Abstract
As a step toward the elucidation of mechanisms in vesicle budding, a cell-free assay that measures cytosol-induced vesicle generation from liposomes was established. This assay then was used to explore the role of phosphoinositides in vesicle formation. Liposomes incubated with brain cytosol in the presence of ATP and GTP massively generated small vesicles, as assessed both quantitatively and qualitatively by a dynamic light-scattering assay. Both ATP and GTP were required. Vesicle formation was inhibited greatly by the immunodepletion of dynamin 1 from the cytosol, indicating a major contribution of this GTPase in this reaction and suggesting that it mimics endocytic vesicle fission. Increasing the concentration of l-α-phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] but not of l-α-phosphatidylinositol 4-monophosphate or l-α-phosphatidylinositol in the lipid membranes enhanced vesicle formation. Lipid analysis revealed rapid degradation of PtdIns(4,5)P2 to l-α-phosphatidylinositol during the incubation with the reaction reaching a maximum within 5 sec, whereas vesicle formation proceeded with a longer time course. PtdIns(4,5)P2 degradation was independent of vesicle formation and occurred also in the presence of guanosine 5′-O-(thiotriphosphate), where few vesicle formations occurred. These results suggest that PtdIns(4,5)P2 plays a critical role in the early step of vesicle formation, possibly in the recruitment of coats and fission factors to membranes.
In eukaryotic cells, transport vesicles are implicated in a variety of intracellular traffic events in both the secretory and endocytic pathways. The formation of a carrier vesicle is initiated by the recruitment of cytosolic coat proteins to the cytoplasmic surface of the donor membrane. One of the functions of the coat is to provide a scaffold for the formation of a high-curvature membrane bud. The coated bud then is pinched off as a coated vesicle, which eventually sheds the coat before fusion with its acceptor compartment. Several types of coats including the COPI, COPII, and clathrin coats have been characterized extensively (reviewed in refs. 1–7).
Recently, the role of lipid factors is becoming a focus of the investigation in the field of membrane–coat interactions (7–11). Growing evidence suggests a key function of phosphoinositides and in particular phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] in the recruitment of endocytic coats. PtdIns(4,5)P2 binds to a variety of proteins that function in endocytic traffic including the α-subunit of clathrin adaptor protein (AP)2 (12), AP180, CALM (13), dynamin (14), and epsin (15). Phosphoinositides affect AP2's self-assembly (12, 16) and stimulate dynamin's GTPase activity (17, 18). Furthermore, disruption of these interactions in living cells resulted in inhibition of endocytosis (15, 19). Strong evidence for a physiological role of phosphoinositide metabolism in endocytosis came from the identification and functional characterization of a synaptic polyphosphoinositide phosphatase, synaptojanin 1 (20). Genetic deletion of synaptojanin in mice (21) and Caenorhabditis elegans (22) or disruption of the interactions of synaptojanin with its functional partners (23) resulted in a partial impairment of synaptic vesicle recycling and an increase of clathrin-coated vesicles and the coated pits. Conversely, an enzyme that synthesizes PtdIns(4,5)P2 from PtdIns 4-monophosphate [PtdIns (4)P], PtdIns (4)P 5-kinase Iγ, was shown recently to be concentrated at the synapse (24). Thus, the PtdIns(4,5)P2 level at the synapse is likely to result from a balance of the enzymatic activities of synaptojanin and PtdIns (4)P 5-kinase.
In vitro reconstitution of specific steps of vesicular transport reactions represents an increasingly important strategy to elucidate molecular mechanisms involved in vesicular traffic. Liposomes can support the formation of clathrin-, COPI-, or COPII-coated buds as well as dynamin-coated tubules (25–29). Previously, we reconstituted clathrin- and dynamin-coated structures by incubating liposomes with brain cytosol and nucleotides, thus demonstrating that brain cytosol has a potent activity in the generation of these coated intermediates (29). In the presence of guanosine 5′-O-(thiotriphosphate) (GTPγS), fragmentation of liposomes by the GTPase dynamin is prevented, and the coated intermediates, which are very transient in vivo, are stabilized. This experimental system has been used for the functional characterization of dynamin, amphiphysin, and endophilin (29–31). The use of liposomes also allows the determination of crucial lipid requirements for the formation of the coated structures (26, 27, 29).
We now have developed an assay to monitor vesicle formation from liposomes incubated in the presence of ATP and GTP. The formation of vesicles was analyzed both quantitatively and qualitatively by dynamic light scattering (DLS), a method that allows the determination of vesicle size as well as relative numbers and weight distribution at each vesicle size range. By using this cell-free system, we demonstrate that vesicle formation is enhanced by the presence of PtdIns(4,5)P2 and dynamin plays a major role in this reaction.
Experimental Procedures
Materials.
Bovine brain lipid extract (Folch fraction type I), PtdIns(4,5)P2, PtdIns (4)P, and gangliosides (type III) from bovine brain were purchased from Sigma. l- α -phosphatidylserine and l-α-phosphatidylethanolamine were from Avanti Polar Lipids. Creatine phosphokinase (type I from rabbit muscle), hexokinase (type III), concanavalin A-Sepharose 4B beads and PMSF were from Sigma. 1,6-Diphenyl-1,3,5-hexatriene (DPH) was from Molecular Probes. [inositol-2-3H]Phosphatidylinositol 4,5-bisphosphate {[3H]PtdIns(4,5)P2, specific activity of 510.6 GBq/mmol} was purchased from NEN Life Science Products. Rabbit polyclonal antibodies against amino acids residues 633–647 of rat dynamin 1 were purchased from Affinity Bioreagents (Golden, CO). Protein A Sepharose 4 Fast Flow was from Amersham Pharmacia.
Preparation of Brain Cytosol.
Brain cytosol was prepared by minor modification of the method of Malhotra et al. (29, 32). Two bovine brains were obtained freshly at the slaughterhouse and kept in 25 mM Tris⋅HCl (pH 7.4) containing 320 mM sucrose. Forebrain and cerebellum (660 g) were homogenized in 1,650 ml of 25 mM Tris⋅HCl (pH 8) containing 2 mM EGTA, 500 mM KCl, 10 mM MgCl2, 250 mM sucrose, 1 mM DTT in the presence of PMSF and a protease inhibitor mixture, a mixture of 0.9 μg/ml each of benzamidine, leupeptin, antipain, and pepstatin A, and 0.45 μg/ml of aprotinin. The homogenate was centrifuged at 5,000 × g for 1 h, 10,000 × g for 1 h, and finally at 171,000 × g for 2 h to obtain the supernatant. The resulting supernatant was dialyzed against a dialysis buffer [25 mM Tris⋅HCl (pH 8) containing 50 mM KCl and 1 mM DTT]. After centrifugation at 171,000 × g for 2 h, cytosol proteins were salted out from the supernatant by adding ammonium sulfate at the 60% saturation. The precipitates were collected by centrifugation at 10,000 × g for 30 min and resuspended in the dialysis buffer (50 ml). The suspension was dialyzed against the dialysis buffer twice (20 liters in each) and dialyzed further against the buffer without DTT. After centrifugation at 100,000 × g for 2 h, a solution of proteins (86 ml) termed the brain cytosol was obtained and stored at −80°C. The brain cytosol (21.6 mg/ml protein) was thawed at 37°C before use.
Depletion of Dynamin 1 from Brain Cytosol.
Brain cytosol was incubated with dynamin antibodies for 30 min at 37°C followed by additional incubation with protein A Sepharose for 30 min at room temperature. After centrifugation at 1,000 × g for 10 min, the supernatant was used as the “dynamin-depleted cytosol” at a concentration described below. The efficiency of the immunodepletion was assessed by Western blotting followed by quantitative densitometry of the dynamin immunoreactive band.
Formation of Small Vesicles from Liposomes.
Large unilamellar liposomes (1 mg/ml) were prepared as described (33). Vesicle formation was performed by incubation of 200 μg of liposomes in 1 ml of cytosolic buffer [25 mM Hepes-KOH (pH 7.2)/25 mM KCl/2.5 mM magnesium acetate/100 mM potassium glutamate] with brain cytosol (6 mg/ml protein) and various nucleotides as indicated. The final concentrations of nucleotides were: 2.3 mM ATP, 200 μM GTP, and 200 μM GTPγS. Samples containing ATP were supplemented also with an ATP-regenerating system consisting of 18.1 mM creatine phosphate and 18.1 units/ml creatine phosphokinase. In samples with brain cytosol and GTP but not with ATP, an ATP-depleting system consisting of 5 units/ml of hexokinase and 10 mM glucose was supplemented. These mixtures were incubated for 15 min at 37°C.
DLS Assay.
The sizes, relative numbers, and weight distribution in each size of vesicles formed were measured by DLS with a DLS-7000 AR-III spectrophotometer (Otsuka Electronics, Osaka, Japan). To prepare the sample for the DLS assay, 400 μl of the incubation mixture was diluted with 1 ml of 300 mM sucrose. As for liposomes, 200 μl of the liposome suspension was diluted with 1.2 ml of 300 mM sucrose. The measurement range for DLS was set from 20 to 10,000 nm to avoid protein particles. As negative controls, a mixture of cytosol, ATP, and GTP without liposomes were measured, and no peak was detected at this setting.
Electron Microscopy (EM).
Incubation mixtures were applied to a dry minicolumn of Sephadex G50 and centrifuged at 800 × g for 3 min to remove cytosolic proteins (34). Liposomes and small vesicles obtained in the void volume were negatively stained with 2% uranyl acetate for EM observation.
Detection of Lipid Membranes by DPH.
Lipid membranes were labeled with DPH, a lipophilic fluorescent dye. An excess of large unilamellar liposomes composed of a brain lipid extract (1.56 mg), cholesterol (400 μg), and gangliosides (40 μg) was immobilized on concanavalin A-Sepharose 4B beads (250 μl) by incubation at room temperature for 30 min followed by washes (3 times) with cytosolic buffer to remove unbound liposomes. Bead-bound liposomes were incubated with brain cytosol in the presence or absence of the nucleotides. After the reaction, newly formed vesicles were separated by centrifugation and tagged with 5 μl of 0.2% DPH in tetrahydrofran. DPH fluorescence (excitation at 360 nm and emission at 430 nm) of samples was observed with an Image Freezer AE-6905 equipped with in an Atto charge-coupled device video camera.
Lipid Extraction.
Lipids were extracted from 1 ml of the incubation mixture essentially in accordance with Bligh and Dyer's method (35). After the reaction, 2 ml of 2% KCl, 7.5 ml of methanol, and 3.75 ml of chloroform were added to the incubation mixture in this order. The suspension then was mixed vigorously, incubated further at room temperature for 10 min; 3.75 ml of chloroform were added and mixed for 30 sec followed by further addition of 3.75 ml of 2% KCl and mixing for 30 sec. This material was centrifuged at 1,200 × g for 5 min, and a lipid extract was obtained in the chloroform layer. To complete the extraction, the aqueous layer was treated further with 3 ml of chloroform, and then the chloroform layer was combined to the extract and evaporated to dryness with nitrogen. The residue of the evaporation was dissolved in 50 μl of chloroform and used as a sample for chromatography.
High-Performance TLC (HPTLC).
HPTLC was carried out on a silica gel 60 F-254 plate (Merck, Darmstadt, Germany) that was impregnated with 1% (wt/vol) potassium oxalate in methanol water [2:3 (vol/vol)] for 30 min (36). The plate was allowed to dry at room temperature and activated by heating at 110°C for 15 min. The developing solvent system used was chloroform/methanol/4 M NH3 [45:35:10 (vol/vol)]. Phospholipids were detected by spraying Ryu-MacCoss reagent (37). The 3H-labeled lipids were detected by autoradiography using a Fuji film BAS-TR2040 imaging plate and a Bio-imaging analyzer, BAS-2000 II.
HPLC.
HPLC was carried out by a Tosoh HPLC system (Tokyo). The system consisted of a Tosoh (Tokyo) CCPM pump, a UV-8010 detector, a Chromatocorder 12 integrator, and a TSKgel Silica-60 column (4.6 × 250-mm i.d.) with a guard column of a TSKgel Silica-60 (4.6 × 35-mm i.d.). Elution was performed with a solvent system of hexane/2-propanol/85% phosphate [850:145:5 (vol/vol)] at a flow rate of 1 ml/min, and the change of absorbance was recorded at 203 nm.
Results
Formation of Small Vesicles from Large Unilamellar Liposomes.
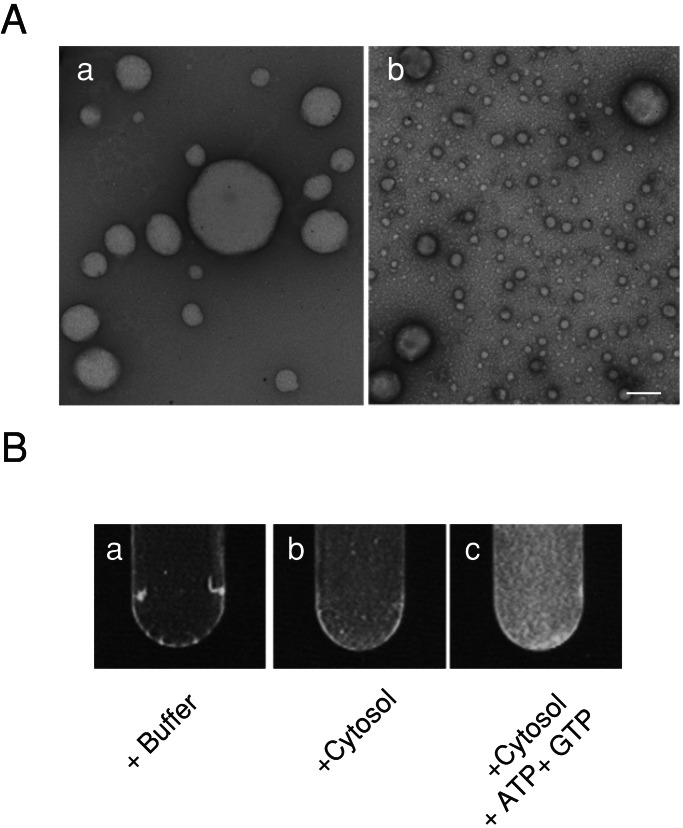
It was shown previously that liposomes incubated with brain cytosol, ATP, and GTPγS generated numerous coated endocytic intermediates including dynamin-coated tubules and clathrin-coated buds (29). We have now investigated whether replacement of GTPγS with GTP allowed the reaction to proceed to vesicle formation. To test this possibility, large unilamellar liposomes composed of 80% (wt/wt) lipid extract and 20% cholesterol were incubated with brain cytosol, ATP, and GTP. As observed by EM, most of the liposomes prepared were large in size (Fig. 1A-a), some exceeding 1 μm in diameter. After the incubation, massive formation of small vesicles was observed (Fig. 1A-b), and semiquantitative morphometry revealed that more than 90% of the vesicles were smaller than 100 nm in diameter.
Figure 1.
Formation of small vesicles from liposomes. (A) EM of negatively stained large unilamellar liposomes prepared from the lipid film containing 20% (wt/wt) cholesterol and 80% (wt/wt) brain lipid extract (a) and vesicles after incubation of the liposomes with brain cytosol for 15 min at 37°C in the presence of ATP and GTP (b). (Bar, 200 nm.) (B) Detection of the lipid membrane in the formed small vesicles. Liposomes immobilized on Con A-Sepharose 4B beads were incubated under the conditions indicated. After the reaction, beads were removed by spin, and then DPH was added to the supernatant. Note that intense fluorescence was observed when liposomes were incubated with brain cytosol, ATP, and GTP (c).
To confirm that small vesicles observed by EM represented lipid vesicles, starting liposomes were immobilized on concanavalin A-Sepharose 4B beads and sedimented at low speed after the incubation. The small vesicles recovered in the supernatant were labeled with DPH to allow detection of membrane lipids by fluorescence. As shown in Fig. 1B, fluorescence was evident when liposomes were reacted with brain cytosol, ATP, and GTP, whereas no fluorescence was detected when liposomes were incubated only with buffer. Fluorescence was very faint when liposomes were incubated with brain cytosol only, indicating that little formation of vesicles occurred without ATP and GTP.
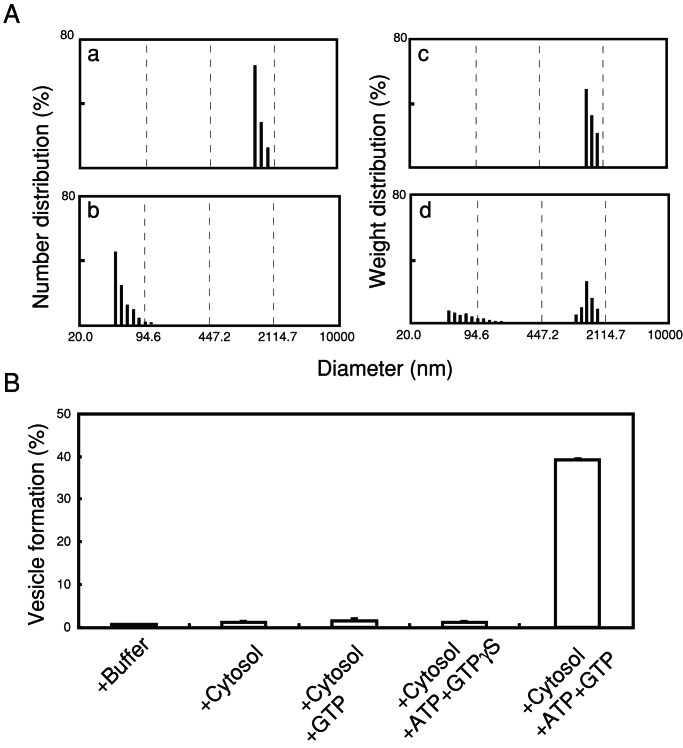
To analyze vesicle formation quantitatively and qualitatively, we measured DLS of the incubation mixture. This method allowed us to determine the size of liposomes and small vesicles as well as the relative number and weight distribution at each size range. Consistent with EM observations, DLS measurement (Fig. 2A-a) yielded a diameter of 1,545 ± 175 nm for the starting liposomes and the weight distribution shown in Fig. 2A-c. After incubation with brain cytosol in the presence of ATP and GTP, DLS analysis revealed small vesicles with an average diameter of 57 ± 14 nm (Fig. 2A-b), which represented 40% of the starting liposomes in weight (Fig. 2A-d). DLS measurement of the incubation mixture also detected residual larger liposomes, 1,082 ± 203 nm, which were negligible in number (Fig. 2A-b) but represented 60% in weight (Fig. 2A-d). The presence of these two membrane populations after the incubation strongly indicates that the formation of small vesicles is reconstituted in our cell-free system, and that vesicle formation can be quantified by DLS analysis of the weight distribution of the smaller vesicles against total particles.
Figure 2.
DLS assay of the vesicle formation. (A) Distribution of the liposomes in numbers and in weight before incubation (a and c, respectively) and of vesicles in the reaction mixture after incubating the liposomes with brain cytosol, ATP, and GTP for 15 min at 37°C (b and d, respectively). (B) Vesicle formation evaluated from weight percent of the small vesicles (less than 200 nm) that were formed by incubating the liposomes with brain cytosol under various nucleotide conditions as indicated. The composition of liposomes used was as described for Fig. 1. The data indicated are the mean ± SD (n = 4).
To identify optimal conditions for vesicle formation, a fixed amount of liposomes was reacted in the presence of ATP and GTP with different concentrations of cytosolic proteins. As quantified by DLS, optimal vesicle formation was obtained with 6 mg/ml cytosol with a protein/liposome ratio of 30:1 (wt/wt; data not shown). We next examined the nucleotide requirement of the reaction. As shown in Fig. 2B, little vesicle formation was detected when liposomes were incubated with cytosol only. Depletion of ATP or substitution of GTP with GTPγS in the reaction mixtures also reduced the vesicle formation. No vesicle formation was observed after incubation of liposomes with buffer alone (Fig. 2B) or with ATP without guanine nucleotides (data not shown). These results are consistent with EM observations and DPH analyses and demonstrate that both ATP and GTP are required for the vesicle formation. Thus, we have established the occurrence of vesicle formation from liposomes incubated with brain cytosol and nucleotides, and we have developed a DLS-based method to monitor vesicle formation qualitatively and quantitatively.
Implication of Dynamin 1 in Vesicle Formation.
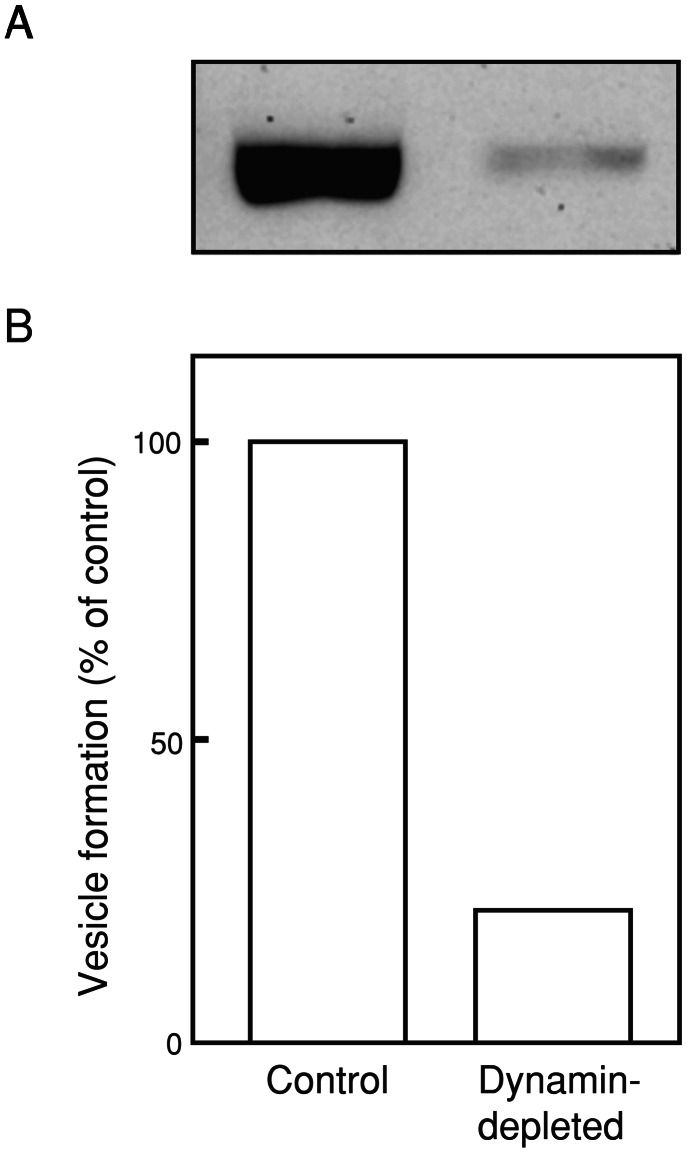
Dynamin 1, an abundant brain GTPase with a putative role in vesicle fission is likely to be implicated in the vesicle formation reaction. To assess the contribution of dynamin to the process, the effect of normal brain cytosol was compared in the DLS assay to that of dynamin 1-immunodepleted brain cytosol. As revealed by Western blotting (Fig. 3A), ≈90% of dynamin 1 was immunodepleted. Incubation of liposomes composed of 80% (wt/wt) lipid extract and 20% cholesterol with the dynamin-depleted cytosol in the presence of ATP and GTP for 15 min at 37°C resulted in a reduction of vesicle formation reaction to 22% of control (Fig. 3B). These results indicate a major contribution of dynamin 1 to vesicle formation under these experimental conditions.
Figure 3.
Contribution of dynamin 1 to vesicle formation. (A) Western blot analysis of dynamin 1 immunoreactivity of control (Left) and dynamin-depleted (Right) brain cytosol. (B) Reduction of vesicle formation with dynamin-depleted cytosol. Liposomes were incubated with control or dynamin-depleted cytosol in the presence of ATP and GTP for 15 min at 37°C. Vesicle formation was detected by the DLS assay. The composition of liposomes was the same as that described for Fig. 1. Results were normalized to control values.
Effects of Phosphoinositides on Vesicle Formation.
Growing evidence implicates phosphoinositides and their metabolism in vesicular traffic including the fission reaction mediated by dynamin 1. To determine the effect of phosphoinositides on vesicle formation, DLS analysis was performed on liposomes containing various phosphoinositide species at different concentrations after they were incubated with brain cytosol in the presence of ATP and GTP. Liposomes containing 20% cholesterol, 40% l-α-phosphatidylserine, 34–40% of l-α-phosphatidylethanolamine, and 0–6% of either PtdIns, PtdIns (4)P, or PtdIns(4,5)P2 were used.
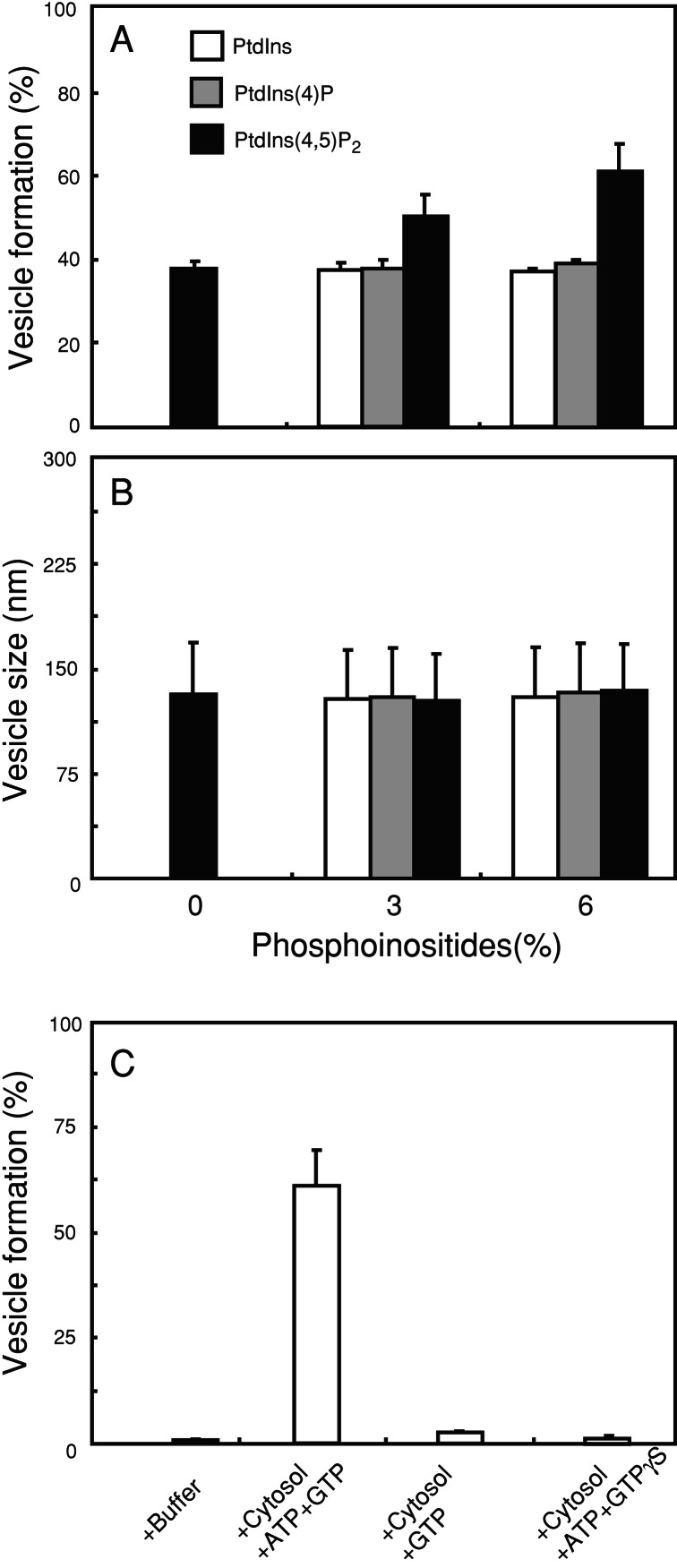
Increasing the amount of PtdIns(4,5)P2 from 0 to 6% stimulated the vesicle formation from 38 to 62% (Fig. 4A, black bars), whereas either PtdIns or PtdIns (4)P had little effect (Fig. 4A). In all cases vesicles formed had the same size (Fig. 4B). Vesicle formation from these liposomes also required both ATP and GTP (Fig. 4C), which was consistent with the results obtained by the reaction with the crude brain lipid extract (see Fig. 2B).
Figure 4.
Effects of phosphoinositides on the vesicle formation from liposomes. (A) Quantities of small vesicle formation from liposomes containing 20% cholesterol, 40% l-α-phosphatidylserine, 34–40% l-α-phosphatidylethanolamine, and PtdIns, PtdIns (4)P, or PtdIns(4,5)P2 at concentrations indicated after incubation with brain cytosol, ATP, and GTP for 15 min at 37°C. (B) The average diameter of the formed vesicles. (C) Vesicle formation from liposomes containing 20% cholesterol, 40% l-α-phosphatidylserine, 34% l-α-phosphatidylethanolamine, and 6% PtdIns(4,5)P2 by incubation with brain cytosol under various nucleotide conditions as indicated. Values represent the mean ± SD (n = 4). Similar results were obtained by incubation of liposomes containing either 6% PtdIns or PtdIns (4)P instead of PtdIns(4,5)P2 with brain cytosol under the same nucleotide conditions (data not shown).
Rapid Degradation of PtdIns(4,5)P2 During Incubation with the Cytosol.
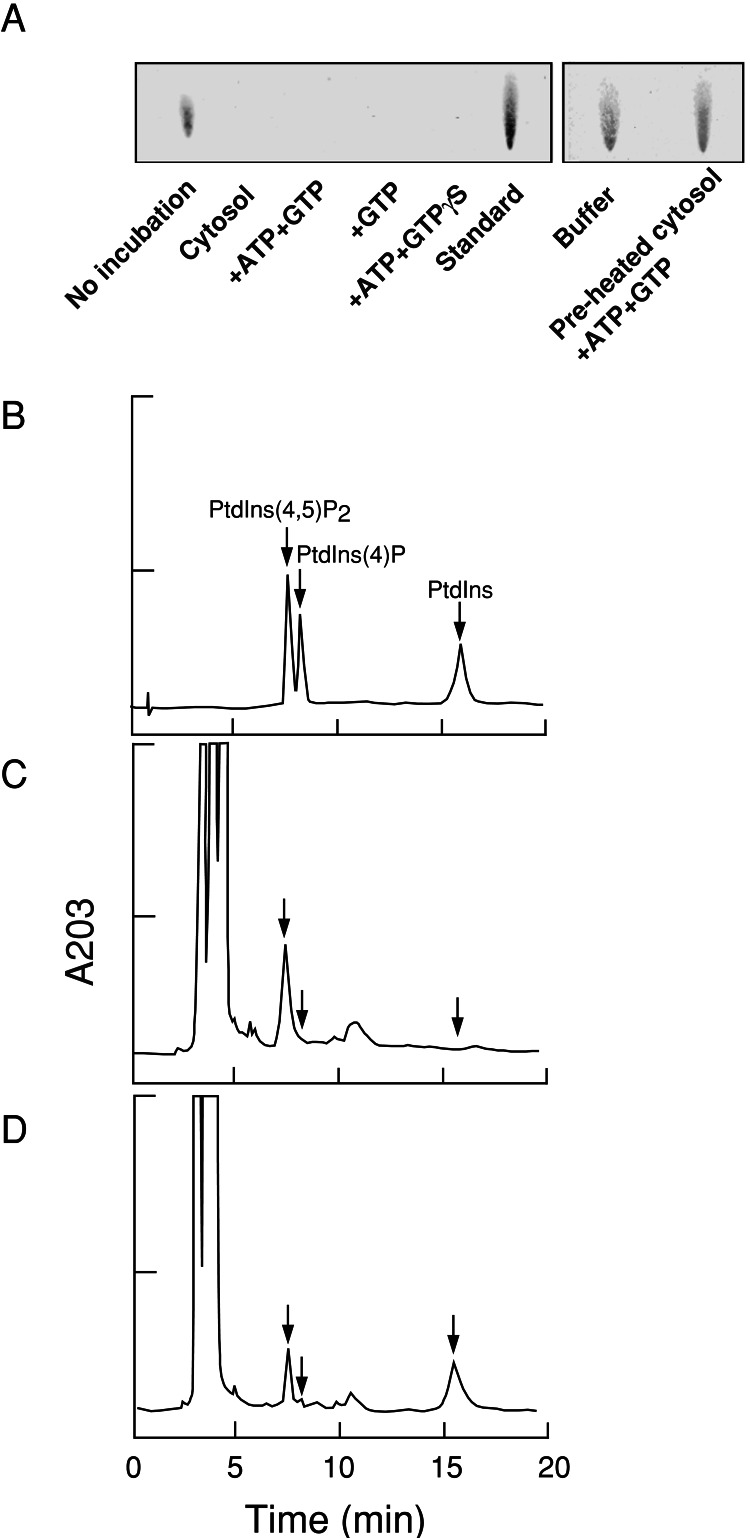
To monitor PtdIns(4,5)P2 metabolism during vesicle formation, membrane lipids of the reactant were extracted and then analyzed by HPTLC and HPLC. By HPTLC analysis, the levels of PtdIns(4,5)P2 were below the limit of detectability when liposomes were incubated with brain cytosol, ATP, and GTP (Fig. 5A). PtdIns(4,5)P2 was undetected also when liposomes were incubated in the absence of ATP or presence of GTPγS (Fig. 5A), conditions under which few vesicles were formed (see Fig. 4C). No degradation of PtdIns(4,5)P2 occurred when the liposomes were incubated with buffer alone or with brain cytosol preheated at 100°C for 5 min to denature cytosolic enzymes (Fig. 5A Right). By HPLC analysis (Fig. 5 B–D), the major product of PtdIns(4,5)P2 degradation was determined to be PtdIns. Other possible metabolites of PtdIns(4,5)P2 such as 1,2-diacylglycerol and phosphatidic acid were not detected by HPTLC or HPLC (data not shown). By HPLC, it was revealed that ≈50% of PtdIns(4,5)P2 remained after incubating with cytosol, ATP, and GTP (Fig. 5D).
Figure 5.
HPTLC and HPLC showing the degradation of PtdIns(4,5)P2. Liposomes containing PtdIns(4,5)P2 [6% (wt/wt)] were as described for Fig. 4C. (A) HPTLC detected with Ryu–MacCoss reagent. Liposomes were incubated for 15 min at 37°C with brain cytosol and various nucleotide conditions (Left) or with buffer alone or buffer supplemented with brain cytosol preheated at 100°C for 5 min in the presence of ATP and GTP (Right). Lipids were extracted from the reaction mixtures and analyzed. Lipid extracts from the liposomes alone or cytosol alone without liposomes are shown as controls (Left, first two lanes). Note that PtdIns(4,5)P2 degradation occurred under conditions in which few vesicles were formed (see Fig. 4C). (B–D) HPLC recording of the absorbance at 203 nm (A203) of standard phosphoinositides (B), lipid extract from liposomes (C), and lipid extract from the reaction mixture after incubation with brain cytosol, ATP, and GTP for 15 min at 37°C (D).
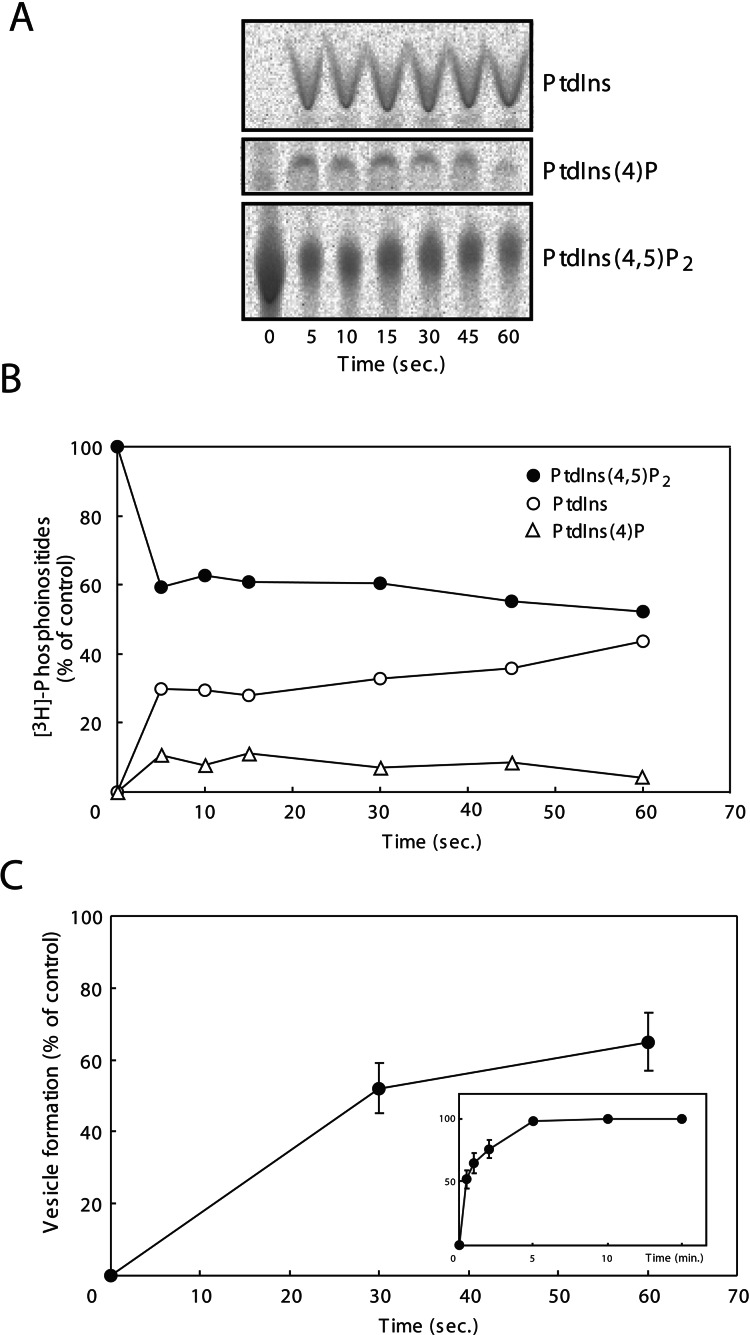
To analyze the time course of PtdIns(4,5)P2 degradation during vesicle formation, liposomes containing [3H]PtdIns(4,5)P2 (1,850 Bq) were reacted with brain cytosol, ATP, and GTP for various periods of time, and then the metabolites were analyzed by HPTLC followed by autoradiography. Degradation of PtdIns(4,5)P2 reached a maximum by as early as 5 sec. After this time there was little change of PtdIns(4,5)P2 up to 2 min (Fig. 6 A and B and data not shown). These results indicate that PtdIns(4,5)P2 was degraded rapidly to PtdIns, possibly through PtdIns (4)P as an intermediate during the vesicle formation. When liposomes were reacted under the same conditions and for the same times, the rate of vesicle formation lagged much behind the degradation kinetics of PtdIns(4,5)P2. It was so slow that vesicle formation was only 52% complete at 30 sec, and nearly completed only at 5 min (Fig. 6C Inset).
Figure 6.
Comparison in time courses of PtdIns(4,5)P2 degradation and of vesicle formation. Liposomes containing [3H]PtdIns(4,5)P2 were incubated with brain cytosol in the presence of ATP and GTP for the time periods indicated. Lipid extracts from the reaction mixtures were applied to HPTLC and analyzed by BAS-2000 autoradiography (A). (B) Radioactivity of the spot for [3H]PtdIns(4,5)P2 and its metabolites, [3H]PtdIns (4)P and [3H]PtdIns, was normalized against the radioactivity obtained before the incubation and blotted in percent. (C) Liposomes were incubated with brain cytosol in the presence of ATP and GTP for the time periods indicated, and the vesicle formations were quantified by DLS assay. The data obtained were normalized against the vesicle formation at 15 min as 100% (Inset). Values represent the mean ± SD (n = 4).
Discussion
In the present study, we established a cell-free system in which the formation of small vesicles was reconstituted by incubating liposomes with brain cytosol in the presence of ATP and GTP. Liposomes have been shown to support the formation of various coated structures implicated in vesicular traffic. The formation of COPI- or COPII-coated vesicles can be achieved by incubating liposomes with coat components (26, 27). Incubation of liposomes with brain cytosol in the presence of GTPγS generates clathrin-coated pits and dynamin-coated tubules (29). Furthermore, purified dynamin forms a coat on liposomes and then deforms the liposomes into dynamin-coated tubules, which are vesiculated in the presence of GTP (25, 29). Considering the very high concentration of proteins implicated in synaptic vesicle endocytosis in brain and the abundance of coated intermediates of synaptic vesicle endocytosis on liposomes incubated with brain cytosol, ATP, and GTPγS (29), it is plausible that a majority of vesicle formation obtained with brain cytosol reflects clathrin- and dynamin-mediated synaptic endocytosis. Consistent with this hypothesis, dynamin 1 depletion from the brain cytosol greatly reduced vesicle formation in our experimental system (Fig. 3).
Vesicle formation in the present study required both ATP and GTP. The requirement of GTP is in agreement with the involvement of dynamin, whereas the need for ATP remains to be explained. Both the recruitment and assembly of the clathrin coat and dynamin can occur in the absence of nucleotides in vitro (29, 30). However, ATP hydrolysis was shown to be required for coat assembly, vesicle budding (19, 38), and decoating by the heat-shock cognate protein hsc70 ATPase (39, 40) in clathrin-mediated endocytosis. ATP may be needed, at least in part, for refolding reactions as well as for the cycle of phosphorylation-dephosphorylation of the endocytic proteins (41). Physiologically, ATP may be needed also to synthesize new PtdIns(4,5)P2 (21), but this requirement is bypassed in our assays by the presence of exogenous PtdIns(4,5)P2.
Increasing levels of PtdIns(4,5)P2 in liposomes significantly enhanced vesicle formation (Fig. 4A). This result is consistent with growing evidence for a role of phosphoinositides, especially PtdIns(4,5)P2, in membrane traffic mediated by coated vesicles. Specific interactions between PtdIns(4,5)P2 and coat proteins have been documented. PtdIns(4,5)P2 binds to the α-subunit of AP2 via a positively charged lysine triad at its N terminus (42) and also to the pleckstrin homology (PH) domain of dynamin (17, 18). The epsin N-terminal homology (ENTH) domain, a binding motif for PtdIns(4,5)P2, is conserved in several endocytic proteins including epsin, AP180/CALM, and Hip1R (13, 15). Disruptions of these interactions by mutations in the phosphoinositide binding modules or application of neomycin, a PtdIns(4,5)P2 binding reagent, inhibits recruitment of these proteins to the membrane (43) as well as endocytosis (15, 19, 44, 45). PtdIns(4,5)P2 also enhances the formation of COPII-coated vesicles on liposomes (27). Considering the importance of dynamin 1 in the reaction measured in our study (Fig. 3), the presence of PtdIns(4,5)P2 in liposomes may help to recruit this GTPase. In addition, it may help to recruit factors upstream (for example clathrin coats) or downstream (accessory factors for clathrin- and dynamin-mediated endocytosis) of dynamin.
Rapid degradation of PtdIns(4,5)P2 was observed during the vesicle-forming reaction (Fig. 6). Such degradation is likely to be catalyzed to a significant extent by synaptojanin 1, a major polyphosphoinositide phosphatase in brain. Synaptojanin 1 dephosphorylates PtdIns(4,5)P2 and PtdIns (4)P via its inositol 5-phosphatase domain and Sac1 domain, respectively (16, 17, 46, 47). It therefore is thought to represent a negative regulator of membrane-clathrin coat interactions. Accordingly, disruption of synaptojanin 1 in mice resulted in the accumulation of clathrin-coated vesicles at synapses (21), and mutations of the unc-26/synaptojanin gene of C. elegans lead to a depletion of vesicles at synapses as well as to the accumulation of endocytic pits and coated vesicles (22). Similar results were obtained in the giant reticulospinal axon of the lamprey after disruption of synaptojanin's function and/or recruitment to membranes by microinjection of antibodies and peptides (23). These findings suggest multiple roles for a synaptojanin-sensitive PtdIns(4,5)P2 pool at multiple steps of the endocytic reaction. This pool, in turn, may be synthesized by type I PtdInsP kinases, most likely type 1γ (24), which function primarily as PtdIns (4)P 5-kinases. These lipid kinases are stimulated by phosphatidic acid, which in turn is generated primarily by the action of phospholipase D (PLD; refs. 48 and 49). PLD is inhibited by endocytic proteins such as synaptojanin, AP180, and amphiphysin. Although AP180 and amphiphysin directly interact with PLD (50, 51), the inhibition of PLD by synaptojanin is attributed to its ability to dephosphorylate PtdIns(4,5)P2 (20, 46), which is a cofactor for PLD (51).
In the assay that we have developed, PtdIns(4,5)P2 was needed to stimulate vesicle formation, but its degradation preceded vesicle formation (Fig. 6). Therefore, it is likely that the requirement for PtdIns(4,5)P2 underlies its role in recruitment of endocytic proteins. Once these proteins are bound to liposomes, their assembly into scaffolds may enhance the affinity for the membrane, thus making the interaction with the membrane independent of PtdIns(4,5)P2. The requirement of PtdIns(4,5)P2 for the recruitment of coat proteins in the early step of vesicle formation is supported by several studies (12–15). Furthermore, as shown for COPII, recruitment of the coat components to membrane occurs instantly (less than 1 sec; ref. 52).
Formation of small vesicles in vitro generally has been assayed by a combination of EM and static light-scattering measurements (30, 52). In the present study, we have used a DLS assay to determine vesicle formation both quantitatively and qualitatively at the same time. The results obtained by the DLS assay agree well with those obtained from a morphometric analysis using EM. Thus, this DLS-based cell-free system promises to be a powerful assay to analyze molecular mechanisms in vesicle formation including the role of lipid metabolism.
Acknowledgments
This work was supported by a grant-in-aid from the Ministry of Education, Sciences, Sports, Culture and Technology of Japan, by Uehara Memorial Foundation, and by the Japan Brain Foundation.
Abbreviations
- PtdIns
l-α-phosphatidylinositol
- PtdIns(4,5)P2
PtdIns 4,5-bisphosphate
- AP
clathrin adaptor protein
- PtdIns (4)P
PtdIns 4-monophosphate
- GTPγS
guanosine 5′-O-(thiotriphosphate)
- DLS
dynamic light scattering
- DPH
1,6-diphenyl-1,3,5-hexatriene
- EM
electron microscopy
- HPTLC
high-performance TLC
- PLD
phospholipase D
References
- 1.Slepnev V I, De Camilli P. Nat Rev Neurosci. 2000;1:161–172. doi: 10.1038/35044540. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhausen T. Nat Rev Mol Cell Biol. 2000;1:187–198. doi: 10.1038/35043117. [DOI] [PubMed] [Google Scholar]
- 3.Barlowe C. Traffic. 2000;1:371–377. doi: 10.1034/j.1600-0854.2000.010501.x. [DOI] [PubMed] [Google Scholar]
- 4.Antonny B, Schekman R. Curr Opin Cell Biol. 2001;13:438–443. doi: 10.1016/s0955-0674(00)00234-9. [DOI] [PubMed] [Google Scholar]
- 5.Hirst J, Robinson M S. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 6.Schmid S L. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 7.Takei K, Haucke V. Trends Cell Biol. 2001;11:385–391. doi: 10.1016/s0962-8924(01)02082-7. [DOI] [PubMed] [Google Scholar]
- 8.Corvera S, D'Arrigo A, Stenmark H. Curr Opin Cell Biol. 1999;11:460–465. doi: 10.1016/S0955-0674(99)80066-0. [DOI] [PubMed] [Google Scholar]
- 9.Huijbregts R P, Topalof L, Bankaitis V A. Traffic. 2000;1:195–202. doi: 10.1034/j.1600-0854.2000.010301.x. [DOI] [PubMed] [Google Scholar]
- 10.Cremona O, De Camilli P. J Cell Sci. 2001;114:1041–1052. doi: 10.1242/jcs.114.6.1041. [DOI] [PubMed] [Google Scholar]
- 11.De Camilli P, Emr S D, McPherson P S, Novick P. Science. 1996;271:1533–1539. doi: 10.1126/science.271.5255.1533. [DOI] [PubMed] [Google Scholar]
- 12.Beck K A, Keen J H. J Biol Chem. 1991;266:4442–4447. [PubMed] [Google Scholar]
- 13.Ford M G J, Pearse B M F, Higgins M K, Vallis Y, Owen D J, Gibson A, Hopkins C R, Evans P R, McMahon H T. Science. 2001;291:1051–1055. doi: 10.1126/science.291.5506.1051. [DOI] [PubMed] [Google Scholar]
- 14.Salim K, Bottomley M J, Querfurth E, Zvelebil M J, Gout I, Scaife R, Margolis R L, Gigg R, Smith C I, Driscoll P C, Waterfield M D, Panayotou G. EMBO J. 1996;15:6241–6250. [PMC free article] [PubMed] [Google Scholar]
- 15.Itoh T, Koshiba S, Kigawa T, Kikuchi A, Yokoyama S, Takenawa T. Science. 2001;291:1047–1051. doi: 10.1126/science.291.5506.1047. [DOI] [PubMed] [Google Scholar]
- 16.Gaidarov I, Chen Q, Falck J R, Reddy K K, Keen J H. J Biol Chem. 1996;271:20922–20929. doi: 10.1074/jbc.271.34.20922. [DOI] [PubMed] [Google Scholar]
- 17.Lin H C, Gilman A G. J Biol Chem. 1996;271:27979–27982. doi: 10.1074/jbc.271.45.27979. [DOI] [PubMed] [Google Scholar]
- 18.Lin H C, Barylko B, Achiriloaie M, Albanesi J P. J Biol Chem. 1997;272:25999–26004. doi: 10.1074/jbc.272.41.25999. [DOI] [PubMed] [Google Scholar]
- 19.Jost M, Simpson F, Kavran J M, Lemmon M A, Schmid S L. Curr Biol. 1998;8:1399–1402. doi: 10.1016/s0960-9822(98)00022-0. [DOI] [PubMed] [Google Scholar]
- 20.McPherson P S, Garcia E P, Slepnev V I, David C, Zhang X, Grabs D, Sossin W S, Bauerfeind R, Nemoto Y, De Camilli P. Nature (London) 1996;379:353–357. doi: 10.1038/379353a0. [DOI] [PubMed] [Google Scholar]
- 21.Cremona O, Di Paolo G, Wenk M R, Luthi A, Kim W T, Takei K, Daniell L, Nemoto Y, Shears S B, Flavell R A, McCormick D A, De Camilli P. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 22.Harris T W, Hartwieg E, Horvitz H R, Jorgensen E M. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gad H, Ringstad N, Low P, Kjaerulff O, Gustafsson J, Wenk M, Di Paolo G, Nemoto Y, Crun J, Ellisman M H, De Camilli P, Shupliakov O, Brodin L. Neuron. 2000;27:301–312. doi: 10.1016/s0896-6273(00)00038-6. [DOI] [PubMed] [Google Scholar]
- 24.Wenk M R, Pellegrini L, Klenchin V A, Di Paolo G, Chang S, Daniell L, Arioka M, Martin T, De Camilli P. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 25.Sweitzer S M, Hinshaw J E. Cell. 1998;93:1021–1029. doi: 10.1016/s0092-8674(00)81207-6. [DOI] [PubMed] [Google Scholar]
- 26.Spang A, Matsuoka K, Hamamoto S, Schekman R, Orci L. Proc Natl Acad Sci USA. 1998;95:11199–11204. doi: 10.1073/pnas.95.19.11199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuoka K, Orci L, Amherdt M, Bednarek S Y, Hamamoto S, Schekman R, Yeung T. Cell. 1998;93:263–275. doi: 10.1016/s0092-8674(00)81577-9. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Drake M T, Kornfeld S. Methods Enzymol. 2001;329:379–387. doi: 10.1016/s0076-6879(01)29099-5. [DOI] [PubMed] [Google Scholar]
- 29.Takei K, Haucke V, Slepnev V, Farsad K, Salazar M, Chen H, De Camilli P. Cell. 1998;94:131–141. doi: 10.1016/s0092-8674(00)81228-3. [DOI] [PubMed] [Google Scholar]
- 30.Takei K, Slepnev V I, Haucke V, De Camilli P. Nat Cell Biol. 1999;1:33–39. doi: 10.1038/9004. [DOI] [PubMed] [Google Scholar]
- 31.Farsad K, Ringstad N, Takei K, Floyd S R, Rose K, De Camilli P. J Cell Biol. 2001;155:193–200. doi: 10.1083/jcb.200107075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhotra V, Serafini T, Orci L, Shepherd J C, Rothman J E. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- 33.Takei K, Slepnev V I, De Camilli P. Methods Enzymol. 2001;329:478–486. doi: 10.1016/s0076-6879(01)29109-5. [DOI] [PubMed] [Google Scholar]
- 34.Fry D W, White C, Goldman D J. Anal Biochem. 1978;90:809–815. doi: 10.1016/0003-2697(78)90172-0. [DOI] [PubMed] [Google Scholar]
- 35.Bligh E G, Dyer W J. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 36.Horwitz J, Perlman R L. Methods Enzymol. 1987;141:169–175. doi: 10.1016/0076-6879(87)41065-3. [DOI] [PubMed] [Google Scholar]
- 37.Ryu E K, MacCoss M. J Lipid Res. 1979;20:561–563. [PubMed] [Google Scholar]
- 38.Schmid S L, Smythe E. J Cell Biol. 1991;114:869–880. doi: 10.1083/jcb.114.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schlossman D M, Schmid S L, Braell W A, Rothman J E. J Cell Biol. 1984;99:723–733. doi: 10.1083/jcb.99.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ungewickell E, Ungewickell H, Holstein S E, Lindner R, Preasad K, Barouch W, Martin B, Greene L E, Elsenberg E. Nature (London) 1995;378:632–635. doi: 10.1038/378632a0. [DOI] [PubMed] [Google Scholar]
- 41.Powell K A, Valova V A, Malladi C S, Jensen O N, Larsen M R, Robinson P. J Biol Chem. 2000;275:11610–11617. doi: 10.1074/jbc.275.16.11610. [DOI] [PubMed] [Google Scholar]
- 42.Gaidarov I, Keen J H. J Cell Biol. 1999;146:755–764. doi: 10.1083/jcb.146.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.West M A, Bright N A, Robinson M S. J Cell Biol. 1997;138:1239–1254. doi: 10.1083/jcb.138.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee A, Frank D W, Marks M S, Lemmon M A. Curr Biol. 1999;9:261–264. doi: 10.1016/s0960-9822(99)80115-8. [DOI] [PubMed] [Google Scholar]
- 45.Achiriloaie M, Barylko B, Albanesi J P. Mol Cell Biol. 1999;19:1410–1415. doi: 10.1128/mcb.19.2.1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung J-K, Sekiya F, Kang H-S, Lee C, Han J-S, Kim S R, Bae Y S, Morris A J, Rhee S G. J Biol Chem. 1997;272:15980–15985. doi: 10.1074/jbc.272.25.15980. [DOI] [PubMed] [Google Scholar]
- 47.Guo S, Stolz L E, Lemrow S M, York J D. J Biol Chem. 1999;274:12990–12995. doi: 10.1074/jbc.274.19.12990. [DOI] [PubMed] [Google Scholar]
- 48.Exton J H. Biochim Biophys Acta. 1999;1439:121–133. doi: 10.1016/s1388-1981(99)00089-x. [DOI] [PubMed] [Google Scholar]
- 49.Honda A, Nogami M, Yokozeki T, Yamazaki M, Nakamura H, Kamamoto K, Nakayama K, Morris A J, Frohman M, Kanaho Y. Cell. 1999;99:521–532. doi: 10.1016/s0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 50.Lee C, Kang H-S, Chung J K, Sekiya F, Han J-S, Kim S R, Bac Y S, Morris A J, Rhee S G. J Biol Chem. 1997;272:15986–15992. doi: 10.1074/jbc.272.25.15986. [DOI] [PubMed] [Google Scholar]
- 51.Lee C, Kim S R, Chung J K, Frohman M A, Kilimann M W, Rhee S G. J Biol Chem. 2000;275:18751–18758. doi: 10.1074/jbc.M001695200. [DOI] [PubMed] [Google Scholar]
- 52.Antonny B, Madden D, Hamamoto S, Orci L, Schekman R. Nat Cell Biol. 2001;3:531–537. doi: 10.1038/35078500. [DOI] [PubMed] [Google Scholar]