Abstract
Multiple cytokines, including IL-2, can affect T cell proliferation and survival. However, IL-2 can lead to apoptosis as well as proliferation, making unclear whether IL-2 receptor (IL-2R) signals ultimately have a predominantly positive or negative effect. To address this issue, we examined the effect of enhancing IL-2R signals in CD8+ T cells after antigen stimulation by engineering a transgenic (Tg) mouse strain with CD8+ T cells capable of augmented, regulated, autocrine IL-2R signaling after target recognition by means of expression of a chimeric granulocyte-macrophage colony-stimulating factor (GM-CSF)/IL-2R. The Tg CD8+ T cells can bind the granulocyte-macrophage colony-stimulating GM-CSF produced by antigen stimulation, but the GM-CSF binding results in delivery of an IL-2R signal. After antigen stimulation in vivo, the Tg T cells demonstrated marked increases in the initial proliferative response and cell expansion and displayed continued increases in cell expansion after repeated antigen exposure. These data suggest that the predominant role of IL-2R signals delivered to responding CD8+ T cells is to set the size of the initial response to antigen by promoting T cell proliferation and survival and not cell death.
During the acute phase of a CD8+ T cell response to an antigen, naive CD8+ T cells can undergo massive proliferation and expansion (1, 2), resulting in a large population of effector CD8+ T cells expressing intracellular perforin and capable of cytolytic activity. The overall effectiveness of a T cell response to a pathogen is in part determined by the magnitude of this expansion. For example, in HIV infection, control of acute virus infection has been linked to the magnitude of the antiviral CD8+ T cell response (3).
The magnitude of the T cell response is regulated in part by signals delivered to T cells through cytokine receptors. The IL-2 receptor (IL-2R) complex is a heterotrimer comprised of a unique α chain (CD25), a β chain shared with the IL-15 receptor, and a γ chain shared with the IL-4, IL-7, IL-9, and IL-15 receptors, all of which also can deliver proliferative signals (4). CD25 is required for high affinity binding of IL-2 but is dispensable for signal transduction by the receptor (5, 6). IL-2 was first described as a growth and survival factor capable of inducing T cell proliferation, and the elimination of IL-2 from proliferating T cells can lead to cytokine withdrawal-mediated cell death. However, IL-2 has since been shown to also have negative regulatory effects on T cell responses. IL-2 enhances activation-induced cell death (AICD) in T cells (7) and may inhibit T cell expansion during the acute response to antigen by down-regulating the shared γc chain, resulting in decreased T cell proliferation, reduced bcl-2 expression, and increased T cell death (8). The potential negative regulatory role of IL-2R signaling in T cell responses is particularly evident in mice deficient in IL-2. IL-2−/− mice exhibit only mild immunodeficiency (9), but T cells from IL-2 or CD25-knockout mice exhibit decreased cell death after activation (8), and the mice demonstrate marked accumulation of lymphocytes, generally succumbing to fatal T cell-mediated autoimmune diseases (10, 11). However, concluding the physiologic role of IL-2 in peripheral T cell responses from these mice may be misleading, because such mice may also have confounding defects in thymic selection and/or production of regulatory CD4+ and CD8+ T cells (12, 13).
The mild impairment in T cell expansion in IL-2−/− mice may be caused by compensatory positive contributions of other cytokines, particularly IL-15, to T cell proliferation during the acute phase of a T cell response after antigen encounter. IL-15 promotes T cell proliferation and appears to protect T cells from AICD in vitro (14, 15). Furthermore, IL-15 recently has been shown to drive CD4+ T cell expansion after antigen stimulation in vivo and play a critical role in the maintenance of memory CD8+ T cells (8, 16). Thus, it has been suggested that IL-15 rather than IL-2 may be the essential cytokine for expanding T cells as well as establishing and maintaining T cell memory.
CD8+ T cells generally lose the ability to produce IL-2 after differentiation into effector T cells (17, 18). Based on the two opposing effects of IL-2R signaling, this might not only result in tighter regulation of the CD8+ T cell response by increasing the dependence for continued expansion on CD4+ T helper (Th) cell production of IL-2, but also represent a mechanism to shield CD8+ T cells from IL-2-mediated cell death. One approach to examine the role of IL-2 in CD8+ T cell responses has been to provide excess exogenous IL-2, which can increase IL-2R signaling after antigen stimulation. Enhanced CD8+ T cell responses after IL-2 administration have been observed in both mice and humans (19, 20). However, exogenous IL-2 could act indirectly by activating CD4+ Th cells, which in turn enhance CD8+ T cell responses by means of alternative pathways (21). Additionally, the effects of exogenous IL-2 may vary depending on the dominant sites of the immune response, as IL-2 administered systemically may variably penetrate and persist in peripheral tissues. Finally, IL-2 administration cannot be synchronized with T cell activation, and thus might have unpredictable effects on CD8+ T cell function and viability. Therefore, our laboratory has been exploring strategies to genetically modify CD8+ T cells to provide an antigen-regulated, autocrine IL-2 signal in effector/memory as well as in naive CD8+ T cells.
Differentiated effector CD8+ T cells retain the capacity to secrete many cytokines including granulocyte-macrophage colony-stimulating factor (GM-CSF) (17), but do not express the GM-CSF receptor (22). Our laboratory has developed chimeric GM-CSF/IL-2Rs (GMIL2R) consisting of the ligand-binding ectodomains of the GM-CSF receptor α and β chains fused with the signaling, cytoplasmic endodomains of the IL-2R γ and β chains, respectively. GM-CSF induces dimerization of the chimeric GMIL2R chains, resulting in delivery of the authentic IL-2 signal (6). Because GM-CSF is not detectable in the serum (23), and production by T cells after T cell receptor (TCR) ligation follows similar kinetics to IL-2 production by T cells (24), functional signals to T cells expressing the GMIL2R should be limited primarily to T cells responding to antigen.
To study the effect of augmented signaling by means of the IL-2R in responding CD8+ T cells in vivo, we have generated transgenic (Tg) mice expressing the murine GMIL2R under the control of a CD8 enhancer/promoter element (25). CD8+ T cells from GMIL2R Tg mice exhibited GM-CSF-mediated autocrine growth in vitro and increased T cell proliferation and expansion in vivo, without a proportional increase in T cell death after antigen stimulation either in vitro or in vivo. The results suggest that the availability of IL-2R signals to CD8+ T cells limits the size of T cell responses and regulates CD8+ T cell expansion predominantly by a positive influence on the magnitude of T cell proliferation and expansion.
Materials and Methods
Mice.
All mice were maintained in a specific pathogen-free facility according to University of Washington guidelines. B6, RAG−/−, and Thy1.1 congenic mice all were obtained from The Jackson Laboratories. TCRαFBL Tg mice were generated in our laboratory, and >98% of CD8+ T cells in these mice express an H-2Db-restricted TCR (Vα3/Vβ12) specific for the gag protein of Friend virus-induced leukemia (FBL) cells (C.Ö., unpublished work).
Generation of Chimeric GMIL2R Genes and GMIL2R Tg Mice.
Murine GMαIL2Rγ and GMβIL2Rβ genes were constructed in a similar manner to human chimeric receptors previously generated in our laboratory (6), and each chimeric receptor chain was separately cloned into the CD8 enhancer/promoter vector Tg-D, kindly provided by Dan Littman (Skirball Institute, New York). The GMαIL2Rγ and GMβIL2Rβ transgene cassettes were then isolated, combined at an ≈1:1 molar ratio, and microinjected into fertilized B6 eggs. Genotyping of founder mice was performed by Southern blot of tail DNA, digested with BamHI, and probed with a portion of the CD4 gene, which is also part of the transgene construct.
Immunoblot.
Cells (5 × 106) were resuspended in 200 μl of lysis buffer (0.1 M Tris⋅HCl pH 8.0/0.1 M NaCl/0.5% Nonidet P-40). Approximately 30 μg of extract was loaded onto 8% SDS/PAGE minigels (Hoefer) and transferred to nitrocellulose (Schleicher and Schuell). Blots were then probed with rabbit anti-mouse antibodies specific for the intracellular portion of IL-2Rγ or IL-2Rβ (Santa Cruz Biotechnology).
Cell Culture.
FBL-specific CD8+ T cells were maintained in vitro by weekly stimulation of 106 T cells with 2 × 106 irradiated FBL cells (10,000 rad), 5 × 106 irradiated syngeneic splenocytes (3,000 rad), and 20 units/ml IL-2 in RPMI supplemented with 10% FBS (10% RPMI). For proliferation assays, effector T cells were harvested 7 days after stimulation and washed, and 2 × 105 T cells were plated in triplicate into 96-well round bottom plates with 5 × 105 irradiated splenocytes and 2 × 104 irradiated FBL. [3H]thymidine (1 μCi) was added 3 days after stimulation and plates were harvested 18 h later. For analysis of growth, effector T cells were harvested 7 days after stimulation and washed, and 105 T cells were plated with 106 irradiated splenocytes and 105 FBL. Cells were enumerated in the presence of Trypan blue. To block the effects of GM-CSF, 20 μg/ml of anti-GM-CSF-neutralizing Ab MP1–22E9 (PharMingen) was added at the initiation of culture.
Carboxyfluorescein Diacetate-Succinimidyl Ester (CFSE) Labeling.
T cells were labeled by incubation with 20 μg/ml CFSE (Molecular Probes) in 10% RPMI for 45–60 min at 37°C.
T Cell Depletion.
Splenocytes were CD4-depleted by using anti-CD4 magnetic beads (Dynal) before in vitro culture or adoptive transfer. Such depletions typically eliminated >98% of CD4+ T cells as determined by flow cytometry.
Cytokine ELISAs.
TCRαFBL splenocytes or effector T cells (105) were stimulated with 104 irradiated FBL in a 96-well round bottom plate. Seventy two hours later, 50 μl of culture supernatant was harvested and cytokines were measured with ELISA reagents specific for murine IL-2 or GM-CSF (Endogen, Woburn, MA).
Purification of Anti-CD4 mAb.
The anti-CD4 mAb secreting GK1.5 hybridoma was grown in DMEM supplemented with 10% FBS. Supernatant was collected and precipitated with 50% NH4Cl. The precipitate was reconstituted in 1× PBS and dialyzed against 1× PBS before use.
Antibodies and Flow Cytometry.
Antibodies (conjugated to various fluorochromes or biotin) against the following molecules were used: CD4, CD8, CD25, CD44, CD62L, CD69, Ly6c, Vα3.2, and Thy1.2 (PharMingen). Cell death was assessed by surface binding of annexin V and uptake of 7AAD (PharMingen). Antibody staining was performed in RPMI supplemented with 1–10% FBS and annexin V in annexin V binding buffer. Cells were analyzed by using a FACSCalibur and cellquest software (Becton Dickinson).
Results
Naive CD8+ T Cells Lose the Ability to Produce IL-2 But Not GM-CSF After Differentiation into Effector T Cells.
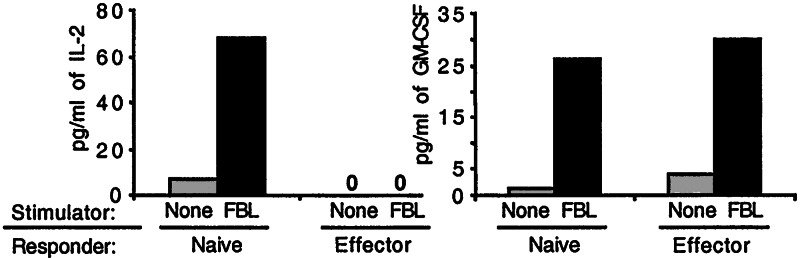
To analyze the cytokine secretion profile of naive and effector CD8+ T cells after antigen stimulation, large numbers of antigen-specific CD8+ T cells bearing a defined TCR specificity were required. Thus, we stimulated either naive CD8+ T cells from spleens of TCRαFBL Tg mice with irradiated FBL-3 cells, or effector TCRαFBL cells generated by repetitive in vitro stimulation of TCRαFBL Tg CD8+ T cells, and compared GM-CSF and IL-2 production. The weekly stimulation of TCRαFBL cells with antigen for >5 weeks differentiates all T cells into effector cells, which display direct cytolytic activity, and are uniformly CD25+, CD69+, Ly6chi, CD44hi, and CD62L− (data not shown). Analysis of culture supernatants from stimulated naive and effector TCRαFBL cells at 72 h by ELISA revealed that freshly isolated, naive TCRαFBL cells produced both IL-2 and GM-CSF (Fig. 1). By contrast, effector TCRαFBL cells no longer produced measurable levels of IL-2 but did produce equivalent amounts of GM-CSF as naive T cells. Analysis of culture supernatants at multiple time points revealed similar comparative results (data not shown).
Figure 1.
GM-CSF and IL-2 production by naive and effector CD8+ T cells. Naive and effector TCRαFBL CD8+ T cells were stimulated with FBL and supernatants were examined for IL-2 and GM-CSF secretion 72 h later.
Generation and Characterization of GMIL2R Tg Mice.
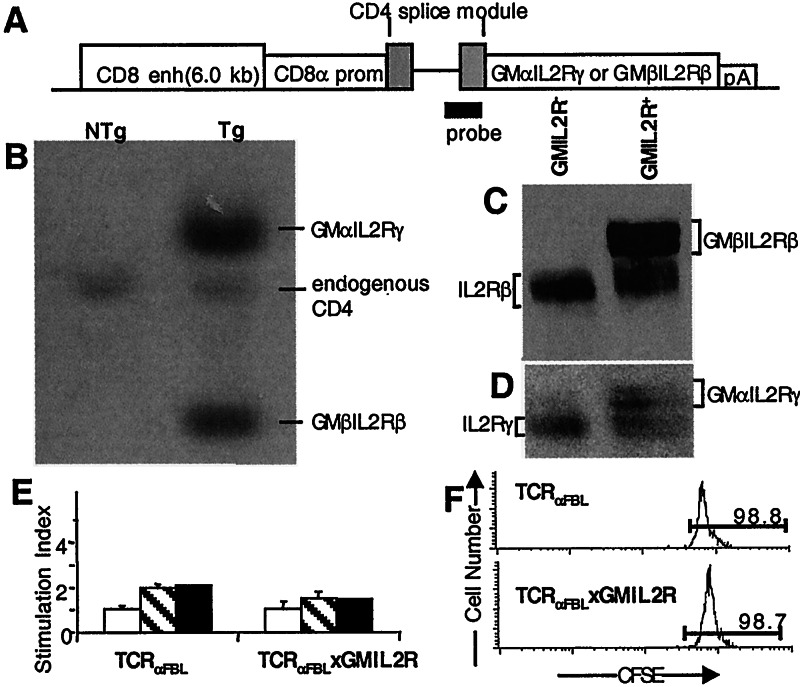
Tg mice expressing the GMIL2R in CD8+ T cells were constructed with the GMαIL2Rγ and GMβIL2Rβ transgenes under the control of a 6.0-kbp CD8 enhancer/promoter element that drives transgene expression exclusively in mature CD4−CD8+ single positive thymocytes and peripheral CD8+ T cells (25). Thus, the transgenes are not expressed during thymocyte maturation and selection. Founder mice were analyzed for transgene integration by Southern blot analysis using a probe derived from a portion of the CD4 sequence (Fig. 2A). One mouse (F9) of 16 founder mice had integrated both transgenes (Fig. 2B). Comparison of the relative intensity of each chimeric transgene band with the endogenous CD4 band by densitometry suggested F9 carried ≈6 copies of the GMβIL2Rβ transgene and 12 copies of the GMαIL2Rγ transgene. The two transgenes appeared to have integrated at a single locus as genotyping of offspring revealed cosegregation of the transgenes and stable transmission of the relative transgene copy number (data not shown).
Figure 2.
Construction and identification of GMIL2R Tg mice. (A) Schematic diagram of the transgene constructs and probe. (B) Southern Blot of tail DNA from GMIL2R Tg mice. (C and D) Immunoblot analysis of GMIL2R− and GMIL2R+ CD8+ T cells stained with antibodies specific for (C) the intracellular domain of the IL-2Rβ or (D) the IL-2Rγ. (E) 2 × 105 TCRαFBL and TCRαFBL×GMIL2R splenocytes were placed in culture for 3 days with either media alone (open bars), 5 ng/ml GM-CSF (hatched bars), or 20 units/ml IL-2 (closed bars) and then pulsed for 18 h with 1 μCi [3H]thymidine. (F) 3 × 106 CFSE loaded TCRαFBL and TCRαFBL×GMIL2R T cells were transferred into B6 hosts i.v., and 7 days later, the TCR+CD8+ T cells were analyzed for CFSE content.
Examination of transgene expression by reverse transcription–PCR demonstrated that the chimeric receptors were expressed only in CD8+ cells (Fig. 6, which is published as supporting information on the PNAS web site, www.pnas.org), and mice expressing the GMIL2R transgenes did not exhibit any gross changes in central or peripheral lymphoid T cell populations. Thymi, lymph nodes, and spleens from GMIL2R Tg mice showed no discernible difference in total cell number or in the size of the CD4 and CD8 T cell populations as compared with B6 mice (data not shown).
Chimeric protein expression was assessed by immunoblot of T cell lysates with antibodies specific for the intracellular portions of the IL-2Rβ and IL-2Rγ chains. GMIL2R+ T cells expressed significant amounts of both transgenes relative to the endogenous IL-2R chains (Fig. 2 C and D). By densitometry, GMIL2R+ T cells expressed equivalent levels of the GMβIL2Rβ and GMαIL2Rγ proteins compared with endogenous IL-2Rβ and γ chain expression.
The GMIL2R is expressed in a constitutive fashion. Thus, the possibility that GMIL2R+ T cells were inappropriately activated or proliferated in the absence of antigen was tested. Similar to control TCRαFBL T cells, fresh CD8+ splenic T cells from TCRαFBL×GMIL2R mice were predominantly CD25−, CD69−, CD44lo/int, Ly6clo, and CD62L+, suggesting that TCRαFBL×GMIL2R cells were phenotypically naive (data not shown). To examine the potential for antigen-independent proliferation, freshly isolated TCRαFBL×GMIL2R splenic T cells were exposed to 5 ng/ml GM-CSF, which is 10-fold higher than the concentration required to induce proliferation of transfected CTLL-2 cells expressing the chimeric GMIL2R (data not shown). Seventy two hours later, the T cells were pulsed for 18 h with [3H]thymidine. No increase in proliferation was observed in TCRαFBL×GMIL2R cells compared with TCRαFBL cells exposed to 5 ng/ml GM-CSF in the absence of antigen stimulation (Fig. 2E). Similarly, neither TCRαFBL nor TCRαFBL×GMIL2R T cells showed a significant increase in proliferation above background when exposed to 20 units/ml IL-2, consistent with reports that naive T cells do not respond to even pharmacologic doses of IL-2 in the absence of a TCR trigger (26).
The potential for antigen-independent proliferation was also examined in vivo. Naive TCRαFBL and TCRαFBL×GMIL2R T cells were labeled ex vivo with CFSE to monitor cell divisions and inoculated i.v. into B6 hosts. After 7 days in vivo, nearly all GMIL2R+ and GMIL2R− T cells had failed to divide (Fig. 2F). Additionally, similar numbers of TCRαFBL and TCRαFBL×GMIL2R cells were recovered at 7 days, suggesting there was not increased retention (or loss) of TCRαFBL× GMIL2R cells (data not shown).
GMIL2R+ T Cells Display Autocrine Proliferation and GM-CSF-Dependent Autocrine Growth in Vitro After Antigen Stimulation.
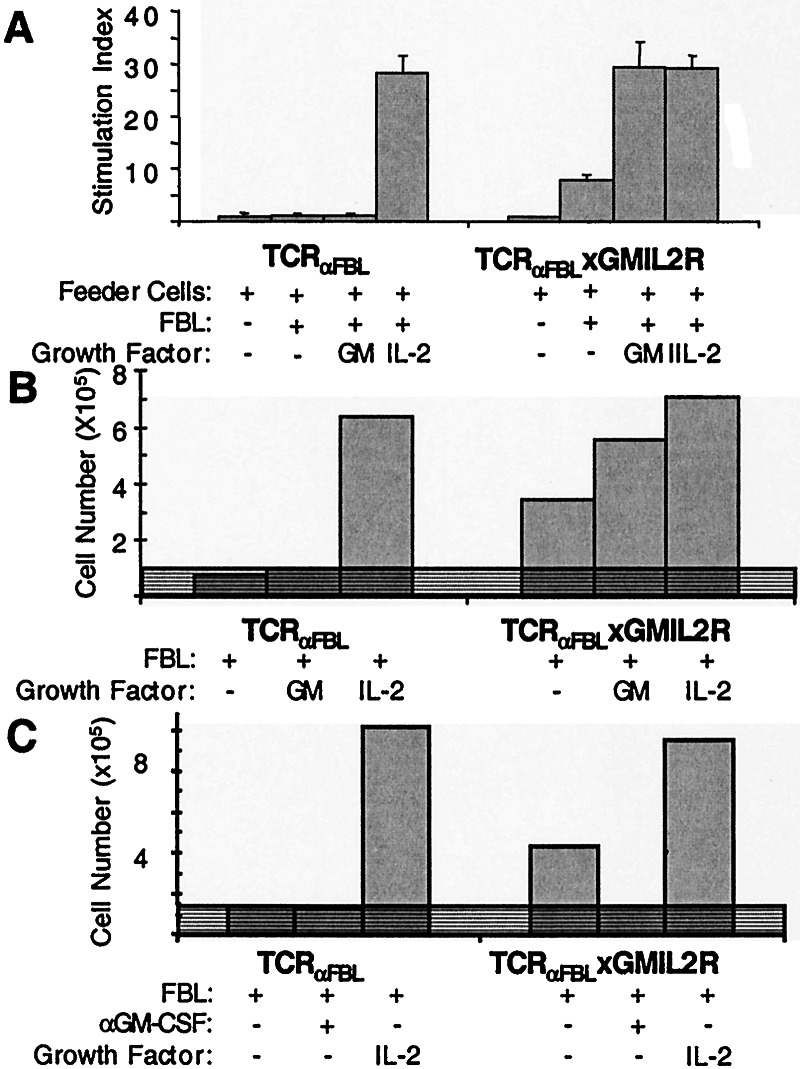
We next determined whether expression of the GMIL2R bypassed the requirement of effector T cells for exogenous IL-2 and resulted in autocrine proliferation and expansion. Effector TCRαFBL and TCRαFBL×GMIL2R T cells previously differentiated in vitro by weekly antigen stimulation with irradiated FBL cells and exogenous IL-2 were stimulated with FBL in wells supplemented with either media alone, 1 ng/ml GM-CSF, or 20 units/ml IL-2, and pulsed with [3H]thymidine 72 h later (Fig. 3A). Both TCRαFBL and TCRαFBL×GMIL2R cells proliferated equally after recognition of FBL in the presence of exogenous IL-2, but no proliferation was observed in TCRαFBL cells stimulated with FBL alone or supplemental GM-CSF. By contrast, TCRαFBL× GMIL2R cells proliferated in response to FBL in the absence of exogenous cytokines. The addition of exogenous GM-CSF further increased proliferation in response to antigen to levels equivalent to that observed with the addition of exogenous IL-2, suggesting that the amount of available cytokine, and not the level of GMIL2R expression, limited the degree of autocrine proliferation.
Figure 3.
Autocrine proliferation and growth of GMIL2R+ CD8+ T cells. (A) [3H]thymidine uptake of effector TCRαFBL×GMIL2R and control TCRαFBL T cells was measured 3 days after stimulation with antigen plus either media, GM-CSF, or IL-2. (B) Viable TCRαFBL×GMIL2R and control TCRαFBL T cells were enumerated 4 days after stimulation with FBL with the addition of media alone, GM-CSF, or IL-2. (C) Viable TCRαFBL×GMIL2R T cells were enumerated 4 days after FBL stimulation in the presence of neutralizing anti-GM-CSF mAb. Shaded regions represent the input cell number at initiation of culture. Data in B and C are representative of >5 separate experiments.
Cellular expansion resulting from the autocrine proliferation observed in TCRαFBL×GMIL2R cells was assessed. Effector TCRαFBL and TCRαFBL×GMIL2R cells were stimulated with irradiated FBL in the presence or absence of 20 units/ml IL-2, and viable cells excluding trypan blue were counted after 4 days. Both wild-type and GMIL2R+ T cells displayed significant numerical expansion in the presence of IL-2 (Fig. 3B). However, in the absence of supplemental exogenous IL-2 after antigen stimulation, TCRαFBL cells did not expand, whereas TCRαFBL×GMIL2R T cells expanded approximately 4-fold. This response was approximately 40% of the expansion seen with cells supplemented with pharmacologic doses of 20 units/ml IL-2. The addition of 1 ng/ml of exogenous GM-CSF increased growth of TCRαFBL×GMIL2R T cells to levels similar to those observed with IL-2 supplementation. To confirm that the observed autocrine growth was GM-CSF-dependent, anti-GM-CSF-neutralizing mAb was added at the initiation of culture. The mAb completely inhibited the expansion of TCRαFBL×GMIL2R T cells after FBL stimulation (Fig. 3C). The mAb itself was not toxic, as the addition of IL-2 in the presence of anti-GM-CSF mAb restored T cell expansion (data not shown).
GMIL2R+ T Cells Do Not Display Enhanced Susceptibility to AICD.
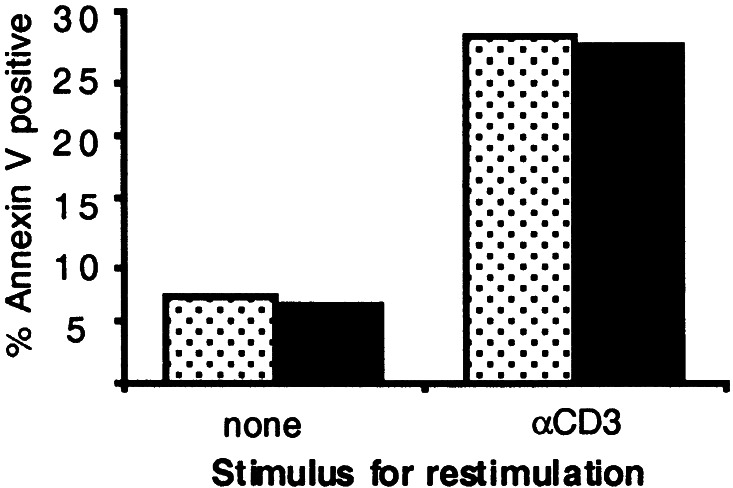
IL-2R signals can be associated with augmented AICD in CD8+ T cells, particularly if T cells are exposed to repetitive TCR activation (7). Therefore, fresh naive splenic TCRαFBL and TCRαFBL×GMIL2R T cells were stimulated with irradiated FBL at a 1:1 responder/stimulator ratio. After 72 h in culture, nearly all CD8+ T cells expressed an activated CD69+CD44hi phenotype (data not shown). The activated T cells were then washed and restimulated in αCD3-coated or control (uncoated) plates to initiate triggering of all T cells, and 20 h later, apoptosis was examined by staining with annexin V (Fig. 4). The restimulation of activated T cells led to apoptosis of 28% of the TCRαFBL cells, which represented a 4- to 5-fold increase compared with cells that did not receive a secondary restimulation. Apoptosis in TCRαFBL×GMIL2R cells was similar to TCRαFBL cells, suggesting that the increased proliferation resulting from endogenous production of GM-CSF and delivered by means of the GMIL2R did not lead to increased susceptibility to AICD.
Figure 4.
Examination of AICD in GMIL2R+ and control CD8+ T cells. TCRαFBL (speckled bars) and TCRαFBLxGMIL2R (closed bars) T cells were stimulated with FBL on day 0, and 72 h later, plated onto anti-CD3-coated or uncoated plates. Twenty hours after restimulation, the percentage of CD8+ T cell death was determined by flow cytometry using the apoptotic marker annexin V and viability marker 7AAD. Results are representative of two independent experiments.
GMIL2R+ T Cells Display Increased Proliferation, Expansion, and Memory Formation After Antigen Stimulation in Vivo.
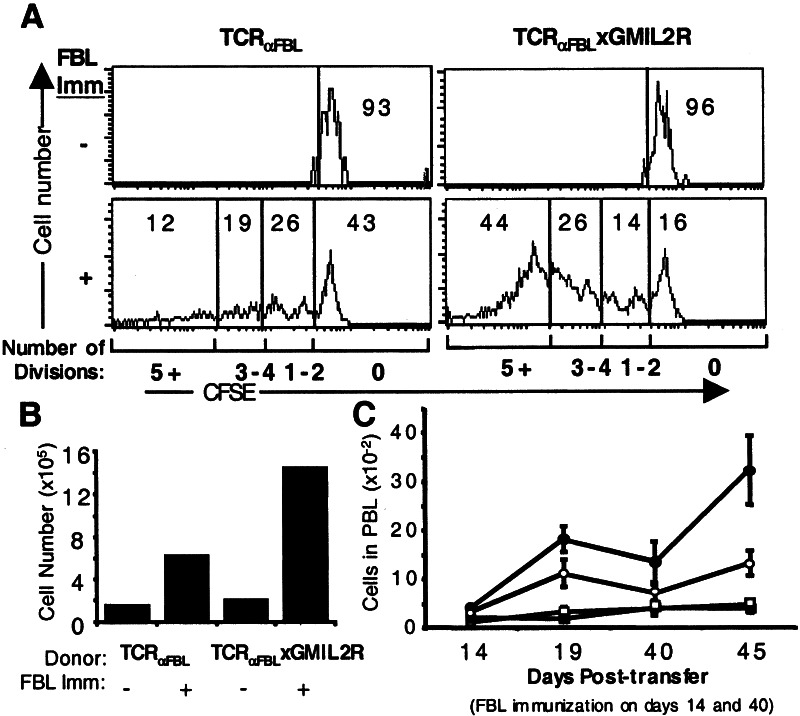
The autocrine growth of GMIL2R+ T cells in vitro after stimulation suggested that providing CD8+ T cells with increased antigen-regulated IL-2R signals can lead to enhanced proliferation and expansion after antigen stimulation. To determine whether this occurs in vivo, naive TCRαFBL and TCRαFBL×GMIL2R cells were labeled with CFSE and transferred into congenic Thy1.1 hosts. To remove the potentially confounding effects of CD4+ T cell help, Thy1.1 hosts were depleted of CD4+ T cells by injection with anti-CD4 mAb (GK1.5) purified from hybridoma supernatants, which eliminates >99% of CD4+ T cells by the third day after injection. Three days after CD4 depletion, mice were injected i.v. with 107 irradiated FBL-3 cells, and 5 days after antigen challenge, splenic T cells were examined for CFSE content and the transferred T cells were enumerated. In the absence of antigen stimulation, both TCRαFBL and TCRαFBL×GMIL2R cells showed little proliferation with similar numbers of T cells recovered (Fig. 5 A and B). After immunization with FBL, 57% of the recovered TCRαFBL cells had undergone at least one round of proliferation, but only 12% of cells had achieved five or more divisions (Fig. 5A). By contrast, 84% of recovered TCRαFBL×GMIL2R cells had completed at least one round of cell division with nearly half (44%) of the cells having undergone five or more divisions. The reduction in percent of cells that had failed to undergo proliferation reflected numerical dilution from the increased proliferative response because 2.3 times the number of TCRαFBL×GMIL2R cells were recovered as compared with TCRαFBL cells (Fig. 5B). The increased expansion of TCRαFBL×GMIL2R cells was underscored when T cell numbers were broken down into each division. In division 0, nearly equal numbers of TCRαFBL and TCRαFBL×GMIL2R cells were recovered. By contrast, there was a nearly 4-fold increase in recovery of TCRαFBL×GMIL2R cells having divided one or more times, and a greater than 8-fold difference between the number of TCRαFBL and TCRαFBL×GMIL2R cells dividing five or more times.
Figure 5.
Proliferation and expansion of adoptively transferred TCRαFBL and TCRαFBL×GMIL2R cells in vivo after antigen challenge. (A and B) 3 × 106 CFSE-labeled Thy1.2+ TCRαFBL and TCRαFBL×GMIL2R cells were transferred into CD4-depleted Thy1.1 congenic mice i.p. immunized with 107 irradiated FBL-3 cells i.v., and splenic T cells were analyzed 5 days after immunization. (A) CFSE profiles of TCRαFBL and TCRαFBL×GMIL2R T cells from mice immunized and not immunized with FBL gated on Thy1.2+/Vα3+ T cells. The percent of cells having completed 0, 1–2, 3–4, or 5+ divisions is denoted within the histograms. (B) Total number of Thy1.2+/Vα3+ T cells found in spleens from immunized and unimmunized animals. (C) 1 × 106 TCRαFBL (open symbols) and TCRαFBL×GMIL2R (closed symbols) T cells were transferred into RAG−/− hosts i.p. and left unimmunized (squares) or immunized (circles) with 107 irradiated FBL-3 cells i.p. three times on days 0, 14, and 40. The number of TCRαFBL and TCRαFBL×GMIL2R T cells present in peripheral blood on days 14, 19, 40, and 45 from immunized and unimmunized mice is indicated along with the SEM at each time point. Results are compiled from two independent experiments with 3–5 mice bled at each time point.
To determine whether the increase in IL-2 signals delivered by means of the GMIL2R led to sustained expansion after antigen exposure or an accompanying increase in cell loss from AICD and/or cytokine withdrawal, TCRαFBL and TCRαFBL×GMIL2R cells were transferred into T cell-deficient RAG−/− hosts, and the mice were primed with 107 irradiated FBL (day 0). The RAG−/− mice provided a setting in which endogenous T cell responses were absent, facilitating detection and enumeration of donor T cells for the duration of the experiment. Mice were bled and given a second immunization on day 14, and bled again 5 days later (day 19), a time point demonstrated to be the peak of expansion (27). On day 40, nearly 4 weeks after the second immunization, mice were immunized again and peripheral blood lymphocytes were analyzed on days 40 and 45 (Fig. 5C). Equivalent numbers of TCRαFBL and TCRαFBL×GMIL2R cells were recovered from unimmunized controls at each time point, consistent with the lack of homeostatic expansion of T cells transferred into antigen-negative RAG−/− hosts (28). FBL immunization increased the number of T cells detected at all time points. Fourteen days after the first immunization, there was a slight 1.3-fold increase in the number of TCRαFBL×GMIL2R cells compared with TCRαFBL cells (4.2 to 3.1). This ratio increased at the peak of expansion after the second immunization with 1.6-fold more TCRαFBL×GMIL2R cells than TCRαFBL cells on day 19. On day 40, a proportional decrease from the peak on day 19 in the number of expanded TCRαFBL and TCRαFBL×GMIL2R cells was observed, suggesting a similar survival capacity of TCRαFBL and TCRαFBL×GMIL2R T cells. At day 45, 5 days after the third immunization, the number of TCRαFBL cells increased by 65% from the day 40 total, whereas the number of TCRαFBL×GMIL2R cells increased more than 200%. Thus, TCRαFBL×GMIL2R cells also possessed an enhanced capacity to expand after repeated antigen stimulation in vivo.
Discussion
IL-2 can promote T cell proliferation and survival as well as lead to T cell death, making it difficult to define the precise role of IL-2R signals in CD8+ T cell responses. Approaches to address this issue in vivo have included modulating IL-2R activity by interfering with IL-2R function, disrupting IL-2 or IL-2R genes, or administering pharmacologic doses of exogenous IL-2, but interpretation of the results may be confounded by the multiple developmental stages and distinct functions of T cell subsets that can be impacted by global alterations in IL-2. Therefore, we examined the effect of enhanced, regulated IL-2R signaling during CD8+ T cell responses by generating Tg mice that express a chimeric GMIL2R under the control of a CD8 enhancer/promoter element, as this model mimics the effects of increased availability of IL-2 to trigger the IL-2R selectively in responding CD8+ T cells. In particular, the native GM-CSF receptor and IL-2R have nearly identical Kd (≈30 pM) as well as on/off rates and t1/2 values of ligand release (29, 30), and ligand binding by the GMIL2R transduces a signal that is indistinguishable from the native IL-2R kinetically and functionally (31–33).
Upon antigen encounter, GMIL2R+ T cells displayed significantly greater proliferation than wild-type T cells in vivo with a 2- to 4-fold increase in the number of progeny T cells, suggesting the proliferative response to the increased IL-2 signals delivered by means of the GMIL2R exceeded any potential concurrent apoptotic response. The increased proliferation, expansion, and persistence of GMIL2R+ T cells support a model in which the predominant role of regulated IL-2R signals after antigen encounter is to promote T cell expansion and survival.
The observed enhanced T cell proliferation and expansion after antigen stimulation in the presence of augmented IL-2R signals are consistent with a proposed model in which IL-2 provided early in the response can increase T cell proliferation (34), but directly contrast with observations suggesting that IL-2 inhibits T cell expansion and enhances T cell death after antigen encounter in vivo (8). These latter studies demonstrated that IL-2-deficient CD4+ T cells proliferated more than wild-type T cells in response to alloantigen stimulation in vivo (8). The difference from our results may be attributed to several factors. First, IL-2R signals play a role in T cell development (35), and the loss of IL-2R signals during thymic development could lead to an altered peripheral T cell compartment that responds differently to antigen. For example, the peripheral CD4+ and CD8+ T cells of IL-2−/− mice display increased expression of CD69 and CD44 (36), suggesting these cells are not physiologically naive. Thus, the increased proliferation observed after antigen stimulation of IL-2-deficient CD4+ T cells may be caused in part by the increased proliferative response of effector and memory T cells after antigen stimulation (37). The absence of IL-2 in IL-2−/− mice also interferes with the development and function of CD4+CD25+ T regulatory cells (12, 13), which could contribute to a hyperproliferative T cell response to antigen (13). Finally, the contrasting results could potentially reflect inherent differences in the responses of CD4+ and CD8+ T cells to IL-2R signaling after antigen stimulation.
IL-15 rather than IL-2 has been proposed as the critical growth and survival factor for T cells after antigen stimulation in vivo. Blockade or disruption of IL-15 signaling in vivo results in reduced numbers of CD8+ T cells with memory phenotype and in a reduced proliferative response to alloantigen, and in some models, IL-2 blockade can have the opposite effect, leading to the suggestion that IL-2 and IL-15 have distinct and perhaps contrasting effects on T cell survival (8, 16, 38). The molecular basis for these observations is unclear given the common use by the IL-2R and IL-15R of the two receptor subunits that signal the IL-2Rβ and γc chains (5, 6). However, the IL-15Rα chain can bind TRAF2 intracellularly, which may provide an additional antiapoptotic signal (39), but the ability of this potential IL-15R signaling domain to protect T cells from cell death has not yet been demonstrated. GM-CSF is not detectable in serum and is expressed by CD8+ T cells only after antigen stimulation with expression kinetics similar to IL-2 and CD25 (24). Therefore, the GMIL2R should transduce signals in the Tg mice primarily at sites of antigen presentation, similar to the activity of the native IL-2R in CD8+ T cells when engaged by IL-2. Thus, the observed increased proliferation and expansion of GMIL2R+ T cells after antigen stimulation in vivo suggests that rather than having opposing effects, IL-2R and IL-15R signaling may have similar biological outcomes with the unique primary functions of the cytokines more likely attributable to distinct patterns of α chain and cytokine expression (38).
GMIL2R+ T cells exhibited increased proliferation and expansion after antigen stimulation in vitro, but surprisingly, this increase in IL-2R signaling did not lead to an accompanying increase in AICD. The IL-2R activates multiple signaling molecules, including Stat5, Shc, and Akt/protein kinase B (PKB). Each of these molecules has distinct effects on T cell function and leads to transcription of specific target genes. Stat5 is critical for IL-2R-mediated proliferation but is also required for Fas-mediated apoptosis (33, 40, 41). By contrast, Shc and Akt/PKB do not enhance T cell death but together can increase proliferation and survival by means of up-regulation of c-myc, bcl-2, and bcl-xL (41–43). Akt/PKB also activates the antiapoptotic factor NF-κB in T cells (44). As NF-κB, bcl-2, and bcl-xL are capable of inhibiting Fas/tumor necrosis factor receptor I (TNFRI)-mediated T cell death (45–47), the lack of increased susceptibility to AICD observed in GMIL2R+ T cells, despite increases in IL-2R and Stat5 signaling, may reflect the relatively enhanced activity of one or more of these antiapoptotic factors and/or may be a consequence of the regulated timing and kinetics at which the increased IL-2R signals are delivered. Additionally, failure to observe increased AICD in responding GMIL2R+ cells may reflect a physiologic level of cytokine rather than the pharmacologic amounts needed to induce high-level expression of Fas/FasL and TNFRI/tumor necrosis factor (48).
After the peak level of T cell expansion and antigen clearance, CD8+ T cells become increasingly sensitive to elimination by means of apoptosis, which may occur by means of AICD, growth factor withdrawal-mediated cell death (37). Increased cell cycling driven by augmented IL-2R signals during the response to antigen could potentially lead to a higher number of cells at the peak of expansion, but subsequently to a greater loss of functional responding T cells. However, 4 weeks after immunization, GMIL2R+ T cells showed a similar proportional loss of T cells from the peak of expansion as compared with GMIL2R− T cells (Fig. 5C). Moreover, the resulting memory T cells were functional as restimulation led to further expansion of GMIL2R+ T cells compared with controls.
IL-2R signals can potentially result in T cell expansion or death. The autoimmune syndromes of mice bearing targeted disruptions in IL-2 or IL-2R components and the increase in CD8+ T cells with memory phenotype after IL-2 blockade in vivo highlight the potential importance of the negative regulatory role of IL-2R signaling, but if IL-2R signaling is predominantly involved in the negative regulation of T cell responses augmented IL-2R signals in responding T cells would be expected to lead to an overall decrease in the size of the response with loss of the responding T cells. Increased IL-2R signaling by means of the GMIL2R regulated by target recognition had the opposite effect, suggesting a dominant positive regulatory role for IL-2 in responding CD8+ T cells. Thus, the availability of IL-2 to responding CD8+ T cells likely plays an important positive role in determining the overall size and effectiveness of the T cell response.
Supplementary Material
Acknowledgments
This work was supported by a scholarship award from the Poncin Foundation to L.E.C. and grants from the National Institutes of Health (CA33084 and AI43650) to P.D.G.
Abbreviations
- TCR
T cell receptor
- IL-2R
IL-2 receptor
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GMIL2R
GM-CSF/IL-2 receptor
- AICD
activation-induced cell death
- Tg
transgenic
- CFSE
carboxyfluorescein diacetate-succinimidyl ester
- FBL
Friend virus-induced leukemia
References
- 1.Butz E A, Bevan M J. Immunity. 1998;8:167–175. doi: 10.1016/s1074-7613(00)80469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murali-Krishna K, Altman J D, Suresh M, Sourdive D J, Zajac A J, Miller J D, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 3.McMichael A J, Callan M, Appay V, Hanke T, Ogg G, Rowland-Jones S. Philos Trans R Soc London B. 2000;355:1007–1011. doi: 10.1098/rstb.2000.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson B H, Willerford D M. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Russell S M, Mess S A, Friedmann M, Erdos M, Francois C, Jacques Y, Adelstein S, Leonard W J. Nature (London) 1994;369:330–333. doi: 10.1038/369330a0. [DOI] [PubMed] [Google Scholar]
- 6.Nelson B H, Lord J D, Greenberg P D. Nature (London) 1994;369:333–336. doi: 10.1038/369333a0. [DOI] [PubMed] [Google Scholar]
- 7.Lenardo M, Chan K M, Hornung F, McFarland H, Siegel R, Wang J, Zheng L. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 8.Li X C, Demirci G, Ferrari-Lacraz S, Groves C, Coyle A, Malek T R, Strom T B. Nat Med. 2001;7:114–118. doi: 10.1038/83253. [DOI] [PubMed] [Google Scholar]
- 9.Schorle H, Holtschke T, Hunig T, Schimpl A, Horak I. Nature (London) 1991;352:621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- 10.Sadlack B, Merz H, Schorle H, Schimpl A, Feller A C, Horak I. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 11.Willerford D M, Chen J, Ferry J A, Davidson L, Ma A, Alt F W. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 12.Papiernik M, de Moraes M L, Pontoux C, Vasseur F, Penit C. Int Immunol. 1998;10:371–378. doi: 10.1093/intimm/10.4.371. [DOI] [PubMed] [Google Scholar]
- 13.Wolf M, Schimpl A, Hunig T. Eur J Immunol. 2001;31:1637–1645. doi: 10.1002/1521-4141(200106)31:6<1637::aid-immu1637>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 14.Grabstein K H, Eisenman J, Shanebeck K, Rauch C, Srinivasan S, Fung V, Beers C, Richardson J, Schoenborn M A, Ahdieh M, et al. Science. 1994;264:965–968. doi: 10.1126/science.8178155. [DOI] [PubMed] [Google Scholar]
- 15.Marks-Konczalik J, Dubois S, Losi J M, Sabzevari H, Yamada N, Feigenbaum L, Waldmann T A, Tagaya Y. Proc Natl Acad Sci USA. 2000;97:11445–11450. doi: 10.1073/pnas.200363097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ku C C, Murakami M, Sakamoto A, Kappler J, Marrack P. Science. 2000;288:675–678. doi: 10.1126/science.288.5466.675. [DOI] [PubMed] [Google Scholar]
- 17.Aruga A, Aruga E, Cameron M J, Chang A E. J Leukocyte Biol. 1997;61:507–516. doi: 10.1002/jlb.61.4.507. [DOI] [PubMed] [Google Scholar]
- 18.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Nature (London) 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg P D. J Immunol. 1986;136:1917–1922. [PubMed] [Google Scholar]
- 20.Rosenberg S A, Lotze M T, Muul L M, Chang A E, Avis F P, Leitman S, Linehan W M, Robertson C N, Lee R E, Rubin J T, et al. N Engl J Med. 1987;316:889–897. doi: 10.1056/NEJM198704093161501. [DOI] [PubMed] [Google Scholar]
- 21.Lanzavecchia A. Nature (London) 1998;393:413–414. doi: 10.1038/30845. [DOI] [PubMed] [Google Scholar]
- 22.Park L S, Martin U, Sorensen R, Luhr S, Morrissey P J, Cosman D, Larsen A. Proc Natl Acad Sci USA. 1992;89:4295–4299. doi: 10.1073/pnas.89.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metcalf D, Begley C G, Williamson D J, Nice E C, De Lamarter J, Mermod J J, Thatcher D, Schmidt A. Exp Hematol. 1987;15:1–9. [PubMed] [Google Scholar]
- 24.Troutt A B, Maraskovsky E, Rogers L A, Pech M H, Kelso A. Immunol Cell Biol. 1992;70:51–57. doi: 10.1038/icb.1992.8. [DOI] [PubMed] [Google Scholar]
- 25.Ellmeier W, Sunshine M J, Losos K, Hatam F, Littman D R. Immunity. 1997;7:537–547. doi: 10.1016/s1074-7613(00)80375-1. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Sun S, Hwang I, Tough D F, Sprent J. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 27.Cho B K, Wang C, Sugawa S, Eisen H N, Chen J. Proc Natl Acad Sci USA. 1999;96:2976–2981. doi: 10.1073/pnas.96.6.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldrath A W, Bogatzki L Y, Bevan M J. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuoka M, Takeshita T, Ishii N, Nakamura M, Ohkubo T, Sugamura K. Eur J Immunol. 1993;23:2472–2476. doi: 10.1002/eji.1830231014. [DOI] [PubMed] [Google Scholar]
- 30.Niu L, Golde D W, Vera J C, Heaney M L. Blood. 1999;94:3748–3753. [PubMed] [Google Scholar]
- 31.Nelson B H, Lord J D, Greenberg P D. Mol Cell Biol. 1996;16:309–317. doi: 10.1128/mcb.16.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson B H, McIntosh B C, Rosencrans L L, Greenberg P D. Proc Natl Acad Sci USA. 1997;94:1878–1883. doi: 10.1073/pnas.94.5.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord J D, McIntosh B C, Greenberg P D, Nelson B H. J Immunol. 2000;164:2533–2541. doi: 10.4049/jimmunol.164.5.2533. [DOI] [PubMed] [Google Scholar]
- 34.Van Parijs L, Abbas A K. Science. 1998;280:243–248. doi: 10.1126/science.280.5361.243. [DOI] [PubMed] [Google Scholar]
- 35.Bassiri H, Carding S R. J Immunol. 2001;166:5945–5954. doi: 10.4049/jimmunol.166.10.5945. [DOI] [PubMed] [Google Scholar]
- 36.Sadlack B, Lohler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle R J, Horak I. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 37.Ahmed R, Gray D. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 38.Ma A, Boone D L, Lodolce J P. J Exp Med. 2000;191:753–756. doi: 10.1084/jem.191.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulfone-Pau S S, Bulanova E, Pohl T, Budagian V, Durkop H, Ruckert R, Kunzendorf U, Paus R, Krause H. FASEB J. 1999;13:1575–1585. doi: 10.1096/fasebj.13.12.1575. [DOI] [PubMed] [Google Scholar]
- 40.Moriggl R, Topham D J, Teglund S, Sexl V, McKay C, Wang D, Hoffmeyer A, van Deursen J, Sangster M Y, Bunting K D, et al. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- 41.Van Parijs L, Refaeli Y, Lord J D, Nelson B H, Abbas A K, Baltimore D. Immunity. 1999;11:281–288. doi: 10.1016/s1074-7613(00)80103-x. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed N N, Grimes H L, Bellacosa A, Chan T O, Tsichlis P N. Proc Natl Acad Sci USA. 1997;94:3627–3632. doi: 10.1073/pnas.94.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lord J D, McIntosh B C, Greenberg P D, Nelson B H. J Immunol. 1998;161:4627–4633. [PubMed] [Google Scholar]
- 44.Kane L P, Shapiro V S, Stokoe D, Weiss A. Curr Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z G, Hsu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 46.Hausler P, Papoff G, Eramo A, Reif K, Cantrell D A, Ruberti G. Eur J Immunol. 1998;28:57–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<57::AID-IMMU57>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 47.Boise L H, Thompson C B. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng L, Trageser C L, Willerford D M, Lenardo M J. J Immunol. 1998;160:763–769. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.