Abstract
Lateral root initiation is strongly repressed in Arabidopsis by the combination of high external sucrose and low external nitrate. A previously isolated mutant, lin1, can overcome this repression. Here, we show that lin1 carries a missense mutation in the NRT2.1 gene. Several allelic mutants, including one in which the NRT2.1 gene is completely deleted, show similar phenotypes to lin1 and fail to complement lin1. NRT2.1 encodes a putative high-affinity nitrate transporter that functions at low external nitrate concentrations. Direct measurement of nitrate uptake and nitrate content in the lin1 mutant seedlings established that both are indeed reduced. Because repression of lateral root initiation in WT plants can be relieved by increased concentrations of external nitrate, it is surprising to find that repression is also relieved by a defect in a component of the high-affinity nitrate uptake system. Furthermore, lateral root initiation is increased in lin1 relative to WT even when seedlings are grown on nitrate-free media, suggesting that the mutant phenotype is nitrate-independent. These results indicate that NRT2.1 is a repressor of lateral root initiation and that this role is independent of nitrate uptake. We propose that Arabidopsis NRT2.1 acts either as a nitrate sensor or signal transducer to coordinate the development of the root system with nutritional cues.
Keywords: carbon, nitrogen, nutrition, lin1
The plant root system develops through the formation of lateral roots along the length of the primary root. As these lateral roots grow, they also give rise to new lateral roots. Unlike the preordained placement of most organs in animals, the number and distribution of lateral roots are strongly influenced by environmental conditions (1-3). The plasticity of lateral root formation serves an important function, as it allows the architecture of the root system to be optimized to the unique distribution of water and nutrients in the soil. Little is known about how nutrient signals are sensed and transduced to regulate plant development.
We are studying lateral root initiation to determine how environmental cues can modulate plant developmental processes. Lateral roots originate from an internal layer of cells called the pericycle. Lateral root initiation involves the stimulation of mature, quiescent pericycle cells to divide and become founder cells for a new lateral root (1, 2). Only pericycle cells situated at the protoxylem poles are competent to become founder cells, and only a small percentage of these cells ever participate in lateral root initiation. We have little understanding about how specific competent pericycle cells are stimulated to initiate lateral root formation or how environmental cues modulate the number and location of lateral root initiations in the root system. This process must require sensing of external conditions and a signal transduction system that terminates in targeted pericycle cell division.
In a previous paper (4) it was demonstrated that lateral root initiation is repressed in seedlings grown on high ratios of sucrose to nitrogen. To further understand the coordination of nutrient cues and lateral root initiation, a mutant that is able to initiate large numbers of lateral roots when grown on these conditions was isolated (4). The existence of the lateral root initiation mutant phenotype (lin1) indicates that the low rate of lateral root initiation in WT plants under repressive conditions is not caused by inadequate nutrients to complete the essential cell divisions. Instead, the high sucrose/low nitrogen condition must trigger a signal that negatively regulates the lateral root initiation program, and this negative regulation must require LIN1. Lateral root initiation in the lin1 mutant only differs from WT under specific growth conditions. Therefore, LIN1 is a component of a signaling pathway connecting specific nutritional cues with lateral root initiation.
Based on the mutant phenotype, we can postulate two alternative models for LIN1 function. In the first model, LIN1 represses lateral root initiation by sensing sucrose and/or nitrogen or by transducing a signal from the actual sensor. In this case, lin1 would fail to perceive the repressive nutritional condition and therefore would fail to repress lateral root initiation. In the second model, LIN1 modifies nutrient uptake or assimilation. For example, if the lin1 mutant obtains less sucrose or more nitrogen from the media, the lateral root repression pathway would not be triggered.
In this article, we report that a change in a conserved amino acid of NRT2.1 is responsible for the lin1 phenotype. NRT2 proteins are members of the major facilitator superfamily (MFS) of transporter and transporter-like proteins (5). Higher plant NRT2 proteins are predicted to be nitrate transporters based on their homology to the fungal nitrate transporter CRNA and on the changes in nitrate uptake kinetics observed in Arabidopsis plants with mutations in NRT2 genes (6-8). Direct studies of nitrate uptake kinetics in higher plants have revealed two distinct systems: the low-affinity transport system that is responsible for uptake when nitrate is plentiful (>1 mM) and the high-affinity transport system (HATS) that is able to scavenge nitrate from the soil at concentrations between 1 μM and 1 mM. Molecular and physiological studies in a number of plant species indicate that the NRT2 proteins are required for the HATS (6-8).
In Arabidopsis, there are seven NRT2 genes (6-10). Of these, the NRT2.1 protein appears to be the most critical for high-affinity nitrate uptake based on the following findings: (i) the activity of the HATS is strongly reduced (Vmax = 27% of WT) in an NRT2.1/NRT2.2 deletion mutant (11); (ii) NRT2.1 is strongly expressed in external cell layers of the root, the main site for nitrate uptake from the soil (9, 10, 12, 13); and (iii) the expression profile of NRT2.1 is consistent with studies of HATS activity. For example, HATS-dependent nitrate uptake is stimulated by low external nitrate and inhibited in response to downstream products of nitrate assimilation such as ammonium and certain amino acids. Similarly, low levels of external nitrate induce NRT2.1 expression, whereas expression is repressed by downstream nitrate metabolites (6-14).
The cloning and identification of the LIN1 gene as NRT2.1 indicates that this component of the high-affinity nitrate uptake system also plays a role in repressing lateral root initiation in response to high sucrose/low nitrogen. Because repression of lateral root initiation can be relieved by increased concentrations of external nitrate, it is surprising to find that it is also relieved by a defect in the HATS, which would be expected to decrease nitrate uptake. However, in this article we demonstrate that the role of NRT2.1 in repressing lateral root initiation is independent of its role in nitrate uptake. Therefore, we propose that the putative nitrate transporter NRT2.1 acts as a nitrate sensor or a downstream signal transducer in a pathway that coordinates nitrate availability with the development of the root system.
Materials and Methods
Plant Materials. Both T-DNA insertion mutants [SALK_035429 (nrt2.1-2) and SALK_008253 (nrt2.1-3)] were obtained from the Arabidopsis Biological Resource Center (Ohio State University, Columbus).
Plant Growth Conditions. All seeds were sterilized in 100% bleach containing Tween 20 (three drops per 10 ml) for 3 min and then rinsed four times with sterile distilled water. The seeds were then stratified in water at 4°C for 2-4 days. Seeds were sown on modified Murashige and Skoog (MS) media (described below) and grown on vertically oriented plates in a growth chamber set at 22°C, 16-h light, 8-h dark.
Nitrogen-free MS basal medium (GIBCO/BRL, custom ordered) was supplemented with 0.5 g/liter 2-(N-morpholino)ethanesulfonic acid and 75 g/liter sucrose. Nitrogen was added in the form of NH4Cl, KNO3, and/or NH4NO3. KCl was used to equalize the total salt concentration among the treatments within an experiment. The pH was adjusted to 5.7 with 1 M KOH. Agar (7 g/liter; Sigma A-7921) was added before autoclaving.
Microscopic Analysis of Lateral Root Initiation. The number of lateral root initiation events was determined in cleared roots as described (4). All lateral roots and lateral root primordia were counted to obtain the total number of lateral root initiations.
Cloning of the LIN1 Gene. The lin1 mutant was crossed to a WT Arabidopsis var. WS to fine-map the gene. Polymorphic markers on chromosome 1 were obtained from the public chromosome maps (www.arabidopsis.org) or identified by the Malamy laboratory. Breakpoint analysis of recombinant F2 plants delimited the mutation to an ≈110-kb region flanked by two simple sequence length polymorphism markers, PHY4 and HY6. The PCR primers used to identify the PHY4 polymorphism were: forward, 5′-ttgtaccagtcaaggttgg-3′; and reverse, 5′-agtttcacatcaacattgtgg-3′. The primers for the HY6 polymorphism were: forward, 5′-aagatagagacatataacccc-3′; and reverse, 5′-tcaaacacagagagaaaaacc-3′.
Determination of Nitrate Uptake and Accumulation. Influx rates of 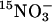 were tested for WT and lin1 plants grown for 14 days on MS media (as described). The seedlings were transferred to 0.1 mM CaSO4 for 1 min, to a nutrient solution containing 7.5% sucrose, 0.1 mM
were tested for WT and lin1 plants grown for 14 days on MS media (as described). The seedlings were transferred to 0.1 mM CaSO4 for 1 min, to a nutrient solution containing 7.5% sucrose, 0.1 mM 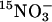 (atom% 15N: 99% in the form of NH4NO3) for 5 min, and finally to 0.1 mM CaSO4 for 1 min. Roots were separated from the shoots immediately after the final transfer to CaSO4, dried for 48 h at 70°C, and analyzed by using the ANCA-MS system (PDZ Europa, Crewe, U.K.). Influx of
(atom% 15N: 99% in the form of NH4NO3) for 5 min, and finally to 0.1 mM CaSO4 for 1 min. Roots were separated from the shoots immediately after the final transfer to CaSO4, dried for 48 h at 70°C, and analyzed by using the ANCA-MS system (PDZ Europa, Crewe, U.K.). Influx of 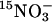 was calculated from the 15N content of the roots (μmol/hr per g dry weight). For determination of nitrate accumulation (μmol/g fresh weight), ethanol extracts, made as described (15), were evaporated and diluted in water before analysis by ion exchange chromatography with a DX-120 column (Dionex, Sunnyvale, CA).
was calculated from the 15N content of the roots (μmol/hr per g dry weight). For determination of nitrate accumulation (μmol/g fresh weight), ethanol extracts, made as described (15), were evaporated and diluted in water before analysis by ion exchange chromatography with a DX-120 column (Dionex, Sunnyvale, CA).
Results
Lateral Root Initiation Is Repressed by a High Sucrose/Nitrate Ratio. When WT plants are grown on standard growth media, lateral root initiation can always be seen along the length of the primary root. In contrast, we previously showed that WT plants initiate few, if any, lateral roots when grown with high sucrose (4.5%) and low nitrogen (0.01 mM NH4NO3). Decreasing the sucrose concentration to 0.5% or increasing the nitrogen to 60 mM restores lateral root initiation (4), indicating that neither the high sucrose nor the limited nitrogen alone is responsible for the repression of lateral root initiation. Therefore, we hypothesized that the repressive element in these conditions is the coincidence of limiting nitrogen supplies and high sucrose concentration, creating a high ratio of sucrose/nitrogen.
If the high sucrose/low nitrogen condition is indeed the critical parameter in repressing lateral root initiation, we would expect to be able to increase sucrose and nitrogen concentrations together and maintain repression of lateral root initiation. Indeed, when WT seedlings were grown with 7.5% sucrose, 0.1 mM NH4Cl, and 0.1 mM KNO3, initiation was significantly repressed compared with seedlings grown with higher concentrations of KNO3 (P < 0.001) (Fig. 1, first two bars). This finding provides further evidence that the coincidence of high sucrose and low nitrogen concentrations is a signal for repression of lateral root initiation. For all further experiments, we used 7.5% sucrose, 0.1 mM NH4Cl, and 0.1 mM KNO3 as our lateral root initiation assay condition. This condition was preferred to our previous assay because of the higher concentration of nitrogen salts. This improvement relieved our concern that trace amounts of contaminating nitrate could contribute significantly to the final nitrate concentration in our experiments. The higher concentrations of nitrogen salts used in this assay also showed that severe nitrogen limitation is not essential for repression of lateral root initiation. Finally, the use of NH4Cl and KNO3, as opposed to NH4NO3, allowed us to assess the affects of nitrate while holding ammonia concentrations constant. Our results demonstrate that, within the context of this assay, lateral root initiation is directly correlated with exogenous nitrate concentrations.
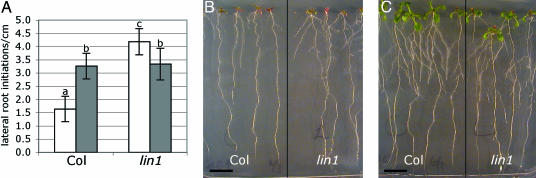
Fig. 1.
High sucrose/low nitrate represses lateral root initiation in WT but not lin1. (A) WT (Col) and lin1 seedlings were grown for 14 days on MS media with high sucrose/low nitrate (7.5% sucrose, 0.1 mM NH4Cl, and 0.1 mM KNO3, empty bars) or high sucrose/high nitrate (7.5% sucrose, 0.1 mM NH4Cl, and 5 mM KNO3, filled bars). KCl was used to balance total salt concentrations between media. Shown are means of the number of initiation events per cm of primary root length. Error bars = SD. Different letters indicate means that are significantly different based on Student's t test (P < 0.005). n = 8-10. (B) lin1 is more highly branched than WT under high sucrose/low nitrate conditions. Shown are representative images of seedlings from A. (C) lin1 is similar to WT under high sucrose/high nitrate conditions. Shown are representative images of seedlings from A. (Scale bar: 1 cm.)
The lin1 mutant was isolated based on its ability to overcome the repression of lateral root initiation under high sucrose/low nitrate conditions (4). The lin1 mutant also showed significantly increased lateral root initiation when compared with WT seedlings under the new assay conditions (P < 0.001) (Fig. 1 A and B). In contrast, lateral root initiation levels in lin1 and WT plants were similar when the external nitrate concentration was increased (P > 0.75) (Fig. 1 A and C). Hence, the role of the LIN1 gene in repressing lateral root initiation is specific to the high sucrose/low nitrate conditions. This finding confirms our previous prediction that LIN1 is a component of a signaling mechanism that coordinates lateral root initiation with external nutritional cues.
The LIN1 Gene Encodes a Putative High-Affinity Nitrate Transporter, NRT2.1. We previously demonstrated that the lin1 mutation is recessive and mapped the mutation to the top of chromosome I (4). To identify the gene associated with the mutation, the lin1 mutant (in a Columbia background) was crossed to a WT Arabidopsis var. WS-0 plant. F2 progeny showing the lin1 phenotype were analyzed by using cleaved amplified polymorphic sequences and simple sequence length polymorphism markers in the region (www.arabidopsis.org). Mapping of chromosomal breakpoints in the F2 recombinants delimited the mutation to an ≈110-kb region between 2,456 and 2,567 kb on chromosome I (see Materials and Methods). This region contains two putative high-affinity nitrate transporters, NRT2.1 (At1g08090) and NRT2.2 (At1g08100), which occur in tandem in a tail-to-tail arrangement. By comparing the sequences of the coding regions between WT Columbia and the lin1 mutant, we discovered a base-pair difference in the NRT2.1 gene (Fig. 2). The G at position 355 is changed to an A in lin1, which is predicted to change Gly-119 to arginine in the NRT2.1 protein. NRT2 proteins are predicted to have 12 membrane spanning domains; Gly-119 is predicted to be located either at the end of transmembrane region 2 or between transmembrane regions 2 and 3 on the cytoplasmic side (7). Gly-119 is part of a signature sequence of proteins in the MFS (5, 7) and is conserved in the nitrate/nitrite porter family of proteins to which the NRT2 family belongs (7). Therefore, it is likely that Gly-119 is critical for protein function.
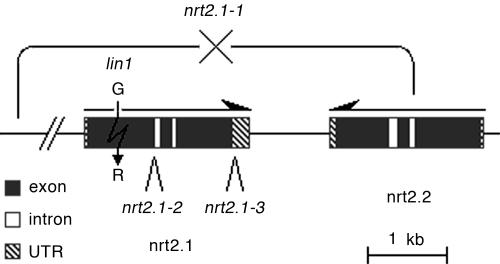
Fig. 2.
Representation of genomic region of chromosome I containing NRT2.1 and NRT2.2. Arrows indicate orientation of transcript. Gene structure predictions are based on annotation from The Arabidopsis Information Resource (www.arabidopsis.org). nrt2.1-1: deletion of indicated region. nrt2.1-2: T-DNA insertion in first intron of NRT2.1 (Salk_035429). nrt2.1-3: T-DNA insertion in 3′ UTR of NRT2.1 (Salk_008278). lin1(nrt2.1-4): Gly-119 missense mutation in NRT2.1
To further confirm that the mutation in NRT2.1 is responsible for the phenotype of lin1, we characterized several additional mutants with defects in this gene. Two T-DNA insertion alleles are available with inserts located in the first intron and 3′ UTR of NRT2.1 (Salk_035429 and Salk_008278, respectively) (Fig. 2). An additional mutant, atnrt2a, has a deletion that covers the entire NRT2.1 gene and a portion of the adjacent NRT2.2 gene (Fig. 2); this mutant is null for both gene transcripts (11). For simplicity, we refer to the alleles in the order in which they were isolated. The deletion line is nrt2.1-1 and the T-DNA insert lines are nrt2.1-2 and nrt2.1-3. When the three nrt2.1 mutants were grown under repressive conditions for 13-14 days (with 7.5% sucrose, 0.1 mM NH4Cl, and 0.1 mM KNO3) they all showed significantly increased initiation of lateral roots compared with their respective WT controls, and thus resembled the lin1 mutant (Table 1). Furthermore, when each of these mutants was crossed to lin1, no complementation was seen in the F1 generation (see Table 2, which is published as supporting information on the PNAS web site). Hence, we conclude that the lin1 mutant phenotype is caused by a loss of function of NRT2.1 and rename the lin1 mutant nrt2.1-4. We further conclude that NRT2.1 represses lateral root initiation under specific nutritional conditions.
Table 1. Three mutants in NRT2.1 phenocopy the lin1 mutant.
| Genotype | Initiations per cm | SD | P value |
|---|---|---|---|
| WT (Col) | 2.1 | 0.89 | |
| WT (WS) | 3.12 | 1.46 | |
| nrt2.1-1 | 5.98 | 0.78 | <0.001 |
| nrt2.1-2 | 4.01 | 1.06 | <0.001 |
| nrt2.1-3 | 4.08 | 1.13 | 0.001 |
| lin1 | 4.62 | 0.97 | <0.001 |
The nrt2.1-1 mutant is in the WS background; all other mutants are in the Col background. Seedlings were grown for 14 days on media containing 7.5% sucrose, 0.1 mM NH4Cl, and 0.1 mM KNO3. The number of lateral root initiations per cm primary root were scored as described in Materials and Methods. P values (Student's t test) for each genotype compared with WT control.
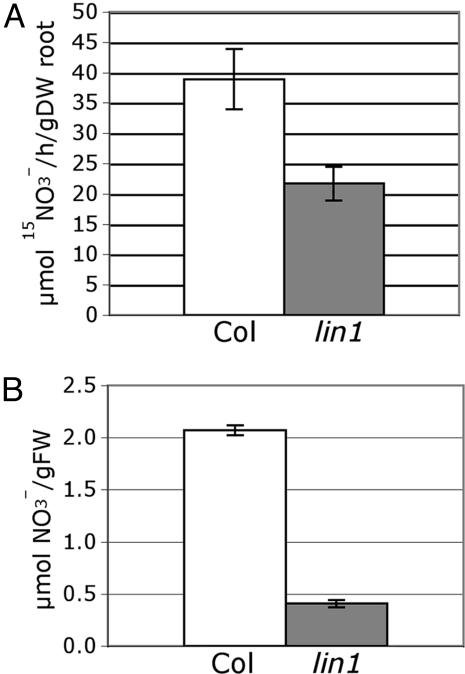
lin1(nrt2.1-4) Has Decreased Nitrate Uptake and Accumulation. The discovery that lin1(nrt2.1-4) carries a mutation in a predicted nitrate transporter, known to play a key role in high-affinity nitrate uptake, immediately suggested that the phenotype is related to changes in nitrate uptake. Indeed, mutation of the same conserved glycine in the Aspergillus nidulans high-affinity nitrate transporter CRNA as in lin1(nrt2.1-4), and mutation of other amino acids in this signature sequence in several prokaryotic MFS proteins, lead to reduced transport activity (5, 7, 16-19). To directly assess the effect of the lin1(nrt2.1-4) mutation on nitrate uptake, seedlings were grown under the high sucrose/low nitrate conditions that repress lateral root initiation in WT, and then briefly exposed to 15NO3. Nitrate influx rates were then measured (see Materials and Methods). The lin1(nrt2.1-4) mutant had a significantly reduced rate of nitrate influx compared with WT (Fig. 3A), indicating that the mutation of Gly-119 compromises the function of NRT2.1 in nitrate uptake. Total nitrate accumulation was also measured in roots of plants grown for 14 days under repressive conditions. This experiment revealed a reduction in nitrate content in the lin1(nrt2.1-4) mutant compared with WT (Fig. 3B). Nitrate accumulation in shoot tissues, although much lower, was also slightly reduced in the lin1(nrt2.1-4) mutant compared with WT, and total amino acid content in lin1(nrt2.1-4) was similar or slightly reduced compared with WT (data not shown). Together, these results indicate that the mutation in lin1(nrt2.1-4) indeed reduces nitrate uptake activity and that there are no compensatory mechanisms in the plant that increase nitrate uptake or accumulation.
Fig. 3.
lin1 mutants are impaired in 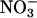 uptake and accumulation. WT (Col) and lin1(nrt2.1-4) seedlings were grown on media containing 7.5% sucrose and 0.1 mM NH4NO3 for 14 days. (A)
uptake and accumulation. WT (Col) and lin1(nrt2.1-4) seedlings were grown on media containing 7.5% sucrose and 0.1 mM NH4NO3 for 14 days. (A) 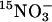 uptake was measured after 5 min in growth media containing 0.1 mM
uptake was measured after 5 min in growth media containing 0.1 mM 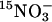 Uptake in root tissues is shown. The values are means of five replicates of three plants each. gDW, gram dry weight. (B) Total
Uptake in root tissues is shown. The values are means of five replicates of three plants each. gDW, gram dry weight. (B) Total 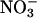 content of roots. The values are means of five replicates of three plants each. gFW, gram fresh weight. Error bars = SD.
content of roots. The values are means of five replicates of three plants each. gFW, gram fresh weight. Error bars = SD.
The Decrease in Nitrate Uptake Does Not Explain the Derepression of Lateral Root Initiation in lin1(nrt2.1-4). We next tested whether a reduction in nitrate uptake and content in the specific range of our assay conditions could cause increased lateral root initiation. To test this possibility, we made the assumption that if we decrease external nitrate, this will result in a decrease in nitrate uptake by the plant, and therefore simulate the effects of a defective transporter. The correlation of external nitrate concentrations with nitrate uptake has been confirmed many times in studies of nitrate transport kinetics (i.e., ref. 11). An increase in lateral root initiation with decreasing nitrate uptake would be unexpected given our previous observation that low levels of external nitrate repress lateral root initiation in our assay. However, it was possible that analysis of lateral root initiation over a range of nitrate concentrations would reveal a more complex relationship between nitrate and initiation, with a “spike” in initiation at some nitrate concentration.
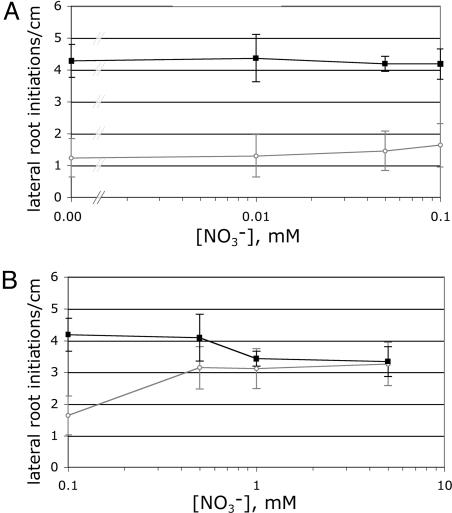
WT and mutant seedlings were grown on media containing 7.5% sucrose, 0.1 mM NH4Cl, and KNO3 concentrations equal to (0.1 mM) or less than our original assay conditions (0.05, 0.01, or 0 mM) (Fig. 4A). Lateral root initiation in WT plants either stayed constant or gradually decreased with decreasing external nitrate in this concentration range (Fig. 4A). None of the nitrate concentrations tested caused the WT seedlings to phenocopy lin1(nrt2.1-4), providing no support for the initiation spike model.
Fig. 4.
The phenotype of the lin1 mutant is not explained by its decreased nitrate uptake. Lateral root initiation in WT (Col, ○) and lin1(nrt2.1-4) (▪) seedlings at various 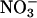 concentrations are shown. Seedlings were grown on media containing 7.5% sucrose, 0.1 mM NH4Cl, and various concentrations of KNO3 (0, 0.01, 0.05, 0.1, 0.5, 1, or 5 mM) for 13 days. Total salt concentrations were balanced with KCl. Shown are means of the number of initiation events per cm of primary root length. Error bars = SD. n = 10.
concentrations are shown. Seedlings were grown on media containing 7.5% sucrose, 0.1 mM NH4Cl, and various concentrations of KNO3 (0, 0.01, 0.05, 0.1, 0.5, 1, or 5 mM) for 13 days. Total salt concentrations were balanced with KCl. Shown are means of the number of initiation events per cm of primary root length. Error bars = SD. n = 10.
As another approach to determine whether decreases in nitrate uptake could cause increased lateral root initiation, we tested whether the lin1(nrt2.1-4) mutant phenotype could be reversed by exogenous nitrate. Although uptake rates are compromised in the mutant, it still shows the capacity to maintain a nitrate influx (Fig. 3A). Therefore, if increases in lateral root initiation in lin1(nrt2.1-4) are the result of decreased nitrate uptake, we should be able to reduce lateral root initiation to the levels of repressed WT seedlings by increasing exogenous nitrate. WT and mutant seedlings were therefore grown on media containing 7.5% sucrose, 0.1 mM NH4Cl, and KNO3 at concentrations equal to (0.1 mM) or greater than our original assay conditions (0.5, 1, or 5 mM) (Fig. 4B). However, exogenous nitrate was unable to repress lateral root initiation in the lin1(nrt2.1-4) mutant to the levels seen in WT under repressive conditions (Fig. 4B). Indeed, lin1(nrt2.1-4) showed higher lateral root initiation rates than WT until sufficient nitrate was added to eliminate the high sucrose/low nitrate repression in WT (1 mM) (Fig. 4B).
Together, these results indicate that the reduction in nitrate uptake observed in lin1(nrt2.1-4) does not explain the increase in lateral root initiation. Instead, these data support the model that mutations in NRT2.1 increase lateral root initiation by abrogating a function of the protein that is unrelated to nitrate uptake.
The lin1(nrt2.1-4) Mutant Phenotype Is Independent of Exogenous Nitrate. Changes in nitrate uptake in the lin1(nrt2.1-4) mutant do not appear to account for the increased lateral root initiation phenotype. This raises the question of whether the lin1(nrt2.1-4) phenotype is nitrate-independent. Indeed, in the above experiment the lin1(nrt2.1-4) mutant had increased lateral root initiation compared with WT even when no nitrate was added to the media, both at 7.5% sucrose (Fig. 4A, P < 0.0001) and 4.5% sucrose (data not shown). Because initiation in both genotypes is unaltered within a range of 0-0.05 mM external nitrate (Fig. 4A), trace contaminant nitrate is unlikely to be affecting these results. This finding confirms the hypothesis that the differences in lateral root initiation between WT and the lin1 mutant must be independent of differences in nitrate uptake activity and indicates that NRT2.1 must have a second function in addition to its role in high-affinity nitrate uptake. Therefore, we propose that NRT2.1 functions as a nitrate sensor or signal transducer and participates in a signaling pathway that culminates in the repression of lateral root initiation.
Discussion
A Signaling Role for Arabididopsis NRT2.1 and Other Putative Nitrate Transporters. The genetic and physiological experiments presented here indicate that NRT2.1 is essential for repression of lateral root initiation under high sucrose/low nitrate growth conditions. NRT2.1 clearly also regulates or participates in high-affinity nitrate uptake. However, our studies demonstrate a regulatory role for NRT2.1 in developmental signaling that is independent of its role in nitrate uptake.
A regulatory role for NRT2 was suggested previously in Chlamydomonas, when it was demonstrated that the NRT2.1-mediated HATS was essential for regulating NR (nitrate reductase) gene transcription in nitrate-free media (20). However, Rexach et al. (20) ascribed this effect to the NRT2.1-dependent uptake of trace nitrate contaminants from the media. In addition, mutations in NRT1 proteins have been reported to cause developmental phenotypes (21, 22). NRT1 proteins have been demonstrated both biochemically and genetically to be low-affinity nitrate transporters (although the Arabidopsis NRT1.1 appears to function in both low-affinity transport system and HATS) (3). Although they are unrelated to the NRT2 proteins at the nucleotide or amino acid sequence level, NRT1 proteins are predicted to be members of the MFS and share an overall structure and membrane topology with the NRT2 family (6-8). Significantly, inhibition of primary root growth in an Arabidopsis nrt1.1 mutant was observed even in the absence of nitrate, leading Guo et al. (22) to suggest that NRT1.1 may contribute to growth “in a way that goes beyond the simple uptake of nitrate,” perhaps through developmental signaling. However, they were unable to exclude the possibility that their observation was caused by low levels of contaminating nitrate. Taken together with our studies of the Arabidopsis NRT2.1 protein, these observations now make it tempting to speculate that members of both the NRT1 and NRT2 families play roles in signaling pathways in addition to their roles in nitrate uptake.
NRT2.1 Acts as a Sensor or Signal Transduction Protein. Under conditions of high sucrose, lateral root initiation levels are inversely correlated with external nitrate concentrations. The data presented here suggest that NRT2.1 functions either as a sensor of low nitrate or as a transducer of the signal emanating from a sensor. In this model, the low nitrate condition in our assay represses lateral root initiation in a pathway mediated by LIN1. The lin1 mutant is not able to recognize/respond to low nitrate, and therefore initiation is not repressed. Because changes in external nitrate concentrations are reflected by changes in internal nitrate and nitrate assimilates, it is impossible from our data to predict which of these pools is serving as the signal in the NRT2.1-mediated pathway.
There are extensive examples of dual-function nutrient transporter/sensor and transporter/signal transduction proteins in bacteria and yeasts (23). Most of them are involved in sensing external nutrients, perhaps because of the optimal location of these transporters in the plasma membrane. For example, the yeast hexose sensor GCR1 and the Escherichia coli Glc6p sensor UhpC (24-26) both activate transport in response to external levels of their substrates. The yeast MEP2 protein functions as an ammonium transporter/sensor and also plays an essential role in coordinating a morphology shift from colonial to pseudohyphal growth when nitrogen is limiting (24, 27). In contrast, there have been no examples of transporter/sensor or transporter/signal transduction proteins reported in plants to our knowledge.
An alternative possibility is that NRT2.1 is not itself a transporter at all, but acts as a nitrate sensor or signal transducer to activate nitrate uptake and repress lateral root initiation in response to specific growth conditions. Despite strong correlative evidence, transport activity has not been demonstrated biochemically for the higher-plant NRT2 proteins. The exception is the barley NRT2.1 protein, which was shown to have nitrate uptake activity in Xenopus oocytes. However, coexpression of an associated protein, NAR2.3, was required for nitrate uptake in this assay (28). Therefore, although it is highly likely that the Arabidopsis NRT2.1 protein is a structural component of the HATS, it remains formally possible that NRT2.1 is required to activate nitrate transport rather than act as an actual transporter. There are other transporter-like MFS proteins that have poor transport activity themselves but regulate the activity of other transporters (5). For example, the yeast proteins SNF3 and RGT2 resemble glucose transporters, have little transport activity, and regulate expression of hexose transporter genes (23, 24). The plant SUC3/SUT2 sucrose transporter was hypothesized to fall into this category, based on initial reports of low sucrose transport activity and structural similarity to SNF3 and RGT2 (29). However, more recent studies demonstrating that SUC3/SUT2 can transport sucrose efficiently have called this hypothesis into question (30). No other examples of putative transporter-like facilitator/sensors have been reported to our knowledge in plants.
Transcriptional Regulation of NRT2.1 May Link High Sucrose/Nitrate Ratios with Lateral Root Initiation. If NRT2.1 indeed functions as a nitrate sensor or signal transducer, it remains unclear why the nrt2.1 mutant phenotype is only observed under conditions of high sucrose. Carbon and nitrogen signaling pathways clearly interact in plants, as in other organisms, to coordinate nutrient uptake, metabolism, and utilization (23, 31-34). Hence, there are many examples in which the effects of combined external carbon and nitrogen cannot be explained by the additive effects of each nutrient alone. For example, in a genomewide survey in Arabidopsis, >300 genes were identified whose expression appeared to be regulated by carbon/nitrogen ratios (33). Furthermore, carbon/nitrogen ratios also appear to regulate many morphophysiological events during early seedling growth (32). The mechanisms underlying the interactions of carbon and nitrogen signaling and carbon/nitrogen ratio sensing remain to be elucidated.
One of the best pieces of evidence indicating that a mechanism exists in plants for sensing sucrose and nitrate levels is the regulation of expression of genes involved in carbon and nitrogen acquisition and metabolism (31, 33), including the NRT2.1 gene itself. NRT2.1 gene regulation integrates external nitrate concentrations with nitrate metabolism and the plant's carbon status. NRT2.1 transcription is induced by external nitrate levels as low as 10 μm but repressed when products of nitrogen assimilation accumulate in the plant (6-10). Furthermore, sucrose and light increase NRT2.1 transcription under low nitrate conditions, suggesting that photoysnthate levels contribute to the regulation of NRT2.1 expression (14).
It is unclear how the internal and external nutritional cues are sensed or how NRT2.1 expression is regulated in response to these cues, but a precise mechanism clearly exists. The efficiency of exploiting NRT2.1 expression levels to coordinate nutritional cues with both nitrate uptake and development would be extremely elegant. It also provides a model for our experimental results. In this model, an unknown sensor and signal transduction pathway would activate NRT2.1 expression in response to high sucrose/low nitrate conditions. (Sensing may involve the NRT2.1 protein itself, which is expressed at some basal level under all conditions examined.) Increased NRT2.1 gene expression would result in increased NRT2.1 protein accumulation. NRT2.1 protein would serve as a signal transducer to activate nitrate uptake and repress lateral root initiation. The activation of nitrate uptake may simply be a result of increased amount of the NRT2.1 protein, if this protein is actually a transporter. In contrast, both activation of nitrate uptake and repression of lateral root initiation may require a signal transduction pathway that is triggered by NRT2.1.
Transcriptional regulation of the NRT2.1 gene could explain why repression of lateral root initiation is seen only under certain conditions: (i) lateral root repression is specific to high sucrose/low nitrate conditions (Fig. 1), consistent with the expectation that these conditions induce transcription of the NRT2.1 gene (6-10, 12-14); and (ii) increases in exogenous nitrate levels relieve the repression of lateral root initiation (Figs. 1 and 4B), consistent with a reported decrease in NRT2.1 expression in response to high nitrate (6-10, 12-14). Regulation of NRT2.1 expression could also explain why the phenotype of the lin1(nrt2.1-4) mutant is condition-specific; WT plants may only differ phenotypically from a nrt2.1 mutant under conditions where NRT2.1 is expressed above a certain threshold level.
Developmental Plasticity and Nutrient Sensing in the Plant Root System. The number and location of lateral roots in the plant root system determine its depth, breadth, and shape. Decisions about root system architecture are made throughout the lifetime of the plant, allowing each plant to continually optimize its structure in accordance with growth conditions. External nitrate, sulfate, and phosphate all have been shown to have dramatic effects on root system architecture (3). For example, localized patches of nitrate lead to localized increases in lateral root elongation and effective capture of the nutrient in several plants studied (3). In another example, Arabidopsis root systems sensing phosphate limitation increase lateral root formation at the expense of primary root elongation, perhaps to maximize the acquisition of phosphate in shallow soil layers (3). It is more difficult to rationalize why plants would repress both primary root growth (4) and lateral root initiation in response to high sucrose/low nitrate. We can speculate that our assay conditions may simulate a stress situation, where it would be in the best interest of the plant to conserve energy and limit growth. Alternatively, our high sucrose/low nitrate assay conditions may simulate a situation that would normally occur only in a small portion of the root system, where a photosynthetically active plant is providing carbon to lateral roots that are not acquiring nitrate. In this case, it might be advantageous to limit growth and root proliferation of that portion of the root system and preferentially sustain other tissues.
The mechanisms for nutrient sensing and developmental responses remain poorly understood in plants. The idea that transporter or transporter-like proteins can modulate downstream events in response to nutrient cues has been substantiated in bacteria and yeast. NRT2.1-mediated repression of lateral root initiation now provides a paradigm for a similar strategy in plants. The ability to modify root system morphology in response to nutrients has been shown to confer a selective advantage in many plants (3). Therefore, it is likely that, as in yeast and bacteria, the nutrient transporters have diversified to take on additional roles in nutrient signaling. In the case of lateral root initiation, the signaling pathway culminates in the regulation of specific cell divisions that give rise to lateral root founder cells in the pericycle. Understanding the mechanism by which the putative transporter NRT2.1 regulates pericycle cell divisions in response to nutritional cues would represent a significant step forward in understanding plant developmental plasticity.
Supplementary Material
Acknowledgments
This work was supported by National Science Foundation Grant 0131690 (to J.E.M.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: MFS, major facilitator superfamily; HATS, high-affinity transport system; MS, Murashige and Skoog.
References
- 1.Malamy, J. E. & Benfey, P. N. (1997) Development (Cambridge, U.K.) 124, 33-44. [DOI] [PubMed] [Google Scholar]
- 2.Casimiro, I., Beeckman, T., Graham, N., Bhalerao, R., Zhang, H., Casero, P., Sandberg, G. & Bennett, M. J. (2003) Trends Plant Sci. 8, 165-171. [DOI] [PubMed] [Google Scholar]
- 3.Malamy, J. E. (2005) Cell Environ. 28, 67-77. [DOI] [PubMed] [Google Scholar]
- 4.Malamy, J. E. & Ryan, K. S. (2001) Plant Physiol. 127, 899-909. [PMC free article] [PubMed] [Google Scholar]
- 5.Pao, S. S., Paulsen, I. T. & Saier, M. H., Jr. (1998) Microbiol. Mol. Biol Rev. 62, 1-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass, A. D. M., Britto, D. T., Kaiser, B. N., Kinghorn, J. R., Kronzucker, H. J., Kumar, A., Okamoto, M., Rawat, S., Siddiqi, M. Y., Unkles, S. E. & Vidmar, J. J. (2002) J. Exp. Bot. 53, 855-864. [DOI] [PubMed] [Google Scholar]
- 7.Forde, B. G. (2000) Biochim. Biophys. Acta 1465, 219-235. [DOI] [PubMed] [Google Scholar]
- 8.Galvan, A. & Fernandez, E. (2001) Cell Mol. Life Sci. 58, 225-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orsel, M., Krapp, A. & Daniel-Vedele, F. (2002) Plant Physiol. 129, 886-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orsel, M., Filleur, S., Fraisier, V. & Daniel-Vedele, F. (2002) J. Exp. Bot. 53, 825-833. [DOI] [PubMed] [Google Scholar]
- 11.Filleur, S., Dorbe, M. F., Cerezo, M., Orsel, M., Granier, F., Gojon, A. & Daniel-Vedele, F. (2001) FEBS Lett. 489, 220-224. [DOI] [PubMed] [Google Scholar]
- 12.Nazoa, P., Vidmar, J. J., Tranbarger, T.J., Mouline, K., Damiani, I., Tillard, P., Zhuo, D., Glass, A. D. & Touraine, B. (2003) Plant Mol. Biol. 52, 689-703. [DOI] [PubMed] [Google Scholar]
- 13.Zhuo, D., Okamoto, M., Vidmar, J. J. & Glass, A. D. (1999) Plant J. 17, 563-568. [DOI] [PubMed] [Google Scholar]
- 14.Lejay, L., Tillard, P., Lepetit, M., Olive, F., Filleur, S., Daniel-Vedele, F. & Gojon, A. (1999) Plant J. 18, 509-519. [DOI] [PubMed] [Google Scholar]
- 15.Orsel, M., Eulenburg, K., Krapp, A. & Daniel-Vedele, F. (2004) Planta 219, 714-721. [DOI] [PubMed] [Google Scholar]
- 16.Unkles, S. E., Rouch, D. A., Wang, Y., Siddiqi, M. Y., Glass, A. D. & Kinghorn, J. R. (2004) Proc. Natl. Acad. Sci. USA 101, 17549-17554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kinghorn, J. R., Sloan, J., Kana'n, G. J. M., DaSilva, E. R., Rouch, D. A. & Unkles, S. E. (2005) Genetics 169, 1369-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamaguchi, A., Someya, Y. & Sawai, T. (1992) J. Biol. Chem. 267, 19155-19162. [PubMed] [Google Scholar]
- 19.Jessen-Marshall, A. E., Paul, N. J. & Brooker, R. J. (1995) J. Biol. Chem. 270, 16251-16257. [DOI] [PubMed] [Google Scholar]
- 20.Rexach, J., Llamas, A. & Fernandez, E. (2002) Planta 215, 606-611. [DOI] [PubMed] [Google Scholar]
- 21.Chiu, C. C., Lin, C. S., Hsia, A. P., Su, R. C., Lin, H. L. & Tsay, Y. F. (2004) Plant Cell Physiol. 45, 1139-1148. [DOI] [PubMed] [Google Scholar]
- 22.Guo, F. Q., Wang, R., Chen, M. & Crawford, N. M. (2001) Plant Cell 13, 1761-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fosberg, H. & Ljungdahl, P. O. (2001) Curr. Genet. 40, 91-109. [DOI] [PubMed] [Google Scholar]
- 24.Lalonde, S., Boles, E., Hellman, H., Barker, L., Patrick, J. W., Frommer, W. B. & Ward, J. M. (1999) Plant Cell 11, 707-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stasyk, O. V., Stasyk, O. G., Komduur, J., Veenhuis, M., Cregg, J. M. & Sibirny, A. A. (2004) J. Biol. Chem. 279, 8116-8125. [DOI] [PubMed] [Google Scholar]
- 26.Schwoppe, C., Winkler, H. H. & Neuhaus, H. E. (2003) Eur. J. Biochem. 270, 1450-1457. [DOI] [PubMed] [Google Scholar]
- 27.Javelle, A., Andre, B., Marini, A.-M. & Chalot, M. (2003) Trends Microbiol. 11, 53-55. [DOI] [PubMed] [Google Scholar]
- 28.Tong, Y., Zhou, J. J., Li, Z. & Miller, A. J. (2005) Plant J. 41, 442-450. [DOI] [PubMed] [Google Scholar]
- 29.Barker, L., Kuhn, C., Weise, A., Schulz, A., Gebhardt, C., Hirner, B., Hellmann, H., Schulze, W., Ward, J. M. & Frommer, W. B. (2000) Plant Cell 12, 1153-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barth, I., Meyer, S. & Sauer, N. (2003) Plant Cell 15, 1375-1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coruzzi, G. M. & Zhou, L. (2001) Curr. Opin. Plant Biol. 4, 247-253. [DOI] [PubMed] [Google Scholar]
- 32.Martin, T., Oswald, O. & Graham, I. A. (2002) Plant Phys. 128, 472-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palechar, P. M., Kouranov, A., Lejay, L. V. & Coruzzi, G. M. (2004) Genome Biol. 5, R91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneper, L., Duvel, K. & Broach, J. R. (2004) Curr. Opin. Microbiol. 7, 624-630. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.