Abstract
Commensal and enteroinvasive microbes in the human gut release bacterial flagellin, a specific microbial ligand of Toll-like receptor 5 (TLR5). However, the pathophysiological role of bacterial flagellin in gastrointestinal inflammation has not been determined. Here we evaluated the role of bacterial flagellin using native human colonic mucosa and the mouse colitis model of dextran sulfate sodium (DSS). We demonstrate that, in intact human colonic mucosa, the flagellin/TLR5 response occurs only after exposure to the basolateral, not the apical, surface, implying a basolaterally polarized TLR5 response in human colonic mucosa. In this context, flagellin exposure to injured colonic mucosa due to DSS administration in mice resulted in a TLR5-associated response evaluated by in vivo activation of mitogen-activated protein kinase/extracellular signal-related kinase 1/2 (MEK1/2) and elevated IL-6, TNF-α, and keratinocyte-derived chemokine production, whereas intact colonic mucosa did not respond to flagellin. Moreover, flagellin exposure to injured mouse colon in vivo, but not to intact colon, also significantly aggravated colonic inflammation, increased mouse mortality, and enhanced histopathological damage in the colonic mucosa. However, the TLR2-specific agonist, peptidoglycan or lipoteichoic acid, did not cause an inflammatory response in intact or DSS-injured mouse colon. Furthermore, intracolonic flagellin administration in mice causes severe apoptosis in colonic epithelium disrupted by DSS administration. These data suggest that intracolonic flagellin via TLR5 engagement is able to elicit inflammatory responses in disrupted colon, whereas the normal colon is not responsive to bacterial flagellin. These results demonstrate that bacterial flagellin plays an important role in the development and progress of colitis.
Keywords: innate immunity, colitis, commensal bacteria
Toll-like receptors (TLRs) represent a family of receptors that recognize a wide array of conserved microbial products, such as LPS, bacterial lipoprotein, bacterial CpG DNA, and bacterial flagellin, present within various microorganisms (1). TLRs recognize such microbial ligands produced by either pathogenic or commensal microorganisms and trigger inflammatory and immune responses (2, 3).
A large number of microbes comprising the gut microflora that colonizes the human gut release microbial products such as flagellin, representing a specific ligand for TLR5 (4, 5). These intestinal microbiota are compartmentalized in the intestinal lumen by the intact epithelium that functions as a shield against microbial molecules released from various luminal microbes. A recent study suggested that a TLR response in the mouse intestine might have a beneficial effect in maintaining intestinal homeostasis (6, 7). However, despite the lack of direct evidence, several clinical observations and animal experiments suggest that intestinal bacteria play a major role in the pathogenesis of bowel inflammation, including inflammatory bowel disease (IBD) (5, 8-10). Moreover, inappropriate and ongoing activation of mucosal immune responses by intestinal bacteria is likely to be a significant component of chronic intestinal inflammation (11).
Flagellin specifically stimulates TLR5 that is highly expressed in intestinal epithelial cells. TLR5 engagement by bacterial flagellin activates mitogen-activated protein kinases and NF-κB-related pathways, leading to macrophage inflammatory protein 3α (MIP3α) and IL-8 production (12). Because colonic inflammation in IBD patients is characterized by increased MIP3α and IL-8 production (13, 14), we hypothesized that TLR5-mediated responses are involved in colonic inflammation. Indeed, bacterial flagellin recently was described as a dominant antigen in IBD patients, and flagellin-specific CD4+ T cells were able to induce colonic inflammation in an adoptive CD4+ T cell transfer model of colitis (15). However, the pathophysiologic relationship between bacterial flagellin and colonic inflammation has yet to be determined. In this study, we demonstrate that bacterial flagellin exposed to the injured colon enhances ongoing colonic inflammation and causes apoptosis of colonocytes in the dextran sulfate sodium- (DSS) induced mouse colitis model. These results represent in vivo evidence that bacterial flagellin participates in the pathophysiology of colitis.
Materials and Methods
Mice and Reagents. Eight-week-old male mice (C3H/HeJ and CD-1) were from The Jackson Laboratory. The Institutional Animal Care and Use Committee of Beth Israel Deaconess Medical Center approved all animal procedures. Flagellin from Salmonella typhimurium (Apotech, Epalinges, Switzerland), peptidoglycan (PGN) from Staphylococcus aureus, and lipoteichoic acid (LTA) from S. aureus (InvivoGen, San Diego) were dissolved in LPS-free water (Sigma-Aldrich).
Ussing Chamber Experiments in Human Colon. Specimens of tumor-free left-sided colon were used. After removal of the seromuscular layer by blunt dissection, two to six mucosal preparations (1.0-cm2 surface area) from each specimen measuring 5-10 cm2 were vertically mounted in Ussing chambers (DCTSYS, Precision Instrument Design, Lake Tahoe, CA), as described (16, 17). The Ethics Committee of the University Clinic of Vienna approved the protocol for use of human tissues. Informed consent was also obtained from all subjects.
Mouse Colitis. Mice were fed 2.5% DSS (ICN) dissolved in regular tap water during the entire experimental period. Water consumption per mouse per day was evaluated and found to be similar among the experimental groups.
Clinical Assessment of Colitis. Animals were observed three times per day for morbidity and mortality, stool consistency, weight, and rectal bleeding, as described (18), for the duration of the experiment.
Histology. At day 7 postflagellin administration, the entire mouse colon was excised, and segments of the transverse colon (1 cm) were fixed in 10% buffered formalin, paraffin-embedded, and stained with hematoxylin/eosin. The histologic severity of colitis was graded in a “blinded” fashion on a scale of 0-3 by a gastrointestinal pathologist (M.O.).
Colon Organ Culture. C3H/HeJ mice that received either water or DSS (2.5%) for 7 days without flagellin were killed, and full-thickness colon segments (1 × 0.5 cm) were removed, cut open longitudinally, and washed in PBS containing penicillin and streptomycin. Colon tissue segments were then incubated with 1 ml of RPMI medium 1640 supplemented with penicillin and streptomycin with or without flagellin (500 ng/ml) for 15 h, and cytokine levels were measured in the tissue supernatants.
Cytokine ELISAs. ELISA was performed to measure the level of human IL-8 and mouse keratinocyte-derived chemokine, IL-6, and TNF-α using the appropriate kits from R & D Systems and Biosource International (Camarillo, CA), respectively, by following the manufacturer's instructions. All assays were performed in triplicate, and data were expressed as mean ± SEM.
Western Blot Analyses. Total protein extracts from homogenized mouse colon tissues were prepared in lysis buffer (150 mM NaCl/50 mM Tris·Cl, pH 8.0/5 mM EDTA/1% Nonidet P-40) with a protease inhibitor mixture (Roche Applied Science, Indianapolis). Equal amounts of total protein were subjected to Western blot analysis, as described (12, 19).
Immunofluorescence Microscopy. Mouse intestinal tissue sections (6 μm) were fixed in 10% formalin and permeabilized by using 0.5% Triton X-100. Slides were washed with PBS-glycine (100 mM) and blocked with IgG-free BSA (1%) plus IgG-free rabbit serum (1%) in PBS for 10 min at room temperature. Slides were then be incubated for 1 h with primary antibodies (1:100 dilution) against phospho-mitogen-activated protein kinase/extracellular signal-related kinase (MEK) 1/2 (Cell Signaling Technology, Beverly, MA). Samples were then washed with PBS-glycine and incubated with FITC-conjugated anti-rabbit secondary antibody (1:200) for 1 h. The slides were then rinsed and mounted with DAPI mounting solution (Vector Laboratories). Images were analyzed with a Zeiss Axioskop-2 microscope.
TUNEL Assays. Colon tissues removed from each mouse group were snap-frozen in OCT compound (Sakura Finetek, Torrance, CA). Sections (6 μm) were subjected to TUNEL assays by using the TUNEL Apoptosis Detection Kit (Upstate Biotechnology, Lake Placid, NY), according to the manufacturer's protocol. Briefly, tissue sections were fixed by using paraformaldehyde (4%) in 0.1 M NaH2PO4 (pH 7.4) for 15 min followed by washing with PBS. Slides were incubated with Proteinase-K at 37°C for 30 min and then incubated with Biotin-dUTP and terminal deoxynucleotidyl transferase for 60 min. Avidin-FITC was applied to the sample section for 30 min. The sections were then counterstained with DAPI followed by mounting with coverslips and analyzed by using a Zeiss Axioskop-2 microscope.
Statistical Analysis. Statistical analyses were performed by using statview 4.51 software (Abacus Concepts, Berkeley, CA). Data are expressed as mean ± SEM. ANOVA was used for intergroup comparisons.
Results
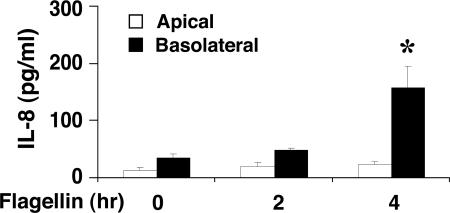
Flagellin/TLR5 Response Is Restricted to the Basolateral Aspect of Native Human Colonic Mucosa. Studies with several colon adenocarcinoma cell lines indicate that flagellin/TLR5-mediated proinflammatory responses are basolaterally localized (20, 21). Moreover, in murine intestine, expression of the murine IL-8 homologue keratinocyte-derived chemokine is more evident when flagellin prepared from commensal Escherichia coli MG1655 is added to the basolateral, rather than the apical, compartment (22). However, whether a similar “different responsiveness” to bacterial flagellin also takes place in native human colon is not known. To address this question, mucosal preparations of normal human colon were mounted in Ussing chambers for electrophysiologic studies (16, 17) and exposed either apically or basolaterally to flagellin (100 ng/ml). Apical or basolateral flagellin administration had no effect on colonic transepithelial resistance during 4 h of exposure (Fig. 7, which is published as supporting information on the PNAS web site). After 2 and 4 h incubation with or without flagellin, IL-8 production was measured in the apical or basolateral bathing solution. ELISA analysis showed that basolateral stimulation with flagellin increased IL-8 production, whereas apically exposed flagellin resulted in negligible IL-8 secretion (Fig. 1). These results indicate that the flagellin/TLR5 response is restricted to the basolateral side of native human colonic epithelium.
Fig. 1.
The basolateral, but not apical, aspect of human colonic mucosa is responsive to flagellin stimulation. Human colonic mucosa mounted in Ussing chambers was stimulated either apically or basolaterally with flagellin (100 ng/ml) for 2 and 4 h. IL-8 production was evaluated in the respective supernatants by ELISA. Data are expressed as mean ± SEM. Results are representative of six separate experiments, each with triplicate determinations. *, P < 0.05.
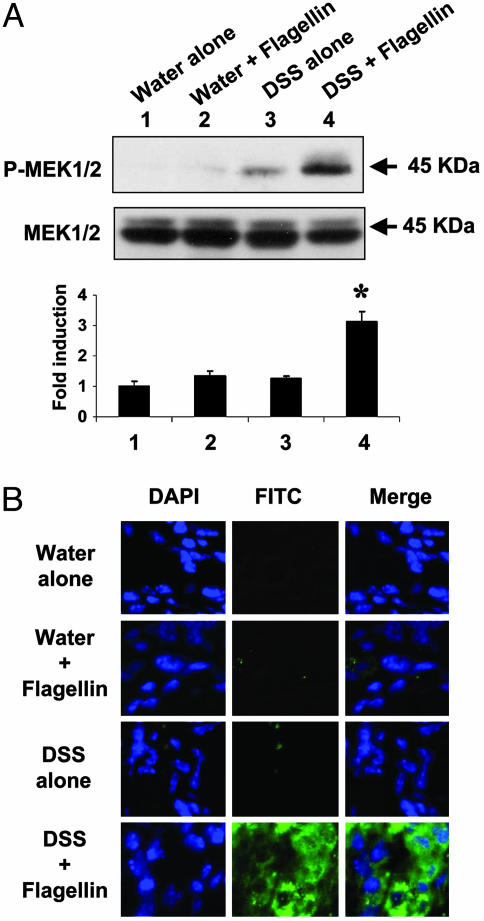
Flagellin Exposure to the Injured Colonic Mucosa Induces in Vivo Activation of MEK1/2 in Colonic Mucosa. The results presented in Fig. 1, together with previous reports (22), led us to hypothesize that an intact intestinal epithelium prevents TLR5 activation by luminal flagellin, whereas a disrupted epithelium would permit flagellin to access the basolateral side, triggering a TLR5 response. To address this hypothesis, we used the DSS model of colitis, because DSS is known to directly disrupt the colonic epithelium, leading to a colonic inflammatory response (23). To compromise the integrity of intestinal epithelium, mice were given water containing a low dose of DSS (2.5%) or water alone during the entire experimental period. Four days after DSS administration, flagellin (0.8-1.0 μg per mouse) was administered daily by rectal enema for 7 days, and then total protein extract and frozen sections of colonic tissues were prepared from all mouse groups. In addition, we recently reported that activation of MEK1/2 is critical for flagellin-induced IL-8 and macrophage inflammatory protein 3α (MIP3α) expression in non-transformed human colonocytes (12). In this context, by evaluating in vivo activation of MEK1/2 in response to colonic flagellin in the colonic mucosa, we determined whether flagellin is able to trigger flagellin/TLR5-associated responses in disrupted or intact colonic mucosa. To study this, we used LPS-resistant C3H/HeJ mice (24, 25) to exclude the possibility that our findings may be confounded by low levels of LPS included in our flagellin preparation (<1pg/μg LPS content as determined by the Limulus Amebocyte Lysate Assay). Total protein extracts of colonic tissues were subjected to Western blot analysis to determine in vivo MEK1/2 activation in response to flagellin. As presented in Fig. 2A, MEK1/2 phosphorylation was substantially enhanced in colon tissues from flagellin plus DSS-treated mice, compared with colonic samples from mice receiving water alone, water plus flagellin, or DSS alone (Fig. 2A). These results indicate that flagellin exposure to injured colonic mucosa induces MEK1/2 activation in vivo. Moreover, we also determined the in vivo activation of MEK1/2 in colonic mucosa by immunofluorescence staining with an antibody recognizing phospho-MEK1/2. Tissues from mice that received water alone, water plus flagellin, or DSS alone showed no evidence of activated MEK1/2, whereas tissues from mice exposed to both DSS and flagellin were positively stained for phosphorylated MEK1/2, shown in green (Fig. 2B). The counterstaining of nuclei was shown by DAPI staining in blue. The merged photomicrographs show that MEK1/2 activation is localized around nuclei inside the cell, confirming its cytoplasmic presence (Fig. 2B). Therefore, these data show that bacterial flagellin is able to initiate cellular events in disrupted colon. However, it remains to be determined which cell type is primarily responsive to flagellin in colonic mucosa. In vivo activation of MEK1/2 by flagellin in compromised mouse colon indicates that disruption of the apical intestinal epithelium enables flagellin to diffuse to the basolateral aspect of epithelium, allowing flagellin/TLR5-mediated responses. These data support the basolaterally confined flagellin/TLR5 response in native colon, as shown in Fig. 1.
Fig. 2.
Flagellin exposure to disrupted colon induces in vivo activation of MEK1/2 in colonic mucosa. C3H/HeJ mice received either water alone or water containing 2.5% DSS through the entire experimental period. Four days after initiation of DSS treatment, mice were colorectally exposed to flagellin for 7 days. (A) Total protein prepared from colonic tissue extracts was subjected to Western blot analysis to determine MEK1/2 activation by using a phospho-MEK1/2 antibody. OD values obtained from x-ray films were expressed as relative ratio of phosphorylated to inactive MEK1/2. Data are expressed as mean ± SEM of five mice per group. *, P < 0.01. (B) Frozen colonic tissue sections obtained from the same mouse groups were exposed to a primary antibody recognizing phosphorylated MEK1/2, followed by a FITC-conjugated anti-rabbit antibody (green). The sections were counterstained with DAPI to localize cell nuclei (blue). Shown here are representative images from each experimental group.
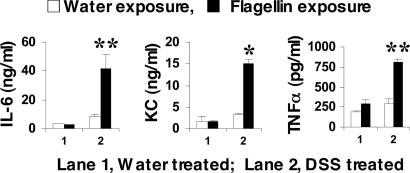
TLR5 Engagement by Flagellin Induces Proinflammatory Cytokine Production in the Injured Colon Tissue. We next examined the effect of flagellin in proinflammatory cytokine production in mouse colon. C3H/HeJ mice that received either water or DSS (2.5%) for 7 days without flagellin administration were killed, and full-thickness colon segments were placed in organ culture followed by flagellin (500 ng/ml) stimulation. After 15 h, the levels of the proinflammatory cytokines IL-6, TNF-α, and keratinocyte-derived chemokine were evaluated in the culture supernatants by ELISA. We found that flagellin induced cytokine production only in colonic explants from DSS-exposed mice. In contrast, flagellin failed to induce cytokine production in intact colon tissues from mice that received water alone (Fig. 3). These results reveal that colonic injury in response to a low dose of DSS alone is not sufficient to induce colonic proinflammatory cytokine production, but the addition of bacterial flagellin to the disrupted colonic epithelium results in significantly increased cytokine production. These results are also consistent with the notion that TLR5 engagement is basolaterally restricted suggested by the results shown in Figs. 1 and 2.
Fig. 3.
Flagellin stimulates cytokine production in colonic explants obtained from DSS-treated mice. C3H/HeJ mice received either water alone or water containing 2.5% DSS for 7 days. Colon segments (1 × 0.5 cm) were then taken and incubated with flagellin (500 ng/ml) for 15 h. The level of IL-6, keratinocyte-derived chemokine (KC), and TNF-α was measured in the supernatants by ELISA. Data are expressed as mean ± SEM (n = 4 mice per group, each with triplicate determinations). *, P < 0.01; **, P < 0.05.
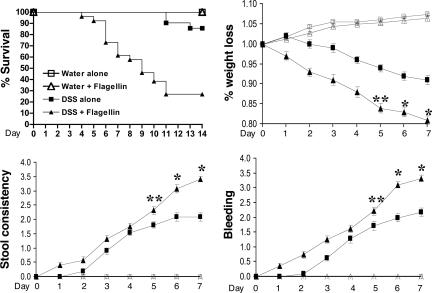
Flagellin Exposed to Injured Colonic Mucosa Significantly Aggravates the Clinical Symptoms of Colitis. We next evaluated the pathophysiological effect of bacterial flagellin in compromised colon. To study this, in the first set of experiments, LPS-resistant C3H/HeJ mice (24, 25) were administered a low dose of DSS (2.5%) in drinking water for the duration of the experiment. Four days after DSS administration, flagellin (0.8-1.0 μg per mouse) was administered daily by rectal enema for a total of 14 days, and several clinical parameters of colitis such as mortality, weight loss, diarrhea, and rectal bleeding were assessed daily, as described (18).
We found that the administration of flagellin together with DSS significantly aggravated all symptoms of colonic inflammation, whereas mice that received DSS alone showed mild colitis-associated symptoms (Fig. 4A). Administration of water alone or water plus flagellin, in the absence of DSS, exhibited no symptoms (Fig. 4). Compared with mice that received DSS alone, animals that received flagellin in the presence of DSS showed substantially reduced survival rates (DSS plus flagellin group, 26%, vs. DSS alone, 85% survival; P < 0.001), increased diarrhea (P < 0.01), marked weight loss (DSS plus flagellin group, 19% vs. DSS alone, 9% weight loss; P < 0.001), and increased rectal bleeding (P < 0.001) (Fig. 4).
Fig. 4.
Flagellin aggravates clinical symptoms of colonic inflammation in DSS-induced colitis. C3H/HeJ mice received either water alone or water containing 2.5% DSS for the entire experimental period. Four days after the beginning of DSS administration, mice were injected intracolonically with flagellin (0.8-1.0 μg per mouse) daily for 14 days. Kaplan-Meier survival analysis showed that flagellin administration increased mortality of C3H/HeJ (C3H/HeJ, P < 0.001; log rank). Several clinical parameters of colonic inflammation were also increased by flagellin exposure to injured colon by DSS administration [C3H/HeJ mice: water alone (n = 22), water plus flagellin (n = 24), DSS alone (n = 21), and DSS plus flagellin (n = 26).
A recent study suggested that LPS might have a beneficial effect on maintaining the intestinal epithelial homeostasis (6). Because our in vivo experiments described above were performed in LPS-resistant C3H/HeJ mice, we performed additional experiments with LPS-responsive CD-1 mice using identical experimental conditions. Similar to the results obtained from C3H/HeJ mice, flagellin administered to CD-1 mice in the presence of DSS substantially enhanced colonic inflammation. Compared with DSS alone, mice that received flagellin together with DSS had substantially reduced survival rates (DSS plus flagellin group, 65%, vs. DSS alone, 94% survival; P < 0.001), as well as increased diarrhea, weight loss, and rectal bleeding (Fig. 8A, which is published as supporting information on the PNAS web site). These results are consistent with the data obtained from C3H/HeJ mice (Fig. 4). As expected, CD-1 mice that received water alone or water plus flagellin without DSS administration were unaffected. These results indicate that colonic flagellin enhances inflammation in injured colonic mucosa, whereas in intact colon, flagellin is unable to induce a colonic inflammatory response.
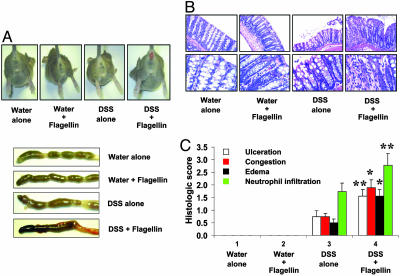
Flagellin Administration to Injured Colon Causes Severe Histopathological Damage in Colonic Mucosa. Next, we evaluated flagellin-induced histological changes in the colonic mucosa in C3H/HeJ mice administered with a low dose of DSS (2.5%) in their drinking water during the entire experimental period. Four days after DSS administration, mice were exposed colorectally to flagellin for 7 days, as described above. At the end of the experiment, frozen colonic tissue sections prepared from each group were stained with hematoxylin/eosin for histological assessment of colitis. Flagellin administration to DSS-treated mice resulted in hemorrhagic colon that was not evident in the control groups (water alone, water plus flagellin, and DSS alone; Fig. 5A). Histologic examination of mice that received flagellin together with DSS revealed severe erosive lesions and increased neutrophil infiltration of the colonic mucosa compared with mice treated with DSS alone. Mice exposed to DSS alone had mildly erosive epithelium, whereas mice that received water alone or water plus flagellin without DSS showed normal colonic histology (Fig. 5B). Next, we evaluated several histopathological parameters of colonic inflammation (ulceration, congestion, edema, and neutrophil infiltration). Colonic tissue sections of flagellin plus DSS-exposed mice showed much higher histopathologic scores of colonic inflammation compared with mice that received only DSS (P < 0.001-0.01). As expected, mice that received water alone or water plus flagellin had intact colonic epithelium (Fig. 5C). These data suggest that bacterial flagellin, in a disrupted colonic mucosa, is able to potently aggravate colitis-associated pathophysiology, although it has no effect in intact colonic mucosa.
Fig. 5.
Flagellin exposed to injured colon causes severe histopathological damage in colonic mucosa. C3H/HeJ mice received either water alone or water containing 2.5% DSS through the entire experimental period. Four days after initiation of DSS treatment, mice were injected intracolonically with flagellin (0.8-1.0 μg per mouse) daily for 7 days. (A) Mice exposed to flagellin and DSS showed severe rectal bleeding and hemorrhagic colon (representative of ≈20 mice per experimental condition). (B) Colonic tissue sections obtained from mice at day 7 after flagellin administration were fixed in formalin, paraffin embedded, and stained with hematoxylin/eosin (×200, Upper; ×600, Lower) (C) Histopathologic scoring for several parameters of colonic inflammation was evaluated as described in Materials and Methods [water alone (n = 14), water plus flagellin (n = 14), DSS alone (n = 15), DSS plus flagellin (n = 19)]. *, P < 0.001; **, P < 0.01.
We also confirmed the results obtained from C3H/HeJ mice in LPS-responsive CD-1 mice under identical experimental conditions. Similar to the results obtained from C3H/HeJ mice, we found that flagellin exposure of CD-1 mice, in the presence of DSS, resulted in significantly increased colonic mucosal damage compared with animals that received DSS alone (Fig. 8B). Moreover, CD-1 mice that received water alone or water plus flagellin without DSS showed intact colonic architecture (Fig. 8B). Histopathologic scoring of several colonic inflammatory parameters also revealed that colonic flagellin exposure in DSS-administered mice gave rise to substantially enhanced colonic inflammation compared to DSS alone (Fig. 8C), consistent with the data obtained from C3H/HeJ mice. Together, these results strongly suggest that flagellin exposure to the disrupted colonic mucosa substantially worsens ongoing colonic inflammation.
In addition, we investigated whether engagement of another TLR, such as TLR2 by PGN and LTA, affects the outcome of colonic inflammation. To test this, we performed similar experiments with PGN, using the same protocol as with our flagellin study. As shown in Fig. 9A, which is published as supporting information on the PNAS web site, unlike flagellin, intracolonic administration of PGN to DSS- or water-treated CD-1 mice did not affect the pathophysiology of colonic inflammation. Moreover, we did not observe any significant histopathologic difference in the colon of mice exposed to PGN (Fig. 9B). In addition, because PGN has been suggested to stimulate nucleotide oligomerization domain 2 (NOD2) as well as TLR2 (26), we tested whether another TLR2 ligand, LTA, is able to affect the pathophysiology of colonic inflammation. As shown in Fig. 9C, LTA did not change the pathophysiology of the colonic inflammation in DSS-colitis mouse model or in intact mouse colon. These results support the relative specificity of the flagellin/TLR5 engagement in the development of colitis, as presented above.
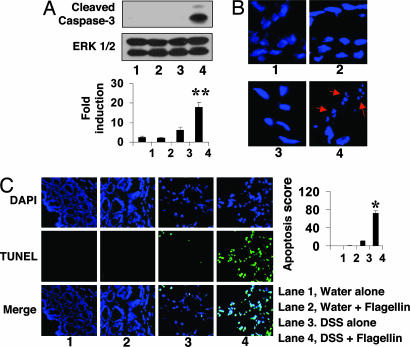
Flagellin Exposure to Injured Colon Causes Apoptosis in Colonic Epithelium. Because our results indicate that flagellin triggers proinflammatory responses and erosive damage in the colon, and evidence suggests a relationship between inflammation and apoptosis in the gut, we investigated whether flagellin exposure to the injured colon induces apoptosis in colonic mucosa. Total colonic protein extracts were prepared from C3H/HeJ mice groups, described in Fig. 2, and subjected to Western blot analysis with an antibody recognizing the apoptotic marker cleaved Caspase-3. Flagellin exposure to injured colon due to DSS administration resulted in cleavage of Caspase-3, compared with levels obtained in mice administered with DSS alone. Colonic protein extracts obtained from mice that received water alone or water plus flagellin did not show cleaved Caspase-3 (Fig. 6A).
Fig. 6.
Flagellin induces apoptosis in the compromised colonic mucosa. C3H/HeJ mice received either water alone or water containing 2.5% DSS through the entire experimental period. Four days after initiation of DSS treatment, mice were colorectally exposed to flagellin for 7 days. (A) Total protein prepared from colon tissues of each mice group was subjected to Western blot analysis with an antibody recognizing cleaved Caspase-3. OD values obtained from the x-ray films were expressed as relative ratio of cleaved Caspase-3 to inactive ERK1/2 serving as a loading control. Data are expressed as mean ± SEM of six mice per group. (B) Frozen colonic tissue sections obtained from the same mice groups were stained with DAPI to examine formation of apoptotic bodies, indicated by red arrows. (C) TUNEL assays were performed in frozen tissue sections followed by DAPI counterstaining of the nuclei. Slides were visualized and photographed under a fluorescent microscope. Values represent mean scores for the TUNEL-positive stain (overlapped TUNEL with DAPI-stained nuclei) ± SEM. *, P < 0.001; **, P < 0.01.
Furthermore, frozen sections of colon tissues obtained from the C3H/HeJ mice groups described in Fig. 2B were stained with DAPI to observe apoptotic nuclear body fragments.We found that flagellin exposure to colonic mucosa disrupted by DSS administration caused substantially increased apoptotic bodies in the colonic mucosa, compared with mice that received water alone, water plus flagellin, or DSS alone (Fig. 6B).
To confirm flagellin-induced apoptosis in colonic mucosa, we performed TUNEL assays in frozen colon tissue sections obtained from C3H/HeJ mice treated with flagellin or DSS, as described in Fig. 2B. TUNEL assays revealed massive apoptosis (Fig. 6C), indicated by TUNEL-positive cells (overlapped TUNEL with DAPI-stained nuclei) in the colonic mucosa of DSS plus flagellin mice compared with the control mice groups (water alone, water plus flagellin, and DSS alone). Although flagellin exposure to intact colon failed to induce apoptosis in the colonic mucosa, in DSS-injured colon, flagellin significantly increased the number of TUNEL-positive cells to ≈75% from ≈10% seen in mice that received DSS alone (Fig. 6C). DAPI counterstaining also showed intact colonic epithelium in water alone and water plus flagellin-exposed mice. However, DSS administration with or without flagellin caused mild colonic damage (Fig. 6C). Interestingly, the merged images with TUNEL and DAPI showed that TUNEL-positive cells are widely distributed in the colonic mucosa and appear to be highly prevalent in epithelium, implying that flagellin-induced apoptosis occurs mainly at the colonocyte level. Together, our findings strongly suggest that flagellin exposure to compromised colonic mucosa causes apoptosis. However, whether this is a direct flagellin/TLR5-associated response or a general proinflammatory effect of this bacterial product remains to be investigated.
Discussion
Several clinical observations and animal experiments strongly implied that intestinal bacteria might contribute to chronic bowel inflammation (5, 9, 10). Thus, an emerging concept is that a dysregulated or aberrant immune response between the host and the intestinal microbiota is implicated or results in IBD.
Either commensal or pathogenic microbes contain or release conserved molecular patterns such as LPS, PGN, and flagellin that are recognized by pattern recognition receptors of the TLR family members, followed by activation of innate or adaptive immune responses. In this context, the TLR response in the colonic mucosa appears to be important in the host-microbiota interaction and intestinal homeostasis. Among various TLRs that are expressed in the intestinal epithelium (28, 29), TLR5 is strongly expressed in intestinal epithelial cells (12). Moreover, flagellin from commensal or enteroinvasive microbes is able to stimulate TLR5, resulting in elevated proinflammatory cytokine production in vivo (22, 27, 30). Therefore, it is important to determine the role of the TLR5-associated responses activated by bacterial flagellin in the pathogenesis of colonic inflammation.
We demonstrated here that the flagellin/TLR5 response is confined to the basolateral, not the apical, aspect of the intestinal mucosa, indicating that the intact colonic mucosa is not responsive to luminal bacterial flagellin (Fig. 1). This confined TLR5 response in colonocytes allows the intestine to compartmentalize the large concentration of foreign bacterial antigens, including flagellin in the colonic lumen. However, our data imply that once the intact colon is compromised, the disrupted colonic mucosa becomes highly susceptible to colonic flagellin, resulting in initiation of an inflammatory response. Several studies with colon adenocarcinoma cell lines, however, demonstrated that flagellin/TLR5 responses are basolaterally localized (20, 21), supporting our results obtained from human mucosa preparations mounted on Ussing chambers. Indeed, our results (Figs. 2 and 3) demonstrate that only the disrupted colonic mucosa is responsive to flagellin as it relates to MEK1/2 activation and cytokine production. Furthermore, the recent study of Lodes et al. (15) demonstrating that bacterial flagellin is a dominant antigen in Crohn's disease patients, a disease characterized with transmural inflammation throughout the gastrointestinal track, but not control patients, provides support to our conclusion. However, we cannot exclude the possibility that immune cells, such as lymphocytes or macrophages, in the submucosa might be activated by flagellin in our Ussing chamber experiments presented in Fig. 1, although studies by Smythies et al. (31) showed that human intestinal macrophages are not responsive to various inflammatory stimuli, including LPS.
We show that flagellin exposure to disrupted colon increases proinflammatory cytokine production and substantially aggravates colonic inflammation. Because our results can be reproduced in both TLR4-deficient and normal mice, it is unlikely that LPS, a relatively common contaminant in preparations of microbial products, is responsible for the flagellin-induced responses observed in our study. Furthermore, the flagellin preparation used in our study failed to induce NF-κB activation in RAW264.7 cells (Fig. 10, which is published as supporting information on the PNAS web site), a murine macrophage cell line highly sensitive to various TLR ligands but not to flagellin, due to the lack of TLR5 (32), indicating that the flagellin preparation does not seem to contain other TLR ligands that might trigger engagement of other TLRs. Moreover, intracolonic administration of PGN or LTA had no effect in water- or DSS-administered mice (Fig. 9 A-C). A combination of TLR2 and nucleotide oligomerization domain 2 (NOD2) stimulation by PGN did not affect the pathophysiology of colitis in the DSS-colitis mouse model (Fig. 9 A and B). These results indicate that TLR5 plays an important and relatively specific role in the pathophysiology of colitis in DSS-injured mucosa.
Interestingly, however, flagellin exposure to DSS-exposed CD-1 mice caused less mortality and had less severe colitis compared with C3H/HeJ mice (see Fig. 4). These differences could be explained by a protective effect of LPS in LPS-responsive CD-1 mice, as Rakoff-Nahoum et al. (6) recently suggested. In addition, flagellin exposure to disrupted colon causes apoptosis in the colonic mucosa, probably involved in the severe colonic erosive lesions shown in Figs. 5 and 6.
Based on our evidence, it is reasonable to suggest that flagellin released from commensal or enteroinvasive bacteria in the gut would be able to aggravate preexisting colonic damage in patients with IBD or other forms of colonic inflammation. Our results also provide important clues to help understand the development and progress of colonic inflammation.
Supplementary Material
Acknowledgments
We thank Dr. J. Thomas LaMont for critical reading of the manuscript. This work was supported by a Research Fellowship Award from the Crohn's and Colitis Foundation of America, Inc.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: DSS, dextran sulfate sodium; IBD, inflammatory bowel disease; LTA, lipoteichoic acid; PGN, peptidoglycan; TLR, Toll-like receptor; MEK, mitogen-activated protein kinase/extracellular signal-related kinase.
References
- 1.Underhill, D. M. & Ozinsky, A. (2002) Curr. Opin. Immunol. 14, 103-110. [DOI] [PubMed] [Google Scholar]
- 2.Kopp, E. & Medzhitov, R. (2003) Curr. Opin. Immunol. 15, 396-401. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S. (2003) Curr. Opin. Immunol. 15, 5-11. [DOI] [PubMed] [Google Scholar]
- 4.Hooper, L. V., Wong, M. H., Thelin, A., Hansson, L., Falk, P. G. & Gordon, J. I. (2001) Science 291, 881-884. [DOI] [PubMed] [Google Scholar]
- 5.Swidsinski, A., Ladhoff, A., Pernthaler, A., Swidsinski, S., Loening-Baucke, V., Ortner, M., Weber, J., Hoffmann, U., Schreiber, S., Dietel, M., et al. (2002) Gastroenterology 122, 44-54. [DOI] [PubMed] [Google Scholar]
- 6.Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. (2004) Cell 118, 229-241. [DOI] [PubMed] [Google Scholar]
- 7.Strober, W. (2004) Nat. Med. 10, 898-900. [DOI] [PubMed] [Google Scholar]
- 8.Stagg, A. J., Hart, A. L., Knight, S. C. & Kamm, M. A. (2003) Gut 52, 1522-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hart, A. L., Stagg, A. J. & Kamm, M. A. (2003) J. Clin. Gastroenterol. 36, 111-119. [DOI] [PubMed] [Google Scholar]
- 10.Cummings, J. H., Macfarlane, G. T. & Macfarlane, S. (2003) Curr. Issues Intest. Microbiol. 4, 9-20. [PubMed] [Google Scholar]
- 11.Beutler, B. (2001) Immunity 15, 5-14. [DOI] [PubMed] [Google Scholar]
- 12.Rhee, S. H., Keates, A. C., Moyer, M. P. & Pothoulakis, C. (2004) J. Biol. Chem. 279, 25179-25188. [DOI] [PubMed] [Google Scholar]
- 13.Kwon, J. H., Keates, S., Bassani, L., Mayer, L. F. & Keates, A. C. (2002) Gut 51, 818-826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banks, C., Bateman, A., Payne, R., Johnson, P. & Sheron, N. (2003) J. Pathol. 199, 28-35. [DOI] [PubMed] [Google Scholar]
- 15.Lodes, M. J., Cong, Y., Elson, C. O., Mohamath, R., Landers, C. J., Targan, S. R., Fort, M. & Hershberg, R. M. (2004) J. Clin. Invest. 113, 1296-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riegler, M., Castagliuolo, I., Wang, C., Wlk, M., Sogukoglu, T., Wenzl, E., Matthews, J. B. & Pothoulakis, C. (2000) Gastroenterology 119, 348-357. [DOI] [PubMed] [Google Scholar]
- 17.Mall, M., Kreda, S. M., Mengos, A., Jensen, T. J., Hirtz, S., Seydewitz, H. H., Yankaskas, J., Kunzelmann, K., Riordan, J. R. & Boucher, R. C. (2004) Gastroenterology 126, 32-41. [DOI] [PubMed] [Google Scholar]
- 18.Siegmund, B., Lehr, H. A. & Fantuzzi, G. (2002) Gastroenterology 122, 2011-2025. [DOI] [PubMed] [Google Scholar]
- 19.Rhee, S. H., Jones, B. W., Toshchakov, V., Vogel, S. N. & Fenton, M. J. (2003) J. Biol. Chem. 278, 22506-22512. [DOI] [PubMed] [Google Scholar]
- 20.Gewirtz, A. T., Navas, T. A., Lyons, S., Godowski, P. J. & Madara, J. L. (2001) J. Immunol. 167, 1882-1885. [DOI] [PubMed] [Google Scholar]
- 21.Reed, K. A., Hobert, M. E., Kolenda, C. E., Sands, K. A., Rathman, M., O'Connor, M., Lyons, S., Gewirtz, A. T., Sansonetti, P. J. & Madara, J. L. (2002) J. Biol. Chem. 277, 13346-13353. [DOI] [PubMed] [Google Scholar]
- 22.Bambou, J. C., Giraud, A., Menard, S., Begue, B., Rakotobe, S., Heyman, M., Taddei, F., Cerf-Bensussan, N. & Gaboriau-Routhiau, V. (2004) J. Biol. Chem. 279, 42984-42992. [DOI] [PubMed] [Google Scholar]
- 23.Kitajima, S., Takuma, S. & Morimoto, M. (1999) Exp. Anim. 48, 137-143. [DOI] [PubMed] [Google Scholar]
- 24.Poltorak, A., He, X., Smirnova, I., Liu, M. Y., Van Huffel, C., Du, X., Birdwell, D., Alejos, E., Silva, M., Galanos, C., et al. (1998) Science 282, 2085-2088. [DOI] [PubMed] [Google Scholar]
- 25.Rhee, S. H. & Hwang, D. (2000) J. Biol. Chem. 275, 34035-34040. [DOI] [PubMed] [Google Scholar]
- 26.Visser, L., Jan de Heer, H., Boven, L. A., van Riel, D., van Meurs, M., Melief, M. J., Zahringer, U., van Strijp, J., Lambrecht, B. N., Nieuwenhuis, E. E., et al. (2005) J. Immunol. 174, 808-816. [DOI] [PubMed] [Google Scholar]
- 27.Murthy, K. G., Deb, A., Goonesekera, S., Szabo, C. & Salzman, A. L. (2004) J. Biol. Chem. 279, 5667-5675. [DOI] [PubMed] [Google Scholar]
- 28.Cario, E. & Podolsky, D. K. (2000) Infect. Immun. 68, 7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Otte, J. M., Cario, E. & Podolsky, D. K. (2004) Gastroenterology 126, 1054-1070. [DOI] [PubMed] [Google Scholar]
- 30.Didierlaurent, A., Ferrero, I., Otten, L. A., Dubois, B., Reinhardt, M., Carlsen, H., Blomhoff, R., Akira, S., Kraehenbuhl, J. P. & Sirard, J. C. (2004) J. Immunol. 172, 6922-6930. [DOI] [PubMed] [Google Scholar]
- 31.Smythies, L. E., Sellers, M., Clements, R. H., Mosteller-Barnum, M., Meng, G., Benjamin, W. H., Orenstein, J. M. & Smith, P. D. (2005) J. Clin. Invest. 115, 66-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West, A. P., Dancho, B. A. & Mizel, S. B. (2005) J. Biol. Chem. 280, 9482-9488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.