Abstract
Background
The incidence of autoimmune diseases in cold environments has been a topic of interest due to the observed geographical patterns and potential environmental influences on disease development. We aimed to investigate the prevalence of five main autoimmune diseases in 201 countries according to average annual temperatures.
Methods
Linear regression analysis was performed for 201 countries by analyzing average annual temperatures and age-standardized rates (prevalence) of five autoimmune diseases: alopecia areata, diabetes mellitus (DM) type 1, inflammatory bowel disease (IBD), psoriasis and rheumatoid arthritis (RA). A systematic review was also conducted to evaluate whether the observed correlations were supported by published original studies.
Results
The linear regression analysis showed a strong correlation between average annual temperatures and age-standardized prevalence rates (p < 0.0001) across 201 countries. The systematic review analysis indicated that certain autoimmune diseases, such as DM type 1, RA, psoriasis and IBD, demonstrate robust associations with geographic and climatic factors. However, there were no available published data for alopecia areata.
Conclusions
These findings underscore the complexity of interactions between environmental, and genetic factors in the development of autoimmune diseases. Further investigation is required to better understand the association between temperature and prevalence of autoimmune diseases and to identify any additional epidemiological factors that contribute to autoimmune pathogenesis.
Supplementary Information
The online version contains supplementary material available at 10.1186/s41927-025-00532-9.
Keywords: Autoimmunity, Rheumatic disease, Multiple sclerosis, Iincidence, Low temperatures, Prevalence, Cold, Climate
Introduction
The incidence of autoimmune diseases in cold environments has been a topic of interest due to the observed geographical patterns and potential environmental influences on disease development [1]. Cold environments, characterized by lower temperatures and reduced sunlight exposure, are often associated with higher incidence rates of certain autoimmune diseases. Multiple sclerosis (MS), for example, shows a higher prevalence in regions with colder climates [2]. This pattern is thought to be linked to reduced ultraviolet (UV) radiation exposure, which can lead to lower vitamin D synthesis. Vitamin D is essential for immune function, and its deficiency has been implicated in increased susceptibility to autoimmune disorders [3]. However, a recent study provided compelling evidence about the north–south incidence gradient of MS in Europe, showing that this is related to ancient population movements and positive selection on certain genes driven by pathogenic challenges, as well as changes in diet, lifestyle and population density [4]. For instance, the increased prevalence of infections in colder climates could act as triggers for autoimmune responses in genetically susceptible individuals [5]. Moreover, lifestyle factors related to colder environments, such as dietary habits and physical activity levels, could also contribute to the observed patterns. A systematic review displayed that countries closer to the equator have a lower prevalence of psoriasis [6]. Another review study indicated that the latitude plays a significant role in the prevalence of psoriasis, as the prevalence increases up to 3% with an increase in latitude [7].
Nonetheless, the relationship between cold environments and autoimmune disease incidence is complex and influenced by a multitude of genetic, environmental, and socio-economic factors. Further research is needed to understand these interactions comprehensively and to develop effective public health strategies.
In this study, we hypothesized that annual average temperature is associated with the prevalence of autoimmune diseases. We performed linear regression analysis looking for any association between the annual average temperature of 201 countries and the incidence of five autoimmune diseases. Additionally, we performed a systematic review to identify and synthesize published evidence that support the association between average annual temperature (AAT) or latitude with the prevalence of autoimmune diseases.
Materials and methods
Temperature and prevalence data
Average Annual Temperature (AAT) by country (years 1991–2020) is based on gridded climatologies from the Climatic Research Unit [8]. Age-standardized rates (prevalence) of autoimmune diseases were adopted by the Global Burden of Disease Study [9] data of 2019. Temperature and prevalence data can be found in Supplementary Table S1. All data were collected in February of 2024.
In this study, we selected 5 autoimmune diseases—type 1 diabetes mellitus, rheumatoid arthritis, inflammatory bowel disease, psoriasis, and alopecia areata—for the regression analysis based on the availability of reliable, age-standardized prevalence data across 201 countries from the Global Burden of Disease Study 2019. These diseases were also chosen due to their substantial prevalence and global burden, which improve the accuracy and comparability of cross-national epidemiological analysis.
Statistical analysis
Statistical analysis was performed by the statistical package STATAv.13 (StataCorp LLC, Texas, USA). A linear regression analysis was performed for AAT and prevalence (age-standardized rates (ASR)) of autoimmune diseases (Alopecia areata, Inflammatory bowel disease, Diabetes mellitus type 1, Psoriasis, Rheumatoid arthritis). Significant level alpha was set to 0.01.
Systematic review method
We conducted a systematic literature review to identify the correlation between specific autoimmune diseases and geographic latitude and temperature as per PRISMA guidelines. We included all studies focusing on this relationship, published in English. We excluded studies with less than 10 patients, systematic and narrative review articles, abstracts, case reports and case series.
A search was performed across Medline/PubMed, Scopus, Embase from their inception until September 2024.
We included the following search terms ‘Geographic latitude’, ‘North-south incidence gradient’, ‘Geographic variation’, ‘Geographic location’, ‘Latitudinal gradient’, ‘Temperature’ and inflammatory bowel disease prevalence, type 1 diabetes mellitus prevalence, psoriasis prevalence, alopecia aerate prevalence, rheumatoid arthritis prevalence, inflammatory bowel disease, type 1 diabetes mellitus, celiac disease, Grave’s disease, Hashimoto’s thyroiditis, psoriasis, psoriatic arthritis, alopecia areata, rheumatoid arthritis, Crohn’s disease, Ankylosing spondylitis, ANCA associated vasculitides, Addison’s disease, Sjogren’s syndrome, Dermatomyositis, Polymyositis, systemic sclerosis, giant cell arteritis or temporal arteritis, polymyalgia rheumatica, Takayasu arteritis, IGA vasculitis.
We have also searched the following terms: ‘Geographic latitude’, ‘North-south incidence gradient’, ‘Geographic variation’, ‘Geographic location’, ‘Latitudinal gradient’, ‘Temperature’ in three pre-print databeses (VeriXiv, medRxiv and Research Square) for psoriasis and alopecia acreata.
Two reviewers extracted the data collaboratively based on the inclusion and exclusion criteria. No automation tools were used.
The main outcome was the effect of geographic latitude and temperature on specific autoimmune diseases. The diagnosis of these autoimmune diseases was based on clinical criteria and medical records. The results were summarized from the included studies, and no meta-analysis was performed.
The broader list of autoimmune diseases included in our search strategy reflects an effort to comprehensively capture existing evidence linking environmental or geographic factors (such as latitude and temperature) with autoimmune disease prevalence. This broader scope allowed us to identify areas of evidence concentration (e.g., DM type 1, IBD) as well as knowledge gaps (e.g., alopecia areata), which we highlight in our results and discussion. The list of diseases for the systematic review was therefore intentionally wider than those selected for the regression analysis.
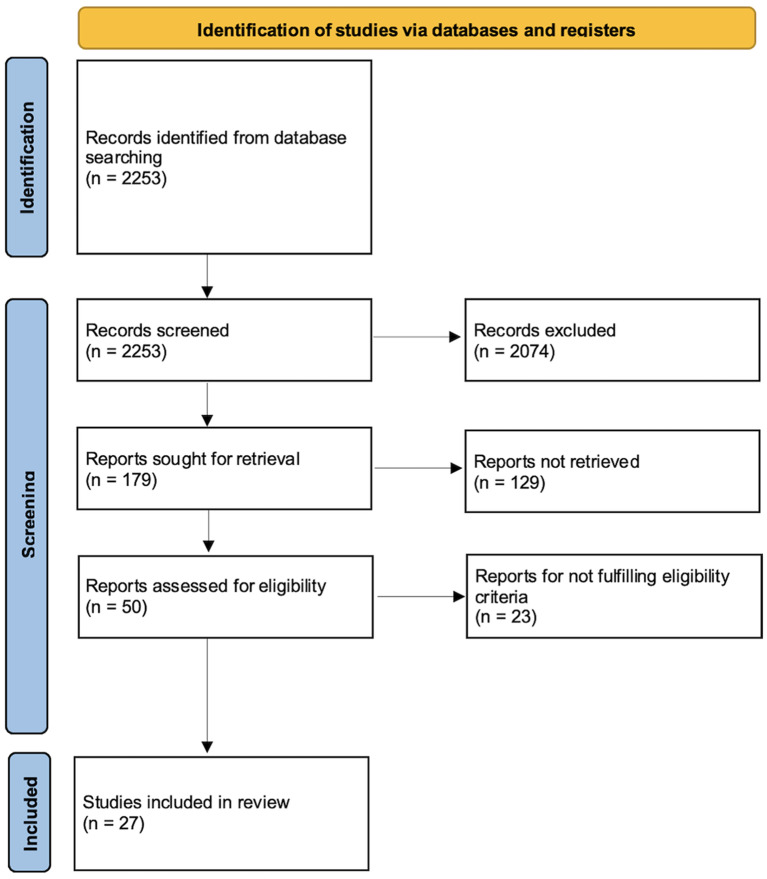
In total, we identified 2253 studies from our search, of which 179 were screened to assess their eligibility. After excluding systematic reviews and those including less than 10 patients, 50 met our eligibility criteria. Following a full text review and the removal of duplicates, 27 studies were included in the final analysis.
Results
Average Annual Temperatures (AAT) and the prevalence of autoimmune diseases
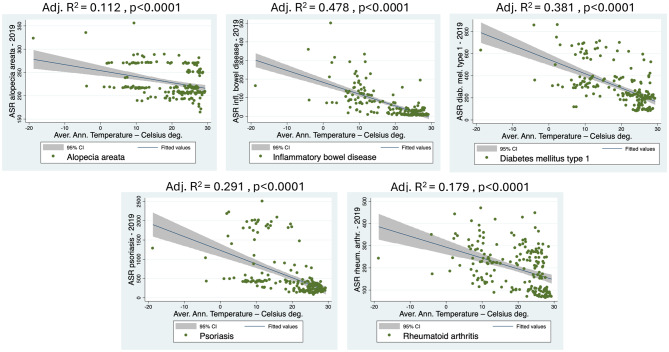
To investigate the relationship between prevalence of autoimmune disease and AAT, a linear regression analysis was performed using AAT and age-standardized rates (ASR). The analysis include data on five autoimmune diseases across 201 countries (Fig. 1). Results showed a highly significant linear association (P < 0.0001) for all five autoimmune diseases: alopecia areata, inflammatory bowel disease (IBD), diabetes mellitus type 1 (DM type 1), psoriasis and rheumatoid arthritis (RA). Among the five diseases analyzed, DM1 type 1 exhibited the strongest association (Adj. R² = 0.478), followed by inflammatory bowel disease (IBD) (Adj. R² = 0.381) and psoriasis (Adj. R² = 0.291). RA demonstrated a weaker association (Adj. R² = 0.179), while alopecia areata had the weakest correlation (Adj. R² = 0.112). These findings suggest that temperature may play a role in the epidemiology of DM type 1, IBD, and psoriasis, whereas its influence on RA and alopecia areata appears to be more modest (Fig. 1).
Fig. 1.
Linear regression analysis showing the strong association between the autoimmune diseases’ prevalence and the average annual temperatures of 201 countries. ASR: Age-standardized rate (prevalence)
Systematic review results
In this review, 27 studies meet out eligibility criteria and were therefore included in the analysis. The process is described in a flow diagram (Fig. 2).
Fig. 2.
A PRISMA chart describing the inclusion/exclusion process
The results focus on the relationship between the prevalence of specific autoimmune diseases and geographic latitude and temperature. Τhe number of articles included for each autoimmune is as follows: 8 for DM type 1, 11 for IBD, 4 for RA, and 4 for psoriasis. Mainly the studies are observational. The range of sample size was from 500 to more than 150.000. The studies investigate the effect of geographical latitude and the following autoimmune diseases. Regarding risk of bias, sample size showed a wide range among studies and this may lead to heterogeneity. Also, the age range was not mentioned in some studies. Details of selected studies can be found in Table 1.
Table 1.
Characteristics of selected studies
| Study 1st author | Publication year | Country | Autoimmune disease | Age | Number of patients and gender | Results |
|---|---|---|---|---|---|---|
| Hong Yang [10] | 2022 | China | IBD | ≥ 20 years old |
CD: 9003 M/5076 F UC: 13 113 M/9615 F |
Eastern China: higher prevalence and incidence of UC and CDWestern China: lowest rates |
| A Sonnenberg [11] | 1991 | USA | IBD | ≥ 65 years old | n/a | Increased prevalence in most of the US northern states compared to southern states |
| John Betteridge [12] | 2013 | USA | IBD | n/a | 35,404 | Lower prevalence in the Western USA |
| Adam Stein [13] | 2016 | USA | IBD | n/a |
CD 14 3495 UC 76 608 |
Lower discharge rates with higher UV index. |
| Emma Armitage [14] | 2004 | Scotland | CD | < 16 years old | 560 | Increased incidence of juvenile onset Crohn’s disease in Northern Scotland. |
| Geir Aamodt [15] | 2013 | Norway | UC | n/a | 370 | Incidence and prevalence of UC follow a north–south gradient |
| Hamed Khalili [16] | 2012 | USA | IBD | Time of birth, 15 years old, 30 years old | 175 912 F (257 CD and 313 UC) | Increased incidence of CD and UC with increasing latitude |
| Virginie Nerich [17] | 2010 | France | IBD | n/a | n/a | North–south gradient of CD |
| Christophe Declercq [18] | 2010 | France | IBD | n/a | 8171 | Higher incidence of UC in the south |
| Johanna Hartwedt Larsen [19] | 2024 | Norway | IBD | < 18 years old | 541,036 (432 IBD) | Higher incidence of IBD in the north |
| Airi Jussila [20] | 2013 | Finland | IBD | N/a | 42,661 | Higher incidence of IBD and UC in the north. No variation in CD |
| Scott Sloka [21] | 2009 | Newfoundland | DM type 1 | n/a | 732 | Negative correlation between UVB radiation and the prevalence of DM type 1 |
| Stephen Ball [22] | 2014 | Australia | DM type 1 | < 18 years old | 1571 | Increased risk of DM type 1 per degree south of the Equator |
| G Joner [23] | 1991 | Norway | DM type 1 | 15–29 years old | 784 | Increased incidence in some southern regions |
| M Rytkonen [24] | 2001 | Finland | DM type 1 | < 14 years old | 3613 | Increased risk in: central Finland, eastermost part, in midwestern |
| G Joner [25] | 1981 | Norway | DM type 1 | 0–14 years old | 845 | Lowest rate in north and highest in South-eastern |
| Z Yang [26] | 1998 | China | DM type 1 | < 15 years old | 592 | Increased incidence rate at northern lattitudes |
| Judith A Staples [27] | 2003 | Australia | DM type 1 | n/a | 23 800 | Increased prevalence over the north-south latitude gradient |
| X H Li [28] | 2000 | China | DM type 1 | < 15 years old | 903 | Increased incidence rate in northern latitude |
| Verónica M. Vieira [29] | 2010 | USA | RA | 30–55 years old | 9681 | Increased risk in women living in higher latitudes |
| G Rosati [30] | 1978 | Sardinia | RA | 5–83 years old | 2252 | No association was found in RA |
| Karen H Costenbader [31] | 2008 | USA | RA | 30–55 years old | 83 546 | Higher risk in women in Midwest and Northeast. |
| Eriksson, Jonas K. [32] | 2013 | Sweden | RA | (mean age) 60.4 ± 15.9 years | 8826 | No geographical trends were noted |
| Valerie Andrees [33] | 2021 | Germany | Psoriasis | n/a | 72.8 million (1.2 million cases of psoriasis in 2017) | Increased prevalence in northern ans eastern parts of Germany |
| D A Springate [34] | 2017 | UK | Psoriasis | All ages | About 1.5 million cases | Increased risk associated with increased latitude |
| C Ferrandiz [35] | 2008 | Spain | Psoriasis | n/a | 12 938 | Increased risk in central parts of Spain |
| Richard O Leder [36] | 2008 | Sub-Saharan Africa | Psoriasis | n/a | n/a | Higher prevalence among Eastern Africa |
DM type 1
A study of 732 patients revealed an increased incidence of DM type1 during the winter months, as lower levels of ultra-violet (UV) radiation have been associated with an increased risk of developing DM [21]. Similar seasonal variation was also observed by another study, which reported higher prevalence of DM type 1 during autumn and winter [25].
Additionally, a number of studies have demonstrated that a north-south gradient plays a role in the incidence of DM type1. Specifically, a study by Ball et al. [22] examined the incidence of DM type 1 in Western Australia from 1991 to 2010 and found that the incidence was less pronounced in northern regions. On the other hand, elevated UV exposure was linked to a lower frequency of DM. Similarly, Joner et al., demonstrated that in Norway there was a higher prevalence in south-eastern areas when compared to the ones in the north [23]. This is consistent with the findings of Yang et al., where DM was more common in the more northern parts of China [26]. In an Australian study [27], the incidence of DM was correlated with latitude and specifically, as the latitude decreases toward south, the incidence increases accordingly. In a Norwegian study of 784 patients, DM was more prevalent in the south, in contrast to the north with an incidence of 18.3 per 100,000 people vs 13.9, respectively [13]. Similarly, a study which examined the incidence of DM in China, reported a higher incidence rate in northern China [28] Additionally, a study by Rytknonen et al., showed that the central part of Finland had a higher incidence of DM, even when compared to areas in the south with a higher population density [24]. On the other hand, increased UV radiation was inversely correlated with DM prevalence. That being said, studies on DM type 1 from various countries, including Australia, China, Norway, and Finland provide valuable insights but may have limited generalizability due to geographic constraints. The absence of detailed data from southern latitudes and equatorial regions may limit conclusions on latitude-related prevalence patterns. While each study reports higher DM type 1 incidence in northern areas, this trend may not apply globally. Future research should incorporate multi-country datasets across diverse latitudes.
Rheumatoid arthritis
A study analyzing individual-level data from the Nurses’ Health Study included USA female nurses and explored the association between geographic location and RA risk. The analysis included 461 incident RA cases and 9,220 controls, with geocoded addresses tracked from 1988 to 2002. Results revealed a statistically significant area of increased RA risk in the northeastern United States (p = 0.034), with a higher risk being observed at the northern latitudes compared to the southern [29].
Another study using the same database, the Nurses’ Health Study, investigated geographic variation in RA risk among 83,546 USA women. Among 706 confirmed RA cases (1976–2004), women in New England had a 37–45% higher RA risk compared to those in the West (rate ratio [RR] 1.42; 95% CI, 1.10–1.82). Higher RA risk was also observed in the Midwest and New England, for women residing in the same regions over time [31].
In the study by Eriksson et al., conducted in Sweden, no clear geographic gradient in RA incidence was observed from north to south within the country. However, a notable limitation of this study is its focus on a single northern European country, which did not include patients from southern latitudes, such as those in the southern European countries [32]. On the other hand, a study of Rosati et al, showed no association between latitude and RA [30].
Psoriasis/Psoriatic arthritis
In a prospective study the prevalence of psoriasis was higher in the northern and eastern regions of Germany, whereas it was notably lower in the southern parts. Specifically, the prevalence increases with every 100 km further north (Pearson’s r = 0.72; 95% CI 0.57 to 0.76; p < 0.05) [33]. Similarly, a study conducted in the United Kingdom showed a significant association between latitude and psoriasis with prevalence increasing by 201 cases per 100,000 for each degree of latitude. One potential mechanism underlying this association, is vitamin D metabolism, as topical treatment has been shown to be effective in the management of psoriasis [34]. Additionally, a Spanish study revealed heterogeneity in psoriasis prevalence, with higher rates observed in the central parts of Spain [35]. A latitudinal difference was also prevalent in African countries, as psoriasis was more common in Eastern Africa compared to Western parts [36]. The observed geographic heterogeneity in Spain and Africa contrasts with the more consistent north-south gradient seen in countries like Germany and the United Kingdom, suggesting that factors beyond latitude influence psoriasis prevalence. These findings highlight that while latitude and UV exposure play a role in some regions, other factors, including climate, and genetics, can lead to distinct geographic patterns of psoriasis prevalence.
Inflammatory bowel disease (IBD)
A study conducted in China utilizing data from a medical insurance database found that the prevalence and incidence rates of both ulcerative colitis (UC) and Crohn’s disease (CD) were significantly higher in the eastern regions compared to western regions. The study demonstrated an evident east-to-west gradient, with higher rates of these conditions observed in the eastern part of the country [10]. A study by Sonnenberg et al conducted in 1990, analyzed 17.5 million hospital discharges in the USA. Medicare beneficiaries, alongside mortality data, and revealed significant geographic variation in IBD occurrence. CD and UC were more prevalent in northern compared to southern regions and in urban areas compared to rural ones, with consistent trends across gender and racial groups [11]. In contrast, a 2013 study using data from the military health care system found no significant differences between northern and southern regions, with the lowest prevalence observed in the West. However, it is important to note that the military patient population may not be fully representative, potentially limiting the generalizability of these findings [12]. In the study of Larsen et al., IBD incidence was higher in the northern parts of Norway with a ratio of 17.4/100,000 person-years in comparison to 8.7/100,000 person-years in southern regions [19]. Similar results were observed in a Finnish study as the prevalence of UC was higher in the north. However, no geographical variation was observed in CD [20]. A USA study examined the relationship between geographic location, seasonal variation, and UV light exposure on the severity of IBD, using nationwide hospital discharge data. The analysis revealed that hospital discharge rates due to IBD were lower during the summer months in both northern and southern regions of the USA demonstrating a statistically significant inverse correlation between a high UV index and discharge rates [13]. The study showed consistently higher IBD-related hospitalization rates in northern states compared to southern states for both UC and CD.
Additionally, a retrospective study conducted in Norway demonstrated a significant relationship between temperature and the likelihood of developing UC. The study found that for each one-degree Celsius increase in temperature, there was an approximately 9% reduction in the risk of UC. The results of this study suggested that environmental factors, such as temperature, may play a role in modulating the risk of UC, highlighting the potential influence of climate on disease prevalence [15].
In a Scottish study, the incidence of juvenile-onset CD in Scotland between 1981 and 1995 was 2.3 cases per 100,000 individuals with a significantly higher incidence observed in the northern Scotland (3.1, 95% CI: 2.6–3.8) compared to the southern Scotland (2.1, 95% CI: 1.9–2.4; P < 0.001). In contrast, the incidence of juvenile-onset UC exhibited no significant North-South variation (P = 0.677) [14]. This is in line with a study utilizing the French National Health Insurance databases that revealed significant geographic variations in the incidence of CD but not for UC [17]. In another French study, CD was more profound in the southern part of the study area, whereas UC was more prevalent in urban and rural areas [18].
On the other hand, a study utilizing data from the Nurses’ Health Study I and II demonstrated a significant association between latitude of residence and the incidence of both conditions, CD and UC. The findings revealed that higher latitude at the age 30s was associated with increased risk of CD and UC, with multivariate-adjusted hazard ratios (HR) of 0.48 (95% CI 0.30–0.77) for CD and 0.62 (95% CI 0.42–0.90) for UC among women residing in southern compared to northern latitudes [16].
Alopecia areata
No published data have been identified that follow the specific procedures outlined in the methods section.
Discussion
This study is the first to integrate a linear regression analysis with a systematic literature review to explore the association between environmental temperature and the prevalence of autoimmune diseases across diverse global setting. It highlights a significant inverse association between lower annual average temperatures, higher geographic latitude, and the increased prevalence of autoimmune diseases, including alopecia areata, IBD, DM type 1, psoriasis, and RA. Findings from the systematic review further emphasize pronounced geographic and seasonal variations in autoimmune disease incidence, with particularly robust evidence supporting a north–south gradient for DM type 1 and IBD. These results suggest that latitude may play a critical role in modulating autoimmune disease prevalence on a global scale.
Several hypotheses have been proposed to explain the link between environmental temperature, latitude, and autoimmune disease prevalence. One possible explanation is that in colder, higher-latitude regions, there is reduced sunlight exposure, leading to reduced vitamin D synthesis in humans. Vitamin D plays a crucial role in immune regulation, and deficiency in this nutrient has been associated with a range of autoimmune conditions, including multiple sclerosis, type 1 diabetes, and rheumatoid arthritis [37].
Another contributing factor could be differences in hygiene, urbanization, and pathogen across geographic regions. The “hygiene hypothesis” suggests that reduced exposure to infectious agents during early childhood — more common in wealthier, colder countries with high standards of sanitation — may impair proper immune system development, making individuals more susceptible to autoimmune conditions [38]. In contrast, warmer countries often have higher exposure to microbial agents, which may stimulate the immune system in ways that protect against autoimmunity. Additionally, genetic and lifestyle differences, such as dietary patterns and levels of physical activity, may also play a role, potentially interacting with environmental factors to influence disease incidence.
It is worth noting that several confounding factors may influence the study results. Our study did not adjust for each country’s median annual income, gross domestic product (GDP), or level of industrialization. Prior research has established a link between economic development and autoimmune disease prevalence [39]. For instance, a population-based cohort study of 22 million individuals in the UK found significant associations between deprived socioeconomic status and higher incidence of autoimmune disorders, suggesting that industrialization and associated lifestyle changes may influence disease risk. Further, differences in healthcare infrastructure and diagnostic capabilities may influence the reported prevalence of autoimmune diseases. Several studies found worldwide inequities and gaps in access to healthcare lead to significant variations in the diagnosis and treatment of autoimmune diseases, which may result in underreporting in resource-limited settings and detection bias in more developed healthcare systems [40, 41] While our study focuses on average annual temperature, other environmental factors such as air pollution may play crucial roles in autoimmune disease prevalence. Recent research has uncovered a significant link between long-term exposure to air pollution and an increased risk of autoimmune diseases suggesting that environmental pollutants can trigger autoimmune responses [42].
Further, genetic predisposition significantly influences the development of autoimmune diseases, with certain genetic variants increasing susceptibility to these conditions. For instance, specific alleles of the human leukocyte antigen (HLA) system have been associated with various autoimmune disorders, such as multiple sclerosis and rheumatoid arthritis [43]. These genetic factors can confound studies examining environmental influences on autoimmune disease prevalence.
Our study contains certain limitations. Firstly, only 27 studies in total met our eligibility criteria, and none for alopecia areata. As a result, this may affect the generalizability of our results. The absence of relevant studies regarding latitude and alopecia areata prevalence shows that further research is needed in this area. Moreover, a notable limitation is that some of the included studies summarize the results of specific countries. Hence, the systematic review results may not be universally applicable.
In general, our findings suggest that geography plays a critical role in autoimmune disease prevalence, with both latitude-related (north/south) and regional (east/west) variations observed. While north/south patterns align with expected climatic influences, such as temperature and UV exposure, some east/west variations may reflect non-climatic factors like industrialization, environmental pollutants, or healthcare access. Our findings underscore the complexity of interactions between environmental, geographic and genetic factors in autoimmune disease etiology. This gap in the literature highlights the need for further research employing robust methodologies to explore potential geographic patterns and their implications.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable
List of abreviations
- CD
Crohn’s disease
- CU
uncreative colitis
- DM type 1
diabetes mellitus type 1
- IBD
inflammatory bowel disease
- RA
rheumatoid arthritis
- USA
United States of America
- UV
ultra-violent
Author contributions
KV perceived the research idea, performed the statistical analysis and wrote the first draft of the manuscript. KP supervised the systematic review analysis and had a major contribution to the paper writing. MH performed part of the systematic review. SP had a major contribution to the systematic review analysis and contributed also to the paper writing. All authors read and approved the final manuscript.
Funding
Not applicable
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Davidson A, Diamond B Autoimmune diseases. N Engl J Med. 2001;345(5):340–50. 10.1056/NEJM200108023450506. [DOI] [PubMed] [Google Scholar]
- 2.Simpson S, Blizzard L, Otahal P, Van der Mei I, Taylor B Latitude is significantly associated with the prevalence of multiple sclerosis: a meta-analysis. J Neurol, Neurosurg, Psychiatry. 2011;82(10):1132–41. 10.1136/jnnp.2011.240432. [DOI] [PubMed] [Google Scholar]
- 3.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296(23):2832–38. 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 4.Barrie W, Yang Y, Irving-Pease EK, Attfield KE, Scorrano G, Jensen LT, et al. Elevated genetic risk for multiple sclerosis emerged in steppe pastoralist populations. Nat. 2024;625(7994625):321–28. 10.1038/s41586-023-06618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bach JF The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med. 2002;347(12):911–20. 10.1056/NEJMra020100. [DOI] [PubMed] [Google Scholar]
- 6.Rosa P, Symmons DPM, Griffiths GEM, Ashcroft DM Global epidemiology of psoriasis: A systematic review of incidence and prevalence. J Invest Dermatol. 2013; 133(2): 377–85. 10.1038/jid.2012.339. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson C, Kumar S, Kimball A Latitude and psoriasis prevalence. J Am Acad Dermatol. 2011; 65(4): 870–73. 10.1016/j.jaad.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 8.Current climate: Climatology. Climate Change Knowledge Portal. Available from: https://climateknowledgeportal.worldbank.org/watershed/161/climate-data-historical; 2024 10 August 2024
- 9.Global Health Data Exchange (GHDx). Institute for Health Metrics and Evaluation. Available from https://vizhub.healthdata.org/gbd-results2023 10 August 2024
- 10.Yang H, Zhou R, Bai X, Guo M, Ruan G, Wang L, et al. Trend and geographic variation in incidence and prevalence of inflammatory bowel disease in regions across China: A nationwide employee study between 2013 and 2016. Frontiers Med (Lausanne). 2022;9:900251. 10.3389/fmed.2022.900251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnenberg A, McCarty DJ, Jacobsen SJ Geographic variation of inflammatory bowel disease within the United States. Gastroenterology. 1991;100(1):143–49. 10.1016/0016-5085(91)90594-b. [DOI] [PubMed] [Google Scholar]
- 12.Betteridge JD, Armbruster SP, Maydonovitch C, Veerappan GR Inflammatory bowel disease prevalence by age, gender, race, and geographic location in the U.S. military health care population. Inflamm Bowel Dis. 2013;19(7):1421–27. 10.1097/MIB.0b013e318281334d. [DOI] [PubMed] [Google Scholar]
- 13.Stein AC, Gaetano JN, Jacobs J, Kunnavakkam R, Bissonnette M, Pekow J Northern latitude but not season is associated with increased rates of hospitalizations related to inflammatory bowel disease: Results of a multi-year analysis of a national Cohort. PLoS One. 2016;11(8):e0161523. 10.1371/journal.pone.0161523.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armitage EL, Aldhous MC, Anderson N, Drummond HE, Riemersma RA, Ghosh S, et al. Incidence of juvenile-onset Crohn’s disease in Scotland: Association with northern latitude and affluence. Gastroenterology. 2004;127(4):1051–57. 10.1053/j.gastro.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 15.Aamodt G, Bengtson MB, Vatn MH Can temperature explain the latitudinal gradient of ulcerative colitis? Cohort of Norway. BMC Public Health. 2013;13:530. 10.1186/1471-2458-13-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalili H, Huang ES, Ananthakrishnan AN, Higuchi L, Richter JM, Fuchs CS, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012;61(12):1686–92. 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nerich V, Monnet E, Weill A, Vallier N, Vanbockstael V, Auleley GR, et al. Fine-scale geographic variations of inflammatory bowel disease in France: Correlation with socioeconomic and house equipment variables. Inflamm Bowel Dis. 2010;16(5):813–21. 10.1002/ibd.21122. [DOI] [PubMed] [Google Scholar]
- 18.Declercq C, Gower-Rousseau C, Vernier-Massouille G, Salleron J, Baldé M, Poirier G, et al. Mapping of inflammatory bowel disease in northern France: Spatial variations and relation to affluence. Inflamm Bowel Dis. 2010;16(5):807–12. 10.1002/ibd.21111. [DOI] [PubMed] [Google Scholar]
- 19.Larsen JH, Andersen S, Perminow G, Mundal HS, Mårild K, Stabell N, et al. Higher incidence of paediatric inflammatory bowel disease by increasing latitude in Norway, but stable incidence by age. Acta Paediatr. 2024 Jul;113(7):1720–27. 10.1111/apa.17222. [DOI] [PubMed] [Google Scholar]
- 20.Jussila A, Virta LJ, Salomaa V, Mäki J, Jula A, Färkkilä MA High and increasing prevalence of inflammatory bowel disease in Finland with a clear North-South difference. J Crohns Colitis. 2013Aug;7(7):e256–62. 10.1016/j.crohns.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Sloka S, Grant M, Nwehook LA The geospatial relation between uv solar radiation and type 1 diabetes in newfoundland. Acta Diabetol. 2009;47(1):73–78. 10.1007/s00592-009-0100-0. [DOI] [PubMed] [Google Scholar]
- 22.Ball SJ, Haynes A, Jacoby P, Pereira G, Miller LJ, Bower C, et al. Spatial and temporal variation in type 1 diabetes incidence in Western Australia from 1991 to 2010: Increased risk at higher latitudes and over time. Health Place. 2014;28:194–204. 10.1016/j.healthplace.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Joner G, Sοvik O The incidence of type 1 (insulin-dependent) diabetes mellitus 15?29 years in Norway 1978?1982. Diabetologia. 1991;34(4):271–74. 10.1007/BF00405087. [DOI] [PubMed] [Google Scholar]
- 24.Rytkönen M, Ranta J, Tuomilehto J, Karvonen M Bayesian analysis of geographical variation in the incidence of type I diabetes in Finland. Diabetologia. 2001;44(S3):B37–44. 10.1007/pl00002952. [DOI] [PubMed] [Google Scholar]
- 25.Joner G, Søvik O Incidence, age at onset and seasonal variation of diabetes mellitus in norwegian children, 1973–1977. Acta Paediatr Scand. 1981;70(3):329–35. 10.1111/j.1651-2227.1981.tb16560.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Wang K, Li T, Sun W, Li Y, Chang YF, et al. Childhood diabetes in China: Enormous variation by place and ethnic group. Diab Care. 1998;21(4):525–29. 10.2337/diacare.21.4.525. [DOI] [PubMed] [Google Scholar]
- 27.Staples JA, Ponsonby AL, Lim–, McMichael AJ Ecologic analysis of some immune-related disorders, including type 1 diabetes, in Australia: latitude, regional ultraviolet radiation, and disease prevalence. Environ Health Perspect. 2003;111(4):518–23. 10.1289/ehp.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XH, Li TL, Yang Z, Liu ZY, Wei YD, Jin SX, et al. A nine-year prospective study on the incidence of childhood type 1 diabetes mellitus in China. Biomed Environ Sci. 2000 Dec;13(4):263–70. [PubMed] [Google Scholar]
- 29.Vieira VM, Hart JE, Webster TF, Weinberg J, Puett R, Laden F, et al. Association between residences in U.S. Northern latitudes and rheumatoid arthritis: A spatial analysis of the nurses’ health study. Environ Health Perspect. 2010;118(7):957–61. 10.1289/ehp.0901861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosati G, Granieri E, Pinna L, Aiello I, De Bastiani P, Tola R The geographical distribution of multiple sclerosis, rheumatoid arthritis, rheumatic heart disease and poststreptococcal nephritis in Sardinia: Climatic and socioeconomic factors. J Neurol. 1978 Jan 1;219(1):27–35. 10.1007/BF00313366. [DOI] [PubMed]
- 31.Costenbader KH, Chang SC, Laden F, Puett R, Karlson EW Geographic variation in rheumatoid arthritis incidence among women in the United States. Arch Intern Med. 2008;168(15):1664. 10.1001/archinte.168.15.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J Incidence of rheumatoid arthritis in Sweden: A nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res (Hoboken). 2013;65(6):870–78. 10.1002/acr.21900. [DOI] [PubMed] [Google Scholar]
- 33.Andrees V, Wolf S, Augustin M, Mohr N, Augustin J Regional variations and prevalence of psoriasis in Germany from 2010 to 2017: A cross-sectional, spatio-epidemiological study on ambulatory claims data. BMJ Open. 2021Nov;11(11):10.1136/bmjopen-2020-047806. [DOI] [PMC free article] [PubMed]
- 34.Springate DA, Parisi R, Kontopantelis E, Reeves D, Griffiths CEM, Ashcroft DM Incidence, prevalence and mortality of patients with psoriasis: A U.K. population-based cohort study. Br J Dermatol. 2016;176(3):650–58. 10.1111/bjd.15021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrandiz C, Bordas X, Patos VG, Puig S, Pujol R, Smandia A Prevalence of psoriasis in Spain (epiderma project: Phase I). J Eur Acad Dermatol Venereol. 2001Jan;15(1):20–23. 10.1046/j.1468-3083.2001.00191.x. [DOI] [PubMed] [Google Scholar]
- 36.Leder R, Farber M The variable incidence of psoriasis in sub-Saharan Africa. Int J Dermatol. 2008;36(12):911–19. 10.1046/j.1365-4362.1997.00251.x. [DOI] [PubMed] [Google Scholar]
- 37.Mehta S, Patel V, Agarwal S, Pant N, Suman S, Sohliya AEL Vitamin D deficiency and immune health in polar populations: a systematic review and hypothesis-driven narrative analysis. Immunol Res. 2025;73(1):84. 10.1007/s12026-025-09640-7. [DOI] [PubMed] [Google Scholar]
- 38.Murdaca G, Greco M, Borro M, Gangemi S Hygiene hypothesis and autoimmune diseases: a narrative review of clinical evidences and mechanisms. Autoimmun Rev. 2021;20(7):102845. 10.1016/j.autrev.2021.102845. [DOI] [PubMed] [Google Scholar]
- 39.Conrad N, Misra S, Verbakel JY, Verbeke G, Molenberghs G, Taylor PN, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–90. 10.1016/S0140-6736(23)00457-9. [DOI] [PubMed] [Google Scholar]
- 40.Shapira Y, Agmon-Levin N, Shoenfeld Y Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34(3):J168–77. 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 41.Cooper GS, Bynum ML, Somers EC Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33(3–4):197–207. 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adami G, Pontalti M, Cattani G, Rossini M, Viapiana O, Orsolini G, et al. Association between long-term exposure to air pollution and immune-mediated diseases: a population-based cohort study. RMD Open. 2022;8(1):e002055. 10.1136/rmdopen-2021-002055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis JA, Kemp AS, Ponsonby AL Gene-environment interaction in autoimmune disease. Expert Rev Mol Med. 2014 Mar 7; 16: e 4 10.1017/erm.2014.5. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.