Abstract
The induction of antigen-specific tolerance is critical for the prevention of autoimmunity and maintenance of immune tolerance. In addition to their classical role as sentinels of the immune response-inducing T cell reactivity, dendritic cells (DCs) play an important role in maintaining peripheral tolerance through the induction/activation of regulatory T cells (Tr). The possibility to generate tolerogenic DCs opens new therapeutic perspectives in autoimmune/inflammatory diseases. Therefore, the characterization of the endogenous factors that contribute to the development of tolerogenic DCs is highly relevant. In this study, we report on the use of the known immunosuppressive neuropeptide, the vasoactive intestinal peptide, as a new approach to induce tolerogenic DCs with capacity to generate Tr cells, to restore tolerance in vivo, and to reduce the progression of rheumatoid arthritis and experimental autoimmune encephalomyelitis.
Keywords: autoimmunity, regulatory T cell, tolerance
The immune system is faced with the daunting job of protecting the host from an array of pathogens, while maintaining tolerance to self-antigens (Ags). The induction of Ag-specific tolerance is essential to maintain immune homeostasis, to control autoreactive T cells, preventing the onset of autoimmune diseases, and to achieve tolerance toward transplants. Both thymic and peripheral mechanisms account for the ability of the immune system to induce tolerance. Attention has been focused recently on induction of active suppression by regulatory T cells (Tr) (1), and dendritic cells (DCs) have been shown to contribute to T cell tolerance (2, 3). The maturation/activation state of DCs might be the control point for the induction of peripheral tolerance, by promoting Tr differentiation. Thus, whereas mature DCs (mDCs) are potent Ag-presenting cells enhancing T cell immunity, immature DCs (iDCs) are involved in the induction of peripheral T cell tolerance under steady-state conditions (2-6). However, the clinical use of iDCs may not be suitable for the treatment of autoimmune diseases, because iDCs are likely to mature in inflammatory conditions (6), emphasizing the need to develop tolerogenic DCs with a strong potential to induce Tr. Immunosuppressive therapy, traditionally focused on lymphocytes, has been revolutionized by targeting the development and key functions of DC, and the generation of tolerogenic DCs in the laboratory has become the focus of new therapies (7).
Vasoactive intestinal peptide (VIP) is a neuropeptide released by both innervation and immune cells, particularly T helper (Th)2 cells, in response to Ag stimulation and under inflammatory/autoimmune conditions (8). VIP elicits a broad spectrum of biological functions, including immunomodulation, predominantly acting as a potent antiinflammatory factor and a suppressive agent for Th1 responses (9). Therefore, VIP has emerged as a promising therapeutic factor for the treatment of autoimmune/inflammatory diseases, including rheumatoid arthritis (RA), ulcerative colitis, uveoretinitis, and experimental autoimmune encephalomyelitis (EAE) (10-12). In this study, we investigated whether the presence of VIP during the early phases of DC differentiation induces the generation of regulatory DCs with the capacity to induce Tr and to prevent autoimmunity.
Materials and Methods
Cell Isolation and Cultures. Bone marrow (BM)-derived DCs (BM-DCs) were generated as described in ref. 13. Briefly, BM cells (2 × 106) obtained from BALB/c (H-2d), C57BL/6 (H-2b), or DBA/1 (H-2q) mice were incubated in complete medium (RPMI medium 1640 supplemented with 100 units/ml penicillin/strectomycin, 2 mM l-glutamine, 50 μM 2-mercaptoethanol, and 10% heat-inactivated FCS) containing 20 ng/ml granulocyte macrophage colony-stimulating factor in the presence or absence of VIP (10-8 M). At day 6, nonadherent cells were collected (routinely containing 80-90% CD11c+ cells) and stimulated for 48 h with LPS (1 μg/ml) to induce activation/maturation. In some experiments, DCs were pulsed with ovoalbumin (OVA), collagen II (CII), or myelin oligodendrocyte glycoprotein (MOG) (20 μg/ml) for 12 h. Allogeneic naïve CD4 T cells were purified from C57BL/6 mice by positive immunomagnetic selection (MACS, Miltenyi Biotec, Auburn, CA).
Flow Cytometry. Cells were incubated with various peridinin-chlorophyll-protein complex (PerCP)-, FITC- and phycoerythrin (PE)-labeled mAbs (BD Pharmingen), diluted at optimal concentration for immunostaining, fixed in 1% paraformaldehyde, and analyzed on a FACSCalibur flow cytometer (Becton Dickinson). We used isotype-matched Abs as controls and IgG block (Sigma) to avoid the nonspecific binding to Fc-receptors.
Cytokine Assays. Cytokine contents in the culture supernatants were determined by specific sandwich ELISAs by using capture/biotinylated detection Abs from BD Pharmingen. For intracellular analysis of cytokines in restimulated CD4 T cells, 106 cells per ml were collected and stimulated with phorbol 12-myristate 13-acetate (1 ng/ml) plus ionomycin (20 ng/ml) for 8 h in the presence of monensin. Cells were stained with PerCP-anti-CD4 mAbs for 30 min at 4°C, washed, fixed/saponin-permeabilized with Cytofix/Cytoperm, stained with 0.5 μg per sample FITC- and PE-conjugated anticytokine-specific mAbs, and analyzed by flow cytometry. To distinguish between DC and T cell sources, intracellular cytokine analysis was done exclusively in the PerCP-labeled CD4 T cell population.
mRNA Analysis. Total RNA was isolated from CD4 T cells, and real-time PCR was used to determine Foxp3 and neuropilin mRNA expression, as described in refs. 14 and 15.
Mixed Leukocyte Reaction and Analysis of Tr Cell Function. Naïve CD4 T cells (2 × 105) were cultured with allogeneic DCcontrol or DCVIP at various T:DC ratios in the presence of IL-2 (100 units/ml) for 3 d. Cell proliferation was evaluated by using a cell-proliferation assay (BrdUrd) from Roche Diagnostics (Mannheim, Germany), and intracellular cytokine content was determined as described above. In some experiments, DCs (105) were cultured with purified allogeneic CD4 T cells (5 × 105). One week later, CD4 T cells were recovered by immuodepletion of CD11c+ DCs and cultured in different numbers with syngeneic CD4 T cells (5 × 105) in the presence of allogeneic mDC (105), and the proliferative response was determined. Some cultures were performed in the presence of blocking anti-IL-10 (10 μg/ml) and/or anti-TGFβ1 (40 μg/ml) mAbs. To determine the cell-contact-dependence of the regulatory response, we placed responder CD4 T cells (5 × 105) with LPS-matured DC (105) in the bottom well of a Transwell system (Millipore) and syngeneic TrVIP (2 × 105) with allogeneic mDC (105) in the upper Transwell chamber. After 72 h, we measured the proliferative response of the bystander reactive CD4 T cells in the bottom well. To generate CII- and MOG-specific Tr cells, DBA1/J and C57BL/6 DCs (105) pulsed with CII or MOG, respectively, were cultured with syngeneic CD4 T cells (5 × 105) for 1week in the presence of CII or MOG (20 μg/ml).
Immunization Model. BALB/c mice were injected s.c. with different numbers (from 50 to 5 × 105) of cells of methylated BSA (mBSA)- pulsed DCcontrol or DCVIP, followed a week later by s.c. immunization with the Ags mBSA or OVA (60 μg) in complete Freund's adjuvant. Five days after Ag immunization, serum Ag-specific Ab, draining lymph nodes (DLN) T cell proliferative responses, and delayed type hypersensitivity (DTH) responses were measured. For the DTH responses, mice were injected i.d. with Ag (5 μg) or saline into the ears, and ear swelling was measured 24 h later by using a caliper. Ag-specific T cell proliferative responses were measured after ex vivo stimulation of DLN cells (4 × 105) with 10 μM Ag. Levels of mBSA-specific IgG in serum were determined by ELISA, as described in ref. 16.
Model for RA and EAE. RA was induced in DBA1/J mice by s.c. injection of CII, as described in ref. 10. Chronic EAE was induced in C57BL/6 mice by s.c. immunization with MOG35-55, as described in ref. 17. Mice with established arthritis (with a clinical score of 2) were injected i.v. with different numbers of syngeneic CII-pulsed DCcontrol or DCVIP or with CII-specific Trcontrol or TrVIP. Mice with established EAE (with a clinical score of 1) were injected i.v. with different numbers of syngeneic MOG35-55-pulsed DCcontrol or DCVIP or with MOG35-55-specific Trcontrol or TrVIP. The clinical score was determined daily, based on joint inflammation for RA and tail/leg paralysis for EAE, as described in ref. 17. DLN cells were isolated at the peak of the diseases, stimulated with CII or MOG35-55 (20 μg/ml), and assayed for proliferation and cytokine production, as described above. The content of serum anti-CII or anti-MOG35-55 IgG antibodies was determined by ELISA, as described in refs. 10 and 18. To assess Ag-specificity, arthritic mice were injected with unpulsed, OVA-pulsed, or CII- or MOG35-55- pulsed DCcontrol or DCVIP and immunized s.c. with OVA, CII, or MOG35-55 (150 μg of Ag in complete Freund's adjuvant) one week later. After 5 d, mice received 5 μg of Ag i.d. in the ear pinna, and the DTH response was determined, as described above. In some experiments, collagen-induced arthritis (CIA) and EAE mice received i.v. injections of neutralizing anti-IL-10 polyclonal Ab, neutralizing anti-TGFβ mAb, or preimmune rat IgG used as control Ig (500 μg of Ab per mouse) on alternate days up to 8 d after onset of disease.
Results and Discussion
The induction of Ag-specific tolerance is critical for the prevention of autoimmunity and maintenance of immune tolerance. In addition to their classical role as sentinels of the immune response inducing T cell reactivity, increasing evidence now indicates that DCs can induce specific T cell tolerance. Although underlying mechanisms are not fully elucidated, the capacity to induce Tr cells is an important property of tolerogenic/regulatory DCs. The generation of “designer” DCs with tolerogenic properties in the laboratory by using specific cytokines or immunologic and pharmacologic reagents is a desirable goal and represents the subject of intensive investigations. Because of its immunosuppressive action, VIP is a candidate for the induction of regulatory DCs with capacity to generate Tr. In a previous study, we showed that VIP treatment of activated DCs reduces their capacity to activate allogeneic and syngeneic T cells, an effect associated with the prevention of CD80/CD86 up-regulation (19). VIP treatment of iDC in the absence of activation resulted in DCs with increased capacity to induce Th2 responses (19). However, other immunomodulatory factors with capacity to induce tolerogenic DCs have been found to be effective when administered during the differentiation of DCs (6, 7). Therefore, we determined whether exposure to VIP during DC differentiation results in DC phenotypic and functional changes.
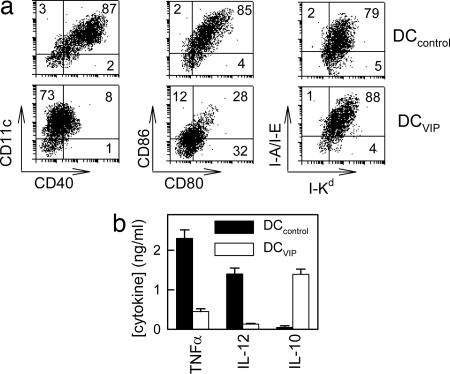
BM-DC Differentiated with VIP Induce Regulatory Tr1-Like Cells and Tolerance in Vivo. We first compared murine BM-derived DCs generated in the presence or absence of VIP in terms of surface markers and cytokine production. As previously described, BM cells cultured with granulocyte macrophage colony-stimulating factor for 6 d differentiate into iDCs (data not shown). Upon LPS stimulation, iDCs mature to DCs expressing high levels of DC markers (CD11c), MHC molecules (class I and class II), and costimulatory molecules (CD40, CD80, and CD86) (Fig. 1a, DCcontrol). However, DCs generated in the presence of VIP (DCVIP) were resistant to the LPS-induced up-regulation of the costimulatory molecules (CD40, CD80, and CD86) (Fig. 1a). Upon toll-like receptor activation, iDCs mature into cells capable of producing high levels of inflammatory cytokines. In contrast to DCcontrol, which produce TNF and IL-12, and low levels of IL-10, DCVIP produce very low levels of proinflammatory cytokines (TNF and IL-12) but secrete significant levels of the antiinflammatory cytokine IL-10 (Fig. 1b). Taken together, these results indicate that the DCs generated in the presence of VIP are resistant to LPS-induced up-regulation of costimulatory molecules and produce IL-10. These characteristics are quite similar to those reported for tolerogenic DCs generated with other immunomodulatory factors, such as IL-10 or the activated form of vitamin D 1,25(OH)2D3 (2-4, 20-24).
Fig. 1.
VIP induces a stable “semimature” phenotype in BM-DCs. DCs were generated from mouse BM cells in the absence (DCcontrol) or presence (DCVIP) of VIP and activated with LPS to induce DC maturation. (a)DCcontrol and DCVIP were double-labeled for different markers and analyzed by flow cytometry. Numbers represent the percentage of positive cells (n = 4). (b) Cytokine content in the DC supernatants was determined by ELISA (n = 4).
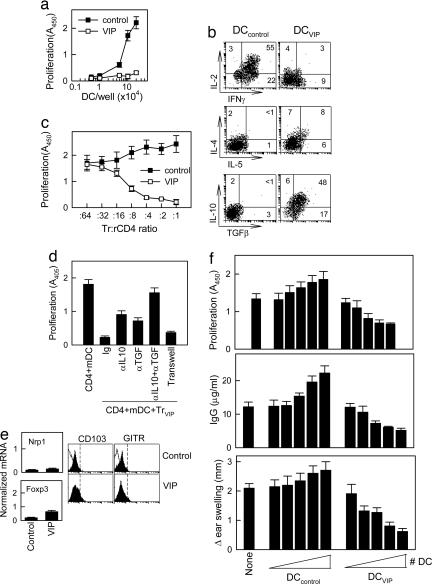
Tolerogenic DCs are poor stimulators of T cell proliferation and cytokine production (20, 25-28). To examine the capacity of the DCVIP to stimulate T cells, we cocultured DCcontrol or DCVIP with alloreactive CD4 T cells. Priming with DCcontrol results in a strong proliferation of allogeneic CD4 T cells, whereas DCVIP induce only weak proliferation (Fig. 2a). In addition, CD4 T cells primed with DCVIP reexposed to fresh LPS-stimulated allogeneic DCs (mDC) did not proliferate (data not shown), indicating that DCVIP induces anergic T cells and/or Tr. Although Tr generated by exposure to regulatory/tolerogenic DCs do not proliferate in response to the Ag, they can release antiinflammatory cytokines, such as IL-10 and TGFβ. Therefore, we assessed the cytokine profile of T cells cocultured with DCVIP. In contrast to T cells exposed to DCcontrol, which show a predominant Th1 cytokine profile, with high levels of IFNγ and IL-2, CD4 T cells primed with allogeneic DCVIP exhibit a Tr1-like phenotype, characterized by IL-10 and TGFβ but not IL-2 and IFNγ production (Fig. 2b).
Fig. 2.
Murine DCs differentiated in the presence of VIP induce regulatory Tr1 cells and tolerance in vivo. DCs were generated from mouse BM cells in the absence (DCcontrol) or presence (DCVIP) of VIP and activated with LPS to induce DC maturation. (a)DCcontrol or DCVIP was added to allogeneic CD4 T cells (5 × 105), and the proliferative response was determined. DCcontrol or DCVIP without T cells did not proliferate. Each result is the mean ± SD of three experiments performed in duplicate. (b) Purified CD4 T cells were exposed to allogeneic DCcontrol or DCVIP and activated with phorbol 12-myristate 13-acetate plus ionomycin. Intracellular cytokines were determined in CD4-gated cells by flow cytometry. Numbers represent percentage of positive cells (n = 5). (c) Purified CD4 T cells were stimulated for 1 week with allogeneic DCcontrol or DCVIP. The resulting regulatory CD4 T cells (Tr) were incubated with syngeneic responder CD4 T cells (rCD4) in the presence of allogeneic mDCs, and the proliferative response was determined (n = 4). (d) Isolated CD4 T cells were cocultured with syngeneic TrVIP and allogeneic mDCs in the presence or absence of blocking anti-IL10 and/or anti-TGFβ. Additionally, CD4+mDCs were separated from TrVIP+mDC in a Transwell system. The proliferative response of responder CD4 T cells was determined (n = 4). (e) Sorted CD4 T cells generated with DCcontrol or DCVIP were analyzed for neuropilin 1 and Foxp3 mRNA expression by real-time RT-PCR and for surface CD103 and glucocorticoid-induced TNF receptor (GITR) expression by flow cytometry. Open histograms and dashed lines represent isotype controls. One representative experiment of two is shown. (f) Mice were injected s.c. with increasing numbers (from 50 to 5 × 105 cells) of Ag-pulsed DCcontrol or DCVIP 1 week before priming with Ag. Five days later, mice were tested for DLN Ag-specific T cell proliferation, serum antibody levels, and DTH responses. Mice injected with Ag alone (None) were used as controls. Results are the mean ± SD for each group (n = 4) tested separately and are representative of three experiments.
After TCR stimulation, Tr cells suppress the proliferation and IL-2 production of Ag-specific effector T cells. To determine whether T cells exposed to DCVIP become functional Tr, we restimulated CD4 T cells with allogeneic mDCs in the presence of syngeneic CD4 T cells previously exposed to allogeneic DCcontrol (Trcontrol) or DCVIP (TrVIP). TrVIP inhibit the proliferation of syngeneic responder CD4 cells in response to allogeneic mDCs in a dose-dependent manner, whereas Trcontrol are not suppressive (Fig. 2c). Similar results were obtained in respect to IL-2 production (data not shown). Therefore, the phenotype of TrVIP correlates with their regulatory T cell activity.
The observation that TrVIP produce high levels of the immunosuppressive cytokines IL-10 and TGFβ suggests that the inhibitory effect of TrVIP on responder CD4 T cell proliferation might be mediated through soluble factors produced. When TrVIP and responder CD4 T cells were separated in transwell experiments by a semipermeable membrane that allows the free exchange of soluble factors but excludes direct cell contact of responder CD4 T cells and TrVIP, the proliferation of effector CD4 cells was still inhibited, indicating that soluble factors mediate the inhibitory effect (Fig. 2d). In regular cocultures, the addition of anti-TGFβ,or anti-IL-10 Abs reversed inhibition modestly. However, the addition of both anti-IL-10 and anti-TGFβ Abs reverses the inhibitory effect almost completely (Fig. 2d).
Several populations of CD4 Tr have been described and characterized, including the naturally occurring thymic-born CD4+CD25+ Tr and the induced peripheral Tr, consisting of IL-10-producing Tr1 and TGFβ-secreting Th3/Tr2 (29). Regulatory DCs do not participate in the generation of naturally occurring CD4+CD25+ Tr; however, they play an important role in the differentiation of peripherally induced Tr1 and Th3/Tr2 Tr (30-32). Although the CD4+CD25+ population is slightly increased in TrVIP, the fact that TrVIP did not express significant levels of the CD4+CD25+ Tr markers Foxp3, neuropilin-1, glucocorticoid-induced TNF-receptor-family-related gene, and CD103 (Fig. 2e), argues against the possibility that DCVIP induce the generation of CD4+CD25+ Tr cells. There are no reports on the expression of neuropilin-1 in IL-10-induced Tr1 cells. However, in contrast to CD4+CD25+ Tr, and in agreement with our results, Tr1 cells generated by repetitive stimulation with IL-10-secreting regulatory DCs have been shown to express low levels of CD25 and Foxp3 (33).
Although the precise mechanisms remain unknown, several possibilities may account for the generation of Tr cells by DCVIP. The activation of naïve CD4 T lymphocytes requires several signals delivered by mDCs and mediated through Ag/MHCII-TCR, CD80/CD86-CD28, and CD40-CD40L interactions. Costimulatory molecules, especially CD40, appear to be key determinants of the decision between tolerance and immunity (34). The characteristic phenotype of DCVIP, i.e., high levels of MHC plus poor expression of costimulatory molecules, which will deliver stimulatory but not costimulatory signals, is in agreement with DCVIP's tolerance-inducing ability. In addition, the observation that DCVIP secrete IL-10 may be linked to the stability of DCVIP's tolerogenic-like phenotype (20, 35-37). Previously, VIP has been reported to inhibit NF-κBp65 nuclear translocation, DNA-binding, and transactivating activity in macrophages (9), and we have recently found that both NF-κBp65 nuclear translocation and IkB phosphorylation are inhibited in DCVIP (M.D., E.G.-R., and D.G., unpublished data). The connection among NF-κB transactivating activity, CD40 expression, and DC function (including TNF-α and IL-12 production) has been established in a number of recent studies. The association between tolerance, particularly tolerogenic DCs, and lack of CD40 expression or signaling has been demonstrated both in vivo and in vitro (28). Expression of CD40 depends on NF-κBp65 (38), and the inhibition of NF-κB in DCs leads to failure of CD40, CD80, and CD86 expression upon LPS-stimulation and to the generation of tolerogenic DCs (39). In addition, a recent study suggests that VIP treatment induced a decrease of toll-like receptors (TLR-2/4) expressions in DCs in a murine model of Crohn's disease by a mechanism that would involve a decrease of NF-κB activation (40). Therefore, we would like to propose that the mechanism by which VIP induces tolerogenic DCs involves the cAMP/PKA-mediated inhibition of IκB phosphorylation and NF-κBp65 nuclear translocation, leading to lack of CD40 expression, TLR-2/4 signaling, and inflammatory cytokine production.
Because DCVIP appear to have a predominantly negative effect on Th1 cells, we determined the effect of DCVIP in an in vivo model of DTH. Ag (methylated-BSA)-pulsed DCVIP and DCcontrol were administered i.v., followed a week later by s.c. antigenic immunization. We determined T cell proliferation in response to ex vivo restimulation, Ab production, and DTH after a secondary s.c. Ag administration (Fig. 2f). Mice that received DCcontrol developed DTH reactions higher than controls (no DCs), whereas those receiving DCVIP exhibited reduced DTH. In addition, DLN T cells from mice inoculated with DCcontrol proliferated at higher levels than controls (no DCs), and, again, inoculation of DCVIP resulted in a substantial reduction in T cell proliferation after ex vivo restimulation with the Ag. Similarly, mice inoculated with DCcontrol produced high levels of anti-mBSA Abs, whereas those inoculated with DCVIP had anti-mBSA Ab levels below control (no DCs) (Fig. 2f). These results indicate that DCVIP induce tolerance in vivo. The induction of tolerance is restricted to the Ag presented by the inoculated DC, because we did not observe reduction in DTH in mice injected with mBSA-pulsed DCVIP when we used an unrelated Ag (OVA) for immunization and ex vivo T cell restimulation (data not shown). These experiments suggest the possibility of using a VIP in vitro system to generate Ag-specific tolerogenic DCs, followed by in vivo administration of these cells to patients with autoimmune diseases.
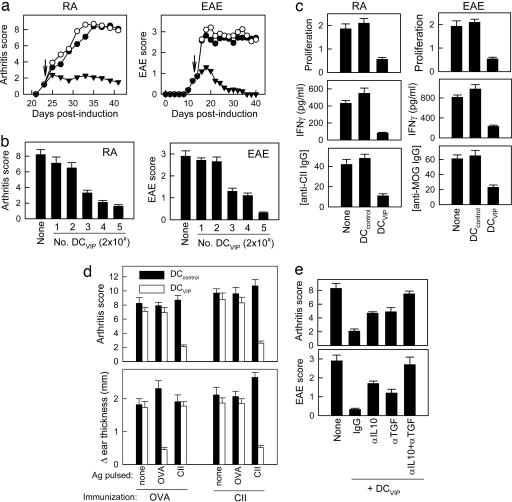
Therapeutic Effect of DCVIP in Autoimmunity. Several reports have recently proposed the possibility of using regulatory/tolerogenic DCs generated ex vivo as a therapeutic tool to prevent organ-specific autoimmune diseases (3, 7, 21). Interestingly, DCVIP retained their T cell regulatory capacity in vitro and in vivo under inflammatory conditions. This observation is particularly relevant for conditions in which ongoing Ag presentation is associated with chronic inflammation, including autoimmune diseases. Therefore, we tested the therapeutic effect of DCVIP in two murine models of RA and multiple sclerosis (MS). For RA, we used the CIA, an experimental disease model induced by immunization with CII, which shares a number of clinical, histologic, and immunological features with RA. For MS, we used the EAE model induced by MOG35-55 in C57BL/6 mice that mirror different clinical characteristics of MS. Inoculation of DCcontrol does not ameliorate arthritis (i.e., joint inflammation, cartilage destruction, and bone erosion) or EAE (i.e., tail and leg paralysis) (Fig. 3a). In contrast, administration of syngeneic DCVIP after the onset of disease abrogates arthritis and EAE progression in a dose-dependent manner (Fig. 3 a and b). The therapeutic effect of DCVIP was associated with the down-regulation of the autoimmune component of both diseases, because DLN T cells from DCVIP-treated mice showed weak proliferation and IFNγ production in response to the autoantigen (Fig. 3c). Furthermore, this inhibition of the Th1-type autoreactive response correlates with decreased levels of CII- and MOG-specific autoantibodies (Fig. 3c). The effect of DCVIP was Ag-specific. Unpulsed or OVA-pulsed DCVIP showed a weak therapeutic effect on arthritis, while reducing OVA-specific, but not CII-specific, DTH responses. In contrast, CII-pulsed DCVIP inhibit arthritis and DTH response toward CII but not toward OVA (Fig. 3d). Similar Ag-dependence was observed in the EAE model (data not shown). These results indicate that DCVIP generated ex vivo could prevent organ-specific autoimmune disorders in matched subjects, probably by inducing Ag-specific Tr cells, which suppress the ongoing autoreactive/inflammatory response. The participation of Tr cells in the therapeutic effect of DCVIP correlated with the fact that DCVIP induce in vitro the generation of IL-10/TGFβ-producing regulatory CD4 T cells (Fig. 2). Therefore, we further examined the role of Tr in the therapeutic effect of DCVIP on both CIA and EAE. In vivo blockade experiments showed that treatment with anti-IL-10 or anti-TGFβ Abs significantly decrease disease amelioration, and treatment with both Abs abrogates the beneficial effects exerted by DCVIP (Fig. 3e), suggesting the partial involvement of newly generated Tr cells in such action.
Fig. 3.
Therapeutic effect of DC differentiated with VIP in RA and EAE. (a) DBA1/J mice (H-2q) with established CIA or C57BL/6 mice (H-2b) with established EAE were treated (arrows) with syngeneic CII-pulsed DCs or MOG-pulsed DCs, respectively, generated in the absence (DCcontrol, ○) or presence (DCVIP, ▾ of VIP. Untreated CIA and EAE mice (none, •) were used as controls. Clinical score was monitored (n = 12). (b) CII- and MOG-pulsed DCVIP were injected at different doses. (c) CII-induced proliferation and IFNγ production by spleen T cells, and the levels of anti-CII IgG in sera were determined in CIA mice injected with DCcontrol or DCVIP (n = 5). (d) The effect of DCVIP is Ag-specific. Arthritic mice were treated with unpulsed, CII-pulsed, or OVA-pulsed DCcontrol or DCVIP after disease onset. One week later, mice were immunized s.c. with OVA or CII and challenged i.d. in the ear pinna with the respective Ag 5 d later. Clinical score and DTH responses were determined 24 h later (n = 5). (e) Untreated CIA or EAE mice or animals injected with DCVIP and treated with control Ig, anti-IL10, anti-TGFβ, or a combination of both mAbs (10 mice per group).
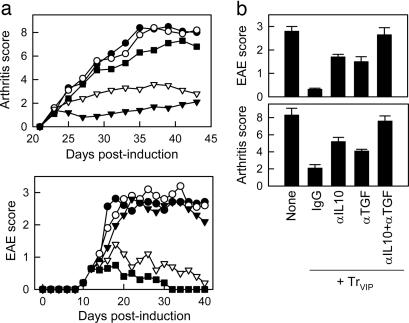
DCVIP-Induced Tr Ameliorate Autoimmunity. In certain circumstances, successful suppression of an autoimmune response might require high numbers of Tr, and the in vivo administration of DCVIP might not be sufficient for a complete and rapid suppression. Therefore, we decided to generate in vitro Ag-specific DCVIP-induced Tr cells and to subsequently determine their suppressive capacity in vivo in both the CIA and EAE models. We generated CII- or MOG-specific TrVIP through stimulations of CD4 T cells with syngeneic CII- or MOG-pulsed DCVIP. Trcontrol were generated in the same manner with DCcontrol. Treatment with TrVIP, but not Trcontrol, of mice with established CIA or EAE prevented disease progression in a dose-dependent manner (Fig. 4a). This effect was mainly mediated through TGFβ and IL-10, because in vivo administration of anti-IL10 and/or anti-TGFβ Abs abrogated the protective effect (Fig. 4b). In both models, the protective effect of TrVIP was Ag-specific, because OVA-specific TrVIP did not efficiently ameliorate arthritis or paralysis (data not shown). These results indicate that Ag-specific Tr1-like cells generated in vitro with DCVIP can efficiently modulate pathogenic immune responses in vivo.
Fig. 4.
DCVIP-induced Tr prevent autoimmunity. (a) Therapeutic effect on arthritis. CIA (H-2q) or EAE mice (H-2b) with established disease were treated with syngeneic CII- and MOG-specific Trcontrol (○) or with different doses of CII- or MOG-specific TrVIP (106 cells, ▾ ;5 × 105 cells, ▿;5 × 104 cells, ▪). Untreated mice (•) were used as CIA and EAE controls. Clinical score was determined (n=10). (b) Untreated CIA/EAE mice or CIA/EAE mice injected with TrVIP and treated with control Ig, anti-IL10, anti-TGFβ, or anti-IL10 plus anti-TGFβ Abs. Clinical score was measured at the peak of the disease (n = 10).
VIP has been previously found to ameliorate CIA and EAE, mainly by down-regulating the two components of both diseases, inflammation and Th1-mediated autoimmunity (ref. 10 and E.G.-R., A.F.-M., A.C., D.P., D.G., and M.D., unpublished results). The involvement of Tr cells in the therapeutic effect of VIP was demonstrated by the fact that CD4 T cells isolated by VIP-treated CIA or EAE mice showed an increased regulatory/suppressive activity against self-reactive Th1 cells. Phenotypic analysis of these Tr cells indicated that they consist of a mix of Foxp3+CD4+CD25+ and IL-10+Tr1-like cells (E.G.-R., A.C., A.F.-M., D.G., and M.D., unpublished results). In addition, by using a transgenic TCR murine model, we found that VIP induces the in vivo generation of Ag-specific tolerogenic IL-10-producing DCs with capacity to generate/activate Tr1-like cells (M.D., E.G.-R., and D.G., unpublished results). These findings validate the data obtained in this study, demonstrating that the pharmacological use of VIP in the treatment of autoimmunity is exerted partially through the induction of tolerogenic DCs and Tr1-like cells.
It has been proposed that tolerance induction by DCs requires maturation signals different from microbial or inflammatory stimuli. In steady-state conditions, VIP could represent one of the endogenous maturation signals driving the differentiation of tolerogenic DCs with a regulatory phenotype. VIP is secreted in the lymphoid microenvironment, mainly by Th2 cells, after Ag stimulation, and VIP levels are increased in immunopathologic conditions, such as autoimmunity and inflammation (8, 9). Therefore, DCVIP may represent a population of DCs that have matured to display a stable tolerogenic phenotype. Under steady-state conditions, DCVIP could be loaded with self- and commonly encountered Ags, and, after migration to the lymphoid organs, they could induce Tr1 differentiation and tolerance. Interestingly, in subjects with various autoimmune disorders, reduced serum VIP levels and increased VIP-specific autoantibodies have been reported (41).
Numerous strategies based on immunosuppressive agents, such as vitamin-D3, IL-10, TGFβ, glucocorticoids, and N-acetyl-l-cysteine, alone or in combinations, have been used to induce tolerogenic DCs (7). However, in the case of regulatory DCs induced with vitamin D analogs, it looks as if these regulatory DCs induce CD4+CD25+ Tr cells rather than Tr1-like cells (7). Our data demonstrate that VIP is very efficient at the induction of regulatory DCs, in comparison with current strategies, and we propose that the addition of VIP to cocktails of immunomodulatory agents will increase their effectiveness.
In conclusion, the possibility of generating tolerogenic DCVIP opens therapeutic perspectives for the treatment of autoimmune/inflammatory diseases and in allogeneic transplantation. In vitro pulsing of tolerogenic DCVIP with self-Ags, followed by in vivo injection, leads to the differentiation of Ag-specific Tr cells. Therefore, the inclusion of tolerogenic DCVIP in future therapeutic regimens may minimize the dependence on nonspecific immunosuppressive drugs used currently for autoimmune disorders.
Acknowledgments
This work was supported by Spanish Ministry of Health Grant PI04/0674 (to M.D.), National Institutes of Health Grant 2R01A047325 (to D.G. and M.D.), a grant from the Ramon Areces Foundation (to M.D.), and by fellowships from Junta de Andalucia (to M.D. and E.G.-R.) and the Spanish Ministry of Education and Science (to M.D.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Ag, antigen; BM, bone marrow; DC, dedritic cell; BM-DC, BM-derived DC; CIA, collagen-induced arthritis; CII, collagen II; DLN, draining lymph nodes; DTH, delayed type hypersensitivity; EAE, experimental autoimmune encephalomyelitis; iDC, immature DC; mDC, mature DC; MOG, myelin oligodendrocyte glycoprotein; OVA, ovalbumin; RA, rheumatoid arthritis; Tr, regulatory T cells; Th, T helper; VIP, vasoactive intestinal peptide
References
- 1.Jonuleit, H. & Schmitt, E. (2003) J. Immunol. 171, 6323-6327. [DOI] [PubMed] [Google Scholar]
- 2.Steinman, R. M., Hawinger, D. & Nussenzweig, M. C. (2003) Annu. Rev. Immunol. 21, 685-711. [DOI] [PubMed] [Google Scholar]
- 3.Rutella, S. & Lemoli, R. M. (2004) Immunol. Lett. 94, 11-26. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., Briere, F., Caux, C., Davoust, J., Lebecque, S., Liu, Y. J., Pulendran, B. & Palucka, K. (2000) Annu. Rev. Immunol. 18, 767-811. [DOI] [PubMed] [Google Scholar]
- 5.Hawiger, D., Inaba, K., Dorsett, Y., Guo, M., Mahnke, K., Rivera, M., Ravetch, J. V., Steinman, R. M. & Nussenzweig, M. C. (2001) J. Exp. Med. 194, 769-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roncarolo, M. C., Levings, M. K. & Traverari, C. (2001) J. Exp. Med. 193, F5-F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackstein, H. & Thomson, A. W. (2004) Nat. Rev. Immunol. 4, 24-34. [DOI] [PubMed] [Google Scholar]
- 8.Pozo, D. & Delgado, M. (2004) FASEB J. 18, 1325-1334. [DOI] [PubMed] [Google Scholar]
- 9.Delgado, M., Pozo, D. & Ganea, D. (2004) Pharmacol. Rev. 56, 249-290. [DOI] [PubMed] [Google Scholar]
- 10.Delgado, M., Abad, C., Martinez, C., Leceta, J. & Gomariz, R. P. (2001) Nat. Med. 7, 563-568. [DOI] [PubMed] [Google Scholar]
- 11.Abad, C., Martinez, C., Juarranz, M. G., Arranz, A., Leceta, J., Delgado, M. & Gomariz, R. P. (2003) Gastroenterology 124, 961-971. [DOI] [PubMed] [Google Scholar]
- 12.Keino, H., Kezuka, T., Takeuchi, M., Yamakawa, N., Hattori, T. & Usui, M. (2004) Arch. Ophthalmol. 122, 1179-1184. [DOI] [PubMed] [Google Scholar]
- 13.Inaba, K., Inaba, M., Romani, N., Aya, H., Deguchi, M., Ikehara, S., Muramatsu, S. & Steinman, R. M. (1992) J. Exp. Med. 176, 1693-1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavin, M. A., Clarke, S. R., Negrou, E., Gallegos, E. & Rudensky, A. (2002) Nat. Immunol. 3, 33-39. [DOI] [PubMed] [Google Scholar]
- 15.Bruder, D., Probst-Kepper, M., Westendorf, A. M., Geffers, R., Beissert, S., Loser, K., von Boehmer, H., Buer, J. & Hansen, W. (2004) Eur. J. Immunol. 34, 623-630. [DOI] [PubMed] [Google Scholar]
- 16.Moseman, E. A., Liang, X., Dawson, A. J., Panoskaltsis-Mortari, A., Krieg, A. M., Liu, Y. J., Blazar, B. R. & Chen, W. (2004) J. Immunol. 173, 4433-4442. [DOI] [PubMed] [Google Scholar]
- 17.Kohm, A. P., Carpentier, P. A., Anger, H. A. & Miller, S. D. (2002) J. Immunol. 169, 4712-4716. [DOI] [PubMed] [Google Scholar]
- 18.Subramanian, S., Matejuk, A., Zamora, A., Vanderbark, A. A. & Offner, H. (2003) J. Immunol. 170, 1548-1555. [DOI] [PubMed] [Google Scholar]
- 19.Delgado, M., Reduta, A., Sharma, V. & Ganea, D. (2004) J. Leukocyte Biol. 75, 1122-1130. [DOI] [PubMed] [Google Scholar]
- 20.Wakkach, A., Fournier, N., Brun, V., Breittmayer, J. P., Cottrez, F. & Groux, H. (2003) Immunity 18, 605-617. [DOI] [PubMed] [Google Scholar]
- 21.Morelli, A. E. & Thomson, A. W. (2003) Immunol. Rev. 196, 125-146. [DOI] [PubMed] [Google Scholar]
- 22.Piemonti, L., Monti, P., Sironi, M., Fraticelli, P., Leone, B. E., Dal Cin, E. Allvera, P. & Di Carlo, V. (2000) J. Immunol. 164, 4443-4451. [DOI] [PubMed] [Google Scholar]
- 23.Griffin, M. D., Lutz, W. H., Phan, V. A., Bachman, L. A., McKean, D. J. & Kumar, R. (2000) Biochem. Biophys. Res. Commun. 270, 701-708. [DOI] [PubMed] [Google Scholar]
- 24.Gregori, S., Casorati, M., Amuchastegui, S., Smiroldo, S., Davalli, A. M. & Adorini, L. (2001) J. Immunol. 167, 1945-1953. [DOI] [PubMed] [Google Scholar]
- 25.Jonuleit, H., Schmitt, E., Schuler, G., Knop, J. & Enk, A. H. (2000) J. Exp. Med. 192, 1213-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato, K., Yamashita, N. & Matsuyama, T. (2002) Cell. Immunol. 215, 186-194. [DOI] [PubMed] [Google Scholar]
- 27.Sato, K., Yamashita, N., Yamashita, N., Baba, M. & Matsuyama, T. (2003) Immunity 18, 367-379. [DOI] [PubMed] [Google Scholar]
- 28.Martin, E., O′Sullivan, B., Low, P. & Thomas, R. (2003) Immunity 18, 155-167. [DOI] [PubMed] [Google Scholar]
- 29.Fehervari, Z. & Sakaguchi, S. (2004) Curr. Opin. Immunol. 16, 203-208. [DOI] [PubMed] [Google Scholar]
- 30.Akbari, O., DeKruyff, R. H. & Umetsu, D. T. (2001) Nat. Immunol. 2, 725-731. [DOI] [PubMed] [Google Scholar]
- 31.Tang, Q., Boden, E. K., Henriksen, K. J., Bour-Jordan, H., Bi, M. & Bluestone, J. A. (2004) Eur. J. Immunol. 34, 2996-3005. [DOI] [PubMed] [Google Scholar]
- 32.Mills, K. H. & McGuirk, P. (2004) Semin. Immunol. 16, 107-117. [DOI] [PubMed] [Google Scholar]
- 33.Levings, M. K., Gregori, S., Tresoldi, E., Cazzaniga, S., Bonini, C. & Roncarolo, M. G. (2005) Blood 105, 1162-1169. [DOI] [PubMed] [Google Scholar]
- 34.Diehl, L., Den Boer, A. T., van der Voort, E. I., Melief, C. J., Offringa, R. & Toes, R. E. (2000) J. Mol. Med. 78, 363-371. [DOI] [PubMed] [Google Scholar]
- 35.Koppelman, B., Neefjes, J. J., de Vries, J. E. & de Waal Malefyt, T. (1997) Immunity 7, 861-871. [DOI] [PubMed] [Google Scholar]
- 36.Ding, L., Linsley, P. S., Huang, L. Y., Germain, R. N. & Shevach, E. M. (1993) J. Immunol. 151, 1224-1234. [PubMed] [Google Scholar]
- 37.Groux, H., O'Garra, A., Bigler, M., Rouleau, M., Antonenko, S., de Vries, J. E. & Roncarolo, M. G. (1997) Nature 389, 737-742. [DOI] [PubMed] [Google Scholar]
- 38.Ouaaz, F., Li, M. & Beg, A. A. (1999) J. Exp. Med. 189, 999-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang, J., Bernier, S. M., Ichim, T. E., Li, M., Xia, X., Zhou, D., Huang, X., Strejan, G. H., White, D. J., Zhong, R. & Min, W. P. (2003) J. Leukocyte Biol. 74, 438-447. [DOI] [PubMed] [Google Scholar]
- 40.Gomariz, R. P., Arranz, A., Abad, C., Torroba, M., Martinez, C., Rosignoli, F., Garcia-Gomez, M., Leceta, J. & Juarranz, Y. (2005) J. Leukocyte Biol. 78, 491-502. [DOI] [PubMed] [Google Scholar]
- 41.Bangale, Y., Karle, S., Planque, S., Zhou, Y. X., Taguchi, H., Nishiyama, Y., Li, L., Kalaga, R. & Paul, S. (2003) FASEB J. 17, 628-635. [DOI] [PubMed] [Google Scholar]