Abstract
Suberoylanilide hydroxamic acid (SAHA) is a hydroxamic acid-containing hybrid polar molecule; SAHA specifically binds to and inhibits the activity of histone deacetylase. Although SAHA, like other inhibitors of histone deacetylase, exhibits antitumor effects by increasing expression of genes regulating tumor survival, we found that SAHA reduces the production of proinflammatory cytokines in vivo and in vitro. A single oral administration of SAHA to mice dose-dependently reduced circulating TNF-α, IL-1-β, IL-6, and IFN-γ induced by lipopolysaccharide (LPS). Administration of SAHA also reduced hepatic cellular injury in mice following i.v. injection of Con A. SAHA inhibited nitric oxide release in mouse macrophages stimulated by the combination of TNF-α plus IFN-γ. Human peripheral blood mononuclear cells stimulated with LPS in the presence of SAHA released less TNF-α, IL-1-β, IL-12, and IFN-γ (50% reduction at 100–200 nM). The production of IFN-γ stimulated by IL-18 plus IL-12 was also inhibited by SAHA (85% at 200 nM). However, SAHA did not affect LPS-induced synthesis of the IL-1-β precursor, the IL-1 receptor antagonist, or the chemokine IL-8. In addition, IFN-γ induced by anti-CD3 was not suppressed by SAHA. Steady-state mRNA levels for LPS-induced TNF-α and IFN-γ in peripheral blood mononuclear cells were markedly decreased, whereas IL-8 and IL-1-β mRNA levels were unaffected. Because SAHA exhibits antiinflammatory properties in vivo and in vitro, inhibitors of histone deacetylase may stimulate the expression of genes that control the synthesis of cytokines and nitric oxide or hyperacetylate other targets.
Suberoylanilide hydroxamic acid (SAHA), like trichostatin, belongs to the class of hydroxamic acid-containing hybrid polar molecules that suppress the proliferation of cancer cells in vitro and reduce the growth of experimental tumors in vivo (1–3). There are ongoing clinical trials of SAHA (3), and patients with cancer have been injected with increasing doses of SAHA (300–600 mg/m2) intravenously.¶ SAHA, trichostatin, and butyrate are inhibitors of nuclear histone deacetylases (HDAC) (3, 5). Inhibitors of HDAC increase the expression of a variety of genes, accounting for their antitumor effects. For example, SAHA increases the expression of genes driving cell cycle, tumor suppression, differentiation, and apoptosis (6, 7). SAHA also binds to S3 protein in the cytosol, a component of the ribosome (8). In addition to suppressing tumor growth, inhibitors of HDAC may affect other intracellular regulatory pathways. Trichostatin, for example, increases viral expression, including that of HIV-1 (9).
In the present study, we assessed the in vivo effect of SAHA on lipopolysaccharide (LPS)-induced circulating cytokines in mice and on the hepatic injury following i.v. Con A. In addition, we studied the in vitro effects of SAHA on NO production from cytokine-stimulated mouse macrophages and gene expression and secretion of cytokines from human peripheral blood mononuclear cells (PBMC).
Materials and Methods
Reagents.
SAHA was synthesized by the Chemical Department of Italfarmaco as described in WO 93/07148 PTC/US92/08454 and WO 95/31977 PTC/US95/06554. After synthesis, the structure of SAHA was confirmed by mass spectrometry and proton NMR spectroscopy. The compound was >99% pure as assessed by HPLC. SAHA was added to water at 10 mg/ml, heated to 95°C until dissolved, and kept at −70°C. RPMI medium1640, ficoll-hypaque, MEM Earle, trypsin, Percoll, penicillin and streptomycin, and DMEM with glucose 4,500 mg/liter were purchased from Biochrom (Berlin), KG (Berlin), or Sigma. Agarose was purchased from Life Technologies (Rockville, MD). FCS was purchased from HyClone. Lipopolysaccharide (LPS, Escherichia coli 055:B5), Griess reagent, ficoll, polyvinylpyrolidone, dextran sulfate, trypsin, thioglycolate broth, Con A, and salmon sperm DNA were from Sigma (or Sigma, Milan). Monoclonal antibody OKT3 was purchased from Ortho Diagnostics. Guanidine isothiocyanate, SDS, formamide, and 2-mercaptoethanol were purchased Bio-Rad, 3H-thymidine and [α-32P]dCTP from Amersham Pharmacia and nick translation kit from Promega. Human recombinant IL-18 was purchased from R & D Systems, and recombinant human IL-12, IFN-γ, TNF-α, IL-8, and IL-1-β as well as recombinant murine IFN-γ were from PeproTech (Rocky Hill, NJ).
Tumor Cell Proliferation Assays.
The human lung carcinoma A549 cell line was a gift of the Mario Negri Institute, Milan, and MDA-MB-435 cells, derived from a human breast carcinoma, were a gift from R. Giavazzi, Mario Negri Institute, Bergamo, Italy. Cell lines were maintained in MEM Earle supplemented with 10% FCS, 100 units/ml penicillin, and 100 μg/ml streptomycin. Following trypsinization, the cells were seeded in 96-well flat-bottom microtiter plates, grown to 40% confluence, and incubated with SAHA. Incorporation of thymidine was assessed after 48 and 72 h.
LPS Injection into Mice.
SAHA was administered by oral gavage (in 200 μl) to BALB/c mice (Charles River Breeding Laboratories, Calco, Italy) or C57Bl6 (The Jackson Laboratory). Vehicle-treated mice received 200 μl of water. After 1 h, mice were injected with LPS (30 mg/kg) i.p. and killed at different time intervals, and serum was obtained. The Animal Investigations Committee of the University of Colorado approved these studies.
Con A-Induced Liver Injury.
C57Bl6 mice were injected i.p. with either water vehicle or SAHA and after 1 h were injected i.v. with Con A as described (10). After 24 h, serum amino-alanine transferase was measured.
Human PBMC Cultures.
The Colorado Multiple Institutional Review Board approved the study. Venous blood was obtained from consenting adults and separated over Ficoll-Hypaque. The PBMC fraction was washed and adjusted to five million cells per ml. Five hundred microliters was aliquoted into each well of 24-well flat-bottom plates, 100 μl of SAHA was added, and the plates were incubated for 1 h at 37°C. The cells were stimulated with LPS, soluble OKT3, or cytokines, and after 24 or 48 h at 37°C the supernatant was removed and frozen for cytokine assays. For IL-12 studies, monocytes were isolated by centrifugation over Percoll, washed, suspended in RPMI with 10% FCS, and aliquoted at 2 million cells per ml in Petriperm Teflon-coated culture dishes (Sigma).
For Northern blot hybridization, the PBMC were added to 50-ml conical tubes at a concentration of 50 million cells in 20 ml of RPMI medium 1640 with 1% FCS and incubated at 37°C with SAHA (100 nM or 25 nM). After 1 h, LPS (10 ng/ml) was added, the cultures incubated for 6 and 24 h at 37°C, and the tubes centrifuged at 350 × g. The supernatant was removed and the cell pellet lysed in 750 μl of 4 M guanidine isothiocyanate/25 mM sodium citrate/0.5% N-lauroylsarcosine/0.1 M 2-mercaptoethanol. RNA was prepared as described (11).
Northern Blot Analysis.
The methods used for Northern blot analysis have been reported (12). The cDNA probes for human TNF-α, IL-1-β, IFN-γ, and IL-8 were obtained from American Type Culture Collection (ATCC): for TNF-α, clone pFC54.t (ATCC no. 53007); for IL-1-β, a 1.047-kb fragment cloned in PBR322 (ATCC no. 39925); for IFN-γ, a 1.1-kb fragment cloned in BamHI site of pSV529 (ATCC no. 39046); for human β-actin, a 2-kb fragment cloned at the EcoRI site of pBluescript SK (ATCC no. 769559); and for IL-8, a 1.2-kb fragment cloned at the EcoRI site of Bluescript (kindly supplied by N. Polentarutti, Mario Negri Institute).
Nitric Oxide Production from Mouse Peritoneal Macrophages.
C57BL/6 mice were injected i.p. with 1 ml of sterile thioglycolate broth and killed after 5 days, and macrophages were isolated using instillation of 10 ml of ice-cold PBS into the peritoneal cavity. The cells were centrifuged (350 × g) and 3 ml of erythrocyte lysing reagent (PharMingen) was added for 10 min. Seven milliliters of DMEM containing 5% FCS was added and the cells were centrifuged at 4°C. The cells were resuspended in DMEM at 1 million per ml and 0.5 ml were added to wells of a 48-well plate. SAHA was added for 60 min at 37°C and then stimulated with the combination of TNF-α plus IFN-γ. After 24 h, NO levels in the supernatant were determined using the Griess reagent as described (13).
Cytokine Assays.
Specific antibodies and cytokine standards for the cytokine electrochemiluminescence (ECL) assays were purchased from R & D Systems and have been reported (14, 15). The Origen 1.5 Analyzer (Igen, Gaithersburg, MD) was used. The ELISA for proIL-1-β (R & D Systems) recognizes less than 10% of mature IL-1-β. The ELISA for human IL-12 (p70) was purchased from Endogen (Woburn, MA).
Results
Effect of SAHA on Tumor Cell Proliferation.
A549 and MDA cells were cultured with increasing concentrations of SAHA and, as shown in Fig. 1, there was a dose-dependent reduction in proliferation. At 5 μM, SAHA reduced proliferation of A549 cells by 50% and at 1 μM a similar reduction was observed in MDA cells. These effective concentrations of SAHA are similar to those reported in a variety of transformed cells (1, 7).
Figure 1.
Anti-proliferative property of SAHA. (A) A549 cells. (B) MA cells. (Mean ± SE, n = 3.)
SAHA Reduces LPS-Induced Circulating Cytokines.
BALB/c mice received increasing doses of SAHA by gavage and after 1 h were injected with LPS i.p. Blood was obtained after 90 min. As shown in Fig. 2, serum TNF-α in saline-injected mice was 250 pg/ml, but in LPS-injected mice TNF-α increased 4-fold to 1 ng/ml. In mice pretreated with 10 mg/kg SAHA, LPS-induced TNF-α decreased to 0.5 ng/ml, which represents a 50% reduction compared with the TNF-α level in mice receiving LPS alone. At 25 mg/kg SAHA, there was no further reduction. In mice pretreated with 10 mg/kg of dexamethasone, TNF-α decreased to the level of saline-injected mice.
Figure 2.
TNF-α levels during endotoxemia. SAHA or water (vehicle) was administered to BALB/c mice by gavage (100 μl). Dexamethasone (dex, 10 mg/kg) was injected i.p. After 1 h, LPS (30 mg/kg) or saline was injected i.p. Serum TNF-α (mean ± SE) was measured after 90 min. n = 10 per group; *, P < 0.05; **, P < 0.01.
Circulating levels of IL-1-β, IL-6, and macrophage inflammatory protein-2 (MIP-2) were also elevated in these 90 min sera. As shown in Table 1, LPS-induced IL-1-β increased from 3.22 pg/ml to 33.9 ± 7.9 (mean pg/ml ± SE) and IL-6 from 16 pg/ml to 21.2 ± 0.9 ng/ml. Similar to TNF-α levels, SAHA pretreatment dose-dependently decreased serum levels of these cytokines. For SAHA at 50 mg/kg, IL-1-β decreased to 18 ± 2.8 pg/ml and IL-6 decreased to 15.1 ± 0.9 ng/ml (P < 0.05 for both cytokines). The chemokine MIP-2 was also elevated at 90 min, but was reduced by only 22% at the highest dose of SAHA (50 mg/kg).
Table 1.
Serum cytokines in SAHA-treated mice after LPS
| Treatment | IL-1-β | % | IL-6 | % | MIP-2 | % |
|---|---|---|---|---|---|---|
| Saline | 3.2 ± 0.7 | NA | 16.0 ± 2 | NA | 52 ± 4.13 | NA |
| Veh + LPS | 33.9 ± 7.9 | 0 | 21,197 ± 921 | 0 | 12,597 ± 770 | NA |
| SAHA + LPS | ||||||
| 0.12 | 29.85 ± 6.9 | 13 | 19,047 ± 1,894 | 10 | 31,551 ± 7,101 | 0 |
| 1.0 | 29.13 ± 4.7 | 15 | 20,529 ± 1,157 | 3 | 25,091 ± 4,947 | 0 |
| 10 | 22.98 ± 3.6 | 35 | 19,773 ± 1,427 | 7 | 11,881 ± 1,830 | 6 |
| 25 | 22.75 ± 3.2 | 37 | 15,948 ± 866 | 25 | 12,799 ± 1,474 | 0 |
| 50 | 18.42 ± 2.8 | 51 | 15,054 ± 875 | 29 | 9,774 ± 2,138 | 22 |
| Dex | 19.38 ± 1.5 | 47 | 8,879 ± 702 | 58 | 13,462 ± 2,106 | 0 |
BALB/C mice (ten per group) were treated with 2SAHA (indicated in mg/kg) by gavage or dexamethasone (dex) (10 mg/kg, i.p.), and after 60 min injected with LPS (30 mg/kg, i.p.). Blood was taken 90 min after LPS and serum prepared. Serum cytokine values are mean ± SEM (in pg/ml). %, Percent reduction from LPS (set at 100%). NA, non-applicable; Veh, vehicle; MIP-2, macrophage inflammatory protein-2.
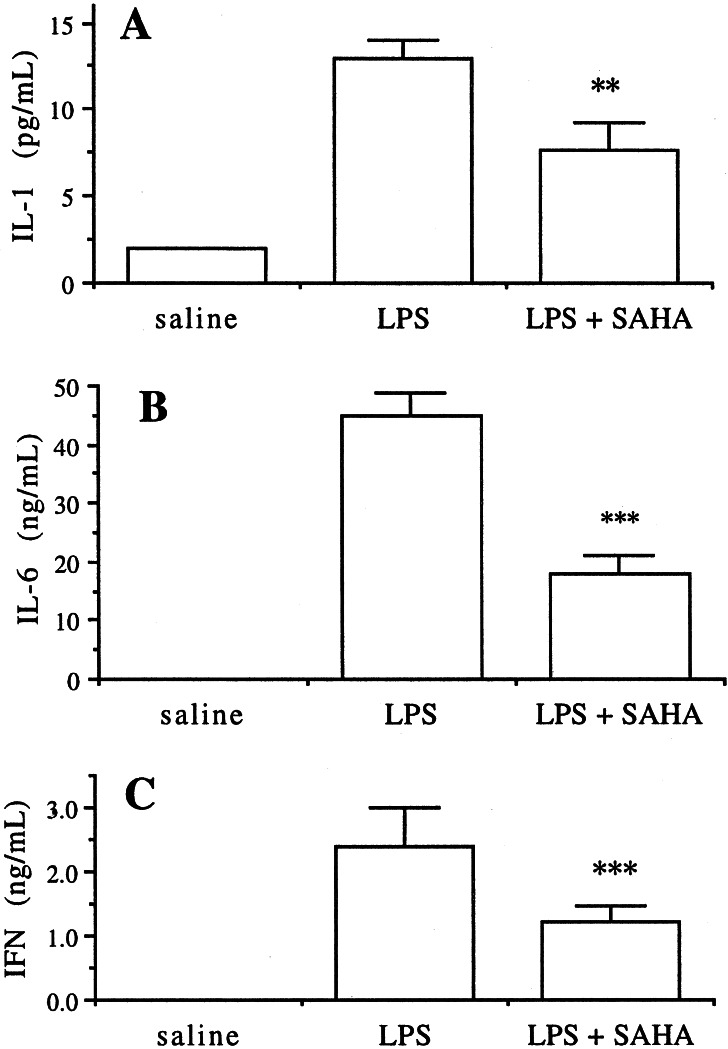
The experiment was repeated using SAHA pretreatment of 50 mg/kg by gavage in C57Bl6 mice and blood was taken after 6 h. Serum IL-1-β levels decreased by 50% (Fig. 3A). Similarly, IL-6 levels in these sera fell from 42 ± 3.2 to 18.5 ± 2.3 ng/ml, P < 0.001 (Fig. 3B). Serum IFN-γ was elevated in water-treated control mice given LPS to 2 ng/ml but decreased to 1.25 ± 0.33 ng/ml in SAHA-treated mice (P < 0.001). In contrast, there was no change in 6-h serum IL-10 or MIP-2 (data not shown).
Figure 3.
SAHA reduces LPS-induced IL-1-β, IL-6, and IFN-γ serum levels. Mice were treated with 50 mg/kg SAHA by gavage as described in Fig. 2. Serum was taken at 6 h (n = 6 per group). The means ± SE are indicated. **, P < 0.01; ***, P < 0.001.
SAHA Attenuates Con A-Induced Hepatic Injury.
Intravenous injection of Con A results in hepatic cell death within 12–24 h with markedly elevated serum levels of hepatic enzymes such as alanine amino transaminase (ALT). In mice pretreated with a single dose of SAHA (50 mg/kg) given i.p. 1 h before Con A, the 24-h level of serum ALT (mean ± SE) was 8,144 ± 2,091 units/liter compared with 15,190 ± 2,580 in vehicle-treated mice (n = 6 per group).
TNF-α/IFN-γ-Stimulated Nitric Oxide Production from Murine Peritoneal Macrophages Is Suppressed by SAHA.
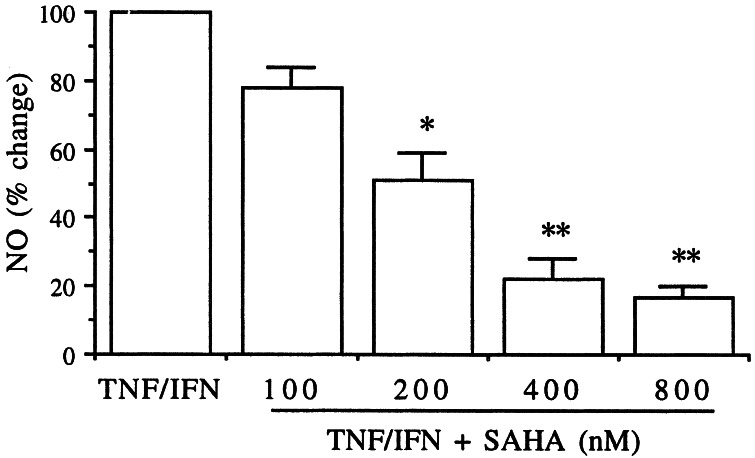
Elicited macrophages were incubated with increasing concentrations of SAHA for 1 h and then stimulated with a mixture of TNF-α plus IFN-γ. After 24 h at 37°C, the level of NO was measured in the supernatants. As shown in Fig. 4, SAHA inhibited NO production; at 200 nM, there was a 50% reduction (P < 0.05). Further reductions of 80 and 85% were observed at 400 and 800 nM, respectively.
Figure 4.
Production of NO from mouse peritoneal macrophages. Thioglycolate-elicited peritoneal macrophages were incubated in vitro with increasing concentrations of SAHA for 1 h and then stimulated with the combination of human TNF-α (10 ng/ml) plus murine IFN-γ (100 units/ml). After 24 h, the supernatants were assayed for NO. Mean ± SE of four separate experiments. *, P < 0.05; **, P < 0.01.
SAHA Inhibits the Secretion of TNF-α, IL-1-β, and IFN-γ in LPS-Stimulated Human PBMC.
Increasing concentrations of SAHA were added to freshly isolated PBMC for 1 h followed by stimulation with LPS. After 24 h at 37°C, the supernatants were removed and assayed for cytokines. As shown in Fig. 5A there was a dose-dependent decrease in the level of TNF-α (55% reduction at 400 nM). The release of IL-1-β was also reduced (45% at 100 nM). However, at concentrations of 200 nM and 400 nM, the reduction in supernatant IL-1-β was less than that at 100 nM. Precursor IL-1-β was measured by specific ELISA in the cell lysates of these cultures. The precursor IL-1-β was increased (mean fold increase of 11 ± 2.8) in LPS-stimulated PBMC compared with nonstimulated cultures; however, there was no reduction in the levels of precursor IL-1-β at any concentration of SAHA. In these same cultures, LPS-induced increase in IL-1Ra (intracellular or secreted) by 8–12-fold but the levels did not change at any concentration of SAHA (data not shown). However, there was dose-dependent decrease in LPS-induced secretion of IFN-γ (Fig. 5B). The reduction in IFN-γ was observed in PBMC stimulated with either LPS or the combination of IL-12/IL-18. At approximately 50 nM, there was a 50% reduction. In the supernatants of PBMC stimulated with LPS, the level of the chemokine IL-8 was increased 6–10-fold but did not change at any concentration of SAHA.
Figure 5.
Inhibition of cytokine secretion from human PBMC. (A) PBMC were stimulated with LPS (10 ng/ml) 1 h after preincubation with increasing concentrations of SAHA. IL-1-β and TNF-α were measured in the supernatant after 24 h. Mean percent change from LPS control (set at 100%) ± SE of five separate blood donors. *, P < 0.05. (B) IFN-γ levels in PBMC stimulated with either LPS (10 ng/ml) or the combination of IL-12 (1 ng/ml) plus IL-18 (10 ng/ml). After 24 h, IFN-γ was measured in the supernatants. Mean ± SE percent change from stimuli (set at 100%) is shown. Reductions in IFN-γ was analyzed by ANOVA and indicated for both stimuli compared with stimulus alone (no SAHA).
SAHA Does Not Affect T Cell-Receptor-Stimulated IFN-γ Production.
The anti-CD3 agonistic antibody (OKT3 at 500 ng/ml) was used to stimulate IFN-γ production in PBMC in the presence of increasing concentrations of SAHA (25–1,000 nM). After 24 h at 37°C, there was no change in IFN-γ levels. However, as shown in Fig. 5B, when IFN-γ was induced by the combination of IL-12 plus IL-18, there was a dose-dependent reduction with 50% inhibition observed at 25 nM SAHA and a 75% at 200 nM.
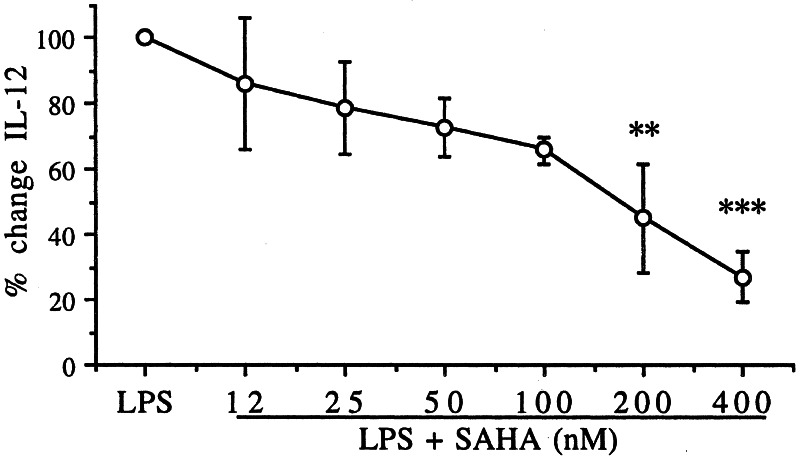
The Induction of IL-12 by IFN-γ and LPS Is Suppressed by SAHA.
As shown in Fig. 6, there was a dose-dependent reduction in LPS/IFN-γ-induced IL-12 production in nonadherent human monocytes. At 200 nM, the reduction was 55% (P < 0.01) and at 86% at 400 nM (P < 0.001).
Figure 6.
SAHA inhibits IL-12 production. Monocytes were separated from venous blood, cultured on Petriperm plates, and incubated with increasing concentrations of SAHA. After 1 h, the combination of LPS (10 ng/ml) plus IFN-γ (500 units/ml) was added, and after 24 h the supernatant was removed and frozen. The percent change (mean ± SE) in IL-12 is shown for three separate blood donors after setting the stimulus-induced IL-12 at 100%. **, P < 0.01; ***, P < 0.001.
Levels of Steady-State mRNA in LPS-Stimulated PBMC Treated with SAHA.
Steady-state mRNA levels were examined 6 and 24 h after LPS stimulation in PBMC by using Northern blot hybridization. As shown in Fig. 7, compared with unstimulated cells after 6 h of culture, LPS induced mRNA for each of the cytokines studied. Pretreatment with 100 nM SAHA resulted in a reduction (85% by densitometry) in the 6-h TNF-α mRNA level; at 25 nM, suppression was approximately 45%. Not unexpectedly, the 24-h levels of TNF-α mRNA were barely detectable. Using the same blot, SAHA did not change IL-1-β mRNA levels at either 25 or 100 nM after 6 or 24 h of LPS stimulation. However, as shown in Fig. 7, IFN-γ levels were dose-dependently reduced at 6 h; after 24 h, there was a near total suppression of IFN-γ mRNA levels. There was no change in IL-8 mRNA in these same cultures.
Figure 7.
Effect of SAHA on steady-state mRNA levels for TNF-α, IL-1-β, IFN-γ, and IL-8. PBMC were preincubated with water vehicle or SAHA at the indicated concentrations and after 1 h, and stimulated with LPS (10 ng/ml; third, fourth, seventh, and eighth lanes). Control PBMC were incubated without stimulus (first and fifth lanes) for the entire experiment or LPS only (second and sixth lanes). After 6 and 24 h, total RNA was isolated and by Northern blot hybridization of TNF-α, IL-1-β, IFN-γ, and IL-8.
Discussion
SAHA as an Antitumor Agent.
A large number of studies have revealed that inhibitors of HDAC reduce the proliferation of transformed cells in vitro as well as the growth of experimental tumors in vivo (5). The HDAC inhibitor depsipeptide has been administered to three patients with cutaneous T-cell lymphoma associated with clinical responses (16). SAHA has also advanced to clinical studies in patients with prostatic cancer and lymphoma (2, ¶). The physical property of SAHA to inhibit HDAC is its binding of the hydroxamic acid moiety to the zinc-containing pocket of HDAC (17), resulting in increased acetylated histones. SAHA inhibits HDAC 1 and 3 and there is hyperacetylation of histones 3 and 4 (7). As a consequence of hyperacetylation, SAHA and other inhibitors of HDAC increase the expression of ≈1–2% of genes (5). Increased gene expression for the cell cycle kinase inhibitor p21 (6), as well as other mechanisms of tumor cell apoptosis, account for the antitumor properties of SAHA (18, 19). In vitro, micromolar concentrations of SAHA result in selective apoptotic cell death, terminal differentiation, and growth arrest for tumor cells, without toxicity on normal cells (1, 6). In mice, growth of transplanted human prostatic cancer cells was suppressed by 97% by daily administration of SAHA at 50 mg/kg/day (1).
The Antiinflammatory Portfolio of SAHA.
By virtue of reducing circulating cytokines during endotoxemia in mice, preventing hepatocellular damage in Con A-injected mice, suppressing cytokine-induced nitric oxide in mouse macrophages, and inhibiting the secretion of proinflammatory cytokines in LPS- and cytokine-stimulated human PBMC, the present studies reveal the antiinflammatory properties of SAHA. In animal experiments, the antitumor effects of SAHA require doses of 50–200 mg/kg/day (1). Using lower than antitumor doses of SAHA (10–50 mg/kg, per os), we observed greater than 50% decreased in circulating cytokines (TNF-α, IL-1-β, IL-6, and IFN-γ) during experimental endotoxemia. In the in vivo model of Con A-induced hepatic injury, a model that is TNF-α- and IL-18-dependent (10), a single dose of 50 mg/kg SAHA reduced the injury by 50%. We have also observed that daily oral treatment of mice with 50 mg/kg SAHA significantly reduced the clinical and cytokine abnormalities in dextran sulfate sodium-induced colitis (B.S., unpublished observations), a model that is IL-1-β converting enzyme (ICE)- and IL-18-dependent (20, 21). Production of LPS-induced IFN-γ in PBMC is also ICE- and IL-18-dependent (22).
It is interesting to note that the inhibitory effect of SAHA appears to be particularly effective for proinflammatory cytokines. In fact, in this report, the in vitro concentrations of SAHA that inhibited proliferation of tumor cell lines by 50% were 1–5 μM, similar to those described by others (1); however, at nanomolar concentrations (50–200 nM), we observed 50–85% reductions in LPS-induced secretion of TNF-α, IL-1-β, IFN-γ, and IL-12 in freshly isolated human PBMC. In humans injected with 300–600 mg/m2 SAHA, acetylated histones were found in the PBMC fraction for up to 4 h after an i.v. infusion.¶ Similar to the reduction in protein levels of TNF-α and IFN-γ, the steady-state mRNA levels for these two cytokines were reduced, particularly those of IFN-γ. In addition, the in vitro production of NO in primary mouse peritoneal macrophages stimulated with the combination of TNF-α and IFN-γ was suppressed by SAHA at 200–400 nM. Therefore, the antiinflammatory effect of SAHA is evident at concentrations lower than those needed to suppress tumor cell growth in vitro and in vivo.
IFN-γ production triggered by the T-cell receptor, using anti-CD3, was unaffected by SAHA. In contrast, when stimulated by either LPS or the combination of IL-18 plus IL-12, IFN-γ production was markedly reduced by SAHA. The intracellular and extracellular levels of the antiinflammatory cytokine IL-1Ra were unaffected by SAHA in LPS-stimulated human PBMC and there was no reduction in circulating IL-10 levels in mice injected with LPS. SAHA also had no effect of the production of the proinflammatory chemokine IL-8. Consistent with this finding, steady-state mRNA for IL-8 was unaffected by SAHA.
Effect of SAHA on Secretion of IL-1-β.
Pretreatment with SAHA resulted in reduced secretion of mature IL-1-β from PBMC and the level of circulating IL-1-β in LPS-injected mice. However, SAHA did not affect steady-state levels of IL-1-β mRNA or the intracellular levels of precursor IL-1-β. In the same PBMC cultures, SAHA reduced both the secretion of TNF-α and IFN-γ as well as their mRNA levels. Therefore, the reduction in IL-1-β by SAHA appears to be primarily at the level of secretion of mature IL-1-β. Although the pathway(s) for secretion of IL-1-β remains unclear, there is no dearth of evidence that inhibition of the ICE results in decreased secretion of mature IL-1-β, particularly in LPS-stimulated human monocytes (reviewed in ref. 23). When tested, SAHA at concentrations as high as 10 μM did not inhibit ICE enzymatic activity when using a synthetic substrate (unpublished observations). However, SAHA may increase gene expression and synthesis of the intracellular inhibitor of ICE, serpin proteinase inhibitor 9 (24). Alternatively, SAHA may decrease the function of the adenosine receptor P2X-7 (25) or induce the level of endogenous inhibitors of ATP-binding cassette transporters (26), which are needed for secretion of mature IL-1-β. The secretion of IL-1-β also includes transport via exocytosis of preterminal endocytic vesicles (27). It is unclear which, whether any, of these pathways are affected by SAHA.
Effects of Other Inhibitors of HDAC.
Although the present studies appear to be the first to examine the antiinflammatory effects of SAHA in vivo, butyrate, and trichostatin, inhibitors of HDAC also have antitumor properties and affect cytokine production. For example, butyrate reduces IL-12 production but up-regulates IL-10 in human blood monocytes (28), although millimolar concentrations are needed. Butyrate also inhibits the proliferation of the Caco-2 colon cancer epithelial cell and reduces spontaneous gene expression of IL-8 in these cells (29). Using the same cell line, others have reported that butyrate or trichostatin increase IL-8 production (30). It is not uncommon for trichostatin and butyrate to have paradoxical effects on IL-8 production by Caco-2 cells; these paradoxical effects are likely due to the state of cell activation. For example, IL-8 secretion was increased by 145% by trichostatin and butyrate alone, whereas in TNF-α-stimulated cells IL-8 was inhibited to levels below unstimulated levels (31). In our studies, we did not observe reduction in LPS-induced IL-8 gene expression or protein synthesis in LPS-stimulated PBMC.
In purified T-cells from patients with systemic lupus erythematosus, trichostatin significantly reduced gene expression of CD40L and IL-10 stimulated by phorbol esters and ionomycin, but up-regulated expression of IFN-γ (32). In contrast, we stimulated PBMC from healthy subjects with LPS or the combination of IL-12/IL-18, and consistently observed a marked reduction in gene expression and synthesis of IFN-γ. Other investigators have reported that at apoptosis-inducing concentrations of trichostatin and butyrate, IFN-γ production in T lymphocytes is reduced (33). In addition, butyrate or trichostatin inhibited IL-1-β-dependent induction of the acute phase protein gene haptoglobin as well as the binding of transcription factors to the haptoglobin promoter (34). Butyrate and trichostatin also suppress IL-2 induction of c-myc, bag-1, and LC-PTP gene expression (35). The IL-2 enhancer and promoter were suppressed by trichostatin by 50% at 73 nM, whereas at the same concentration there was increased expression directed by the c-fos enhancer and promoter (36).
The transcription of most eukaryotic genes is silenced by tight packaging of DNA into chromatin structures; however, repression of the basal expression of many genes is also controlled by the level of deacetylation of histones. Therefore, it is not surprising that the action of inhibitors of HDAC includes an increase in gene expression of silenced genes. For example, treatment of Caco cells with trichostatin increases the expression of the proinflammatory chemokine IL-8 (37). We have also observed an increase in IL-1-induced IL-8 production by SAHA in the COS cell line (unpublished observations). However, most in vitro studies on inhibitors of HDAC have been carried out using cell lines, particularly cell lines of malignant origin. In contrast, we have used primary cells from mice or freshly obtained blood cells healthy from human subjects and have examined the effects of SAHA under in vitro conditions of inflammatory stimuli such as LPS or cytokines themselves. In the absence of exogenous stimuli, we did not observe an increase in cytokines or steady-state levels of cytokines studied in this report. Even under conditions of stimulation, there was no increase, for example, of IL-8 or IL-1-β mRNA. In contrast, LPS-induced TNF-α and IFN-γ mRNA were decreased by treatment with SAHA. Therefore, the antiinflammatory effects of SAHA and possibly other inhibitors of HDAC is selective and likely dependent on the level of activation, particularly in primary cells. Alternatively, SAHA may have antiinflammatory properties independent of its ability to inhibit nuclear HDAC, for example, hyperacetylation of nonhistone proteins such as ribosomal S3 (8) or the Rel A subunit of NF-κB (4).
Acknowledgments
We thank Dr. B. Vergani and Mr. G. Pavich for the synthesis and purification of SAHA, and Marisa Vulcano for assistance with the IL-12 studies. This work was supported by National Institutes of Health Grant AI 15614.
Abbreviations
- SAHA
suberoylanilide hydroxamic acid
- TNF-α
tumor necrosis factor-α
- HDAC
histone deacetylase
- PBMC
peripheral blood mononuclear cells
- ICE
IL-1-β converting enzyme
- LPS
lipopolysaccharide
Footnotes
O'Connor, O. A., Kelly, W., Wang, E. S., Richon, V., Moskowitz, C., Tong, W., Donnelly, G., Zelenetz, A. D., Moore, M. A. S., Rifkind, R. A. & Marks, P. A. (2001) Blood 98,611.a (abstr.).
References
- 1.Butler L M, Agus D B, Scher H I, Higgins B, Rose A, Cordon-Cardo C, Thaler H T, Rifkind R A, Marks P A, Richon V M. Cancer Res. 2000;60:5165–5170. [PubMed] [Google Scholar]
- 2.Richon V M, Zhou X, Rifkind R A, Marks P A. Blood Cells Mol Dis. 2001;27:260–264. doi: 10.1006/bcmd.2000.0376. [DOI] [PubMed] [Google Scholar]
- 3.Marks P A, Rifkind R A, Richon V M, Breslow R, Miller T, Kelly W K. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Fischle W, Verdin E, Greene W C. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- 5.Marks P A, Richon V M, Rifkind R A. J Natl Cancer Inst. 2000;92:1210–1216. doi: 10.1093/jnci/92.15.1210. [DOI] [PubMed] [Google Scholar]
- 6.Richon V M, Sandhoff T W, Rifkind R A, Marks P A. Proc Natl Acad Sci USA. 2000;97:10014–10019. doi: 10.1073/pnas.180316197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richon V M, Emiliani S, Verdin E, Webb Y, Breslow R, Rifkind R A, Marks P A. Proc Natl Acad Sci USA. 1998;95:3003–3007. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb Y, Zhou X, Ngo L, Cornish V, Stahl J, Erdjument-Bromage H, Tempst P, Rifkind R A, Marks P A, Breslow R, Richon V M. J Biol Chem. 1999;274:14280–14287. doi: 10.1074/jbc.274.20.14280. [DOI] [PubMed] [Google Scholar]
- 9.Van Lint C, Emiliani S, Ott M, Verdin E. EMBO J. 1996;15:1112–1120. [PMC free article] [PubMed] [Google Scholar]
- 10.Faggioni R, Jones-Carson J, Reed D A, Dinarello C A, Feingold K R, Grunfeld C, Fantuzzi G. Proc Natl Acad Sci USA. 2000;97:2367–2372. doi: 10.1073/pnas.040561297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erroi A, Pagani P, Sironi M, Salmona M. Am J Physiol. 1996;271:L132–L138. doi: 10.1152/ajplung.1996.271.1.L132. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski P, Sacchi N. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 13.Chan E D, Riches D W. Am J Physiol Cell Physiol. 2001;280:C441–C450. doi: 10.1152/ajpcell.2001.280.3.C441. [DOI] [PubMed] [Google Scholar]
- 14.Puren A J, Fantuzzi G, Gu Y, Su M S-S, Dinarello C A. J Clin Invest. 1998;101:711–724. doi: 10.1172/JCI1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Puren A J, Razeghi P, Fantuzzi G, Dinarello C A. J Infect Dis. 1998;178:1830–1834. doi: 10.1086/314481. [DOI] [PubMed] [Google Scholar]
- 16.Piekarz R L, Robey R, Sandor V, Bakke S, Wilson W H, Dahmoush L, Kingma D M, Turner M L, Altemus R, Bates S E. Blood. 2001;98:2865–2868. doi: 10.1182/blood.v98.9.2865. [DOI] [PubMed] [Google Scholar]
- 17.Finnin M S, Donigian J R, Cohen A, Richon V M, Rifkind R A, Marks P A, Breslow R, Pavletich N P. Nature (London) 1999;401:188–193. doi: 10.1038/43710. [DOI] [PubMed] [Google Scholar]
- 18.Said T K, Moraes R C, Sinha R, Medina D. Breast Cancer Res. 2001;3:122–133. doi: 10.1186/bcr284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vrana J A, Decker R H, Johnson C R, Wang Z, Jarvis W D, Richon V M, Ehinger M, Fisher P B, Grant S. Oncogene. 1999;18:7016–7025. doi: 10.1038/sj.onc.1203176. [DOI] [PubMed] [Google Scholar]
- 20.Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr H A, Hartmann G, Dinarello C A, Endres S, Eigler A. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–R1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- 21.Siegmund B, Lehr H A, Fantuzzi G, Dinarello C A. Proc Natl Acad Sci USA. 2001;98:13249–13254. doi: 10.1073/pnas.231473998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dinarello C A. J Allergy Clin Immunol. 1999;103:11–24. doi: 10.1016/s0091-6749(99)70518-x. [DOI] [PubMed] [Google Scholar]
- 23.Dinarello C A. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 24.Young J L, Sukhova G K, Foster D, Kisiel W, Libby P, Schonbeck U. J Exp Med. 2000;191:1535–1544. doi: 10.1084/jem.191.9.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solle M, Labasi J, Perregaux D G, Stam E, Petrushova N, Koller B H, Griffiths R J, Gabel C A. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 26.Hamon Y, Luciani M F, Becq F, Verrier B, Rubartelli A, Chimini G. Blood. 1997;90:2911–2915. [PubMed] [Google Scholar]
- 27.Andrei C, Dazzi C, Lotti L, Torrisi M R, Chimini G, Rubartelli A. Mol Biol Cell. 1999;10:1463–1475. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saemann M D, Bohmig G A, Osterreicher C H, Burtscher H, Parolini O, Diakos C, Stockl J, Horl W H, Zlabinger G J. FASEB J. 2000;14:2380–2382. doi: 10.1096/fj.00-0359fje. [DOI] [PubMed] [Google Scholar]
- 29.Huang N, Katz J P, Martin D R, Wu G D. Cytokine. 1997;9:27–36. doi: 10.1006/cyto.1996.0132. [DOI] [PubMed] [Google Scholar]
- 30.Fusunyan R D, Quinn J J, Fujimoto M, MacDermott R P, Sanderson I R. Mol Med. 1999;5:631–640. [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson P R, Rosella O, Wilson A J, Mariadason J M, Rickard K, Byron K, Barkla D H. Carcinogenesis. 1999;20:539–544. doi: 10.1093/carcin/20.4.539. [DOI] [PubMed] [Google Scholar]
- 32.Mishra N, Brown D R, Olorenshaw I M, Kammer G M. Proc Natl Acad Sci USA. 2001;98:2628–2633. doi: 10.1073/pnas.051507098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dangond F, Gullans S R. Biochem Biophys Res Commun. 1998;247:833–837. doi: 10.1006/bbrc.1998.8891. [DOI] [PubMed] [Google Scholar]
- 34.Desilets A, Gheorghiu I, Yu S J, Seidman E G, Asselin C. Biochem Biophys Res Commun. 2000;276:673–679. doi: 10.1006/bbrc.2000.3531. [DOI] [PubMed] [Google Scholar]
- 35.Koyama Y, Adachi M, Sekiya M, Takekawa M, Imai K. Blood. 2000;96:1490–1495. [PubMed] [Google Scholar]
- 36.Takahashi I, Miyaji H, Yoshida T, Sato S, Mizukami T. J Antibiot. 1996;49:453–457. doi: 10.7164/antibiotics.49.453. [DOI] [PubMed] [Google Scholar]
- 37.Wen X, Wu G D. J Immunol. 2001;166:7290–7299. doi: 10.4049/jimmunol.166.12.7290. [DOI] [PubMed] [Google Scholar]