Abstract
We have previously reported that the immediate early (IE)-86 protein of human cytomegalovirus (HCMV) pushes the cell cycle toward S phase but inhibits cell division [Murphy, E. A., Streblow, D. N., Nelson, J. A. & Stinski, M. F. (2000) J. Virol. 74, 7108–7118]. We determined the cellular genes activated by the IE86 protein in permissive human fibroblast cells. A 4-fold or greater increase in the steady-state RNA from many cellular genes that regulate the cell cycle, the enzymes for DNA precursor synthesis, and the initiation of cellular DNA replication was detected by high-density DNA microarray analysis. Northern blot analysis confirmed the DNA microarray data. The viral IE86 protein induced a significant increase in the cellular steady-state RNA level from the B-myb, cyclin E, cdk-2, E2F-1, ribonucleotide reductase 1, ribonucleotide reductase 2, thymidylate synthetase, MCM3, and MCM7 genes, but actin RNA was not affected. Cellular genes regulated by the E2F transcription factors were strongly activated by the IE86 protein. In most cases, the cellular genes induced by the IE86 protein were also induced by HCMV infection. This study demonstrates the global array of cellular genes activated by the IE86 protein that pushes progression of the cell cycle from G0/G1 toward the G1/S transition point.
Cytomegaloviruses (CMVs) productively infect terminally differentiated cells of the host that are in the G0 phase of the cell cycle. Human foreskin fibroblast (HFF) cells are widely used for permissive HCMV infection in tissue culture. These cells withdraw from the cell cycle on contact inhibition and are in G0 arrest or G1 phase. Because CMVs do not encode many of the biosynthetic enzymes for DNA precursor synthesis, the virus requires a mechanism to overcome cellular quiescence. HCMV infection induces the progression of quiescent cells toward the G1/S transition point (reviewed in ref. 1) and activates cellular genes required for DNA replication such as thymidine kinase (TK) (2), dihydrofolate reductase (DHFR) (3), cyclin E (4), and DNA polymerase α (5). Mouse CMV induces the expression of ribonucleotide reductase (RR) (6) and thymidylate synthetase (TS) (7).
The retinoblastoma (Rb) family of proteins prevents cell cycle progression into S phase by binding to the E2F family of transcription factors and inhibiting the activity of E2Fs. The cyclin-dependent kinases (cdks) phosphorylate Rb family proteins, which free the E2F family of transcription factors (8, 9). The E2F transcription factors activate the promoters for DNA synthesis and G1/S regulatory genes (10). HCMV infection has been shown to cause hyperphosphorylation of Rb (4, 11).
HCMV gene expression occurs in three temporal phases designated immediate early (IE), early, and late. IE proteins are transactivators of viral as well as cellular gene expression (reviewed in refs. 12 and 13). The IE86 protein encoded by the IE2 gene (UL122) is a strong transactivator that interacts with factors of the basal-transcription machinery (14–17). The IE86 protein activates cyclin E gene expression (18), which is essential for progression of the cell cycle into the S phase. Cyclin E and cdks are highly activated after HCMV infection of HFF cells (4, 18–20). In addition, GST-IE86 fusion protein interacts in vitro with a variety of transcription factors that include histone acetyltransferase, CREB, TBP, TEF-1, TFIIB, and TAFII-130, and cell cycle regulatory factors that include p53, RB, and p21 (11, 14, 16, 21–27). The Rb protein has also been reported to bind the IE86 protein in vivo (22, 28).
The IE86 protein alone pushes quiescent, serum-starved cells into S phase, but mutant IE86 protein does not (11, 29, 30). In IE86 protein expressing cells, cell division does not occur (11, 30, 31). Because HCMV may require cell cycle progression for replication in terminally differentiated cells, and the IE86 protein alone induces cyclin E expression and cell cycle progression to S phase, we determined the effect of the IE86 protein on global cellular gene expression.
Here we present the cellular genes activated by the IE86 protein in permissive human fibroblast cells. We demonstrate that HCMV infection induces multiple E2F-responsive genes for entry into the S phase. The viral IE86 protein has a major role in activation of these cellular genes. The cellular genes that regulate the cell cycle, the enzymes for DNA precursor synthesis and the initiation of cellular DNA replication were significantly activated by the IE86 protein. The IE86 protein induces the cell cycle progression to the G1/S transition point by up-regulating the expression of E2F-responsive genes.
Materials and Methods
Cell Culture, Virus, and Adenovirus Vectors.
Maintenance and propagation of primary HFF and the HCMV Towne strain has been described (32). Replication-defective E1a−, E1b− and E3− adenovirus vectors expressing either the “Tet-off” transactivator (Ad-Trans), the green fluorescent protein (Ad-GFP), or the IE86 protein (Ad-IE86) were grown in 293 cells as described (30). The titers of the various recombinant adenovirus vectors were determined by plaque assay on 293 cells. For transduction experiments, 10 plaque-forming units (pfu) per cell of recombinant adenovirus vectors in Eagle's MEM containing 3 μl of Lipofectamine reagent (Life Technologies, Gaithersburg, MD)/ml was used. After 1 h at 37°C, the inoculum was removed, and the cells were maintained in media.
Slot Blot Analysis.
Cytoplasmic RNAs (4, 2, 1, 0.5, and 0.25 μg) were blotted onto nytran membranes (Schleicher & Schuell) by using the Minifold II Slot Blotter (Schleicher & Schuell). The plasmid containing Ad-E4 (pE4), a gift from R. Anderson (University of Iowa, Vector Core), was subcloned into the pBluescript II KS+ Hind III-Nar I sites to generate plasmid pAd-E4 orf3. 32P-labeled random-primed DNA probes of Ad-E4 orf3 or actin were synthesized using Ready-To-Go DNA Labeling Beads (Amersham Pharmacia) and purified by NucTrap Probe purification columns (Stratagene). Membranes were probed overnight at 60°C in hybridization buffer containing 10% dextransulfate, 1× standard saline phosphate/EDTA (SSPE; 0.18 M NaCl/10 mM phosphate, pH 7.4/1 mM EDTA), and 2% SDS, and then washed twice at room temperature and once at 60°C in 2× SSC (1× SSC = 0.15 M sodium chloride/0.015 M sodium citrate, pH 7) and 0.1% SDS.
DNA Microarray.
Affymetrix (Santa Clara, CA) DNA microarray analysis was done according to the manufacturer's instructions. Cytoplasmic RNAs (40 μg) were reverse transcribed to cDNA by using T7-(dT)24 primer and Super Script Double-stranded cDNA Synthesis Kit (Life Technologies). Biotin-labeled cRNA was synthesized from cDNA by using ENZO BioArray High Yield RNA Transcript Labeling Kit (Enzo Diagnostics). cRNA was fragmented to an average size of 50–100 nucleotides by incubating at 94°C for 35 min in 40 mM Tris-acetate (pH 8.1) containing 100 mM potassium acetate and 30 mM magnesium acetate, and then hybridized to human GENECHIP arrays (Human Genome U95A, Affymetrix) containing approximately 12,000 human genes. The DNA microarrays were scanned using a confocal scanner (Affymetrix Molecular Dynamics). The data were processed for average difference values by using GENECHIP software (Affymetrix). A 4-fold or higher level of activated cellular expression induced by Ad-IE86 compared with Ad-GFP was considered significant.
Northern Blots.
Ten μg of cytoplasmic RNA was subjected to electrophoresis in a 1.5% agarose-formaldehyde gel and transferred to nytran membranes (Schleicher & Schuell). DNA probes for Northern blot analysis were kindly provided by the following investigators with assistance from J. Nevins (Duke University, Durham, NC): human E2F-1 and cdk2 (E. Harlow), human cyclin E (X.-F. Wang), and human B-myb (B. Calabretta). The cDNA for mouse thymidylate synthetase, mouse RR1 and RR2, and MCM3 and MCM7 were provided by L. Johnson (Ohio State University, Columbus, OH), L. Thelander (Umea University, Sweden), and H. Kimura (Mitsubishi Kasei Institute of Life Sciences, Tokyo), respectively. 32P-labeled random-primed DNA probes were synthesized using Ready-To-Go DNA Labeling Beads (Amersham Pharmacia), and purified using NucTrap Probe purification columns (Stratagene). Hybridization was performed overnight at 58°C in buffer containing 10% dextransulfate, 1× SSPE, and 2% SDS. The membranes were washed twice at room temperature and once at 58°C in 0.2× SSC and 0.1% SDS. Blots were stripped by boiling for 15 min in 0.1× SSPE and 0.5% SDS.
Results
Up-Regulation of Cellular Genes in HFF Cells Infected with HCMV Towne.
HCMV induces cell cycle progression of quiescent HFF cells but stops the cell cycle at the G1/S transition point (33–35). Because HCMV infection induces the expression of thymidine kinase, dihydrofolate reductase, cyclin E, and DNA polymerase α (2–5), we determined whether HCMV up-regulates transcription from other cellular genes for progression to the S phase of the cell cycle. HFF cells were infected with HCMV Towne strain, and cytoplasmic RNAs were harvested at 6 and 24 h after infection. The RNAs were harvested from the same cell densities and were analyzed by Northern blot analysis as described in Materials and Methods. There was an increase in steady-state RNA levels of the following genes: B-myb, E2F-1, MCM3, MCM7, RR1, RR2, and TS (Fig. 1). These results suggested that HCMV induces the expression of many genes for cell cycle progression to the G1/S transition point. These data are in agreement with Browne et al. (ref. 36; posted at http://www.molbio. princeton.edu/labs/shenk/browneetal2001).
Figure 1.
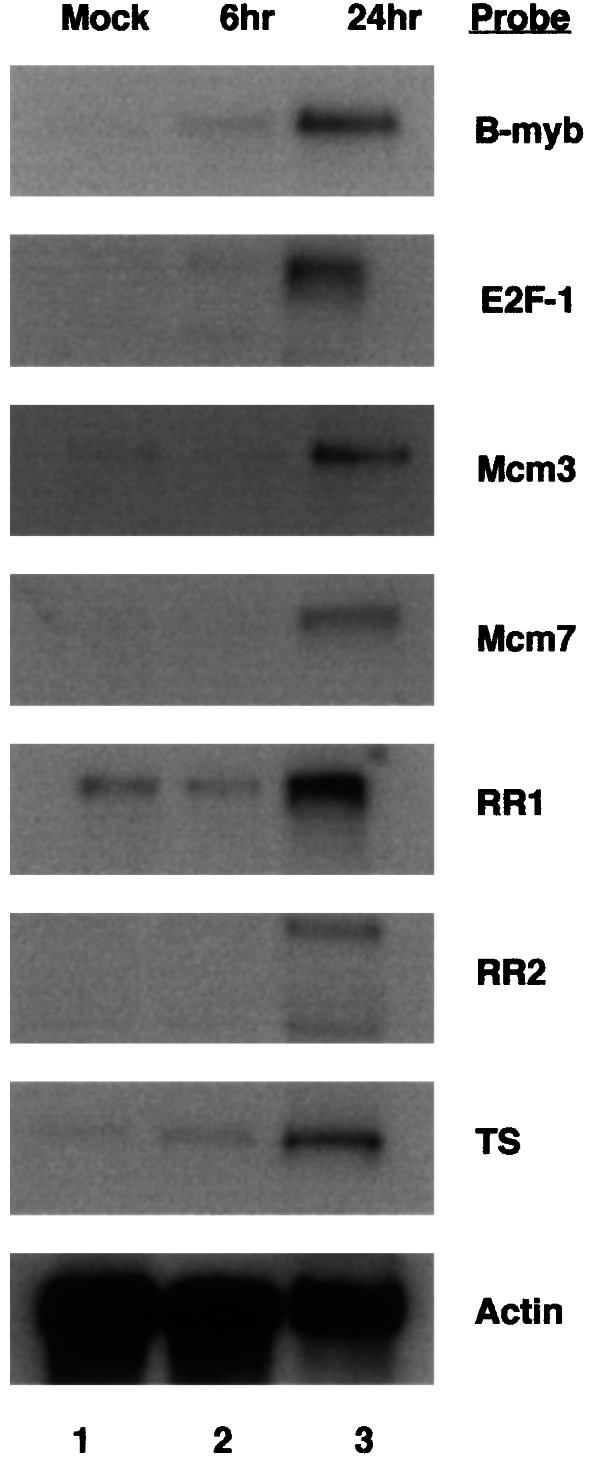
Increase in steady-state RNAs from E2F-responsive genes in HCMV-infected cells. HFF cells were infected with HCMV Towne, and cytoplasmic RNA was harvested at 6 and 24 h after infection. RNAs were fractionated by electrophoresis in 1.5% agarose-formaldehyde gels and analyzed by Northern blot hybridization with 32P-labeled random-primed DNA probes as described in Materials and Methods. The 32P-labeled DNA probes for specific cellular genes are designated. Lane 1, mock-infected; lane 2, 6 h after infection; lane 3, 24 h after infection.
Up-Regulation of Cellular Genes in HFF Cells Transduced with Ad-IE86.
The IE86 protein is a transactivator of viral as well as cellular gene expression (reviewed in refs. 12 and 13) and activates cellular cyclin E expression (18). To determine whether the IE86 protein is involved in up-regulation of S-phase genes, we determined the effect of the IE86 protein on global cellular gene expression by using DNA microarray analysis. Because in transient transfection assays with an IE86 expression plasmid, the viral IE86 protein is expressed in a small percentage of cells, we used recombinant adenovirus vectors for expressing the IE86 protein in greater than 95% of the HFF cells. To do this, we used a replication-defective E1a−, E1b−, and E3− knock-out adenovirus vector expressing the IE86 protein (Ad-IE86) or GFP (Ad-GFP). The expression of the IE86 protein with the adenovirus vector system was activated by Ad-Trans and turned off by tetracycline as described (30).
Because the E4 transcription unit of adenovirus has an effect on the cell cycle (37), we determined whether the IE86 protein increased E4 expression. HFF cells were transduced with 10 pfu per cell of either Ad-IE86 plus Ad-Trans or Ad-GFP plus Ad-Trans, and cytoplasmic RNA was harvested after 24 h. To determine the level of E4 expression in transduced HFFs, cytoplasmic RNAs were blotted onto nytran membranes for slot blot hybridization with 32P-labeled random-primed E4 ORF3 DNA probe. We selected an ORF3 probe because it is in the middle of the E4 transcription unit. Mock-transduced cells and adenovirus wild type (Ad-wt) infected cells were used as controls. Ad-GFP and Ad-IE86 transduced HFF cells had similar levels of E4 expression, but E4 expression was significantly higher in Ad-wt infected HFFs because of the presence of the E1a and E1b genes (Fig. 2). We conclude that E4 expression was not induced by the IE86 protein.
Figure 2.
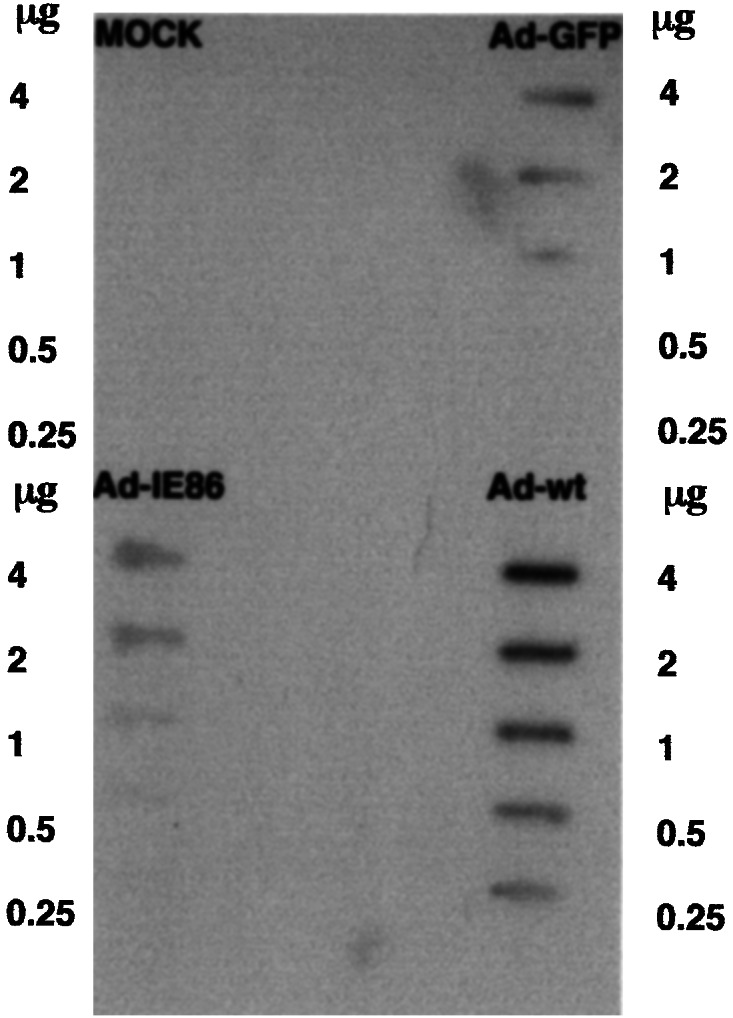
Ad-E4 gene expression in Ad-GFP- or Ad-IE86-transduced cells. Cytoplasmic RNAs ranging from 0.25 to 4.0 μg were blotted onto nytran membranes for slot blot hybridization with 32P-labeled random-primed E4 ORF3 DNA as described in Materials and Methods. Controls were mock-transduced cells and adenovirus wild-type (Ad-wt) infected cells.
To determine the cellular genes up-regulated by the IE86 protein, HFF cells were transduced with 10 pfu per cell of either Ad-IE86 plus Ad-Trans or Ad-GFP plus Ad-Trans. The level of IE86 protein in these cells was similar to HCMV-infected cells when using a multiplicity of infection (moi) of 5 pfu per cell. Cytoplasmic RNAs were harvested after 24 h and were reverse transcribed to cDNA. Biotin-labeled cRNA was synthesized from cDNA, and biotin-labeled cRNA was fragmented and hybridized to human GENECHIP arrays containing over 12,000 human genes as described in Materials and Methods.
Ad-GFP induced the expression of INF-responsive genes relative to mock-transduced cells (data not shown). We performed two separate DNA microarray analysis with HFF cellular RNAs from cells transduced on two separate occasions with Ad-IE86 vectors. Table 1 lists the cellular genes up-regulated 4-fold or higher by Ad-IE86 compared with Ad-GFP. We set 4-fold or higher as a significant increase in steady-state cellular RNA level. Sixty-four of approximately 12,000 genes were activated 4-fold or higher by Ad-IE86 compared with Ad-GFP (Table 1). Up-regulated cellular genes were categorized into genes related to DNA replication, cell cycle/proliferation, DNA repair/stability, oncogene/tumor, apoptosis, transcription regulation, G protein, splicing, and expressed sequence tags (ESTs). One-half of the genes up-regulated by Ad-IE86 are involved in DNA replication and cell cycle/proliferation (32 of 64). Genes such as replication factor C, dihydrofolate reductase (DHFR), replication protein A, proliferating cell nuclear antigen (PCNA), RR, TS, MCM3, MCM5, MCM6, MCM7, DNA polymerase α, thymidine kinase (TK), B-myb, cyclin E, and E2F-1 were significantly up-regulated. These cellular genes contain E2F-responsive elements upstream of their promoters. The fold-activation of cellular genes involved in regulation of the cell cycle (Fig. 3A), DNA precursor synthesis (Fig. 3B), and initiation of cellular DNA replication (Fig. 3C) ranged from 6- to 30-fold. These results demonstrate the array of cellular genes up-regulated significantly by the IE86 protein. Other genes involved in oncogenesis, apoptosis, transcription, and signal transduction were also significantly up-regulated by IE86 protein expression. Only two genes were significantly repressed and they were acute myelogenous leukemia (AML) 1B and AML 1C proteins.
Table 1.
RNAs of cellular genes upregulated 4-fold or higher by the IE86 protein
| GenBank accession nos. | Description |
|---|---|
| DNA replication | |
| M87338 | Human replication factor C, 40-kDa subunit (A1) mRNA |
| M87339 | Human replication factor C, 37-kDa subunit mRNA |
| M63488 | Human replication protein A, 70-kDa subunit mRNA |
| J05614 | Human proliferating cell nuclear antigen (PCNA) gene, promoter region |
| M97856 | Homo sapiens histone-binding protein mRNA |
| U81375 | Human placental equilibrative nucleoside transporter 1 (hENT1) mRNA |
| X59543 | Human mRNA for M1 subunit of ribonucleotide reductase |
| X02308 | Human mRNA for thymidylate synthase (EC 2.1.1.45) |
| J00140 | Human dihydrofolate reductase gene |
| L07541 | Human replication factor C, 38-kDa subunit mRNA |
| D84557 | H. sapiens mRNA for HsMCM6 |
| D38073 | Human mRNA for hRlf beta subunit (p102 protein) (MCM3) |
| X14850 | Human H2A.X mRNA encoding histone H2A.X |
| X00737 | Human mRNA for purine nucleoside phosphorylase (PNP; EC 2.4.2.1) |
| X06745 | Human mRNA for DNA polymerase alpha subunit |
| M15205 | Human thymidine kinase gene with clustered Alu repeats in introns |
| D55716 | Human mRNA for P1cdc47 (MCM7) |
| X74795 | H. sapiens P1-Cdc46 mRNA (MCM5) |
| J03626 | Human UMP synthase mRNA |
| Cell cycle/proliferation | |
| U65410 | Human Mad2 (hsMAD2) mRNA |
| X05360 | Human CDC2 gene involved in cell cycle control |
| X13293 | Human mRNA for B-myb gene |
| M15796 | Human cyclin protein gene, complete cds |
| M73812 | Human cyclin E mRNA sequence |
| M96577 | H. sapiens (E2F-1) pRB-binding protein mRNA |
| U18271 | Human thymopoietin (TMPO) gene |
| D88357 | H. sapiens mRNA for CDC2 delta T |
| AF067656 | H. sapiens ZW10 interactor Zwint mRNA |
| D64110 | H. sapiens mRNA for ANA |
| AJ000186 | H. sapiens mRNA for MAD2 protein |
| U37426 | Human kinesin-like spindle protein HKSP (HKSP) mRNA |
| DNA repair/stability | |
| Y09008 | H. sapiens mRNA for uracil-DNA glycosylase |
| M60527 | Human deoxycytidine kinase mRNA |
| HG4074-HT4344 | Rad2 |
| U39817 | Human Blooms syndrome protein (BLM) mRNA |
| X62534 | H. sapiens HMG-2 mRNA |
| AF029669 | H. sapiens Rad51C (RAD51C) mRNA |
| AC004770 | H. sapiens chromosome 11, BAC CIT-HSP-311e8 (BC269730) containing the hFEN1 gene |
| U03911 | Human mutator gene (hMSH2) mRNA |
| Oncogene/Tumor associated | |
| U76638 | Human BRCA1-associated RING domain protein (BARD1) mRNA |
| Y15227 | H. sapiens mRNA for leukemia-associated gene 1 |
| X64228 | H. sapiens can mRNA |
| AB024704 | H. sapiens mRNA for fls353 |
| Apoptosis | |
| U13737 | Human cysteine protease CPP32 isoform alpha mRNA |
| Transcription regulator | |
| U74612 | Human hepatocyte nuclear factor-3/fork head homolog 11A (HFH-11A) mRNA |
| M62810 | Human mitochondrial transcription factor 1 mRNA |
| U61145 | Human enhancer of zeste homolog 2 (EZH2) mRNA |
| G protein | |
| U57094 | Human small GTP-binding protein mRNA |
| Splicing | |
| AF016371 | H. sapiens U-snRNP-associated cyclophilin (USA-CyP) mRNA |
| AF026402 | H. sapiens U5 snRNP 100 kD protein mRNA |
| Unknown (ESTs) | |
| L38933 | H. sapiens GT198 mRNA |
| AF070552 | H. sapiens clone 24767 mRNA sequence |
| D21063 | Human mRNA for KIAA0030 gene |
| N30625 | H. sapiens cDNA clone IMAGE:257795 3′, mRNA sequence |
| D26018 | Human mRNA for KIAA0039 gene |
| D14657 | Human mRNA for KIAA0101 gene |
| AI525633 | PT1.3_04_A08.r tumor1 Homo sapiens cDNA 5′, mRNA sequence |
| D87448 | Human mRNA for KIAA0259 gene |
| U79241 | Human clone 23759 mRNA |
| AF059274 | H. sapiens neuroglycan C mRNA |
| AW016815 | H. sapiens cDNA clone IMAGE:2709880 3′, mRNA sequence |
| AA203476 | H. sapiens cDNA clone IMAGE:446424 5′, mRNA sequence |
| AI680675 | H. sapiens cDNA clone IMAGE:2272022 3′, mRNA sequence |
RNA levels are expressed relative to the Ad-GFP control and were upregulated 4-fold or higher in two separate DNA microarrays.
Figure 3.
Effect of the IE86 protein on the steady-state RNA from E2F-responsive genes that regulate the cell cycle, the enzymes for DNA precursor synthesis, and the initiation of cellular DNA replication. Cytoplasmic RNAs from Ad-GFP- or Ad-IE86-transduced HFF cells were analyzed using human gene chips containing over 12,000 genes. The DNA microarrays were scanned by using a confocal scanner and the data are expressed as fold-change in steady-state RNA levels relative to the Ad-GFP control as described in Materials and Methods. (A) Cell cycle regulatory genes. (B) Cellular enzymes for DNA precursor synthesis. (C) Initiation of cellular DNA replication genes. DHFR, dihydrofolate reductase; DNA pol α, DNA polymerase α; MCM, minichromosome maintenance; RR1, M1 subunit of RR; PCNA, proliferating cell nuclear antigen; TK, thymidine kinase.
Northern Blot Analysis.
Because some of E2F-responsive genes were activated less than 4-fold and DNA microarray analysis alone can produce some false-positive or -negative results, we determined the reproducibility of the DNA microarray data by Northern blot analysis. HFF cells were transduced with 10 pfu per cell of either Ad-IE86 plus Ad-Trans or Ad-GFP plus Ad-Trans, and cytoplasmic RNA was harvested after 24 h. Cytoplasmic RNAs were fractionated by electrophoresis in 1.5% agarose-formaldehyde gels and analyzed by Northern blot hybridization with 32P-labeled random-primed DNA probes for B-myb, cdk-2, cyclin E, E2F-1, MCM3, MCM7, RR1, RR2, and TS. An increase in the level of steady-state RNA from E2F-responsive genes was detected by Northern blot analysis in Ad-IE86 transduced cells but not in Ad-GFP transduced cells (Fig. 4). E2F-responsive genes such as A-myb, cdk-2, RR2, MCM4, and Cdc7 were not included in Table 1 because these genes were up-regulated 4-fold or higher in one microarray but not in both microarrays (data not shown). Even though the cdk-2 and RR2 genes were not included in Table 1, RNAs from these genes were determined to be significantly up-regulated by Ad-IE86a or Ad-IE86b, which were two separate transductions of HFFs (Fig. 4). These DNA microarray and Northern blot analysis demonstrate that the RNAs from E2F-responsive genes were up-regulated by the IE86 protein. The up-regulation of non-E2F-responsive genes requires further investigation.
Figure 4.
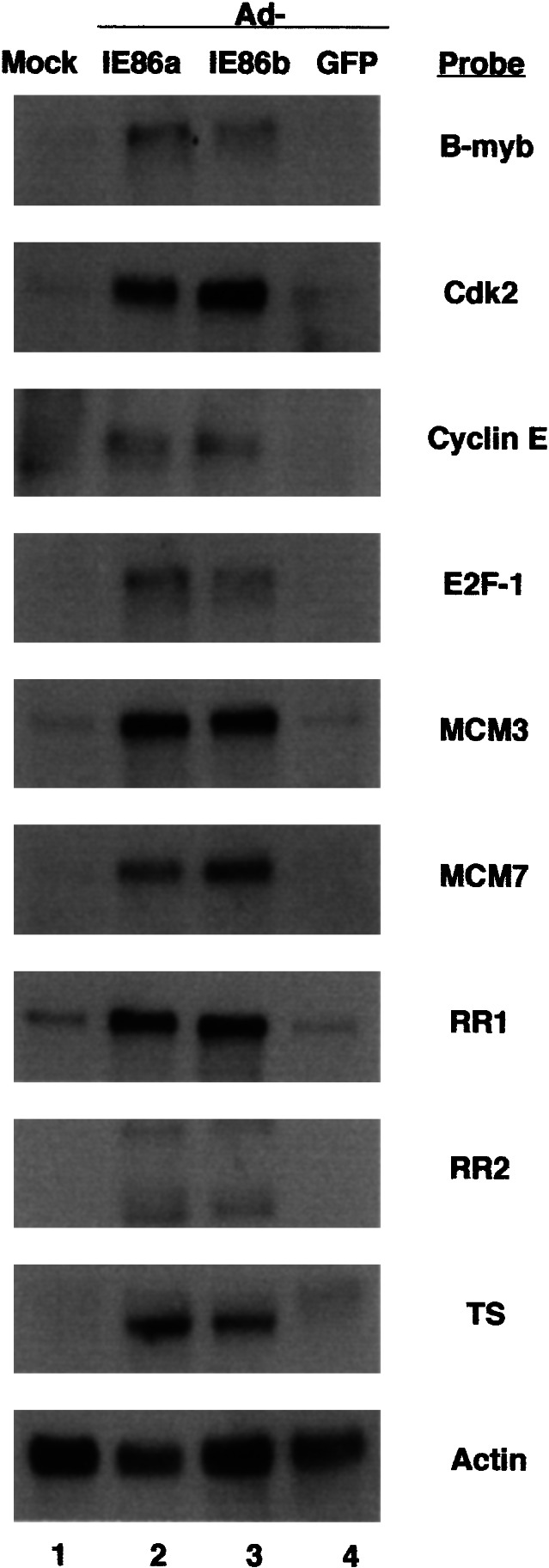
Northern blot analysis of RNAs from E2F-1-responsive genes in Ad-GFP- or Ad-IE86-transduced HFF cells. Cytoplasmic RNAs were fractionated by electrophoresis in 1.5% agarose-formaldehyde gels and analyzed by Northern blot hybridization with 32P-labeled random-primed DNA probes as described in Materials and Methods. Ad-IE86a and Ad-IE86b represent two separate transductions of HFF cells. Lanes 1, mock; lane 2, Ad-IE86a; lane 3, Ad-IE86b; lane 4, Ad-GFP. The 32P-labeled probes for specific cellular genes are designated.
Discussion
Differentiated HFF cells withdraw from the cell cycle on contact inhibition and are in G0 arrest or G1 phase. After infection with HCMV, the steady-state RNA levels of multiple E2F-responsive genes were highly increased. The up-regulation of E2F-responsive genes indicates cell cycle progression from G0/G1 to S phase. Because the viral IE86 protein alone induces cell cycle progression into S phase and cellular DNA synthesis (11, 29–31) and transactivates the expression of cellular as well as viral genes (14–17, 22, 24), we assayed the effect of the IE86 protein on global cellular gene expression. HFF cells were transduced with recombinant adenovirus vectors expressing either the IE86 protein or GFP, and cytoplasmic RNAs were assayed by DNA microarray. The DNA microarray allows one to assay a very large number of target genes in an unbiased manner.
Because the E4 gene of adenovirus has been implicated in affecting the cell cycle (37) and inactivating cellular p53 protein (38–41), we determined whether the IE86 protein activated transcription of the adenovirus E4 gene. Similar levels of E4 expression were detected with the control Ad-GFP or with Ad-IE86 in transduced HFF cells. The levels of E4 expression were weak compared with adenovirus wild type infected HFF cells. The IE86 protein did not induce the expression of the E4 gene. Therefore, it is unlikely that the effects observed were caused by IE86 protein induction of adenovirus E4 proteins. However, we cannot eliminate the possibility of a change of E4 activity in the presence of the IE86 protein.
Most of the genes up-regulated 4-fold or higher by the IE86 protein were genes required for DNA replication and cell cycle progression to S phase. Whereas some of these cellular genes were activated 4-fold or less, others were activated as much as 25- to 30-fold as determined by Affymetrix GENECHIP software. Differences in the level of activation of S-phase genes may be related to differences in the individual promoter strengths. Cellular genes required for progression of the cell cycle from G0 or G1 to S phase are transactivated by the E2F family of transcription factors. Many E2F-1-responsive genes, such as cyclin E, E2F-1, B-myb, dihydrofolate reductase (DHFR), PCNA, TK, TS, RR, and MCMs, were strongly up-regulated by the IE86 protein. The expression of these cellular genes indicates cell cycle progression to the G1/S transition point.
Some of E2F-responsive genes were up-regulated 4-fold or higher in one experiment and less than 4-fold in the other. However, the up-regulation of the genes cdk-2 and RR2 was qualitatively confirmed by Northern blot analysis. Lastly, preliminary data indicate that there is a correlation between an increase in cellular mRNA and cellular protein in HFF cells expressing the IE86 protein.
These results demonstrate that the IE86 protein activates the expression of genes required for entry into S phase and DNA replication. The IE86 protein induces a cellular environment favorable for DNA synthesis. The up-regulation of other cellular genes not directly involved in DNA replication and cell cycle control may be due to activation of cellular transcription resulting from cell cycle progression. How the IE86 protein induces cell cycle progression toward S phase and transactivates E2F-responsive genes is still unclear. GST-IE86 fusion protein interacts with Rb (22, 26). The interaction of the IE86 protein with Rb was also detected by coimmunoprecipitation assays (22). The interaction of the IE86 protein with Rb may inactivate the Rb protein and free E2Fs for activation of transcription.
It has also been shown that the IE86 protein also activates the cyclin E promoter through direct binding (18). An increase in cyclin E levels activates cdk-2 to phosphorylate Rb, and the phosphorylation of Rb dissociates Rb-E2F complexes. Therefore, it is also possible that the IE86 protein activates cell cycle progression to the G1/S transition point by inducing the expression of cyclin E. The IE86 protein is also reported to interact with p53 and p21 (11, 27, 42). A decrease in activities of cdk inhibitor, such as p21, would increase cdk-2 activity and the activity of E2F transcription factors.
The up-regulation of cellular genes required for DNA replication and cell cycle progression to the G1/S transition point by the IE86 protein indicates that the IE86 protein is one of the critical viral proteins that allows the virus to replicate in quiescent cells. The IE86 protein activates the expression of cellular genes to increase nucleotide pools and to acquire cellular DNA replication machinery for viral DNA replication. However, HCMV and the viral IE86 protein inhibit cell cycle progression at either the G1/S transition point or during S phase (30, 31), and the infected cell specializes in viral DNA synthesis. How the IE86 protein inhibits cellular DNA synthesis is unclear and is the subject of future studies.
Acknowledgments
We thank members of the laboratory for helpful discussion and Al Klingelhutz and Jay Nelson for critical reading of the manuscript. We are grateful to Philip Lashmit for assistance. This work was supported by National Institutes of Health Grant AI-13562 (to M.F.S.) and Training Grant AI-07533 to the University of Iowa (to Y.-J.S.).
Abbreviations
- RR
ribonucleotide reductase
- CMV
cytomegalovirus
- HCMV
human CMV
- HFF
human foreskin fibroblast
- cdk
cyclin-dependent kinases
- TS
thymidylate synthetase
- Rb
retinoblastoma
- pfu
plaque-forming units
- IE
immediate early
References
- 1.Stinski M F, Song Y-J. In: Structure-Function Relationships of Human Pathogenic Viruses. Bogner E, Holzenburg A, editors. New York: Kluwer Academic/Plenum; 2001. [Google Scholar]
- 2.Colberg-Poley A M, Santomenna L D. Virology. 1988;166:217–228. doi: 10.1016/0042-6822(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 3.Wade M, Kowalik T F, Mudryj M, Huang E-S, Azizkhan J C. Mol Cell Biol. 1992;12:4364–4374. doi: 10.1128/mcb.12.10.4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francoise M J, Jault J, Ruchti F, Fortunato E A, Clark C, Corbeil J, Richman D D, Spector D H. J Virol. 1995;69:6697–6704. doi: 10.1128/jvi.69.11.6697-6704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albrecht T, Boldogh I, Fons M, Lee C H, AbuBakar S, Russell J M, Au W W. Subcell Biochem. 1989;15:157–202. [PubMed] [Google Scholar]
- 6.Lembo D, Gribaudo G, Hofer A, Riera L, Cornaglia M, Mondo A, Angeretti A, Gariglio M, Thelander L, Landolfo S. J Virol. 2000;74:11557–11565. doi: 10.1128/jvi.74.24.11557-11565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gribaudo G, Riera L, Lembo D, De Andrea M, Gariglio M, Rudge T L, Johnson L F, Landolfo S. J Virol. 2000;74:4979–4887. doi: 10.1128/jvi.74.11.4979-4987.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nevins J R. Science. 1992;258:424–429. doi: 10.1126/science.1411535. [DOI] [PubMed] [Google Scholar]
- 9.Weinberg R. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 10.DeGregori J, Kowalik T, Nevins J R. Mol. Cell. Biol. 1995. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinclair J, Baillie J, Bryant L, Caswell R. J Gen Virol. 2000;6:1553–1565. doi: 10.1099/0022-1317-81-6-1553. [DOI] [PubMed] [Google Scholar]
- 12.Spector D H. Intervirology. 1996;39:361–377. doi: 10.1159/000150507. [DOI] [PubMed] [Google Scholar]
- 13.Stenberg R M. Intervirology. 1996;39:343–349. doi: 10.1159/000150505. [DOI] [PubMed] [Google Scholar]
- 14.Caswell R, Hagemeier C, Chiou C-J, Hayward G, Kouzarides T, Sinclair J. J Gen Virol. 1993;74:2691–2698. doi: 10.1099/0022-1317-74-12-2691. [DOI] [PubMed] [Google Scholar]
- 15.Jupp R, Hoffmann S, Stenberg R M, Nelson J A, Ghazal P. J Virol. 1993;67:7539–7546. doi: 10.1128/jvi.67.12.7539-7546.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukac D M, Harel N Y, Tanese N, Alwine J C. J Virol. 1997;71:7227–7239. doi: 10.1128/jvi.71.10.7227-7239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukac D M, Manuppello J R, Alwine J C. J Virol. 1994;68:5184–5193. doi: 10.1128/jvi.68.8.5184-5193.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bresnahan W A, Albrecht T, Thompson E A. J Biol Chem. 1998;273:22075–22082. doi: 10.1074/jbc.273.34.22075. [DOI] [PubMed] [Google Scholar]
- 19.Bresnahan W A, Boldogh I, Chi P, Thompson E A, Albrecht T. Virology. 1997;231:239–247. doi: 10.1006/viro.1997.8489. [DOI] [PubMed] [Google Scholar]
- 20.Salvant B S, Fortunato E A, Spector D H. J Virol. 1998;72:3729–3741. doi: 10.1128/jvi.72.5.3729-3741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryant L A, Mixon P, Davidson M, Bannister A J, Kouzarides T, Sinclair J H. J Virol. 2000;74:7230–7237. doi: 10.1128/jvi.74.16.7230-7237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hagemeier C, Caswell R, Hayhurst G, Sinclair J, Kouzarides T. EMBO J. 1994;13:2897–2903. doi: 10.1002/j.1460-2075.1994.tb06584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagemeier C, Walker S, Caswell R, Kouzarides T, Sinclair J. J Virol. 1992;66:4452–4456. doi: 10.1128/jvi.66.7.4452-4456.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lang D, Gebert S, Arlt H, Stamminger T. J Virol. 1995;69:6030–6037. doi: 10.1128/jvi.69.10.6030-6037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scully A L, Sommer M H, Schwartz R, Spector D H. J Virol. 1995;69:6533–6540. doi: 10.1128/jvi.69.10.6533-6540.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sommer M H, Scully A L, Spector D H. J Virol. 1994;68:6223–6231. doi: 10.1128/jvi.68.10.6223-6231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speir E, Modali R, Huang E, Leon M B, Shawl F, Finkel T, Epstein S E. Science. 1994;265:391–394. doi: 10.1126/science.8023160. [DOI] [PubMed] [Google Scholar]
- 28.Fortunato E A, Sommer M H, Yoder K, Spector D H. J Virol. 1997;71:8176–8185. doi: 10.1128/jvi.71.11.8176-8185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castillo J P, Yurochko A D, Kowalik T F. J Virol. 2000;74:8028–8037. doi: 10.1128/jvi.74.17.8028-8037.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murphy E A, Streblow D N, Nelson J A, Stinski M F. J Virol. 2000;74:7108–7118. doi: 10.1128/jvi.74.15.7108-7118.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiebusch L, Hagemeier C. EMBO J. 2001;20:1086–1098. doi: 10.1093/emboj/20.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stinski M F. J Virol. 1977;23:751–767. doi: 10.1128/jvi.23.3.751-767.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bresnahan W A, Boldogh I, Thompson E A, Albrecht T. Virology. 1996;224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 34.Dittmer D, Mocarski E S. J Virol. 1997;71:1629–1634. doi: 10.1128/jvi.71.2.1629-1634.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu M, Shenk T. J Virol. 1996;70:8850–8857. doi: 10.1128/jvi.70.12.8850-8857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Browne E P, Wing B, Coleman D, Shenk T. J Virol. 2001;75:12319–12330. doi: 10.1128/JVI.75.24.12319-12330.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wersto R P, Rosenthal E R, Seth P K, Eissa N T, Donahue R E. J Virol. 1998;72:9491–9502. doi: 10.1128/jvi.72.12.9491-9502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cathomen T, Weitzman M D. J Virol. 2000;74:11407–11412. doi: 10.1128/jvi.74.23.11407-11412.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higashino F, Pipas J M, Shenk T. Proc Natl Acad Sci USA. 1998;95:15683–15687. doi: 10.1073/pnas.95.26.15683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roth J, Konig C, Wienzek S, Weigel S, Ristea S, Dobbelstein M. J Virol. 1998;72:8510–8516. doi: 10.1128/jvi.72.11.8510-8516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steegenga W T, Shvarts A, Riteco N, Bos J L, Jochemsen A G. Mol Cell Biol. 1999;19:3885–3894. doi: 10.1128/mcb.19.5.3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonin L R, McDougall J K. J Virol. 1997;71:5861–5870. doi: 10.1128/jvi.71.8.5861-5870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]



