Abstract
The factors that control replication rate of the intracellular bacterium Wolbachia pipientis in its insect hosts are unknown and difficult to explore, given the complex interaction of symbiont and host genotypes. Using a strain of Wolbachia that is known to over-replicate and shorten the lifespan of its Drosophila melanogaster host, we have tracked the evolution of replication control in both somatic and reproductive tissues in a novel host/Wolbachia association. After transinfection (the transfer of a Wolbachia strain into a different species) of the over-replicating Wolbachia popcorn strain from D. melanogaster to Drosophila simulans, we demonstrated that initial high densities in the ovaries were in excess of what was required for perfect maternal transmission, and were likely causing reductions in reproductive fitness. Both densities and fitness costs associated with ovary infection rapidly declined in the generations after transinfection. The early death effect in D. simulans attenuated only slightly and was comparable to that induced in D. melanogaster. This study reveals a strong host involvement in Wolbachia replication rates, the independence of density control responses in different tissues, and the strength of natural selection acting on reproductive fitness.
Wolbachia pipientis are common bacterial endosymbionts of arthropods and filarial nematodes. They occur intracellularly in the ovaries of all described hosts, as well as in a number of other tissues depending on the particular Wolbachia/host combination (1, 2). Infection of the host germ line enables transovarial transmission as well as induction of a number of reproductive abnormalities. These include cytoplasmic incompatibility (CI), parthenogenesis, feminization, and male killing, all of which enhance the spread of Wolbachia in host populations (3).
In Drosophila simulans and Drosophila melanogaster, most infections induce CI (4), where Wolbachia modify developing sperm such that only the presence of the same Wolbachia strain in the egg can rescue the modification, allowing successful completion of karyogamy (5, 6) and the subsequent normal development of the embryo. Uninfected females cannot rescue the sperm modification, and so the development of their offspring is blocked. The result is that with each subsequent generation, infection frequencies rise and fewer and fewer uninfected females can successfully reproduce (7).
For infections to be maintained in host populations, Wolbachia replication rates must be sufficiently high to ensure fidelity of transovarial transmission, while being low enough to not cause overt host pathology. This replication control can be considered a defining feature that separates vertically transmitted symbionts from horizontally transmitted pathogens. The mechanisms that underlie control of Wolbachia replication and the extent to which this control is influenced by host or bacterial genotype are not well understood. Studies exploring the link between CI expression and Wolbachia infection densities have revealed that it is possible to select for increased Wolbachia densities in both D. melanogaster (8) and Nasonia. In the latter case, however, heritability was limited, and densities appeared to autocorrect around a mean from one generation to the next (9). The results of transinfection experiments in Drosophila have argued for the presence of a host component by demonstrating that the same Wolbachia strain is maintained at higher densities in D. simulans than in D. melanogaster (8, 10), and that there may also be species-specific colonization patterns of different tissues (11). Variation in the behavior of different Wolbachia strains that infect the same host species (15) supports the role of bacterial genomic contributions. As such, it appears that control of Wolbachia replication is a complex interplay of host and bacterial components.
In nearly all reported cases, direct fitness costs suffered by the host because of Wolbachia infection appear to be negligible (12) and in some instances Wolbachia infections appear to be beneficial (13, 14). An exception to this rule was recently described in D. melanogaster (15), in which a Wolbachia strain was identified that caused a dramatic reduction in the lifespan of its host. The strain, named popcorn (wMelPop), replicates to abnormally high densities, especially in adult nervous and muscle tissue, resulting in host cell pathology. Interactions between the popcorn strain and its host provide an ideal system for studying the factors that regulate Wolbachia replication rate. We used embryonic microinjection to transfer wMelPop from its natural D. melanogaster host into an uninfected D. simulans genetic background. We were then able to determine the role of the popcorn genotype in the over-replication phenotype and temporally track the attenuation of fitness costs associated with popcorn in the newly infected host.
Materials and Methods
Strain Nomenclature.
Wolbachia/host species combinations are named with a two-part designation, the first referring to the host species (Dmel or Dsim for D. melanogaster or D. simulans, respectively) and the second indicating the bacterial strain (i.e., wMelPop and wRi for the popcorn and Riverside infections, respectively). Any strain name followed by a “T” (e.g., Dsim wRiT) indicates that the original host has been cured of its infection by standard methods of tetracycline treatment (16).
Embryonic Microinjection.
Egg cytoplasm from Dmel wMelPop (15) was microinjected into Dsim wRiT (16) by standard techniques (8, 17, 18). Dsim wRiT was chosen as the recipient host strain, because the infection it harbored before tetracycline treatment was known to cause only slight reproductive costs to its host (19) and to be capable of inducing strong CI expression (16, 19). Embryos were incubated at 27°C for 48 h. Larvae were transferred to vials containing fruit fly diet (Sigma) and moved to 21°C for all subsequent rearing except where noted.
Rearing and Screening for Infection Status.
Surviving G0 females were crossed with Dsim wRiT males and used to establish isofemale lines. After we found evidence of viable G1 offspring, females were killed and DNA was extracted by using the STE/boil method (STE = 100 mM NaCl/10 mM Tris⋅HCl, pH 8.0/1 mM EDTA) (20). Presence of Wolbachia in the samples was detected by PCR using primers for the wsp gene (81F and 691R) (21). Amplification of DNA was carried out in 50 mM KCl/10 mM Tris⋅HCl (pH 9.0)/0.1% Triton X-100/2.5 mM MgCl2/0.25 mM each dNTP/0.5 μM primers. The thermal cycling regime was as follows; 3-min denaturation at 94°C, 35 cycles of denaturation at 94°C, annealing at 55°C, and extension at 72°C each for 1 min, followed by an extra 10-min extension step at 72°C. Reactions were run in either a PTC-200 Thermal Cycler (MJ Research) or a Mastercycler (Eppendorf). Roughly 350 embryos were injected. All (9 of 9) surviving G0 males and 7 of 12 surviving G0 females were PCR positive for Wolbachia.
To ensure the stability of the infection in early generations, offspring from females that tested positive for Wolbachia by PCR screening were used as parental stock. After 11 generations, we removed the selection regime and began rearing populations of flies in bottles. Several lines were lost during selection, such that descendents of a single original female comprised the entire line.
Tests for CI Rescue and Fitness Measurements.
Single mating pairs of 1- to 2-day-old virgin flies were placed in empty food bottles with molasses plate lids that were dotted with a yeast suspension. Plates were collected every 24 h. The first set of plates containing 15 or more eggs was counted and saved to measure egg hatchability. If females did not produce eggs within 3 days, the cross was discarded from the experiment. The number of unhatched eggs was recorded 72 h after collection, and the percentage of egg hatch was calculated. Dsim wMelPop was assayed at generation 20 after transinfection for CI rescue by mating infected males to uninfected (tetracycline control) females (n = 75). Fecundity was simply a summation of the first 3 days of egg production. Productivity was the same measure, extended for 10 days. Fecundity was measured from the same crosses used to measure hatch rate, and is therefore likely to be an overestimate, given that all nonproducing crosses were removed. All estimates were compared with parallel measurements from control crosses (uninfected × uninfected flies generated by tetracycline treatment, for each line). Statistical significance of hatch rates for various crosses was determined by using a Kruskal–Wallis test followed by comparisons using the Simultaneous Test Procedure method (22). Fecundity measurements were compared with a general one-way ANOVA and a sequential Bonferroni adjusted α value for planned comparisons.
Maternal Transmission.
We measured the transmission efficiency of Dsim wRi, Dmel wMelPop, and Dsim wMelPop at generations 15 and 22 by selecting 10 PCR positive females (mated to uninfected control males) from each line and by assaying, with PCR, the proportion of their offspring (10 each) that were infected with Wolbachia.
Real-Time Quantitative PCR.
DNA from either head or ovary dissections was extracted as above. All hybridization probes, primers, and PCR procedures were as reported (11). In short, PCR amplification was carried out independently for both the single-copy wsp and su(fk)C genes of bacterial and host origin, respectively, in the presence of specific molecular beacon hybridization probes (23). Initial gene copy number was estimated by comparison to a standard curve using Roche lightcycler data analysis software v3.1.02. The copy number of the host gene was used to normalize estimates of Wolbachia gene copy number. Three replicates were run and averaged for each sample before construction of relative copy number ratios. For each strain at each time point, we collected measurements on 10 samples. Because variances tended to be correlated with means, we log transformed all ratio data before performing t tests. If variances remained unequal (F test) we then used the nonparametric Mann–Whitney U test. A Bonferroni correction was used to compensate for multiple comparisons.
Fluorescence Microscopy.
Embryos less than 45 min old were collected from 3-day-old females and dechorionated in 50% bleach (0.352 M NaClO) for 5 min, shaking periodically, followed by rinses in cold Drosophila saline (0.11 mM NaCl/1.8 mM KCl/2.3 mM NaHCO3/1.0 mM CaCl2/0.055 mM NaH2PO4) until a bleach odor could no longer be detected. Embryos were then exposed to equal parts fixative (4% formaldehyde in PBS) and heptane for 20 min, shaking vigorously every 5 min. All fixative was then removed, and the eggs were washed with fresh heptane. Devitellinization was achieved by adding an equal volume of 100% methanol to the heptane followed by shaking for 1 min. All heptane was removed, and the eggs were washed with 100% methanol. Eggs were washed three times, 5 min each with PBS containing 0.25% BSA and 0.4% Triton-X (PBT), and then exposed to a blocking agent [normal goat serum (NGS) diluted in PBT] for 1 h at room temperature. After removing PBT–NGS, the embryos were incubated in primary antibody (Hsp60, LK2; from Sigma) (2) overnight at 4°C. The primary antibody was removed with four 10-min rinses in PBT. Eggs were incubated in mouse FITC-conjugated anti-IgG for 3 h at room temperature. The secondary antibody was removed by two washes in PBT, 10 min each. Eggs were then stained with a 1 μg/ml 4′,6-diamidino-2-phenylindole (DAPI) solution in 80% glycerol for 5 min. After four 10-min rinses in PBS, the embryos were removed to an 80% glycerol solution. After slide mounting, embryos were examined by using a Zeiss Axioskop FS microscope equipped with epifluorescence optics. Images were recorded with a Cohu gray-scale charge-coupled device camera, digitized with a Scion framegrabber under control of SCION IMAGE 1.62, and compiled by using PHOTOSHOP version 5.5.
Lifespan Measurements.
For each strain included in the analysis (Dsim wRi, Dsim wRiT, Dsim wMelPop, Dsim wMelPopT, Dmel wMelPop, and Dmel wMelPopT), four vials of 20 flies each of virgin males and females (24, 25) (total 80 flies per strain for each gender treatment) were reared at 26°C with a relative humidity of 40% in standard yeast-inoculated food vials. Each day we recorded the number of new deaths. Flies were moved to fresh food vials every 3 days. When all flies in all lines were dead, survival curves of the various treatment groups were compared with a Cox Proportional Hazard regression model of survival analysis (STATVIEW 5.01) followed by Z tests for individual comparisons.
Results
CI Characteristics of wMelPop Infection in D. simulans.
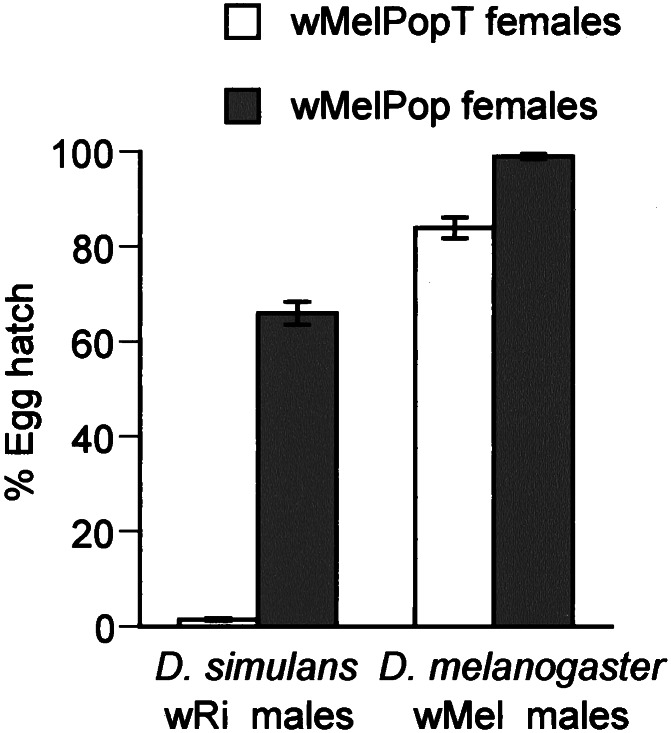
The initial characterization of wMelPop (based on matings to 3-day-old males) revealed that it was incapable of modifying sperm in a D. melanogaster background and as such could be considered a mod− strain (15). After transinfection into D. simulans, however, the association gained the ability to cause CI, indicating that sperm modification was host dependent (11). We also wished to compare the ability of the infection in the two hosts to rescue sperm defects induced by mod+ Wolbachia variants carried by the same host species (Fig. 1). Dsim wMelPop females at generation 20 mated to Dsim wRi (strong CI inducer) males produced embryos with a mean hatch rate of 65.93% (SE ± 2.39%; n = 50), which was significantly greater than nonrescuing uninfected controls (0.49% ± 0.29%; n = 50, P < 0.01). D. melanogaster (yw67C23) males infected with wMel induced only moderate CI expression (83.9% ± 2.2%; n = 50), which was fully rescued by wMelPop-infected females (98.9% ± 0.53%, n = 50). As such, it appears that the wMelPop infection behaves as a mod−, resc+ strain in a D. melanogaster background and as a mod+, resc+ strain in a D. simulans host background.
Figure 1.
Ability of the wMelPop infection to rescue CI. Mean percentage egg hatch (±SE, n = 50 per cross) from wMelPop and wMelPopT females in both D. simulans and D. melanogaster hosts mated to Dsim wRi or Dmel wMel, respectively. White columns indicate hatch rates from crosses involving uninfected females that cannot rescue sperm modification, and gray columns represent crosses involving wMelPop-infected females which show partial rescue of the wRi infection and complete rescue of the wMel infection.
wMelPop Replication in D. simulans.
Ovaries.
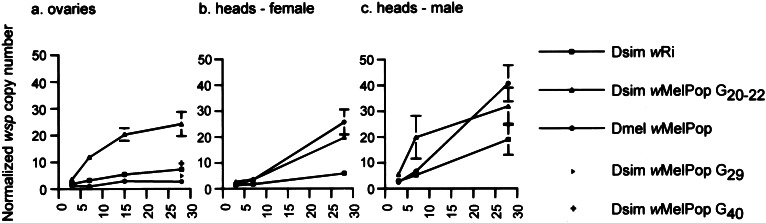
All measurements of Wolbachia densities in ovary tissue for the transinfected line were taken between G20–22 from four ages of adults, and again at G29 for 28-day-old flies only (Fig. 2a). Ovary density estimates from Dsim wRi and Dmel wMelPop were comparatively low and increased very little during the lifespan of the fly. In contrast, Wolbachia densities (as determined by wsp gene relative copy number) in the ovaries of Dsim wMelPop rose rapidly with age, and by 28 days were roughly 5-fold greater (24.2 ± 4.5 copies per cell; n = 10) on average than either Dsim wRi (7.37 ± 1.6; n = 10) or Dmel wMelPop (2.7 ± 0.54; n = 10). Fluorescent microscopy using both an anti-Hsp60 antibody (2) and the DNA-specific dye (DAPI) (11, 26, 27) corroborate the data collected from the real-time quantitative PCR assay. The transinfected line shows much higher densities of Wolbachia at G20 in comparison to Dsim wRi (Fig. 3). By G29, densities of wMelPop in the ovaries were greatly reduced (2.4 ± 0.54; n = 8) to a level that was no longer greater than densities associated with the wRi infection (P > 0.05) indicating a rapid attenuation of replication rate. At G40 we again assayed 28-day-old females for wMelPop densities in ovaries, and found that they had risen slightly (8.4 ± 0.21; n = 12) compared with G29 (P < 0.05), but were still well below mean densities found at G20 (P < 0.01).
Figure 2.
Mean Wolbachia densities in ovaries and heads (±SE, n = 10 per each point) as determined by real-time quantitative PCR for three lines of infected flies of various ages plus a set of measurements for both G29 and G40 Dsim wMelPop at 28 days of age. Measurements are of ovaries dissected from virgin females.
Figure 3.
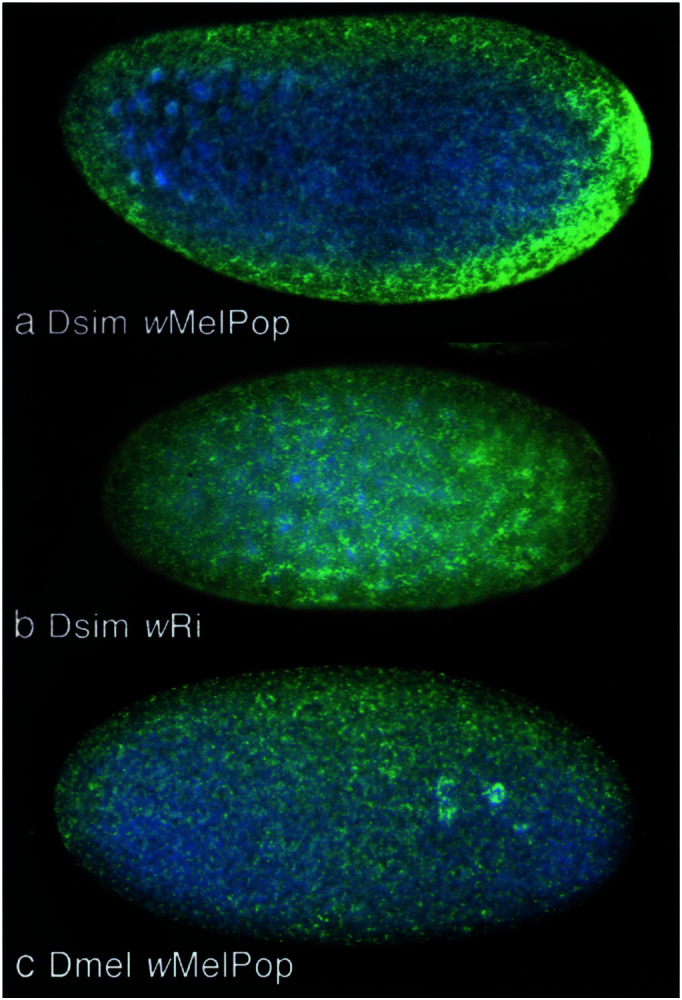
Wolbachia distribution in embryos from 3-day-old infected females. Embryos were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue) and Hsp60 immunostaining (green). (a) D. simulans embryos infected with wMelPop. (b) D. simulans infected with wRi. (c) D. melanogaster infected with wMelPop. In all cases Wolbachia are distributed throughout the periphery of the embryo. Densities appear the highest in Dsim wMelPop embryos.
Heads.
Wolbachia densities in heads were similar in young flies for all three lines compared, Dsim wRi, Dsim wMelPop, and Dmel wMelPop (Fig. 2 b and c). All infections increased in density with age of the fly, particularly wMelPop. We focused on comparisons between heads collected from older flies because they are more relevant to the early death phenotype. Our primary interest was to determine whether the density of wMelPop was higher in D. simulans G20 (females: 19.7 ± 2.0, n = 10; males: 29.5 ± 5.8, n = 18) than in D. melanogaster (females: 25.6 ± 4.8, n = 14; males: 40.7 ± 7.0, n = 10). For females, we determined that the densities of wMelPop were not different in the two hosts (P > 0.05), but that the density of wMelPop in both hosts was significantly higher (P < 0.01) than Dsim wRi (5.8 ± 0.60, n = 10). The pattern was the same for densities in males, with no differences between the wMelPop infections in D. simulans and D. melanogaster (P > 0.05), but wMelPop having significantly higher densities than wRi (15.5 ± 5.6; n = 12; P < 0.01). Variability in our density measurements was, in general, greatest for the wMelPop infection, most especially for male heads.
Fitness Costs Associated with wMelPop Infection.
Fecundity and hatch rate.
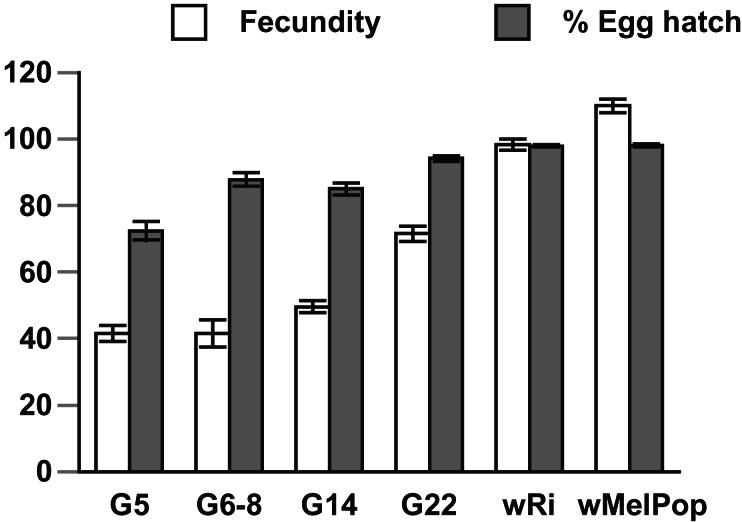
Because Wolbachia are maternally transmitted, bacterial densities in the ovaries have direct bearing on transmission efficiencies. Higher densities of Wolbachia in the ovaries that favor greater transmission may also confer fitness costs. We estimated the effects of the popcorn infection on the reproductive fitness of females in both hosts by measuring fecundity and hatch rates. The same measures were taken for D. simulans infected with the wRi infection as well as uninfected D. simulans and D. melanogaster lines for comparison (Fig. 4). Surprisingly, wMelPop in its native D. melanogaster has no effect on female reproductive fitness, with mean egg hatch rate (98.2 ± 0.5%, n = 75) and fecundity (114.1 ± 2.0 eggs in 3 days, n = 75) indistinguishable from uninfected controls (data not shown). The wRi infection demonstrated mild, but significant, reductions (P < 0.01) in both egg hatch (92.6%, ± 1.0%, n = 75) and in fecundity (96.0 ± 3.3, n = 75) when compared with uninfected D. simulans (98.0% ± 0.5, n = 75; and 110.0 ± 2.0, n = 75, respectively). Reduced fecundity in laboratory populations of Dsim wRi has been reported (28). Egg hatch rates for the transinfected line measured at G5 were much reduced (72.4% ± 2.8%, n = 27), but by G22 they had risen (P < 0.01) to 94.1% (± 0.77%, n = 75) (Fig. 4), with the most significant increase occurring immediately after G5 (P < 0.05). Fecundity of Dsim wMelPop was also significantly (P < 0.01) lower at G5 (41.5 ± 2.3%, n = 27), less than half that of uninfected controls (98.3 ± 2.5%, n = 75). The effect lessened most significantly between G14 and G22 (P < 0.05), but had not disappeared by G22 (71.5 ± 2.2%, n = 75), with fecundities still below controls (P < 0.05).
Figure 4.
Mean egg hatch (%) and fecundities (number of eggs in first 3 days) (±SE) assayed by mating females of each line to uninfected males. Data are presented for several generations after transinfection in Dsim wMelPop, Dsim wRi, and Dmel wMelPop.
The tetracycline-treated transinfected control line (Dsim wMelPopT) showed normal levels of hatch rate and fecundity, which indicated that the observed reductions in Dsim wMelPop were not host effects associated with either inbreeding or genetic drift. The reduced fecundity in the transinfected line could also have been explained by delayed egg production, and therefore we compared productivity from G14 Dsim wMelPop and Dsim wRi lines. Mean productivity for the transinfected line (130.0 ± 60.3, n = 10) was significantly lower (P < 0.01) than Dsim wRi (337.6 ± 20.6, n = 10). The disparity in numbers could be explained by a decline in egg production of the transinfected line to near zero after only 5 days of collection.
Maternal transmission.
We hypothesized that the higher Wolbachia densities in the ovaries of Dsim wMelPop might translate into greater maternal transmission efficiencies. Modeling by Turelli (29) has suggested that significant reductions in host fitness may be stable in populations if they are linked to gains in Wolbachia transmission and high CI expression. The transinfected line demonstrated 100% transmission efficiency as assayed by PCR at generations 15 and 22. Both Dsim wRi and Dmel wMelPop also displayed perfect transmission. Although our results are preliminary, given the small sample sizes, it is clear that a comparison of the three lines that levels of popcorn Wolbachia in the ovaries are in excess of what is required for perfect transmission in the laboratory.
Longevity.
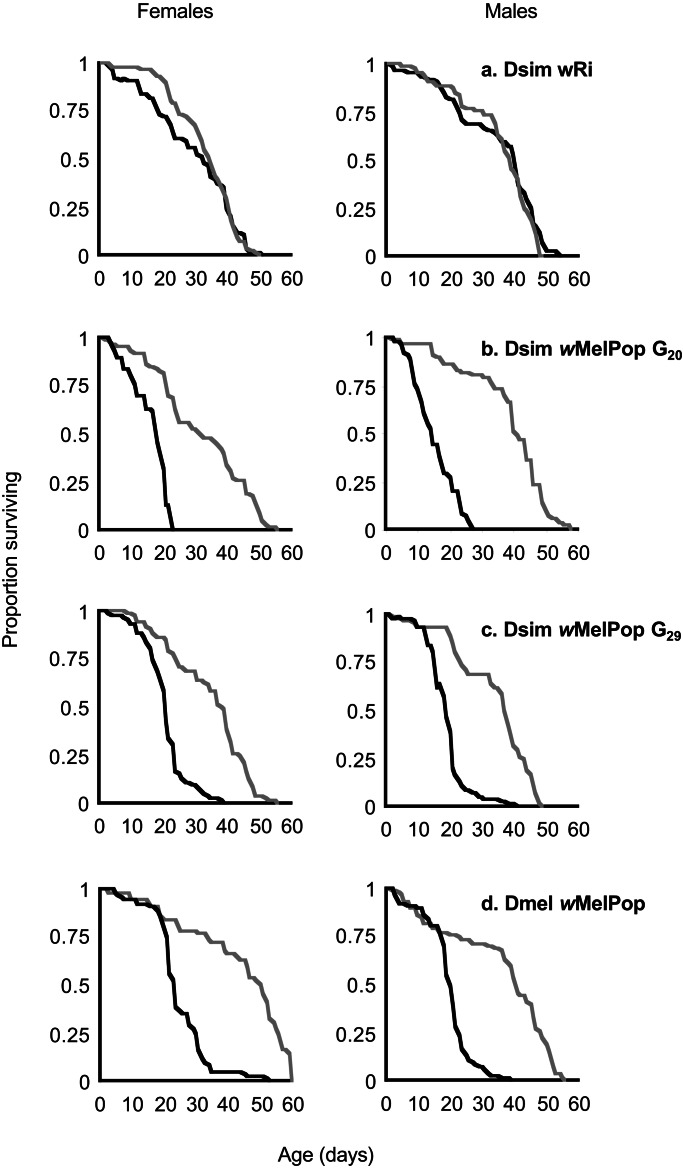
The effect of the wMelPop infection on lifespan of D. simulans was tested at G20 and G29 post transinfection (Fig. 5). For both experiments, survival of Dsim wRi and Dmel wMelPop populations was tracked simultaneously for comparison, as well as all uninfected counterpart strains. Survival curves for males and females of each treatment group were measured independently. The Dsim wMelPop line demonstrated a reduced lifespan in comparison with both uninfected controls (Fig. 5b) and to Dsim wRi (Fig. 5a), confirming the dominant role of the wMelPop genome in the phenotypic expression of shortened host lifespan. The proportional hazard of death caused by wMelPop infection for D. simulans at G20 versus the nonvirulent wRi infection (Fig. 5 a vs. b) was significantly greater for both females (Z = 25.3, P < 0.01) and males (Z = 16.2, P < 0.01), with relative risk ratios of 6.42 (95% confidence interval, 5.76–7.08) and 11.35 (95% confidence interval, 11.05–11.65), respectively. The risk ratio demonstrates the degree to which either variable (in this case infection type) causes a greater hazard of dying. Were the wMelPop and wRi infections associated with identical death risk in males and females, their relative risk ratios would have been 1.0. The effects of early death in the new host compared with the native host were no more extreme for males at either G20 (Fig 5 b vs. d) or G29 (Fig. 5 c vs. d) (Z = 1.0 and 0.25, P > 0.05). Females at G20 did demonstrate a more extreme reduction in lifespan (Z = 17.9, P < 0.01) with a relative risk ratio 4.95 times as great (95% confidence interval, 4.39–5.51) (Fig. 5 b vs. d). The effect had nearly disappeared but was still significant by G29 (Z = 2.3, P < 0.05) with a relative risk ratio of 1.18 (95% confidence interval, 0.64–1.7) (Fig. 5 c vs. d). The latter result indicates that transinfection initially led to heightened virulence in females but over multiple generations this effect attenuated back to levels observed in its natural host. Comparisons of death hazards for Dsim wMelPop G20 versus G29 (Fig. 5 b vs. c) demonstrated a significant difference for females only (Z = 8.9, P < 0.01). The relative risk ratio was 2.23 (95% confidence interval, 1.68–2.81). Mean time to death lengthened from 14.8 days at G20 to 20.8 days at G29. For males the mean time to death at G20 was 15.0 days, and for G29 it was 18.3 days. This differential was not large enough to produce a significant difference (Z = 0.84, P > 0.05) in relative risk (1.06, 95% confidence interval, 0.55–1.57). For both genders, survival curves at G29 appear less steep with an increasing variability in survival time, with a subset of flies living longer.
Figure 5.
Survival curves of populations of female and male flies of various Wolbachia strain/host species combinations. Black lines represent infected flies and gray lines represent uninfected tetracycline-treated counterparts.
Discussion
A comparison of the CI expression of wMelPop in D. simulans and D. melanogaster indicates the complexity of host and bacterial genotypic interactions that determine expression of reproductive phenotypes. Previously we reported that wMelPop could not modify sperm in D. melanogaster, but gained that ability in a D. simulans host (11). The cause appeared to be differential tissue tropism in the two hosts. In D. simulans, the bacterium localized to both testes sheath cells and developing sperm bundles, whereas in D. melanogaster, wMelPop was found only in the sheath cells. The strain's ability to rescue was more stable, expressing itself in two host backgrounds and in response to modification by two different infections. This stability is likely to be a byproduct of the need for Wolbachia strains to maintain themselves in the female germ line to be transovarially transmitted. This observation, in turn, places them in the correct cellular compartment for CI rescue. The same restriction is not placed on testes infections because the male germ line is not directly involved in vertical transmission.
The ability of the wMelPop infection to completely rescue the wMel infection is not unexpected, given that wMelPop is very closely related (they share identical wsp sequences; ref. 11). The incomplete rescue of the wRi infection is in keeping with previous estimates of hatch rates from Dsim wMel × Dsim wRi crosses (10). Both results indicate the dominant role of bacterial genotype in determination of CI crossing type.
wMelPop densities in ovaries of D. melanogaster were surprisingly low and constant throughout the host's lifespan. We expected the gonads to be a site of over-replication and to experience increasing densities with age as observed by Min and Benzer in other tissues (15). But if Wolbachia density correlates with reduced fitness, then lower titers explain the complete lack of fecundity and hatch rate reductions in D. melanogaster. In contrast, the same infection transinfected into D. simulans initially infects ovaries at much higher densities and in a manner consistent with the observed reductions in reproductive fitness. The attenuation of such fitness costs could be explained by parallel reductions in Wolbachia replication rates in the ovaries. Comparisons of maternal transmission rates for the infections indicate that the low ovary densities in the first few days of egg production are sufficient to secure perfect transmission in the laboratory.
Patterns of bacterial densities in heads for the different strain/host combinations did not parallel those for the ovaries. The wMelPop infection increased in density with age, regardless of the host species, and was clearly maintained at higher densities than wRi. The simultaneous increases in variability associated with wMelPop may be an inherent property of such high titers or may reflect a recently established association with low selection pressure for optimal bacterial densities in nervous tissue (30).
The host-dependent differences in both wMelPop ovary densities and reproductive fitness costs can be explained by two hypotheses, which may not be mutually exclusive. The first is that there may be intrinsic host-species-specific differences in control of wMelPop replication. The differences in tissue tropism of wMelPop in the two hosts (11) and the observation that Wolbachia densities are generally higher in D. simulans than in D. melanogaster (8) suggest this is quite likely. The second explanation is that the older Dmel wMelPop association has coevolved to a point where the replication rate in the ovaries has been reduced to where it does not induce reproductive fitness costs, but is still high enough to guarantee perfect transmission. Such endpoints are predictable in a host–parasite system that relies on vertical transmission (31). The rapid attenuation of fecundity and hatch rate in association with reductions in Wolbachia density demonstrate that the establishment of the Wolbachia symbiosis is an active process. This finding is in contrast to the image of Wolbachia as a stealthy manipulator of the host implied by the results of transfer experiments that show little host involvement in phenotypic outcomes (32) or by what appears to be the lack of any interaction between Wolbachia and the innate immune system of the host (33).
The reduction of adult lifespan observed in the novel host indicates the strong involvement of the wMelPop genome in expression of the early death phenotype. In contrast to fecundity and egg hatch, the reduced lifespan phenotype did not attenuate. This reduced attenuation may be explained by a number of reasons. First, the level of selection on adult lifespan may be negligible under most laboratory rearing conditions, in which flies are typically maintained at low temperatures and passaged when young. Second, the characteristics of the wMelPop genome that confer loss of replication control in nervous tissue may not be easily reversible by compensatory or back mutations. Interestingly, the progressive attenuation of virulence in ovaries compared with maintenance of virulence in nervous tissue suggests that there are tissue-specific controls on Wolbachia replication. Again these results point to the complexity of the interaction between Wolbachia and the host. It should be possible in the future to determine the loci involved in replication control now that complete genome sequence is available for both the Drosophila host and the Wolbachia strain that infects it.
It has been proposed that Wolbachia-induced longevity reductions might be able to be used as a form of vector-borne disease control because old insects are responsible for the vast majority of disease transmission (34). Such a scenario would rely on a Wolbachia strain that is capable of inducing strong CI as well as reducing the adult lifespan of its host. The behavior of wMelPop in D. simulans indicates that this strain might be able to be used in transinfections of mosquitoes or other disease vectors because the strain is capable of inducing both strong CI expression and life shortening in a novel host. Moreover, the reproductive costs associated with the infection quickly attenuated, but the life-shortening phenotype appeared stable under laboratory conditions. Alternatively, understanding the genetic basis of longevity reduction by wMelPop might allow for the future manipulation of natural mosquito/Wolbachia associations so as to produce the same phenotype.
Acknowledgments
We thank John Brownstein for his assistance with measuring fitness characteristics, Ros Schumacher for help with the fluorescence microscopy, and Oleg Kruglov for aid in Drosophila care and rearing. This work was supported by National Institutes of Health Grant AI40620 (to S.L.O.), University of Queensland Grant 01/UQESEG009 (to D.J.M.), and National Science Foundation Postdoctoral Fellowship 0074396 (to E.A.M.).
Abbreviation
- CI
cytoplasmic incompatibility
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dobson S L, Bourtzis K, Braig H R, Jones B F, Zhou W, Rousset F, O'Neill S L. Insect Biochem Mol Biol. 1999;29:153–160. doi: 10.1016/s0965-1748(98)00119-2. [DOI] [PubMed] [Google Scholar]
- 2.Taylor M J, Hoerauf A. Parasitol Today. 1999;15:437–442. doi: 10.1016/s0169-4758(99)01533-1. [DOI] [PubMed] [Google Scholar]
- 3.O'Neill S L, Hoffmann A A, Werren J H, editors. Influential Passengers: Inherited Microorganisms and Invertebrate Reproduction. Oxford: Oxford Univ. Press; 1997. [Google Scholar]
- 4.Hoffmann A A, Turelli M. In: Influential Passengers: inherited Microorganisms and Invertebrate Reproduction. O'Neill S L, Hoffmann A A, Werren J H, editors. Oxford: Oxford Univ. Press; 1997. pp. 42–80. [Google Scholar]
- 5.Callaini G, Dallai R, Riparbelli M G. J Cell Sci. 1997;110:271–280. doi: 10.1242/jcs.110.2.271. [DOI] [PubMed] [Google Scholar]
- 6.Lassy C W, Karr T L. Mech Dev. 1996;57:47–58. doi: 10.1016/0925-4773(96)00527-8. [DOI] [PubMed] [Google Scholar]
- 7.Turelli M, Hoffmann A A. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyle L, O'Neill S L, Robertson H M, Karr T L. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 9.Perrot-Minnot M J, Werren J H. J Evol Biol. 1999;12:272–282. [Google Scholar]
- 10.Poinsot D, Bourtzis K, Markakis G, Savakis C, Mercot H. Genetics. 1998;150:227–237. doi: 10.1093/genetics/150.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGraw E A, Merritt D J, Droller J N, O'Neill S L. Proc R Soc London Ser B. 2001;268:2565–2570. doi: 10.1098/rspb.2001.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poinsot D, Mercot H. Evolution (Lawrence, Kans) 1997;51:180–186. doi: 10.1111/j.1558-5646.1997.tb02399.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoerauf A, Nissen-Pahle K, Schmetz C, Henkle-Duhrsen K, Blaxter M L, Buttner D W, Gallin M Y, Al-Qaoud K M, Lucius R, Fleischer B. J Clin Invest. 1999;103:11–18. doi: 10.1172/JCI4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dedeine F, Vavre F, Fleury F, Loppin B, Hochberg M E, Bouletreau M. Proc Natl Acad Sci USA. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min K, Benzer S. Proc Natl Acad Sci USA. 1997;94:10792–10796. doi: 10.1073/pnas.94.20.10792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann A A, Turelli M, Simmons G M. Evolution (Lawrence, Kans) 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 17.Ashburner M. Drosophila: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 18.Sinkins S P, Braig H R, O'Neill S L. Proc R Soc London Ser B. 1995;261:325–330. doi: 10.1098/rspb.1995.0154. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann A A, Turelli M. Genetics. 1988;119:435–444. doi: 10.1093/genetics/119.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neill S L, Giordano R, Colbert A M E, Karr T L, Robertson H M. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braig H R, Zhou W, Dobson S L, O'Neill S L. J Bacteriol. 1998;180:2373–2378. doi: 10.1128/jb.180.9.2373-2378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sokal R R, Rohlf F J. Biometry. New York: Freeman; 1995. [Google Scholar]
- 23.Tyagi S, Kramer F R. Nat Biotechnol. 1996;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- 24.Partridge L, Andrews R. J. Insect Physiol. 1985. , 393–395. [Google Scholar]
- 25.Partridge L, Fowler K, Trevitt S, Sharp W. J Insect Physiol. 1986;32:925–930. [Google Scholar]
- 26.Bressac C, Rousset F. J Invert Pathol. 1993;61:226–230. doi: 10.1006/jipa.1993.1044. [DOI] [PubMed] [Google Scholar]
- 27.O'Neill S L, Karr T L. Nature (London) 1990;348:178–180. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann A A, Turelli M, Harshman L G. Genetics. 1990;126:933–948. doi: 10.1093/genetics/126.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turelli M. Evolution (Lawrence, Kans) 1994;48:1500–1513. doi: 10.1111/j.1558-5646.1994.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 30.Ebert D. Science. 1994;265:1084–1086. doi: 10.1126/science.265.5175.1084. [DOI] [PubMed] [Google Scholar]
- 31.Anderson R M, May R M. Parasitology. 1982;85:411–426. doi: 10.1017/s0031182000055360. [DOI] [PubMed] [Google Scholar]
- 32.Giordano R, O'Neill S L, Robertson H M. Genetics. 1995;140:1307–1317. doi: 10.1093/genetics/140.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bourtzis K, Pettigrew M M, O'Neill S L. Insect Mol Biol. 2000;9:635–639. doi: 10.1046/j.1365-2583.2000.00224.x. [DOI] [PubMed] [Google Scholar]
- 34.Sinkins S P, O'Neill S L. In: Insect Transgenesis Methods and Applications. Handler A M, James A A, editors. New York: CRC Press; 2000. [Google Scholar]