Abstract
Introduction
Patients receiving the standard prophylaxis dose of colchicine for gout flares are at increased risk for developing toxicity if there are pre-existing renal impairment or drug–drug interactions. Guidelines recommend exercising caution, deferring dose adjustment to the clinician’s discretion.
Methods
Pharmacokinetic study data for colchicine oral solution in healthy subjects was used to build a pharmacokinetic model. Using the derived pharmacokinetic disposition parameters from the best fit model and the derived parameters of clearance in patients with renal impairment, simulation of colchicine plasma levels to target 0.5–3 ng/mL with colchicine oral solution was undertaken for various dose levels in different degrees of renal impairment.
Results
With the standard colchicine 0.6 mg daily dose, plasma levels are expected to be therapeutic in patients with mild renal impairment (estimated glomerular filtration rate [eGFR] 60–89 mL/min/1.73 m2). However, with this same 0.6 mg daily dose, patients with moderate renal impairment (eGFR 30–59 mL/min/1.73 m2) and severe renal impairment (eGFR of 15–29 mL/min/1.73 m2) would have excursions up to 10% and 36%, respectively, above the maximum tolerated level. Administering a lower dose such as 0.3 mg daily by splitting the conventional 0.6 mg tablet or by administering 0.6 mg once every-other-day (QOD) in moderate renal impairment would result in plasma colchicine levels in subtherapeutic range (< 0.5 ng/mL) for 20–70% of the dosing interval.
Conclusion
Analysis of pharmacokinetic model data confirms that the majority of patients with renal impairment taking colchicine solid dosage formulations will be above or below therapeutic levels, exposing them to potential side effects. However, more precise dosing with colchicine oral solution of 0.48 mg (4 mL) or 0.5 mg tablet available in certain countries for moderate renal impairment and 0.3 mg (2.5 mL) for severe renal impairment are associated with optimal levels and safer for patients with renal impairment. No dosage adjustment is needed for patients with mild renal impairment.
Keywords: Colchicine, Glomerular filtration rate, Gout, Narrow therapeutic index, Oral solution, Renal impairment, Toxicity
Plain Language Summary
Patients taking colchicine for gout may have troubling diarrhea, nausea, and vomiting side effects if their kidneys do not work well. The goal of this analysis is to figure out a reasonable way to decrease the colchicine dose to avoid these issues. We evaluated this in two phases. First, we used data from a study of colchicine oral solution in healthy adults to create a model to understand how the drug moves through the body. Then we adjusted the model for different levels of kidney function impairment. We used this model to simulate what each dosage schedule of colchicine is expected to do. Patients with mild kidney damage can take the standard 0.6 mg dose of colchicine once a day. However, this standard 0.6 mg dose will likely cause side effects in patients with moderate or severe kidney damage. Patients with moderate kidney damage should take only 0.48 mg (4 mL) of colchicine oral solution or 0.5 mg tablet (available in certain countries) once a day. Patients with severe kidney damage should take only 0.3 mg (2.5 mL) of colchicine oral solution once a day. These reduced doses can easily be taken using the liquid formulation of colchicine, rather than the tablet or capsule. We acknowledge that medication adherence is a challenge in patients with gout (Fontanet et al. BMJ Open 11:e055930, 2021), and the use of an oral solution may provide an additional barrier; nonetheless, providing an appropriate dose that is tolerable may help mitigate this issue.
Key Summary Points
| Why carry out this study? |
| Among the 12.1 million US adults afflicted with gout, over 70% of patients have renal impairment stage 2 or worse. |
| Gastrointestinal side effects are often the first sign of colchicine toxicity, as colchicine has a narrow therapeutic index of 0.5–3 ng/mL. |
| Simulations from a pharmacokinetic model developed based on oral solution data were used to determine appropriate dosage adjustments for colchicine oral solution to avoid the risk of toxicity in patients with gout and renal impairment. |
| What was learned from the study? |
| Decrease the colchicine dose to 0.48 mg (4 mL) oral solution or 0.5 mg tablet (where available) daily for patients with moderate renal impairment, and to 0.3 mg (2.5 mL) daily for patients with severe renal impairment. |
| Colchicine dosage adjustments are precisely achievable using the oral solution. |
Introduction
Gout is the most common inflammatory arthritis, afflicting 5% of US adults representing 12.1 million people, and its incidence has been increasing over the past 40 years [1]. Gout is highly prevalent in individuals with pre-existing chronic kidney disease (CKD) [2]. About 70% of adults with gout have CKD stage ≥ 2, 20–24% have CKD stage ≥ 3, and 24% develop nephrolithiasis [2]. The prevalence of CKD amongst individuals with gout increases with increasing serum urate (sU) levels [3]. Thus, the risk of gout increases with decreasing renal function, including end-stage renal disease (ESRD) [4].
Treatment of gout focuses on two different aspects of disease management [5]. Whereas the first involves treating the pain and inflammation of flares, the second involves lowering sU levels to prevent future flares [5]. Urate lowering therapy (ULT) with either allopurinol, febuxostat, or probenecid is recommended by the American College of Rheumatology (ACR) for patients with gout [5]. Colchicine is recommended by ACR guidelines as a first-line anti-inflammatory prophylaxis agent for patients with gout when ULT is initiated [5]. Prophylaxis is to be continued for at least 3–6 months, or longer if flares continue [5]. There is an early increase in flares during the initial phase of ULT, which is thought to be due to remodeling of articular urate crystal deposits because of rapid and substantial lowering of ambient urate concentrations [6].
Colchicine binds to tubulin on a single high-affinity site [7]. In blood, colchicine is sequestered in red blood cells and polymorphonuclear leukocytes, in a concentration- and temperature-independent way [8]. After oral administration, colchicine is rapidly absorbed, with a bioavailability of up to 50% [9]. It undergoes metabolism by the liver, primarily by cytochrome P450 3A4 (CYP3A4) and P‑glycoprotein (PgP) and its metabolites are excreted by renal and biliary-intestinal routes [10, 11]. Up to 20% of the active drug is excreted by the kidneys [9]. Colchicine’s clearance is significantly reduced in patients with renal or hepatic insufficiency, and the drug may accumulate in cells, with resultant toxicity [10]. Colchicine-induced toxicity has been observed when the drug was used for acute treatment, as well as for chronic prophylaxis of gout in patients with CKD [11]. Dosing adjustment based on estimated glomerular filtration rate (eGFR) is often required in individuals with CKD, although there is no specific evidence-based guidance in monitoring the efficacy and safety of the treatment used.
Colchicine has a low therapeutic index [12]. The effective steady state plasma concentrations after acute colchicine treatment are 0.5–3 ng/mL, with toxic effects potentially occurring at or above a level of approximately 3 ng/mL [12]. The most commonly observed side effects with colchicine are gastrointestinal (GI) in nature (e.g., diarrhea, nausea, vomiting, abdominal pain) and are dose-related, often appearing as the first signs of toxicity [13, 14]. Rarer side effects that have been reported with colchicine include neurological, hematological, hepatobiliary, musculoskeletal, and reproduction. These have been generally reversible upon treatment interruption or lowering of colchicine dose [15]. A recent meta-analysis of randomized controlled colchicine trials found that gastrointestinal events were reported in 17.6% of colchicine users, but did not detect an increase in the rate of other adverse events, including liver, muscle, hematology, sensory, or infectious events, though this is likely due to the study’s inability to detect these rarer events [16].
The objective of this analysis is to utilize simulations from a pharmacokinetic model developed based on the plasma concentration data of a single dose colchicine oral solution to determine appropriate dosage adjustments of colchicine in patients with gout and reduced colchicine clearance due to renal impairment.
Methods
A randomized single-dose bioavailability and food effect study with colchicine oral solution vs. probenecid/colchicine tablet, which was submitted for US Food and Drug Administration (FDA) approval of colchicine oral solution for prophylaxis of gout flares, was used as the source data [17]. The mean plasma colchicine concentration data of oral solution fasted treatment data from the study was chosen to determine the disposition characteristics of the drug in the body.
To create a pharmacokinetic model for drug plasma concentration data, we relied on standard PK models based on compartmental modeling, which involves the drug distribution across one or more compartments.
Mean observed plasma concentration data were fitted to several models (with and without lag time) using nonlinear regression techniques (e.g., least square fitting to minimize the difference between predicted and observed drug concentrations over time). This was achieved by using Phoenix 64® WinNonlin® software version 8.1 (Certara, USA). The best fit to the colchicine oral solution data was obtained using a two-compartment oral pharmacokinetic model with lag time (Fig. 1). In this model, colchicine is distributed in both the central and the peripheral compartments after oral administration with a lag time and first-order intercompartmental transfer from the central to the peripheral compartment as well as the reverse. Both renal and non-renal clearance occurred from the central compartment.
Fig. 1.
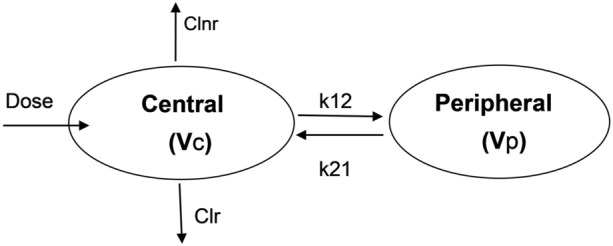
A schematic representation of the colchicine oral solution pharmacokinetic model. Clnr nonrenal clearance, Clr renal clearance, k12 first-order intercompartmental transfer constant from the central to the peripheral compartment, k21 first order intercompartmental transfer constant from the peripheral to the central compartment, Vc volume of distribution of the central compartment, Vp volume of distribution of the peripheral compartment
A renal impairment pharmacokinetic study with colchicine showed that exposure of colchicine (peak concentration and area under the curve) increased by 1.5-fold and twofold with increased severity of renal impairment compared to no renal impairment. Also, the apparent oral clearance (CL/F) was decreased by 0.5- to 0.6-fold in moderate and severe renal impairment [18].
Utilizing these findings, the model fit disposition parameters obtained from colchicine solution data in our pharmacokinetic analysis were adjusted for impairment in colchicine clearance (30%, 50%, and 70%) to simulate plasma drug concentration data for mild, moderate, and severe renal impairment, similar to an approach as used by Karatza et al. [19]. The reduction in colchicine clearance may be due to renal and/or hepatic impairment and drug interactions. Colchicine dose regimens were evaluated through simulations intended to be used in patients with several levels of clearance impairment. The simulated colchicine concentrations were assessed in terms of the desired levels to achieve efficacy and avoid toxicity.
This analysis utilized data based on previously conducted studies and did not contain any new studies with human participants or animals performed by any of the authors.
Results
The mean colchicine pharmacokinetic parameters following administration of colchicine oral solution in the fasted state are presented in Table 1.
Table 1.
Mean colchicine pharmacokinetic parameters in healthy, fasting adults [15]
| PK parameters, mean (SD) | Colchicine 0.6 mg oral solution Normal (healthy, fasting adults) N = 34 |
|---|---|
| Cmax (ng/mL) | 2.161 (0.874) |
| T1/2 (h) | 31.00 (6.090) |
| AUCinf (h·ng/mL) | 19.90 (4.736) |
| CL/F (L/h/kg) | 30.15 |
AUCinf area under the concentration vs time curve from time zero to infinity, CL/F apparent oral clearance, Cmax peak concentration, PK pharmacokinetic, SD standard deviation, t1/2 half-life
The pharmacokinetic model that best fitted the mean plasma concentration time profile for colchicine oral solution was a two-compartment model with a lag time. Model appropriateness was based on a few model selection criteria including the measure of goodness of fit using metrics including R2, residual plots, Akaike’s information criterion (AIC), and Schwartz Bayesian criterion (SBC).
The disposition parameters of colchicine derived from the single-dose oral solution data in healthy subjects with normal renal function were then used to estimate the corresponding model parameters for impaired total colchicine clearance (30%, 50%, and 70% representing mild, moderate, and severe renal impairment, respectively). Figure 2 presents the anticipated plasma colchicine concentration versus time profiles in patients with three levels of impaired total clearance (i.e., 30%, 50%, and 70%).
Fig. 2.
Simulation of colchicine plasma concentrations with varying levels of renal impairment. BID twice daily, QD once daily, QOD once every-other-day
For a decrease in clearance of 30% (mild renal impairment), colchicine 0.6 mg once daily (QD) and 0.5 mg QD are expected to be within the therapeutic range for the entire dosing interval with minimal excursion below the lower range once steady state is achieved, whereas, for the 0.6 mg twice daily (BID) dosing, the colchicine concentrations will exceed the upper safety range by approximately 23% (Fig. 2a).
For a decrease in clearance of 50% (moderate renal impairment), colchicine 0.48 mg QD is expected to be within therapeutic range for the entire dosing interval at steady state, while 0.6 mg QD will result in concentrations above the safety range by approximately 10%, and 0.3 mg QD may result in subtherapeutic levels for 30–90% of the dosing interval until steady state is reached with steady state levels consistently in the lower boundary of the therapeutic range (Fig. 2b).
For a decrease in clearance of 70% (severe renal impairment), colchicine 0.3 mg QD is expected to be within therapeutic range for the entire dosing interval at steady state, whereas 0.48 mg and 0.6 mg QD will result in excursions above the safety range by 15–36% (Fig. 2c).
Visual inspection of Fig. 2 reveals that several regimens lead to increased colchicine plasma levels (Fig. 2a, c), while some others (Fig. 2b, c) result in levels that are lower than the minimum concentration of the therapeutic range. With colchicine concentrations exceeding the upper limit of the therapeutic range, there is a higher chance for the induction of toxic effects, whereas concentrations below the range may adversely impact efficacy.
Discussion
In this study, pharmacokinetic simulations were performed to determine what dosing regimens, with colchicine oral solution in different levels of reduced renal clearance, support plasma levels to be within the safe and efficacious therapeutic range (0.5–3 ng/mL). Our analysis shows that in patients with normal to mildly impaired renal function, dosing with the currently recommended colchicine dose of 0.6 mg QD would contain the steady state colchicine plasma concentration within the therapeutic range (Fig. 2a). For patients in the moderate renal impairment group based on simulation from Fig. 2b, a dose of 0.48 mg (4 mL) QD of colchicine oral solution or 0.5 mg of colchicine tablet (in countries where available) will result in steady state concentrations contained within the target range. For patients in the severe renal impairment group, dosing 0.3 mg (2.5 mL) QD of colchicine oral solution seems to be optimal (Fig. 2c) as the steady state levels are contained in the target range, and this concurs with the dosing recommendation on the product label. QOD dosing with 0.6 mg tablet/capsule formulations has been used by clinicians, but QOD dosing may pose adherence challenges to the colchicine regimen in a patient population with already low compliance rates compared to daily dosing [20]. In addition, 0.6 mg QOD regimen will essentially leave patients with moderate renal impairment essentially untreated half the time as their colchicine levels will be below the lower therapeutic range of 0.5 ng/mL in the second half of the dosing interval based on the simulation (Fig. 2b).
Colchicine’s cellular effects depend predominantly on its ability to bind tubulin, particularly within leukocytes [7]. Although the anti-inflammatory effects of colchicine are largely determined by intracellular leukocyte accumulation, and serum concentrations may relate inadequately to efficacy, serum levels are useful to model drug safety which may depend on colchicine actions in cells other than or in addition to leukocytes [21]. At steady state, the safe upper limit of colchicine concentration is generally considered to be 3.0 ng/mL [12]. The plasma half-life of colchicine is prolonged in patients with renal failure, especially in those with combined renal and liver diseases [22]. Patients with renal or liver diseases who take colchicine should be monitored carefully for possible toxic effects of the drug. The ACR guidelines recommend exercising caution and instituting colchicine dose adjustment in renal impairment at the discretion of the treating clinician [6]. Pharmacodynamic studies confirm that doses of 0.5 mg BID and 0.6 mg QD do not sustain serum levels beyond this threshold in healthy individuals, and that doses of 0.5 mg QD do not sustain serum levels beyond this range even in patients with mild to moderate renal impairment or with concomitant use of most interacting medications [13].
Apart from avoiding the use of colchicine with adversely interacting drugs, colchicine dose reductions should be considered in patients with renal or hepatic impairment, and in the elderly. Some recommendations suggest reducing the colchicine dose by 50% in patients with creatinine clearance below 50 mL/min [23]. However, there is a paucity of data and very little guidance regarding colchicine dosing regimens for patients with gout and CKD. In addition, as most studies on colchicine have excluded individuals with CKD, there is insufficient data to inform on the safety and efficacy of colchicine in these patients [24]. In patients with gout and CKD, it is challenging to maintain optimum sU levels, as uric acid is largely excreted renally. As a result of decreased renal function, hyperuricemia is more common in patients with moderate-severe CKD [3]. An eGFR of 60 mL/min/1.73 m2 (the borderline between mild and moderate renal impairment) appears to be a threshold for the dramatic increase in the prevalence of gout, with the risk of gout increasing 10-fold in patients with moderate CKD [3]. Colchicine doses of 0.5 mg or 0.6 mg orally QD or BID are recommended per international and ACR guidelines, respectively, for prophylaxis use in treatment of gout, in subjects with normal renal function, with downward dose adjustments recommended for patients with moderate to severe renal impairment or due to potential drug–drug interactions [5, 6]. Unfortunately, there is a substantial knowledge gap around the safe use and dosing of colchicine in patients with both gout and CKD [25, 26].
Prior to the availability of colchicine oral solution, there existed an unmet need in treatment of gout in patients with CKD (particularly in stage ≥ 3) as non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and other anti-inflammatory drugs are relatively contraindicated for gout prophylaxis in CKD [27, 28]. NSAIDs can negatively impact kidney function and prolonged use is not recommended for patients with eGFR below 60 mL/min/1.73 m2 [26, 27]. Similarly, known risks associated with prolonged use of corticosteroids include effects on glycemic control, weight gain, and cardiovascular adverse effects [28].
Use of solid dosage forms of colchicine may be challenging when dose reductions are needed, such as for renal impairment or drug–drug interactions. Colchicine oral solution offers a convenient choice for patients with CKD and gout as precise dosing is possible. The availability of colchicine oral solution as a liquid formulation allows for precise dosing adjustments using a graduated oral syringe to help balance efficacy with the risk of toxicities.
Study Limitations
Modeling assumptions were made. The level of renal impairment was based on creatinine clearance, which is assumed to be directly proportional to the impairment in colchicine clearance and estimated parameters of clearance for patients with mild, moderate, and severe renal impairment used in simulation. We did not perform analyses on sex and therefore could not determine if there were any differences in men vs. women.
Conclusions
On the basis of the simulations from the pharmacokinetic model, physicians treating patients with gout should consider starting doses of 0.48 mg (4 mL) colchicine oral solution or 0.5 mg tablet (where available) QD in patients with moderate renal function impairment and 0.3 mg (2.5 mL) colchicine oral solution QD in patients with severe renal function impairment. These dosage adjustments are easily and precisely achievable when using colchicine oral solution.
Acknowledgements
The authors gratefully acknowledge and thank Angelo L. Gaffo, MD for his critical review of the manuscript.
Medical Writing/Editorial Assistance
Medical writing assistance was provided by LoAn K. Ho, PharmD, of Forward WE Go, a division of Wesley Enterprise, Inc. (Huntington Beach, CA) based on authors’ input and direction, and in accordance with Good Publication Practice (GPP) 2022 guidelines (https://www.ismpp.org/gpp-2022), which was funded by Scilex Holding Company. Scilex Holding Company reviewed and provided feedback on the manuscript. The authors had full editorial control of the manuscript and provided their final approval of all content.
Author Contributions
Conceptualization: Jaymin Shah, Elaine K. Chan and Dmitri Lissin; Methodology, formal analysis and investigation: Jaymin Shah; Writing—original draft preparation: Jaymin Shah; Writing—revising and critically reviewing the manuscript for important intellectual content: Jaymin Shah, Michael H. Pillinger, Elaine K. Chan, Dmitri Lissin; Funding acquisition, resources, supervision: Dmitri Lissin. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work, and have given their approval for this version to be published.
Funding
Medical writing support and the Rapid Service Fee for this publication was funded by Scilex Holding Company. Pharmacokinetic modeling and analysis was funded by Scilex Holding Company.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Declarations
Conflict of Interest
Jaymin Shah is a consultant to Scilex Holding Company. Michael H. Pillinger is a consultant for Amgen, Fortress Bioscience, Convergence Bio and Federation Bio and has received investigator-initiated grants paid to NYU Grossman School of Medicine from Hikma Pharmaceuticals and Horizon Pharma. Elaine K. Chan and Dmitri Lissin are employees of Scilex Holding Company.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Footnotes
Prior Presentation: Shah J, Chan EK, Lissin D. Prophylaxis of Gout Flares in Patients with Renal Impairment: Dosing Adjustments with Colchicine Oral Solution Informed by a Pharmacokinetic Model. Poster Presentation at American College of Rheumatology (ACR) Convergence, Nov 14–19, 2024, Washington, DC.
References
- 1.Yokose C, McCormick N, Lu N, et al. Trends in prevalence of gout among US Asian adults, 2011–2018. JAMA Netw Open. 2023;6:e239501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu Y, Pandya B, Choi H. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007–2008. Am J Med. 2012;125:679–87. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan E. Reduced glomerular function and prevalence of gout: NHANES 2009–10. PLoS ONE. 2012;7:e50046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen SD, Kimmel PL, Neff R, Agodoa L, Abbott KC. Association of incident gout and mortality in dialysis patients. J Am Soc Nephrol. 2008;19:2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FitzGerald JD, Dalbeth N, Mikuls T, et al. American College of Rheumatology Guideline for the management of gout. Arthritis Rheumatol. 2020;72:879–95. [DOI] [PubMed] [Google Scholar]
- 6.Khanna D, Khanna PP, Fitzgerald JD, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and antiinflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz JF, Andreu JM. Kinetics of dissociation of the tubulin-colchicine complex. J Biol Chem. 1991;266:2890–6. [PubMed] [Google Scholar]
- 8.Chappey ON, Niel E, Wautier J-L, et al. Colchicine disposition in human leukocytes after single and multiple oral administration. Clin Pharmacol Ther. 1993;54:360–7. [DOI] [PubMed] [Google Scholar]
- 9.Achtert G, Scherrmann JM, Christen MO. Pharmacokinetics/bioavailability of colchicine in healthy male volunteers. Eur J Drug Metab Pharmacokinet. 1989;14:317–22. [DOI] [PubMed] [Google Scholar]
- 10.Ben-Chetrit E, Scherrmann JM, Zylber-Katz E, Levy M. Colchicine disposition in patients with familial Mediterranean fever with renal impairment. J Rheumatol. 1994;21:710–3. [PubMed] [Google Scholar]
- 11.Putterman C, Ben-Chetrit E, Caraco Y, Levy M. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum. 1991;21:143–55. [DOI] [PubMed] [Google Scholar]
- 12.Molad Y. Update on colchicine and its mechanism of action. Curr Rheumatol Rep. 2002;4:252–6. [DOI] [PubMed] [Google Scholar]
- 13.Robinson PC, Terkeltaub R, Pillinger MH, et al. Consensus statement regarding the efficacy and safety of long-term low-dose colchicine in gout and cardiovascular disease. Am J Med. 2022;135:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borstad GC, Bryant LR, Abel MP, Scroggie DA, Harris MD, Alloway JA. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429–32. [PubMed] [Google Scholar]
- 15.Colcrys [Package Insert]. Philadelphia, PA; Mutual Pharmaceutical Company, Inc.; 2009.
- 16.Stewart S, Yang K, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2020;22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin J, Vishnupad N, Willett M, et al. Pharmacokinetic study of colchicine oral solution (0.6 mg) and exposure comparison with probenecid/colchicine tablets USP under fasted and fed conditions in healthy adult subjects. Poster Number: 020. Clin Pharmacol Drug Dev. 2020;9(S2):14–5. [Google Scholar]
- 18.Wason S, Mount D, Faulkner R. Single-dose, open-label study of the differences in pharmacokinetics of colchicine in subjects with renal impairment, including end-stage renal disease. Clin Drug Investig. 2014;34:845–55. [DOI] [PubMed] [Google Scholar]
- 19.Karatza E, Ismailos G, Karalis V. Colchicine for the treatment of COVID-19 patients: efficacy, safety, and model informed dosage regimens. Xenobiotica. 2021;51:643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adher. 2013;7:419–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochdi M, Sabouraud A, Girre C, Venet R, Scherrmann JM. Pharmacokinetics and absolute bioavailability of colchicine after i.v. and oral administration in healthy human volunteers and elderly subjects. Eur J Clin Pharmacol. 1994;46(4):351–4. [DOI] [PubMed] [Google Scholar]
- 22.Terkeltaub R, Furst D, Bennett K, Kook K, Crockett RS, Davis WM. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomized, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis Rheum. 2010;62:1060–8. [DOI] [PubMed] [Google Scholar]
- 23.Terkeltaub RA. Colchicine update: 2008. Semin Arthritis Rheum. 2009;38:411–9. [DOI] [PubMed] [Google Scholar]
- 24.Pisaniello HL, Fisher MC, Farquhar H, et al. Efficacy and safety of gout flare prophylaxis and therapy use in people with chronic kidney disease: a Gout, Hyperuricemia and Crystal-Associated Disease Network (G-CAN)-initiated literature review. Arthritis Res Ther. 2021;23:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kannuthurai V, Gaffo A. Management of patients with gout and kidney disease: a review of available therapies and common missteps. Kidney360. 2023;4:e1332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamp LK, Farquhar H, Pisaniello HL, et al. Management of gout in chronic kidney disease: a G-CAN consensus statement on the research priorities. Nat Rev Rheumatol. 2021;17:633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vargas-Santos AB, Neogi T. Management of gout and hyperuricemia in CKD. Am J Kidney Dis. 2017;70:422–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curiel RV, Guzman NJ. Challenges associated with the management of gouty arthritis in patients with chronic kidney disease: a systematic review. Semin Arthritis Rheum. 2012;42:166–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.



