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ning Wang, Chengyang Sun, Yijie Yang and Dandan Zhang contributed equally to this work.
Contributor Information
Hao Sun, Email: sunhao6666@126.com.
Wenyi Guo, Email: wyguo9h@163.com.
References
- 1.Jayaram H, Kolko M, Friedman DS, Gazzard G. Glaucoma: now and beyond. Lancet. 2023;402:1788–801. 10.1016/S0140-6736(23)01289-8 [DOI] [PubMed] [Google Scholar]
- 2.Jonas JB, Aung T, Bourne RR, Bron AM, Ritch R, Panda-Jonas S, Glaucoma. Lancet. 2017;390:2183–93. 10.1016/s0140-6736(17)31469-1 [DOI] [PubMed] [Google Scholar]
- 3.Jameson KG, Olson CA, Kazmi SA, Hsiao EY. Toward Understanding Microbiome-Neuronal signaling. Mol Cell. 2020;78:577–83. 10.1016/j.molcel.2020.03.006 [DOI] [PubMed] [Google Scholar]
- 4.Niso-Santano M, Fuentes JM, Galluzzi L. Immunological aspects of central neurodegeneration. Cell Discov. 2024;10. 10.1038/s41421-024-00666-z [DOI] [PMC free article] [PubMed]
- 5.Hassan A, Huang Y, Mahjabina, Sudiro OH, Wang X, He J, Wei Y, Liao X, Wang G. Emerging role of gut microbiota extracellular vesicle in neurodegenerative disorders and insights on their therapeutic management. iMetaOmics 1. 10.1002/imo2.33 (2024).
- 6.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9:44–53. 10.1038/nrneurol.2012.227 [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Chen DF, Cho K-S. The role of gut microbiota in Glaucoma progression and other retinal diseases. Am J Pathol. 2023;193:1662–8. 10.1016/j.ajpath.2023.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, Zhou X, Lu Y. Gut microbiota and derived metabolomic profiling in glaucoma with progressive neurodegeneration. Front Cell Infect Microbiol. 2022;12. 10.3389/fcimb.2022.968992 [DOI] [PMC free article] [PubMed]
- 9.Li C, Lu P. Association of gut microbiota with Age-Related macular degeneration and glaucoma: A bidirectional Mendelian randomization study. Nutrients. 2023;15. 10.3390/nu15214646 [DOI] [PMC free article] [PubMed]
- 10.Gong H, Zhang S, Li Q, Zuo C, Gao X, Zheng B, Lin M. Gut microbiota compositional profile and serum metabolic phenotype in patients with primary open-angle glaucoma. Exp Eye Res. 2020;191:107921. 10.1016/j.exer.2020.107921 [DOI] [PubMed] [Google Scholar]
- 11.Wang N, Sun C, Ju Y, Huang L, Liu Y, Gu M, Xu C, Wang M, Wu Y, Zhang D, Xu L, Guo W. Gut microbiota compositional profile in patients with posner-schlossman syndrome. Exp Eye Res. 2024;240. 10.1016/j.exer.2024.109825 [DOI] [PubMed]
- 12.Lin X, Liu ZYY, Li C, Hu H, Liu J-CH et al. Gut–X axis. iMeta 4, e270. 10.1002/imt2.270 (2025). [DOI] [PMC free article] [PubMed]
- 13.Rothhammer V. The Aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–97. 10.1038/s41577-019-0125-8 [DOI] [PubMed] [Google Scholar]
- 14.Li D, Yu S, Long Y, Shi A, Deng J, Ma Y, Wen J, Li X, Liu S, Zhang Y, Wan J, Li N, Ao R. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front Immunol. 2022;13. 10.3389/fimmu.2022.985378 [DOI] [PMC free article] [PubMed]
- 15.Aburto MR, Cryan JF. Gastrointestinal and brain barriers: unlocking gates of communication across the microbiota–gut–brain axis. Nat Reviews Gastroenterol Hepatol. 2024;21:222–47. 10.1038/s41575-023-00890-0 [DOI] [PubMed] [Google Scholar]
- 16.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C, Li T, Yin Y. Impact of the gut microbiota on intestinal immunity mediated by Tryptophan metabolism. Front Cell Infect Microbiol. 2018;8:13. 10.3389/fcimb.2018.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M, Alvarez JI, Kebir H, Anandasabapathy N, Izquierdo G, Jung S, Obholzer N, Pochet N, Clish CB, Prinz M, Prat A, Antel J, Quintana FJ. Type I interferons and microbial metabolites of Tryptophan modulate astrocyte activity and central nervous system inflammation via the Aryl hydrocarbon receptor. Nat Med. 2016;22:586–97. 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo X, Li C, Zhang J, Sun M, Xu J, Xu C, Kuang H, Xu L. Chiral nanoparticle-remodeled gut microbiota alleviates neurodegeneration via the gut-brain axis. Nat Aging. 2023;3:1415–29. 10.1038/s43587-023-00516-9 [DOI] [PubMed] [Google Scholar]
- 19.Li H, Du Y, Cheng K, Chen Y, Wei L, Pei Y, Wang X, Wang L, Zhang Y, Hu X, Lu Y, Zhu X. Gut microbiota-derived indole-3-acetic acid suppresses high myopia progression by promoting type I collagen synthesis. Cell Discov. 2024;10:89. 10.1038/s41421-024-00709-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Wang Y, Liu Y, Li F, Chen Y, Fang X, Wen T, Xu S, Kermany D, Deng S, Li G, Zhang K, Zhang X. Dysbiosis of gut Microbiome contributes to glaucoma pathogenesis. MedComm – Future Med. 2022;1. 10.1002/mef2.28
- 21.Roager HM, Licht TR. Microbial Tryptophan catabolites in health and disease. Nat Commun. 2018;9. 10.1038/s41467-018-05470-4 [DOI] [PMC free article] [PubMed]
- 22.Agus A, Planchais J, Sokol H. Gut microbiota regulation of Tryptophan metabolism in health and disease. Cell Host Microbe. 2018;23:716–24. 10.1016/j.chom.2018.05.003 [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Hu XY, Zhao Y, You X, Zou J. A blend of formic acid, benzoic acid, and tributyrin alleviates ETEC K88-induced intestinal barrier dysfunction by regulating intestinal inflammation and gut microbiota in a murine model. Int Immunopharmacol. 2023;114:109538. 10.1016/j.intimp.2022.109538 [DOI] [PubMed] [Google Scholar]
- 24.Quaranta L, Bruttini C, Micheletti E, Konstas AGP, Michelessi M, Oddone F, Katsanos A, Sbardella D, De Angelis G, Riva I. Glaucoma and neuroinflammation: an overview. Surv Ophthalmol. 2021;66:693–713. 10.1016/j.survophthal.2021.02.003 [DOI] [PubMed] [Google Scholar]
- 25.Baudouin C, Kolko M, Melik-Parsadaniantz S, Messmer EM. Inflammation in glaucoma: from the back to the front of the eye, and beyond. Prog Retin Eye Res. 2021;83:100916. 10.1016/j.preteyeres.2020.100916 [DOI] [PubMed] [Google Scholar]
- 26.Smith EA. Enumeration of human colonic bacteria producing phenolic and Indolic compounds: effects of pH, carbohydrate availability and retention time on dissimilatory aromatic amino acid metabolism. J Appl Bacteriol. 1996;81:288–302. 10.1111/j.1365-2672.1996.tb04331.x [DOI] [PubMed] [Google Scholar]
- 27.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, Lee K. Gut Microbiota-Derived Tryptophan metabolites modulate inflammatory response in hepatocytes and macrophages. Cell Rep. 2018;23:1099–111. 10.1016/j.celrep.2018.03.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong J, Wang X, Zhang N, Fu H, Li W. D-ribose induces nephropathy through RAGE-dependent NF-kappaB inflammation. Arch Pharm Res. 2018;41:838–47. 10.1007/s12272-018-1061-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou W, Wu WH, Si ZL, Liu HL, Wang H, Jiang H, Liu YF, Alolga RN, Chen C, Liu SJ, Bian XY, Shan JJ, Li J, Tan NH, Zhang ZH. The gut microbe Bacteroides fragilis ameliorates renal fibrosis in mice. Nat Commun. 2022;13:6081. 10.1038/s41467-022-33824-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Sun D, Zhao X, Luo Y, Yu H, Zhou Y, Gao Y, Han X, Duan Y, Fang N, Duan X, Li T, Zhang S, Gong Y, Li Y. Bacteroides fragilis prevents aging-related atrial fibrillation in rats via regulatory T cells-mediated regulation of inflammation. Pharmacol Res. 2022;177:106141. 10.1016/j.phrs.2022.106141 [DOI] [PubMed] [Google Scholar]
- 31.Chen Z, Chen H, Huang W, Guo X, Yu L, Shan J, Deng X, Liu J, Li W, Shen W, Fan H. Bacteroides fragilis alleviates necrotizing Enterocolitis through restoring bile acid metabolism balance using bile salt hydrolase and inhibiting FXR-NLRP3 signaling pathway. Gut Microbes. 2024;16:2379566. 10.1080/19490976.2024.2379566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng Y, Chiu ATG, Li VWY, Zhang X, Yeung WL, Chan SHS, Tun HM. The role of the gut-microbiome-brain axis in metabolic remodeling amongst children with cerebral palsy and epilepsy. Front Neurol. 2023;14. 10.3389/fneur.2023.1109469 [DOI] [PMC free article] [PubMed]
- 33.Ochoa-Repáraz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper L. H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–95. 10.1038/mi.2010.29 [DOI] [PubMed] [Google Scholar]
- 34.Sun F, Zhang Q, Zhao J, Zhang H, Zhai Q, Chen W. A potential species of next-generation probiotics? The dark and light sides of Bacteroides fragilis in health. Food Res Int. 2019;126:108590. 10.1016/j.foodres.2019.108590 [DOI] [PubMed] [Google Scholar]
- 35.Tintelnot J, Xu Y, Lesker TR, Schonlein M, Konczalla L, Giannou AD, Pelczar P, Kylies D, Puelles VG, Bielecka AA, Peschka M, Cortesi F, Riecken K, Jung M, Amend L, Broring TS, Trajkovic-Arsic M, Siveke JT, Renne T, Zhang D, Boeck S, Strowig T, Uzunoglu FG, Gungor C, Stein A, Izbicki JR, Bokemeyer C, Sinn M, Kimmelman AC, Huber S, Gagliani N. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature. 2023;615:168–74. 10.1038/s41586-023-05728-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ji Y, Gao Y, Chen H, Yin Y, Zhang Y. Indole-3-Acetic acid alleviates nonalcoholic fatty liver disease in mice via Attenuation of hepatic lipogenesis, and oxidative and inflammatory stress. Nutrients. 2019;11. 10.3390/nu11092062 [DOI] [PMC free article] [PubMed]
- 37.Li S, Cai Y, Guan T, Zhang Y, Huang K, Zhang Z, Cao W, Guan X. Quinic acid alleviates high-fat diet-induced neuroinflammation by inhibiting DR3/IKK/NF-kappaB signaling via gut microbial Tryptophan metabolites. Gut Microbes. 2024;16:2374608. 10.1080/19490976.2024.2374608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tezel G. Glial modulation of retinal ganglion cell death in glaucoma. J Glaucoma. 2003;12:63–8. 10.1097/00061198-200302000-00014 [DOI] [PubMed] [Google Scholar]
- 39.Yuan L. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64:523–32. 10.1002/jnr.1104 [DOI] [PubMed] [Google Scholar]
- 40.You Y, Graham S, Yan P, Mirzaei M, Parilla GE, Chitranshi N, Basavarajappa D, Wall RV, Salkar A. Glial cell activation and immune responses in glaucoma: A systematic review of human postmortem studies of the retina and optic nerve. Aging Disease. 2024;15. 10.14336/ad.2024.0103 [DOI] [PMC free article] [PubMed]
- 41.Hudson BI, Lippman ME. Targeting RAGE signaling in inflammatory disease. Annu Rev Med. 2018;69:349–64. 10.1146/annurev-med-041316-085215 [DOI] [PubMed] [Google Scholar]
- 42.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108:949–55. 10.1172/jci200114002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S, Hu L, Jiang J, Li H, Wu Q, Ooi K, Wang J, Feng Y, Zhu D, Xia C. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J Neuroinflamm. 2020;17. 10.1186/s12974-019-1673-3 [DOI] [PMC free article] [PubMed]
- 44.Tezel G, Yang LC. Accelerated aging in glaucoma: immunohistochemical assessment of advanced glycation end products in the human retina and optic nerve head. Invest Ophthalmol Vis Sci. 2007;48:1201–11. 10.1167/iovs.06-0737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mi XS, Feng Q, Lo ACY, Chang RC, Chung SK, So KF. Lycium barbarum polysaccharides related RAGE and Abeta levels in the retina of mice with acute ocular hypertension and promote maintenance of blood retinal barrier. Neural Regen Res. 2020;15:2344–52. 10.4103/1673-5374.284998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen M, Glenn JV, Dasari S, McVicar C, Ward M, Colhoun L, Quinn M, Bierhaus A, Xu H, Stitt A. W. RAGE regulates immune cell infiltration and angiogenesis in choroidal neovascularization. PLoS ONE. 2014;9:e89548. 10.1371/journal.pone.0089548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tezel G. Molecular regulation of neuroinflammation in glaucoma: current knowledge and the ongoing search for new treatment targets. Prog Retin Eye Res. 2022;87:100998. 10.1016/j.preteyeres.2021.100998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nikitina AS, Kharlampieva DD, Babenko VV, Shirokov DA, Vakhitova MT, Manolov AI, Shkoporov AN, Taraskina AE, Manuvera VA, Lazarev VN, Kostryukova ES. Complete genome sequence of an enterotoxigenic Bacteroides fragilis clinical isolate. Genome Announc. 2015;3. 10.1128/genomeA.00450-15 [DOI] [PMC free article] [PubMed]
- 49.Suez J, Elinav E. The path towards microbiome-based metabolite treatment. Nat Microbiol. 2017;2:17075. 10.1038/nmicrobiol.2017.75 [DOI] [PubMed] [Google Scholar]
- 50.Schalnus R. Topical nonsteroidal anti-inflammatory therapy in ophthalmology. Ophthalmologica. 2003;217:89–98. 10.1159/000068563 [DOI] [PubMed] [Google Scholar]
- 51.Davis BM, Normando EM, Guo L, Turner LA, Nizari S, O’Shea P, Moss SE, Somavarapu S, Cordeiro MF. Topical delivery of Avastin to the posterior segment of the eye in vivo using Annexin A5-associated liposomes. Small. 2014;10:1575–84. 10.1002/smll.201303433 [DOI] [PubMed] [Google Scholar]
- 52.Xu X, Sun L, Zhou L, Cheng Y, Cao F. Functional Chitosan oligosaccharide nanomicelles for topical ocular drug delivery of dexamethasone. Carbohydr Polym. 2020;227:115356. 10.1016/j.carbpol.2019.115356 [DOI] [PubMed] [Google Scholar]
- 53.Mandal A, Cholkar K, Khurana V, Shah A, Agrahari V, Bisht R, Pal D, Mitra AK. Topical formulation of Self-Assembled antiviral prodrug nanomicelles for targeted retinal delivery. Mol Pharm. 2017;14:2056–69. 10.1021/acs.molpharmaceut.7b00128 [DOI] [PubMed] [Google Scholar]
- 54.Wang D, Wu J, Zhu P, Xie H, Lu L, Bai W, Pan W, Shi R, Ye J, Xia B, Zhao Z, Wang Y, Liu X, Zhao B. Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: the potential involvement of gut-brain axis. Food Res Int. 2022;157:111289. 10.1016/j.foodres.2022.111289 [DOI] [PubMed] [Google Scholar]
- 55.Zhu H, Yang X, Zhao Y. Recent advances in current uptake situation, metabolic and nutritional characteristics, health, and safety of dietary Tryptophan. J Agric Food Chem. 2024;72:6787–802. 10.1021/acs.jafc.3c06419 [DOI] [PubMed] [Google Scholar]
- 56.Chen H, Cho KS, Vu THK, Shen CH, Kaur M, Chen G, Mathew R, McHam ML, Fazelat A, Lashkari K, Au NPB, Tse JKY, Li Y, Yu H, Yang L, Stein-Streilein J, Ma CHE, Woolf CJ, Whary MT, Jager MJ, Fox JG, Chen J, Chen DF. Commensal microflora-induced T cell responses mediate progressive neurodegeneration in glaucoma. Nat Commun. 2018;9:3209. 10.1038/s41467-018-05681-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E. Potential roles of gut Microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–80. 10.1038/s41586-019-1443-5 [DOI] [PubMed] [Google Scholar]
- 58.Nikitina AS, Kharlampieva DD, Babenko VV, Shirokov DA, Vakhitova MT, Manolov AI, Shkoporov AN, Taraskina AE, Manuvera VA, Lazarev VN, Kostryukova ES. Complete genome sequence of an enterotoxigenic Bacteroides fragilis clinical isolate. Genome Announcements. 2015;3. 10.1128/genomeA.00450-15 [DOI] [PMC free article] [PubMed]
- 59.Yang Y, Wang N, Xu L, Liu Y, Huang L, Gu M, Wu Y, Guo W, Sun H. Aryl hydrocarbon receptor dependent anti-inflammation and neuroprotective effects of Tryptophan metabolites on retinal ischemia/reperfusion injury. Cell Death Dis. 2023;14:92. 10.1038/s41419-023-05616-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Zhao D, Wang H, Wang L, Liu X, Zhang H. FPS-ZM1 inhibits LPS-induced microglial inflammation by suppressing JAK/STAT signaling pathway. Int Immunopharmacol. 2021;100. 10.1016/j.intimp.2021.108117 [DOI] [PubMed]
- 61.Wang X, Wang Z, Cao J, Dong Y, Chen Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 2023;11:17. 10.1186/s40168-022-01452-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kitamei H, Iwabuchi K, Namba K, Yoshida K, Yanagawa Y, Kitaichi N, Kitamura M, Ohno S, Onoe K. Amelioration of experimental autoimmune uveoretinitis (EAU) with an inhibitor of nuclear factor-kappaB (NF-kappaB), pyrrolidine dithiocarbamate. J Leukoc Biol. 2006;79:1193–201. 10.1189/jlb.0805453 [DOI] [PubMed] [Google Scholar]
- 63.Khan AS. Indole-3-carbinol regulates microglia homeostasis and protects the retina from degeneration. J Neuroinflammation. 2020;17. 10.1186/s12974-020-01999-8 [DOI] [PMC free article] [PubMed]
- 64.Wang Z, Chen W-H, Li S-X, He Z-M, Zhu W-L, Ji Y-B, Wang Z, Zhu X-M, Yuan K, Bao Y-P, Shi L, Meng S-Q, Xue Y-X, Xie W, Shi J, Yan W, Wei H, Lu L, Han Y. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol Psychiatry. 2021;26:6277–92. 10.1038/s41380-021-01113-1 [DOI] [PubMed] [Google Scholar]
- 65.Gramlich OW, Zhu DQ, Cook W, Anderson A, Kuehn MG. Adoptive transfer of immune cells from glaucomatous mice provokes retinal ganglion cell loss in recipients. Acta Neuropathol Commun. 2015;3. 10.1186/s40478-015-0234-y [DOI] [PMC free article] [PubMed]
- 66.Cuny CS, Joachim SC, Gramlich OW, Gottschling PF, Pfeiffer N, Grus FH. Repeated intraocular pressure measurement in awake Lewis rats does not Bias retinal ganglion cell survival. Curr Eye Res. 2010;35:1034–9. 10.3109/02713683.2010.498654 [DOI] [PubMed] [Google Scholar]
- 67.Ju Y, Dai X, Tang Z, Ming Z, Ni N, Zhu D, Zhang J, Ma B, Wang J, Huang R, Zhao S, Pang Y, Gu P. Verteporfin-mediated on/off photoswitching functions synergistically to treat choroidal vascular diseases. Bioact Mater. 2022;14:402–15. 10.1016/j.bioactmat.2022.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Q, Tang XJ, Wu J, Liu S, Wu Z, Liu C, Liu C, Lin Y, Han J, Liu J, Chen L, Yang Y, Li J, Zhao Z, Wei L, Li Y, Zhuo Y. Modulating Amacrine cell-derived dopamine signaling promotes optic nerve regeneration and preserves visual function. Sci Adv. 2024;10:eado0866. 10.1126/sciadv.ado0866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jeong GU, Kwon HJ, Ng WH, Liu X, Moon HW, Yoon GY, Shin HJ, Lee IC, Ling ZL, Spiteri AG, King NJC, Taylor A, Chae JS, Kim C, Ahn DG, Kim KD, Ryu YB, Kim SJ, Mahalingam S, Kwon YC. Ocular tropism of SARS-CoV-2 in animal models with retinal inflammation via neuronal invasion following intranasal inoculation. Nat Commun. 2022;13:7675. 10.1038/s41467-022-35225-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang Y, Xu Y, Sun Q, Xue S, Guan H, Ji M. Activation of P2X(7)R- NLRP3 pathway in Retinal microglia contribute to Retinal Ganglion Cells death in chronic ocular hypertension (COH). Exp Eye Res. 2019;188:107771. 10.1016/j.exer.2019.107771 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.