Abstract
We tested the hypothesis that chronically ischemic (IS) myocardium induces autophagy, a cellular degradation process responsible for the turnover of unnecessary or dysfunctional organelles and cytoplasmic proteins, which could protect against the consequences of further ischemia. Chronically instrumented pigs were studied with repetitive myocardial ischemia produced by one, three, or six episodes of 90 min of coronary stenosis (30% reduction in baseline coronary flow followed by reperfusion every 12 h) with the non-IS region as control. In this model, wall thickening in the IS region was chronically depressed by ≈37%. Using a nonbiased proteomic approach combining 2D gel electrophoresis with in-gel proteolysis, peptide mapping by MS, and sequence database searches for protein identification, we demonstrated increased expression of cathepsin D, a protein known to mediate autophagy. Additional autophagic proteins, cathepsin B, heat shock cognate protein Hsc73 (a key protein marker for chaperone-mediated autophagy), beclin 1 (a mammalian autophagy gene), and the processed form of microtubule-associated protein 1 light chain 3 (a marker for autophagosomes), were also increased. These changes, not evident after one episode, began to appear after two or three episodes and were most marked after six episodes of ischemia, when EM demonstrated autophagic vacuoles in chronically IS myocytes. Conversely, apoptosis, which was most marked after three episodes, decreased strikingly after six episodes, when autophagy had increased. Immunohistochemistry staining for cathepsin B was more intense in areas where apoptosis was absent. Thus, autophagy, triggered by ischemia, could be a homeostatic mechanism, by which apoptosis is inhibited and the deleterious effects of chronic ischemia are limited.
Keywords: proteomics, lyposomal proteins, apoptosis, hibernating myocardium, myocardial protection
Autophagy is a cellular degradation process responsible for the turnover of unnecessary or dysfunctional organelles and cytoplasmic proteins and has been studied extensively in lower organisms such as yeast, Caenorhabditis elegans, and Drosophila (1–4). Autophagy has been suggested to be an essential function for cell homeostasis and cell defense and adaptation to an adverse environment (1, 2, 5). Autophagy is typically activated by starvation, when the cytoplasmic proteins or organelles are delivered to the lysosome and degraded (1–4). In autophagy, cytoplasmic proteins or dysfunctional organelles are sequestrated in a double-membrane-bound vesicle, termed autophagosome, delivered to the lysosome by fusion, and then degraded. Autophagy allows the cell not only to recycle amino acids but also to remove damaged organelles, thereby eliminating oxidative stress and allowing cellular remodeling for survival (2, 6). In fact, autophagy is a cellular mechanism essential for dauer development and lifespan extension in C. elegans (1). It can also prevent accumulation of misfolded and aggregated proteins in Parkinson's, Huntington's, and Alzheimer's diseases (7). It should be noted, however, that formation of autophagosomes and degradation of the bulk of cytoplasm are also observed in cells undergoing cell death. Thus, programmed cell death could be another general function of autophagy, namely the inducible mechanism for massive degradation of cytoplasmic components leading to caspase-independent cell death.
Although autophagy, as well as necrosis and apoptosis, can disrupt myocytes, the mechanisms are quite different; autophagy can be considered protective, because the salvaged amino acids can be used to build new proteins, whereas necrosis and apoptosis result in cell death without regeneration.
Our hypothesis was that autophagy might be invoked in chronically ischemic (IS) myocardium, where it could play a role in cardioprotection. Accordingly, the specific goal of this investigation was to search for pathognomonic features of autophagy in chronically IS myocardium, e.g., characteristic vacuoles by EM, and using a proteomic approach, we found an increase in proteins known to be involved in autophagy, e.g., beclin 1, cathepsins B and D, heat shock cognate protein (Hsc73), and the processed form of microtubule-associated protein 1 light chain 3 (LC3). The model of chronic ischemia we used was developed in conscious swine, where repetitive myocardial and ischemia and reperfusion resulted in chronic stunning, resembling the phenotype of hibernating myocardium in patients with chronic IS heart disease, including persistently reduced regional function, myolysis, and increased glycogen deposition in the IS region of the heart (8–10). In this model, apoptosis is activated after one episode of ischemia, reaches a peak after three episodes of ischemia, and then declines significantly after six episodes (8, 10). If autophagy is indeed involved in the protection against ischemia and apoptosis, then this mechanism should be most evident after six episodes of ischemia/reperfusion, when the incidence of apoptosis actually declines. Furthermore, we predicted that apoptosis would be less frequent in myocytes demonstrating evidence of autophagic proteins than in myocytes without autophagy.
Methods
Twenty-eight female domestic swine were instrumented chronically to measure global and regional myocardial function as described (10–13). After 1 week of postoperative recovery, ischemia was induced regionally by one, three, or six episodes of 90 min of left anterior descending coronary stenosis (CS) (30% reduction in baseline coronary flow) followed by full reperfusion repeated every 12 h, while the posterior region served as a control (10). These pigs were killed 1 h after one (n = 6), three (n = 6), or six (n = 6) episodes, and samples were taken for either protein analysis or histopathology from the chronically IS region or the control, non-IS (NI) region. Proteins were extracted and defined with 2D gel electrophoresis and quantitated with Western blot analysis. The TUNEL technique was used to quantitate apoptosis, and autophagic vacuoles were identified by using EM.
For comparison of the data among one vs. three vs. six episodes, a repeated one-way ANOVA with Student-Newman-Keuls test (SAS Institute, Cary, NC) was used. For comparison of data between chronically IS and NI responses, Student's paired t test with significant differences taken at P < 0.05 was used.
More detailed methodology can be found at Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Results
Regional Myocardial Function. We created a conscious swine model of chronic ischemia by inducing six episodes of CS (30% reduction of baseline blood flow for 90 min followed by full reperfusion) every 12 h. Regional myocardial function as reflected by anterior wall thickening fell during the initial stenosis, reversed only partially after reperfusion because of myocardial stunning, and remained depressed before and after the sixth episode similarly, i.e., by ≈37%. Regional left ventricular (LV) function in the contralateral NI region remained at normal levels, i.e., did not change from values before the first episode of CS.
Proteomic Analysis. On 2D gel maps, as shown in Fig. 1, two spots (≈34 kDa) with distinct pH (6.2 and 6.3) were observed after six episodes. Both spots were identified as cathepsin D by using peptide mapping by MS and sequence database searches. One of these spots with more acidic pH (6.2) that was detected after six episodes was not present either in the NI region or after three episodes, which could be posttranslational modification or another isoform of cathepsin D. Thus, proteomic analysis demonstrated an increase in the level of total cathepsin D after six episodes. Because cathepsin D is a key protein involved in autophagy, other markers of autophagy were examined.
Fig. 1.
Alteration of cathepsin D in chronically IS myocardium. (Upper)2Dgel map of protein extracts from chronically IS region after six episodes. (Lower) A magnified gel region from the NI region (Left) and the IS after either three (Center) or six (Right) episodes of CS. Arrows indicate the spots after six episodes that were all later identified as cathepsin D. MW, molecular weight. Each lower image is a 6-fold magnification from the original gel.
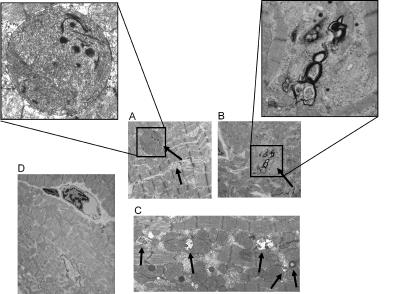
Additional Evidence of Autophagy. EM. Autophagic vacuoles (AVs) were observed by EM only in the chronically IS region after six episodes of CS. Fig. 2 shows the different types of AVs found in the six-episode IS region. These AVs containing heterogeneous organelles ranging from mitochondria to multivesicular bodies surrounded by a sequestering membrane were observed in myocytes that were not necrotic but showed some disfigurement, i.e., there was minor disarray of myofilaments and slightly enlarged extracellular spaces.
Fig. 2.
Electron micrographs of different types of AVs observed in the six-episode chronically IS region (A–C) and six-episode NI region (D). (A) AVs containing remnants of mitochondria are demonstrated. (B) Double-membrane AVs containing recognizable cytoplasmic contents are displayed. (C) AVs containing multivesicular bodies surrounded by a sequestering membrane are demonstrated. (D) These AVs were not observed in the six-episode NI. Arrows indicate AVs. (Magnifications: ×1,400–2,000, A–D; ×5,000, A and B Insets.)
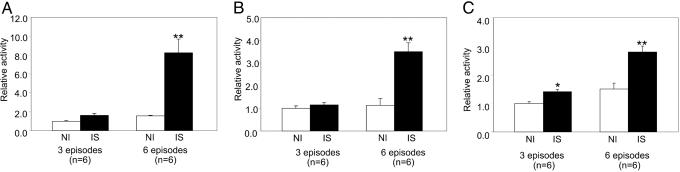
Biochemical markers. Lysosomal proteins cathepsins B and D. In addition to protein levels of cathepsin D (Fig. 3 A and C), protein levels of cathepsin B (Fig. 3 B and D) were increased in the chronically IS region after six episodes of CS compared with three episodes or the NI region (Fig. 3). The elevated expression of cathepsins B and D was confirmed in myocytes isolated from the chronically IS region after six episodes (Fig. 3D), indicating that the macrophages were not the source of the autophagic protein. This increased expression was accompanied by an increase in the activity of cathepsin B and D and lysosomal protein β-hexosaminidase after six episodes (Fig. 4).
Fig. 3.
Comparison of expression level of cathepsin B and D in chronically IS myocardium at different episodes. (A and B) Western blot analysis of cathepsin D (A) and cathepsin B (B) at six episodes chronically IS vs. NI regions (Upper) and three episodes (Lower). The expression of cathepsins B and D significantly increased at six episodes (**, P < 0.01; *, P < 0.05 vs. NI). (C) The direct comparison of levels of cathepsin B (CB) and cathepsin D (CD) in chronically IS in one, three, and six episodes, again showing marked increased expression of both proteins after six episodes. (D) The elevated expression of cathepsins B and D was confirmed to occur in myocytes from the chronically IS myocardium. ADU, arbitrary densitometric units.
Fig. 4.
Enzyme activity assays of cathepsin B (A), cathepsin D (B), and β-hexosaminidase (C). Activity of both cathepsin B and D and β-hexosaminidase was much higher at six episodes in chronically IS region vs. NI region compared with three episodes vs. NI. **, P < 0.01 vs. NI; *, P < 0.05 vs NI.
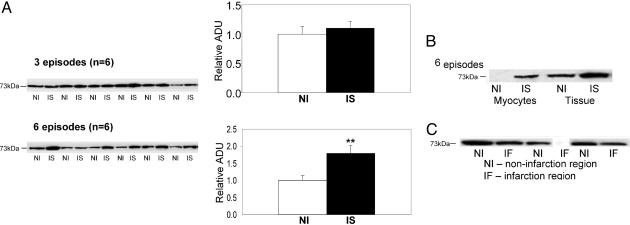
Hsc73. Hsc73 has been identified as a key protein marker for chaperone-mediated autophagy (14, 15). A significant increase (P < 0.01) in the expression levels of Hsc73 was observed in the chronically IS region after six episodes, but not after three episodes or in the NI region (Fig. 5A). This elevation after six episodes was confirmed in isolated myocytes (Fig. 5B). In contrast, myocardial infarction showed a reduced level of Hsc73, indicating that the increase in this protein occurred in reversibly IS, but not irreversibly IS, myocardium (Fig. 5C).
Fig. 5.
Western blot analysis of Hsc73 at six episodes chronically IS vs. NI myocardium (A) and at three episodes in isolated myocytes (B) and comparing effects in necrotic tissue after myocardial infarction (IF) vs. NI regions (C). The expression of Hsc73 increased significantly in the chronically IS of six episodes (**, P < 0.01 vs. NI), but not in the IS after three episodes compared with NI. The elevation of Hsc73 after six episodes was confirmed in isolated myocytes. In contrast, the expression of Hsc73 decreased in the infarcted region. ADU, arbitrary densitometric units.
Beclin 1. Beclin 1 has been identified as a mammalian autophagy gene (1, 16). Its protein expression level was increased (P < 0.05) only after six episodes in the chronically IS region, i.e., there was no significant increase after three episodes (Fig. 6A). The elevation after six episodes was confirmed in isolated cardiac myocytes (Fig. 6B). Beclin 1 was also found to be reduced in the infarcted myocardium (Fig. 6C).
Fig. 6.
Western blot analysis of beclin 1 at three and six episodes (chronically IS vs. NI regions) (A) and in isolated myocytes from the chronically IS (B) and comparing effects in necrotic tissue after myocardial infarction (IF) vs. NI regions (C). The expression of beclin 1 was increased significantly in the chronically IS after six episodes (*, P < 0.05 vs. NI), but not after three episodes. The elevation of beclin 1 after six episodes was confirmed in isolated myocytes. In contrast, the expression of beclin 1 decreased in the infarcted region. ADU, arbitrary densitometric units.
LC3. The rat microtubule-associated protein LC3, a mammalian homolog of yeast Atg8 (essential for yeast autophagy), was identified as the first mammalian protein localized in the autophagosome membrane and therefore has been suggested as an excellent marker for the detection of autophagosomes (17). Endogenous LC3 is present in two forms, LC3-I (cystolic form) and LC3-II (processed form located on the autophagosomal membrane). The ratio of LC3-II/LC3-I is correlated with the extent of autophagosome formation (18). Here, the LC3-II/LC3-I ratio was significantly increased after six episodes in the chronically IS myocardium (Fig. 7), further supporting the presence of autophagy in chronic ischemia.
Fig. 7.
Western blot analysis of LC3 at six episodes chronically IS vs. NI myocardium. The LC3-II/LC3-I ratio was significantly increased in the chronically IS (**, P < 0.01 vs. NI). Western blotting of actin showed equal loading of the samples.
Extent of Colocalization of Cathepsin B Immunostaining and Apoptosis. Cathepsin B fluorescent immunostaining further demonstrates the accumulation of this protein in myocytes after six episodes (Fig. 8A). Apoptosis was not distributed evenly throughout the chronically IS myocardium. First of all, the distribution of apoptosis was preferential to the subendocardium, consistent with the well known effects of more intense ischemia in the subendocardium after CS. Furthermore, the apoptosis was localized to discrete, patchy areas, which were also characterized by patchy necrosis. Necrosis was identified by morphological changes, e.g., nuclear or cytoplasmic degeneration. There were other patchy areas of necrosis, essentially devoid of apoptosis. The majority of the subendocardium was free from evidence of increased apoptosis. To quantify data for apoptosis and autophagy, we performed dual labeling of apoptosis (TUNEL) and cathepsin B (Fig. 8 B and C). Interestingly, immunostaining for cathepsin B was much more prevalent in areas of the subendocardium where there was no apoptosis or in those patchy areas of necrosis where apoptosis was absent. Conversely, myocytes in those patchy areas of necrosis associated with increased apoptosis exhibited much less cathepsin B immunostaining. The data supporting this conclusion were collected from the examination of 3,000 myocytes in 49 fields, corresponding to the three areas described above. In the patchy areas of necrosis with apoptosis, the frequency of myocytes staining positive for TUNEL was 3.3 ± 0.5%, whereas there was almost no apoptosis in the remaining areas, i.e., <0.1%. The frequency of myocytes staining positive for cathepsin B was significantly higher (19.9 ± 3.3%), P < 0.05, in patchy necrotic areas without apoptosis or in areas of the subendocardium distant from areas of necrosis and apoptosis (5.4 ± 1.0%), compared with myocytes in areas of patchy necrosis with apoptosis (1.8 ± 0.8%).
Fig. 8.
Localization of cathepsin B and dual labeling of cathepsin B and apoptosis. (A) Fluorescent immunostaining for detection of cathepsin B in myocytes after six episodes. Cathepsin B (green) was localized to myocytes. (B and C) Dual labeling of apoptosis (TUNEL) and cathepsin B. (B) Staining for cathepsin B (blue) was more intense in areas where apoptosis was absent. (C) Conversely, myocytes associated with increased apoptosis (green) exhibited much less cathepsin B (indicated by blue and the arrow) immunostaining. (Magnification: ×430, A; ×275, B and C.)
Timed Controls. After two episodes of CS, three pigs were followed for the time required for four more episodes and then killed. In these hearts, cathepsin B levels increased only negligibly, less than observed for three episodes, demonstrating that it was the multiple episodes of CS and not simply the time from the initial episodes of CS that invoked the autophagy.
Recovery from Ischemia. After the six episodes of CS, two pigs were allowed to recover. There was no functional deficit in the previously IS region, and the level of all markers of autophagy, including cathepsins B and D, Hsc73, and beclin 1, returned to normal (similar to results in the NI region) (Fig. 9). Evidence of chronic ischemia, e.g., fibrosis, was essentially absent, as observed by trichrome staining.
Fig. 9.
Western blots of cathepsins B and D, Hsc73, and beclin 1 in two pigs with recovery compared after six episodes. The level of all altered proteins returned to normal in the previously IS region compared with the NI region.
Discussion
Patients with chronic IS heart disease and hibernating myocardium often survive for years despite severe atherosclerosis. Furthermore, many of these patients recover myocardial function after chronic bypass surgery, an observation that resulted in the concept of the “smart heart” (8–10, 19–24), i.e., whereby myocardial function and metabolism down-regulate in response to chronic ischemia. The question arises as to whether the ability of chronically IS myocardium to survive totally depends on alterations of myocardial metabolism and contraction or potentially on genomic and proteomic mechanisms of cell survival. If this concept is correct, the smart heart hypothesis (20) has to be re-evaluated to include a component of the “smartness” involving up-regulation of cytoprotective mechanisms. In support of this hypothesis, genes and proteins involved in cytoprotection have been found to be up-regulated in chronically IS myocardium (8, 9), and the current investigation demonstrated the presence of another cytoprotective mechanism, autophagy.
We used a model of chronic myocardial ischemia and chronic myocardial stunning produced by six episodes of CS and 12 h of reperfusion in chronically instrumented, conscious swine (8, 10). This model is characterized by persistently depressed function, a switch to glucose metabolism, and pathognomonic histologic features of hibernating myocardium, i.e., glycogen granules and myolysis in the myocardium (10, 24, 25).
A nonbiased proteomic analysis initially provided the identification of autophagy in the chronically IS myocardium, i.e., after six episodes. 2D gel electrophoresis with in-gel proteolysis, peptide mapping by MS, and sequence database searches for protein identification demonstrated an increase of cathepsin D expression, a protein known to mediate autophagy (26, 27). Several studies reported a role for autophagy in programmed cell death type II (nonapoptotic death) in disease (28, 29). There is also growing evidence that the activation of autophagy could play a protective role in early postnatal life (30) and early stages of diseases (31, 32), and it has been proposed that autophagy delays apoptosis in a human intestinal colon cancer cell line (33) and in sympathetic neurons (34). Another in vitro model demonstrated that the onset of the mitochondrial permeability transition within a cell leads to mitochondrial degradation through autophagy (35, 36). According to this model, elimination of damaged mitochondria by autophagy would prevent the release of proapoptotic substances from mitochondria, thus preventing apoptosis. Results from our study support this position. Interestingly, in this model, apoptosis is activated after one episode of ischemia, reaches a peak after three episodes of ischemia, and then declines significantly after six episodes of ischemia (8). In the current investigation, we observed that peak autophagic activity was concurrent with the decline in apoptosis after six episodes of ischemia, which suggests autophagy may be involved in the protection against apoptosis in the setting of chronic ischemia. In apparent contrast, a recent study suggested that autophagy may be responsible for cell death in hibernating myocardium in humans (37).
The apoptosis, which was distributed primarily in patchy areas of necrosis in the subendocardium in this model of chronically IS myocardium, presented an opportunity to determine whether the autophagic markers were also more or less prominent in these areas. Interestingly, only some patchy areas of necrosis in the subendocardium were associated with increased levels of apoptosis, whereas other areas were essentially devoid of apoptosis. Conversely, the frequency of myocytes immunostaining positive for cathepsin B was inversely related to the frequency of TUNEL-positive myocytes, further supporting our concept that the autophagic process protects against apoptosis.
Our investigation generated multiple lines of evidence supporting the concept that autophagy is up-regulated in the setting of chronic myocardial ischemia. First, the hallmark of autophagy is increased lysosomal activity (38). Increased lysosomal proteins cathepsins B and D (27), β-hexosaminidase, Hsc73 (14), beclin 1 (16, 39), and LC3-II (17, 18) strongly support the presence of autophagy (Figs. 3, 4, 5, 6, 7). These proteins were up-regulated essentially only after six episodes and only in the chronically IS region. Second, histologically, autophagy can be detected by an increase in the number and size of AVs, which contain multivesicular bodies and organelles surrounded by a sequestering membrane (32, 38). EM confirmed that AVs accumulated in myocytes. Importantly, these myocytes were still intact even though there were signs of disfigurement with minor disarray of myofilaments and slightly enlarged extracellular space. All of these results taken together suggest that chronic ischemia elicits autophagy, which was confirmed by the AVs (Fig. 2 A–C).
It was considered important to demonstrate that these histological and biochemical changes attributed to autophagy could not be attributed to other cell types. It has been reported that some autophagy markers, e.g., cathepsins, exist in macrophages (40) and indeed, some macrophages were observed by EM in the chronically IS myocardium. The studies in isolated myocytes, demonstrating similar increases in autophagic markers as were observed in membrane homogenates from myocardial tissue, indicate that the macrophages were not the source of the autophagic markers observed in chronic myocardial ischemia in this study. In addition, immunohistochemistry localized cathepsin B to isolated myocytes. In further support of this point of view, we found unchanged levels of macrophage marker proteins CD14 and CD68 in myocardial homogenates from chronically IS myocardium, indicating that the quantity of the macrophages was not sufficiently high to permit the expression of their markers, CD14 and CD68. Furthermore, in pigs with irreversible ischemia, i.e., infarct, the autophagic markers were observed to decrease.
Lysosomal enzymes play a central role in the degradation of extracellular and intracellular macromolecules (41). Hsc73 was reported as a marker protein for chaperone-mediated autophagy, a selective lysosomal degradation pathway, in which proteins containing the KFERQ motif are bound by Hsc73 and targeted to lysosomes for degradation (14). Although the presence of Hsc73 in heart has been alluded to (42–44), this report identifies its presence and its elevation in IS disease. Beclin 1 was recently reported as a novel autophagic gene in mammalian cells. It inhibits neoplasia (16) and it is also reported to be essential for dauer morphogenesis and lifespan extension in C. elegans (1). The presence and alteration in the levels of beclin 1 have also not been reported previously in the heart.
Autophagy was found previously in hypoxic and reoxygenated rat and rabbit hearts maintained on a Langendorff perfusion apparatus (45, 46) and in organ culture from hearts of late fetal mice with prolonged exposure to nonmetabolizable sugars (47). These models were acute and in vitro. Our swine model is unique in that repetitive myocardial stunning results in the phenotype of hibernating myocardium in patients with chronic IS heart disease. Our investigation demonstrates that autophagy is likely to be a survival mechanism in chronically IS myocardium in vivo in a large animal model.
In summary, autophagy triggered by ischemia could be a homeostatic mechanism, by which apoptosis is inhibited and the deleterious effects of chronic ischemia are limited, based on the following evidence: (i) the myocytes containing AVs observed in chronically IS myocardium were identified in live, but not lysed, cells, and conversely, autophagic markers were down-regulated in infarcted myocardium; (ii) apoptosis that was maximal after three episodes of ischemia declined markedly after six episodes, coincident with the appearance of autophagy, which was just becoming evident after three episodes and was fully manifest after six episodes of ischemia; (iii) immunostaining of the autophagic marker cathepsin B was much more abundant in myocytes in nonapoptotic regions, and conversely, myocytes in apoptotic regions rarely stained positive for cathepsin B; and (iv) the chronically IS myocardium exhibited full functional and histological recovery from the last episode of CS with a decline in markers of autophagy; conversely, infarcted myocardium demonstrated reduced levels of autophagic markers.
Supplementary Material
Acknowledgments
We thank Dr. Tamotsu Yoshimori for providing the LC3 antibody, Dr. Michael Brimacombe for consultation on the statistical analysis, and members of the Electron Microscopy Laboratory Facility and Center for Advanced Proteomics Research at the University of Medicine and Dentistry of New Jersey for critical assistance. This work was supported by National Institutes of Health Grants 1P01HL69020, 2R01AG14121, 2R01HL33107, 2P01HL59139, AG023137, HL65183, and HL65182 and Building Interdisciplinary Research Careers in Women's Health Award HD01457. L.Y. is a Building Interdisciplinary Research Careers in Women's Health Scholar.
Author contributions: L.Y., D.E.V., J.S., and S.F.V. designed research; L.Y., S.-J.K., H.G., M.M., W.H.M., G.Y., and Y.M. performed research; L.Y., D.E.V., J.S., and S.F.V. analyzed data; and L.Y., D.E.V., J.S., and S.F.V. wrote the paper.
Abbreviations: CS, coronary stenosis; LV, left ventricular; IS, ischemic; NI, non-IS; AV, autophagic vacuole; LC3, microtubule-associated protein 1 light chain 3.
References
- 1.Melendez, A., Talloczy, Z., Seaman, M., Eskelinen, E. L., Hall, D. H. & Levine, B. (2003) Science 301, 1387–1391. [DOI] [PubMed] [Google Scholar]
- 2.Stromhaug, P. E. & Klionsky, D. J. (2001) Traffic 2, 524–531. [DOI] [PubMed] [Google Scholar]
- 3.Huang, W. P. & Klionsky, D. J. (2002) Cell Struct. Funct. 27, 409–420. [DOI] [PubMed] [Google Scholar]
- 4.Ohsumi, Y. (2001) Nat. Rev. Mol. Cell. Biol. 2, 211–216. [DOI] [PubMed] [Google Scholar]
- 5.Otto, G. P., Wu, M. Y., Kazgan, N., Anderson, O. R. & Kessin, R. H. (2003) J. Biol. Chem. 278, 17636–17645. [DOI] [PubMed] [Google Scholar]
- 6.Klionsky, D. J. & Emr, S. D. (2000) Science 290, 1717–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shintani, T. & Klionsky, D. J. (2004) Science 306, 990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Depre, C., Kim, S. J., John, A. S., Huang, Y., Rimoldi, O. E., Pepper, J. R., Dreyfus, G. D., Gaussin, V., Pennell, D. J., Vatner, D. E., et al. (2004) Circ. Res. 95, 433–440. [DOI] [PubMed] [Google Scholar]
- 9.Kim, S. J., Depre, C. & Vatner, S. F. (2003) Heart Failure Rev. 8, 143–153. [DOI] [PubMed] [Google Scholar]
- 10.Kim, S. J., Peppas, A., Hong, S. K., Yang, G., Huang, Y., Diaz, G., Sadoshima, J., Vatner, D. E. & Vatner, S. F. (2003) Circ. Res. 92, 1233–1239. [DOI] [PubMed] [Google Scholar]
- 11.Camici, P. G., Wijns, W., Borgers, M., De Silva, R., Ferrari, R., Knuuti, J., Lammertsma, A. A., Liedtke, A. J., Paternostro, G. & Vatner, S. F. (1997) Circulation 96, 3205–3214. [DOI] [PubMed] [Google Scholar]
- 12.Depre, C., Tomlinson, J. E., Kudej, R. K., Gaussin, V., Thompson, E., Kim, S. J., Vatner, D. E., Topper, J. N. & Vatner, S. F. (2001) Proc. Natl. Acad. Sci. USA 98, 9336–9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudej, R. K., Kim, S. J., Shen, Y. T., Jackson, J. B., Kudej, A. B., Yang, G. P., Bishop, S. P. & Vatner, S. F. (2000) Am. J. Physiol. 279, H451–H456. [DOI] [PubMed] [Google Scholar]
- 14.Cuervo, A. M., Knecht, E., Terlecky, S. R. & Dice, J. F. (1995) Am. J. Physiol. 269, C1200–C1208. [DOI] [PubMed] [Google Scholar]
- 15.Chiang, H. L., Terlecky, S. R., Plant, C. P. & Dice, J. F. (1989) Science 246, 382–385. [DOI] [PubMed] [Google Scholar]
- 16.Liang, X. H., Jackson, S., Seaman, M., Brown, K., Kempkes, B., Hibshoosh, H. & Levine, B. (1999) Nature 402, 672–676. [DOI] [PubMed] [Google Scholar]
- 17.Kabeya, Y., Mizushima, N., Ueno, T., Yamamoto, A., Kirisako, T., Noda, T., Kominami, E., Ohsumi, Y. & Yoshimori, T. (2000) EMBO J. 19, 5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizushima, N., Yamamoto, A., Matsui, M., Yoshimori, T. & Ohsumi, Y. (2004) Mol. Biol. Cell. 15, 1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahimtoola, S. H. (1996) Circulation 94, 3055–3061. [DOI] [PubMed] [Google Scholar]
- 20.Braunwald, E. & Rutherford, J. D. (1986) J. Am. Coll. Cardiol. 8, 1467–1470. [DOI] [PubMed] [Google Scholar]
- 21.Wijns, W., Vatner, S. F. & Camici, P. G. (1998) N. Engl. J. Med. 339, 173–181. [DOI] [PubMed] [Google Scholar]
- 22.Canty, J. M., Jr. & Fallavollita, J. A. (2000) J. Nucl. Cardiol. 7, 509–527. [DOI] [PubMed] [Google Scholar]
- 23.Heusch, G. (1998) Physiol. Rev. 78, 1055–1085. [DOI] [PubMed] [Google Scholar]
- 24.Elsasser, A., Schlepper, M., Klovekorn, W. P., Cai, W. J., Zimmermann, R., Muller, K. D., Strasser, R., Kostin, S., Gagel, C., Munkel, B., et al. (1997) Circulation 96, 2920–2931. [DOI] [PubMed] [Google Scholar]
- 25.Borgers, M. & Ausma, J. (1995) Basic Res. Cardiol. 90, 44–46. [DOI] [PubMed] [Google Scholar]
- 26.Uchiyama, Y. (2001) Arch. Histol. Cytol. 64, 233–246. [DOI] [PubMed] [Google Scholar]
- 27.Qin, Z. H., Wang, Y., Kegel, K. B., Kazantsev, A., Apostol, B. L., Thompson, L. M., Yoder, J., Aronin, N. & DiFiglia, M. (2003) Hum. Mol. Genet. 12, 3231–3244. [DOI] [PubMed] [Google Scholar]
- 28.Bursch, W. (2001) Cell Death Differ 8, 569–581. [DOI] [PubMed] [Google Scholar]
- 29.Tolkovsky, A. M., Xue, L., Fletcher, G. C. & Borutaite, V. (2002) Biochimie 84, 233–240. [DOI] [PubMed] [Google Scholar]
- 30.Kuma, A., Hatano, M., Matsui, M., Yamamoto, A., Nakaya, H., Yoshimori, T., Ohsumi, Y., Tokuhisa, T. & Mizushima, N. (2004) Nature 432, 1032–1036. [DOI] [PubMed] [Google Scholar]
- 31.Cuervo, A. M. (2004) Trends Cell Biol. 14, 70–77. [DOI] [PubMed] [Google Scholar]
- 32.Larsen, K. E. & Sulzer, D. (2002) Histol. Histopathol. 17, 897–908. [DOI] [PubMed] [Google Scholar]
- 33.Bauvy, C., Gane, P., Arico, S., Codogno, P. & Ogier-Denis, E. (2001) Exp. Cell Res. 268, 139–149. [DOI] [PubMed] [Google Scholar]
- 34.Xue, L., Fletcher, G. C. & Tolkovsky, A. M. (1999) Mol. Cell. Neurosci. 14, 180–198. [DOI] [PubMed] [Google Scholar]
- 35.Lemasters, J. J., Nieminen, A. L., Qian, T., Trost, L. C., Elmore, S. P., Nishimura, Y., Crowe, R. A., Cascio, W. E., Bradham, C. A., Brenner, D. A. & Herman, B. (1998) Biochim. Biophys. Acta 1366, 177–196. [DOI] [PubMed] [Google Scholar]
- 36.Elmore, S. P., Qian, T., Grissom, S. F. & Lemasters, J. J. (2001) FASEB J. 15, 2286–2287. [DOI] [PubMed] [Google Scholar]
- 37.Elsasser, A., Vogt, A. M., Nef, H., Kostin, S., Mollmann, H., Skwara, W., Bode, C., Hamm, C. & Schaper, J. (2004) J. Am. Coll. Cardiol. 43, 2191–2199. [DOI] [PubMed] [Google Scholar]
- 38.Marzella, L., Ahlberg, J. & Glaumann, H. (1982) J. Cell Biol. 93, 144–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yue, Z., Jin, S., Yang, C., Levine, A. J. & Heintz, N. (2003) Proc. Natl. Acad. Sci. USA 100, 15077–15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lesser, M., Chang, J. C. & Orlowski, M. (1985) Mol. Cell. Biochem. 69, 67–73. [DOI] [PubMed] [Google Scholar]
- 41.Dunn, W. A., Jr. (1994) Trends Cell Biol. 4, 139–143. [DOI] [PubMed] [Google Scholar]
- 42.Tanonaka, K., Yoshida, H., Toga, W., Furuhama, K. & Takeo, S. (2001) Biochem. Biophys. Res. Commun. 283, 520–525. [DOI] [PubMed] [Google Scholar]
- 43.Tanonaka, K., Furuhama, K. I., Yoshida, H., Kakuta, K., Miyamoto, Y., Toga, W. & Takeo, S. (2001) Am. J. Physiol. 281, H215–H22. [DOI] [PubMed] [Google Scholar]
- 44.Wing, S. S., Chiang, H. L., Goldberg, A. L. & Dice, J. F. (1991) Biochem. J. 275, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decker, R. S., Poole, A. R., Crie, J. S., Dingle, J. T. & Wildenthal, K. (1980) Am. J. Pathol. 98, 445–456. [PMC free article] [PubMed] [Google Scholar]
- 46.Decker, R. S. & Wildenthal, K. (1980) Am. J. Pathol. 98, 425–444. [PMC free article] [PubMed] [Google Scholar]
- 47.Wildenthal, K., Dees, J. H. & Buja, L. M. (1977) Circ. Res. 40, 26–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.