Abstract
Bulk chondritic meteorites and terrestrial planets show a monotonic depletion in moderately volatile and volatile elements relative to the Sun's photosphere and CI carbonaceous chondrites. Although volatile depletion was the most fundamental chemical process affecting the inner solar nebula, debate continues as to its cause. Carbonaceous chondrites are the most primitive rocks available to us, and fine-grained, volatile-rich matrix is the most primitive component in these rocks. Several volatile depletion models posit a pristine matrix, with uniform CI-like chemistry across the different chondrite groups. To understand the nature of volatile fractionation, we studied minor and trace element abundances in fine-grained matrices of a variety of carbonaceous chondrites. We find that matrix trace element abundances are characteristic for a given chondrite group; they are depleted relative to CI chondrites, but are enriched relative to bulk compositions of their parent meteorites, particularly in volatile siderophile and chalcophile elements. This enrichment produces a highly nonmonotonic trace element pattern that requires a complementary depletion in chondrule compositions to achieve a monotonic bulk. We infer that carbonaceous chondrite matrices are not pristine: they formed from a material reservoir that was already depleted in volatile and moderately volatile elements. Additional thermal processing occurred during chondrule formation, with exchange of volatile siderophile and chalcophile elements between chondrules and matrix. This chemical complementarity shows that these chondritic components formed in the same nebula region.
Keywords: carbonaceous chondrite, chondrule formation, volatile depletion
Carbonaceous chondrites were first recognized as primitive samples of the early solar nebula in the 1960s, when it became apparent that a group of these meteorites contained moderately volatile elements (condensing between ≈1,350 K and 650 K) and volatile elements (condensing at <650 K) in similar abundance to the solar photosphere (1). These elements are severely depleted in the terrestrial planets and in most other meteorite groups. Chondrites are constructed from chondrules, the igneous products of transient heating events, and Ca–Al-rich refractory inclusions (CAIs). These high-temperature components are embedded in a volatile-rich, fine-grained, mineralogically complex matrix, which is a host for presolar grains. In addition to the different compositional groups (e.g., CV, CO, CR, CM, and CI), carbonaceous chondrites are subdivided from types 1 to 6 based on the degree of secondary aqueous and thermal processing that they have experienced, type 3 being the most primitive, with aqueous alteration increasing to type 1, and types 3 to 6 showing increasing thermal alteration. CI1 chondrites, aqueously altered, and composed almost entirely of matrix, have compositions indistinguishable from solar. Other carbonaceous meteorites, containing less matrix (and more chondrules), are depleted in volatiles to varying degrees.
The varying volatile contents in chondritic meteorites prompted Anders (1) to explain volatile element depletion by using a two-component model related to chondrule formation, where a volatile-rich component (matrix, of CI composition) accreted with volatile-depleted chondrules (the assumption being that volatility-dependent evaporative loss occurred during chondrule formation). Wasson and Chou (2), observing a monotonic decrease in volatile abundance with decreasing condensation temperature, proposed the incomplete condensation model, in which a gas of solar composition dissipated during condensation. In this scenario, volatile depletion occurred before chondrule formation and is generally related to cooling of a hot inner disk. Attempts were also made to explain volatile depletion by evaporation during parent body heating (3). Subsequent experimental studies showed that volatile fractionation patterns cannot be reproduced by heating chondritic materials (4), while numerical modeling of nebula conditions further supported incomplete condensation (5). The incomplete condensation model is now broadly favored (6). The two-component model remains alive, however, with the recent suggestion that the primitive chondrites contain fine-grained materials (accretionary rims around chondrules and matrix) of approximately uniform CI-like composition, which escaped high-temperature processing (7). In addition, the X-wind model (8) suggests that CAIs and chondrules formed close to the proto-Sun (≈0.06 a.u.), before being carried out to fall onto a “cold” accretion disk composed of thermally unprocessed fine-grained nebula material. This is a variant of the two-component model: in this scenario, nonfragmental matrix (matrix without chondrule fragments) should be of similar CI-like composition in different unequilibrated meteorites; matrix should not be compositionally related to chondrules in a given meteorite; and bulk volatile depletion should be a function of the chondrule/matrix ratio. Several other models (9, 10) also advocate chondrule formation in the inner solar nebula and later mixing with matrix further out. Huss et al. (11) consider that the survival of presolar grains in carbonaceous chondrites, and the correlations between presolar grain abundance patterns and volatile-element depletions in bulk meteorites, precludes incomplete condensation from a solar gas as the mechanism for bulk volatile depletion. Instead, they propose that the basic chemical characteristics of the different chondrite groups were produced by variable heating and partial evaporation of presolar dust before chondrule formation. Volatile loss during chondrule formation and possibly local condensation were superimposed on this primary evaporative depletion. Finally, Yin proposed that the signature of volatile element depletion in chondritic meteorites predates any nebula process and is instead inherited from the interstellar medium.**
Trace elements have widely varying chemical affinities and condensation temperatures, so their abundance places primary constraints on models describing the formation and evolution of chondritic materials. Yet, while primitive meteorites are composed of materials that have experienced a huge range of thermal environments, models of volatile depletion are based on bulk trace element data, where all components are analyzed together in a bulk. Matrix is the most primitive component in carbonaceous chondrites, but trace element data are available only for four meteorites, with a limited element list: Allende (13, 14), Ornans (15), Mokoia (16), and Renazzo (17). Finally, the extent to which chondrules and matrix are genetically related is the subject of continuing debate. Some evidence of chemical complementarity has been produced for the CR2 chondrites (17), but for other meteorite groups the relationship between chondrules and matrix is less clear.
Materials and Methods
In this study, we used a combination of in situ laser ablation (LA) inductively coupled plasma (ICP)-MS and solution ICP-MS techniques to analyze the trace element composition of matrix in 22 carbonaceous chondrites, spanning the range of compositional types. We obtained data for 43 elements spanning a wide range of condensation temperatures. All but three of the samples analyzed are meteorite falls. Finds represent unusual compositional types: ALH88045 is a CM1; Acfer 094 is a unique, primitive C3; and NWA1152 is a possible CR3. Meteorite falls in our sample set represent ≈50% of available carbonaceous chondrite falls and were chosen to span the range of compositional types.
Samples for LA-ICP-MS were prepared as polished blocks. Additional matrix aliquots (2–5 mg) were hand-picked, using a needle and binocular microscope, from Allende, Vigarano, Murchison, Cold Bokkeveld, Mighei, and Al Rais, for solution analysis by ICP-MS after acid digestion [performed on a VG (Manchester, U.K.) PQ2 ICP-MS spectrometer]. LA-ICP-MS analyses were performed with a New Wave Research (Fremont, CA) UP213 laser (quintupled Nd:YAG delivering a 213-nm UV beam) coupled to a HP7500a ICP-MS spectrometer (Agilent Technologies, Palo Alto, CA). Laser spot size was 80 μm, and between 10 and 20 laser analyses were obtained for each meteorite. Ablations were performed in He atmosphere, and the ICP-MS spectrometer was operated in shield torch mode.
Analyses were normalized to an external glass standard, NIST612. Data were typically ratioed to Yb and CI chondrite, but, to constrain possible refractory enrichment in matrix (see Discussion), we also considered data ratioed to Mg.
Given the focus of this study, a major concern was that volatile fractionation could be an analytical artifact arising from the interaction of the laser with the sample (i.e., a thermal effect). We investigated this possibility by comparing analyses of Alais matrix performed with various spot sizes (60–120 μm) and energy output (0.01–0.1 mJ), thus drastically changing the energy density of the ablation area. In terms of a possible thermal effect, the worst-case scenario is a small spot size coupled with high energy output, yielding a high energy density. Finally, one of the ablation experiments was performed in raster mode, for which thermal effects are strongly reduced. The results of these experiments (Table 1, which is published as supporting information on the PNAS web site) demonstrate that the fractionations that we observe between elements of different volatility are not related to the ablation conditions and are similar for high and low energy density conditions. Finally, we observe a good agreement between solution and LA analysis, further evidence that our LA-ICP-MS data do not suffer from an analytical artifact. We also find good agreement with earlier instrumental neutron activation analysis data for Allende, Ornans, and Renazzo matrix (13, 15, 17).
Results
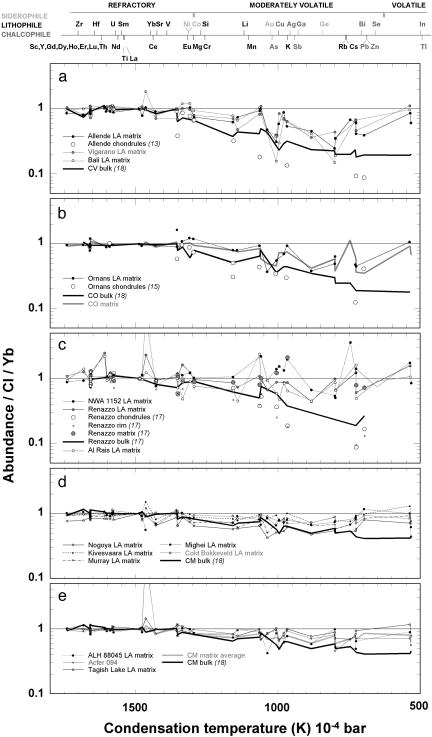
Elemental compositions of matrices in the CV3, CO3, CR2, CM2, and ungrouped carbonaceous chondrites Acfer 094, Tagish Lake, and NWA1152 are plotted in Fig. 1, with abundance vs. condensation temperature. Literature data for bulks and relevant chondrule, matrix, and rim data (13, 15, 17, 18) are also shown (our ICP-MS data, ratioed to CI1 chondrite and Yb, are provided in Tables 2–5, which are published as supporting information on the PNAS web site). Fig. 2 summarizes volatile data from selected meteorites (with elements in order of volatility) and shows average matrix compositions for the different groups and LA-ICP-MS data for the CI1 chondrites Orgueil and Alais.
Fig. 1.
Trace element data derived from LA and solution ICP-MS analyses of matrix in 18 carbonaceous chondrites, ratioed to CI and Yb, with relevant literature data for bulks, rims, chondrules, and matrix (13, 15, 17, 18), ordered in terms of condensation temperature (19). (a) CV3 chondrites. (b) CO3 chondrites. (c) CR2 chondrites and NWA1152. (d) CM2 chondrites. (e) Anomalous and ungrouped chondrites.
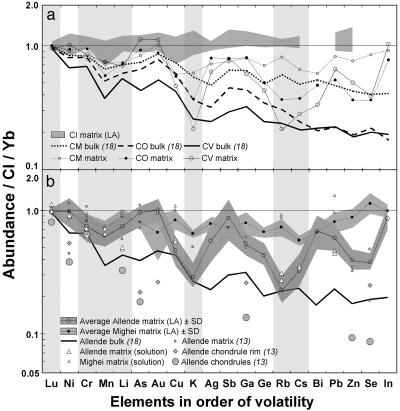
Fig. 2.
Data for moderately volatile elements, ordered in a sequence of increasing volatility (lithophiles are indicated by vertical bars; Ni, Au, and Ge are siderophile; all other elements are chalcophile). (a) Average matrix compositions for CV, CO, and CM chondrites. LA-ICP-MS data (and standard deviation in LA analyses) is also shown for CI1 chondrites (Alais and Orgueil). Different chondrite groups have distinct matrix lithophile abundances, which plot close to the bulk data for those groups. CV and CO matrices have very similar siderophile and chalcophile abundances. The elements most fractionated in C3 matrices are siderophiles and chalcophiles; they are significantly enriched over bulk and over matrix lithophiles. (b) Allende and Mighei have representative matrix compositions for CV3 and CM2 chondrites, respectively. LA-ICP-MS and analysis of separated matrix by solution ICP-MS show that their matrices are compositionally distinct. Allende is generally depleted compared with Mighei, and Mighei shows much less variation between siderophile/chalcophile and lithophile elements. Mighei matrix is depleted in all moderately volatile elements compared with CI. Finally, in Allende, chondrule siderophiles and chalcophiles (13) are substantially depleted compared with bulk, complementing enrichment in matrix in these elements.
The CV3 chondrites are subdivided into oxidized Allende type, oxidized Bali type, and reduced (with Vigarano as the type example). Matrix is anhydrous and dominated by fayalitic olivine. Despite having experienced very different parent body histories (20), Allende, Vigarano, and Bali have matrices with very similar trace element compositions (Fig. 1a). CO3 chondrites have also experienced varying thermal alteration [with minor aqueous (21)]. Ornans matrix is shown in Fig. 1b, together with an averaged CO3 composition that includes partial data for three additional meteorites (Kainsaz, Felix, and Lance). Both CV3 and CO3 matrices show a nonmonotonic decrease in volatile element abundance with decreasing condensation temperature and are enriched relative to bulk compositions of CV and CO chondrites. For both of these groups, this nonmonotonic enrichment requires a complementary nonmonotonic depletion in chondrule compositions. Calculated nonmatrix (chondrule) compositions [based on our ICP-MS analyses of matrix, the abundance of matrix (22–24), and bulk data (17, 18)] for CV, CO, and CR chondrites are close to measured values for Allende (13), Ornans (15), and Renazzo (17).
The CR2 chondrites have abundant large chondrules, with a hydrated matrix dominated by phyllosilicates. Renazzo and Al Rais have very similar matrix compositions (Fig. 1c), with highly nonmonotonic depletion patterns. NWA1152 is an anomalous C3 find with affinities to CRs (25). Matrix in NWA has a similar overall level of depletion to CR but departs even further from a monotonic depletion pattern (enrichment in aqueously mobile Sr in NWA matrix is likely a consequence of terrestrial weathering).
The CM chondrites have experienced varying degrees of aqueous alteration, but significantly more intense alteration than the C3s. Similar to the CR2s, matrix is dominated by phyllosilicate. CM2 chondrites show very little variation in matrix composition (Fig. 1d). Although matrix in CM is the least depleted of all of the major chondrite groups, average CM matrix still shows a consistent depletion compared with CI1.
ALH88045, a CM1, has similar matrix composition to CM2s, whereas Tagish Lake, a unique C2, has the most enriched matrix among the C2 chondrites that we analyzed (Fig. 1e). Acfer 094 (A094) is an ungrouped, type 3 carbonaceous chondrite with mineralogical, petrological, and oxygen isotopic affinities to the CM and CO groups, which, however, shows no evidence for aqueous alteration and thermal metamorphism (26, 27). Its matrix composition plots closer to CM than CO (and as in NWA, Sr is superabundant).
Discussion
Several first-order key observations can be made. First, matrix is enriched in volatile and moderately volatile elements compared with the bulk meteorite, but is not of CI composition in any chondrite group (apart from CI). Even the CMs (commonly assumed to have CI-like matrix), and Tagish Lake, show a consistent depletion compared with CI in moderately volatile element abundance (see also Fig. 2a). Second, in CV3, CO3, and CR2 chondrites, matrix does not show the monotonic decrease in volatile abundance with decreasing condensation temperature characteristic of the bulk composition; elements with similar condensation temperatures show large variations in abundance. Matrix volatile compositions are also specific to a given group. Finally, the presence of significant excursions away from a monotonic depletion pattern in matrix compositions, and an approximate monotonic depletion pattern in the bulk, requires that elemental enrichments in the matrix trace element pattern relative to the bulk composition are mirrored by concomitant depletions in chondrules.
In bulk meteorites, the sequence of volatile depletion would be CV > CO > CR > CM, with Tagish Lake the least depleted C2 chondrite. Despite the observation that matrix frequently does not show a smooth trace element pattern, for most volatile and moderately volatile trace elements we observe the same general sequence of depletion for matrix as we do in bulks (Fig. 2a). This depletion sequence is most clearly seen in moderately volatile lithophile elements (Fig. 2). In addition, matrix lithophiles show similar levels of depletion to the bulk meteorite in these groups; matrix is not significantly enriched over bulk in lithophile elements (Fig. 2). It is clear that the main excursions away from a monotonic depletion pattern in C3 chondrite matrix are in siderophile and chalcophile elements, which are significantly enriched over bulk (Fig. 2). Literature data for Allende chondrules (13) show that siderophiles and chalcophiles are highly depleted compared with bulk (Fig. 2b). Finally, minor deviations in C3 bulk composition away from a monotonic depletion pattern appear to correspond to major deviations in the matrix pattern, with enrichment in bulk matching enrichment in matrix (Fig. 2a).
The fact that chondrite matrix has a composition different from a primordial solar (CI-like) value indicates that matrix material has been processed. Second, as the matrix composition changes across the carbonaceous chondrite groups it is clear that the extent of this processing varied spatially or temporally. Third, the composition of matrix contributes to the overall volatile depletion observed in the bulk, but it typically shows a much more fractionated, nonmonotonic depletion pattern than the bulk. This nonmonotonic pattern requires some complementarity between matrix and chondrules, chiefly in siderophile and chalcophile elements, and suggests that a component of the chemical characteristics of both chondrules and matrix resulted from a common process. That minor deviations in the C3 bulk composition away from a monotonic depletion pattern correspond to major deviations in the matrix pattern may suggest that a minor component of volatile-depleted chondrules were lost from the system or that bulks include a modest enrichment in a matrix component.
Hypotheses to explain the fractionation between chondrules and matrix, and nonmonotonic matrix volatile depletion patterns, can be grouped into three broad categories: (i) mobilization of elements between chondrule and matrix during aqueous alteration on the parent asteroid; (ii) fractionation during chondrule formation, either by evaporation and recondensation (17, 28);†† or (iii) physical separation of metal and sulfide from chondrule melt.
Aqueous Alteration as a Mechanism for Chondrule/Matrix Complementarity. Evidence for aqueous alteration can be found in most chondrites. In ordinary chondrites, aqueous mobility of elements has been proposed to explain trace element enrichments in the outer portions of chondrule mesostasis.‡‡ Similarly, evidence for iron-alkali aqueous metasomatism is found around chondrules and CAIs in CV chondrites (20). It is clear that aqueous mobilization must be considered as a possible mechanism to produce the fractionation we observe in the chondrule/matrix trace element composition. However, a number of observations from our data set militate against aqueous mobility as a significant process in the development of nonmonotonic depletion patterns in matrix and volatile fractionation between chondrules and matrix:
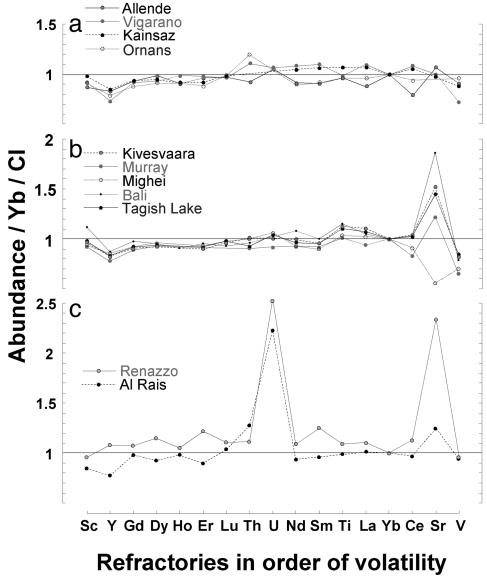
Soluble refractories in matrix. Refractory elements, with condensation temperatures greater than ≈1,350 K, are unaffected by volatile fractionation; soluble refractories would therefore record aqueous redistribution, without the complication of volatility-controlled fractionation. Sr is particularly useful in this discussion, as it is highly mobile in aqueous fluids, and abundant in chondrule mesostasis glass, a component that is rapidly decomposed during aqueous alteration. As such, we would anticipate nonchondritic Sr matrix values if aqueous processes produced the chondrule/matrix fractionation. C3 chondrites show significant fractionation between chondrules and matrix and a matrix composition that departs substantially from a volatility-controlled monotonic depletion pattern, but chondrite-normalized (N) refractory/Yb abundances are flat (Fig. 3a). Of all of the C3 matrices analyzed, only Bali, a highly altered CV3, shows enrichment in Sr (Fig. 3b). In Allende, for instance, matrix (Sr/Yb)N is 1.00; bulk (Sr/Yb)N in Allende is also chondritic (1.00), therefore chondrule values will be chondritic. Values so close to chondritic, in this and other C3s, suggest that <1% of Sr was transferred from chondrules to matrix. Matrix refractory abundances in the C2s tell a different story. Like Bali, CM2 matrix (and matrix in Tagish Lake) shows a clear departure from chondritic values in Sr (Fig. 3b). Of all of the matrices analyzed, CR2s show the most evidence for aqueous exchange between chondrules and matrix (Fig. 3c), with substantial enrichments in Sr and U (another aqueously mobile refractory trace element). This pattern is qualitatively similar to what we would expect based on the chondrite classification, less evidence for aqueous redistribution in type 3s, but more in type 2s. To conclude, a consideration of matrix refractory composition does not support aqueous mobility as the mechanism producing fractionation of volatile siderophiles and chalcophiles in the C3s.
The siderophile and chalcophile elements that are most enriched in C3 matrix relative to the bulk composition (Au, As, Ag, Sb, Ga, Ge, Bi, Pb, Zn, Se, In, and Tl) cover a huge range of solubilities. Their fractionation is not readily explained by mobility in aqueous fluids.
The C3 chondrites analyzed have experienced a wide range of parent body conditions, but their matrices have very similar siderophile and chalcophile abundances; it is unlikely that aqueous alteration could account for the uniformly large fractionation of siderophiles and chalcophiles in all C3 matrices. In addition, CM chondrites experienced the most extensive aqueous alteration, but matrix and chondrule compositions are the least fractionated compared with bulk. CM matrix lithophiles, siderophiles, and chalcophiles also show quite similar overall levels of depletion.
In the C3s (Fig. 2a), we observe minor positive deviations from a monotonic pattern in the bulk matching major positive deviations in matrix: the matrix fractionation is imposing itself on the bulk composition. It is difficult to achieve this relationship through aqueous mobilization.
Chondrule/matrix compositions are fractionated in C3s that experienced negligible aqueous alteration, with intact chondrule glass.
Fig. 3.
Refractory trace element data, ordered in terms of volatility. Note the linear scale, to highlight any interelement variability in abundance. (a) Matrix data from representative C3 chondrites. C3 chondrites show highly nonmonotonic volatile patterns, but chondrite-normalized (N) refractory/Yb abundances are flat. (b) Data from representative CM chondrite matrices, Tagish Lake, and Bali. Of the eight C3 matrices analyzed, only Bali's shows enrichment in Sr. This enrichment is similar to CM matrix (and matrix in Tagish Lake), which typically shows a departure from chondritic values in Sr. (c) Data from CR2 matrices. This group shows the most evidence for aqueous exchange between chondrules and matrix, with substantial enrichments in Sr and U. The absence of fractionated refractories in C3 matrices with highly nonmonotonic volatile patterns, and the presence of fractionated refractories in C2s with monotonic volatile patterns, suggests that aqueous redistribution is not the mechanism of choice to explain siderophile and chalcophile enrichment in C3 matrices.
In summary, it is difficult to explain the observed fractionation in volatile and moderately volatile elements between chondrules and matrix in C3 chondrites, and nonmonotonic trace element patterns in both components, by aqueous alteration. Reconciling the geochemical data with earlier petrographic observations (e.g., ref. 20)‡‡ involves considering the length scale over which aqueous metasomatism occurred. Early analyses of gram samples of meteorite (2, 3, 18) showed that aqueous alteration was isochemical on the scale of these samples. More recent data from 10- to 100-mg aliquots of Allende (4) showed remarkable reproducibility between samples and no evidence for element mobility on that scale. Our data indicate that alteration in most C3s was isochemical at the scale of chondrules and matrix, and that although element mobility occurred, it was probably restricted to zones within ≈100–200 μm of anhydrous chondrules.
Chondrule/Matrix Complementarity by Chondrule Formation. If aqueous alteration did not produce chondrule/matrix fractionation, then complementary compositions existed before accretion: matrix with a nonmonotonic trace element pattern, and fractionated siderophiles and chalcophiles, accreted with a complementary nonmonotonic chondrule composition to maintain a monotonic depletion pattern in the bulk. The obvious time for volatile fractionation between matrix and chondrules to occur was during chondrule formation itself; the suggestion is that chondrules and matrix formed from the same material reservoir, in the same nebula region. Evaporation from chondrules, and recondensation in surrounding matrix, has been suggested to explain chondrule/matrix complementarity in the CR2s (17, 28). In the C3s, matrix lithophiles show minimal enrichment over bulk; evaporation/recondensation should affect lithophiles as well as siderophiles and chalcophiles. Therefore although evaporation/recondensation cannot be ruled out, we prefer physical separation of metal and sulfide from chondrule melt and reaccretion of this material with matrix to explain chondrule/matrix complementarity in siderophiles and chalcophiles. This interpretation is supported by siderophile and chalcophile data from C3 chondrules (13, 15) and also by measured sulfide contents in Allende bulk, matrix, and chondrules (29).
Mechanisms for Bulk Volatile Depletion. Because none of the chondrite groups (with the exception of the CIs themselves) contain a matrix of CI-like composition, and a similar intergroup sequence of overall depletion is observed in matrix and bulks, it appears that depleted chondrules were not added to primordial matrix in varying proportions. Calculated chondrule trace element patterns also do not appear to be consistent with volatility-controlled depletion. In addition, a basic assumption of the two-component model is that essentially all moderately and highly volatile elements are contained in a primitive matrix. In contrast, our data show that moderately volatile lithophiles in matrix have similar abundance to bulk (Fig. 2a). In lithophiles, we cannot clearly discriminate a volatile-enriched and a volatile-depleted component in a chondrite; fundamentally, we do not have two components. Taken together, these observations suggest that variants of the traditional two-component model may not be tenable. In addition, we suggest that the X-wind model for chondrule formation (our data do not constrain X-wind CAI formation), as currently stated, is inadequate; there is complementarity between chondrules and matrix in siderophile and chalcophile elements; matrix composition is specific to a chondrite group; and matrix is evidently not composed of abundant chondrule fragments (see below), suggested as a possible mechanism to explain complementarity. The limited data set on chondrule rim trace element compositions also suggests that matrix is compositionally distinct from chondrule rims; it does not appear to be disaggregated rim material, as has been proposed (8).
Estimating the abundance of chondrule fragments in matrix is problematic, but trace element data provide a constraint. This analysis is relevant not only to the X-wind model, but also in recent versions of the two-component model, where addition of chondrule fragments is suggested as a mechanism for producing non-CI matrix compositions in the other chondrite groups. To modify C3 matrix compositions from a proposed CI-like starting point would require addition of several tens of percent volatile-depleted chondrule (or chondrule-related) materials. If this material was simply fragmented bulk chondrule (e.g., ref. 8) we would expect a matrix trace element pattern that was enriched compared with chondrule compositions, but which essentially paralleled the chondrule pattern. We did not observe this relationship. Our work shows that the matrix in C3 chondrites is typically enriched in siderophiles and chalcophiles, with chondrules depleted in these elements relative to bulk (13, 15, 29): trace element patterns for matrix and chondrules appear to mirror one another.
Alternatively, if instead of simply adding fragmented bulk chondrules, we preferentially add fragmented chondrule glass, we run into additional problems. Setting aside volatile fractionations, chondrule glass in ordinary chondrites is enriched in most refractory elements to ≈×10 CI (30). But we observe no refractory enrichment in matrix. Here, we use Mg as a ratioing element (as Yb would obscure any refractory enrichment). Average matrix (refractory/Mg)N in CVs is 0.91, in COs is 1.02, and in CMs is 1.01. This observation contrasts with bulk data (18) where (refractory/Mg)N in CVs is 1.33, in COs is 1.12, and CMs is 1.16 (refractory transition metals are not included in this matrix and bulk comparison). Refractory enrichment in the bulks relative to CI is caused by addition of CAIs, but CI-like values for matrix suggest no addition of a refractory component (either in the form of CAI fragments, or chondrule glass). Finally, whereas most refractory elements are at ≈×10 CI in chondrule glass (30), Sc and V, substituting in chondrule ferromagnesian silicates, are at ×5 CI and ×0.9 CI, respectively. The refractory trace element signature of chondrule glass (substantial excess in most elements, modest Sc excess, no V excess) has not been detected in C3 chondrite matrix in this work, in previous studies (13, 15), or in the matrix of CR2s (17). In summary, the available trace element data for carbonaceous chondrite matrix do not support addition of a substantial fragmental chondrule component.
Our analysis supports a volatility-controlled mechanism that predates chondrule formation for the overall bulk volatile element depletion in meteorites. Whether this mechanism was incomplete condensation after a large-scale thermal event (2) or inheritance from the interstellar medium** is not constrained by our data. Chondrules and matrix then formed from an already depleted material reservoir in the same nebula region. The major event producing chondrule/matrix fractionation in C3s was the physical separation of metal-sulfide during chondrule formation, with retention of chondrule metal-sulfide in matrix. Preserving chondrule/matrix complementarity requires rapid accretion after chondrule formation. Our data appear most consistent with the shock-wave model for chondrule formation (12), with chondrules forming adjacent to fine-grained materials, and metal-sulfide inclusions in molten chondrules decelerating at different rates from silicate melt within nebular shocks, and separating from that melt,§§ but being retained in matrix.
Supplementary Material
Acknowledgments
This paper benefited substantially from discussions with M. Gounelle, C. M. O. Alexander, B. Zanda, H. Palme, G. R. Huss, and S. S. Russell. We thank R. N. Clayton and two anonymous reviewers for input that considerably improved the final draft. This study was supported by the Particle Physics and Astronomy Research Council under Grant PPA/G/S/2003/00071. P.A.B. and O.A. thank the Royal Society for their support. This article is Impacts and Astromaterials Research Centre paper no. 2005-0727.
Author contributions: P.A.B. designed research; P.A.B., O.A., G.K.B., A.T.K., O.N.M., L.E.W., and N.W.R. performed research; P.A.B., O.A., G.K.B., and A.T.K. analyzed data; and P.A.B. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: CAI, Ca–Al-rich refractory inclusion; LA, laser ablation; ICP, inductively coupled plasma.
Footnotes
Yin, Q.-Z. (2004) in Workshop on Chondrites and the Protoplanetary Disk, School of Ocean and Earth Science and Technology Publication 04-03 (University of Hawaii, Manoa), pp. 227–228 (abstr.).
Grossman, J. N. (1985) Lunar Planet. Sci. Conf. 16, 302–303 (abstr.).
Grossman, J. N., Alexander, C. M. O. & Wang, J. (1997) Lunar Planet. Sci. Conf. 28, 1443 (abstr.).
Uesugi, M., Akaki, T., Sekiya, M. & Nakamura, T. (2003) Meteorit. Planet. Sci. 38, A29 (abstr.).
References
- 1.Anders, E. (1964) Space Sci. Rev. 3, 583–714. [Google Scholar]
- 2.Wasson, J. T. & Chou, C.-L. (1974) Meteoritics 9, 69–84. [Google Scholar]
- 3.Ikramuddin, M. & Lipschutz, M. E. (1975) Geochim. Cosmochim. Acta 39, 363–375. [Google Scholar]
- 4.Wulf, A. V., Palme, H. & Jochum, P. (1995) Planet. Space Sci. 43, 451–468. [Google Scholar]
- 5.Cassen, P. (1996) Meteorit. Planet. Sci. 31, 793–806. [Google Scholar]
- 6.Palme, H. & Jones, A. (2003) in Treatise on Geochemistry: Meteorites, Comets, and Planets, ed. Davis, A. M. (Elsevier, Amsterdam), pp. 41–61.
- 7.Alexander, C. M. O., Boss, A. P. & Carlson, R. W. (2001) Science 293, 64–68. [DOI] [PubMed] [Google Scholar]
- 8.Shu, F. H., Shang, H. & Lee, T. (1996) Science 271, 1545–1552. [Google Scholar]
- 9.Boss, A. P. (1993) Astrophys. J. 417, 351–367. [Google Scholar]
- 10.Liffman, K. & Brown, M. J. I. (1996) in Chondrules and the Protoplanetary Disk, eds. Hewins, R. H., Jones, R. H. & Scott, E. R. D. (Cambridge Univ. Press, Cambridge, U.K.), pp. 285–302.
- 11.Huss, G. R., Meshik, A. P., Smith, J. B. & Hohenberg, C. M. (2003) Geochim. Cosmochim. Acta 67, 4283–4848. [Google Scholar]
- 12.Desch, S. J. & Connolly, H. C., Jr. (2002) Meteorit. Planet. Sci. 37, 183–207. [Google Scholar]
- 13.Rubin, A. E. & Wasson, J. T. (1987) Geochim. Cosmochim. Acta 51, 1923–1937. [Google Scholar]
- 14.Inoue, M., Kimura, M. & Nakamura, N. (2004) Meteorit. Planet. Sci. 39, 599–608. [Google Scholar]
- 15.Rubin, A. E. & Wasson, J. T. (1988) Geochim. Cosmochim. Acta 52, 425–432. [Google Scholar]
- 16.Hua, X., Zinner, E. K. & Buseck, P. R. (1996) Geochim. Cosmochim. Acta 60, 4265–4274. [Google Scholar]
- 17.Kong, P. & Palme, H. (1999) Geochim. Cosmochim. Acta 63, 3673–3682. [Google Scholar]
- 18.Kallemeyn, G. W. & Wasson, J. T. (1981) Geochim. Cosmochim. Acta 45, 1217–1230. [Google Scholar]
- 19.Lodders, K. (2003) Astrophys. J. 591, 1220–1247. [Google Scholar]
- 20.Krot, A. N., Scott, E. R. D. & Zolensky, M. E. (1995) Meteoritics 30, 748–775. [Google Scholar]
- 21.Chizmadia, L. J., Rubin, A. E. & Wasson, J. T. (2002) Meteorit. Planet. Sci. 37, 1781–1796. [Google Scholar]
- 22.McSween, H. Y., Jr. (1977) Geochim. Cosmochim. Acta 41, 1777–1790. [Google Scholar]
- 23.McSween, H. Y., Jr. (1977) Geochim. Cosmochim. Acta 41, 477–491. [DOI] [PubMed] [Google Scholar]
- 24.McSween, H. Y., Jr. (1979) Geochim. Cosmochim. Acta 43, 1761–1770. [DOI] [PubMed] [Google Scholar]
- 25.Smith, C. L., Russell, S. S., Gounelle, M., Greenwood, R. C. & Franchi, I. A. (2004) Meteorit. Planet. Sci. 39, 2009–2032. [Google Scholar]
- 26.Newton, J., Bischoff, A., Arden, J. W., Franchi, I. A., Geiger, T., Greshake, A. & Pillinger, C. T. (1995) Meteoritics 30, 47–56. [Google Scholar]
- 27.Greshake, A. (1997) Geochim. Cosmochim. Acta 61, 437–452. [DOI] [PubMed] [Google Scholar]
- 28.Kong, P., Ebihara, M. & Palme, H. (1999) Geochim. Cosmochim. Acta 63, 2637–2652. [Google Scholar]
- 29.Jarosewich, E. (1990) Meteoritics 25, 323–337. [Google Scholar]
- 30.Alexander, C. M. O. (1994) Geochim. Cosmochim. Acta 58, 3451–3467. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.